Abstract
MICA are major histocompatibility complex class I-related molecules, expressed by endothelial cells (ECs), that may be targets for alloantibodies and NKG2D-expressing natural killer (NK) and T effector cells in organ allografts. This study shows that basal levels of MICA expressed on vascular ECs is sufficient to functionally modulate the expression and activity of the immunoreceptor NKG2D in allogeneic NK cells. We found that MICA expression is differentially regulated at the EC surface in response to cytokines. TNFa upregulates MICA while IFNγ significantly decreases MICA at the EC surface. Both cytokines induce the release of soluble MICA by ECs. Modulation of NKG2D correlates with the MICA level on the EC surface. Glycosylation and metalloproteinase activities account for major post-transcriptional mechanisms controlling MICA level and the function in ECs. Our results indicate that, in addition to the NFγB pathway, the mitogen-activated protein kinase pathways JNK, ERK1/2 and p38 are key signaling pathways in the control of MICA by the cytokines. Finally, we show that EC proliferation mediated by FGF-2 or wound healing increases the MICA level. Together, our data suggest that inflammation and proliferation regulate endothelial MICA expression and shedding, enabling ECs to modulate NKG2D activity on effector NK and T cells, and provide further evidence of a role for ECs in immunoregulation.
Key Words: Endothelial cells, MICA, Cytokines, NKG2D, Signaling
Introduction
The endothelium is a functional barrier between the vessel wall and blood stream, and endothelial cells (ECs) are involved in regulating several key physiological functions, including coagulation, fibrinolysis, vascular tone and growth [1]. Dysfunction of the endothelium disturbs the physiological protective regulatory balance, which is a critical factor in the progression of inflammatory [2] and cardiovascular diseases [3], and in transplant rejection [4]. Upon inflammation, ECs regulate the trafficking and modulate the functions of leucocytes by expressing, in a regulated way, chemokines, adhesion molecules and cytokines [2]. Activated ECs also modulate the immune response through innate and cognate interactions with T and natural killer (NK) cells [5, 6]. Current evidence supports the idea that ECs not only play a critical role in the recruitment of immune cells, but can also influence the outcome of the immune response [7]. Human vascular graft ECs basally display donor class I and II major histocompatibility complex (MHC)-peptide complexes on their surface and are thought to stimulate allogeneic humoral and cellular responses in vivo [6, 7, 8].
The classical human leukocyte antigen (HLA) class I loci within the MHC (HLA-A, B, C) are characterized by their ubiquitous expression and their wide polymorphism [9]. By contrast, the human MHC class I chain-related genes (MICA and MICB), located within the HLA class I region of chromosome 6, show a more restricted cell and tissue distribution. MICA proteins are physiologically expressed on the cell surface of intestinal epithelial cells, and at low levels by ECs and fibroblasts, but are not present on unstimulated T and B lymphocytes [10, 11, 12]. MICA is frequently found expressed on tumor cell lines and primary tumor cells. Moreover, early evidence for heat and virus-induced regulation of MIC protein cell-surface expression has led to the concept that MIC proteins are markers of stress in the epithelia [13]. Today, however, this view is changing, as more and more experimental evidence indicates that MIC can be expressed on a broader set of normal cells and tissues than initially expected [14].
MICA, unlike their classical HLA class I counterparts, do not bind β2-microglobulin and are independent of any transporter-associated protein which exclude a role for MICA in peptide binding and antigen presentation [15]. MICA is a ligand for the activating immunoreceptor NKG2D, a highly conserved C-type lectin-like membrane glycoprotein expressed on essentially all NK cells, as well as on γδ and αβ CD8+ T cells, in humans and mice [for reviews see [16, 17]].
Unlike other nonclassical HLA class I genes (HLAE, F and G), the MIC genes are highly polymorphic [18]. Consistent with the high polymorphism of MIC molecules, specific antibodies (Abs) against MICA have been reported in the serum of patients who had rejected kidney allografts, suggesting a potential role for these molecules in transplant immunopathology [19, 20, 21, 22]. Renal and pancreatic grafts with evidence of both acute and chronic rejection have been shown to express MIC proteins, and anti-MIC Abs have been identified in the serum of these patients. Expression of MICA and MICB in transplanted organs has been demonstrated [23]. It is likely that polymorphic MICA molecules may be targets for specific Abs and T cells in solid organ grafts [20, 23, 24].
We previously reported that alloreactive Abs binding to MHC class I and class II molecules expressed on vascular ECs mediates specific signaling events controlling EC survival and proliferation [25, 26]. Moreover, we showed that activated ECs overexpress the nonclassical MHC class I molecule HLA-E and produce a soluble form of HLA-E [27, 28]. In this study, we show that basal levels of MICA expressed on human vascular ECs are sufficient to modulate NKG2D expression and activity in allogeneic NK effector cells. We show that MICA expression was differentially regulated at the EC surface in response to cytokines (TNFα, IFNγ). We found that cytokines induced the release of soluble MICA in culture supernatant from activated vascular ECs and identified several molecular mechanisms, at post-transcriptional and signaling level, that control MICA regulation and function in ECs. Together, our data suggest that EC activation and proliferation may modulate NKG2D activity by regulating MICA surface expression and release, and thus provide further evidence for a role for ECs in immunomodulation.
Materials and Methods
Reagents and Antibodies
The following monoclonal (m)Abs were used in this study. Anti-pan HLA class I (anti-HLA-A, B, C; clone W6/32; American Type Cell Culture), anti-MICA and MICA/B (AMO1, BAM01, BAMO3), which were kindly provided by Dr. A. Steinle (Eberhard-Karls-University, Tübingen, Germany). Anti-MICA/B (clone 6D4) was from Santa Cruz Biotechnology. Anti-MICA (AF1300 and MAB1300) Abs, anti-ULBP1, ULBP2, ULBP3 and anti-NKG2D mAbs, as well as NKG2D-Fc protein were purchased from R&D Systems (Lille, France). Anti-CD107a and anti-IFNγ Abs were from Miltenyi Biotech (Paris, France). FITC and PE-conjugated anti-mouse F(ab′)2 and anti-goat IgG were from Jackson Immunoresearch Laboratories (West Grove, Pa., USA). For activation, ECs were incubated with the following recombinant human cytokines: TNFα (100 U/ml; Miltenyi Biotech), IFNγ (100 U/ml; Imukin, Boehringer Ingelheim, Ingelheim am Rhein, Germany) and IL-1β (5 ng/ml; R&D Systems). For proliferation, ECs were incubated with recombinant FGF-2 (2.5 ng/ml; R&D Systems) for 24 h after deprivation overnight in culture medium containing 2% fetal calf serum (FCS) without growth factors. Tunicamycin, monensin, brefeldin A, ionomycin, phorbol 12-myristate 13-acetate (PMA), galardin (GM6001), pyrrolidine dithiocarbamate (PDTC), LY294002 (10 μM), wortmannin (100 nM), U0126 (10 µM), SB203580 (10 μM), PD98059 (50 μM) and SP600125 (10 μM) inhibitors were purchased from Calbiochem (St. Quentin Fallavier, France).
Cell Culture
Human arterial ECs (HAEC) were isolated and characterized as we previously described [29]. ECs were cultured in EC basal medium supplemented with 10% FCS, 0.004 ml/ml ECGS/Heparin, 0.1 ng/ml hEGF, 1 ng/ml hbFGF, 1 µg/ml hydrocortisone, 50 µg/ml gentamicin and 50 ng/ml amphotericin B (C-22010; PromoCell, Heidelberg, Germany). For activation, confluent EC monolayers were starved overnight and incubated with recombinant human TNFα or IFNγ for the indicated period of time in EC basal medium supplemented with 2% FCS. ECs were used between passage 2 and 5. MICA typing of EC donors was performed as we previously described [30]. PBMC from random healthy volunteers were purified by Ficoll/Hypaque density centrifugation and NK cells were isolated (>95% of CD3-CD56+ and/or CD16+, as assessed by FACS) by negative selection using NK Cell Isolation kits (Miltenyi Biotec) according to the manufacturer's recommendations. The human NK cell line, NKL, was grown in RPMI 1640 media supplemented with 10% FCS, 4 mM glutamine, 1 mM sodium pyruvate and 200 U/ml of rIL-2 (R&D Systems). HeLa, Raji, U937 and Jurkat cell lines were from ATCC. The NKL cell line was kindly provided by Dr. Eric Vivier (Marseille, France). C1R and CIR transfected with MICA*008 (C1RMICA) cells were kindly provided by Prof. A. Toubert (INSERM Unité 940, Hôpital Saint-Louis, Paris, France) [31].
Immunofluorescence and Confocal Microscopy
ECs were grown to confluence on glass coverslips. Cultures were washed with phosphate-buffered saline (PBS) and fixed for 20 min in 4% paraformaldehyde. Cells were incubated overnight at 4°C with blocking buffer (5% BSA in PBS) and then incubated with an anti-MICA (AMO1) mAbs or NKG2D-Fc (both 10 µg/ml) or IgG isotype controls for 1 h. Cells were then incubated with FITC-conjugated goat anti-mouse or anti-human Abs (5 µg/ml; Jackson Lab.) for 1 h. Endoplasmic reticulum (ER) and Golgi staining were performed after cell permeabilization with 0.1% Triton X-100 using rhodamine-B hexyl ester (2.5 µg/ml; Molecular Probes, Eugene, Oreg., USA) and anti-golgi mAbs (5 µg/ml; anti-golgin-97, clone CDF4, Molecular Probes), respectively. Anti-golgi mAbs were revealed using TRITC-conjugated goat anti-mouse Abs (5 µg/ml; Jackson Lab.). Nuclear staining was performed using propidium iodide (Sigma). Slides were washed in PBS, dried and mounted with ProLong® antifade reagent (Molecular Probes). Fluorescence microscopy was performed with a Leica DM-IRBE laser scanning confocal microscope (Leica AG, Heerbrugg, Switzerland) using a 63 × 1.4 oil p-aplo lens and analyzed using Leica TCS NT software.
Immunoblotting
Cells were lysed on ice in 20 mM Tris-HCl (pH 7.4), 137 mM NaCl, 0.05% Triton X-100, 1 mM supplemented with protease inhibitors (Sigma). Cell lysates (20 µg) were resolved by SDS-PAGE (12%) and subjected to Western immunoblot analysis using specific Abs for MICA (BAMO1) and GAPDH (Chemicon, Val de Fontenay, France) and secondary horseradish peroxidase-labeled anti-mouse Abs (Cell Signaling Technology, St. Quentin-en-Yveline, France). Antibody-bound proteins were detected using an ECL kit (Amersham).
Flow Cytometry
Cells (1-2 × 105 cells/sample) were suspended with Trypsin-EDTA (Gibco BRL), washed twice with PBS containing 1% BSA and 0.1% NaN3, and then incubated on ice for 30 min with a saturating concentration of first Ab. After three washes, cells were incubated with a FITC-labeled goat anti-mouse F(ab′)2 IgG (Jackson Lab.) at 4°C for 30 min. Cells were fixed in 1% paraformaldehyde. Negative controls were performed using an istotype-matched IgG control. Fluorescence was measured on 10,000 cells/sample using a fluorescence-activated cell sorter (FACScalibur® and Facs Canto®, BD Bioscience, Mountain View, Calif., USA) and analyzed using CellQuestPro® software (BD Bioscience) and FlowJo® software (Tree Star Inc., Ashland, Oreg., USA). Data are depicted in histograms or dot plots of mean fluorescence intensity on a 4-decade logarithmic scale (x-axis) versus cell number (y-axis).
Short Interfering RNAs and Silencing
Short interfering RNAs (siRNAs) were designed, synthesized and purchased from Ambion (Applied Biosystems). Cells were transiently transfected with 10 mM of nontargeting (Si scramble) or MICA-, GAPDH-, ULBP2- and ULBP3-specific siRNAs (Ambion, Applied Biosystems) using LipofectAMINE™ RNAiMAX reagent according to the manufacturer's instructions (Invitrogen). The efficiency of silencing, determined by flow cytometry analyses in each experiment, ranged from 70 to 90% for the selected siRNAs.
Cell-Mediated Cytotoxicity Assays and NK Cell Activity
Target cells labeled with 51Cr were incubated with effector cells (freshly isolated NK or NKL cells) for 4 h at various E:T ratios. The supernatants were obtained after the incubation and subjected to gamma counting. The maximum or spontaneous release was defined as counts from samples incubated with 5% Triton X-100 or medium alone, respectively. Cytolytic activity was calculated with the following formula: % lysis = (experimental release - spontaneous release) × 100/(maximum release - spontaneous release). The spontaneous release in all assays was less than 20% of the maximum release. Blocking experiments were performed using anti-NKG2D-specific Abs (R&D Systems) at a concentration from 0.5 to 2 µg/ml and an isotype-matched control IgG. For redirected cytotoxicity assays, NKL cells were incubated with or without ECs for 24 h, harvested and then assayed for cytotoxicity against C1RMICA cells and C1R cells, which were used as controls. Immunostaining for CD107a and IFNγ was performed on purified NK cells from healthy donors, as we previously described [32].
ELISA for Soluble MICA
Detection of soluble (s)MICA was done using a sandwich-ELISA from Immatics (Tubingen, Germany). In brief, for detection of sMICA the mAbs AMO1 and BAMO3 were used for capture and detection at 5 and 1 µg/ml, respectively, with recombinant sMICA*04 as a standard. Assays were processed using anti-mouse IgG2a-horseradish peroxidase (1:4,000) and developed using the TMB Peroxidase Substrate Reagent (R&D Systems). Optical density was measured at 450 nm.
Scratch Wound Assays
Scratch wound assays were performed as we previously described [33]. Briefly, glass coverslips were coated with 1% gelatin and ECs were plated at a density of 2 × 105 cells/35-mm dish. Confluent monolayers were then starved for 24 h in 2% FCS-containing medium. The cell monolayer was scratched with a single pass of a pipette tip, washed twice with PBS, and then incubated in medium supplemented with growth factors and 10% FCS for 24 h. After culture, cells were fixed and stained with anti-MICA Abs (AF1300) followed by Alexa-568-labeled anti-goat F(ab′)2 (Invitrogen). Cells were then permeabilized and stained with an anti-KI-67 mAbs (clone MM1; Novocastra Labs) followed by an FITC-labeled anti-mouse F(ab′)2 (Jackson Lab.), as described above. Nuclear staining was performed using Dapi (Sigma). Slides were washed in PBS, dried and mounted with ProLong® antifade reagent (Molecular Probes). Fluorescence microscopy was performed with a Nikon Diaphot microscope (Nikon, Tokyo, Japan). For immunochemistry, cell monolayers were incubated with primary Ab at room temperature for 30 min and then incubated with the HRP-conjugated secondary Ab for 30 min, also at room temperature. Immunostaining was visualized using the DAB/H202 substrate and cells were counterstained with hematoxylin. The data are representative of experiments repeated three times with similar results.
Statistical Analysis
The data are expressed as the mean ± SD and compared using two-tailed Student's t tests, and analysis of variance when more than two conditions were compared. A value of p < 0.05 was considered statistically significant.
Results
Constitutive Expression of Functional MICA on Vascular ECs
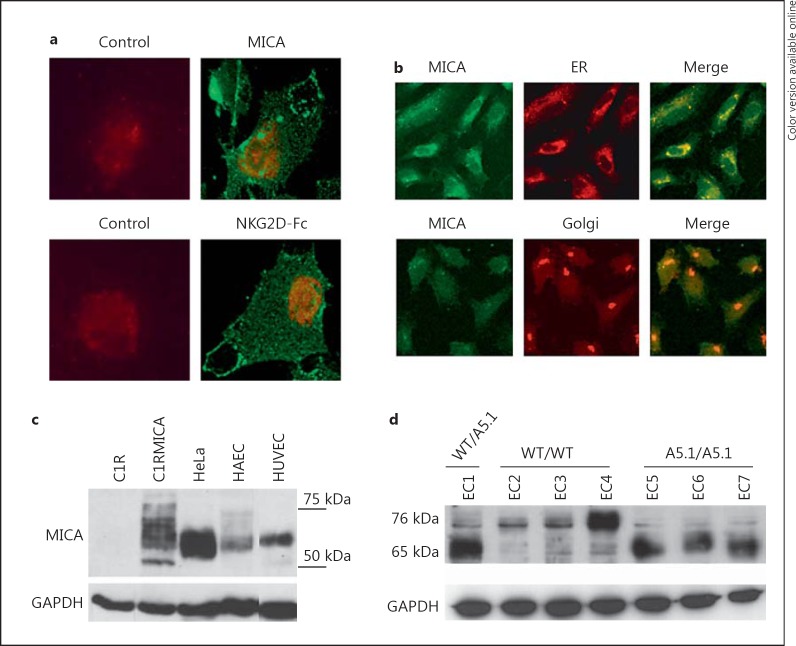
Basal expression for MICA was first examined by indirect immunofluorescence using confocal microscopy (fig. 1). EC labeling, performed on nonpermeabilized cells, using either specific anti-MICA mAbs (AMO1) or a recombinant NKG2D-Fc fusion protein, revealed a similar membrane staining confirming cell surface expression and also indicated that membrane-bound MICA could be functionally relevant due to its ability to bind to NKG2D, its receptor on effector cells (fig. 1a). Figure 1b further shows that intracellular MICA proteins colocalize with the ER while colocalization with the Golgi apparatus was only partial. Western blotting confirmed the expression of MICA in ECs (HAEC and HUVEC; fig. 1c). Immunoblotting also revealed MICA variability among cells that may suggest cell type-specific heterogeneity among the N-linked carbohydrates of mature MICA and/or MICA level. In addition, the MICA gene is highly polymorphic and a mutation in exon 5 (A5.1) causes a frameshift mutation leading to a premature intradomain stop codon [34]. This genetic variant encodes a truncated MICA protein with no cytoplasmic tail detected in ECs at 65 kDa in A5.1 homozygous individuals instead of 75 kDa for wild-type MICA (fig. 1d). We recently demonstrated that MICA A5.1 variant promotes allosensitization in kidney transplantation [30].
Fig. 1.
MICA expression and localization in human vascular ECs. a Immunofluorescence showing cell surface staining for MICA on nonpermeabilized vascular ECs using anti-MICA mAbs (AMO1) or recombinant NKG2D-Fc protein. Nuclei were stained with To-pro-3 (red; colors online version only). Negative controls were performed using IgG isotype controls (left panels). b Confocal microscope images showing the colocalization of MICA (left panel; green), rhodamine-B hexyl ester (for ER staining) or anti-golgin-97 (for Golgi staining, both middle panel; red) on permeabilized ECs. Merged images are shown in the right panel. Colocalization is shown in yellow. Original magnification ×63. c Immunoblotting of MICA in cellular lysates from C1R, C1RMICA, HeLa and primary cultures of human vascular ECs (HUVEC and HAEC). d Immunoblot for MICA showing the impact of A5.1 genetic variant on MICA protein length. Immunoblots were performed on cell lysates from EC cultures issued from individuals without the A5.1 mutation (WT/WT), or hemizygous (WT/A5.1) or homozygous (A5.1/A5.1) individuals bearing the MICA A5.1 genetic variant. c, d Blots were reprobed using anti-GAPDH mAbs to ensure equal loading. Immunoblots shown are representative of 3 independent experiments.
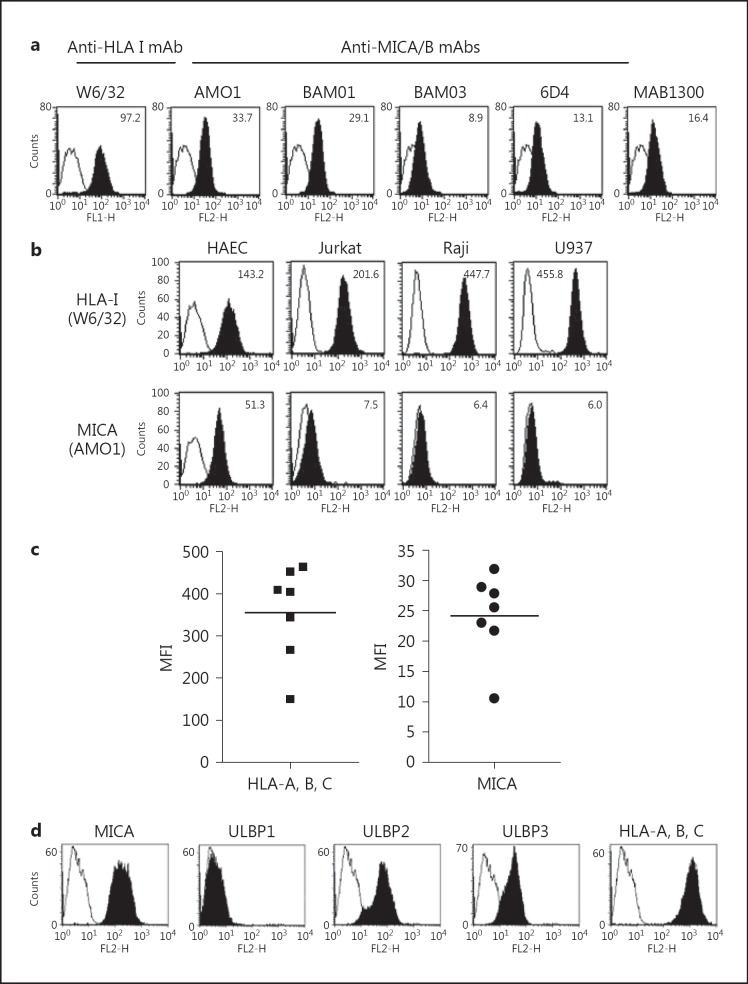
FACS analysis, performed using different anti-MICA-specific mAbs, further confirmed that MICA is expressed by human vascular ECs (HAEC) at the cell surface (fig. 2a). By contrast, no MICB was detected (data not shown). No significant expression for MICA was found on lymphoid (Jurkat and Raji) and monocytoid (U937) cell lines (fig. 2b). When compared to classical HLA, MICA expression was lower than expression for classical HLA class I (HLA-A, B, C) but was significant and consistently observed, at roughly similar levels, on ECs (HAEC) issued from different individuals (n = 7; fig. 2c). No correlation between HLA class I and MICA basal levels was observed. In addition to MICA, ECs also express at least two other NKG2D ligands, ULBP2 and ULBP3 (fig. 2d).
Fig. 2.
Constitutive expression of functional MICA at the EC surface. a Flow cytometry analysis comparing HLA-A, B, C and MICA expression at the cell surface on resting, unstimulated HAEC using various anti-MICA/B mAbs. The mean of specific fluorescence intensities are indicated. b Comparative analysis of basal MICA expression on ECs and lymphoid T (Jurkat), B (Raji) and monocytoid (U937) cell lines. c Profiles of classical HLA class I and MICA expression on vascular ECs isolated from transplant donors. HAEC isolated from normal volunteers (n = 7) were stained with mAbs for HLA-A, B, C (W6/32) or MICA (AMO1) as described in Materials and Methods. Cell surface expression of HLA-A, B, C and MICA cells was examined by flow cytometry and was expressed as the mean of fluorescence intensity (MFI); the horizontal bar represents the mean of the 7 determinations. d Comparative analysis of NKG2D ligands expressed at the EC surface. A representative analysis showing the expression of MICA, ULBP1, ULBP2, ULBP3 and HLA-A, B, C on ECs assessed by flow cytometry. Means of specific fluorescence intensity are indicated. Data are representative of 3 independent experiments.
Function of Endothelial MICA Proteins toward NKG2D Expression, NKG2D-Mediated Cytotoxicity and NK Activity
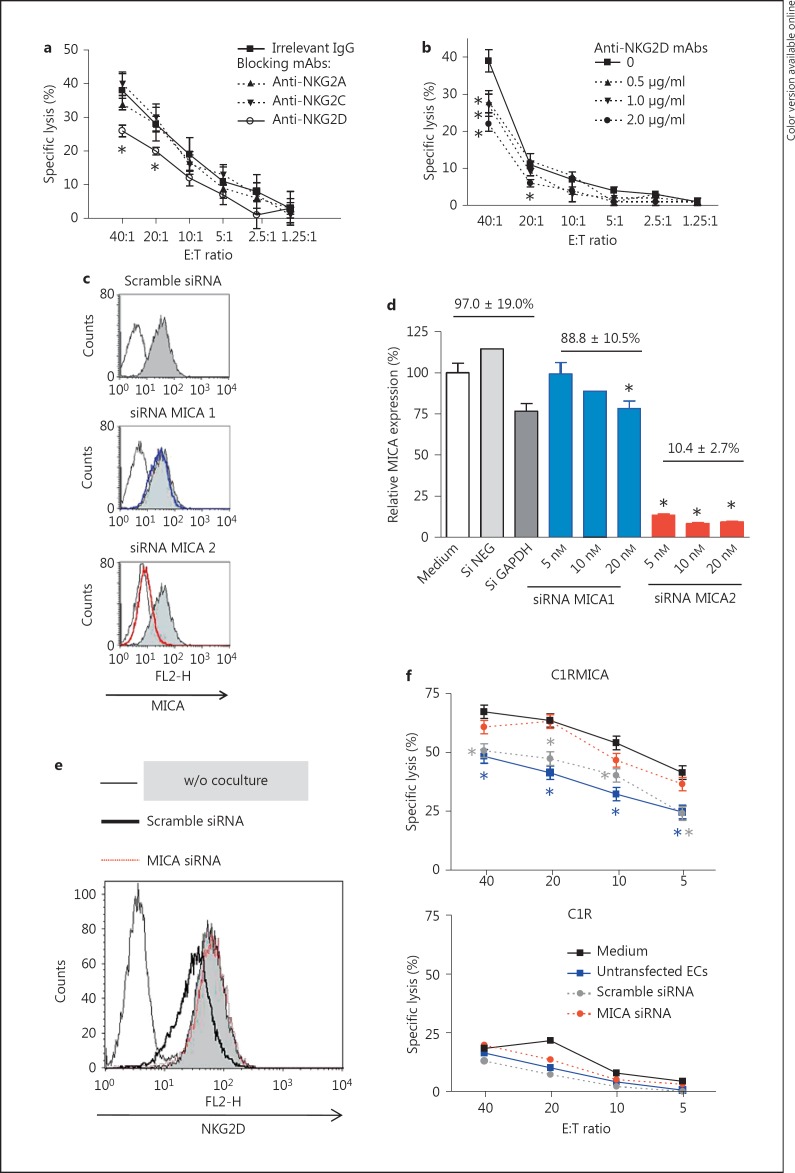
As a first attempt to assess the function of the MICA-mediated signal in NK cell activation and cytotoxic activity, a cytotoxicity assay using freshly isolated NK cells and allogeneic HAEC, as target cells, was performed in the presence of anti-NKG2 receptors blocking mAbs (fig. 3). First, we found that NK cells efficiently kill allogeneic ECs with maximum specific lysis ranging from 40 to 75% at a high E:T (40:1) ratio depending on the effector/target donor combination (data not shown). Figure 3a indicates that, among NKG2 receptors, only NKG2D blockade significantly protects ECs from NK-mediated cytotoxic activity. NKG2D blockade decreases NK-mediated EC lyses with a maximal reduction of 44 ± 4% as compared to controls (p < 0.05), suggesting the ability of basal MICA level on ECs to activate NK cells through a NKG2D-dependent process (fig. 3b). To sustain this finding we sought to silence MICA in EC cultures using specific siRNAs. Figure 3c, d respectively illustrate and quantify the inhibition of MICA at the EC surface using specific and control (Scramble or GAPDH) siRNAs. Up to 90% of inhibition was achieved. Next, transfected ECs were used to address the functional consequences of constitutive MICA expression on vascular ECs, and NKG2D expression and activity. To this aim, NKL cells were cocultured for 24 h with untransfected and siRNA-transfected ECs. Next, NKG2D expression was examined by flow cytometry (fig. 3e) and NKL cells were used as effectors in redirected cytoxicity assays toward C1R or C1RMICA cells (fig. 3f). FACS analysis showed that NKG2D surface levels were reduced on NKL cells cultured with ECs for 24 h when compared with controls. Expression of CD94, used as a control, was found to be unchanged (data not shown). NKG2D downregulation was abrogated using MICA- silenced ECs. Functionally, NKG2D downregulation induced by control ECs (untransfected and scramble siRNA) correlates with a significant increase in cytotoxicity (from 63.6 to 41.3 and 47.3% at a 20:1 ratio for untransfected and scramble siRNA; p < 0.05). Silencing MICA restores redirected NK cytotoxic activity (63.2 vs. 63.6% lysis without coculture at a 20:1 ratio; fig. 3f). The impact of MICA on NK activity was further confirmed by CD107a and IFNγ staining using purified NK cells from independent donors (fig. 4). Together these data suggest that MICA expressed at the endothelial surface modulates NKG2D expression and activity.
Fig. 3.
Basal MICA expressed on ECs triggers a functional duality toward NKG2D-bearing cells. a, b Endothelial MICA activates NK cell cytotoxicity. Cytotoxicity assays were performed using ECs as target cells and freshly isolated polyclonal allogeneic NK cells as effectors. NK cells were preincubated with culture medium, control IgG (mouse IgG1; 10 µg/ml) and anti-NKG2A, NKG2C or anti-NKG2D mAbs (0.5-2 µg/ml) for 20 min at room temperature before the addition of ECs. Results are expressed as the mean of specific lysis ± SD, and are representative of at least 3 independent experiments. * p < 0.05 versus control. c, d Silencing of MICA in vascular ECs by the use of siRNAs. The efficiency of two siRNAs to silence MICA was measured by FACS as illustrated by representative histograms. A quantitative analysis comparing the blocking effect of two MICA-targeting siRNAs (1 and 2) and control siRNAs is shown. Data are expressed in percentages ± SD as relative MICA expression in comparison with mock-transfected ECs (medium). * p < 0.05 versus mock-transfected ECs. e, f Functional regulation NKG2D expression and activity triggered by basal endothelial MICA is abrogated by silencing. e A representative FACS analysis showing NKG2D expression on NKL cells after a coculture period with or without a monolayer of ECs transfected with a nontargeting (scramble) or targeting MICA siRNA. f After a 24-hour coculture period with ECs, NKL cells were used in a redirected cytotoxicity assay using MICA-transfected C1R (C1RMICA; upper panel) or C1R (lower panel) cells as targets. Results are expressed as the mean of specific lysis ± SD, and are representative of 3 independent experiments. * p < 0.05 versus control (i.e. basal NKG2D on NKL before coculture).
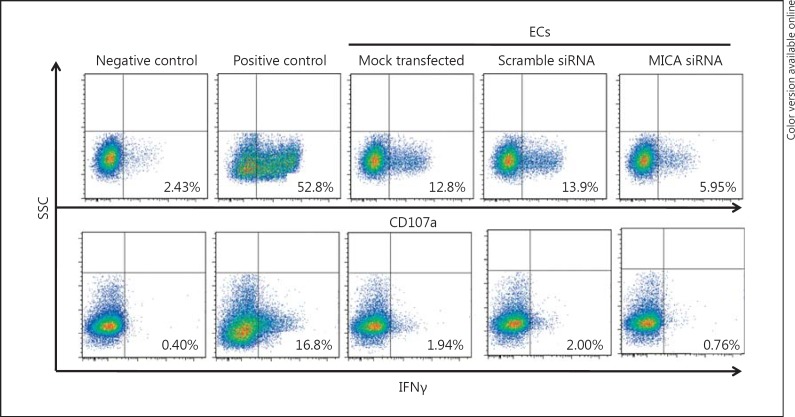
Fig. 4.
MICA silencing in vascular ECs impairs NK cell degranulation and IFNγ production. Primary cultures of ECs were mock transfected or transfected with either nontargeting (scramble) or MICA-targeting siRNAs. Twenty-four hours post-transfection, ECs were incubated with freshly isolated and purified NK cells at E:T ratio 20:1 for 6 h. Immunostaining for CD107a and intracellular IFNγ were performed as described in Materials and Methods. Untreated NK cells and NK cells stimulated with PMA and ionomycin were used as negative and positive controls, respectively. The percentages of positive cells are indicated. Results are representative of 3 independent experiments.
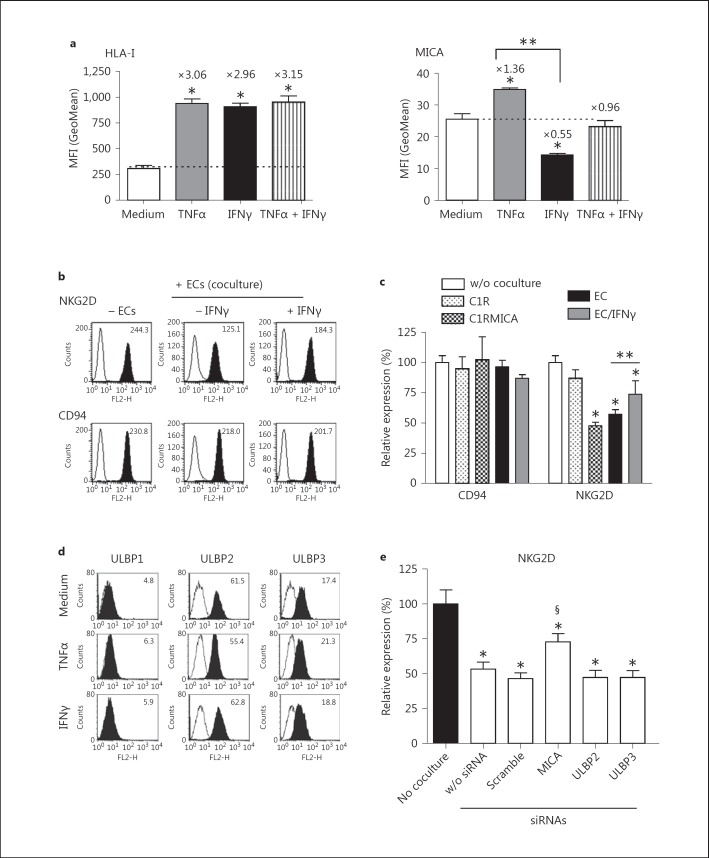
TNFα and IFNγ Trigger Opposite Regulations of MICA at the EC Surface
EC stimulation with proinflammatory cytokines, such as TNFα and IL1β, or IFNγ modify EC phenotype and functions leading to a status referred to as EC activation [8]. As previously reported [35], we observed that both TNFα and IFNγ significantly increase the expression of classical HLA class I (HLA-A, B, C) on human ECs (fig. 5a). At 24 h post-treatment, TNFα and IFNγ induces a 3.06-fold and 2.96-fold increase as compared to the control, respectively (n = 4; p < 0.05). No additive effect was found when TNFα and IFNγ were used in combination. In contrast to HLA class I, figure 5a shows that MICA is differentially regulated at the EC surface in response to TNFα or IFNγ (p < 0.05). TNFα promotes a significant but moderate increase in MICA level (1.36-fold increase as compared to basal level; p < 0.05) while IFNγ reduces MICA level on ECs (0.55-fold increase as compared to basal level; p < 0.05). No significant regulation was found when TNFα and IFNγ were used in combination. The biological relevance of MICA downregulation was assessed by analyzing the capacity of cytokine-treated ECs to downmodulate NKG2D expression in coculture experiments. Our findings indicate that an IFNγ-mediated decrease of cell surface MICA correlates with a partially impaired regulatory effect of ECs on NKG2D expression (fig. 5b, c). No significant impact was observed when cells were treated with TNF alone or in combination with IFNγ (data not shown). Together these findings could suggest that the moderate reduction in MICA level (50%) induced by IFNγ is sufficient to functionally affect NKG2D-bearing cells. In contrast to MICA, no regulatory effect of TNFα or IFNγ was observed for ULBP1, ULBP2 and ULBP3 (fig. 5d) and silencing ULBP2 or ULBP3 in ECs did not restore NKG2D expression (fig. 5e).
Fig. 5.
Comparative effect of cytokines on MICA expression and function. a FACS analysis comparing HLA-A, B, C (left) and MICA (right) expression at the EC surface before and after 48 h of treatment with TNFα and/or IFNγ. Results are expressed as a quantitative analysis from 4 separate experiments. * p < 0.05 versus controls; ** p < 0.05 versus untreated (medium) ECs. b FACS analysis showing NKG2D and CD94 levels expressed on NKL cells after a coculture period with resting or IFNγ-activated ECs. The means of specific fluorescence intensity are indicated above the histograms. Data are representative of 3 separate experiments. c Quantification of CD94 and NKG2D expression on NKL after a 24-hour coculture period with C1R, C1RMICA, HeLa, ECs (HAEC, resting or stimulated for 48 h with 100 U/ml IFNγ). * p < 0.05 versus controls; ** p < 0.05 versus untreated ECs. d FACS analysis comparing ULBP expression at the EC surface before and after 48 h of treatment with TNFα or IFNγ. The means of specific fluorescence intensities are indicated. Data are representative of 3 separate experiments. e Functional impact of NKG2D ligand silencing in EC cultures. ECs were transfected with specific siRNAs targeting MICA, ULBP2 or ULBP3, a scramble siRNA or mock transfected (w/o siRNA). Coculture experiments using transfected ECs and NKL cells were performed and NKG2D expression was analyzed by flow cytometry at 24 h. The results shown are expressed as relative expression (%) ± SD of NKG2D as compared to basal NKG2D levels before coculture from 4 separate experiments. * p < 0.05 versus basal NKG2D level; § p < 0.05 versus NKG2D level in coculture with transfected controls.
Glycosylation, Matrix Metalloproteinase Activity and Shedding Provide a Post-Transcriptional Control to MICA Expression
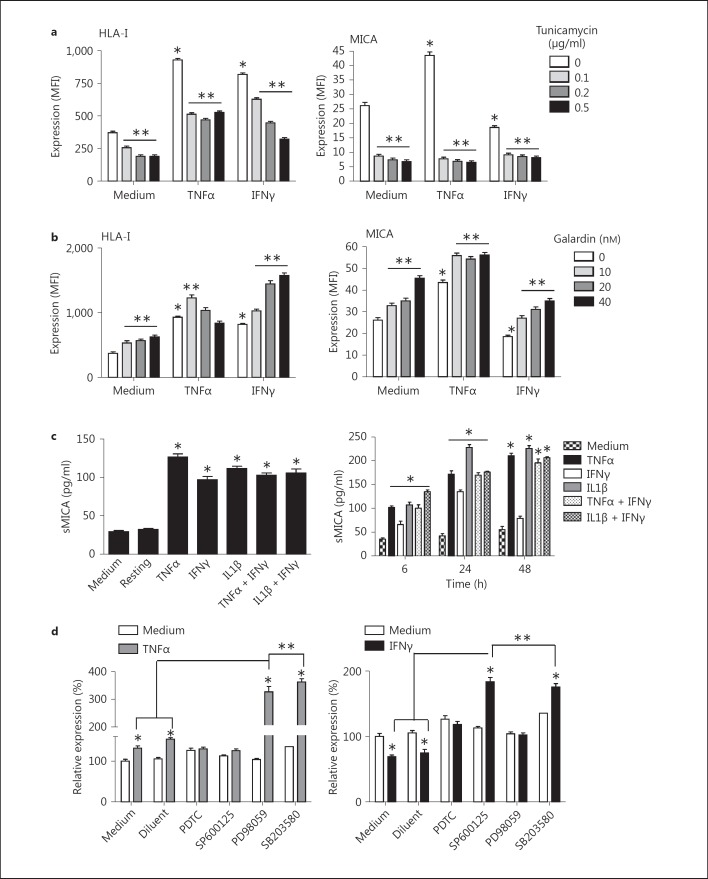
Since, as demonstrated above, only slight modulation in MICA level is sufficient to deeply affect MICA receptor, we sought to investigate the molecular mechanisms controlling MICA expression in ECs. Mechanistically, we observed that cell surface expression was weakly affected by blockade of mRNA or protein synthesis with actinomycin D and cycloheximide, respectively (data not shown), but mostly rely on protein glycosylation and activity of matrix metalloproteinases (MMPs). In figure 6a, we show that inhibition of protein glycosylation with tunicamycin strongly reduces MICA at the cell surface. Inhibition was achieved with a low dose of tunicamycin (0.1 µg/ml) and was similar and consistently observed on both resting and cytokine-activated ECs. As a comparison, tunicamycin also reduced both basal and regulated classical HLA class I expression but did not prevent HLA class I upregulation by TNFα and IFNγ.
Fig. 6.
Post-transcriptional and signaling events controlling MICA expression. a Impact of cell glycosylation. FACS analysis comparing HLA-A, B, C (left) and MICA (right) expression at the EC surface before and after 24 h of treatment with tunicamycin. Results are expressed as a quantitative analysis from 4 separate experiments. b Impact of MMP activity. FACS analysis comparing HLA-A, B, C (left) and MICA (right) expression at the EC surface before and after 24 h of treatment with galardin. Results are expressed as a quantitative analysis from 4 separate experiments. c Release of soluble MICA in conditioned medium from activated ECs. ECs were treated for various periods of time with TNFα, IL1β or IFNγ. After treatment, cell culture supernatants were collected and soluble MICA was quantified using a sandwich ELISA assay. The left panel shows the level of sMICA in supernatants from cytokine-activated ECs at 6 h and the right panel shows a time-course analysis of sMICA release by ECs treated with cytokines. Results are shown as the mean of triplicates and are representative of 3 independent experiments. * p < 0.05 versus controls without cytokines. d Signaling pathways triggering changes in MICA expression in response to cytokines. A quantitative analysis from 4 independent experiments performed by FACS analysis and showing the cell surface level of MICA on ECs treated with TNFα (100 U/ml; left) or IFNγ (100 U/ml; right) for 24 h after a 1-hour preincubation with selective inhibitors of signaling pathways. Results from 4 independent experiments are expressed as percentages of MICA. * p < 0.05 versus controls without cytokines; ** p < 0.05 versus cytokine-treated controls (medium and diluent controls).
Tumor cells of epithelial origin spontaneously release a soluble form of MICA (sMICA) encompassing the three extracellular domains, which is present at high levels in the sera of patients with gastrointestinal malignancies [36]. Soluble MICA downregulates NKG2D receptor expression and thus may account for tumor immune escape [37]. Therefore, we decided to investigate whether shedding of MICA from activated ECs may also occur upon inflammation. For this, cultured ECs were pretreated with galardin, an inhibitor of MMPs, before stimulation with cytokines. MICA expression was analyzed by FACS and compared to the expression of HLA class I molecules. Figure 6b indicates that inhibition of MMPs significantly enhances MICA expression. This effect was dose dependent and a maximal effect was observed at 40 nM. Interestingly, MMP blockade totally prevents the MICA downregulation in response to IFNγ. Similar results were obtained with classical HLA class I molecules. Due to the role of MMP in both basal and regulated expression for MICA, we sought to determine whether ECs could release sMICA using a dedicated ELISA assay. An increased level of sMICA was detected in the culture supernatants from activated ECs as compared to resting ECs (fig. 6c). sMICA levels of IFNγ-treated ECs were significantly lower than in supernatants from cells activated with TNFα or IL1β, and were maximal at 24 h (≤150 ng/ml), indicating that lower expression in response to IFNγ also correlates with a lower shedding of sMICA. However, we cannot rule out the possibility that IFNγ directly impairs MICA shedding.
ERK1/2, p38, JNK Mitogen-Activated Protein Kinase Pathways and NFκB Signaling Pathways Are Involved in Cytokine-Mediated Regulation of MICA in ECs
Modulation of EC functions involves three major signaling pathways: the phosphatidylinositol 3-kinase (PI3-K), NFκB and mitogen-activated protein kinase (MAPK) pathways [38, 39]. The respective involvement of these signaling pathways in MICA expression and regulation by ECs was examined by FACS in comparison to HLA-class I. Hence, ECs were pretreated with or without inhibitors of NFκB (PDTC, IKK2 inhibitor), PI3-K (wortmannin, LY294002), ERK1/2 (U0126, PD98059), p38 (SB203580) and JNK (SP600125) MAPK for 1 h before activation with TNFα or IFNγ for 24 h, a time point leading to maximal MICA regulation. Specific and efficient inhibition of the respective pathways was confirmed for each inhibitor by Western blotting, as we previously described [40] (data not shown). In figure 6d we show that blockade of NFκB and JNK MAPK prevents MICA upregulation by TNFα, while downregulation by IFNγ required NFκB and ERK1/2 MAPK, suggesting a key role for these pathways and a central implication for NFκB, as recently reported [41]. Unexpectedly, inhibition of JNK and p38 MAPKs not only abrogates decrease in MICA by IFNγ, but also strongly enhances MICA in stimulated ECs. Inhibition of ERK1/2and p38 MAPKs strongly increases MICA upregulation in response to TNFα. These findings suggest that, in addition to the NFκB pathway, the MAPKs are key players in both positive and negative control of MICA by IFNγ and TNFα. No significant effect was obtained using PI3-K inhibitors, suggesting that this pathway is not implicated in the regulation of MICA by cytokines in the ECs (data not shown). Interestingly, we found that, in parallel experiments, HLA-A, B, C upregulation by both TNFα and IFNγ was strictly NFκB dependent (data not shown).
EC Proliferation Regulates MICA Expression at Cell Surface
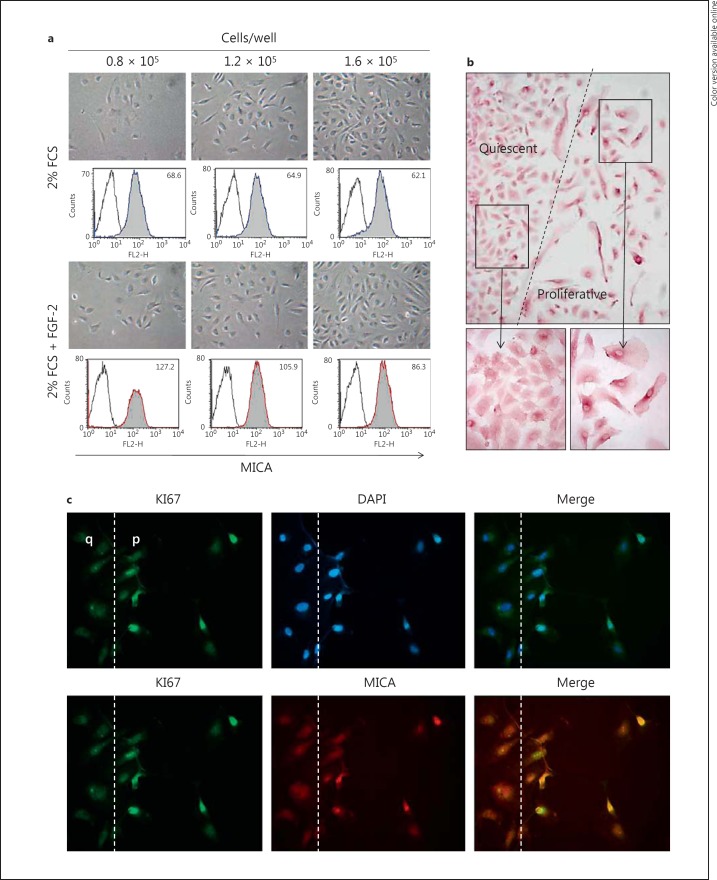
Next, we sought to explore whether cellular events implicated in EC dysfunction or injury, such as loss of endothelial monolayer integrity and EC proliferation, could either promote or impair MICA expression (fig. 7). Firstly, the respective effect of cell density and proliferation was measured by FACS analysis. We found that FGF-2-induced EC proliferation greatly promotes MICA expression at the cell surface as compared to quiescence. Our results also suggest that MICA expression decreases when EC cultures reach confluence and when ECs stop to proliferate (fig. 7a). Functionally, using an NK-mediated cytotoxicity assay, we observed that EC quiescence significantly reduces NK cell activation compared to proliferating ECs, confirming the direct relationship between endothelial MICA level and NKG2D activation established above (data not shown). Scratch and wound healing assays were established in cultured EC monolayers to further investigate the regulation of MICA in endothelium repair processes. After wound induction, ECs were cultured in the presence of culture medium supplemented with growth factors for 24 h before analyses. EC proliferation and wound repair were assessed by immunofluorescence using anti-KI67 staining to identify proliferative ECs and anti-MICA mAbs in double-staining assays in addition to conventional immunostaining for MICA by immunochemistry. Figure 7b, c illustrates our findings that upon vascular repair proliferative ECs expressed a higher level of MICA at the cell surface.
Fig. 7.
Regulatory effect of cell contact inhibition and proliferation on MICA levels. a ECs were plated at various cell densities (0.8, 1.2 and 1.6 × 105 cells/well) and cultured for 12 h with a starvation medium (2% FCS) or were induced to proliferate with FGF-2 (2.5 ng/ml). Cells were then harvested and MICA expression was analyzed by flow cytometry using anti-MICA mAbs (AMO1). Representative photographs of EC monolayers for the various conditions are shown in the upper panels (phase contrast microscope; original magnification ×10). Lower panels show a representative analysis of MICA by flow cytometer. Specific means of fluorescence intensity are indicated. b, c EC monolayers were mechanistically wounded using sterile pipette tips, allowed to repair for 24 h and then fixed. b ECs were stained first using anti-MICA mAbs and then incubated with HRP-conjugated secondary Ab. Immunostaining was visualized using the DAB/H202 substrate and cells were counterstained by HE. c Cells were first stained with an anti-MICA mAbs using a TRITC-labeled secondary Ab (red; colors online version only) and subsequently permeabilized and stained with a FITC-labeled anti-KI67 Ab (green). Nuclei were stained with Dapi (blue). Merged images are shown in the right panel. Original magnification ×40. The wound edge is indicated by a broken line. q = Quiescent; p = proliferative. Data are representative of 5 separate experiments.
Discussion
This study examined the expression of MICA and its regulation on vascular human ECs. Our results confirm that MICA is constitutively expressed at the EC surface and show that basal MICA expression is sufficient to allow a functional interaction with the activating receptor NKG2D expressed on NK cells. Constitutive MICA expression on ECs was consistently observed on ECs from different donors (n = 7) and using different anti-MICA Abs. No correlation between HLA class I and MICA levels was found. When we analyzed the same EC culture with 5 different anti-MICA-specific Abs (fig. 2) we found different degrees of reactivity, with the highest level of MICA obtained using the mAb AMO1 (specific for the α1α2 domains of MICA). These discrepancies in staining may reflect allelic preferences and/or a glycosylation pattern [42]. A high degree of variability in the levels of some NKG2D ligands expressed on B and T cells, monocytes and granulocytes among different donors has been previously reported [43, 44]. In contrast, our results show that basal MICA was low compared to the expression of HLA class I (A, B, C), but was consistently observed on ECs isolated from a set of individual transplant donors (n = 7).
Functionally, we demonstrated that endothelial MICA triggers an activating signal in allogeneic polyclonal NK cells through the immunoreceptor NKG2D that may account for a significant part in EC lysis by allogeneic NK cells. Indeed, basal expression of MICA on the cell surface of resting ECs rendered them susceptible to lysis by allogeneic NK cells since MICA silencing or blockade by an anti-NKG2D mAb decreased EC lysis with a dose-dependent effect. Moreover, we also observed that, in coculture assays in vitro, endothelial MICA interacting with NKG2D provides an immunosuppressive pathway by downregulating NKG2D on the NK cell surface. The C-type lectin-like NKG2D is an activating cell surface receptor expressed on a wide range of effector cells, including NK, NKT, γδT and αβT CD8+ cells [45]. When coexpressed and associated noncovalently with the adaptor protein DAP10, NKG2D transduces signals that activate or costimulate effector functions of these cytotoxic lymphocytes. NKG2D recognizes several families of ligands expressed on interacting stressed, transformed or pathogen-infected cells, including MICA and MICB encoded in the human MHC, and a diverse family of proteins present in both mice and humans, including mouse Rae1 (retinoic acid early transcript 1), H60, Mult1 (murine UL16-binding protein-like transcript 1), and the human UL16-binding proteins (ULBP) or RAET1 proteins. The contribution of sustained basal MICA expression by the endothelium to physiological and pathological events is unknown. Previous studies have established that sustained NKG2D ligand expression, even when restricted to the epithelia, elicits broad impairments in NK cell function. In contrast to the systemic immunosuppression of NK activities, primary T cell responses were mostly unaffected by NKG2D downregulation in Rae-1+ transgenic mice[46]. In addition, the diversity of NKG2D ligands may provide various modes of immunoregulation or activation. Of note, NKG2D ligands differ in their expression patterns and in their membrane associations.
In contrast with recent reports showing a clear induction of MICA on activated T cells [43], we found that the proinflammatory cytokines TNFα and IL1β only slightly increase MICA at the EC membrane (not shown). Moreover, EC stimulation with IFNγ significantly decreases endothelial MICA expression, suggesting that IFNγ may protect ECs from NKG2D-mediated allogeneic NK lysis. Overall, our data provide some evidence for a role for endothelial MICA in cellular alloimmune response beyond a role in alloimmunization. An interesting but poorly explored issue is the fact that MICA is highly polymorphic (more than 70 alleles have so far been described)[47]. Our recent studies investigated the frequency of MICA mismatch between transplant donors and recipients, and propose an association between MICA mismatch, alloimmunization and transplant outcome[30]. In addition, considering that different MICA alleles may vary in their affinity for NKG2D, these variations may affect the thresholds of recognition by NK cells and T lymphocytes, and subsequently may affect the functionality of MICA/NKG2D interaction.
Recently, we demonstrated that IFNγ upregulated the nonclassical MHC class Ib molecule HLA-E. HLA-E is a ligand for the inhibitory receptor CD94/NKG2A on ECs, and thus provides a protective pathway to ECs[27]. Consequently, both the inhibitory and activating NKG2 receptor complexes interact with the nonclassical MHC class I molecules HLA-E and MICA on the surface of target cells. Thus, the ratio of ligands for inhibitory and activating immunoreceptors expressed on the endothelial surface is likely to be an important determinant of the functional status of an NK cell. Competition for binding of activating and inhibitory NKG2 molecules to CD94 may also regulate the balance between receptors expressed on the cell surface. Moreover, our data also indicate that cytokines promote the release of soluble HLA-E and MICA by ECs. Both soluble molecules are biologically active and confer an overall inhibition to NK cytotoxic activity. Together these findings may suggest a role for ECs in a specific immune suppression pathway.
Shedding of MICA could be an additive step of a regulatory process required for a fine tune control of MICA expression at the EC surface. Our study provides the first line of evidence that activated ECs release soluble MICA in the extracellular compartment. We demonstrated that inhibition of MMP increases MICA at the cell surface, suggesting that MMPs contribute to the shedding of sMICA from ECs as reported for tumor cells [36]. The presence of post-transplant sMICA was found to be associated with functioning grafts without acute rejection episodes [48]. The possible contribution of sMICA as a bioactive molecule modulating T and NK cell alloreactivity and/or as a biomarker characterizing graft endothelium status remains to be established.
We also observed that MICA was differentially expressed according to EC density and proliferation. Our findings suggest that MICA level increases on proliferating ECs as compared to resting ECs. These findings are consistent with a recent study showing that NKG2D ligands, including MICA, were expressed on a large fraction of antigen-activated CD4+ and CD8+ proliferating T cells, ranging from 10 to 50% [43]. Similarly, Zou et al. [49] also demonstrated that contact inhibition causes strong downregulation of expression of MICA in human fibroblasts and decreased NK cell killing. Overall, these data suggest a fine tune regulated expression of MICA related to a particular state of activation, proliferation or differentiation that could suggest a role for MICA besides controlling immune functions.
The molecular mechanisms controlling MICA expression in ECs are not well established. The first intron of the MICA gene contains an NFκB-binding site that binds p65 (RelA)/p50 heterodimers and p50/p50 homodimers of the NFκB transcription factor family. A previous report showed that these NFκB complexes play a role in the regulated expression of the stress/activation-inducible MICA molecule [50]. Recently, the role of the proximal −130-bp NFκB site was reported as necessary and sufficient for transcriptional transactivation of MICA in the response to TNFα in primary ECs [41]. Our results confirm a role for the NFκB pathway in the cytokine-regulated expression of MICA. In addition, we demonstrate that the MAPK ERK1/2, p38 and JNK also participate in the regulation of MICA by cytokines. Inhibition of JNK MAPK was also able to prevent both the upregulation by TNFα and downregulation by IFNγ, suggesting a pivotal role for NFκB and JNK MAPK in the molecular processes controlling the MICA level on the endothelium. Consistent with our data, the MAPK inhibitors were able to block MICA/B expression in T [51], MRO87 and HeLa cells [52]. This demonstration of the intracellular pathways involved in activation-induced expression of MICA may reveal potential targets for immune intervention to modulate MICA expression.
To conclude, these results provide a basis for further investigation of the biology underlying the endothelial expression of MICA and the shedding of soluble MICA molecules, as well as their impact in both innate and allospecific immune responses and in transplant outcome.
Disclosure Statement
The authors disclose no financial or commercial conflicts of interest.
Acknowledgments
This work was supported by grants from l'Agence de Biomédecine and La Société de Néphrologie. This work was supported by an EU-funded Integrated Project in Life Sciences, Genomics and Biotechnology for Health LSHB-CT-2006-037377, funding from the Centre Européen des Sciences de la Transplantation et Immunothérapie, the Agence Nationale pour la Recherche, and Laboratoire d'Excellence (LabEx) TRANSPLANTEX. A. Chauveau was supported by a grant from La Société de Néphrologie.
References
- 1.Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Bussolino F, Introna M. Cytokine regulation of endothelial cell function: from molecular level to the bedside. Immunol Today. 1997;18:231–240. doi: 10.1016/s0167-5699(97)81662-3. [DOI] [PubMed] [Google Scholar]
- 3.Dimmeler S, Haendeler J, Zeiher AM. Regulation of endothelial cell apoptosis in atherothrombosis. Curr Opin Lipidol. 2002;13:531–536. doi: 10.1097/00041433-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 5.Choi J, Enis DR, Koh KP, Shiao SL, Pober JS. T lymphocyte-endothelial cell interactions. Annu Rev Immunol. 2004;22:683–709. doi: 10.1146/annurev.immunol.22.012703.104639. [DOI] [PubMed] [Google Scholar]
- 6.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briscoe DM, Sayegh MH. A rendezvous before rejection: where do T cells meet transplant antigens? Nat Med. 2002;8:220–222. doi: 10.1038/nm0302-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pober J, Orosz CG, Rose ML, Savage CO. Can graft endothelial cells initiate a host anti-graft immune response? Transplantation. 1996;61:343–349. doi: 10.1097/00007890-199602150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Mason PM, Parham P. HLA class I region sequences, 1998. Tissue Antigens. 1998;51:417–466. doi: 10.1111/j.1399-0039.1998.tb02983.x. [DOI] [PubMed] [Google Scholar]
- 10.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci USA. 1996;93:12445–12450. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwirner NW, Fernandez-Vina MA, Stastny P. MICA, a new polymorphic HLA-related antigen, is expressed mainly by keratinocytes, endothelial cells, and monocytes. Immunogenetics. 1998;47:139–148. doi: 10.1007/s002510050339. [DOI] [PubMed] [Google Scholar]
- 12.Zwirner NW, Dole K, Stastny P. Differential surface expression of MICA by endothelial cells, fibroblasts, keratinocytes, and monocytes. Hum Immunol. 1999;60:323–330. doi: 10.1016/s0198-8859(98)00128-1. [DOI] [PubMed] [Google Scholar]
- 13.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 14.Schrambach S, Ardizzone M, Leymarie V, Sibilia J, Bahram S. In vivo expression pattern of MICA and MICB and its relevance to auto-immunity and cancer. PLoS ONE. 2007;2:e518. doi: 10.1371/journal.pone.0000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahram S, Mizuki N, Inoko H, Spies T. Nucleotide sequence of the human MHC class I MICA gene. Immunogenetics. 1996;44:80–81. doi: 10.1007/BF02602661. [DOI] [PubMed] [Google Scholar]
- 16.Ogasawara K, Lanier LL. NKG2D in NK and T cell-mediated immunity. J Clin Immunol. 2005;25:534–540. doi: 10.1007/s10875-005-8786-4. [DOI] [PubMed] [Google Scholar]
- 17.Bahram S, Inoko H, Shiina T, Radosavljevic M. MIC and other NKG2D ligands: from none to too many. Curr Opin Immunol. 2005;17:505–509. doi: 10.1016/j.coi.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Stephens HA. MICA and MICB genes: can the enigma of their polymorphism be resolved? Trends Immunol. 2001;22:378–385. doi: 10.1016/s1471-4906(01)01960-3. [DOI] [PubMed] [Google Scholar]
- 19.Zwirner NW, Marcos CY, Mirbaha F, Zou Y, Stastny P. Identification of MICA as a new polymorphic alloantigen recognized by antibodies in sera of organ transplant recipients. Hum Immunol. 2000;61:917–924. doi: 10.1016/s0198-8859(00)00162-2. [DOI] [PubMed] [Google Scholar]
- 20.Sumitran-Holgersson S, Wilczek HE, Holgersson J, Soderstrom K. Identification of the nonclassical HLA molecules, MICA, as targets for humoral immunity associated with irreversible rejection of kidney allografts. Transplantation. 2002;74:268–277. doi: 10.1097/00007890-200207270-00019. [DOI] [PubMed] [Google Scholar]
- 21.Amezaga N, Crespo M, Lopez-Cobos M, Millan MA, Vinas O, Sole M, Oppenheimer F, Martorell J, Ercilla MG. Relevance of MICA antibodies in acute humoral rejection in renal transplant patients. Transpl Immunol. 2006;17:39–42. doi: 10.1016/j.trim.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Terasaki PI, Ozawa M, Castro R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7:408–415. doi: 10.1111/j.1600-6143.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 23.Hankey KG, Drachenberg CB, Papadimitriou JC, Klassen DK, Philosophe B, Bartlett ST, Groh V, Spies T, Mann DL. MIC expression in renal and pancreatic allografts. Transplantation. 2002;73:304–306. doi: 10.1097/00007890-200201270-00029. [DOI] [PubMed] [Google Scholar]
- 24.Collins RW. Human MHC class I chain related (MIC) genes: their biological function and relevance to disease and transplantation. Eur J Immunogenet. 2004;31:105–114. doi: 10.1111/j.1365-2370.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- 25.Coupel S, Leboeuf F, Boulday G, Soulillou JP, Charreau B. RhoA activation mediates phosphatidylinositol 3-kinase-dependent proliferation of human vascular endothelial cells: an alloimmune mechanism of chronic allograft nephropathy. J Am Soc Nephrol. 2004;15:2429–2439. doi: 10.1097/01.ASN.0000138237.42675.45. [DOI] [PubMed] [Google Scholar]
- 26.Le Bas-Bernardet S, Coupel S, Chauveau A, Soulillou JP, Charreau B. Vascular endothelial cells evade apoptosis triggered by human leukocyte antigen-DR ligation mediated by allospecific antibodies. Transplantation. 2004;78:1729–1739. doi: 10.1097/01.tp.0000147339.31581.99. [DOI] [PubMed] [Google Scholar]
- 27.Coupel S, Moreau A, Hamidou M, Horejsi V, Soulillou JP, Charreau B. Expression and release of soluble HLA-E is an immunoregulatory feature of endothelial cell activation. Blood. 2007;109:2806–2814. doi: 10.1182/blood-2006-06-030213. [DOI] [PubMed] [Google Scholar]
- 28.Allard M, Oger R, Vignard V, Percier JM, Fregni G, Perier A, Caignard A, Charreau B, Bernardeau K, Khammari A, Dreno B, Gervois N. Serum soluble HLA-E in melanoma: a new potential immune-related marker in cancer. PLoS One. 2011;6:e21118. doi: 10.1371/journal.pone.0021118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bas-Bernardet S, Hourmant M, Coupel S, Bignon JD, Soulillou JP, Charreau B. Non-HLA-type endothelial cell reactive alloantibodies in pre-transplant sera of kidney recipients trigger apoptosis. Am J Transplant. 2003;3:167–177. doi: 10.1034/j.1600-6143.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 30.Tonnerre P, Gerard N, Chatelais M, Poli C, Allard S, Cury S, Bressollette C, Cesbron-Gautier A, Charreau B. MICA variant promotes allosensitization after kidney transplantation. J Am Soc Nephrol. 2013;24:954–966. doi: 10.1681/ASN.2012080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tieng V, Le Bouguenec C, du Merle L, Bertheau P, Desreumaux P, Janin A, Charron D, Toubert A. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc Natl Acad Sci USA. 2002;99:2977–2982. doi: 10.1073/pnas.032668099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allard M, Tonnerre P, Nedellec S, Oger R, Morice A, Guilloux Y, Houssaint E, Charreau B, Gervois N. HLA-E-restricted cross-recognition of allogeneic endothelial cells by CMV-associated CD8 T cells: a potential risk factor following transplantation. PLoS One. 2012;7:e50951. doi: 10.1371/journal.pone.0050951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devalliere J, Chatelais M, Fitau J, Gerard N, Hulin P, Velazquez L, Turner CE, Charreau B. LNK (SH2B3) is a key regulator of integrin signaling in endothelial cells and targets α-parvin to control cell adhesion and migration. FASEB J. 2012;6:2592–606. doi: 10.1096/fj.11-193383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuki N, Ota M, Kimura M, Ohno S, Ando H, Katsuyama Y, Yamazaki M, Watanabe K, Goto K, Nakamura S, Bahram S, Inoko H. Triplet repeat polymorphism in the transmembrane region of the MICA gene: a strong association of six GCT repetitions with behcet disease. Proc Natl Acad Sci USA. 1997;94:1298–1303. doi: 10.1073/pnas.94.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson DR. Locus-specific constitutive and cytokine-induced HLA class I gene expression. J Immunol. 2003;170:1894–1902. doi: 10.4049/jimmunol.170.4.1894. [DOI] [PubMed] [Google Scholar]
- 36.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 37.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 38.Pober JS. Endothelial activation: intracellular signaling pathways. Arthritis Res. 2002;4((suppl 3)):S109–S116. doi: 10.1186/ar576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 40.Fitau J, Boulday G, Coulon F, Quillard T, Charreau B. The adaptor molecule Lnk negatively regulates tumor necrosis factor-α-dependent VCAM-1 expression in endothelial cells through inhibition of the ERK1 and −2 pathways. J Biol Chem. 2006;281:20148–20159. doi: 10.1074/jbc.M510997200. [DOI] [PubMed] [Google Scholar]
- 41.Lin D, Lavender H, Soilleux EJ, O'Callaghan CA. NF-κB regulates MICA gene transcription in endothelial cell through a genetically inhibitable control site. J Biol Chem. 2012;287:4299–4310. doi: 10.1074/jbc.M111.282152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest. 2004;114:560–568. doi: 10.1172/JCI22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A. Antigen-activated human T lymphocytes express cell surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK cell lysis. 2007;110:606–615. doi: 10.1182/blood-2006-10-052720. [DOI] [PubMed] [Google Scholar]
- 44.Nowbakht P, Ionescu MC, Rohner A, Kalberer CP, Rossy E, Mori L, Cosman D, De Libero G, Wodnar-Filipowicz A. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105:3615–3622. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 45.Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr Opin Immunol. 2002;14:306–311. doi: 10.1016/s0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 46.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 47.Tonnerre P, Gerard N, Chatelais M, Charreau B. MICA gene polymorphism in kidney allografts and possible impact of functionally relevant variants. Transplant Proc. 2010;42:4318–4321. doi: 10.1016/j.transproceed.2010.09.118. [DOI] [PubMed] [Google Scholar]
- 48.Suarez-Alvarez B, Lopez-Vazquez A, Diaz-Pena R, Diaz-Molina B, Blanco-Garcia RM, Alvarez-Lopez MR, Lopez-Larrea C. Post-transplant soluble MICA and MICA antibodies predict subsequent heart graft outcome. Transpl Immunol. 2006;17:43–46. doi: 10.1016/j.trim.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Zou Y, Mirbaha F, Stastny P. Contact inhibition causes strong downregulation of expression of MICA in human fibroblasts and decreased NK cell killing. Hum Immunol. 2006;67:183–187. doi: 10.1016/j.humimm.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Molinero LL, Fuertes MB, Girart MV, Fainboim L, Rabinovich GA, Costas MA, Zwirner NW. NF-κB regulates expression of the MHC class I-related chain A gene in activated T lymphocytes. J Immunol. 2004;173:5583–5590. doi: 10.4049/jimmunol.173.9.5583. [DOI] [PubMed] [Google Scholar]
- 51.Molinero LL, Fuertes MB, Fainboim L, Rabinovich GA, Zwirner NW. Up-regulated expression of MICA on activated T lymphocytes involves Lck and Fyn kinases and signaling through MEK1/ERK, p38 map kinase, and calcineurin. J Leukoc Biol. 2003;73:815–822. doi: 10.1189/jlb.0602329. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Rao G, Gaffud MJ, Ding HG, Maki G, Klingemann HG, Groh V, Spies T, Caillat-Zucman S, Gattuso P, Plate J, Prinz RA. Clinicopathological significance of major histocompatibility complex class I-related chain A and B expression in thyroid cancer. J Clin Endocrinol Metab. 2006;91:2704–2712. doi: 10.1210/jc.2006-0492. [DOI] [PubMed] [Google Scholar]