Abstract
The nasal administration of vaccines directed against diseases caused by upper respiratory tract infections of pathogens, such as the influenza virus, mimics the natural infection of pathogens and induces immunoglobulin A (IgA) production in the nasal cavity to effectively protect viral entry. Therefore, the development of a nasally administered vaccine is a research objective. Because the antigenicity of influenza split vaccines is low, nasal inoculation with the vaccine alone does not induce strong IgA production in the nasal cavity. However, the addition of adjuvants activates the innate immune response, enhancing antigen-specific IgA production and the T-cell response. Although the development of suitable adjuvants for nasal vaccinations is in progress, the mechanism by which adjuvants promote the immune response is still unclear. In this review, we discuss the mucosal immune response, especially in the nasal-associated lymphoid tissue, induced in response to the intranasal inoculation of an influenza vaccine and adjuvants in animal models.
Keywords: Immunoglobulin A, Influenza vaccine, Mucosal immunity, Nasal-associated lymphoid tissue
Introduction
Influenza, caused by the influenza virus, spreads throughout the world every year and mortality in the elderly and infants is high. Therefore, individuals are vaccinated against the virus to prevent the severe pathological conditions it causes. However, subcutaneous inoculation with an influenza vaccine does not prevent the viral infection itself [1]. The influenza virus is susceptible to antigen shift and antigen drift, and the prediction of epidemic strains is also difficult, so many problems must be solved to develop a vaccine that has the desired infection-preventing effect [2].
Nasally administered vaccines that mimic natural infections have been shown to be effective in preventing influenza virus infection [1]. After the intranasal administration of an influenza vaccine, immunoglobulin A (IgA) is secreted into the nasal mucosa and can prevent infection after viral exposure [3]. Furthermore, because IgA is cross-reactive, even if the vaccine strain and the epidemic strain differ, an infection-preventing effect can be induced [4]. However, because safe split vaccines have low antigenicity, it is necessary to administer them intranasally together with an adjuvant to induce IgA production. The selection of an appropriate adjuvant is essential for the development of a safe and effective vaccine, but the mechanism of action of adjuvants in the nasal mucosa is unclear. New findings have recently been reported regarding the adjuvant effect in the nasal-associated lymphoid tissue (NALT), which is considered to regulate the mucosal immune response in the nasal mucosa and upper respiratory tract. In this paper, the mechanisms of antibody production induced by adjuvants after their nasal administration are discussed.
Nasal-Associated Lymphoid Tissue
NALT is one of the mucosal-associated lymphoid tissues, and is located on the nasal cavity side of the upper palate in rodents, including rats, hamsters, and mice [5]. In rodents, the NALT is considered to be equivalent to Waldeyer's ring and the adenoid, tubal, palatine, and lingual tonsils in humans [5]. In contrast to other mucosal lymph nodes (Peyer's patches), the NALT develops after birth. Although the development of Peyer's patches requires lymphotoxin (LT)-a, LT-b, and LT-b receptor, these molecules are essential for the development of the NALT. Id2–/– mice have an impaired NALT structure [6, 7]. The NALT differs from the lymphoid tissues involved in other mucosal immunity in terms of its organizational development. Furthermore, NALT formation is suppressed in germ-free mice compared with wild-type mice, suggesting that the formation of the NALT is established by an interaction with the indigenous bacteria in the nasal cavity after birth [8].
The NALT, which consists of T cells, B cells, dendritic cells (DCs), macrophages, and microfold cells, is located in the nasal cavity and is the first immune tissue exposed to inhalant antigens and pathogens [5, 9, 10]. Inhalant antigens are transported to the NALT by microfold cells located in the epithelium overlying the NALT and are taken up by DCs, resulting in their presentation to T cells [7]. After T-cell activation, a cytokine environment suitable for IgA is considered to form at the inductive site of mucosal immunity, promoting IgA class switching and affinity maturation in the germinal centers formed in the NALT. Activated T cells and IgA-producing B cells reach the effector site to produce antigen-specific IgA in the nasal cavity to protect it against pathogen invasion [11]. Therefore, the NALT is considered to be the inductive site of the mucosal immune response in the upper respiratory tract.
Intranasal vaccination induces strong local and systemic immune responses in the respiratory and genitourinary tracts but induces only weak immune responses in the local intestinal tract (Table 1) [7, 12]. One of the reasons for directivity of mucosal immunity induction by intranasal route is that mucosal B cells induced at the inductive site have different characteristics. B cells induced by NALT express α4β1-integrin and CCR10, and therefore strongly migrate to the respiratory and genitourinary tracts highly expressing their ligands, VACM-1 and CCL28 [13, 14, 15]. Indeed, B cells co-cultured with lung DCs have been shown to more extensively migrate to the lung than to the intestinal tract [16]. On the other hand, B cells induced by intestinal inductive sites, Peyer's patches and isolated lymphoid tissues, express CCR9 and α4β7-integrin, and therefore, preferentially migrate to intestinal tracts expressing their ligands, CCL25 and MAdCAM-1 [15, 17]. Although T cells of NALT show Th0-type dominant cytokine signature and are able to differentiate into Th1 or Th2 cells immediately after antigen inoculation by the nasal route [18], T cells in Peyer's patches show memory phenotype [19]. DCs and macrophages are different between NALT and intestinal tracts (Table 1) [9, 20, 21]. NALT has a low frequency of CD103+ DCs, and CD11b+CD64–F4/80– DCs occupy the majority of DC subsets in NALT. By contrast, Peyer's patches have a high frequency of CD103+ DCs [9]. Both CD103+ DCs in NALT and intestinal tracts are able to incorporate antigens and migrate to draining lymph nodes. Oral vaccination induces a strong mucosal immune response in intestinal tracts but not in the upper airways. Antigens require modification for oral vaccination to be protected from digestion before reaching the intestinal tract, and oral vaccines tend to induce immune tolerance to antigens [22]. Taken together, in several immunological respects nasal vaccines are superior to oral vaccines against pathogens that infect the respiratory tract.
Table 1.
Comparison of nasal and intestinal mucosal immunity
| Nasal mucosal immunity | Intestinal mucosal immunity | Ref. | |
|---|---|---|---|
| Inductive sites | NALT | Peyer's patch and isolated lymphoid follicles | 7, 12 |
| Effective sites | Upper airways, lacrimal, nasal, and salivary glands | Small and large bowels | 7, 12 |
| T cells | Naïve T cell | Memory T cell | 18, 19 |
| B cells | CD62L, α4β1, CCR7, CCR10 | α4β7, α4β1, CCR9, CCR10 | 13–17 |
| DCs | CD103+CD11b– | CD103–CD11b– | 9, 20, 21 |
| CD103+CD11b+ | CD103+CD11b–CD8α+CX3CR1– | ||
| PDCA-1+ pDC | CD103+CD11b+CX3CR1low | ||
| CD11b+CD64–F4/80– | CD103–CD11b+CX3CR1int | ||
| Macrophages | CD11b+CD64+F4/80+ | CD64+CX3CR1hi | 9, 20, 21 |
| Immune response | Th1/Th2 | Th2 > Th1 | |
DCs, dendritic cells; NALT, nasal associated lymphoid tissue.
Mucosal Adjuvants for IgA Production in the Upper Respiratory Tract
Cholera toxin (CT) and Escherichia coli heat-labile enterotoxin are major mucosal adjuvants. These toxins activate the immune response to antigens, and the intranasal inoculation of influenza hemagglutinin (HA) antigen with these toxins strongly induces antigen-specific IgA production in the upper respiratory tract [23, 24, 25, 26]. CT triggers Th2-type cytokine production [27] and enterotoxin increases the Th1- and Th2-type immune responses, leading to the production of IgA in the mucosa [26]. Derivatives of these toxins, the B subunit of enterotoxin, nontoxic enterotoxin K63, and the B subunit of CT, have been developed to improve the safety of the adjuvants [26, 28].
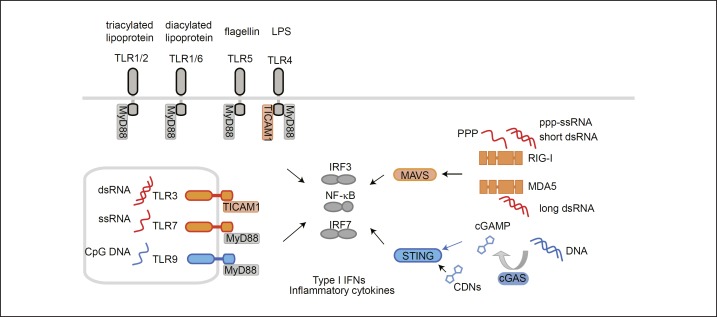
Pathogen-associated molecular patterns (PAMPs) are pathogen molecules sensed by pattern recognition receptors, including the toll-like receptors (TLRs) and RIG-I-like receptors (RLRs), in host cells (Fig. 1). The activation of the innate immune response by PAMPs evokes a subsequent acquired immune response against invading pathogens. Recent studies of the innate immune system have shown that PAMPs function as adjuvants (Table 2). The intranasal inoculation of a split vaccine against the influenza virus together with PAMPs promotes mucosal IgA production. For example, monophosphoryl lipid A, a ligand of toll-like receptor 4 (TLR4), drives the Th1 response and IgA production [29]. Polyinosine-polycytidylic acid (polyI:C), a synthetic analog of double-stranded RNA, is recognized by endosomal TLR3 and cytosolic RLRs, which then activate the innate immune response, resulting in IgA production [4]. Flagellin, a component of bacteria, is recognized by TLR5, which enhances IgA production [30, 31]. Unmethylated CpG oligodeoxynucleotide, a synthetic analog of short single-stranded DNA and a ligand of TLR9, activates DCs and B cells, resulting in the induction of IgA [32, 33]. These PAMPs induce the activation of immune cells and produce type I interferons (IFNs), which confer resistance to viruses in both myeloid and nonmyeloid cells. Therefore, type I IFNs are also used as intranasal mucosal adjuvants to promote the expression of HA antigen-specific IgA [34, 35]. The activation of DCs by IFNs might be one explanation for the mechanisms underlying the adjuvanticity of IFNs [36]. However, studies of IFN-α receptor knockout (KO) mice have shown that the adjuvanticity of TLR ligands does not necessarily depend on type I IFNs [37, 38].
Fig. 1.
Ligands for pattern recognition receptors and signaling pathways. TLRs are present in cell membranes and in endosomes. Surface TLRs recognize components of the bacterial membrane and endosomal TLRs recognize the nucleic acid components of pathogens. TLRs activate the transcription factors NF-κB, IRF3, and IRF7 via the adaptors MyD88 and TICAM1, leading to the production of type I IFNs and inflammatory cytokines. TLR4, which recognizes lipopolysaccharide, uses both MyD88 and TICAM1 as adaptors, whereas TLR3 uses only TICAM1 as an adaptor. Cytosolic RIG-I and MDA5 sense RNA and signal to activate NF-κB and IRF3 via MAVS on the mitochondria. Cytosolic DNA is converted to cGAMP by cyclic GMP-AMP synthase (cGAS) and is sensed by STING, resulting in the activation of NF-κB, IRF3, and IRF7. Orange and blue colors indicate the molecules involved in the RNA and DNA recognition pathways, respectively. CDN, cyclic dinucleotides; cGAMP, cyclic GMP-AMP; cGAS, cGAMP synthase; IFN, interferon; IRF, interferon regulatory factor; MDA, melanoma differentiation-associated protein; MyD, myeloid differentiation primary response gene; RIG-I, retinoic acid-inducible gene I; STING, stimulator of interferon genes; TICAM1, toll-like receptor adaptor molecule 1; TLR, toll-like receptor.
Table 2.
Representative nasally administered influenza vaccine adjuvant and immune responses
| Adjuvant | Receptor | Immune responses | Ref. |
|---|---|---|---|
| Cholera toxin, CTB | Ganglioside | IgA induction Protection of viral challenge T cell response |
23, 24, 26, 27 |
|
Escherichia coli heat-labile enterotoxin Enterotoxin B subunit |
Ganglioside | IgA induction Protection of viral challenge |
25, 26 |
| PolyI:C | TLR3 RIG-I MDA5 |
IgA induction Protection of viral challenge T cell response |
4 |
| Monophosphoryl lipid A | TLR4 | IgA induction T cell response |
29 |
| Flagellin | TLR5 | IgA induction Protection of viral challenge T cell response |
30, 31 |
| Unmethylated CpG oligodeoxynucleotide | TLR9 | IgA induction Protection of viral challenge T cell response |
32, 33 |
| Cyclic-di-nucleotides | STING | IgA induction Protection of viral challenge T cell response |
46 |
| Type I IFN | IFNAR | IgA induction Protection of viral challenge |
34, 35 |
CTB, cholera toxin B subunit; IFN, interferon; IFNAR, interferon alpha receptor; MDA5, melanoma differentiation-associated gene 5; polyI:C, polyinosine-polycytidylic acid; RIG-I, retinoic acid-inducible gene-I; STING, stimulator of interferon genes; TLR, toll-like receptor.
After antigen stimulation, IgA class switching occurs in B cells via T-cell-dependent (TD) and T-cell-independent (TI) mechanisms [39, 40]. Transforming growth factor (TGF)-β signaling in B cells is necessary for both TD and TI pathways. TD IgA class switching requires binding of CD40 on B cells to CD40L on CD4+ T cells. The interaction between CD40 and CD40L leads to the activation of the transcription factor NF-κB, resulting in the transcription of the activation-induced cytidine deaminase (Aicda) gene, which is an essential factor for class switching [39]. TI IgA class switching requires TLR signaling or the binding of B-cell-activating factor (BAFF) or proliferation-inducing ligand (APRIL) to the transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) on B cells rather than the CD40-CD40L interaction required by the TD pathway [39, 40]. TLR signaling and TACI binding induce NF-κB to transcribe the Aicda gene. The IgA induction mechanism in mucosal tissues has been predominantly studied in the intestinal tract, and the mechanism of IgA induction in the nasal mucosa is still largely unclear. However, to develop an efficient vaccine against a virus that enters the host cells in the upper respiratory tract, it is necessary to determine the molecular mechanisms by which the vaccine induces IgA production in the nasal mucosa. The molecular mechanisms by which adjuvants induce IgA in the upper respiratory tract mucosa are becoming clearer.
PolyI:C Induces IgA Production via the TLR3 Pathway
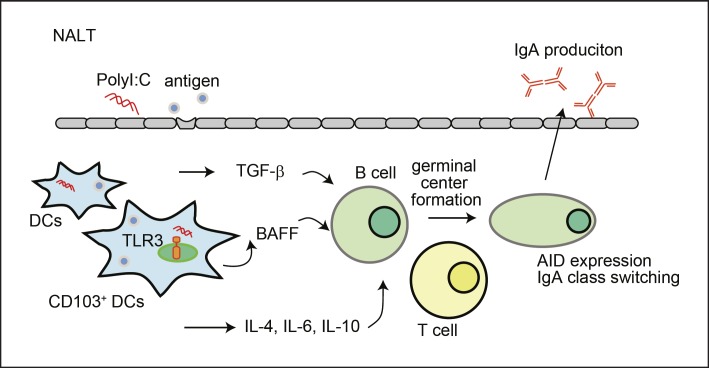
PolyI:C, a synthetic analog of double-stranded RNA, is recognized by endosomal TLR3 and the cytosolic RLRs, RIG-I and MDA5, which induce type I IFN production via their adaptor molecules, TLR adaptor molecule 1 (TICAM1, also called TRIF) and mitochondrial antivi ral signaling protein (MAVS, also called VISA, IPS-1, and Cardif), respectively (Fig. 1) [41]. PolyI:C robustly upregulated the production of antigen-specific IgA in the nasal mucosa when mice were intranasally inoculated with a combination of an HA split vaccine and polyI:C, protecting against viral challenge not only with the same influenza viral strain but also with different strains [4]. The intranasal inoculation of polyI:C upregulates the production of Th2- and Th1-type cytokines in the NALT [4]. A recent study using KO mice clearly showed that the adjuvanticity of polyI:C during vaccination is dependent on the TLR3-TICAM1 pathway, but not on the RLR- MAVS pathway [37]. IgA and IgG production, the T-cell response, the formation of the germinal centers, and expression of the Aicda gene after the intranasal inoculation of a vaccine with polyI:C were significantly impaired in TLR3-KO and TICAM1-KO mice, but not in MAVS-KO mice. The intranasally inoculated polyI:C was incorporated into the NALT CD103+ DCs, which express TLR3, and colocalized with endosomal TLR3. BAFF expression was induced on NALT CD103+ DCs by polyI:C in a TLR3-TICAM1-dependent manner. Mice lacking CD103+ DCs showed reduced vaccine-specific Ig production and a reduced T-cell response. Together, these data indicate that the intranasal inoculation of polyI:C exerts its adjuvanticity by activating the TLR3-TICAM1 pathway in the NALT CD103+ DCs and the expression of cytokines that skew IgA production in the NALT (Fig. 2) [37]. In terms of adverse effects, an adjuvant that activates only TLR3 is expected because the expression of TLR3 is restricted among the myeloid cells to CD103+ and CD8α+ DCs [42, 43]. A TLR3 adjuvant caused the regression of tumor growth in mice without a cytokine stream [44].
Fig. 2.
PolyI:C enhances IgA production in the NALT via the TLR3-TICAM1 pathway. After the intranasal administration of an HA vaccine and polyI:C, CD103+ DCs incorporate polyI:C and are activated through the TLR3-TICAM1 pathway. CD103+ DCs express BAFF and APRIL to activate B cells. PolyI:C also induces Th2-type cytokines, including IL-4, IL-6, and IL-10, in a TLR3-dependent manner. Furthermore, polyI:C enhances the formation of germinal centers and the expression of the Aicda gene, coding AID protein. Th2-type cytokines and TGF-β signaling promote IgA class switching in the NALT. The secretory IgA produced is secreted to the nasal mucosa through the polymeric immunoglobulin receptor expressed on epithelial cells in the NALT. AID, activation-induced cytidine deaminase; BAFF, B-cell-activating factor; DC, dendritic cell; HA, hemagglutinin; TGF-β, transforming growth factor β; TLR, toll-like receptor.
Cyclic-di Nucleotides Induce IgA Production in a STING-Dependent Manner
Cyclic di-nucleotides (CDNs), including cyclic (c) guanosine monophosphate (GMP)–adenosine monophosphate (AMP) (cGAMP), c-di-GMP, and c-di-AMP, are recognized by STING, which is located on the endoplasmic reticulum membrane. CDNs exert potent adjuvanticity on the vaccine effect, including Ig production, the T-cell response, and the formation of germinal centers, via the STING pathway [38, 45, 46, 47]. The adjuvanticity of c-di-GMP is more effective than that of cGAMP because it enhances antigen uptake by DCs and the selective activation of pinocytosis-efficient cells [38]. CD11c-specific STING-KO mice displayed impaired Ig production and a defective T-cell response after intranasal inoculation with c-di-GMP [45], so the adjuvanticity of c-di-GMP mainly depends on the activation of STING-expressing DCs. Although c-di-GMP also induces interleukin 33 and thymic stromal lymphopoietin TSLP in epithelial cells via the STING-independent pathway, the molecular mechanism of STING-independent cytokine production is unknown.
Conclusions
Although intranasal vaccination is less painful and simpler than previous vaccines using syringes and needles, these vaccines need strong adjuvants without side effects. Successful intranasal vaccination requires the development of appropriate adjuvants, but it is essential to clarify the mechanism of action of adjuvants as a preliminary step. Antigen-producing B cells induced by the mucosal vaccine accumulate in the effector site close to the inductive site, so it is very important to vaccinate the site where pathogens are likely to invade. Due to directivity of mucosal immunity induction by intranasal route, the development of a nasal administration vaccine is expected as a vaccine against pathogens causing upper respiratory tract infection such as the influenza virus, RS virus, Streptococcus pneumoniae, and Haemophilus influenzae. Since nasal vaccination also upregulates IgA production in genitourinary tracts, it may be one of the effective routes for the administration of vaccines against HIV and HPV.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgments
This research was supported by the Japan Agency for Medical Research and Development (grant No. 17fk0108212h0302) and the Japan Society for the Promotion of Science (grant No. 16K19134).
References
- 1.Houser K, Subbarao K. Influenza vaccines: challenges and solutions. Cell Host Microbe. 2015;17:295–300. doi: 10.1016/j.chom.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki A. Exploiting mucosal immunity for antiviral vaccines. Annu Rev Immunol. 2016;34:575–608. doi: 10.1146/annurev-immunol-032414-112315. [DOI] [PubMed] [Google Scholar]
- 3.Tamura S, Ainai A, Suzuki T, Kurata T, Hasegawa H. Intranasal inactivated influenza vaccines: a reasonable approach to improve the efficacy of influenza vaccine? Jpn J Infect Dis. 2016;69:165–179. doi: 10.7883/yoken.JJID.2015.560. [DOI] [PubMed] [Google Scholar]
- 4.Ichinohe T, Watanabe I, Ito S, Fujii H, Moriyama M, Tamura S, Takahashi H, Sawa H, Chiba J, Kurata T, Sata T, Hasegawa H. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol. 2005;79:2910–2919. doi: 10.1128/JVI.79.5.2910-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heritage PL, Underdown BJ, Arsenault AL, Snider DP, McDermott MR. Comparison of murine nasal-associated lymphoid tissue and Peyer's patches. Am J Respir Crit Care Med. 1997;156:1256–1262. doi: 10.1164/ajrccm.156.4.97-03017. [DOI] [PubMed] [Google Scholar]
- 6.Krege J, Seth S, Hardtke S, Davalos-Misslitz AC, Förster R. Antigen-dependent rescue of nose-associated lymphoid tissue (NALT) development independent of LTbetaR and CXCR5 signaling. Eur J Immunol. 2009;39:2765–2778. doi: 10.1002/eji.200939422. [DOI] [PubMed] [Google Scholar]
- 7.Kiyono H, Fukuyama S. NALT - versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain R, Waldvogel-Thurlow S, Darveau R, Douglas R. Differences in the paranasal sinuses between germ-free and pathogen-free mice. Int Forum Allergy Rhinol. 2016;6:631–637. doi: 10.1002/alr.21712. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Ruane D, Law K, Ho Y, Garg A, Rahman A, Esterházy D, Cheong C, Goljo E, Sikora AG, Mucida D, Chen BK, Govindraj S, Breton G, Mehandru S. Phenotype and function of nasal dendritic cells. Mucosal Immunol. 2015;8:1083–1098. doi: 10.1038/mi.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutoh M, Kimura S, Takahashi-Iwanaga H, Hisamoto M, Iwanaga T, Iida J. RANKL regulates differentiation of microfold cells in mouse nasopharynx-associated lymphoid tissue (NALT) Cell Tissue Res. 2016;364:175–184. doi: 10.1007/s00441-015-2309-2. [DOI] [PubMed] [Google Scholar]
- 11.Wu HY, Nguyen HH, Russell MW. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 12.Yanagita M, Hiroi T, Kitagaki N, Hamada S, Ito HO, Shimauchi H, Murakami S, Okada H, Kiyono H. Nasopharyngeal-associated lymphoreticular tissue (NALT) immunity: fimbriae-specific Th1 and Th2 cell-regulated IgA responses for the inhibition of bacterial attachment to epithelial cells and subsequent inflammatory cytokine production. J Immunol. 1999;162:3559–3565. [PubMed] [Google Scholar]
- 13.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170:3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 14.Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. 2003;111:1001–1010. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansen FE, Baekkevold ES, Carlsen HS, Farstad IN, Soler D, Brandtzaeg P. Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. Blood. 2005;106:593–600. doi: 10.1182/blood-2004-12-4630. [DOI] [PubMed] [Google Scholar]
- 16.Ruane D, Chorny A, Lee H, Faith J, Pandey G, Shan M, Simchoni N, Rahman A, Garg A, Weinstein EG, Oropallo M, Gaylord M, Ungaro R, Cunningham-Rundles C, Alexandropoulos K, Mucida D, Merad M, Cerutti A, Mehandru S. Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. J Exp Med. 2016;213:53–73. doi: 10.1084/jem.20150567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 18.Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M, Kiyono H. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur J Immunol. 1998;28:3346–3353. doi: 10.1002/(SICI)1521-4141(199810)28:10<3346::AID-IMMU3346>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Chao CC, Sandor M, Dailey MO. Expression and regulation of adhesion molecules by gamma delta T cells from lymphoid tissues and intestinal epithelium. Eur J Immunol. 1994;24:3180–3187. doi: 10.1002/eji.1830241240. [DOI] [PubMed] [Google Scholar]
- 20.Persson EK, Scott CL, Mowat AM, Agace WW. Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. Eur J Immunol. 2013;43:3098–3107. doi: 10.1002/eji.201343740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerovic V, Bain CC, Mowat AM, Milling SW. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol. 2014;35:270–277. doi: 10.1016/j.it.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010;6:e1001147. doi: 10.1371/journal.ppat.1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura S, Samegai Y, Kurata H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6:409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 24.Tamura S, Yamanaka A, Shimohara M, Tomita T, Komase K, Tsuda Y, Suzuki Y, Nagamine T, Kawahara K, Danbara H. Synergistic action of cholera toxin B subunit (and Escherichia coli heat-labile toxin B subunit) and a trace amount of cholera whole toxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994;12:419–426. doi: 10.1016/0264-410x(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 25.Tamura S, Asanuma H, Tomita T, Komase K, Kawahara K, Danbara H, Hattori N, Watanabe K, Suzuki Y, Nagamine T. Escherichia coli heat-labile enterotoxin B subunits supplemented with a trace amount of the holotoxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994;12:1083–1089. doi: 10.1016/0264-410x(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 26.Pizza M, Giuliani MM, Fontana MR, Monaci E, Douce G, Dougan G, Mills KH, Rappuoli R, Del Giudice G. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001;19:2534–2541. doi: 10.1016/s0264-410x(00)00553-3. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo K, Iwasaki T, Asanuma H, Yoshikawa T, Chen Z, Tsujimoto H, Kurata T, Tamura SS. Cytokine mRNAs in the nasal-associated lymphoid tissue during influenza virus infection and nasal vaccination. Vaccine. 2000;18:1344–1350. doi: 10.1016/s0264-410x(99)00401-6. [DOI] [PubMed] [Google Scholar]
- 28.Barchfeld GL, Hessler AL, Chen M, Pizza M, Rappuoli R, Van Nest GA. The adjuvants MF59 and LT-K63 enhance the mucosal and systemic immunogenicity of subunit influenza vaccine administered intranasally in mice. Vaccine. 1999;17:695–704. doi: 10.1016/s0264-410x(98)00252-7. [DOI] [PubMed] [Google Scholar]
- 29.Baldridge JR, Yorgensen Y, Ward JR, Ulrich JT. Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine. 2000;18:2416–2425. doi: 10.1016/s0264-410x(99)00572-1. [DOI] [PubMed] [Google Scholar]
- 30.Wang BZ, Xu R, Quan FS, Kang SM, Wang L, Compans RW. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS One. 2010;5:e13972. doi: 10.1371/journal.pone.0013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong SH, Byun YH, Nguyen CT, Kim SY, Seong BL, Park S, Woo GJ, Yoon Y, Koh JT, Fujihashi K, Rhee JH, Lee SE. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine. 2012;30:466–474. doi: 10.1016/j.vaccine.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 32.Joseph A, Louria-Hayon I, Plis-Finarov A, Zeira E, Zakay-Rones Z, Raz E, Hayashi T, Takabayashi K, Barenholz Y, Kedar E. Liposomal immunostimulatory DNA sequence (ISS-ODN): an efficient parenteral and mucosal adjuvant for influenza and hepatitis B vaccines. Vaccine. 2002;20:3342–3354. doi: 10.1016/s0264-410x(02)00295-5. [DOI] [PubMed] [Google Scholar]
- 33.Moldoveanu Z, Love-Homan L, Huang WQ, Krieg AM. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine. 1998;16:1216–1224. doi: 10.1016/s0264-410x(98)80122-9. [DOI] [PubMed] [Google Scholar]
- 34.Cao M, Sasaki O, Yamada A, Imanishi J. Enhancement of the protective effect of inactivated influenza virus vaccine by cytokines. Vaccine. 1992;10:238–242. doi: 10.1016/0264-410x(92)90158-g. [DOI] [PubMed] [Google Scholar]
- 35.Bracci L, Canini I, Puzelli S, Sestili P, Venditti M, Spada M, Donatelli I, Belardelli F, Proietti E. Type I IFN is a powerful mucosal adjuvant for a selective intranasal vaccination against influenza virus in mice and affects antigen capture at mucosal level. Vaccine. 2005;23:2994–3004. doi: 10.1016/j.vaccine.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Tovey MG, Lallemand C, Meritet JF, Maury C. Adjuvant activity of interferon alpha: mechanism(s) of action. Vaccine. 2006;24((suppl 2)):–46–47. doi: 10.1016/j.vaccine.2005.01.117. [DOI] [PubMed] [Google Scholar]
- 37.Takaki H, Kure S, Oshiumi H, Sakoda Y, Suzuki T, Ainai A, Hasegawa H, Matsumoto M, Seya T. Toll-like receptor 3 in nasal CD103+ dendritic cells is involved in immunoglobulin A production. Mucosal Immunol. 2018;11:82–96–. doi: 10.1038/mi.2017.48. [DOI] [PubMed] [Google Scholar]
- 38.Blaauboer SM, Gabrielle VD, Jin L. MPYS/STING-mediated TNF-α, not type I IFN, is essential for the mucosal adjuvant activity of (3′-5′)-cyclic-di-guanosine-monophosphate in vivo. J Immunol. 2014;192:492–502. doi: 10.4049/jimmunol.1301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bemark M, Boysen P, Lycke NY. Induction of gut IgA production through T cell-dependent and T cell-independent pathways. Ann NY Acad Sci. 2012;1247:97–116. doi: 10.1111/j.1749-6632.2011.06378.x. [DOI] [PubMed] [Google Scholar]
- 41.Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. J Biochem. 2007;141:137–145. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- 42.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reise Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 43.Takaki H, Oshiumi H, Matsumoto M, Seya T. Dendritic cell subsets involved in type I IFN induction in mouse measles virus infection models. Int J Biochem Cell Biol. 2014;53:329–333. doi: 10.1016/j.biocel.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto M, Tatematsu M, Nishikawa F, Azuma M, Ishii N, Morii-Sakai A, Shime H, Seya T. Defined TLR3-specific adjuvant that induces NK and CTL activation without significant cytokine production in vivo. Nat Commun. 2015;6:6280. doi: 10.1038/ncomms7280. [DOI] [PubMed] [Google Scholar]
- 45.Blaauboer SM, Mansouri S, Tucker HR, Wang HL, Gabrielle VD, Jin L. The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo. Elife. 2015:4. doi: 10.7554/eLife.06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madhun AS, Haaheim LR, Nøstbakken JK, Ebensen T, Chichester J, Yusibov V, Guzman CA, Cox RJ. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine. 2011;29:4973–4982. doi: 10.1016/j.vaccine.2011.04.094. [DOI] [PubMed] [Google Scholar]
- 47.Takaki H, Takashima K, Oshiumi H, Ainai A, Suzuki T, Hasegawa H, Matsumoto M, Seya T. cGAMP promotes germinal center formation and production of IgA in nasal-associated lymphoid tissue. Med Sci (Basel) 2017;5 doi: 10.3390/medsci5040035. [DOI] [PMC free article] [PubMed] [Google Scholar]