Abstract
RNA works as a genome and messenger in RNA viruses, and it sends messages in most of the creatures of the Earth, including viruses, bacteria, fungi, plants, and animals. The human innate immune system has evolved to detect single- and double-stranded RNA molecules from microbes by pattern recognition receptors and induce defense reactions against infections such as the production of type I interferons and inflammatory cytokines. To avoid cytokine toxicity causing chronic inflammation or autoimmunity by sensing self-RNA, the activation of RNA sensors is strictly regulated. All of the Toll-like receptors that recognize RNA are localized to endosomes/lysosomes, which require internalization of RNA for sensing through an endocytic pathway. RIG-I-like receptors sense RNA in cytosol. These receptors are expressed in a cell type-specific fashion, enabling sensing of RNA for a wide range of microbial invasions. At the same time, both endosomal and cytoplasmic receptors have strategies to respond only to RNA of pathogenic microorganisms or dying cells. RNA are potential vaccine adjuvants for immune enhancement against cancer and provide a benefit for vaccinations. Understanding the detailed molecular mechanisms of the RNA-sensing system will help us to broaden the clinical utility of RNA adjuvants for patients with incurable diseases.
Keywords: Endosome, Extracellular RNA, Innate immune signaling, RNA uptake, Toll-like receptor
Introduction
Three different types of pattern recognition receptor (PRR) families, i.e., Toll-like receptors (TLR), RIG-I-like receptors (RLR), and nucleotide-binding and oligomerization-like receptors, play key roles in host defense against microbial infection and tissue injury by recognizing the molecular patterns of microorganisms or damaged and dead cells. Nucleic acids are well-studied ligands for these PRR.
Both single-stranded (ss) and double-stranded (ds) RNA can be recognized by PRR in mammalian cells as pathogen-associated molecular patterns or damage-associated molecular patterns. PRR activation leads to the induction of innate immune responses, including the production of type I interferons and inflammatory cytokines. Because excessive production of cytokines can cause harmful chronic inflammation or auto immune responses, hosts have developed strategies to avoid sensing of self-RNA by RNA sensors.
TLR are type I transmembrane proteins. As all nucleic acid-sensing (NAS) TLR are localized to endosomal compartments, extracellular nucleic acids have to be endocytosed to gain access to NAS TLR. Host cells have an apparatus to selectively take up extracellular RNA into endosomes/lysosomes. In contrast, RLR are cytosolic sensors; RIG-I recognizes 5′-di-/triphosphate blunt-ended dsRNA and 5′-triphosphate ssRNA, which are unique patterns to pathogens, and MDA5 recognizes long dsRNA. Non-self-RNA can be segregated from self-RNA by its structures.
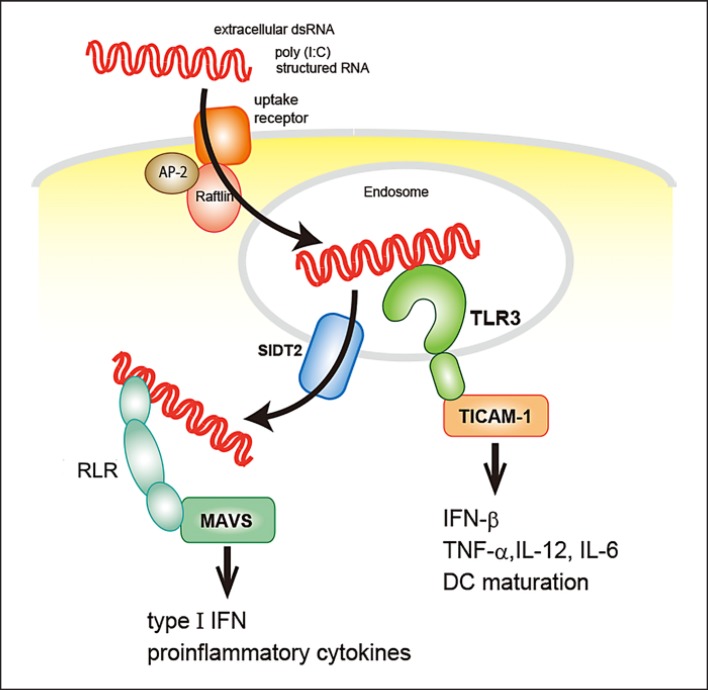
Uptake of extracellular RNA is the first step in recognition by endosomal TLR. To date, some cell type-specific uptake mechanisms have been reported. However, although extracellular RNA activate RLR-mediated signaling, the mechanisms via which RNA reach cytosolic sensors is unclear. One component that may be involved is SIDT2, which was recently reported to play a role in trafficking of dsRNA from the endosome to the cytosol after TLR3 activation [1] (Fig. 1). In this review, we summarize how RNA trigger innate immunity through RNA sensors and how subsequent immune responses are regulated.
Fig. 1.
Internalization of extracellular dsRNA. dsRNA in the extracellular milieu bind to uptake receptors on the cell surface and are endocytosed, mediated by Raftlin, in a clathrin-dependent manner. dsRNA activate TLR3 in endosomes and then SIDT2 urgse them to exit from the endosome to the cytosol. In the cytosol, RLR recognize dsRNA.
RNA Species Activate Innate Immunity
RNA-sensing receptors detect various RNA structures of microorganisms and self-RNA (Table 1). During viral infection, positive-stranded RNA viruses and dsDNA viruses produce dsRNA. These RNA byproducts, as well as genomic RNA, accumulate in the cytoplasm of infected cells and are released into the extracellular space when infected cells are dying.
Table 1.
RNA-sensing pattern recognition receptors
| RNA sensor |
Expression | Localization (signal initiation) | Ligands |
|---|---|---|---|
| TLR3 | cDC, macrophages, neural cells, fibroblasts, epithelial cells | endosome | dsRNA (>40 bp), poly(I:C), structured RNA |
| TLR7 | pDC, B cells | endosome | ssRNA, R848, CL097 |
| TLR8 | monocytes, macrophages, mDC | endosome | ssRNA, R848, CL095, CL097 |
| RIG-I | ubiquitous | cytoplasm | short dsRNA, 5′di-/triphosphate RNA, RNase L cleavage product, circular RNA |
| MDA5 | ubiquitous | cytoplasm | long dsRNA (>1 kb) |
TLR3 senses dsRNA and structured RNA, which contains a partial stem in secondary structures of ssRNA, originating from viruses or from stressed or necrotic cells [2, 3, 4, 5]. The synthetic dsRNA analog poly(I:C), which activates both TLR3 and MDA5, is widely used as an experimental TLR3 ligand.
Endocytosed and phagocytosed ssRNA are sensed by TLR7 and TLR8. These 2 receptors have a high homology and are both activated by GU-rich ssRNA and the synthetic chemical compounds imidazoquinolines [6, 7]. It has been reported that bacterial RNA stimulates TLR7/8, whereas fungal RNA stimulates TLR7 [8, 9, 10, 11, 12]. Viruses invade mammalian cells using endosomal or phagosomal pathways and release their genomic RNA into endosomal compartments, triggering TLR7/8 responses.
dsRNA are sensed by RLR when they are present in the cytoplasm. Especially RIG-I recognizes RNA with 5′-triphosphate or diphosphate ends. Because RNA polymerase III produces transcription products with 5′ triphosphate ends from dsDNA, RIG-I activation by dsDNA in the cytoplasm is thought to occur via an RNA polymerase III-dependent mechanism [13, 14]. However, this mechanism of viral nucleic acid sensing has yet to be confirmed. Furthermore, RNase L cleavage product from self-RNA and circular viral RNA have been reported to activate RIG-I, although it is unclear which motifs in these RNA species are recognized [15, 16, 17, 18].
RIG-I and MDA5 are activated by dsRNA with different lengths; RIG-I detects short dsRNA (minimum 18–19 bp), whereas MDA5 detects long dsRNA (> 1,000 bp) [19]. Similarly, in the laboratory, low- and high-molecular-weight poly(I:C) can be used for different purposes. Mammalian cells also produce short dsRNA of self-origin as miRNA duplexes. Although miRNA can bind to RIG-I, miRNA Dicer processing creates a 2-nucleotide overhang at the 3′ ends of miRNA duplexes [20] that prevents RIG-I from activating the signaling cascade [21]. Thus, the structure of RNA species allows RLR to discriminate between self- and non-self-RNA.
RNA Sensing in the Endosomal Compartment
TLR3, TLR7, and TLR8 can detect extracellular dsRNA or ssRNA in the endosome or lysosome and initiate immune responses. Therefore, endosomal localization must be strictly regulated to avoid autoimmune reactions caused by self-RNA sensing.
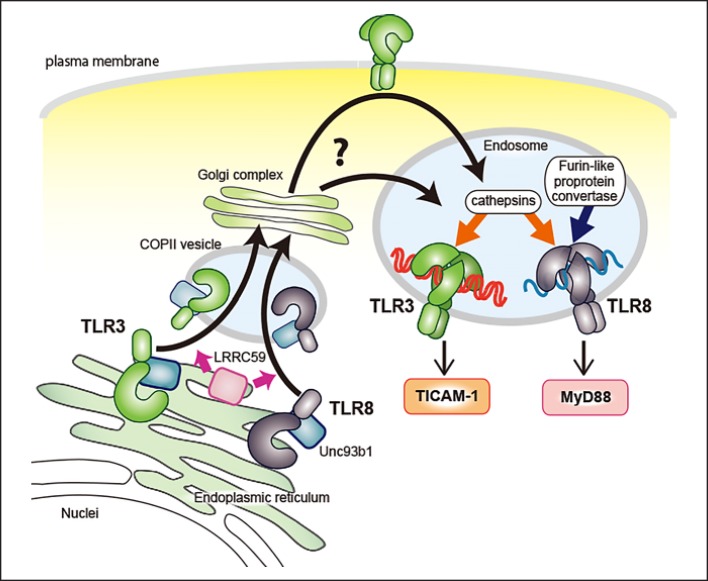
TLR are secreted from the endoplasmic reticulum (ER) and transported to the Golgi apparatus by COPII-coated vesicles in a similar manner to other transmembrane proteins. UNC93B1 is known to play a key role in the ER-to-Golgi trafficking of NAS TLR [22, 23]. UNC93B1 binds to the transmembrane domains of TLR in the ER and aides in their translocation from the ER to the Golgi apparatus. Additionally, LRRC59, a type II transmembrane ER protein, binds to UNC93B1 and promotes the exit of TLR3 and TLR8 from the ER [24] (Fig. 2).
Fig. 2.
Trafficking and maturation in the endosome of TLR3 and TLR8. UNC93B1 interacts with NAS TLR and regulates the COP II-mediated exit from the ER to the Golgi apparatus. Also, LRRC59 promotes the ER-to-Golgi delivery of TLR3 and TLR8. NAS TLR are translocated to the endosomal/lysosomal compartments and cleaved by proteases maturing to recognize ligands effectively.
Different regions of TLR3, TLR7, and TLR8 are responsible for their intracellular localization. The linker region between the transmembrane and Toll-interleukin-1 receptor (TIR) domains of TLR3, the transmembrane domain of TLR7, and both the transmembrane and TIR domains of TLR8 are required for endosomal targeting [25, 26, 27]. Regarding post-Golgi trafficking, TLR7 is sorted from the Golgi apparatus directly to the endosome through the noncanonical endosomal sorting complexes required for transport pathway [28, 29]. However, the mechanisms of post-Golgi trafficking of TLR3 and TLR8 are not known. Considering that TLR3 is expressed on the cell surface of human fibroblasts and some epithelial cells, trafficking via the plasma membrane is conceivable, at least in cells with surface TLR3 [25, 30]. The detailed mechanism via which NAS TLR are targeted to the endosome/lysosome remains to be determined.
NAS TLR are matured through proteolytic processing to bind ligands. This also contributes to the prevention of autoimmunity by limiting the activation site to the endolysosomal compartment. In mouse macrophages and dendritic cells (DC), TLR7 is processed by asparagine endopeptidase and pH-dependent cathepsins in a stepwise manner [31, 32, 33], whereas furin-like proprotein convertase cleaves human TLR7 and TLR8 [34, 35]. Murine TLR7 is thought to be digested at the asparagine endopeptidase-sensitive asparagine residue located between leucine-rich repeat (LRR) 14 and LRR15. However, this putative asparagine cleavage site is located in the C-terminal half of TLR8, suggesting that asparagine endopeptidase is not involved in TLR8 proteolysis. After processing, the N- and C-terminal halves of TLR7 and TLR8 reassociate, and both fragments contribute to ligand recognition. Although TLR3 is cleaved by cathepsins B and H at LRR12 [36], and the N- and C-terminal halves of TLR3 are associated [37], it is unclear whether this is necessary for ligand recognition since intact TLR3 binds dsRNA [38].
Acidic pH is important for the function of TLR3 [39]. TLR3 binds dsRNA more effectively in low-pH environments than at pH 7.0 [40]. The ligand-binding sites of TLR3 are located in the N- and C-terminals of the ectodomain. Upon the binding of dsRNA to both binding sites, TLR3 forms a dimer or oligomer that initiates signal transduction. For TLR3 dimerization, 40–50 bp of dsRNA are required [41]. However, short dsRNA between 21 and 30 bp can also trigger the formation of less stable TLR3 dimers [42]. The flexible recognition of RNA structures by TLR3 might allow a variety of RNA to be TLR3 ligands.
TLR3 and TLR7 are also expressed on the plasma membrane in some cell types including immune cells. Cell surface TLR3 are detected in mouse CD8-positive DC and marginal zone B cells as well as human fibroblasts, and TLR3-mediated signaling can be blocked by anti-TLR3 antibodies [30, 43], suggesting that cell surface TLR3 may have a role in the TLR3 response. However, TLR3 binds to dsRNA efficiently in acidic pH and delivers signals from endosomes, indicating that ligand recognition occurs mainly in endosomes. Also, cell surface expression of mouse TLR7 has been shown in splenic DCs and bone-marrow derived macrophages. Anti-TLR7 antibody inhibits TLR7 responses in vitro and in vivo [44]. TLR3 and mouse TLR7 need to be processed in the endosome, so trafficking between endosomes and the plasma membrane should regulate the cell surface expression of mature TLR3 and mouse TLR7. Cell type-specific surface expression of these TLR regardless of endosomal expression levels indicates the existence of unknown mechanisms of trafficking to control expression sites.
On the other hand, human TLR7 can mature before reaching the endosome since it is proteolytic processed by furin-like proprotein convertase which does not require an acidic pH for its activity. In THP-1 cells, the lack of biological activity of TLR7 forcedly expressed on the cell surface has been demonstrated using chimeric protein of the TLR7 ectodomain and the TLR4 transmembrane and cytosolic domains as well as full-length TLR7 fused to a fragment of Ist2, a yeast protein with a plasma membrane-targeting motif. Both of these proteins containing a TLR7 ectodomain failed to response to R837, a chemical ligand of TLR7, although they were properly processed to N- and C-terminal fragments [45]. Thus, the actual function of NAS TLR on the cell surface remains to be elucidated.
Internalization of Extracellular RNA into the Endosome and Cytosol
Extracellular dsRNA are internalized by cells via clathrin-dependent endocytosis [46]. Raftlin, a lipid raft protein that is known to positively regulate B-cell receptor signaling, mediates the endocytosis of poly(I:C) bound to a clathrin heavy chain [47]. However, raftlin is not involved in all endocytic events and the molecular mechanism of rafltin-mediated nucleic acid internalization is not yet known. It is thought that a specific receptor for dsRNA uptake is expressed on the cell surface and that the relationship between this receptor and raftlin plays a key role in this interaction.
To date, several plasma membrane proteins have been shown to participate in dsRNA uptake in different species and cell types. Although the class A scavenger receptor has been reported to play a role in poly(I:C) internalization [48], it is not involved in TLR3- or RLR-mediated innate immune signaling [49]. CD11b has also been reported to be involved in poly(I:C) uptake by mouse macrophages but it contributed only partially to this function [50]. It seems that multiple mechanisms participate in RNA internalization.
The receptor for advanced glycation end-products (RAGE) has been shown to be a cell surface uptake receptor for CpG oligodeoxynucleotides [51] and was recently shown to function as an RNA uptake receptor as well [52]. In HEK293 cells, RAGE is expressed on the cell surface and enhances the signaling of TLR3, TLR7, TLR8, and TLR13 upon stimulation with their respective ligands, which include dsRNA or ssRNA. Additionally, CD14 directly binds to and mediates internalization of dsRNA in bone marrow-derived macrophages [53]. However, the dsRNA uptake receptor in human myeloid DC, which do not express CD14, is yet to be determined. Since dsRNA must be internalized to reach endosomes, binding to these uptake receptors is the first step for RNA to access RNA sensors and trigger immune responses.
The discovery of a novel machinery that mediates endocytosis of dsRNA and its transport from endosomes to the cytosol is important for our understanding of the host reaction to extracellular RNA that have been internalized into cells. SIDT2, a mammalian ortholog of the Caenorhabditis elegans dsRNA transporter SID-1, localizes to endolysosomes and functions as a transporter of dsRNA, promoting escape from intracellular compartments [1]. Sixty minutes after its addition, poly(I:C) accumulates in the endosomal compartments of Sidt2 knockout bone marrow-derived DC, whereas poly(I:C) is diffuse in wild-type (WT) cells. Notably, there was no difference in the uptake of dsRNA between knockout and WT cells. Moreover, SIDT2 positively regulates MDA-5-mediated defense against encephalomyocarditis virus infection. Additionally, SIDT2 has been reported to be involved in uptake of ssRNA in a human cell line [54]. Further investigations are required to uncover the cell type specificity and selectivity of RNA structures in SIDT2-mediated RNA transport (Fig. 1).
RNA-Sensing Signaling Pathways
TLR3 induces TICAM-1 (TRIF)-dependent pathways, resulting in the production of type I interferon and proinflammatory cytokines via interferon regulatory factor (IRF) 3 and NF-κB activation [55]. TICAM-1 interacts with tumor necrosis factor receptor-associated factors (TRAF) and receptor-interacting protein (RIP) 1 [56]. The recruitment of TRAF3 leads to the activation of TANK-binding kinase (TBK) 1, followed by IRF3 phosphorylation and activation of NF-κB by TRAF6 and RIP1. Type I interferon induction by TLR3 is mediated by IRF3. This pathway also causes apoptosis via caspase 8. Additionally, the activation of DC and the induction of foreign antigen cross-presentation with dsRNA as an adjuvant are important components of the TLR3-TICAM-1 pathway in antitumor immunity [57]. The TLR3-TICAM-1-IRF3-IFN-β axis is indispensable for poly(I:C)-induced cross-presentation in DC. The TLR3-specific ligand ARNAX induces IL-12 and IFN-β production by professional antigen-presenting DC and cross-primes antigen-specific CD8+ T cells, leading to antitumor immunity without inflammatory cytokine production [58].
All TLR, with the exception of TLR3, trigger MyD88-dependent signal transduction. IL-1 receptor-associated kinases, TRAF6, and IRF7 are recruited to Myd88, resulting in NF-κB activation by TRAF6 and type I interferon induction by IRF7.
RLR are ubiquitously expressed in various types of cells and induce robust proinflammatory cytokines and type I interferons. RIG-I and MDA5 bind RNA ligands via their helicase domains, whereas the C-terminal and caspase activation and recruitment (CARD) domains bind to MAVS, an adaptor molecule localized to the mitochondrial-associated membrane, upon ligand recognition. Upon recognition of RNA by RIG-I, the C-terminal domain of RIG-I plays a crucial role in sensing 5′ di- and triphosphates of dsRNA, forming a pocket for binding of the ligand ends [59, 60]. Although MDA5 does bind short dsRNA, the binding of long dsRNA enables MDA5 to form a helical filament and initiate signal transduction via MAVS [61, 62]. Oligomerized MAVS forms a signaling complex with TRAF3, IκB kinase, and TBK1, leading to the activation of NF-κB and IRF3/7. RLR activation is controlled by several regulatory mechanisms [63, 64, 65] and aberrant activation of the MDA5-MAVS pathway can trigger autoimmune disorders [66].
Antiviral Immunity Mediated by RNA Sensors
TLR3 is expressed in immune and nonimmune cells, including conventional DC, macrophages, fibroblasts, and epithelial cells. Based on experiments with Tlr3 knockout mice, TLR3 appears to be involved in immunity against mouse cytomegalovirus [67], encephalomyocarditis virus [68], coxsackievirus [69], and poliovirus [70]. In the case of West Nile virus infection, TLR3 may both benefit and inhibit the virus [71, 72]. Additionally, Tlr3 knockout mice infected with influenza virus A reportedly had higher survival rates than WT mice [73]. It is thought that WT mice produce lethal levels of inflammatory mediators upon IVA infection. Since IVA is a negative-stranded RNA virus, it barely generates stable dsRNA during replication [74], indicating that structured RNA, but not dsRNA, activates TLR3.
In human clinical cases, TLR3-mediated signaling has been shown to be critical for protection against herpes simplex encephalitis in children. Gene mutations in Tlr3, Unc93b1, Ticam-1, Traf3, and Irf3 have been reported in patients with herpes simplex encephalitis [75, 76, 77, 78, 79, 80]. Similarly, TLR3-TICAM-1 pathway-dependent interferon production has been shown to be crucial for human host defense mechanisms against herpes simplex virus 1 infection.
TLR7 is predominantly expressed by plasmacytoid DC, whereas TLR8 is expressed by monocytes, macrophages, and myeloid DC. TLR7 produces type I interferons in plasmacytoid DC infected with ssRNA viruses such as human immunodeficiency virus (HIV)-1, Sendai virus, and flaviviruses [81, 82, 83, 84]. TLR8 is also activated in HIV-1 infection [82]. Unlike TLR7, TLR8 activation mainly results in the production of inflammatory cytokines due to the types of cells in which it is expressed. A recent study demonstrated that recognition of live bacteria by TLR8 induced human monocytes to produce large amounts of IL-12, which drives a follicular helper T-cell response in naive human CD4+ T cells [85].
It is known that RIG-I and MDA5 play different roles during viral infection due to their different ligand specificity. Genomic dsRNA from dsRNA viruses and replication byproducts of positive-stranded ssRNA viruses activate both RIG-I and MDA5. Nonsegmented negative-stranded ssRNA viruses produce copy-back defective interfering particles that can be sensed by RIG-I [86]. Additionally, the genomes of segmented negative-stranded ssRNA viruses provide panhandle structures that can be recognized by RIG-I but not by MDA5 [87].
RIG-I is activated by a variety of viruses, including vesicular stomatitis virus, Newcastle disease virus, IVA, IVB, hepatitis C virus, and the Ebola virus [88, 89, 90, 91, 92, 93, 94]. In contrast, MDA5 responds to infection with encephalomyocarditis virus, Theiler's virus, and Mengo virus [95, 96]. The Sendai virus, the West Nile virus and the dengue virus induce responses by both RIG-I and MDA5 [97, 98, 99]. RLR have been thought to play a role mainly in infected cells because they detect RNA in the cytosol. However, SIDT2-mediated RNA translocation from endosomes to the cytosol may explain how RLR in noninfected bystander cells induce immune responses. Likewise, exosomes may help RNA translocation in some situations including cancer and infections [100, 101].
Concluding Remarks
RNA sensors play different roles in diverse infections and tissue injuries. PRR activated by RNA are expressed on different cell types and they are localized to different areas of cells. This diversity allows the host to tailor its response to diverse infections with microbes that employ different infection routes and strategies to evade host defenses. Multiple levels of regulation of RNA-sensing systems maintains appropriate immune responses to ssRNA and dsRNA from pathogenic microbes and damaged cells and avoids excessive inflammatory responses. Indeed, inappropriate TLR activation is related to the pathogenesis and/or progression of autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis.
In clinical applications, RNA species are considered to be potential adjuvants for cancer immunotherapies and vaccinations. Therefore, it is important to understand the common and unique mechanisms that underlie the activation and regulation of RNA sensors. For example, SIDT2 may be a novel target for selective activation of TLR by RNA trapping in endosomes. New insights into the molecular mechanisms of RNA sensing will uncover novel strategies for the optimization of therapeutic RNA species, lead to clinical benefits, and further our understanding of innate immunity in mammals.
Disclosure Statement
The authors declare no conflict of interests.
Acknowledgement
We thank Drs. H. Oshiumi, H. Shime, H. Takaki, and K. Takashima for invaluable discussions. This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Agency for Medical Research and Development (16nk0101327h0002), the Akiyama Life Science Foundation (M.M), the Uehara Memorial Foundation (T.S.), and the Alexander von Humboldt Foundation (M.T).
References
- 1.Nguyen TA, Smith BR, Tate MD, Belz GT, Barrios MH, Elgass KD, et al. SIDT2 Transports Extracellular dsRNA into the Cytoplasm for Innate Immune Recognition. Immunity. 2017 Sep;47((3)):498–509. doi: 10.1016/j.immuni.2017.08.007. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tatematsu M, Nishikawa F, Seya T, Matsumoto M. Toll-like receptor 3 recognizes incomplete stem structures in single-stranded viral RNA. Nat Commun. 2013;4((1)):1833. doi: 10.1038/ncomms2857. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001 Oct;413((6857)):732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 4.Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004 Mar;279((13)):12542–50. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 5.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008 Oct;205((11)):2609–21. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 7.Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, et al. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2010 Nov;107((46)):19973–8. doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005 Aug;23((2)):165–75. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, et al. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009 Jun;10((6)):587–94. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- 10.Krüger A, Oldenburg M, Chebrolu C, Beisser D, Kolter J, Sigmund AM, et al. Human TLR8 senses UR/URR motifs in bacterial and mitochondrial RNA. EMBO Rep. 2015 Dec;16((12)):1656–63. doi: 10.15252/embr.201540861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eigenbrod T, Pelka K, Latz E, Kreikemeyer B, Dalpke AH. TLR8 Senses Bacterial RNA in Human Monocytes and Plays a Nonredundant Role for Recognition of Streptococcus pyogenes. J Immunol. 2015 Aug;195((3)):1092–9. doi: 10.4049/jimmunol.1403173. [DOI] [PubMed] [Google Scholar]
- 12.Biondo C, Malara A, Costa A, Signorino G, Cardile F, Midiri A, et al. Recognition of fungal RNA by TLR7 has a nonredundant role in host defense against experimental candidiasis. Eur J Immunol. 2012 Oct;42((10)):2632–43. doi: 10.1002/eji.201242532. [DOI] [PubMed] [Google Scholar]
- 13.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009 Aug;138((3)):576–91. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009 Oct;10((10)):1065–72. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007 Aug;448((7155)):816–9. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malathi K, Saito T, Crochet N, Barton DJ, Gale M, Jr, Silverman RH. RNase L releases a small RNA from HCV RNA that refolds into a potent PAMP. RNA. 2010 Nov;16((11)):2108–19. doi: 10.1261/rna.2244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Liu CX, Xue W, Zhang Y, Jiang S, Yin QF, et al. Coordinated circRNA Biogenesis and Function with NF90/NF110 in Viral Infection. Mol Cell. 2017 Jul;67((2)):214–227. doi: 10.1016/j.molcel.2017.05.023. e7. [DOI] [PubMed] [Google Scholar]
- 18.Chen YG, Kim MV, Chen X, Batista PJ, Aoyama S, Wilusz JE, et al. Sensing Self and Foreign Circular RNAs by Intron Identity. Mol Cell. 2017 Jul;67((2)):228–238. doi: 10.1016/j.molcel.2017.05.022. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008 Jul;205((7)):1601–10. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullen BR. Transcription and processing of human microRNA precursors. Mol Cell. 2004 Dec;16((6)):861–5. doi: 10.1016/j.molcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Marques JT, Devosse T, Wang D, Zamanian-Daryoush M, Serbinowski P, Hartmann R, et al. A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat Biotechnol. 2006 May;24((5)):559–65. doi: 10.1038/nbt1205. [DOI] [PubMed] [Google Scholar]
- 22.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008 Mar;452((7184)):234–8. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 23.Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, et al. UNC93B1 mediates differential trafficking of endosomal TLRs. eLife. 2013 Feb;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatematsu M, Funami K, Ishii N, Seya T, Obuse C, Matsumoto M. LRRC59 Regulates Trafficking of Nucleic Acid-Sensing TLRs from the Endoplasmic Reticulum via Association with UNC93B1. J Immunol. 2015 Nov;195((10)):4933–42. doi: 10.4049/jimmunol.1501305. [DOI] [PubMed] [Google Scholar]
- 25.Funami K, Matsumoto M, Oshiumi H, Akazawa T, Yamamoto A, Seya T. The cytoplasmic ‘linker region’ in Toll-like receptor 3 controls receptor localization and signaling. Int Immunol. 2004 Aug;16((8)):1143–54. doi: 10.1093/intimm/dxh115. [DOI] [PubMed] [Google Scholar]
- 26.Nishiya T, DeFranco AL. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J Biol Chem. 2004 Apr;279((18)):19008–17. doi: 10.1074/jbc.M311618200. [DOI] [PubMed] [Google Scholar]
- 27.Itoh H, Tatematsu M, Watanabe A, Iwano K, Funami K, Seya T, et al. UNC93B1 physically associates with human TLR8 and regulates TLR8-mediated signaling. PLoS One. 2011;6((12)):e28500. doi: 10.1371/journal.pone.0028500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BL, Barton GM. Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 2014 Jun;24((6)):360–9. doi: 10.1016/j.tcb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang CY, Engel A, Opaluch AM, Ramos I, Maestre AM, Secundino I, et al. Cofactors required for TLR7- and TLR9-dependent innate immune responses. Cell Host Microbe. 2012 Mar;11((3)):306–18. doi: 10.1016/j.chom.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun. 2002 May;293((5)):1364–9. doi: 10.1016/S0006-291X(02)00380-7. [DOI] [PubMed] [Google Scholar]
- 31.Sepulveda FE, Maschalidi S, Colisson R, Heslop L, Ghirelli C, Sakka E, et al. Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling in dendritic cells. Immunity. 2009 Nov;31((5)):737–48. doi: 10.1016/j.immuni.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011 Apr;208((4)):643–51. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maschalidi S, Hässler S, Blanc F, Sepulveda FE, Tohme M, Chignard M, et al. Asparagine endopeptidase controls anti-influenza virus immune responses through TLR7 activation. PLoS Pathog. 2012;8((8)):e1002841. doi: 10.1371/journal.ppat.1002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hipp MM, Shepherd D, Gileadi U, Aichinger MC, Kessler BM, Edelmann MJ, et al. Processing of human toll-like receptor 7 by furin-like proprotein convertases is required for its accumulation and activity in endosomes. Immunity. 2013 Oct;39((4)):711–21. doi: 10.1016/j.immuni.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishii N, Funami K, Tatematsu M, Seya T, Matsumoto M. Endosomal localization of TLR8 confers distinctive proteolytic processing on human myeloid cells. J Immunol. 2014 Nov;193((10)):5118–28. doi: 10.4049/jimmunol.1401375. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Cattaneo A, Gobert FX, Müller M, Toscano F, Flores M, Lescure A, et al. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc Natl Acad Sci USA. 2012 Jun;109((23)):9053–8. doi: 10.1073/pnas.1115091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toscano F, Estornes Y, Virard F, Garcia-Cattaneo A, Pierrot A, Vanbervliet B, et al. Cleaved/associated TLR3 represents the primary form of the signaling receptor. J Immunol. 2013 Jan;190((2)):764–73. doi: 10.4049/jimmunol.1202173. [DOI] [PubMed] [Google Scholar]
- 38.Qi R, Singh D, Kao CC. Proteolytic processing regulates Toll-like receptor 3 stability and endosomal localization. J Biol Chem. 2012 Sep;287((39)):32617–29. doi: 10.1074/jbc.M112.387803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Bouteiller O, Merck E, Hasan UA, Hubac S, Benguigui B, Trinchieri G, et al. Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem. 2005 Nov;280((46)):38133–45. doi: 10.1074/jbc.M507163200. [DOI] [PubMed] [Google Scholar]
- 40.Leonard JN, Ghirlando R, Askins J, Bell JK, Margulies DH, Davies DR, et al. The TLR3 signaling complex forms by cooperative receptor dimerization. Proc Natl Acad Sci USA. 2008 Jan;105((1)):258–63. doi: 10.1073/pnas.0710779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008 Apr;320((5874)):379–81. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pirher N, Ivicak K, Pohar J, Bencina M, Jerala R. A second binding site for double-stranded RNA in TLR3 and consequences for interferon activation. Nat Struct Mol Biol. 2008 Jul;15((7)):761–3. doi: 10.1038/nsmb.1453. [DOI] [PubMed] [Google Scholar]
- 43.Murakami Y, Fukui R, Motoi Y, Kanno A, Shibata T, Tanimura N, et al. Roles of the cleaved N-terminal TLR3 fragment and cell surface TLR3 in double-stranded RNA sensing. J Immunol. 2014 Nov;193((10)):5208–17. doi: 10.4049/jimmunol.1400386. [DOI] [PubMed] [Google Scholar]
- 44.Kanno A, Tanimura N, Ishizaki M, Ohko K, Motoi Y, Onji M, et al. Targeting cell surface TLR7 for therapeutic intervention in autoimmune diseases. Nat Commun. 2015 Feb;6((1)):6119. doi: 10.1038/ncomms7119. [DOI] [PubMed] [Google Scholar]
- 45.Hipp MM, Shepherd D, Booth S, Waithe D, Reis e Sousa C, Cerundolo V. The Processed Amino-Terminal Fragment of Human TLR7 Acts as a Chaperone To Direct Human TLR7 into Endosomes. J Immunol. 2015 Jun;194((11)):5417–25. doi: 10.4049/jimmunol.1402703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itoh K, Watanabe A, Funami K, Seya T, Matsumoto M. The clathrin-mediated endocytic pathway participates in dsRNA-induced IFN-beta production. J Immunol. 2008 Oct;181((8)):5522–9. doi: 10.4049/jimmunol.181.8.5522. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe A, Tatematsu M, Saeki K, Shibata S, Shime H, Yoshimura A, et al. Raftlin is involved in the nucleocapture complex to induce poly(I:C)-mediated TLR3 activation. J Biol Chem. 2011 Mar;286((12)):10702–11. doi: 10.1074/jbc.M110.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeWitte-Orr SJ, Collins SE, Bauer CM, Bowdish DM, Mossman KL. An accessory to the ‘Trinity’: SR-As are essential pathogen sensors of extracellular dsRNA, mediating entry and leading to subsequent type I IFN responses. PLoS Pathog. 2010 Mar;6((3)):e1000829. doi: 10.1371/journal.ppat.1000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nellimarla S, Baid K, Loo YM, Gale M, Jr, Bowdish DM, Mossman KL. Class A Scavenger Receptor-Mediated Double-Stranded RNA Internalization Is Independent of Innate Antiviral Signaling and Does Not Require Phosphatidylinositol 3-Kinase Activity. J Immunol. 2015 Oct;195((8)):3858–65. doi: 10.4049/jimmunol.1501028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou H, Liao J, Aloor J, Nie H, Wilson BC, Fessler MB, et al. CD11b/CD18 (Mac-1) is a novel surface receptor for extracellular double-stranded RNA to mediate cellular inflammatory responses. J Immunol. 2013 Jan;190((1)):115–25. doi: 10.4049/jimmunol.1202136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sirois CM, Jin T, Miller AL, Bertheloot D, Nakamura H, Horvath GL, et al. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. J Exp Med. 2013 Oct;210((11)):2447–63. doi: 10.1084/jem.20120201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertheloot D, Naumovski AL, Langhoff P, Horvath GL, Jin T, Xiao TS, et al. RAGE Enhances TLR Responses through Binding and Internalization of RNA. J Immunol. 2016 Nov;197((10)):4118–26. doi: 10.4049/jimmunol.1502169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HK, Dunzendorfer S, Soldau K, Tobias PS. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity. 2006 Feb;24((2)):153–63. doi: 10.1016/j.immuni.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi M, Contu VR, Kabuta C, Hase K, Fujiwara Y, Wada K, et al. SIDT2 mediates gymnosis, the uptake of naked single-stranded oligonucleotides into living cells. RNA Biol. 2017 Nov;14((11)):1534–43. doi: 10.1080/15476286.2017.1302641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003 Feb;4((2)):161–7. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 56.Tatematsu M, Ishii A, Oshiumi H, Horiuchi M, Inagaki F, Seya T, et al. A molecular mechanism for Toll-IL-1 receptor domain-containing adaptor molecule-1-mediated IRF-3 activation. J Biol Chem. 2010 Jun;285((26)):20128–36. doi: 10.1074/jbc.M109.099101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto M, Takeda Y, Tatematsu M, Seya T. Toll-Like Receptor 3 Signal in Dendritic Cells Benefits Cancer Immunotherapy. Front Immunol. 2017 Dec;8:1897. doi: 10.3389/fimmu.2017.01897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsumoto M, Tatematsu M, Nishikawa F, Azuma M, Ishii N, Morii-Sakai A, et al. Defined TLR3-specific adjuvant that induces NK and CTL activation without significant cytokine production in vivo. Nat Commun. 2015 Feb;6((1)):6280. doi: 10.1038/ncomms7280. [DOI] [PubMed] [Google Scholar]
- 59.Goubau D, Schlee M, Deddouche S, Pruijssers AJ, Zillinger T, Goldeck M, et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature. 2014 Oct;514((7522)):372–5. doi: 10.1038/nature13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramanathan A, Devarkar SC, Jiang F, Miller MT, Khan AG, Marcotrigiano J, et al. The autoinhibitory CARD2-Hel2i Interface of RIG-I governs RNA selection. Nucleic Acids Res. 2016 Jan;44((2)):896–909. doi: 10.1093/nar/gkv1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peisley A, Lin C, Wu B, Orme-Johnson M, Liu M, Walz T, et al. Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc Natl Acad Sci USA. 2011 Dec;108((52)):21010–5. doi: 10.1073/pnas.1113651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berke IC, Modis Y. MDA5 cooperatively forms dimers and ATP-sensitive filaments upon binding double-stranded RNA. EMBO J. 2012 Apr;31((7)):1714–26. doi: 10.1038/emboj.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011 May;34((5)):680–92. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wies E, Wang MK, Maharaj NP, Chen K, Zhou S, Finberg RW, et al. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity. 2013 Mar;38((3)):437–49. doi: 10.1016/j.immuni.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oshiumi H, Miyashita M, Inoue N, Okabe M, Matsumoto M, Seya T. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe. 2010 Dec;8((6)):496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Funabiki M, Kato H, Miyachi Y, Toki H, Motegi H, Inoue M, et al. Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity. 2014 Feb;40((2)):199–212. doi: 10.1016/j.immuni.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 67.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004 Mar;101((10)):3516–21. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardarson HS, Baker JS, Yang Z, Purevjav E, Huang CH, Alexopoulou L, et al. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am J Physiol Heart Circ Physiol. 2007 Jan;292((1)):H251–8. doi: 10.1152/ajpheart.00398.2006. [DOI] [PubMed] [Google Scholar]
- 69.Negishi H, Osawa T, Ogami K, Ouyang X, Sakaguchi S, Koshiba R, et al. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc Natl Acad Sci USA. 2008 Dec;105((51)):20446–51. doi: 10.1073/pnas.0810372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abe Y, Fujii K, Nagata N, Takeuchi O, Akira S, Oshiumi H, et al. The toll-like receptor 3-mediated antiviral response is important for protection against poliovirus infection in poliovirus receptor transgenic mice. J Virol. 2012 Jan;86((1)):185–94. doi: 10.1128/JVI.05245-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004 Dec;10((12)):1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 72.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008 Nov;82((21)):10349–58. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006 Jun;2((6)):e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006 May;80((10)):5059–64. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007 Sep;317((5844)):1522–7. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 76.Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med. 2011 Sep;208((10)):2083–98. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006 Oct;314((5797)):308–12. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 78.Sancho-Shimizu V, Pérez de Diego R, Lorenzo L, Halwani R, Alangari A, Israelsson E, et al. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J Clin Invest. 2011 Dec;121((12)):4889–902. doi: 10.1172/JCI59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pérez de Diego R, Sancho-Shimizu V, Lorenzo L, Puel A, Plancoulaine S, Picard C, et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity. 2010 Sep;33((3)):400–11. doi: 10.1016/j.immuni.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersen LL, Mørk N, Reinert LS, Kofod-Olsen E, Narita R, Jørgensen SE, et al. Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J Exp Med. 2015 Aug;212((9)):1371–9. doi: 10.1084/jem.20142274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004 Mar;303((5663)):1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 82.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004 Mar;303((5663)):1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 83.Wang JP, Liu P, Latz E, Golenbock DT, Finberg RW, Libraty DH. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J Immunol. 2006 Nov;177((10)):7114–21. doi: 10.4049/jimmunol.177.10.7114. [DOI] [PubMed] [Google Scholar]
- 84.Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, et al. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009 Feb;30((2)):242–53. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ugolini M, Gerhard J, Burkert S, Jensen KJ, Georg P, Ebner F, et al. Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nat Immunol. 2018 Apr;19((4)):386–96. doi: 10.1038/s41590-018-0068-4. [DOI] [PubMed] [Google Scholar]
- 86.Shingai M, Ebihara T, Begum NA, Kato A, Honma T, Matsumoto K, et al. Differential type I IFN-inducing abilities of wild-type versus vaccine strains of measles virus. J Immunol. 2007 Nov;179((9)):6123–33. doi: 10.4049/jimmunol.179.9.6123. [DOI] [PubMed] [Google Scholar]
- 87.Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009 Jul;31((1)):25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006 May;441((7089)):101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 89.Sumpter R, Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005 Mar;79((5)):2689–99. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmolke M, García-Sastre A. Evasion of innate and adaptive immune responses by influenza A virus. Cell Microbiol. 2010 Jul;12((7)):873–80. doi: 10.1111/j.1462-5822.2010.01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Furr SR, Moerdyk-Schauwecker M, Grdzelishvili VZ, Marriott I. RIG-I mediates nonsegmented negative-sense RNA virus-induced inflammatory immune responses of primary human astrocytes. Glia. 2010 Oct;58((13)):1620–9. doi: 10.1002/glia.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cárdenas WB, Loo YM, Gale M, Jr, Hartman AL, Kimberlin CR, Martínez-Sobrido L, et al. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol. 2006 Jun;80((11)):5168–78. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramanan P, Edwards MR, Shabman RS, Leung DW, Endlich-Frazier AC, Borek DM, et al. Structural basis for Marburg virus VP35-mediated immune evasion mechanisms. Proc Natl Acad Sci USA. 2012 Dec;109((50)):20661–6. doi: 10.1073/pnas.1213559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oh SW, Onomoto K, Wakimoto M, Onoguchi K, Ishidate F, Fujiwara T, et al. Leader-Containing Uncapped Viral Transcript Activates RIG-I in Antiviral Stress Granules. PLoS Pathog. 2016 Feb;12((2)):e1005444. doi: 10.1371/journal.ppat.1005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin YH, Kim SJ, So EY, Meng L, Colonna M, Kim BS. Melanoma differentiation-associated gene 5 is critical for protection against Theiler's virus-induced demyelinating disease. J Virol. 2012 Feb;86((3)):1531–43. doi: 10.1128/JVI.06457-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pichlmair A, Schulz O, Tan CP, Rehwinkel J, Kato H, Takeuchi O, et al. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J Virol. 2009 Oct;83((20)):10761–9. doi: 10.1128/JVI.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Errett JS, Suthar MS, McMillan A, Diamond MS, Gale M., Jr The essential, nonredundant roles of RIG-I and MDA5 in detecting and controlling West Nile virus infection. J Virol. 2013 Nov;87((21)):11416–25. doi: 10.1128/JVI.01488-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nasirudeen AM, Wong HH, Thien P, Xu S, Lam KP, Liu DX. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl Trop Dis. 2011 Jan;5((1)):e926. doi: 10.1371/journal.pntd.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baum A, Sachidanandam R, García-Sastre A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc Natl Acad Sci USA. 2010 Sep;107((37)):16303–8. doi: 10.1073/pnas.1005077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012 Oct;12((4)):558–70. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, et al. Exosome RNA Unshielding Couples Stromal Activation to Pattern Recognition Receptor Signaling in Cancer. Cell. 2017 Jul;170((2)):352–366. doi: 10.1016/j.cell.2017.06.031. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]