Abstract
Complement is a complex protein network of plasma, and an integral part of the innate immune system. Complement activation results in the rapid clearance of bacteria by immune cells, and direct bacterial killing via large pore-forming complexes. Here we review important recent discoveries in the complement field, focusing on interactions relevant for the defense against bacteria. Understanding the molecular interplay between complement and bacteria is of great importance for future therapies for infectious and inflammatory diseases. Antibodies that support complement-dependent bacterial killing are of interest for the development of alternative therapies to treat infections with antibiotic-resistant bacteria. Furthermore, a variety of novel therapeutic complement inhibitors have been developed to prevent unwanted complement activation in autoimmune inflammatory diseases. A better understanding of how such inhibitors may increase the risk of bacterial infections is essential if such therapies are to be successful.
Keywords: Complement, Bacteria, Infections, Antibody therapy, Antibiotic resistance, Inflammatory diseases, Eculizumab, Neisseria, Membrane attack complex
Complement in Innate Immune Defenses Against Bacteria
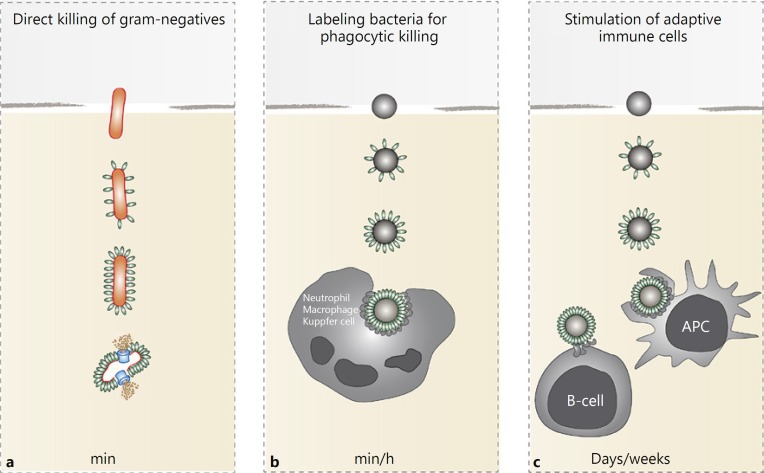
The human body is constantly exposed to bacteria that may be present in the environment, shed by other individuals, or living in symbiosis with the host. Normally, physical barriers (skin or epithelial cell layers) successfully protect the body from bacterial infections. However, when bacteria cross these barriers and invade the human body, the innate immune system provides the first line of response, capable of clearing bacteria within minutes to hours upon infection [1]. Complement is essential for this rapid elimination of invading bacteria. Complement proteins are present in the blood and body fluids as inactive precursors but are rapidly activated upon contact with bacterial cells. An activated complement cascade on the bacterial surface triggers a variety of responses that help to kill the bacterium. The most rapid response is the formation of ring-structured pores (the membrane attack complex, MAC) that directly kill Gram-negative bacteria within minutes (Fig. 1a) [2, 3, 4]. This potent bacteriolytic activity was recognized in 1895 by Nobel laureate Jules Bordet, who discovered complement as a system in serum that allows antibodies in vaccinated animals to kill bacteria without the help of immune cells [5]. Nowadays, we understand that complement is not only essential for the direct killing of Gram-negatives, but that it also triggers many other innate processes such as the production of chemoattractants, and the labeling of bacteria for phagocytosis and intracellular killing by professional phagocytes [2, 3, 4] (Fig. 1).
Fig. 1.
Antibacterial effector functions of complement. a Complement activation results in formation of the membrane attack complex (MAC or C5b-9; blue) that rapidly kills Gram-negative bacteria (orange) without the help of immune cells. Gram-positive bacteria are resistant to MAC. b Complement labels bacteria with C3-derived products (C3b and C3bi; green) that stimulate engulfment of bacteria by phagocytes. Release of complement peptide C5a is crucial for attraction of phagocytes to the site of infection. c Bacterial labeling with C3-derived products also enhances antigen presentation to B cells and thereby triggers the development of an adaptive immune response.
The main effector functions of complement are driven by the cleavage of 2 central complement proteins: C3 and C5 [2, 3]. The complement cascade is triggered by the recognition of bacteria via soluble pattern-recognition molecules or antibodies that bind both Gram-positive and Gram-negative bacteria (separated based on different cell wall composition) [4, 6]. All recognition pathways converge in the formation of convertase enzymes on the surface of the bacterium. First, C3 convertases cleave complement protein C3 to generate C3b that exposes a reactive thioester bond; this can covalently attach to hydroxyl groups of carbohydrates on the bacterial surface [7, 8]. When C3b molecules are covalently deposited onto the bacterial surface, these efficiently trigger and facilitate phagocytosis by immune cells. C3b (and its breakdown product, iC3b) are recognized by complement receptors (CR) on myeloid (CR1, CR3, and CR4) and Kupffer cells (CRIg), and enhance the engulfment of opsonized particles, leading to intracellular (microbial) killing [9, 10] (Fig. 1b). The labeling of bacterial cells with C3-derived activation products also stimulates an adaptive immune response (review [11]) by directing the transport of bacteria to lymphoid organs and by enhancing antigen presentation to adaptive immune cells (Fig. 1c) [11, 12, 13]. Another role of the deposited C3b molecules is to alter the specificity of the C3 convertase. At high local C3b densities, C3 convertases change into C5 convertases, meaning that they switch substrate from C3 to C5 [14].
Activation of C5 results in the release of peptide C5a, a strong chemoattractant that helps to recruit phagocytes towards the site of infection and induces an oxidative burst. Additionally, C5a-mediated stimulation of basophils and mast cells triggers the production of histamine and subsequent vasodilatation (review [15]). Concomitant generation of C5b triggers the assembly of the MAC (C5b-9) (Fig. 1a); this specifically kills Gram-negative bacteria. Gram-positive bacteria are protected from MAC-dependent killing, likely because their thick peptidoglycan outer layer prevents insertion of the MAC into the cell membrane [16].
Complement-dependent bacterial killing is one of the most rapid ways to kill an invading bacterium (Fig. 1). While both the labeling of bacteria with C3b and the MAC-dependent killing of Gram-negatives occur within minutes, phagocyte attraction and subsequent intracellular killing takes longer (we estimate 30 min to 1 h). The importance of complement in the clearance of bacterial infections is clearly illustrated by the recurrent infections in patients with genetic complement deficiencies [17]. Furthermore, the fact that pathogenic bacteria have evolved mechanisms to resist various steps in the complement cascade strongly supports the crucial role of complement in human defense against bacteria [18].
Molecular Insights into Complement Activation
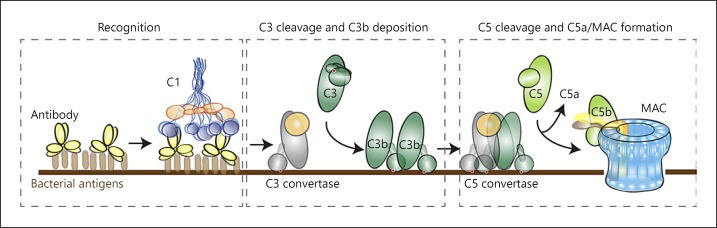
In the past years, many excellent studies have opened up our molecular view of complement activation mechanisms. As outlined in Figure 2, the complement cascade is a step-wise reaction that occurs in a defined order. The sequence of events is mostly determined by protein binding and enzymatic cleavage reactions [3, 19]. Thanks to advances in structural biology, the structures of many complement proteins and their activation products have now been revealed, and these explain how conformational changes in complement proteins are crucial to control the order of the complement cascade. The complement cascade can be divided into 3 main steps: first, different recognition molecules bind “foreign” elements on the microbial surface (“recognition”); second, recognition molecules drive the formation of convertase enzymes that cleave the major proteins C3 and C5 (“convertase formation”); third, newly generated C5b molecules initiate the formation of MAC (“MAC assembly”). Below, we explain in more detail how complement reactions occur and highlight some of the recent insights into complement activation mechanisms that, in our view, are important to understand the activity of complement molecules on bacteria.
Fig. 2.
The complement reaction. Recognition of bacterial cells occurs via soluble pattern-recognition molecules (lectin pathway) or antibodies (classical pathway). Antibody-mediated complement activation is depicted here. C1 binds to antibodies on the surface and triggers formation of a C3 convertase enzyme that converts C3 into C3b. At high C3b densities on the surface, the C3 convertase switches substrate, from C3 to C5, and is now called a C5 convertase. C5 convertases convert C5 into the chemoattractant C5a and C5b that trigger formation of the MAC (C5b-9).
Recognition
An important route to trigger complement activation on a bacterial surface is via antibodies. Since antibody molecules, or immunoglobulins, are produced by the cells of the adaptive immune system, this so-called “classical pathway” (CP) can be viewed as an integrated response of both innate and adaptive immunity. While antibodies bound to surface epitopes can directly bind Fc receptors on phagocytes, the engulfment of bacterial cells is strongly enhanced in the presence of C3-derived opsonization [20, 21].
Complement activation via antibodies depends on the large C1 complex, that consists of the recognition molecule C1q and the serine proteases C1r and C1s (ratio 1: 2: 2). C1q has 6 globular head residues that each can bind an antibody molecule [22]; C1r and C1s are proenzymes that are activated when the complex binds to a target surface. Activated C1s subsequently cleave C4 and C2 to deposit a C3 convertase enzyme (C4b2a) onto the target surface. Among the different immunoglobulin subclasses, IgM is the strongest complement activator. Likely, this is due to the structure of the IgM molecule, which comprises a multimer of 5 (pentamers) or 6 (hexamers) immunoglobulins [23] that allow the 6 globular C1q heads to bind multiple antibody subunits at the same time. Interestingly, recent cryo-electron tomography (cryo-ET) studies revealed that IgG molecules also need to form higher ordered structures (hexamers) to induce complement activation [24]. Structural analyses of C1q bound to antigen-coated liposomes revealed that the 6 antibody-binding headpieces of C1q could simultaneously bind to hexameric IgG, which is held together by noncovalent Fc-Fc interactions [24]. Since clustering of antibodies is most efficient on a target surface (where local antigen densities are high), these insights help to understand how surface-bound antibodies efficiently trigger complement activation while these interactions are prevented in the circulation.
Similarly, recognition of foreign microbes in the “lectin pathway” (LP) is a surface-specific process. Recognition molecules of the lectin pathway are collectins (collectin 11 and mannose-binding lectin, MBL) and ficolins (Ficolin-1, also named M-Ficolin or p35-related protein; Ficolin-2, also named L-Ficolin, p35, or Hucolin; and Ficolin-3, also named H-Ficolin, HAKA1, or hakata-antigen). Collectins are bundles of different polypeptide chains that each have a carbohydrate recognition domain to bind terminal monosaccharides exposing horizontal 3′- and 4′-OH groups (e.g., mannose, glucose, and N-acetyl-glucosamine). Although the individual recognition domains bind with low affinity to monosaccharides, the simultaneous binding of multiple head groups to repeated sugar/acetyl patterns generates a stable interaction [25, 26]. Since such repeated sugar/acetyl groups are uniquely present on bacteria and fungi, the collectins specifically recognize microbial surfaces. Similarly, ficolins use their fibrinogen-like domain to recognize repeated acetylated structures (e.g., GlcNAc), which are commonly found on bacterial and fungal cell walls.
Just like C1q associates with C1r and C1s in the classical pathway, collectins and ficolins are in complex with MBL-associated serine proteases (MASPs) that cleave C4 and C2 and form the C3 convertase C4b2a. Although it was previously thought that activated MASP-1 can cleave both C2 and C4, its ability to cleave C4 was later disproven [27]. Activation of MASP-2 turned out to be essential for the cleavage of C4 and thus for C4b2a C3-convertase formation. The exact activation route of the MBL-MASP complex is strongly debated; some believe that autoactivated MASP-1 induces MASP-2 activation via transactivation of the proenzymes [26]. Others speculate that MASP-2 can activate via cis-activation in the absence of MASP-1, leading to the cleavage of C2 and C4. The lower complement activating efficiency of MBL-MASP-2 complexes in the absence of MASP-1 still implies that MASP-1 is important for lectin pathway activation [28].
The “alternative pathway” (AP) plays 2 different roles in the complement reaction. First, the alternative pathway proteins, factor B and factor D, play a well-defined role in amplifying the number of C3b molecules deposited via the classical and lectin pathways. As outlined below, factor B and factor D can react with deposited C3b molecules to make an alternative pathway C3 convertase (C3bBb) that has functional properties similar to C4b2a. Ongoing C3 cleavage by C3bBb and the formation of new C3bBb convertases create an “amplification loop” that dramatically increases the density of C3b molecules on the bacterial surface. Second, some also consider the alternative pathway as a third “recognition” pathway, next to the classical and lectin routes. However, the exact molecular mechanism by which the alternative pathway discriminates foreign surfaces from self-surfaces is not as clear as that of the other pathways and is often disputed. One route by which the alternative pathway can be directly activated is the spontaneous hydrolysis of circulating C3 into “hydrolyzed C3” (C3H2O). Since C3H2O adopts a structure similar to deposited C3b, it can react with factor B and factor D to make a convertase. Although such low-level hydrolysis of C3 occurs in vitro, it remains uncertain (and difficult to prove) whether this also occurs in vivo. Furthermore, CP and LP independent complement activation may be triggered when other serum-derived (less specific) proteases convert C3 into C3b-like molecules that are deposited on the bacterial surface [29].
Lastly, it has been proposed that alternative pathway activation could be triggered via the molecule properdin. While multimeric properdin functions as a stabilizer of inherently labile C3bBb convertase, some studies suggest that properdin may bind directly to apoptotic, necrotic, and microbial cells. Properdin binding to the proteoglycans on apoptotic T cells and the DNA of late apoptotic and necrotic cells has been claimed to stimulate C3 convertase formation in a serum environment, in the absence of CP or LP activation [30, 31]. These results should be interpreted carefully, given that the experiments were all done in a serum environment in the presence of intact C3. Others have shown that, in serum, properdin binding to multiple targets is fully dependent on C3 [32].
In a non-serum environment, binding of purified properdin to Neisseria gonorrhoeae and Chlamydophila pneumoniae was claimed to stimulate alternative pathway activation by recruiting C3b and C3H2O, which, upon addition of FB and FD, formed C3bBb convertases [33, 34]. Whereas properdin did not bind to wild-type Escherichia coli or Salmonella enterica serovar Typhimurium strains, it did bind to mutants lacking the O-antigens of their lipopolysaccharides (LPS). N. gonorrhoeae expresses lipooligosaccharides (LOS) on its surface (that also lacks O-antigens), suggesting that properdin binding sites on the bacterial surface are shielded in the presence of LPS. Indeed, shorter LPS has been correlated with higher properdin binding and faster AP activation [35]. However, obtaining clear mechanistic results with purified properdin is hampered by the fact that this protein is prone to aggregation [36]. The physiological, non-aggregated properdin form turned out not to bind to Neisseria, even when LOS hexose extensions (that could prevent binding of native properdin) were mutated [37]. Altogether, whereas it is generally accepted that properdin functions as a stabilizer of the C3bBb convertase, the role of properdin as an innate recognition molecule is strongly debated.
Convertases
Central to the complement cascade are the convertase enzymes that generate the main complement effectors via the cleavage of C3 and C5. In recent years, significant progress has been made in understanding the molecular activation mechanisms of alternative pathway C3 convertases, which consist of the noncatalytic subunit C3b that is reversibly bound to protease fragment Bb. First, (crystal) structures of C3 and activated C3b [38, 39] highlighted how C3 activation results in a large conformational change that translocates the thioester domain of C3b 85 Å away from its original position and allows it to covalently bind the target surfaces [38, 39]. Later on, it became clear how the C3b molecule reacts with factor B to form the proconvertase C3bB, which can then be cleaved by factor D to generate C3bBb [40]. The structure of C3bBb, stabilized by an immune evasion protein SCIN, suggests that C3b forms a dimer with its substrate C3, and, since Bb is bound to a flexible domain in C3b, it can swing towards the substrate and cleave the scissile bond in C3 to release C3a and generate more C3b [41]. The structures of C4b2a [42] revealed that the classical/lectin C3 convertase is very similar to the alternative pathway C3 convertase C3bBb.
Ongoing C3 cleavage by C4b2a and C3bBb increases the density of C3b molecules on the bacterial surface. The noncatalytic subunits of C3 convertases (C4b or C3b) are thought to associate with extra C3b molecules and form multimeric C4b-C3bn or C3b-C3bn complexes that have an increased affinity for C5 [14]. These complexes (C3bBbC3b and C4b2aC3b) are known as C5 convertases, and they cleave C5 into C5a and C5b [43]. The exact molecular arrangement of C5 convertases is unclear and complicated to study due to their surface-specific conformation.
The MAC
Following conversion of C5 by convertases, newly formed C5b associates with components C6, C7, and C8, and multiple copies of C9 to form the lytic MAC. Recent cryo-ET maps of the structures of MAC pores on liposomes revealed that the MAC indeed consists of single copies of C5b, C6, C7, and C8, and 18 C9 molecules [44, 45]. Together, these molecules form a heterogeneous pore with an inner diameter of 100 Å, an outer diameter of 25 nm, and a trans-membrane domain of < 10 nm high [44]. Although these structural insights increase our knowledge on the composition of the MAC, it remains unclear how this pore disrupts the complex cell envelope of Gram-negative bacteria in which the cytoplasmic membrane (inner membrane) is protected by a thin peptidoglycan layer and an additional outer membrane with LPS [6].
Complement Evasion Strategies by Pathogenic Organisms
The important role of complement in the clearance of invading microbes is shown by the fact that pathogenic bacteria have evolved mechanisms to resist complement attack [4, 46, 47, 48]. There are several ways by which bacterial pathogens block the complement system; these include capsule production [49, 50], modification of LPS [51, 52], recruitment of human complement regulators to the bacterial surface (C4BP, factor H, and FHL-1) and production of proteases that cleave complement components [46]. Furthermore, bacteria produce specific complement inhibitory molecules that block specific steps in the complement cascade; for example, bacteria can frustrate the activation of C1s [53, 54], block C3 and C5 convertases [20, 55], block C5 cleavage [56, 57], prevent C5aR activation [58], or inhibit MAC formation [59]. For more detailed information on these evasion mechanisms, we refer to an extensive review on this topic [46].
Exploiting the Action of Complement in Immune Therapies against Bacteria
Because complement activation can be specifically triggered via antibodies, the development of complement-enhancing antibodies represents an attractive strategy for antibacterial therapies. The accelerated emergence of antibiotic resistance underscores the need to examine nontraditional antibacterial treatment strategies that offer more specific eradication of a certain pathogen. Thanks to the success of antibody therapy in cancer treatment [60], there is now growing interest in the use of monoclonal antibodies for treating bacterial infections. Complement-enhancing antibodies could offer several advantages over the currently existing antibodies in clinical human studies that mainly function by neutralization of bacterial virulence factors. Since bacterial virulence factors are often associated with certain disease conditions (one bacterium can cause several different diseases), these neutralizing antibodies cannot be used to treat all infections by a certain pathogen. Complement-enhancing antibodies would not be condition-specific and could potentially be used as a global therapy. Furthermore, since complement is an enzymatic cascade with several amplification loops, it is expected that one complement-enhancing antibody will provide stronger protection than an antibody neutralizing a single bacterial virulence factor (since bacteria express hundreds of different virulence factors).
The main challenge is still to identify antibodies and bacterial antigens that drive potent complement activation, but the first successes have been made in the field of Klebsiella pneumoniae [61, 62] and N. gonorrhoeae [63]. With the advancement of new antibody-discovery technologies (immune receptor identification), we believe that this promises to be a rapidly growing field in the near future. Generation of complement-activating antibodies is also considered important for the protective action of multivalent Neisseria vaccines that trigger the formation of antibodies that kill bacteria via MAC or phagocytosis [64, 65]. A potent vaccine candidate in N. meningitidis is factor H-binding protein (fHBP), an outer membrane-associated lipoprotein that recruits factor H and thereby downregulates complement activation. Two fHBP-targeting vaccines were recently approved when vaccine-generated antibodies were demonstrated to kill Neisseria isolates in a serum bactericidal assay.
Therapeutic Complement Inhibitors in Inflammatory Diseases
Understanding the interplay between complement and bacteria is also relevant for the inflammatory disease therapeutic market. There is a large list of autoimmune diseases in which dysregulated complement activity causes damage to the body's own cells. In the past decade, many promising complement inhibitors have been developed; 2 of these (the C5 blocking antibody eculizumab and a C1-inhibitor) have been approved for clinical use, and others are currently being evaluated in clinical trials [66]. Since these inhibitors target essential elements of the host response to bacteria and other pathogens, the use of complement-inhibitory therapies has raised concerns about the increased risk of infections [29, 67]. Below, we discuss the associated risks and recommended preventive measures of eculizumab therapy.
Complement-Related Diseases
Excessive complement activation on host cells may be caused by four different mechanisms. First, the presence of autoantibodies may drive classical pathway activation on host cells (e.g., autoimmune hemolytic anemia, AIHA [68]; neuromyelitis optica [69]; and systemic lupus erythematosus, SLE [70]). Second, “change-of-function” mutations in specific complement proteins can make the system hyperactive. An example of this is atypical hemolytic-uremic syndrome (aHUS), which is often characterized by mutations in alternative pathway regulators (loss-of-function mutations) or components of the amplification loop such as C3 and factor B (gain-of-function mutations) [71]. Third, autoantibodies to specific complement components can trigger both loss of function (e.g., anti-factor H in aHUS) or gain of function (e.g., C3 nephritic factors) of their target. Finally, deficiencies in surface-bound complement regulators result in complement attack on human cells. This is evident in patients with paroxysmal nocturnal hemoglobinuria (PNH), where a deficiency for glycosylphosphatidylinositol-anchored regulators of convertases (CD55) and the MAC (CD59) renders erythrocytes susceptible to uncontrolled MAC-dependent hemolysis [72].
Eculizumab
In 2007, the FDA approved eculizumab (Soliris®, Alexion Pharmaceuticals, USA), a recombinant humanized monoclonal antibody targeting C5, for the treatment of patients with PNH [73]. Eculizumab forms a 1: 1 complex with C5, and sterically hinders convertases from binding and cleaving C5 into C5a and C5b [74]. Eculizumab successfully prevents intravascular hemolysis and thrombosis and has revolutionized disease management of PNH [75]. It has also been approved for the treatment of aHUS [76] and, more recently, myasthenia gravis [77]. Furthermore, the use of eculizumab in other clinical disorders is currently being explored [67]. Since patients receiving eculizumab are at risk for infections with Neisseria, the FDA has requested the implementation of a risk evaluation and mitigation strategy to minimize these infections.
Neisseria meningitidis (or meningococcus) is a Gram-negative bacterium that colonizes the nasopharynx in up to 35% of the population [78, 79]. While colonization is often harmless, N. meningitidis occasionally penetrates mucosal barriers to enter the bloodstream and cause life-threatening sepsis [80], and/or cross the blood-brain barrier to cause meningitis [81]. The incidence of meningococcal disease is higher for children < 5 years and teenagers. Meningococcal meningitis and sepsis cause devastating consequences if not treated immediately; death or permanent disability occurs in 20–50% of the patients within 24 h after the first recognizable symptoms. Because of the known risk of Neisseria infection in individuals with C5 or later-acting MAC component deficiencies, vaccination against Neisseria, together with the education of patients and other elements of a risk and mitigation program, has always been mandatory for subjects treated with eculizumab. Vaccination with meningococcal vaccines (MenACWY and MenB) before starting eculizumab therapy is regarded as the most important measure. In addition, it is recommended that patients receive antibiotic prophylaxis (penicillin V or ciprofloxacin) until 2 weeks after vaccination, and patients are monitored for early signs of meningococcal infections. Furthermore, for patients < 18 years (and certain risk groups) vaccination against Haemophilus influenzae and Streptococcus pneumoniae infections is recommended. The exact relevance of meningococcal vaccination is still under debate because it is still not certain whether antibodies can really support Neisseria killing in a patient treated with C5 inhibitors. Certainly, the “protective” antibody titer based on bactericidal killing assays with C5-sufficient serum will not reflect the activity of the antibody under eculizumab therapy that blocks MAC formation.
In theory, the only way antibodies could function under eculizumab therapy is via triggering Fc-receptor and/or C3b-dependent opsonization of Neisseria and subsequent phagocytic killing. However, it is often questioned whether phagocytes contribute to the killing of Neisseria in vivo. The fact that genetic MAC deficiencies lead predominantly to an increased risk of Neisseria infection has led many immunologists to believe that Neisseria is only cleared by the MAC. Rather, we think that phagocytes can contribute to the killing of Neisseria, but that the rapid progression of meningococcal disease (with > 50% fatality if untreated) makes the fast action of the MAC more important than the (relatively slower) killing by neutrophils. This idea is supported by the reported link between CD32 and CD16b polymorphisms and efficacy in clearing Neisseria in MAC-deficient individuals [82]. Furthermore, the finding that vaccination against Neisseria in MAC-deficient patients protects the majority against recurrent infections [83] suggests that boosting phagocytic killing via antibodies helps to clear this bacterium more rapidly. However, physicians should be careful and not fully rely on the action of the vaccine. Despite the current precautions, a significant increase in Neisseria infections can be seen in eculizumab-treated individuals [67, 84]. It is anticipated that the neutrophil response under eculizumab therapy is less effective since the patient is not able to form C5a, which is crucial to attract neutrophils [85].
Vaccine efficacy is further complicated by the fact that eculizumab and MAC-deficient patients can be infected with less invasive meningococcal strains that are not included in current vaccines [67, 86]. Also, in acute cases of PNH or aHUS, postponing eculizumab therapy to await the response to vaccination may be detrimental and outweigh the risks of developing a meningococcal infection. Whereas the FDA recommends concomitant use of prophylactic antibiotics until antibody titers may be protective, it is often advised to continue prophylactic antibiotics for the duration of the eculizumab treatment [87, 88, 89]. Assessment of the function of anti-Neisseria antibodies under C5 inhibitory therapies, e.g., by developing standardized laboratory assays to assess phagocyte dependent bacterial killing, while currently not feasible on a routine basis, is something that could be considered in the future and would add to confidence in vaccine effectiveness.
Finally, it is anticipated that complement inhibitory treatment might also predispose patients to bacteria other than Neisseria, especially if the use is extended to more immunocompromised patients. In general, there is a clear difference in a person's susceptibility to bacterial infections if we compare community settings to hospitalized patients. “True” bacterial pathogens like N. meningitidis and S. pneumoniae can cause serious diseases in nonhospital settings. However, in the hospital, patients are much more susceptible to infections by “opportunistic” microorganisms that are part of the patient's own microbiota or have colonized during hospitalization. Opportunistic bacteria more readily infect immunocompromised patients who undergo medical procedures than they would immune-competent individuals (e.g., via surgery, intravenous catheterization, or ventilation). An example is Staphylococcus aureus, well-known for causing outbreaks in health care centers, but rarely causing an epidemic in the healthy population. In infected patients, > 80% of disease is caused by endogenous carriage strains [90, 91]. Although it is often stated that C5 and the MAC are only crucial to protect against Neisseria, we believe that the overall immune status of the patient receiving complement inhibitory therapy will determine their susceptibility to additional infections. Indeed, recent case reports showed that eculizumab treatment also predisposes patients to bacteria other than Neisseria (Pseudomonas aeruginosa [92, 93], E. coli, and Enterococcus faecium [94]), thus underscoring the eminent role of C5 and MAC in defense against all invading bacteria.
Disclosure Statement
S.H.M.R. is coauthor on a patent entitled “Antibodies and methods of use thereof in treatment of bacteria” (WO17198731); R.A.H. holds shares in Novartis Pharma AG.
Acknowledgements
The work was funded by an ERC Starting grant (639209-ComBact) and the EMBO Young Investigator Program (to S.H.M.R). The authors thank Dr. Helen Leavis for critically reviewing the manuscript.
References
- 1.Medzhitov R, Janeway C. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 3.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berends ETM, Kuipers A, Ravesloot MM, Urbanus RT, Rooijakkers SHM. Bacteria under stress by complement and coagulation. FEMS Microbiol Rev. 2014;38:1146–1171. doi: 10.1111/1574-6976.12080. [DOI] [PubMed] [Google Scholar]
- 5.Schmalstieg FC, Goldman AS. Jules Bordet (1870–1961): a bridge between early and modern immunology. J Med Biogr. 2009;17:217–224. doi: 10.1258/jmb.2009.009061. [DOI] [PubMed] [Google Scholar]
- 6.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:1–17. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mortensen S, Kidmose RT, Petersen SV, Szilágyi Á, Prohászka Z, Andersen GR. Structural basis for the function of complement component C4 within the classical and lectin pathways of complement. J Immunol. 2015;194:5488–5496. doi: 10.4049/jimmunol.1500087. [DOI] [PubMed] [Google Scholar]
- 8.Law SKA, Dodds AW. The internal thioester and the covalent binding properties of the complement proteins C3 and C4. Protein Sci. 2008;6:263–274. doi: 10.1002/pro.5560060201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He JQ, Wiesmann C, van Lookeren Campagne M. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol Immunol. 2008;45:4041–4047. doi: 10.1016/j.molimm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll MC. Complement and humoral immunity. Vaccine. 2008;26((suppl 8)):I28–I33. doi: 10.1016/j.vaccine.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broadley SP, Plaumann A, Coletti R, Lehmann C, Wanisch A, Seidlmeier A, et al. Dual-track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host Microbe. 2016;20:36–48. doi: 10.1016/j.chom.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Verschoor A, Neuenhahn M, Navarini AA, Graef P, Plaumann A, Seidlmeier A, et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α + dendritic cells depends on glycoprotein GPIb and complement C3. Nat Immunol. 2011;12:1194–1201. doi: 10.1038/ni.2140. [DOI] [PubMed] [Google Scholar]
- 14.Rawal N, Pangburn MK. Structure/function of C5 convertases of complement. Int Immunopharmacol. 2001;1:415–422. doi: 10.1016/s1567-5769(00)00039-4. [DOI] [PubMed] [Google Scholar]
- 15.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement system part II: role in immunity. Front Immunol. 2015;6:1–26. doi: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berends ETM, Dekkers JF, Nijland R, Kuipers A, Soppe JA, van Strijp JAG, et al. Distinct localization of the complement C5b-9 complex on Gram-positive bacteria. Cell Microbiol. 2013;15:1955–1968. doi: 10.1111/cmi.12170. [DOI] [PubMed] [Google Scholar]
- 17.Bousfiha A, Jeddane L, Picard C, Ailal F, Bobby Gaspar H, Al-Herz W, et al. The 2017 IUIS Phenotypic Classification for Primary Immunodeficiencies. J Clin Immunol. 2018;38:129–143. doi: 10.1007/s10875-017-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potempa M, Potempa J. Protease dependent mechanisms of complement evasion by bacterial pathogens. 2013;393:873–888. doi: 10.1515/hsz-2012-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gros P, Milder FJ, Janssen BJC. Complement driven by conformational changes. Nat Rev Immunol. 2008;8:48–58. doi: 10.1038/nri2231. [DOI] [PubMed] [Google Scholar]
- 20.Rooijakkers SHM, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, et al. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6:920–927. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- 21.Laarman AJ, Ruyken M, Malone CL, van Strijp JAG, Horswill AR, Rooijakkers SHM. Staphylococcus aureus metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J Immunol. 2011;186:6445–6453. doi: 10.4049/jimmunol.1002948. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, de Jong RN, van den Bremer ETJ, Beurskens FJ, Labrijn AF, Ugurlar D, et al. Molecular basis of assembly and activation of complement component C1 in complex with immunoglobulin G1 and antigen. Mol Cell. 2016;63:135–145. doi: 10.1016/j.molcel.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Hughey CT, Brewer JW, Colosia AD, Rosse WF, Corley RB. Production of IgM hexamers by normal and autoimmune B cells: implications for the physiologic role of hexameric IgM. J Immunol. 1998;161:4091–4097. [PubMed] [Google Scholar]
- 24.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensenius H, Klein DCG, van Hecke M, Oosterkamp TH, Schmidt T, Jensenius JC. Mannan-binding lectin: structure, oligomerization, and flexibility studied by atomic force microscopy. J Mol Biol. 2009;391:246–259. doi: 10.1016/j.jmb.2009.05.083. [DOI] [PubMed] [Google Scholar]
- 26.Kjaer TR, Thiel S, Andersen GR. Toward a structure-based comprehension of the lectin pathway of complement. Mol Immunol. 2013;56:413–422. doi: 10.1016/j.molimm.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, Poulsen K, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 28.Vorup-Jensen T, Petersen S V., Hansen AG, Poulsen K, Schwaeble W, Sim RB, et al. Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J Immunol. 2000;165:2093–2100. doi: 10.4049/jimmunol.165.4.2093. [DOI] [PubMed] [Google Scholar]
- 29.Harrison RA. The properdin pathway: an “alternative activation pathway” or a “critical amplification loop” for C3 and C5 activation? Semin Immunopathol. 2018;40:15–35. doi: 10.1007/s00281-017-0661-x. [DOI] [PubMed] [Google Scholar]
- 30.Kemper C, Mitchell LM, Zhang L, Hourcade DE. The complement protein properdin binds apoptotic T cells and promotes complement activation and phagocytosis. Proc Natl Acad Sci. 2008;105:9023–9028. doi: 10.1073/pnas.0801015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W, Berger SP, Trouw LA, de Boer HC, Schlagwein N, Mutsaers C, et al. Properdin binds to late apoptotic and necrotic cells independently of C3b and regulates alternative pathway complement activation. J Immunol. 2008;180:7613–7621. doi: 10.4049/jimmunol.180.11.7613. [DOI] [PubMed] [Google Scholar]
- 32.Harboe M, Johnson C, Nymo S, Ekholt K, Schjalm C, Lindstad JK, et al. Properdin binding to complement activating surfaces depends on initial C3b deposition. Proc Natl Acad Sci USA. 2017;114:E534–E539. doi: 10.1073/pnas.1612385114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 34.Cortes C, Ferreira VP, Pangburn MK. Native properdin binds to Chlamydia pneumoniae and promotes complement activation. Infect Immun. 2011;79:724–731. doi: 10.1128/IAI.00980-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 36.Farries TC, Finch JT, Lachmann PJ, Harrison R. Resolution and analysis of “native” and “activated” properdin. Biochem J. 1987;243:507–517. doi: 10.1042/bj2430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal S, Ferreira VP, Cortes C, Pangburn MK, Peter A, Ram S. An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae. J Immunol. 2010;185:507–516. doi: 10.4049/jimmunol.0903598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssen BJC, Huizinga EG, Raaijmakers HCA, Roos A, Daha MR, Nilsson-Ekdahl K, et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437:505–511. doi: 10.1038/nature04005. [DOI] [PubMed] [Google Scholar]
- 39.Janssen BJC, Christodoulidou A, McCarthy A, Lambris JD, Gros P. Structure of C3b reveals conformational changes that underlie complement activity. Nature. 2006;444:213–216. doi: 10.1038/nature05172. [DOI] [PubMed] [Google Scholar]
- 40.Forneris F, Ricklin D, Wu J, Tzekou A, Wallace RS, Lambris JD, et al. Structures of C3b in complex with factors B and D give insight into complement convertase formation. Science. 2010;330:1816–1820. doi: 10.1126/science.1195821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooijakkers SHM, Wu J, Ruyken M, van Domselaar R, Planken KL, Tzekou A, et al. Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat Immunol. 2009;10:721–727. doi: 10.1038/ni.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortensen S, Jensen JK, Andersen GR. Solution structures of complement C2 and its C4 complexes propose pathway-specific mechanisms for control and activation of the complement proconvertases. J Biol Chem. 2016;291:16494–16507. doi: 10.1074/jbc.M116.722017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berends ETM, Gorham RD, Ruyken M, Soppe JA, Orhan H, Aerts PC, et al. Molecular insights into the surface-specific arrangement of complement C5 convertase enzymes. BMC Biol. 2015;13:93. doi: 10.1186/s12915-015-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serna M, Giles JL, Morgan BP, Bubeck D. Structural basis of complement membrane attack complex formation. Nat Commun. 2016;7:1–22. doi: 10.1038/ncomms10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharp TH, Koster AJ, Gros P. Heterogeneous MAC initiator and pore structures in a lipid bilayer by phase-plate cryo-electron tomography. Cell Rep. 2016;15:1–8. doi: 10.1016/j.celrep.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zipfel PF, Hallström T, Riesbeck K. Human complement control and complement evasion by pathogenic microbes - tipping the balance. Mol Immunol. 2013;56:152–160. doi: 10.1016/j.molimm.2013.05.222. [DOI] [PubMed] [Google Scholar]
- 48.Geisbrecht BV. Staphylococcal complement inhibitors: biological functions, recognition of complement components, and potential therapeutic implications. Adv Exp Med Biol. 2008;632:221–236. [PubMed] [Google Scholar]
- 49.Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immun. 2001;69:6796–6803. doi: 10.1128/IAI.69.11.6796-6803.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuura M. Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front Immunol. 2013;4:1–9. doi: 10.3389/fimmu.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray GL, Attridge SR, Morona R. Altering the length of the lipopolysaccharide O antigen has an impact on the interaction of Salmonella enterica serovar Typhimurium with macrophages and complement. J Bacteriol. 2006;188:2735–2739. doi: 10.1128/JB.188.7.2735-2739.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang M, Ko YP, Liang X, Ross CL, Liu Q, Murray BE, et al. Collagen-binding microbial surface components recognizing adhesive matrix molecule (MSCRAMM) of gram-positive bacteria inhibit complement activation via the classical pathway. J Biol Chem. 2013;288:20520–20531. doi: 10.1074/jbc.M113.454462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia BL, Zhi H, Wager B, Höök M, Skare JT. Borrelia burgdorferi bbk32 inhibits the classical pathway by blocking activation of the C1 complement complex. PLoS Pathog. 2016;12:1–28. doi: 10.1371/journal.ppat.1005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hammel M, Sfyroera G, Pyrpassopoulos S, Ricklin D, Ramyar KX, Pop M, et al. Characterization of Ehp, a secreted complement inhibitory protein from Staphylococcus aureus. J Biol Chem. 2007;282:30051–30061. doi: 10.1074/jbc.M704247200. [DOI] [PubMed] [Google Scholar]
- 56.Langley R, Wines B, Willoughby N, Basu I, Proft T, Fraser JD. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-FcRI binding and serum killing of bacteria. J Immunol. 2005;174:2926–2933. doi: 10.4049/jimmunol.174.5.2926. [DOI] [PubMed] [Google Scholar]
- 57.Bestebroer J, Aerts PC, Rooijakkers SHM, Pandey MK, Köhl J, Van Strijp JAG, et al. Functional basis for complement evasion by staphylococcal superantigen-like 7. Cell Microbiol. 2010;12:1506–1516. doi: 10.1111/j.1462-5822.2010.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Haas CJC, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJB, Heezius ECJM, et al. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial anti-inflammatory agent. J Exp Med. 2004;199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernie-King BA, Seilly DJ, Willers C, Würzner R, Davies A, Lachmann PJ. Streptococcal inhibitor of complement (SIC) inhibits the membrane attack complex by preventing uptake of C567 onto cell membranes. Immunology. 2001;103:390–398. doi: 10.1046/j.1365-2567.2001.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 61.Diago-Navarro E, Motley MP, Ruiz-Peréz G, Yu W, Austin J, Seco BMS, et al. Novel, broadly reactive anticapsular antibodies against carbapenem-resistant Klebsiella pneumoniae protect from infection. M Bio. 2018;9:1–20. doi: 10.1128/mBio.00091-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rollenske T, Szijarto V, Lukasiewicz J, Guachalla LM, Stojkovic K, Hartl K, et al. Cross-specificity of protective human antibodies against Klebsiella pneumoniae LPS O-antigen. Nat Immunol. 2018;19:1. doi: 10.1038/s41590-018-0106-2. [DOI] [PubMed] [Google Scholar]
- 63.Chakraborti S, Lewis LA, Cox AD, St. Michael F, Li J, Rice PA, et al. Phase-variable heptose i glycan extensions modulate efficacy of 2C7 vaccine antibody directed against Neisseria gonorrhoeae lipooligosaccharide. J Immunol. 2016;196:4576–4586. doi: 10.4049/jimmunol.1600374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNeil LK, Donald RGK, Gribenko A, French R, Lambert N, Harris SL, et al. Predicting the susceptibility of meningococcal serogroup B isolates to bactericidal antibodies elicited by bivalent rLP2086, a novel prophylactic vaccine. MBio. 2018;9:e00036–18. doi: 10.1128/mBio.00036-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dretler AW, Rouphael NG, Stephens DS. Progress toward the global control of Neisseria meningitidis: 21st century vaccines, current guidelines, and challenges for future vaccine development. Hum Vaccin Immunother. 2018:1–35. doi: 10.1080/21645515.2018.1451810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ricklin D, Mastellos DC, Reis ES, Lambris JD. The renaissance of complement therapeutics. Nat Rev Nephrol. 2017;14:26–47. doi: 10.1038/nrneph.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winthrop KL, Mariette X, Silva JT, Benamu E, Calabrese LH, Dumusc A, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules (II): agents targeting interleukins, immunoglobuli. Clin Microbiol Infect. 2018 doi: 10.1016/j.cmi.2018.02.002. DOI 10.1016/j.cmi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Wouters D, Zeerleder S. Complement inhibitors to treat IgM-mediated autoimmune hemolysis. Haematologica. 2015;100:1388–1395. doi: 10.3324/haematol.2015.128538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nytrova P, Potlukova E, Kemlink D, Woodhall M, Horakova D, Waters P, et al. Complement activation in patients with neuromyelitis optica. J Neuroimmunol. 2014;274:185–191. doi: 10.1016/j.jneuroim.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Giles BM, Boackle SA. Linking complement and anti-dsDNA antibodies in the pathogenesis of systemic lupus erythematosus. Immunol Res. 2013;55:10–21. doi: 10.1007/s12026-012-8345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kavanagh D, Goodship TH, Richards A. Atypical hemolytic uremic syndrome. Semin Nephrol. 2013;33:508–530. doi: 10.1016/j.semnephrol.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boccuni P, Del Vecchio L, Di Noto R, Rotoli B. Glycosyl phosphatidylinositol (GPI)-anchored molecules and the pathogenesis of paroxysmal nocturnal hemoglobinuria. Crit Rev Oncol Hematol. 2000;33:25–43. doi: 10.1016/s1040-8428(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 73.Hillmen P, Hall C, Marsh JCW, Elebute M, Bombara MP, Petro BE, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350:552–559. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 74.Schatz-Jakobsen JA, Zhang Y, Johnson K, Neill A, Sheridan D, Andersen GR. Structural basis for eculizumab-mediated inhibition of the complement terminal pathway. J Immunol. 2016;197:337–344. doi: 10.4049/jimmunol.1600280. [DOI] [PubMed] [Google Scholar]
- 75.Devalet B, Mullier F, Chatelain B, Dogné J-M, Chatelain C. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190–198. doi: 10.1111/ejh.12543. [DOI] [PubMed] [Google Scholar]
- 76.Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Food and Drug Administration (FDA) Advancing health through innovation 2017. New drug therapy approvals (2018) www.fda.gov.
- 78.Caugant DA, Maiden MCJ. Meningococcal carriage and disease-population biology and evolution. Vaccine. 2009;27:B64–B70. doi: 10.1016/j.vaccine.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caugant DA, Tzanakaki G, Kriz P. Lessons from meningococcal carriage studies. FEMS Microbiol Rev. 2007;31:52–63. doi: 10.1111/j.1574-6976.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 80.Coureuil M, Join-Lambert O, Lécuyer H, Bourdoulous S, Marullo S, Nassif X. Pathogenesis of meningococcemia. Cold Spring Harb Perspect Med. 2013:3. doi: 10.1101/cshperspect.a012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–1388. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 82.Fijen CAP, Bredius RGM, Kuijper EJ, Out TA, De Haas M, De Wit APM, et al. The role of Fcγ receptor polymorphisms and C3 in the immune defence against Neisseria meningitidis in complement-deficient individuals. Clin Exp Immunol. 2000;120:338–345. doi: 10.1046/j.1365-2249.2000.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fijen CAP, Kuijper EJ, Drogari-Apiranthitou M, Van Leeuwen Y, Daha MR, Dankert J. Protection against meningococcal serogroup ACYW disease in complement-deficient individuals vaccinated with the tetravalent meningococcal capsular polysaccharide vaccine. Clin Exp Immunol. 1998;114:362–369. doi: 10.1046/j.1365-2249.1998.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Struijk GH, Bouts AHM, Rijkers GT, Kuin EAC, Ten Berge IJM, Bemelman FJ. Meningococcal sepsis complicating eculizumab treatment despite prior vaccination. Am J Transplant. 2013;13:819–820. doi: 10.1111/ajt.12032. [DOI] [PubMed] [Google Scholar]
- 85.Granoff DM. Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease. Vaccine. 2009;27((suppl 2)):B117–B125. doi: 10.1016/j.vaccine.2009.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouts A, Monnens L, Davin JC, Struijk G, Spanjaard L. Insufficient protection by Neisseria meningitidis vaccination alone during eculizumab therapy. Pediatr Nephrol. 2011;26:1919–1920. doi: 10.1007/s00467-011-1929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Ani F, Chin-Yee I, Lazo-Langner A. Eculizumab in the management of paroxysmal nocturnal hemoglobinuria: patient selection and special considerations. Ther Clin Risk Manag. 2016;12:1161–1170. doi: 10.2147/TCRM.S96720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benamu E, Montoya JG. Infections associated with the use of eculizumab: recommendations for prevention and prophylaxis. Curr Opin Infect Dis. 2016;29:319–329. doi: 10.1097/QCO.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 89.Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Mitchell LD, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117:6786–6792. doi: 10.1182/blood-2011-02-333997. [DOI] [PubMed] [Google Scholar]
- 90.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh H, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 91.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 92.Kawakami T, Nakazawa H, Kurasawa Y, Sakai H, Nishina S, Senoo N, et al. Severe infection of Pseudomonas aeruginosa during eculizumab therapy for paroxysmal nocturnal hemoglobinuria. Intern Med. 2018;57:127–130. doi: 10.2169/internalmedicine.9151-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Webb BJ, Healy R, Child B, Majers J, Anand S, Gouw L. Recurrent infection with Pseudomonas aeruginosa during eculizumab therapy in an allogeneic hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2016;18:312–314. doi: 10.1111/tid.12517. [DOI] [PubMed] [Google Scholar]
- 94.Vellanki VS, Bargman JM. Aspergillus niger peritonitis in a peritoneal dialysis patient treated with eculizumab. Ren Fail. 2014;36:631–633. doi: 10.3109/0886022X.2014.882712. [DOI] [PubMed] [Google Scholar]