Abstract
Clostridium perfringens type A causes gas gangrene characterized by myonecrosis and development of an effective therapy for treating affected patients is of clinical importance. It was recently reported that the expression of granulocyte colony-stimulating factor (G-CSF) is greatly up-regulated by C. perfringens infection. However, the role of G-CSF in C. perfringens-mediated myonecrosis is still unclear. Here, we assessed the destructive changes in C. perfringens-infected skeletal muscles and tested whether inhibition of G-CSF receptor (G-CSFR) signaling or administration of recombinant G-CSF affects the tissue injury. Severe edema, contraction of muscle fiber diameter, and increased plasma creatine kinase activity were observed in mice intramuscularly injected with C. perfringens type A, and the destructive changes were α-toxin-dependent, indicating that infection induces the destruction of skeletal muscle in an α-toxin-dependent manner. G-CSF plays important roles in the protection of tissue against damage and in the regeneration of injured tissue. However, administration of a neutralizing antibody against G-CSFR had no profound impact on the destructive changes to skeletal muscle. Moreover, administration of recombinant human G-CSF, filgrastim, imparted no inhibitory effect against the destructive changes caused by C. perfringens. Together, these results indicate that G-CSF is not beneficial for treating C. perfringens α-toxin-mediated myonecrosis, but highlight the importance of revealing the mechanism by which C. perfringens negates the protective effects of G-CSF in skeletal muscle.
Keywords: Clostridial myonecrosis, phospholipase C, host-pathogen interaction, granulocyte colony-stimulating factor
1. Introduction
Clostridium perfringens type A is a Gram-positive anaerobic bacterium that causes gas gangrene [1,2]. The disease is characterized by myonecrosis, shock, multiple organ failure, and death of patients [3]. The infection progresses so rapidly that death precedes diagnosis in some patients and, thus, the development of an effective therapy for treating patients with C. perfringens-mediated myonecrosis is of utmost importance.
The well-known virulence factors produced by C. perfringens type A are α-toxin (or phospholipase C), which has both phospholipase C (PLC) and sphingomyelinase (SMase) activities [3,4], and θ-toxin (or perfringlysin O), which is a pore-forming and cholesterol-dependent cytolysin [5,6]. Using a mouse model of C. perfringens-mediated myonecrosis, it was reported that α-toxin-deficient and θ-toxin-deficient strains delay the spread of muscle necrosis [7]. θ-toxin is cytotoxic to polymorphonuclear leukocytes and macrophages [8,9,10]. α-Toxin and θ-toxin enhance intravascular cell aggregation, leading to vascular occlusion, and impair the host immune response by impeding inflammatory cell infiltration to the site of the infection, which is a hallmark of clostridial myonecrosis [11,12,13,14,15]. Additionally, we have reported that α-toxin interferes with the production of neutrophils by inhibiting their differentiation in bone marrow, which is also related to the impairment of innate host immunity [16,17]. Furthermore, C. perfringens produces other toxins and enzymes, including a collagenase, hyaluronicdase, sialidases, and the cysteine protease α-clostripain [5,18]. Thus, various toxins involved in C. perfringens-mediated myonecrosis have been identified and the infection process has become increasingly clear. However, understanding of the recovery process remains limited.
Low et al. recently analyzed the host transcriptome from C. peringens-infected muscle and found that expression of Csf3, the gene encoding granulocyte colony-stimulating factor (G-CSF), is greatly up-regulated by the infection [19]. G-CSF is a cytokine that stimulates the mobilization of hematopoietic stem cells and the production of neutrophils and exerts its effects by binding to G-CSF receptor (G-CSFR) [20]. G-CSFR is not only expressed on hematopoietic cells, but also non-hematopoietic cells, including oval cells, myoblasts, cardiomyocytes, and neural tissue [21,22,23,24]. G-CSF was reported to be involved in the development of cardiac muscle and skeletal muscle by promoting their cell proliferation [23,25]. Moreover, a growing body of scientific evidence has indicated that treatment with G-CSF ameliorates tissue injury in various organs, as follows: A neuroprotective effect of G-CSF in cerebral ischemia was reported [26]; G-CSF improves outcomes in mouse models of amyotrophic lateral sclerosis and doxorubicin-induced cardiomyopathy [27,28]; anti-apoptotic effects of G-CSF were observed on neurons [26,29]; G-CSF enhances muscle cell proliferation and strength following muscle injury in rats [30]; and G-CSF promotes liver repair by enhancing oval cell proliferation [21]. Thus, G-CSF plays important roles in protecting tissue against damage and in regenerating injured tissue. However, it is still unknown how the amplified production of G-CSF following C. perfringens infection affects the progression of C. perfringens-mediated myonecrosis. Here, we assessed the destructive changes in C. perfringens-infected skeletal muscles and tested whether inhibition of G-CSFR signaling or administration of recombinant G-CSF affects the tissue injury.
2. Results
2.1. C. perfringens Induces Myonecrosis in a Toxin-Dependent Manner
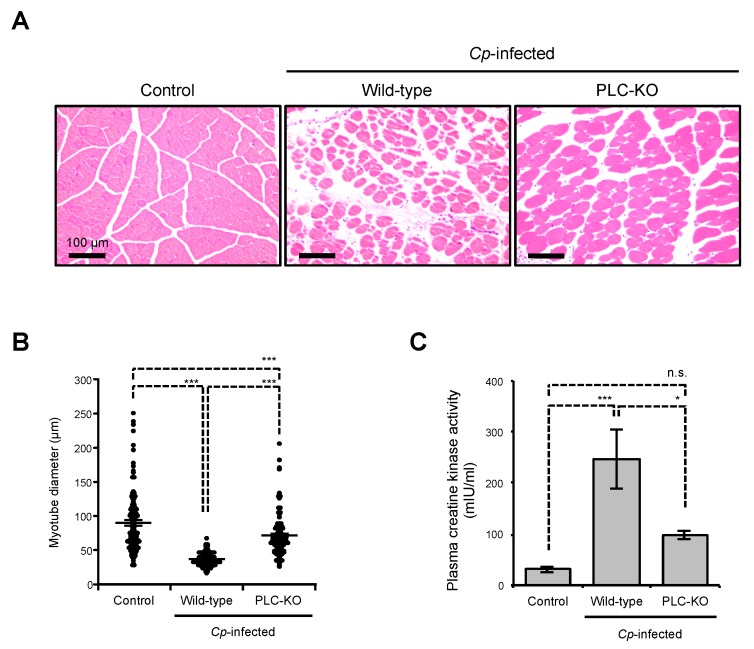
It was previously reported that C. perfringens infection causes myonecrosis in mice and the severity of skeletal muscle necrosis decreased in mice injected with the α-toxin-deficient strain [7]. At 24 h post-infection, severe edema and a contraction of muscle fiber diameter were observed in mice intramuscularly injected with wild-type (WT) C. perfringens type A (Figure 1A,B). The release of creatine kinase, which is a marker of muscle damage, into the circulation following C. perfringens infection or inoculation of α-toxin has been reported [31]. As shown in Figure 1C, plasma creatine kinase activity increased in C. perfringens-infected mice. These results indicate that C. perfringens infection induces the destruction of skeletal muscle. The destructive changes observed in a plc gene-knockout mutant of C. perfringens (PLC-KO)-infected mice were lower than those in mice injected with WT C. perfringens (Figure 1A–C), demonstrating that C. perfringens induces myonecrosis in an α-toxin-dependent manner.
Figure 1.
Destructive changes in C. perfringens-infected skeletal muscle. Mice were intramuscularly injected with 1 × 107 CFU of C. perfringens Strain 13 (Wild-type), PLC-KO (PLC-KO), or TGY (tryptone, glucose, and yeast extract) medium as a control (Control). (A) Muscles were isolated 24 h after infection. Hematoxylin and eosin (H&E)-stained sections are shown. Representative H&E-stained sections of three independent experiments are shown. (B) The diameters of at least 100 muscle fibers of three independent experiments were measured. (C) Peripheral blood was isolated 24 h after infection and plasma creatine kinase activities were determined using a creatine kinase activity assay kit (n = 8 per condition). One-way ANOVA was employed to assess significance. Values are the mean ± standard error. * p < 0.05; *** p < 0.001; n.s., not significant.
2.2. Inhibition of G-CSFR Does Not Affect C. perfringens α-Toxin-Induced Myonecrosis In Vivo
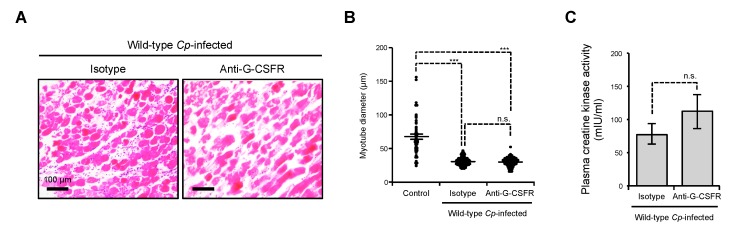
The augmented production of G-CSF in C. perfringens-infected skeletal muscle was observed in two independent experiments [19,32], but the role of G-CSF in C. perfringens-induced myonecrosis has not been elucidated. To reveal how the amplified production of G-CSF affects the progression of C. perfringens-mediated myonecrosis, we treated C. perfringens-infected mice with a neutralizing antibody against G-CSFR shortly after the injection of C. perfringens and assessed the destructive changes in skeletal muscles at 24 h after infection. The dose of the antibody was 10 times higher than that in the previous report [33]. Many studies have indicated that treatment with G-CSF ameliorates tissue injury in various organs, such as skeletal muscle, brain, and cardiac muscle [26,27,28,29,30]. Therefore, we expected that the administration of a neutralizing antibody against G-CSFR exacerbates the destructive changes, i.e., severe edema, contraction of muscle fiber diameter, and release of creatine kinase. Contrary to our expectation, the administration of the neutralizing antibody had no profound impact on the destructive changes (Figure 2A–C). Thus, inhibition of G-CSFR signaling did not affect C. perfringens α-toxin-induced myonecrosis in vivo.
Figure 2.
Neutralization of G-CSFR does not influence C. perfringens α-toxin-induced myonecrosis. Mice were intramuscularly injected with 1 × 107 CFU of C. perfringens Strain 13 (Wild-type Cp-infected) or TGY medium as a control (Control). To neutralize G-CSFR, a specific antibody against mouse G-CSFR (Anti-G-CSFR) or an isotype control antibody (Isotype) was intraperitoneally administered to the C. perfringens-infected mice shortly after the injection of C. perfringens. (A) Representative H&E-stained sections of three independent experiments are shown. (B) The diameters of at least 100 muscle fibers of three independent experiments were measured. (C) Plasma creatine kinase activities were determined using a creatine kinase activity assay kit (n = 11 per condition). One-way ANOVA or the two-tailed Student’s t-test was employed to assess significance. Values are the mean ± standard error. *** p < 0.001; n.s., not significant.
2.3. Filgrastim Has no Protective Effect on Skeletal Muscle During C. perfringens α-Toxin-Mediated Myonecrosis
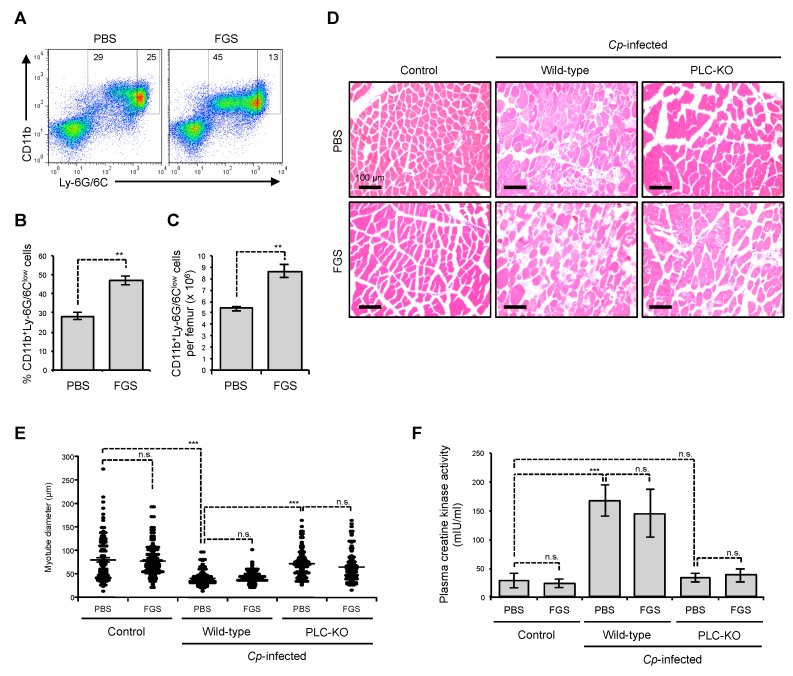
G-CSF represents antiapoptotic effects on neurons [26,29]. Additionally, G-CSF was reported to promote skeletal muscle regeneration and development by stimulating myoblast proliferation [25]. In the clinical setting, recombinant G-CSF is widely used to treat neutropenia caused by cancer treatment, to stimulate production of neutrophils [34]. A Neuroprotective effect of recombinant human G-CSF in transient focal ischemia of mice has been reported [26], suggesting that human G-CSF and murine G-CSF show biological cross reactivity. To test whether treatment with G-CSF is beneficial for C. perfringens α-toxin-mediated myonecrosis, we administered recombinant human G-CSF, filgrastim, to C. perfringens-infected mice and assessed the destructive changes. As shown in Figure 3A–C, administration of filgrastim increased the proportion and number of CD11b+Ly-6G/6Clow immature neutrophils in bone marrow at 24 h after the injection, demonstrating that the injection protocol is appropriate for assessing the efficacy of filgrastim. However, filgrastim had no profound impact on the severe edema and contraction of muscle fiber diameter at 24 h after infection with C. perfringens (Figure 3D,E). Moreover, the administration did not diminish the release of creatine kinase (Figure 3F). Together, our results suggest that filgrastim has no protective effect on skeletal muscle during C. perfringens α-toxin-mediated myonecrosis.
Figure 3.
Filgrastim has no protective effect against C. perfringens infection in skeletal muscle. (A–C) Mice were subcutaneously injected with 1.5 µg of filgrastim (FGS) or the same volume of phosphate buffered saline (PBS), bone marrow cells (BMCs) were isolated after 24 h, and flow cytometry analysis was performed. A representative flow cytometry profile of three independent experiments (A) and the proportion (B, n = 3) and absolute number (C, n = 3) of CD11b+Ly-6G/6Clow immature neutrophils are shown. (D–F) Mice were intramuscularly injected with 1 × 107 CFU of C. perfringens Strain 13 (Wild-type), PLC-KO (PLC-KO), or TGY medium as a control (Control). Shortly after the injection, 1.5 µg of filgrastim (FGS) or the same volume of PBS was administered subcutaneously and muscles were isolated 24 h after infection. (D) Representative H&E-stained sections of three independent experiments are shown. (E) The diameters of at least 100 muscle fibers of three independent experiments were measured. (F) Plasma creatine kinase activities were determined using a creatine kinase activity assay kit (n = 5–8). One-way ANOVA or the two-tailed Student’s t-test was employed to assess significance. Values are the mean ± standard error. ** p < 0.01;*** p < 0.001; n.s., not significant.
3. Discussion
In the present study, we showed that C. perfringens induces myonecrosis in an α-toxin dependent manner, but PLC-KO infection still induces the destruction of skeletal muscle. Using a mouse model of C. perfringens-mediated myonecrosis, it was reported that θ-toxin-deficient strains delay the spread of muscle necrosis [7], meaning that θ-toxin is also involved in C. perfringens-mediated myonecrosis. Furthermore, C. perfringens produces other toxins and enzymes including a collagenase, hyaluronicdase, sialidases, and the cysteine protease α-clostripain [5,18]. Thus, various toxins involved in C. perfringens-mediated myonecrosis have been identified. Therefore, myonecrosis caused by PLC-KO infection could be explained by the existence of the other toxins.
A growing body of scientific evidence indicates that treatment with G-CSF ameliorates tissue injury in various organs [26,27,28,29,30]. However, our results demonstrated that G-CSF has no influence on C. perfringens-induced myonecrosis in mice. In the present study, we evaluated the destructive changes at 24 h after C. perfringens infection. Low et al. reported that C. perfringens-mediated clostridial myonecrosis progresses extremely rapidly; early signs of the disease in mouse skeletal muscle are observed from two hours after infection [19]. We previously reported that secretion of G-CSF starts to be up-regulated more than three hours after stimulation of toll-like receptor 2 by its agonist, peptidoglycan [35]. It is thus possible that tissue injury by C. perfringens infection commences before the increased production of G-CSF.
In the present study, C. perfringens-infected mice were administrated with the G-CSFR antibody or recombinant G-CSF shortly after the infection for the following reasons. One is that G-CSFR-expressing cells are absent in non-injured skeletal muscle while G-CSFR is clearly expressed in injured skeletal muscle [25], and then we thought that pre-treatment with the G-CSFR antibody or recombinant G-CSF does not represent any effect on skeletal muscle. Moreover, pre-treatment might increase the chance of detecting off-target effects for the same reason. The second is that pre-treatment with G-CSFR antibody or recombinant G-CSF should affect granulopoiesis and change the total number of neutrophils in the whole body. Neutrophils have been shown to be involved in the elimination of injected C. perfringens in skeletal muscle [16], so the change of the number of neutrophils could influence the survival of C. perfringens. The difference of the survival probably affects destructive changes by C. perfringens infection. Thus, it would be difficult to evaluate the results of pre-treatment experiments. However, it would still be worth testing if pre-treatment with G-CSFR antibody or G-CSF prior to C. perfringens infection improves the outcome of C. perfringens infection.
Recently, we reported that α-toxin augmented the production of G-CSF from endothelial cells only in the presence of TLR2 agonists, suggesting that α-toxin enhances Toll-like receptor 2 (TLR2) signaling [32]. During C. perfringens infection, bacterial components stimulate TLR2. The increased G-CSF could act on myeloid progenitors to promote granulopoiesis, which is one of the host defense mechanisms against pathogenic bacterial infection, but α-toxin disturbs G-CSF-mediated granulopoiesis by reducing the expression of G-CSFR on neutrophils [32]. Thus, C. perfringens overwhelm the host defense by producing α-toxin. G-CSF represents antiapoptotic effects on various tissues including skeletal muscle, brain, and cardiac muscle [26,27,28,29,30], which is another host defense mechanism, but no protective effect by G-CSF treatment was observed during C. perfringens infection in the present study. Further investigation is necessary to elucidate the detailed mechanism, but it is possible that α-toxin is involved in the negation of the tissue protective effect of G-CSF on skeletal muscles.
C. perfringens type A is known to produce several toxins related to the destruction of skeletal muscle. α-Toxin, which is a cytotoxic bacterial phospholipase C, plays a key role in the pathogenesis of myonecrosis [3,4]. In the steady state, G-CSFR is expressed in proliferating mouse myoblasts, C2C12 cells, differentiated C2C12 myotubes, and human and mouse primary skeletal muscle [22]. However, we recently reported that α-toxin desensitizes neutrophils to G-CSF by inducing the degradation of G-CSFR [32]. Additionally, C. perfringens type A produces θ-toxin, which is a pore-forming cholesterol-dependent cytolysin [5,6]. Muscle tissue from mice infected with a θ-toxin-deficient strain displays a delayed spread of muscle necrosis [7]. Thus, cytotoxic bacterial toxins produced by C. perfringens type A are involved in the pathogenesis of clostridial myonecrosis. It is possible that these toxins disturb and impair the antiapoptotic effects of G-CSF, or that treatment with G-CSF has no protective effect against muscle cell necrosis. Furthermore, G-CSFR expression increases in muscle cells after injury by injection of cardiotoxin [25], suggesting that damage to skeletal muscles makes the muscle cells more sensitive to G-CSF. Further studies are needed to clarify whether and how the increased G-CSF production affects skeletal muscle regeneration during the recovery period.
4. Conclusions
In conclusion, our results suggest that G-CSF is not beneficial for the treatment of C. perfringens α-toxin-mediated myonecrosis, but they emphasize the importance of revealing the mechanism by which C. perfringens negates the protective effects of G-CSF in skeletal muscle. We hope that further studies will unveil this mechanism and that our results contribute to the development of new therapeutic strategies for treating patients with clostridial myonecrosis.
5. Materials and Methods
5.1. Mice
For all experiments, mice aged more than 8 weeks old were used. The animal experiments were approved by the Animal Care and Use Committee of Tokushima Bunri University and procedures were performed in accordance with institutional guidelines (Approval code: 17-3 Date: 1 April 2018). C57BL/6J mice were from SLC (Shizuoka, Japan).
5.2. Reagents and Strains
Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated specific antibodies against mouse CD11b (clone M1/70) or Ly-6G (clone 1A8) and purified rat anti-mouse CD16/CD32 (Fc Block) were purchased from BD Biosciences (San Jose, CA, USA). Recombinant human G-CSF, filgrastim, was from Mochida Pharmaceutical Co., Ltd. (Tokyo, Japan). All other chemicals were of the highest grade available from commercial sources. C. perfringens wild-type (WT) Strain 13 was used in this study. The preparation of a plc gene-knockout mutant of C. perfringens (PLC-KO) was as described in our previous report [16].
5.3. Flow Cytometry Analysis
Mice were subcutaneously injected with 1.5 µg of filgrastim or the same volume of PBS and bone marrow cells (BMCs) were isolated after 24 h, as previously described [16]. The antibodies described above were used to label cells after blocking Fc-receptors with purified rat anti-mouse CD16/CD32. Antibodies were diluted with PBS containing 2% fetal bovine serum (FBS; AusGeneX, Molendinar, QLD, Australia). The labeled cells were analyzed using a Guava easyCyte (Millipore, Billerica, MA, USA). FlowJo software (Tree Star, Ashland, OR, USA) was used to analyze the data.
5.4. Bacterial Culture and Infection
Bacterial culture and infection were performed as previously described [16]. Briefly, C. perfringens WT strain 13 or PLC-KO were grown in TGY (tryptone, glucose, and yeast extract) medium under anaerobic conditions at 37 °C, and exponentially-growing bacteria were harvested, washed, and re-suspended in TGY medium. The bacteria were injected into the femoral muscles of the mice. Residual bacteria were serially diluted, plated on brain heart infusion agar plates, and cultured anaerobically at 37 °C to quantify the colony-forming units (CFU).
5.5. Myotube Morphology Analysis
C. perfringens-infected muscles were isolated 24 h after infection. The isolated tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Paraffin sections were cut from the tissue and stained with hematoxylin and eosin to visualize muscle fibers. Pictures of the muscle fibers were taken using a digital camera and the diameters of muscle fibers were measured using a DS-L4 (Nikon, Tokyo, Japan). The diameters of at least 100 muscle fibers were measured for each condition.
5.6. Administration of G-CSF and Inhibition of G-CSFR In Vivo
Mice were subcutaneously injected with 1.5 µg of filgrastim or the same volume of PBS, shortly after the injection of C. perfringens. To inhibit G-CSFR, a neutralizing antibody against G-CSFR (R&D systems, Minneapolis, MN, USA) was used as described in a previous report [33]. The neutralizing antibody or Rat IgG2B isotype control (R&D systems, Minneapolis, MN, USA) was intraperitoneally administered to C. perfringens-infected mice at 5 µg per mouse shortly after the injection of C. perfringens. This dose was 10 times higher than that in the previous report [33].
5.7. Measurement of Plasma Creatine Kinase
Using heparinized syringes, peripheral blood was obtained via the vena cava from mice 24 h after C. perfringens injection. To assess plasma creatine kinase activity, a commercial creatine kinase activity assay kit (Abcam, Cambridge, MA, USA) was used, and measurements were performed in accordance with the manufacturer’s instructions.
5.8. Statistical Analysis
All statistical analyses were performed with Easy R (Version 1.38, Saitama Medical Center, Jichi Medical University, Saitama, Saitama, Japan, 2018) [36]. Differences between the two groups were evaluated using the two-tailed Student’s t-test. One-way analysis of variance (ANOVA) followed by Tukey’s test was used to evaluate differences among three or more groups. Differences were considered to be significant for values of p < 0.05.
Acknowledgments
The authors thank Satono Takada and Sachiko Minakuchi for providing technical assistance.
Author Contributions
M.T. and M.N. supervised the experiments. M.T. performed experiments and analyses, designed the study, and wrote the manuscript. Y.S. and H.B. contributed to murine studies. K.K. contributed to the design of the study.
Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
G-CSF is not beneficial for treating C. perfringens α-toxin-mediated myonecrosis.
References
- 1.Songer J.G. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 1996;9:216–234. doi: 10.1128/CMR.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petit L., Gibert M., Popoff M.R. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999;7:104–110. doi: 10.1016/S0966-842X(98)01430-9. [DOI] [PubMed] [Google Scholar]
- 3.Bryant A.E. Biology and pathogenesis of thrombosis and procoagulant activity in invasive infections caused by group A streptococci and Clostridium perfringens. Clin. Microbiol. Rev. 2003;16:451–462. doi: 10.1128/CMR.16.3.451-462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai J., Nagahama M., Oda M. Clostridium perfringens alpha-toxin: Characterization and mode of action. J. Biochem. 2004;136:569–574. doi: 10.1093/jb/mvh161. [DOI] [PubMed] [Google Scholar]
- 5.Rood J.I. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 1998;52:333–360. doi: 10.1146/annurev.micro.52.1.333. [DOI] [PubMed] [Google Scholar]
- 6.Verherstraeten S., Goossens E., Valgaeren B., Pardon B., Timbermont L., Haesebrouck F., Ducatelle R., Deprez P., Wade K.R., Tweten R., et al. Perfringolysin O: The Underrated Clostridium perfringens Toxin? Toxins. 2015;7:1702–1721. doi: 10.3390/toxins7051702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellemor D.M., Baird R.N., Awad M.M., Boyd R.L., Rood J.I., Emmins J.J. Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene. Infect. Immun. 1999;67:4902–4907. doi: 10.1128/iai.67.9.4902-4907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens D.L., Mitten J., Henry C. Effects of alpha and theta toxins from Clostridium perfringens on human polymorphonuclear leukocytes. J. Infect. Dis. 1987;156:324–333. doi: 10.1093/infdis/156.2.324. [DOI] [PubMed] [Google Scholar]
- 9.Stevens D.L., Bryant A.E. Role of theta toxin, a sulfhydryl-activated cytolysin, in the pathogenesis of clostridial gas gangrene. Clin. Infect. Dis. 1993;16:195–199. doi: 10.1093/clinids/16.Supplement_4.S195. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien D.K., Melville S.B. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infect. Immun. 2004;72:5204–5215. doi: 10.1128/IAI.72.9.5204-5215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant A.E., Bayer C.R., Aldape M.J., Wallace R.J., Titball R.W., Stevens D.L. Clostridium perfringens phospholipase C-induced platelet/leukocyte interactions impede neutrophil diapedesis. J. Med. Microbiol. 2006;55:495–504. doi: 10.1099/jmm.0.46390-0. [DOI] [PubMed] [Google Scholar]
- 12.Ochi S., Miyawaki T., Matsuda H., Oda M., Nagahama M., Sakurai J. Clostridium perfringens alpha-toxin induces rabbit neutrophil adhesion. Microbiology. 2002;148:237–245. doi: 10.1099/00221287-148-1-237. [DOI] [PubMed] [Google Scholar]
- 13.Bryant A.E., Chen R.Y., Nagata Y., Wang Y., Lee C.H., Finegold S., Guth P.H., Stevens D.L. Clostridial gas gangrene. II. Phospholipase C-induced activation of platelet gpIIbIIIa mediates vascular occlusion and myonecrosis in Clostridium perfringens gas gangrene. J. Infect. Dis. 2000;182:808–815. doi: 10.1086/315757. [DOI] [PubMed] [Google Scholar]
- 14.Stevens D.L., Tweten R.K., Awad M.M., Rood J.I., Bryant A.E. Clostridial gas gangrene: Evidence that alpha and theta toxins differentially modulate the immune response and induce acute tissue necrosis. J. Infect. Dis. 1997;176:189–195. doi: 10.1086/514022. [DOI] [PubMed] [Google Scholar]
- 15.Bahl H., Dürre P. Clostridia: Biotechnology and Medical Applications. Wiley-VCH; Weinheim, Germany: Chichester, UK: 2001. p. xii.279p [Google Scholar]
- 16.Takehara M., Takagishi T., Seike S., Ohtani K., Kobayashi K., Miyamoto K., Shimizu T., Nagahama M. Clostridium perfringens α-toxin impairs innate immunity via inhibition of neutrophil differentiation. Sci. Rep. 2016;6:28192. doi: 10.1038/srep28192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takehara M., Takagishi T., Seike S., Oishi K., Fujihara Y., Miyamoto K., Kobayashi K., Nagahama M. Clostridium perfringens α-toxin impairs lipid raft integrity in neutrophils. Biol. Pharm. Bull. 2016;39:1694–1700. doi: 10.1248/bpb.b16-00444. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu T., Ohtani K., Hirakawa H., Ohshima K., Yamashita A., Shiba T., Ogasawara N., Hattori M., Kuhara S., Hayashi H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low L.Y., Harrison P.F., Gould J., Powell D.R., Choo J.M., Forster S.C., Chapman R., Gearing L.J., Cheung J.K., Hertzog P., et al. Concurrent Host-Pathogen Transcriptional Responses in a Clostridium perfringens Murine Myonecrosis Infection. MBio. 2018;9:e00473-18. doi: 10.1128/mBio.00473-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts A.W. G-CSF: A key regulator of neutrophil production, but that’s not all! Growth Factors. 2005;23:33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 21.Piscaglia A.C., Shupe T.D., Oh S.H., Gasbarrini A., Petersen B.E. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology. 2007;133:619–631. doi: 10.1053/j.gastro.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright C.R., Brown E.L., Della-Gatta P.A., Ward A.C., Lynch G.S., Russell A.P. G-CSF does not influence C2C12 myogenesis despite receptor expression in healthy and dystrophic skeletal muscle. Front. Physiol. 2014;5:170. doi: 10.3389/fphys.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimoji K., Yuasa S., Onizuka T., Hattori F., Tanaka T., Hara M., Ohno Y., Chen H., Egasgira T., Seki T., et al. G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs. Cell Stem Cell. 2010;6:227–237. doi: 10.1016/j.stem.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Yata K., Matchett G.A., Tsubokawa T., Tang J., Kanamaru K., Zhang J.H. Granulocyte-colony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxia-ischemia in rats. Brain Res. 2007;1145:227–238. doi: 10.1016/j.brainres.2007.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara M., Yuasa S., Shimoji K., Onizuka T., Hayashiji N., Ohno Y., Arai T., Hattori F., Kaneda R., Kimura K., et al. G-CSF influences mouse skeletal muscle development and regeneration by stimulating myoblast proliferation. J. Exp. Med. 2011;208:715–727. doi: 10.1084/jem.20101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komine-Kobayashi M., Zhang N., Liu M., Tanaka R., Hara H., Osaka A., Mochizuki H., Mizuno Y., Urabe T. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J. Cereb. Blood Flow Metab. 2006;26:402–413. doi: 10.1038/sj.jcbfm.9600195. [DOI] [PubMed] [Google Scholar]
- 27.Pitzer C., Kruger C., Plaas C., Kirsch F., Dittgen T., Muller R., Laage R., Kastner S., Suess S., Spoelgen R., et al. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain. 2008;131:3335–3347. doi: 10.1093/brain/awn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L., Takemura G., Li Y., Miyata S., Esaki M., Okada H., Kanamori H., Ogino A., Maruyama R., Nakagawa M., et al. Granulocyte colony-stimulating factor improves left ventricular function of doxorubicin-induced cardiomyopathy. Lab. Investig. 2007;87:440–455. doi: 10.1038/labinvest.3700530. [DOI] [PubMed] [Google Scholar]
- 29.Solaroglu I., Cahill J., Jadhav V., Zhang J.H. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 2006;37:1123–1128. doi: 10.1161/01.STR.0000208205.26253.96. [DOI] [PubMed] [Google Scholar]
- 30.Stratos I., Rotter R., Eipel C., Mittlmeier T., Vollmar B. Granulocyte-colony stimulating factor enhances muscle proliferation and strength following skeletal muscle injury in rats. J. Appl. Physiol. 2007;103:1857–1863. doi: 10.1152/japplphysiol.00066.2007. [DOI] [PubMed] [Google Scholar]
- 31.Monturiol-Gross L., Flores-Diaz M., Araya-Castillo C., Pineda-Padilla M.J., Clark G.C., Titball R.W., Alape-Giron A. Reactive oxygen species and the MEK/ERK pathway are involved in the toxicity of clostridium perfringens alpha-toxin, a prototype bacterial phospholipase C. J. Infect. Dis. 2012;206:1218–1226. doi: 10.1093/infdis/jis496. [DOI] [PubMed] [Google Scholar]
- 32.Takehara M., Seike S., Sonobe Y., Bandou H., Yokoyama S., Takagishi T., Miyamoto K., Kobayashi K., Nagahama M. Clostridium perfringens alpha-toxin impairs granulocyte colony-stimulating factor receptor-mediated granulocyte production while triggering septic shock. Commun. Biol. 2019;2:45. doi: 10.1038/s42003-019-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Files D.C., Liu C., Pereyra A., Wang Z.M., Aggarwal N.R., D’Alessio F.R., Garibaldi B.T., Mock J.R., Singer B.D., Feng X., et al. Therapeutic exercise attenuates neutrophilic lung injury and skeletal muscle wasting. Sci. Transl. Med. 2015;7:278ra232. doi: 10.1126/scitranslmed.3010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frampton J.E., Lee C.R., Faulds D.F. A review of its pharmacological properties and therapeutic efficacy in neutropenia. Drugs. 1994;48:731–760. doi: 10.2165/00003495-199448050-00007. [DOI] [PubMed] [Google Scholar]
- 35.Takehara M., Seike S., Takagishi T., Kobayashi K., Nagahama M. Peptidoglycan accelerates granulopoiesis through a TLR2- and MyD88-dependent pathway. Biochem. Biophys. Res. Commun. 2017;487:419–425. doi: 10.1016/j.bbrc.2017.04.077. [DOI] [PubMed] [Google Scholar]
- 36.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]