Abstract
“Ciguatera” fish poisoning (CFP) is one of the well-known food poisoning caused by the ingestion of fish that have accumulated trace amounts of ciguatoxins (CTXs). CFP affects more than 50,000 individuals annually. The difficulty in preventing CFP comes from the lack of reliable methods for analysis of CTXs in contaminated fish, together with the normal appearance, taste, and smell of CTX-contaminated fish. Thus, a sensitive, accurate, routine, and portable analytical method to detect CTXs is urgently required. Monoclonal antibodies (mAbs) specific against either wing of major CTX congeners (CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C) were generated by immunizing mice with rationally designed synthetic haptens-KLH conjugates instead of the CTXs. Haptenic groups with a surface area greater than 400 Å2 are required to produce mAbs that can strongly bind to CTXs. Furthermore, a highly sensitive fluorescence-based sandwich enzyme-linked immunosorbent assay (ELISA) was developed. This protocol can detect and quantify four major CTX congeners (CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C) with a limit of detection (LOD) of less than 1 pg/mL. The LOD determined for this sandwich ELISA is sufficient to detect CTX1B-contaminated fish at the FDA guidance level of 0.01 ppb.
Keywords: ciguatera, ciguatoxin, monoclonal antibody, sandwich ELISA
1. Introduction
“Ciguatera” fish poisoning (CFP) is one of the well-known food-borne diseases caused by the ingestion of various reef fish that have accumulated trace amounts of ciguatoxins (CTXs) [1,2]. Typical human symptoms of CFP are severe neurological, gastrointestinal, and cardiovascular symptoms, which may last week, months, or even years. It is estimated that over 50,000 people throughout the world suffer from CFP each year, making it the most common form of seafood poisoning caused by natural toxins [3,4,5]. The spread of CFP gives rise to a considerable burden on public health systems, and damage to fishery resources and the economies in tropical and subtropical regions [6]. In addition to climate change, globalization of trade might contribute to the expansion of CFP, even in temperate zones. It is difficult to prevent CFP due to the lack of reliable analytical methods to detect causative CTXs, along with the normal appearance, taste, and smell of CTX-contaminated fish.
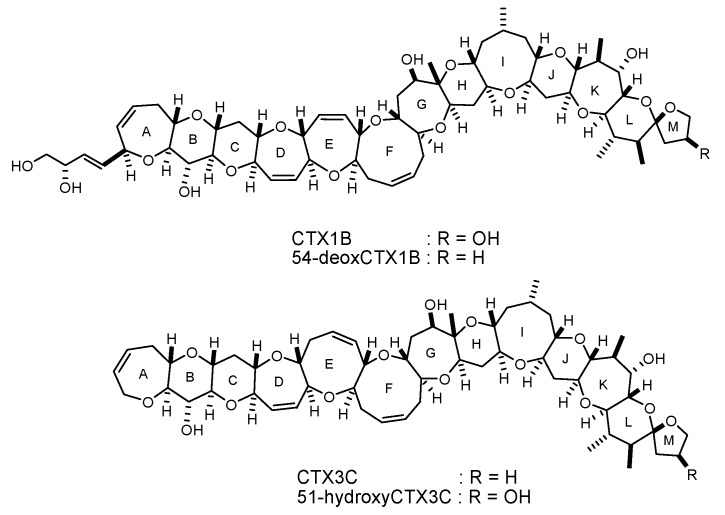
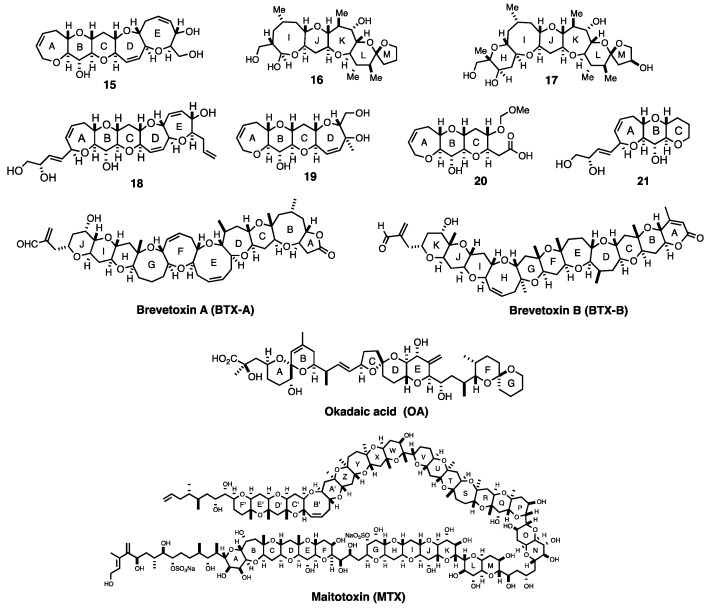
Yasumoto and his coworkers characterized CTXs as 3 nm-long ladder-shaped polycyclic ethers. CTXs were originally produced by Gambierdiscus species of marine dinoflagellates and accumulate in various types of reef fish through bioaccumulation [1,2,7]. CTX1B and its congeners (CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C) (Figure 1) were initially isolated in the Pacific region [8,9,10,11,12], then detected in the Atlantic [13,14]. These CTXs are extremely toxic to mammals and the lethal potencies of CTXs by intraperitoneal injection into mice (median lethal dose (LD50) 0.25–4 μg/kg) are much greater than those of the structurally related red-tide toxins, brevetoxins (LD50 > 100 μg/kg) and the pufferfish toxin, tetrodotoxin (LD50~10 μg/kg) [8,9,10,15,16,17]. The structural differences between the CTX congeners arise from the substituents on the A and M terminal rings as well as the size of the E-ring. The CTX1B series (CTX1B and 54-deoxyCTX1B) possesses a dihydroxybutenyl group on the A-ring and a seven-membered E-ring, while members the CTX3C series (CTX3C and 51-hydroxyCTX3C) lack the dihydroxybutenyl group and have an eight-membered E-ring.
Figure 1.
Chemical structures of the four major ciguatoxin (CTX) congeners: CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C.
Considerable attention has recently been directed to the development of analytical methods for detecting CTXs. To replace the traditional mouse bioassay (MBA), several analytical methods have been developed in recent years to detect CTXs in a contaminated fish. Examples include a neuroblastoma cell-based assay (CBA-N2a) [18], radiolabeled and fluorescent receptor binding assays (RBA) [16,19,20], HPLC [21], MS [12,22,23], and LC-MS/MS assays [13,14,24,25,26,27,28,29]. However, there is no quick and reliable method to detect CTXs in the fishery or even at inspection stations for seafood. MAb-based immunoassays such as ELISA was expected to provide suitable methods for the sensitive, accurate, routine, and portable detection of CTXs. For the generation of anti-CTX antibodies, Hokama et al. claimed that an anti-CTX1B mAb was prepared by immunization with natural CTX1B [30], but the mAb cross-reacted with okadaic acid (OA) [31,32], and the results of immunochemical kit “Cigua-Check®” using this mAb have been controversial [33,34,35]. The minimal amount of CTX isolated from contaminated fish has prevented further development of anti-CTX antibodies. Thus, rationally designed synthetic haptens instead of natural toxins were planned to produce anti-CTX mAbs. Successful syntheses of CTX congeners based on a convergent synthetic strategy unifying right wing (ABCDE) and left wing (HIJKLM) fragments [36,37,38,39,40] allowed to synthesize nontoxic haptens to generate mAbs that recognize the specific structure of CTXs. Here, the current status of the preparation of anti-CTX mAbs and development of highly sensitive sandwich ELISA to detect CTX congeners is summarized (Figure 2) [41,42,43,44,45,46,47,48,49].
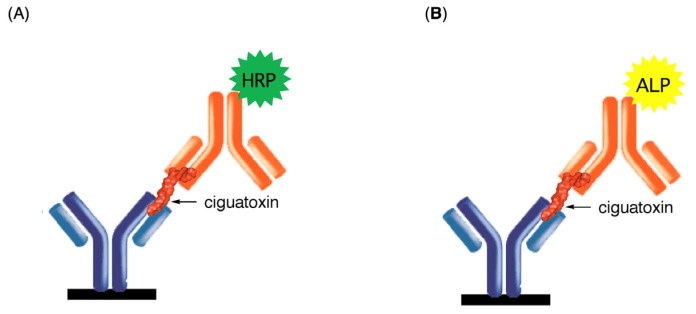
Figure 2.
Schematic representation of the detection of CTXs by sandwich ELISA. Monoclonal antibodies (mAbs) (blue) against the left end of CTXs (red) is adsorbed on the wells of a 96-well microtiter plate and mAb (orange) against the right end is labeled with (A) horseradish peroxidase (HRP, green) or (B) alkaline phosphatase (ALP, yellow).
2. Generation of Anti-CTX MAbs
2.1. Design of Haptens and Preparation of Protein-Conjugates
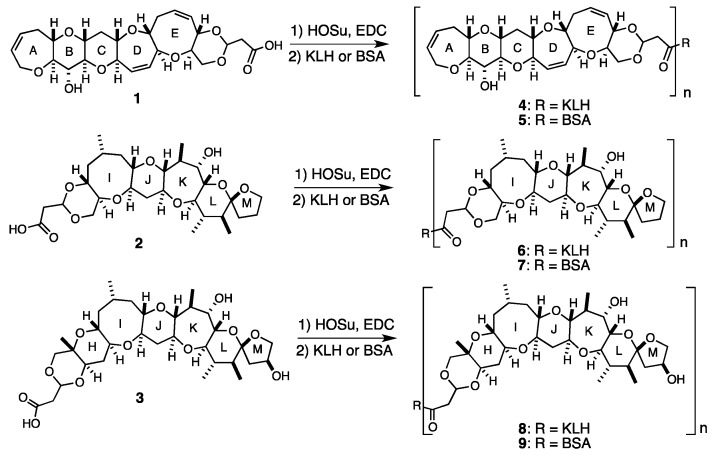
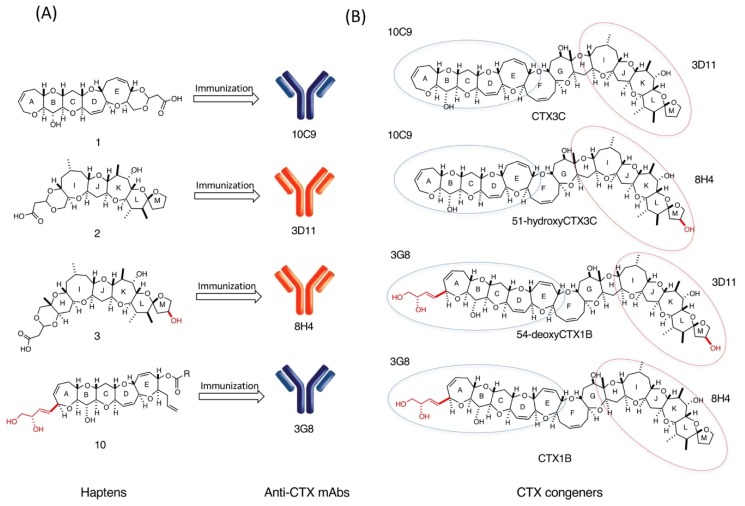
Since the maximum buried surface areas of small haptens in antibody-hapten complexes are estimated to be approximately 400 Å2 [50,51,52,53,54,55,56], it was predicted that hapten 1, consisting of a pentacyclic skeleton (ABCDE ring, calculated water accessible surface area was 398 Å2) and a cyclic acetal connected to a linker, would be sufficiently large to occupy the antibody-combining site, whereas leaving the acetal and linker moiety free from interactions with the antibody-combining site [44]. In addition, hapten 1 was designed to induce mAbs that would bind to the left wing of CTX3C or 51-hydroxyCTX3C in an orientation suitable for sandwich ELISA. Similarly, hapten 2 consisting of a pentacyclic skeleton (IJKLM ring, calculated water accessible surface area was 477 Å2) was designed to induce mAbs against the right wing of CTX3C and 54-deoxyCTX1B [44]. Hapten 3 bearing a hydroxy group on the M ring was designed to generate mAbs for the right wing of 51-hydroxyCTX3C and CTX1B [45]. Since small-molecular-weight haptens such as 1, 2, and 3 can be made immunogenic only by coupling them to carrier proteins, these haptens were linked with keyhole limpet hemocyanin (KLH) and also with bovine serum albumin (BSA) via a conventional active ester method using N-hydroxysuccimide (HOSu) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) to provide the KLH- and BSA-conjugates 4–9 (Figure 3). The KLH-conjugates 4, 6, and 8 were used for immunization and the BSA-conjugates 5, 7, and 9 were used for the binding analysis of hybridoma supernatants by ELISA.
Figure 3.
Structures of synthetic haptens 1–3 and synthesis of protein-conjugates 4–9.
In contrast, the conjugation of synthetic haptens smaller than 1, 2, and 3 with KLH did not elicit competent mAbs [41,42,43] showing that pentacyclic haptens of more than 400 Å2 surface areas are required to elicit mAbs binding strongly to CTXs.
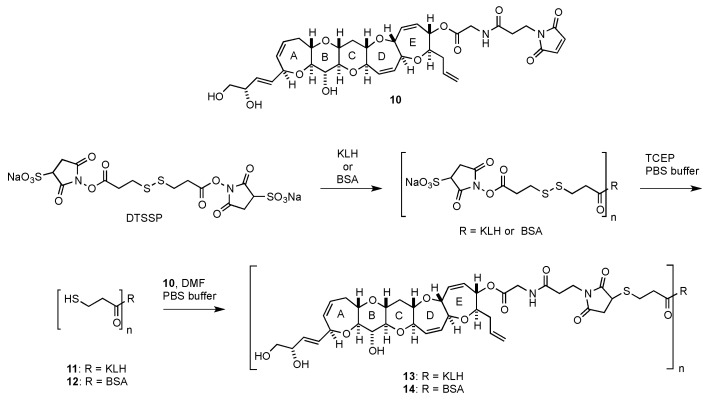
However, it was difficult to raise mAbs against the left wing of the CTX1B series possessing a dihydroxybutenyl group on the A-ring (CTX1B and 54-deoxyCTX1B). After several unsuccessful immunization studies using synthetic hapten-KLH conjugates connected through acetal linkers [41], the conjugation strategy was changed and the pentacyclic hapten 10 was synthesized to prepare mAbs against the left wing of the CTX1B series [47]. The KLH- and BSA-conjugates 13 and 14 were reliably synthesized from maleimide 10, as outlined in Figure 4. KLH and BSA were converted to thiol-enriched KLH 11 and BSA 12, respectively, using 3,3’-dithiobis (sulfosuccinimidyl propionate) (DTSSP) followed by reduction with tris (2-carboxyethyl) phosphine (TCEP). The coupling of 10 with 11 and 12 yielded KLH- and BSA-conjugates 13 and 14, respectively.
Figure 4.
Structure of hapten 10 and synthesis of protein-conjugates 13 and 14 for the left wing of CTX1B and 54-deoxyCTX1B.
2.2. Production and Affinity Measurement of Anti-CTX mAbs
MAbs specific to the left wing of the CTX3C series (CTX3C and 51-hydroxyCTX3C) were produced by immunization of mice with KLH-conjugate 4 according to the standard protocol. After three injections of KLH-conjugate 4, the titer of serum immunoglobulin G (IgG) was determined by ELISA using BSA-conjugate 5. The mouse showing the highest titer was boosted; after 3 days, the spleen was taken from the mouse, and the splenocytes were fused with X63-Ag8.653 myeloma cells using an electro cell fusion apparatus as reported previously [53,54]. All hybridomas producing IgGs that bound to BSA-conjugate 5 were subcloned twice or three times to provide six hybridomas producing BSA-conjugate 5-binding mAbs. Competitive ELISA analyses of hybridoma supernatants showed that one mAb, designated 10C9, was most strongly inhibited by ABCDE fragment 15 and thus 10C9 was chosen for further analysis [44].
Similarly, immunization with KLH-conjugate 6, followed by competitive ELISA with IJKLM-fragment 16, provided mAb 3D11 that specifically bound the right wing of CTX3C and 54-deoxy CTX1B [44].
MAbs specific for the right wing of CTX1B and 51-hydroxyCTX3C were prepared by immunization with KLH-conjugate 8 to elicit mAbs (7C9, 8B9, and 8H4) whose dissociation constants (Kds) for HIJKLM fragment 17 determined by competitive ELISA were 407, 108, and 48 nM, respectively. Thus, mAb 8H4 showing the highest affinity was selected for further studies [45].
KLH-conjugate 13 was immunized to prepare mAbs specific for the left wing of the CTX1B series (CTX1B and 54-deoxyCTX1B). This immunization provided 18 cell lines producing mAbs that bound to BSA-conjugate 14. Competitive ELISA analyses of hybridoma supernatants showed that the binding of mAb 3G8 was strongly inhibited by 0.1 μM ABCDE fragment 18 and thus 3G8 was selected for further analysis [47].
To evaluate the molecular recognition and interaction of four anti-CTX mAbs 10C9, 3D11, 8H4, and 3G8, the Kds of these mAbs against four CTXs, as well as against small synthetic fragments and structurally related marine toxins, were determined by competitive ELISA (Table 1) [57,58]. As expected, mAb 10C9 bound strongly to ABCDE ring fragment 15 with a dissociation constant (Kd) of 0.8 nM (Figure 5). More importantly, 10C9 bound also to CTX3C itself, with a comparable Kd of 2.8 nM. Mab 10C9 showed no cross-reactivity with either the other-wing fragment (IJKLM ring 16) or with the structurally related marine toxins such as brevetoxin A (BTX-A) [59,60], brevetoxin B (BTX-B) [61,62], okadaic acid (OA) [63], and maitotoxin (MTX) [64]. Furthermore, the Kd values of mAb 10C9 for tetracyclic fragment (ABCD ring 19) and tricyclic fragment (ABC ring 20) were determined to be 1.8 and 74 μM, respectively, while that for pentacyclic fragment (ABCDE ring 15) was 0.8 nM (Table 1) [44]. Thus, as the synthetic fragments become smaller, the Kd value of 10C9 increased 2250-fold (5 to 4 rings) or 41-fold (4 to 3 rings). This result suggests that mAb 10C9 recognizes the entire pentacyclic skeleton (ABCDE ring) of CTX3C, and that the surface area required for molecular recognition of 10C9 is consistent with the prediction of 400 Å2. Probable recognition of mAb 10C9 and the pentacyclic ring moiety (ABCDE ring) of CTX3C was confirmed by crystal structure analysis of 10C9 complexed with ABCDE ring 15 [65].
Table 1.
Dissociation constants (Kds) of anti-CTX mAbs against synthetic fragments and marine toxins 1.
| mAb | Kd (nM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 16 | 17 | 18 | CTX1B | CTX3C | 51-hydroxy-CTX3C | BTX-A | BTX-B | OA | MTX | |
| 10C9 | 0.8 | NI | ND | ND | ND | 2.8 | ND | NI | NI | NI | NI |
| 3D11 | NI | 8.6 | ND | ND | ND | 122 | ND | 43000 | NI | NI | NI |
| 8H4 | ND | ND | 48 | ND | 20.4 | 3200 | 13.6 | NI | NI | NI | NI |
| 3G8 | ND | ND | ND | 1.5 | 15 | NI | ND | NI | NI | NI | ND |
1 The Kd’s were determined by competitive ELISA. ND: not determined. NI: no inhibition was observed at the maximum concentration of each inhibitor (for 10C9, 250 μM 16, 100 μM BTX-A, 100 μM BTX-B, 100 μM OA, 25 μM MTX; for 3D11, 250 μM 15, 100 μM BTX-A, 100 μM BTX-B, 100 μM OA, 25 μM MTX; for 8H4, 100 μM BTX-A, 100 μM BTX-B, 100 μM OA, 25 μM MTX; for 3G8, 1.9 μM CTX3C, 10 μM BTX-A, 100 μM BTX-B, 100 μM OA).
Figure 5.
Structures of synthetic fragments 15–21 and structurally related marine toxins.
Similarly, mAb 3D11 also bound strongly to both IJKLM ring 16 (Kd = 8.6 nM) and CTX3C (Kd = 122 nM), and did not show any cross-reactivity with synthetic fragment (ABCDE ring 15) or other marine toxins (Table 1) [44].
MAb 8H4 strongly bound to both CTX1B and 51-hydroxyCTX3C with Kds of 20.4 and 13.6 nM, respectively. The Kd value of 8H4 for CTX3C, which lacks an M-ring hydroxyl group, was 3.2 μM. These results indicate that 8H4 distinguishes between CTXs with and without an M-ring hydroxyl group. This mAb also did not cross-react with the structurally related cyclic ethers BTX-A, BTX-B, OA, and MTX [45].
MAb 3G8 was found to bind very strongly to ABCDE ring fragment 18, with a Kd of 1.5 nM, and also strongly to CTX1B with a Kd of 15 nM. 3G8 did not cross-react with CTX3C, which lacks a dihydroxybutenyl substituent on the A ring, indicating that mAb 3G8 recognizes the A-ring dihydroxybutenyl substituent. In addition, the Kd of 3G8 for ABC-ring fragment 21 was 410 nM, 270 times larger Kd than that for ABCDE ring fragment 18, indicating that mAb 3G8 recognizes the whole pentacyclic ring skeleton (ABCDE ring) of CTX1B [47]. Since CTX1B and 54-deoxyCTX1B share the left end, mAb 3G8 should also bind to 54-deoxyCTX1B. This was confirmed by the successful sandwich ELISA detection of 54-deoxyCTX1B using mAbs 3G8 and 8H4 [49]. MAb 3G8 did not cross-react with the structurally related marine toxins.
3. Sandwich ELISA Detection of CTXs
Four anti-CTX mAbs that bind strongly and specifically to CTXs are now available, as summarized in Figure 6. 10C9 binds to the left end of the CTX3C series (CTX3C and 51-hydroxyCTX3C) while 3D11 binds to the right end of CTX3C and 54-deoxyCTX1B. Similarly, 3G8 binds to the left end of the CTX1B series (CTX1B and 54-deoxyCTX1B) whereas 8H4 binds to the right end of CTX1B and 51-hydroxyCTX3C. Using two of these anti-CTX mAbs, a sandwich ELISA was developed for the detection of the major CTX congeners (Figure 2 and Figure 6). First attempted was detection of CTXs by sandwich ELISA using a combination of two mAbs: a mAb for the left end (10C9 or 3G8) was adsorbed on a microtiter plate as a capture antibody (Ab) and a horseradish peroxidase (HRP)-labeled mAb for the right end (3D11-HRP or 8H4-HRP) was used as a detection Ab. For example, to detect CTX1B, 3G8 was used to capture CTX1B and 8H4 as a detection Ab. 96-well microtiter plate wells were directly coated with 3G8 whereas 8H4 was labeled with HRP. In the sandwich ELISA o-pheylenediamine (OPD) was used as a colorimetric substrate to detect CTX1B specifically as shown in Figure 7 [47]. The limit of detection (LOD) was 0.28 ng/mL. Similar sandwich ELISA protocols also detected other CTXs, CTX3C, 51-hydroxyCTX3C, and 54-deoxyCTX1B: 10C9 and HRP-linked 3D11 were used to detect CTX3C [44], 10C9 and HRP-linked 8H4 were used to detect 51-hydroxyCTX3C [45], and 3G8 and HRP-linked 3D11 were used to detect 54-deoxyCTX1B. The cross-reactivities of the sandwich ELISA were also investigated against structurally related marine natural toxins, such as BTX-A, BTX-B, OA, and MTX. The sandwich ELISA did not show any cross-reactivity with these marine toxins [44,45,47].
Figure 6.
Summary of anti-CTX mAbs produced by immunization with synthetic haptens (A) and their combination for detection of four CTX major congeners by sandwich ELISA (B). The circles in figure (B) indicate the wings of the CTX congeners to which each mAb binds.
Figure 7.
Detection of CTX1B by sandwich ELISA using 3G8 and HRP-labeled 8H4. The values represent mean absorbance (λ = 490 nm) of three experiments. The error bars represent standard deviations.
4. Highly Sensitive Detection of CTXs
4.1. Fluorescent Sandwich ELISA Detection of CTXs
Although a regulatory limit for CTXs in fish has not been established by the United Nations the World Health Organization (WHO) and Food and Agriculture Organization (FAO), the United States Food and Drug Administration (FDA) issued a guidance level of 0.01 ppb CTX1B equivalent toxicity in fish [5,66]. Therefore, the sensitivity of the sandwich ELISA should be adequate to reliably detect CTX1B at the FDA guidance level (0.01 ppb) in contaminated fish. The sensitivity of 0.28 ng/mL observed in the HRP-based sandwich ELISA detection of CTX1B is thus inadequate and so a protocol to improve the sensitivity in the sandwich ELISA was investigated. The combination of alkaline phosphatase (ALP) as a conjugated enzyme and 2’-(2-benzothiazoyl)-6’-hydroxybenzothiazole phosphate (BBTP) as a fluorogenic substrate is optimal (Figure 8). The BBT anion is produced by the reaction of ALP with BBTP and has a large Stokes’ shift, which leads to lower background fluorescence as well as higher detection sensitivity. This new fluorescent sandwich ELISA can detect CTXs with extremely high sensitivity (vide infra) [49].
Figure 8.
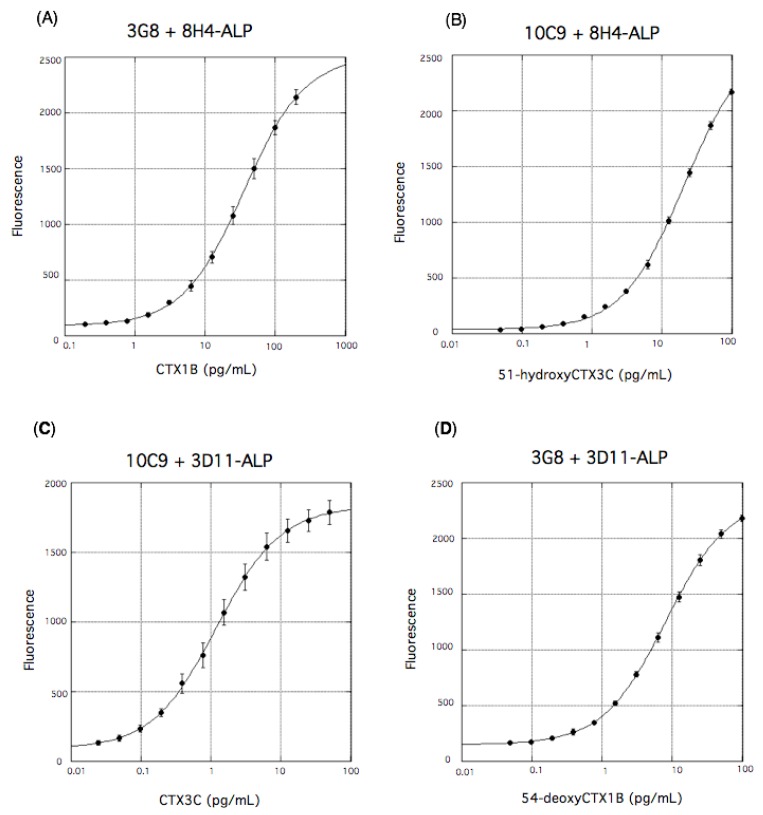
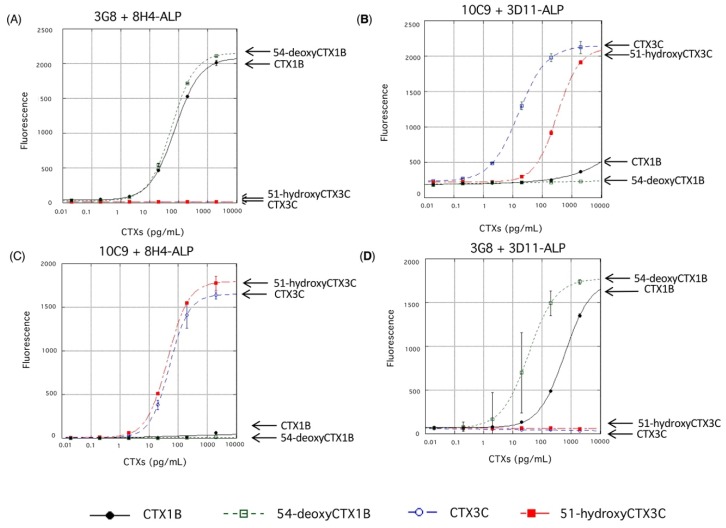
Fluorescent sandwich ELISA detection of CTX1B using 3G8 and ALP-labeled 8H4 (A), 51-hydroxyCTX3C using 10C9 and ALP-labeled 8H4 (B), CTX3C using 10C9 and ALP-labeled 3D11 (C), and 54-deoxyCTX1B using 3G8 and ALP-labeled 3D11 (D). Immunoreaction enhancer solution (Can Get Signal®) was added to detect CTX3C (C) and 54-deoxyCTX1B (D). The values represent mean fluorescence intensities (excitation, λ = 435 nm; emission, λ = 555 nm) of three experiments. The error bars represent standard deviations.
Capture Ab 3G8 was adsorbed on the wells of 96-well microtiter plates and detection Ab 8H4 was labeled with ALP. A general sandwich ELISA protocol using a fluorescent substrate BBTP allowed detection of CTX1B as shown in Figure 8A. The detection sensitivity of this fluorescent sandwich ELISA was improved considerably and the LOD was determined to be 0.16 pg/mL. Similar fluorescent sandwich ELISA using mAb 10C9 and ALP-labeled 8H4 allowed a sensitive detection of 51-hydroxyCTX3C with an LOD of 0.1 pg/mL (Figure 8B). However, fluorescent sandwich ELISA using ALP-labeled 3D11 for the right wing gave unsatisfactory results: sandwich ELISA using mAb 10C9 for the left end and ALP-labeled 3D11 for the right end gave an unsatisfactory LOD for CTX3C (ca. 2 pg/mL); in addition, the background was high, probably due to the relatively weak binding of 3D11 to CTX3C (Kd = 122 nM) [44]. Thus, to improve the detection sensitivity, immunoreaction enhancer solution (Can Get Signal®) was added to the fluorescent sandwich ELISA system. This solution significantly improved the LOD (0.090 pg/mL, Figure 8C) and lowered the background fluorescence in the detection of CTX3C. The addition of this additive to the fluorescent sandwich ELISA using 3G8 and ALP-labeled 3D11 also led to a significant improvement in the detection of 54-deoxyCTX1B with an LOD of 0.11 pg/mL (Figure 8D). In contrast, this enhancer solution did not improve the LOD or the background in fluorescent sandwich ELISAs using 3G8 and ALP-labeled 8H4 to detect CTX1B and 10C9 and ALP-labeled 8H4 to detect 51-hydroxyCTX3C [49].
4.2. Cross-Reactivity of the Fluorescent Sandwich ELISA
The cross-reactivities of the present fluorescent sandwich ELISA protocols were first investigated against other related marine toxins, such as BTX-A, BTX-B, OA, and MTX. The sandwich ELISA showed high specificity to CTXs and did not show any cross-reactivity with the structurally related marine toxins [49].
Next, the cross-reactivity of the fluorescent sandwich ELISA against the other congeners of CTX were examined (Figure 9). Each ELISA protocol strictly differentiate between the CTX1B series and the CTX3C series, namely between CTX congeners with and without the dihydroxybutenyl group on the A ring. Thus, 3G8 specifically recognizes the presence of the dihydroxybutenyl group on the A ring of CTX1B and 54-deoxyCTX1B (Figure 9A,D), whereas 10C9 strictly recognizes the A ring that lacks the substituent in CTX3C and 51-hydroxyCTX3C (Figure 9B,C).
Figure 9.
Cross-reactivity of fluorescent sandwich ELISA against the four major CTX congeners (CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C). (A) Cross-reactivity of the ELISA originally designed for the detection of CTX1B using 3G8 and ALP-labeled 8H4. (B) Cross-reactivity of the ELISA originally designed for the detection of CTX3C using 10C9 and ALP-labeled 3D11. (C) Cross-reactivity of the ELISA originally designed for the detection of 51-hydroxyCTX3C using 10C9 and ALP-labeled 8H4. (D) Cross-reactivity of the ELISA originally designed for the detection of 54-deoxyCTX1B using 3G8 and ALP-labeled 3D11. The values represent mean fluorescence intensities (excitation, λ = 435 nm; emission, λ = 555 nm) of three experiments. The error bars represent standard deviations.
More interesting is that ALP-labeled 8H4 appears to have tolerance for the absence of the hydroxy group on the M ring in sandwich ELISA (CTX1B vs. 54-deoxyCTX1B in Figure 9A; CTX3C vs. 51-hydroxyCTX3C in Figure 9C), while free 8H4 discriminated more strictly 51-hydroxyCTX3C (Kd = 13.8 nM) from CTX3C (Kd = 3.2 μM) in an aqueous media [45]. Meanwhile, ALP-labeled 3D11 still showed some discrimination (Figure 9B,D). Based on the above results ALP-labeled 8H4, rather than ALP-labeled 3D11, should be used for a fluorescent sandwich ELISA designed to bind the right end of CTXs regardless of the presence or absence of the hydroxy group on the M ring as shown in the following section [49].
4.3. Single ELISA Analysis of Four CTX Congeners
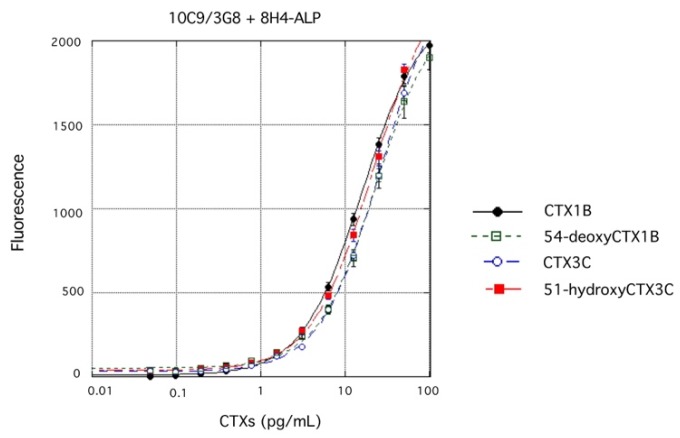
For practical purposes, it is crucial to detect CTXs irrespective of the congener since several CTX congeners are typically present in a contaminated fish. A more convenient sandwich ELISA to detect any of the four CTXs (CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C) was developed using one microtiter plate in a single operation: a 1:1 mixture of 10C9 and 3G8 was adsorbed on the surface of wells of a microtiter plate, while ALP-labeled 8H4 and BBTP were used for detection. All four CTX congeners were detected effectively (Figure 10). It is noteworthy that these four CTXs gave similar fluorescence intensities as the concentrations of CTX changed, indicating that this sandwich ELISA protocol can detect and quantify any of the four CTX congeners in a single operation [49].
Figure 10.
Sandwich ELISA detection of any of four CTX congeners (CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C) using one microtiter plate whose wells were coated with a 1:1 mixture of capture Abs 10C9 and 3G8, ALP-labeled 8H4, and BBTP. The values represent mean fluorescence intensities (excitation, λ = 435 nm; emission, λ = 555 nm) of three experiments. The error bars represent standard deviations.
4.4. Detection of CTX1B from Fish Flesh and Evaluation of the Matrix Effects at Each Extraction/Purification Step
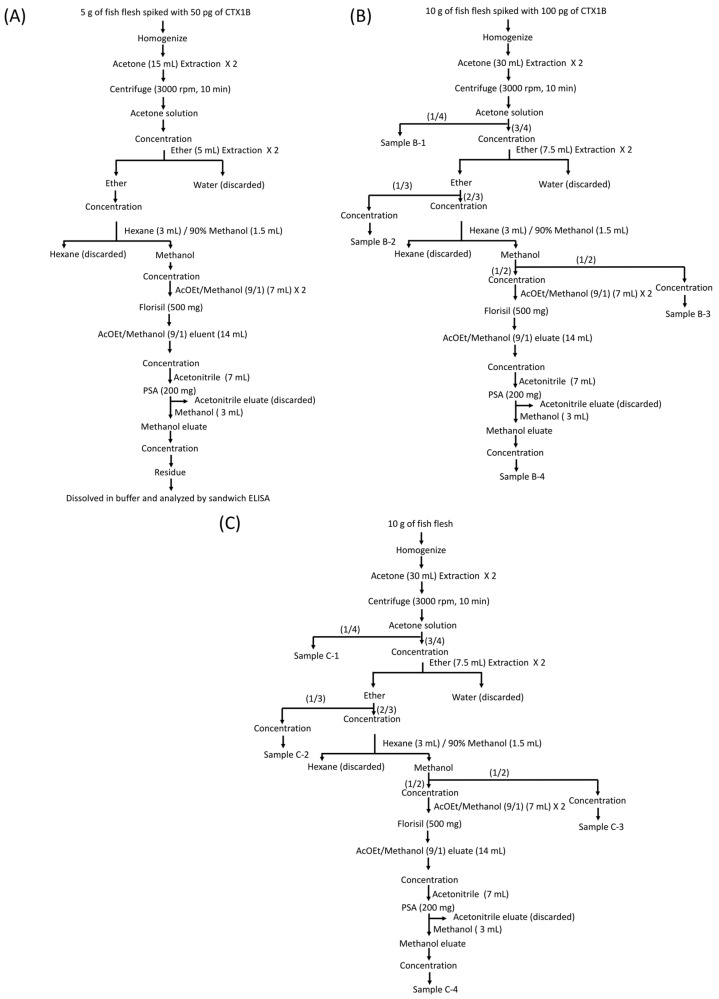
To confirm that the fluorescent sandwich ELISA is applicable to the detection of CTX1B at the FDA guidance level, fish (Variola louti) determined to be free of CTXs by LC-MS/MS analysis [25], was spiked with pure CTX1B (0.01 ppb), the flesh was extracted with acetone, and the extract was purified as shown in Figure 11A. The extraction/purification method was originally developed for the analysis of CTXs by LC-MS/MS [25]. The CTX1B concentrations determined by the fluorescent sandwich ELISA in the final extract and its 10-fold dilution were 17 pg/mL and 20 pg/mL, respectively, after calculation using the dilution factor. These results showed that 34% and 40% of the initially spiked CTX1B was detected. Thus, CTX1B spiked into the flesh of fish at the FDA guidance level of 0.01 ppb was securely detected by the fluorescent sandwich ELISA protocol after extraction/purification using a conventional procedure [49].
Figure 11.
(A) Extraction and purification scheme for CTX1B. Fish flesh was spiked with Food and Drug Administration (FDA) guidance level (0.01 ppb) of pure CTX1B and after extraction/purification, the sample and the tenfold dilution were analyzed by fluorescent sandwich ELISA. (B) Extraction and purification scheme for the evaluation of matrix effects at each step of the extraction/purification procedure. 0.01 ppb of pure CTX1B was spiked into fish flesh and after extraction/purification each extract (Samples B-1~B-4) was analyzed by fluorescent sandwich ELISA. (C) Extraction and purification scheme for direct evaluation of the matrix effects of each extract. 0.01 ppb of pure CTX1B was added to each extract obtained by the extraction/purification procedure (Samples C-1~C-4) and each sample was analyzed by fluorescent sandwich ELISA.
To simplify the extraction/purification procedure, the recovery of CTX1B was then evaluated at every extraction/purification step, because contaminants like lipids and proteins in fish flesh may have an effect on the fluorescent sandwich ELISA assay through their matrix effects. Ten grams of flesh of Variola louti was spiked with CTX1B (100 pg), which is equal to the amount of the FDA guidance level (0.01 ppb). At each extraction/purification step, a portion of the sample was collected for analysis by sandwich ELISA, as shown in Figure 11B. To each sample of B-1~B-4 was added 1 mL of analysis buffer and then each sample was analyzed by sandwich ELISA. The CTX1B concentrations were determined to be 2.3 pg/mL for sample B-1, 2.5 pg/mL for sample B-2, 2.7 pg/mL for sample B-3, and 8.0 pg/mL for sample B-4. The results indicated that 9%, 10%, 11%, and 32% were recovered from the initially spiked CTX1B for samples B-1, B-2, B-3, and B-4, respectively. In the earlier steps of the extraction/purification procedure, the concentrations of CTX1B determined by sandwich ELISA were significantly decreased to about 10% of the initially spiked CTX1B, probably due to the matrix effects [49].
Furthermore, to evaluate the matrix effects of contaminants on the fluorescent sandwich ELISA more directly, extracts were prepared from 10 g of fish flesh (Variola louti) following the above-mentioned procedure without adding CTX1B (Figure 11C), then a solution of pure CTX1B (25 pg) in analysis buffer (1 mL) was added to each extract (samples C-1~C-4 in Figure 11C) before conducting the sandwich ELISA analysis. The concentrations of CTX1B determined by the fluorescent sandwich ELISA were 3.2 pg/mL (13% of added CTX1B) for sample C-1, 5.3 pg/mL (21%) for sample C-2, 7.7 pg/mL (31%) for sample C-3 and 25 pg/mL (100%) for sample C-4. These results clearly showed that the significant matrix effects of contaminants were observed in samples C-1, C-2, and C-3 (in decreasing order). However, due to the high sensitivity of the fluorescent sandwich ELISA, simple extraction by homogenization in acetone and centrifugation is very useful as a pre-treatment procedure to detect CTX1B-contaminated fish at 0.01 ppb (FDA guidance level) [49].
Table 2 summarizes the LOD, specificity, pre-treatment of the sample, and sample-to-answer time of the fluorescent sandwich ELISA and other established methods developed for the detection of CTXs. The fluorescent sandwich ELISA is superior in terms of LOD and specificity compared with the other established methods. Although LC-MS/MS has the shortest sample-to-answer time, it should be noted that this is the time needed to analyze one sample, while the sandwich ELISA, receptor binding and cell-based assays can analyze multiple samples in a single operation.
Table 2.
Comparison of limit of detection (LOD), specificity, sample preparation methods, and sample-to-answer time of the analytical methods developed for the detection of CTXs.
| Methods | Fluorescent Sandwich Elisa | LC-MS/MS | Receptor Binding Assay (RBA) | Cell-Based Assay (CBA-N2a) |
|---|---|---|---|---|
| LOD | 0.09–0.16 pg/mL (18–32 pg/kg) 1 |
20 pg/mL (4 ng/kg) 1 |
0.075 ppb (75 ng/kg) |
0.002 ppb (2 ng/kg) |
| Specificity | high | high | none | none |
| Sample preparation methods | Extraction + solid phase extraction cartridges (Florisil + PSA) |
Extraction + solid phase extraction cartridges (Florisil + PSA) |
Extraction + solid phase extraction cartridge (C18) | Extraction + solid phase extraction cartridge (C18) |
| Sample-to-answer time | 2.5 h | 30 min | <3 h | 2.5 days |
| References | [49] | [25,26] | [16,19,20] | [18,20] |
1 It was assumed that the sample extracted and purified from fish flesh (5 g) was dissolved in buffer or solvent (1 mL) and then analyzed.
5. Conclusions
Recently, great progress has been made in generation of anti-CTX mAbs. Anti-CTX mAbs (10C9, 3D11, 8H4, and 3G8) that specifically bind either wing of CTXs were prepared by immunization with KLH conjugates of designed synthetic haptens instead of scarce natural toxins. The synthetic haptens and the synthetic strategy for preparation of the hapten-KLH conjugates are critical for the production of mAbs that bind strongly and specifically to CTXs. The surface area >400 Å2 of the haptenic groups are required to induce mAbs that strongly bind CTXs [44]. It should be emphasized that organic synthesis played a critical role in the development of these specific mAbs against large natural marine toxins containing ladder-like polycyclic ethers. In addition, a highly sensitive and accurate fluorescent sandwich ELISA for major CTX congeners has been developed. The ELISA show no cross-reactivity with other structurally related marine natural toxins. The fluorescent sandwich ELISA can detect and quantify four major congeners of CTX (CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C) with an LOD of <1 pg/mL. The sensitivity observed in this ELISA is sufficient to detect CTX1B-contaminated fish at the FDA guidance level following a conventional extraction/purification procedure. Moreover, the sandwich ELISA using one microtiter plate whose wells were coated with a mixture of two mAbs 10C9 and 3G8 together with ALP-labeled 8H4, can detect any four major congeners of CTXs in a single operation. It is also demonstrated that simple homogenization in acetone followed by centrifugation without any tedious extraction and purification procedures is satisfactory for the detection of CTX1B in fish at the FDA guidance level by the fluorescent sandwich ELISA [49]. The sandwich ELISA would be helpful for epidemiological and physiological studies as well as for the prevention of CFP. A fluorescent sandwich ELISA kit “CTX-ELISATM 1B” consisting of a 96-well microtiter plate coated with capture mAb 3G8 and ALP-linked 8H4 for detection of the CTX1B series (CTX1B and 54-deoxyCTX1B) can be purchased commercially from Fujifilm Wako Corporation, Japan (382-14341) and another kit for detecting the CTX3C series (CTX3C and 51-hydroxyCTX3C) will be soon available from Fujifilm Wako Corporation, Japan. Portable, easy-to-use ELISA kits for detecting CTX congeners will also be developed.
Acknowledgments
We are most grateful to our collaborators, who are listed in the references, for their experimental and conceptual development of the anti-CTX mAbs and sandwich ELISA detection for CTXs summarized in this article. We also thank the late Koji Nakanishi for the generous gift of BTX-A and BTX-B and Takeshi Yasumoto and Masayuki Satake for valuable discussions. We are very grateful to Naomasa Oshiro for his generous gift of Variola louti flesh and his valuable discussion.
Author Contributions
Written and revised by T.T. and M.H.
Funding
This research was funded by the CREST and SORST programs from Japan Science and Technology Corporation (JST), by Grants-in-Aid for Scientific Research (S), and for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Conflicts of Interest
The authors declare no conflict of interest, while Osaka Prefecture University has a patent P5757497 (JP) for mAb 3G8, which was invented by the authors.
Key Contribution
This review summarizes recent studies on the generation of anti-CTX mAbs by immunizing mice with synthetic haptens and the development of a sandwich ELISA for the specific and reliable detection of four major congeners of CTXs using two mAbs specific for each wing of a CTX.
References
- 1.Yasumoto T., Murata M. Marine toxins. Chem. Rev. 1993;93:1897–1909. doi: 10.1021/cr00021a011. [DOI] [Google Scholar]
- 2.Yasumoto T. The chemistry and biological function of natural marine toxins. Chem. Rec. 2001;1:228–242. doi: 10.1002/tcr.1010. [DOI] [PubMed] [Google Scholar]
- 3.Scheuer P.J. Tetrahedron perspective number 2. Ciguatera and its off-shoots-chance encounters en route to a molecular structures. Tetrahedron. 1994;50:3–18. doi: 10.1016/S0040-4020(01)80733-X. [DOI] [Google Scholar]
- 4.Lewis R.J. The changing face of ciguatera. Toxicon. 2001;39:97–106. doi: 10.1016/S0041-0101(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 5.Dickey R.W., Plakas S.M. Ciguatera: A public health perspective. Toxicon. 2010;56:123–136. doi: 10.1016/j.toxicon.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Bagnis R. Ciguatera in French Polynesian islands: Of coral, fish and men | La ciguatera dans les îles de Polynésie française: Des coraux, des poissons et des hommes. Bull. Soc. Pathol. Exot. 1992;85:412–414. [PubMed] [Google Scholar]
- 7.Murata M., Yasumoto T. The structure elucidation and biological activities of high molecular weight algal toxins: Maitotoxin, prymnesins and zooxanthellatoxins. Nat. Prod. Rep. 2000;17:293–314. doi: 10.1039/a901979k. [DOI] [PubMed] [Google Scholar]
- 8.Murata M., Legrand A.M., Ishibashi Y., Yasumoto T. Structures of Ciguatoxin and Its Congener. J. Am. Chem. Soc. 1989;111:8929–8931. doi: 10.1021/ja00206a032. [DOI] [Google Scholar]
- 9.Murata M., Legrand A.M., Ishibashi Y., Fukui M., Yasumoto T. Structures and Configurations of Ciguatoxin from the Moray Eel Gymnothorax javanicus and Its Likely Precursor from the Dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990;112:4380–4386. doi: 10.1021/ja00167a040. [DOI] [Google Scholar]
- 10.Satake M., Murata M., Yasumoto T. The structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdiscus toxicus. Tetrahedron Lett. 1993;34:1975–1978. doi: 10.1016/S0040-4039(00)91978-6. [DOI] [Google Scholar]
- 11.Satake M., Fukui M., Legrand A.-M., Cruchet P., Yasumoto T. Isolation and structures of new ciguatoxin analogs, 2,3-dihydroxyCTX3C and 51-hydroxyCTX3C, accumulated in tropical reef fish. Tetrahedron Lett. 1998;39:1197–1198. doi: 10.1016/S0040-4039(97)10808-5. [DOI] [Google Scholar]
- 12.Yasumoto T., Igarashi T., Legrand A.-M., Cruchet P., Chinain M., Fujita T., Naoki H. Structural elucidation of ciguatoxin congeners by fast-atom bombardment tandem mass spectroscopy. J. Am. Chem. Soc. 2000;122:4988–4989. doi: 10.1021/ja9944204. [DOI] [Google Scholar]
- 13.Otero P., Pérez S., Alfonso A., Vale C., Rodríguez P., Gouveia N.N., Gouveia N., Delgado J., Vale P., Hirama M., et al. First toxin profile of ciguateric fish in Madeira Arquipelago (Europe) Anal. Chem. 2010;82:6032–6039. doi: 10.1021/ac100516q. [DOI] [PubMed] [Google Scholar]
- 14.Silva M., Rodriguez I., Barreiro A., Kaufmann M., Neto A.I., Hassouani M., Sabour B., Alfonso A., Botana L.M., Vasconcelos V. First report of ciguatoxins in two starfish species: Ophidiaster ophidianus and Marthasterias glacialis. Toxins (Basel) 2015;7:3740–3757. doi: 10.3390/toxins7093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis R.J., Sellin M., Poli M.A., Norton R.S., MacLeod J.K., Sheil M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae) Toxicon. 1991;29:1115–1127. doi: 10.1016/0041-0101(91)90209-A. [DOI] [PubMed] [Google Scholar]
- 16.Dechraoui M.-Y., Naar J., Pauillac S., Legrand A.-M. Ciguatoxins and brevetoxins, neurotoxic polyether compounds active on sodium channels. Toxicon. 1999;37:125–143. doi: 10.1016/S0041-0101(98)00169-X. [DOI] [PubMed] [Google Scholar]
- 17.Lewis R.J. Structure of Caribbean ciguatoxin isolated from Caranx latus. J. Am. Chem. Soc. 1998;120:5914–5920. doi: 10.1021/ja980389e. [DOI] [Google Scholar]
- 18.Manger R.L., Leja L.S., Lee S.Y., Hungerford J.M., Hokama Y., Dickey R.W., Granade H.R., Lewis R., Yasumoto T., Wekell M.M. Detection of sodium channel toxins: Directed cytotoxicity assays of purified ciguatoxins, brevetoxins, saxitoxins, and seafood extracts. J. AOAC Int. 1995;78:521–527. [PubMed] [Google Scholar]
- 19.Poli M.A., Lewis R.J., Dickey R.W., Musser S.M., Buckner C.A., Carpenter L.G. Identification of Caribbean ciguatoxins as the cause of an outbreak of fish poisoning among U.S. soldiers in Haiti. Toxicon. 1997;35:733–741. doi: 10.1016/S0041-0101(96)00166-3. [DOI] [PubMed] [Google Scholar]
- 20.Hardison D.R., Holland W.C., McCall J.R., Bourdelais A.J., Baden D.G., Darius H.T., Chinain M., Tester P.A., Shea D., Quintana H.A.F., et al. Fluorescent receptor binding assay for detecting ciguatoxins in fish. PLoS ONE. 2016;11:e0153348. doi: 10.1371/journal.pone.0153348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasumoto T., Fukui M., Sasaki K., Sugiyama K. Determinations of marine toxins in foods. J. AOAC Int. 1995;78:574–582. [PubMed] [Google Scholar]
- 22.Lewis R.J., Jones A. Characterization of ciguatoxins and ciguatoxin congeners present in ciguateric fish by gradient reverse-phase high-performance liquid chromatography/mass spectrometry. Toxicon. 1997;35:159–168. doi: 10.1016/S0041-0101(96)00132-8. [DOI] [PubMed] [Google Scholar]
- 23.Lewis R.J., Jones A., Vernoux J.-P. HPLC/tandem electrospray mass spectrometry for the determination of Sub-ppb levels of pacific and caribbean ciguatoxins in crude extracts of fish. Anal. Chem. 1999;71:247–250. doi: 10.1021/ac980598h. [DOI] [PubMed] [Google Scholar]
- 24.Lewis R.J., Yang A., Jones A. Rapid extraction combined with LC-tandem mass spectrometry (CREM-LC/MS/MS) for the determination of ciguatoxins in ciguateric fish flesh. Toxicon. 2009;54:62–66. doi: 10.1016/j.toxicon.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Yogi K., Oshiro N., Inafuku Y., Hirama M., Yasumoto T. Detailed LC-MS/MS analysis of ciguatoxins revealing distinct regional and species characteristics in fish and causative alga from the pacific. Anal. Chem. 2011;83:8886–8891. doi: 10.1021/ac200799j. [DOI] [PubMed] [Google Scholar]
- 26.Yogi K., Oshiro N., Matsuda S., Sakugawa S., Matsuo T., Yasumoto T. Toxin profiles in fish implicated in ciguatera fish poisoning in Amami and Kakeroma Islands, Kagoshima prefecture, Japan. J. Food Hyg. Soc. Jpn. 2013;54:385–391. doi: 10.3358/shokueishi.54.385. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton B., Hurbungs M., Vernoux J.-P., Jones A., Lewis R.J. Isolation and characterisation of Indian Ocean ciguatoxin. Toxicon. 2002;40:685–693. doi: 10.1016/S0041-0101(01)00259-8. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton B., Hurbungs M., Jones A., Lewis R.J. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon. 2002;40:1347–1353. doi: 10.1016/S0041-0101(02)00146-0. [DOI] [PubMed] [Google Scholar]
- 29.Diogène J., Reverté L., Rambla-Alegre M., Del Rió V., De La Iglesia P., Campàs M., Palacios O., Flores C., Caixach J., Ralijaona C., et al. Identification of ciguatoxins in a shark involved in a fatal food poisoning in the Indian Ocean. Sci. Rep. 2017;7:8240. doi: 10.1038/s41598-017-08682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hokama Y., Banner A.H.H., Boylan D.B.B. A radioimmunoassay for the detection of ciguatoxin. Toxicon. 1977;15:317–325. doi: 10.1016/0041-0101(77)90014-9. [DOI] [PubMed] [Google Scholar]
- 31.Hokama Y., Hong T.W.P., Isobe M., Ichikawa Y., Yasumoto T. Cross-reactivity of highly purified okadaic acid (OA), synthetic, spiroketal east sphere of OA and ciguatoxin. J. Clin. Lab. Anal. 1992;6:54–58. doi: 10.1002/jcla.1860060111. [DOI] [PubMed] [Google Scholar]
- 32.Hokama Y., Takenaka W.E., Nishimura K.L., Ebesu J.S.M., Bourke R., Sullivan P.K. A Simple Membrane Immunobead Assay for Detecting Ciguatoxin and Related Polyethers from Human Ciguatera Intoxication and Natural Reef Fishes. J. AOAC Int. 1998;81:727–735. [PubMed] [Google Scholar]
- 33.Hokama Y. Immunological studies using monoclonal antibodies for detection of low dalton marine toxins. Food Addit. Contam. 1993;10:83–95. doi: 10.1080/02652039309374132. [DOI] [PubMed] [Google Scholar]
- 34.Lehane L., Lewis R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000;61:91–125. doi: 10.1016/S0168-1605(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 35.Wong C.-K., Hung P., Lee K.L.H., Kam K.-M. Study of an outbreak of ciguatera fish poisoning in Hong Kong. Toxicon. 2005;46:563–571. doi: 10.1016/j.toxicon.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Hirama M., Oishi T., Uehara H., Inoue M., Maruyama M., Oguri H., Satake M. Total synthesis of ciguatoxin CTX3C. Science. 2001;294:1904–1907. doi: 10.1126/science.1065757. [DOI] [PubMed] [Google Scholar]
- 37.Inoue M., Miyazaki K., Uehara H., Maruyama M., Hirama M. First- and second-generation total synthesis of ciguatoxin CTX3C. Proc. Natl. Acad. Sci. USA. 2004;101:12013–12018. doi: 10.1073/pnas.0401684101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue M., Miyazaki K., Ishihara Y., Tatami A., Ohnuma Y., Kawada Y., Komano K., Yamashita S., Lee N., Hirama M. Total synthesis of ciguatoxin and 51-hydroxyCTX3C. J. Am. Chem. Soc. 2006;128:9352–9354. doi: 10.1021/ja063041p. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita S., Ishihara Y., Morita H., Uchiyama J., Takeuchi K., Inoue M., Hirama M. Stereoselective 6-exo radical cyclization using cis-Vinyl sulfoxide: Practical total synthesis of CTX3C. J. Nat. Prod. 2011;74:357–364. doi: 10.1021/np100729d. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita S., Takeuchi K., Koyama T., Inoue M., Hayashi Y., Hirama M. Practical route to the left wing of CTX1B and total syntheses of CTX1B and 54-deoxyCTX1B. Chem.—A Eur. J. 2015;21:2621–2628. doi: 10.1002/chem.201405629. [DOI] [PubMed] [Google Scholar]
- 41.Oguri H., Tanaka S.-I., Hishiyama S., Oishi T., Hirama M., Tsumuraya T., Tomioka Y., Mizugaki M. Designed hapten aimed at anti-ciguatoxin monoclonal antibody: Synthesis, immunization and discrimination of the C2 configuration. Synthesis (Stuttgart) 1999;1999:1431–1436. doi: 10.1055/s-1999-3646. [DOI] [Google Scholar]
- 42.Nagumo Y., Oguri H., Shindo Y., Sasaki S.-Y.S., Oishi T., Hirama M., Tomioka Y., Mizugaki M., Tsumuraya T. Concise Synthesis of Ciguatoxin ABC-Ring Fragments and Surface Plasmon Resonance Study of the Interaction of their BSA Conjugates with Monoclonal Antibodies. Bioorg. Med. Chem. Lett. 2001;11:2037–2040. doi: 10.1016/S0960-894X(01)00358-4. [DOI] [PubMed] [Google Scholar]
- 43.Nagumo Y., Oguri H., Tsumoto K., Shindo Y., Hirama M., Tsumuraya T., Fujii I., Tomioka Y., Mizugaki M., Kumagai I. Phage-display selection of antibodies to the left end of CTX3C using synthetic fragments. J. Immunol. Methods. 2004;289:137–146. doi: 10.1016/j.jim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Oguri H., Hirama M., Tsumuraya T., Fujii I., Maruyama M., Uehara H., Nagumo Y. Synthesis-based approach toward direct sandwich immunoassay for ciguatoxin CTX3C. J. Am. Chem. Soc. 2003;125:7608–7612. doi: 10.1021/ja034990a. [DOI] [PubMed] [Google Scholar]
- 45.Tsumuraya T., Fujii I., Inoue M., Tatami A., Miyazaki K., Hirama M. Production of monoclonal antibodies for sandwich immunoassay detection of ciguatoxin 51-hydroxyCTX3C. Toxicon. 2006;48:287–294. doi: 10.1016/j.toxicon.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Tsumuraya T., Fujii I., Hirama M. Production of monoclonal antibodies for sandwich immunoassay detection of Pacific ciguatoxins. Toxicon. 2010;56:797–803. doi: 10.1016/j.toxicon.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Tsumuraya T., Takeuchi K., Yamashita S., Fujii I., Hirama M. Development of a monoclonal antibody against the left wing of ciguatoxin CTX1B: Thiol strategy and detection using a sandwich ELISA. Toxicon. 2012;60:348–357. doi: 10.1016/j.toxicon.2012.04.347. [DOI] [PubMed] [Google Scholar]
- 48.Tsumuraya T., Fujii I., Hirama M. Preparation of anti-ciguatoxin monoclonal antibodies using synthetic haptens: Sandwich ELISA detection of ciguatoxins. J. AOAC Int. 2014;97:373–379. doi: 10.5740/jaoacint.SGETsumuraya. [DOI] [PubMed] [Google Scholar]
- 49.Tsumuraya T., Sato T., Hirama M., Fujii I. Highly Sensitive and Practical Fluorescent Sandwich ELISA for Ciguatoxins. Anal. Chem. 2018;90:7318–7324. doi: 10.1021/acs.analchem.8b00519. [DOI] [PubMed] [Google Scholar]
- 50.Arevalo J.H., Taussig M.J., Wilson I.A. Molecular basis of crossreactivity and the limits of antibody–antigen complementarity. Nature. 1993;365:859–863. doi: 10.1038/365859a0. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka F., Kinoshita K., Tanimura R., Fujii I. Relaxing Substrate Specificity in Antibody-Catalyzed Reactions: Enantioselective Hydrolysis of N -Cbz-Amino Acid Esters. J. Am. Chem. Soc. 1996;118:2332–2339. doi: 10.1021/ja952220w. [DOI] [Google Scholar]
- 52.Romesberg F.E., Spiller B., Schultz P.G., Stevens R.C. Immunological origins of binding and catalysis in a Diels-Alderase antibody. Science. 1998;279:1929–1933. doi: 10.1126/science.279.5358.1929. [DOI] [PubMed] [Google Scholar]
- 53.Kurihara S., Tsumuraya T., Suzuki K., Kuroda M., Liu L., Takaoka Y., Fujii I. Antibody-catalyzed removal of the p-nitrobenzyl ester protecting group: The molecular basis of broad substrate specificity. Chem. Eur. J. 2000;6:1656–1662. doi: 10.1002/(SICI)1521-3765(20000502)6:9<1656::AID-CHEM1656>3.3.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 54.Tsumuraya T., Takazawa N., Tsunakawa A., Fleck R., Masamune S. Catalytic antibodies induced by a zwitterionic hapten. Chem. Eur. J. 2001;7:3748–3755. doi: 10.1002/1521-3765(20010903)7:17<3748::AID-CHEM3748>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 55.Xu J., Deng Q., Chen J., Houk K.N., Bartek J., Hilvert D., Wilson I.A. Evolution of shape complementarity and catalytic efficiency from a primordial antibody template. Science. 1999;286:2345–2348. doi: 10.1126/science.286.5448.2345. [DOI] [PubMed] [Google Scholar]
- 56.Miyamoto N., Yoshimura M., Okubo Y., Suzuki-Nagata K., Tsumuraya T., Ito N., Fujii I. Structural basis of the broad substrate tolerance of the antibody 7B9-catalyzed hydrolysis of p-nitrobenzyl esters. Bioorganic Med. Chem. 2018;26:1412–1417. doi: 10.1016/j.bmc.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 57.Friguet B., Chaffotte A.F., Djavadi-Ohaniance L., Goldberg M.E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods. 1985;77:305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 58.Suga H., Ersoy O., Tsumuraya T., Lee J., Sinskey A.J., Masamune S., Tsumuraya T., Sinskey A.J., Masamune S. Esterolytic Antibodies Induced to Haptens with a 1,2-Amino Alcohol Functionality. J. Am. Chem. Soc. 1994;116:487–494. doi: 10.1021/ja00081a008. [DOI] [Google Scholar]
- 59.Shimizu Y., Chou H.N., Bando H., Van Duyne G., Clardy J. Structure of brevetoxin A (GB-1 toxin), the most potent toxin in the Florida red tide organism Gymnodinium breve (Ptychodiscus brevis) J. Am. Chem. Soc. 1986;108:514–515. doi: 10.1021/ja00263a031. [DOI] [PubMed] [Google Scholar]
- 60.Pawlak J., Tempesta M.S., Golik J., Zagorski M.G., Lee M.S., Nakanishi K., Iwashita T., Gross M.L., Tomer K.B. Structure of brevetoxin A as constructed from NMR and mass spectral data. J. Am. Chem. Soc. 1987;109:1144–1150. doi: 10.1021/ja00238a025. [DOI] [Google Scholar]
- 61.Lin Y.-Y., Risk M., Ray S.M., Van Engen D., Clardy J., Golik J., James J.C., Nakanishi K. Isolation and structure of brevetoxin B from the “Red Tide” dinoflagellate Ptychodiscus brevis (Gymnodinium breve) J. Am. Chem. Soc. 1981;103:6773–6775. doi: 10.1021/ja00412a053. [DOI] [Google Scholar]
- 62.Lee M.S., Repeta D.J., Nakanishi K., Zagorski M.G. Biosynthetic origins and assignments of carbon 13 NMR peaks of brevetoxin B. J. Am. Chem. Soc. 1986;108:7855–7856. doi: 10.1021/ja00284a072. [DOI] [PubMed] [Google Scholar]
- 63.Tachibana K., Scheuer P.J., Tsukitani Y., Kikuchi H., Van Engen D., Clardy J., Gopichand Y., Schmitz F.J. Okadaic acid, a cytotoxic polyether from two marine sponges of the genus Halichondria. J. Am. Chem. Soc. 1981;103:2469–2471. doi: 10.1021/ja00399a082. [DOI] [Google Scholar]
- 64.Murata M., Iwashita T., Yokoyama A., Sasaki M., Yasumoto T. Partial structures of maitotoxin, the most potent marine toxin from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1992;114:6594–6596. doi: 10.1021/ja00042a070. [DOI] [PubMed] [Google Scholar]
- 65.Ui M., Tanaka Y., Tsumuraya T., Fujii I., Inoue M., Hirama M., Tsumoto K. How protein recognizes ladder-like polycyclic ethers: Interactions between ciguatoxin (CTX3C) fragments and its specific antibody 10C9. J. Biol. Chem. 2008;283:19440–19447. doi: 10.1074/jbc.M801282200. [DOI] [PubMed] [Google Scholar]
- 66.Food and Drug Administration . Fish and Fishery Products Hazards and Controls Guidance. 4th ed. Diane Publishing Co.; Philadelphia, PA, USA: 2011. [Google Scholar]