Abstract
Nearly 15 years after the first description of neutrophil extracellular traps (NETs), our knowledge concerning this structure has expanded considerably. Initially, NETs were considered solely an elaborate function of the innate immune system to combat invading microorganisms. Successively it became clear that NETs have farther-reaching capabilities. They are involved in a series of pathophysiological mechanisms ranging from inflammation to thrombosis where they fulfill essential functions when produced at the right site and the right time but can have a serious impact when generation or clearance of NETs is inadequately controlled. This review provides a concise overview on the far-reaching functions of NETs in health and disease.
Keywords: Autoimmunity, Bacterial infection, Cancer, Coagulation, Extracellular traps, Inflammation
Structure and Generation of Neutrophil Extracellular Traps
In 2004, a paper was published describing that human neutrophils, when treated with the mitogen phorbol myristate acetate (PMA), undergo a characteristic form of cell death that is neither necrosis nor apoptosis [1]. A report on the response of neutrophils treated with PMA had already been published by a Japanese group in 1996 [2]. At that time, it was not apparent that this form of cell death results in the formation of large 3-D structures composed of thin chromatin fibers studded with granular and cytoplasmic proteins and peptides. The main protein components of neutrophil extracellular traps (NETs) are histones, followed by granular enzymes and peptides including neutrophil elastase (NE), myeloperoxidase (MPO), cathepsin G, leukocyte proteinase 3 (PR3), lactoferrin, gelatinase, lysozyme C, calprotectin, neutrophil defensins, and cathelicidins [3]. These structures were named NETs, and the peculiar cell death pathway was termed NETosis, accordingly [4]. A large number of inducers of NETosis have been described, including bacteria or bacterial components [1], fungi [5], protozoa [6, 7], viruses [8], activated platelets [9], complement-derived peptides [10], autoantibodies [11], IL-8 [1], hydrogen peroxide [12], urate crystals [13], cigarette smoke [14], and ionophores [15]. PMA-induced NETosis has been studied in some detail and a number of molecular events leading to NET formation have been identified. PMA directly binds to protein kinase C, calcium is released from intracellular stores, and the Raf-MEK-ERK pathway is activated [16]. Further downstream, the multimeric complex of NADPH oxidase assembles at the phagosomal membrane and generates reactive oxygen species [12]. Patients with chronic granulomatous disease suffer from recurrent infections due to an inactive NADPH oxidase complex. Restoration of its function by gene therapy reestablishes the ability to form NETs and to overcome fungal lung infections [17]. The granular serine protease NE is mobilized together with MPO and it is transported to the nucleus where it processes core histones [18, 19]. The rise in intracellular calcium levels activates also peptidylarginine deiminase 4, which converts arginines in histones to citrullines, resulting in a reduction of the positive charge of these proteins [20, 21, 22]. Together, these molecular events decrease the compaction of chromatin. Consequently, after about 2 h, PMA-stimulated neutrophils lose heterochromatic areas of the nucleus as well as the characteristic nuclear lobuli. As a result, nuclei round up and expand. The nuclear envelope disintegrates into vesicles, granules, and mitochondria break down. Cytoplasm and karyoplasm intermingle and finally the cell membrane ruptures and releases the cellular content, which unfolds in the extracellular space to form NETs [12].
Physiological induction of NETosis is dependent on receptors including integrins and Toll-like receptors [7, 23], but it can also be induced by ionophores like A23187 and nigericin. In this case, activation of protein kinase C is not required [24]. Independently of the stimulus, NET formation does not require transcription [25]. The breakdown of the nuclear envelope is reminiscent of mitosis, and NETosis is strongly induced by PMA, which also acts as a mitogen. Two other mitogens, the plant lectins concanavalin A and phytohemagglutinin, also induce NET formation. In contrast to unstimulated cells, neutrophils treated with these mitogens express the cell division marker Ki-67 in their nuclei [26]. Furthermore, cyclin-dependent kinase (CDK) 6 transiently enters the nucleus after PMA stimulation. CDK6 inhibition blocks NET formation, and neutrophils from a CDK6–/– mouse strain are impaired in NET production. In summary, the pathways for NETosis and mitosis share some components, although the outcome is different.
This series of events is called suicidal NETosis. In contrast, it has been reported that neutrophils can release part of or the entire nucleus without breaching the cell membrane, resulting in anuclear cytoplasts that are still able to move and phagocytose bacteria [27]. When coculturing neutrophils with Staphylococcus aureus, a very rapid release of DNA was monitored [28]. Nuclei underwent massive dilation of the nuclear envelope and vesicle formation. These vesicles contained DNA and finally fused with the cell membrane, releasing their contents without cell lysis. Similar neutrophil behavior was described using intravital imaging and was found to be dependent on TLR2 and C3 [23]. The neutrophils remained viable even after the release of nuclear DNA and continued to move inside the tissue although their crawling pattern differed from that of neutrophils containing nuclei. Since neutrophils survive this form of discharging DNA, it is called vital NETosis.
It has been proposed that upon GM-CSF priming and subsequent stimulation with LPS or complement factor 5a neutrophils release mitochondrial but not nuclear DNA [10]. In the extracellular space, DNA binds to granular enzymes like NE or MPO. The resulting structures differ considerably from NETs since their main protein component, i.e., histones, is missing. Furthermore, no mechanism for the release of mitochondrial DNA has been proposed, and so far it is unclear whether the minor DNA content of mitochondria would suffice for the large amount of DNA-containing structures that have been detected.
NET formation is evolutionarily conserved within the kingdoms of animals and plants and has been described in different mammalian species [1, 6, 29, 30, 31], birds [32], fish [31, 33], invertebrates [34, 35], and plants that protect their root tips against fungal infections with extracellular DNA [36].
Antimicrobial Activity of NETs
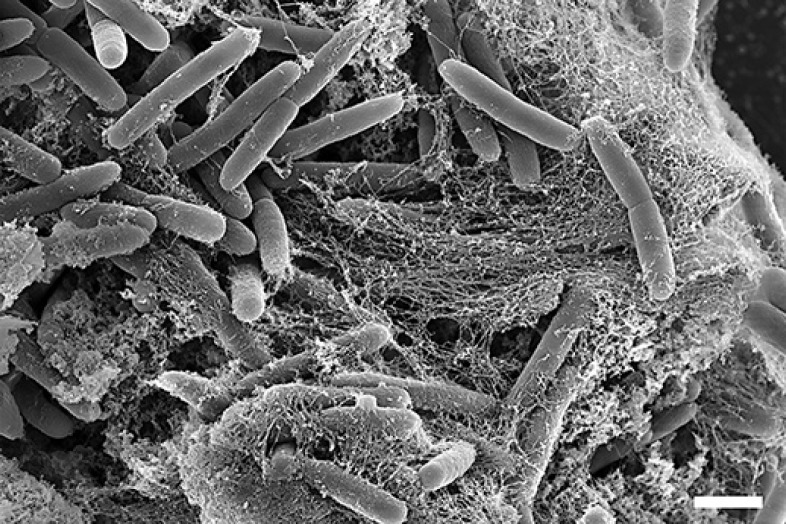
NETs bind gram-positive as well as gram-negative bacteria (Fig. 1). Their antimicrobial activity is direct by killing the pathogens but also indirect by preventing further spread from the entry point. NETs are 3-D structures composed of the following toxic components: DNA [37], histones [38, 39], granular enzymes like NE, MPO, cathepsin G, and antimicrobial peptides like defensins and cathelicidins. Negatively charged DNA serves as a backbone. When this is degraded by DNases, the structural integrity of NETs is impaired [1]. Consequently, bacterial strains that express DNases can be more pathogenic than DNase-negative strains, and conversely the de novo expression of DNases by otherwise harmless bacteria renders them more virulent [40, 41, 42].
Fig. 1.
Scanning electron micrograph of purified human neutrophils that have produced NETs after cocultivation with P. aeruginosa for 3 h. Scale bar, 1 µm.
S. aureus has found a way to degrade NETs and establish a sheltered niche [43]. By converting NETs to deoxyadenosine, S. aureus not only escapes killing by NETs but also avoids phagocytosis, creating abscesses that are devoid of macrophages since they respond to deoxyadenosine by apoptosis.
NETs are also active against fungi, e.g., they can kill both yeast and hyphal forms of Candida albicans [5]. Interestingly, neutrophils can sense the size of pathogens and produce more NETs when in contact with hyphae compared to yeast [44].
When analyzing the fate of different C. albicans strains in lung infections, the same group found that yeast-locked fungi were controlled by a relatively low number of phagocytosing neutrophils, while WT strains that form hyphae in the lung were attacked by large numbers of neutrophils forming clusters around the hyphae. IL-1β signaling was critical for the massive recruitment of neutrophils to hyphae, while it played no role for the neutrophils phagocytosing yeast forms [45].
Pathogenic Potential
NETs are structures with a high local concentration of very active molecules. The strong intermolecular binding prevents individual components from diffusing into neighboring tissue, thus limiting tissue damage. If the amount and the site of NET formation, as well as their timely removal, are not tightly controlled, the presence of NETs can have serious consequences and it is linked to numerous pathophysiological processes, some of which are presented here.
Cardiovascular Diseases
Although the pathogeneses of atherothrombosis and venous thrombosis are very different, NET formation is involved in both. Often induced by hypoxia due to a reduced blood flow, venous endothelium is activated, releases von Willebrand factor, and upregulates P-selectin. Platelets and neutrophils are recruited. Activated platelets can induce neutrophils to release NETs, which serve as a scaffold for further platelets. Erythrocytes bind to the developing thrombus and stabilize it. DNase treatment dissolves the thrombus as does heparin [46], a potent anticoagulant with a high affinity for histones which displaces histones from chromatin [47].
In a flow restriction model of deep vein thrombosis it was found that neutropenic mice develop no or significantly smaller thrombi compared to control mice [48]. In areas of restricted blood flow, neutrophils release large amounts of NETs, which form a dense network with fibrin and platelets. Treatment with DNase dismantled NETs, resulting in significantly smaller thrombi, as did administration of heparin.
A Link between NETs, Thrombosis, and Innate Immunity
It was proposed that thrombosis could be considered a means of the immune system to promote immune defense [49]. By inducing microthrombi in microvessels during infection with blood-borne pathogens, bacteria are killed by NET components or further dissemination into neighboring tissue is limited. Since thrombosis under these conditions is restricted to a small percentage of microvessels, e.g. of the liver, the overall function of the respective organ is not at risk.
Atherothrombosis starts with dysfunctional endothelium. It has been shown that NETs can be toxic to endothelial cells [50]. Activated endothelium induces NET formation [51], which can create a vicious cycle resulting in more damage. Injured endothelium can lead to atherosclerosis, which is characterized by the accumulation of lipoproteins and monocytes in the subendothelial layer. Monocytes differentiate to macrophages that ingest large amounts of lipoprotein and cholesterol, which gives them a “foamy” appearance due to the numerous cytoplasmic vesicles. Gradually, a plaque builds up that increasingly obstructs the blood flow. Rupture of the plaque leads to immediate blood clotting, which can result in complete artery closure inducing hypoxia and tissue damage in downstream organs.
Warnatsch et al. [52] found that NETs play a significant role in the progression to plaque formation. When human neutrophils come in contact with cholesterol crystals, they spontaneously undergo NETosis [52]. They used a mouse model of atherosclerosis characterized by hypercholesterolemia and plaque formation and crossed this strain with mice that were deficient both in NE and PR3 and thus unable to produce NETs. These triple mutant mice developed significantly fewer atherosclerotic lesions than did the parental hypercholesterolemic strain, which showed a similar reduction in lesion size when treated with DNase. Furthermore, it could be shown that in this setting NETs activate macrophages to produce proinflammatory cytokines.
The role of NETs in thrombosis was analyzed in a ferric chloride artery injury model [53]. In mice deficient in NE and cathepsin G, fibrin deposition was markedly reduced 15 min after vessel injury compared to WT animals. This resulted in a reduced thrombus size and a longer time to-occlusion of the carotid artery and a prolonged tail-bleeding time. It could be shown that while thrombus-associated tissue factor pathway inhibitor (TFPI) is markedly degraded in WT mice, while most of the TFPI in thrombi of NE/CatG-deficient mice is noncleaved.
A noncanonical pathway to vascular occlusion has recently been demonstrated [54]. It is assumed that NETs have a rather short lifetime in circulation due to the presence of serum DNases. As will be discussed later, patients with defects in DNase 1 develop autoantibodies against NET components due to the increased exposure of autoantigens in NETs that cannot be dismantled in a timely manner. Mice that not only lack the expression of DNase 1 but are also deficient in DNase 1-like protein 3 (DNas1l3) are apparently normal. Surprisingly, during septicemia or sterile neutrophilia, they produce large amounts of NETs that cannot be degraded and rapidly lead to massive intravascular clot formation inducing organ damage and death. This clearly demonstrates the need of serum-expressed DNases for a timely removal of NETs.
Autoimmune and Autoinflammatory Diseases
Systemic lupus erythematosus (SLE) is characterized by the overproduction of autoantibodies against nuclear antigens like DNA and histones, but also against neutrophil granule proteins. Several studies have found that a subpopulation of systemic lupus erythematosus patients have a limited capacity to degrade NETs, and that this is associated with clinical manifestations [55, 56]. In these patients, NETs could not be degraded because they were shielded by autoantibodies against degradation by DNase 1 or because inhibitors of DNase 1 were present in the sera. This correlation between the incomplete removal of NETs and phases of more severe disease could also explain why infections in these patients initiate flares.
In ANCA-associated small vessel vasculitis, an autoinflammatory disorder leading to necrotic inflammation of small blood vessels, autoantibodies are generated against 2 neutrophil granular proteins, i.e., PR3 and MPO. Purified antibodies isolated from small vessel vasculitis patients and a mouse monoclonal against PR3 induced NET formation in TNF-α-primed neutrophils, in contrast to antibodies from healthy donors [11]. NETs were also found in kidney biopsies of small vessel vasculitis patients. In the absence of microbial infection, antibodies against granular NET components can induce a vicious cycle, which continuously provides more NETs. In a different study, it was shown that IgG from patients with microscopic polyangiitis readily induce NETs and that the amount of NET formation correlates with disease severity [57]. NET induction by IgG purified from patients' sera could be reduced by the addition of recombinant human MPO, indicating that autoantibodies were directed against this enzyme. In general, the NET-degrading capacity of microscopic polyangiitis sera was significantly reduced compared to that of sera from normal donors, further exposing autoantigens.
Rheumatoid arthritis is a chronic autoinflammatory disorder mainly of the joints. Neutrophils are abundant in synovial fluids, especially during flares. Since most of the rheumatoid arthritis autoantibodies are directed against citrullinated proteins, NETs and their citrullinated components could be involved in induction of autoreactive B cells. Compared to neutrophils from healthy donors, enhanced NETosis was observed in neutrophils from rheumatoid arthritis patients, both isolated from circulation and isolated from synovial fluid [58].
Synovial fluids contain large amounts of DNA, partly derived from NETs that contain peptidylarginine deiminases 2 and 4. Both enzymes were also found freely diffused in the fluid [59]. Release of the enzymes by neutrophils undergoing NETosis could explain the generation of citrullinated autoantigens.
An anti-inflammatory function of NETs was proposed for gout. This is a form of inflammatory arthritis caused by high levels of uric acid, which can even crystallize as monosodium urate in the joints and induce severe pain, which lasts for hours or days. Monosodium urate crystals can form large aggregates called tophi, which contain numerous neutrophils and large aggregates of NETs. Similar structures are formed in vitro when neutrophils are seeded at a high density and treated with monosodium urate crystals [60]. Probably due to the activity of enzymes associated to NETs, aggregates of these DNA structures were found to decrease the concentration of proinflammatory cytokines like IL-1β and TNF, which could explain the spontaneous termination of the inflammatory response typical of gout.
Fertility
During insemination of mares, numerous neutrophils are recruited to the fertilization tract in order to eliminate invading microorganisms. Interestingly, stallions with limited fertility lack a DNase-like protein in their seminal plasma. This correlation could be explained when it was found that bacteria in the fertilization tract were constricted by NETs which also bound spermatozoa. These could only be released from NET trapping when DNase was present in seminal plasma [29].
Preeclampsia is a human pregnancy disorder characterized by proteinuria and a sudden rise in blood pressure, which occurs in about 2–8% of pregnancies. One hallmark of the disease is shedding of microparticles from the placental syncytiotrophoblast, which was shown to directly induce NETs in vitro [61]. The amount of NETs found in the intervillous space of the placenta was far higher in preeclampsia than in normal tissue, restricting blood flow and possibly increasing hypoxia [62].
Lung Diseases
Due to mutations in both copies of the gene for the cystic fibrosis (CF) transmembrane conductance regulator, patients with CF suffer from accumulations of highly viscous mucus in the lung, which clog the airways. Lung infections with S. aureus, Haemophilus influenzae, and Pseudomonas aeruginosa are common and induce massive neutrophil infiltration and chronic inflammation. CF mucus contains large amounts of DNA, and most of the mucus DNA is complexed in NETs [63]. The amount of extracellular DNA directly correlates to poor pulmonary function [64]. CF symptoms can be treated by inhalation of recombinant DNase 1, which reduces the viscosity of CF mucus and improves lung function, but carries the risk of liberating highly active enzymes and toxic molecules like histones, which can damage the lung epithelium.
Chronic obstructive pulmonary disease (COPD) is also a persistent neutrophilic inflammation. After long-term exposure to inhaled irritants like tobacco smoke, COPD patients suffer from recurrent bacterial and viral infections leading to repeated exacerbations. Tobacco smoke has been found to directly induce NET formation [14], possibly due to its LPS content. Additionally, nicotine directly induces NETosis [65, 66, 67], and it is not surprising that NETs have been found in COPD lungs [68]. As is the case in CF, also in COPD disease severity correlates to the amount of NETs, which is highest in phases of exacerbations [65].
NETs and Cancer
For a long time, neutrophils have been considered inert bystander cells in cancer formation and metastasis, but this concept has changed, as reviewed recently [69]. In a small study with Ewing sarcoma patients, in 25% of cases NETs were found inside the tumor, and these patients developed metastases, indicating that NETs may promote tumor progression [70]. In a study comparing metastatic and nonmetastatic murine breast cancer cell lines it was found that more neutrophils were recruited to sites of implanted metastatic cells where they induced NET formation, while NETs were not found adjacent to implantation sites of nonmetastatic cells [71]. Further down the metastatic cascade, NETs have been shown to trap circulating tumor cells both in vitro and in vivo, leading to massive formation of micrometastases in the liver [72]. Interestingly, administration of DNase 1 or an inhibitor of NE reduced the metastatic burden to control levels. Although NETs have not been presented directly, in a cohort of patients with advanced cancer elevated levels of citrullinated histone 3 (H3Cit) were found, which was not the case in age-matched individuals [73]. H3Cit could serve as a prognostic marker since in the analyzed cohort high levels of serum H3Cit strongly correlated with a poor clinical outcome.
Conclusion
In recent years, the interest in NETs has shifted from innate immune defense to their involvement in a multitude of diseases ranging from infertility to cancer. It has become clear that uncontrolled NET formation or their insufficient removal can have serious consequences. This is exemplified by the function of NETs during thrombosis: they are essential for timely coagulation in case of vascular trauma, and they are presumably quickly broken down by serum DNase 1 after the wound is sealed. When DNase 1 is not fully active, NET remnants expose autoantigens and may foster the progression to autoimmune disorders like systemic lupus erythematosus, potentially leading to lupus nephritis. When both DNA-degrading serum enzymes, i.e., DNase 1 and DNase 1-like protein 3, are missing, NET formation due to sterile neutrophilia or sepsis has abysmal consequences leading to generalized vessel obstruction with organ failure and rapid death.
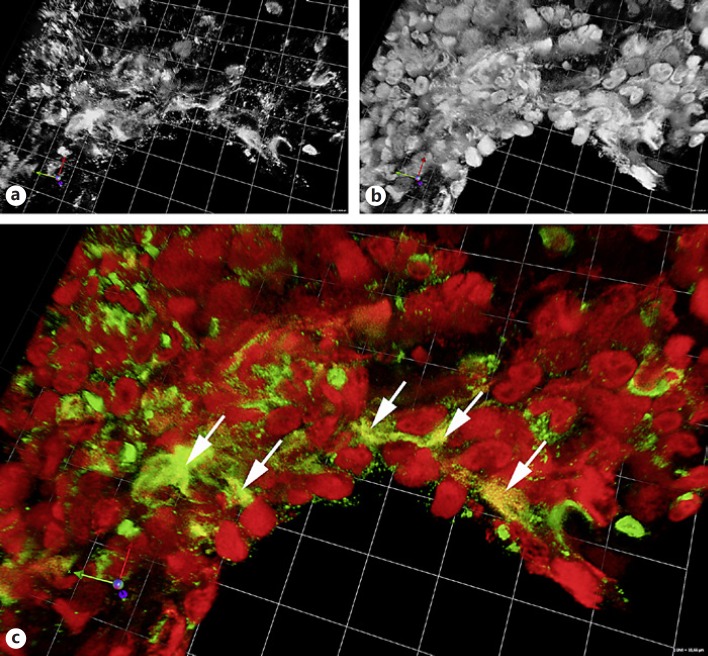
For a number of other diseases the role of NETs still needs to be defined. Many of these studies are limited since they show the presence of neutrophils and extracellular DNA but fail to unequivocally detect NETs. As long as there are no antibodies against epitopes available that are exclusively present in NETs, a complex of DNA/chromatin and granular/cytoplasmic neutrophil components needs to be demonstrated, e.g., by means of immunofluorescence (Fig. 2) or sandwich ELISA.
Fig. 2.
Paraffin section of a C. albicans– infected mouse lung stained for neutrophil elastase (a, green in c) and histone 2B (b, red in c). The depicted area is rich in neutrophils that have partly formed NETs (arrows). The image is a 3-D reconstruction of a confocal stack. The side length of the squares in the background is 10.66 µm.
With the evolving concept of aberrant NET formation or their insufficient removal as a pathomechanism, it becomes even more important to better understand the diverse signaling pathways that finally lead to NETosis and to develop tools to intervene. Recombinant DNase 1 has successfully been administered to liquefy CF sputum, and NE inhibitors are used in COPD. Peptidylarginine deiminase 4 is overexpressed in a majority of human cancers, and inhibitors could improve the clinical outcome. A better comprehension of the molecules involved in induction of NETs and the mechanisms that dismantle them will enable us to successfully modify the pathogenic potential of NETs.
Disclosure Statement
The author declares no conflict of interests.
Acknowledgements
I thank Arturo Zychlinsky, Ulrike Abu Abed, and Christian Goosmann for critically reading this paper. The samples for Figures 1 and 2 were prepared by Christian Goosmann and Ulrike Abu Abed, respectively.
References
- 1.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Takei H, Araki A, Watanabe H, Ichinose A, Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol. 1996;59:229–240. doi: 10.1002/jlb.59.2.229. [DOI] [PubMed] [Google Scholar]
- 3.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE. 2007;2007:pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- 5.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 6.Aulik NA, Hellenbrand KM, Klos H, Czuprynski CJ. Mannheimia haemolytica and its leukotoxin cause neutrophil extracellular trap formation by bovine neutrophils. Infect Immun. 2010;78:4454–4466. doi: 10.1128/IAI.00840-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sousa-Rocha D, Thomaz-Tobias M, Diniz LF, Souza PS, Pinge-Filho P, Toledo KA. Trypanosoma cruzi and its soluble antigens induce NET release by stimulating Toll-like receptors. PLoS One. 2015;10:e0139569. doi: 10.1371/journal.pone.0139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, Yamamoto N, Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 10.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 11.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011;6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu SL, Zhang H, Tang QY, Bai J, He ZY, Zhang JQ, Li MH, Deng JM, Liu GN, Zhong XN. Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. Thorax. 2017;72:1084–1093. doi: 10.1136/thoraxjnl-2016-209887. [DOI] [PubMed] [Google Scholar]
- 15.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol. 2012;92:841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 16.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114:2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8:883–896. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 21.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1:194–201. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, Brinkmann V, Bernuth HV, Zychlinsky A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6:e24437. doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sollberger G, Amulic B, Zychlinsky A. Neutrophil extracellular trap formation is independent of de novo gene expression. PLoS One. 2016;11:e0157454. doi: 10.1371/journal.pone.0157454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amulic B, Knackstedt SL, Abu Abed U, Deigendesch N, Harbort CJ, Caffrey BE, Brinkmann V, Heppner FL, Hinds PW, Zychlinsky A. Cell-cycle proteins control production of neutrophil extracellular traps. Dev Cell. 2017;43:449–462. doi: 10.1016/j.devcel.2017.10.013. e445. [DOI] [PubMed] [Google Scholar]
- 27.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 28.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 29.Alghamdi AS, Foster DN. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol Reprod. 2005;73:1174–1181. doi: 10.1095/biolreprod.105.045666. [DOI] [PubMed] [Google Scholar]
- 30.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1:181–193. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palic D, Ostojic J, Andreasen CB, Roth JA. Fish cast NETs: neutrophil extracellular traps are released from fish neutrophils. Dev Comp Immunol. 2007;31:805–816. doi: 10.1016/j.dci.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Chuammitri P, Ostojic J, Andreasen CB, Redmond SB, Lamont SJ, Palic D. Chicken heterophil extracellular traps (HETs): novel defense mechanism of chicken heterophils. Vet Immunol Immunopathol. 2009;129:126–131. doi: 10.1016/j.vetimm.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Zhao ML, Chi H, Sun L. Neutrophil extracellular traps of Cynoglossus semilaevis: production characteristics and antibacterial effect. Front Immunol. 2017;8:290. doi: 10.3389/fimmu.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng TH, Chang SH, Wu MH, Wang HC. Shrimp hemocytes release extracellular traps that kill bacteria. Dev Comp Immunol. 2013;41:644–651. doi: 10.1016/j.dci.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Poirier AC, Schmitt P, Rosa RD, Vanhove AS, Kieffer-Jaquinod S, Rubio TP, Charriere GM, Destoumieux-Garzon D. Antimicrobial histones and DNA traps in invertebrate immunity: evidences in Crassostrea gigas. J Biol Chem. 2014;289:24821–24831. doi: 10.1074/jbc.M114.576546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawes MC, Curlango-Rivera G, Wen F, White GJ, Vanetten HD, Xiong Z. Extracellular DNA: the tip of root defenses? Plant Sci. 2011;180:741–745. doi: 10.1016/j.plantsci.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Halverson TW, Wilton M, Poon KK, Petri B, Lewenza S. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 2015;11:e1004593. doi: 10.1371/journal.ppat.1004593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch JG. Bactericidal action of histone. J Exp Med. 1958;108:925–944. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 41.Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, Bailey JR, Parnell MJ, Hoe NP, Adams GG, Deleo FR, Musser JM. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci USA. 2005;102:1679–1684. doi: 10.1073/pnas.0406641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schilcher K, Andreoni F, Uchiyama S, Ogawa T, Schuepbach RA, Zinkernagel AS. Increased neutrophil extracellular trap-mediated Staphylococcus aureus clearance through inhibition of nuclease activity by clindamycin and immunoglobulin. J Infect Dis. 2014;210:473–482. doi: 10.1093/infdis/jiu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warnatsch A, Tsourouktsoglou TD, Branzk N, Wang Q, Reincke S, Herbst S, Gutierrez M, Papayannopoulos V. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity. 2017;46:421–432. doi: 10.1016/j.immuni.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Napirei M, Ludwig S, Mezrhab J, Klockl T, Mannherz HG. Murine serum nucleases - contrasting effects of plasmin and heparin on the activities of DNase1 and DNase1-like 3 (DNase1l3) FEBS J. 2009;276:1059–1073. doi: 10.1111/j.1742-4658.2008.06849.x. [DOI] [PubMed] [Google Scholar]
- 48.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 50.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation: neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez-Alcazar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, Bilyy R, Krenn V, Renne C, Renne T, Kluge S, Panzer U, Mizuta R, Mannherz HG, Kitamura D, Herrmann M, Napirei M, Fuchs TA. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358:1202–1206. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- 55.Leffler J, Gullstrand B, Jonsen A, Nilsson JA, Martin M, Blom AM, Bengtsson AA. Degradation of neutrophil extracellular traps co-varies with disease activity in patients with systemic lupus erythematosus. Arthritis Res Ther. 2013;15:R84. doi: 10.1186/ar4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakazawa D, Shida H, Tomaru U, Yoshida M, Nishio S, Atsumi T, Ishizu A. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J Am Soc Nephrol. 2014;25:990–997. doi: 10.1681/ASN.2013060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005580. 178ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spengler J, Lugonja B, Ytterberg AJ, Zubarev RA, Creese AJ, Pearson MJ, Grant MM, Milward M, Lundberg K, Buckley CD, Filer A, Raza K, Cooper PR, Chapple IL, Scheel-Toellner D. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. 2015;67:3135–3145. doi: 10.1002/art.39313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, Holmdahl R, Krenn V, Harrer T, Jeremic I, Bilyy R, Schett G, Hoffmann M, Herrmann M. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 61.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–1154. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Marder W, Knight JS, Kaplan MJ, Somers EC, Zhang X, O'Dell AA, Padmanabhan V, Lieberman RW. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci Med. 2016;3:e000134. doi: 10.1136/lupus-2015-000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dwyer M, Shan Q, D'Ortona S, Maurer R, Mitchell R, Olesen H, Thiel S, Huebner J, Gadjeva M. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J Innate Immun. 2014;6:765–779. doi: 10.1159/000363242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marcos V, Zhou-Suckow Z, Onder Yildirim A, Bohla A, Hector A, Vitkov L, Krautgartner WD, Stoiber W, Griese M, Eickelberg O, Mall MA, Hartl D. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediators Inflamm. 2015;2015:408935. doi: 10.1155/2015/408935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grabcanovic-Musija F, Obermayer A, Stoiber W, Krautgartner WD, Steinbacher P, Winterberg N, Bathke AC, Klappacher M, Studnicka M. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir Res. 2015;16:59. doi: 10.1186/s12931-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. Nicotine induces neutrophil extracellular traps. J Leukoc Biol. 2016;100:1105–1112. doi: 10.1189/jlb.3AB0815-379RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J, Luria A, Rhodes C, Raghu H, Lingampalli N, Sharpe O, Rada B, Sohn DH, Robinson WH, Sokolove J. Nicotine drives neutrophil extracellular traps formation and accelerates collagen-induced arthritis. Rheumatology (Oxford) 2017;56:644–653. doi: 10.1093/rheumatology/kew449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Obermayer A, Stoiber W, Krautgartner WD, Klappacher M, Kofler B, Steinbacher P, Vitkov L, Grabcanovic-Musija F, Studnicka M. New aspects on the structure of neutrophil extracellular traps from chronic obstructive pulmonary disease and in vitro generation. PLoS One. 2014;9:e97784. doi: 10.1371/journal.pone.0097784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 70.Berger-Achituv S, Brinkmann V, Abed UA, Kuhn LI, Ben-Ezra J, Elhasid R, Zychlinsky A. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48. doi: 10.3389/fimmu.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, Nakasone ES, Hearn SA, Kuttner V, Qiu J, Almeida AS, Perurena N, Kessenbrock K, Goldberg MS, Egeblad M. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aag1711. 361ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013 doi: 10.1172/JCI67484. DOI: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thalin C, Lundstrom S, Seignez C, Daleskog M, Lundstrom A, Henriksson P, Helleday T, Phillipson M, Wallen H, Demers M. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One. 2018;13:e0191231. doi: 10.1371/journal.pone.0191231. [DOI] [PMC free article] [PubMed] [Google Scholar]