Abstract
Silicon (Si), the second most predominant element in the earth crust consists of numerous benefits to plant. Beneficial effect of Si has been apparently visible under both abiotic and biotic stress conditions in plants. Supplementation of Si improved physiology and yield on several important agricultural and horticultural crops. Salinity is one of the major abiotic stresses that affect growth and yield. The presence of high concentration of salt in growing medium causes oxidative, osmotic, and ionic stresses to plants. In extreme conditions salinity affects soil, ground water, and limits agricultural production. Si ameliorates salt stress in several plants. The Si mediated stress mitigation involves various regulatory mechanisms such as photosynthesis, detoxification of harmful reactive oxygen species using antioxidant and non-antioxidants, and proper nutrient management. In the present review, Si mediated alleviation of salinity stress in plants through the regulation of photosynthesis, root developmental changes, redox homeostasis equilibrium, and regulation of nutrients have been dealt in detail.
Keywords: agriculture, abiotic stress, nutrients, physiology, photosynthesis, Silicate
1. Introduction
Salinity is one of the predominant abiotic stresses that affect agricultural production. The adverse effects of salinity have damaged at least 20% of crop cultivation around the world [1]. Upon the exposure to high saline conditions, the water absorption rate of plants is reduced drastically. This affects inter- and intra-cellular water level and inhibits the cell expansion followed by decline in stomatal activity. The long term exposure of salinity results in substantial ionic and oxidative stresses that are caused due to the higher amount of NaCl influx [2]. Ionic and osmotic imbalances under salt stress condition impairs the growth and development of plants [3]. Accumulation of salts decreases the concentration of photosynthetic pigments such as chlorophyll and carotenoid followed by the inhibition of ribulose-1,5-bisphophate and degrades the photosynthetic apparatus. Consequently, generation of higher amounts of reactive oxygen species (ROS)surpasses the level of scavenging rate [4]. Moreover, excessive ROS hinders the transpiration, nutrient uptake, damages vital macromolecules such as nucleic acids, proteins and lipids, collapses membrane integrity, and other vital metabolisms [5]. Interference of NaCl in the protein synthesis, activities of enzymes, and photosynthesis leads to the chlorosis, necrosis, and premature senescence of older leaves [3]. To overcome the salt stress plants need to improve ion exclusion, osmotic tolerance, redox homeostasis, and efficient photosynthesis. In order to combat the adverse effect of salinity stress in plants various researches have been initiated and ongoing in myriads fields of plant biology.
Silicon (Si) is the second most abundant element in the earth crust. Previously, Si was considered as beneficial but non-essential element. Recently, the International Plant Nutrition Institute included Si as “quasi-essential” element. Plants uptake Si in the form of soluble silicic acid [Si(OH)4] under less than 9 pH [6]. Supplementation of Si has been proved as beneficial to plants in several ways such as increasing yield, resistance against diseases, and alleviation of abiotic stresses. The amendment of Si nutrition has been reported against various stresses including, salinity [7], higher temperature [8], powdery mildew [9], hyperhydricity [10], and herbivores [11]. In general, Si mediated stress resistance mechanism can be attributed to the following physiological improvements in plants; enhancement of growth and biomass, management of essential nutrients, maintenance of structural rigidity, increased photosynthesis efficiency, lodging resistance, balancing the ion homeostasis, activation of antioxidant system in plants, elicitation of secondary metabolites related to stress resistance, and regulation of genes involved in various physiological processes [12]. Thus, the numerous merits of Si have been exploited in diverse crops to increase the yield and stress tolerance. Although, several reports have evidenced the merits of Si nutrition, the exact molecular mechanism behind the stress alleviation is still under study. However, in the current review the available mechanisms of silicon to alleviate salinity stress have been summarized.
Stress Tolerance Imparted by Silicon
Under salinity condition, Si decreases the apoplastic transportation of Na+ and Cl− [13,14]. In Si accumulators, formation of double-cuticle layers by amorphous-Si increases the photosynthetic rate and decreases the evapo-transpiration. Further, Si improves the rigidity and erectness of leaves to enhance the photosynthetic canopy [15]. Photosynthesis pigment degradation was efficiently prevented by the application of Si. Photosynthesis-related proteins such as photosystem-I, -II, RubisCO, and other chloroplast-related proteins were regulated by the Si during the salinity and hyperhydric condition [10,16]. Several studies reported that Si modulated the redox-homeostasis mechanism. Supplemented Si regulated the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), guaiacolperoxidase (GPX), and ascorbate peroxidase (APX) to overcome the redox imbalance [17,18,19]. In addition, lower molecular non-enzymatic antioxidants such as glutathione and proline content under the stress condition was improved by the Si supplementation [20].
Recent study showed that biochars made from bamboo combined with the Si (Si-biochar) effectively reduced the arsenic (As) bioaccumulation in spinach by 37.7%. Additionally, Si improved the dry biomass of spinach to 67.7% [21]. Under As stress condition, expression of Si transporters such as Lsi1 and Lsi2 was increased in Oryza sativa along with glutaredoxin (GRX) and glutathione-S-transferase (GST) [22]. A study conducted between the drought resistant and drought sensitive lines of tomato showed that Si application resulted on different response in both genotypes. In tolerant line, Si improved sulfur (S) and ammonium (NH4+). This leads to the higher synthesis of amino acids such as arginine, methionine, serine, and glycine. Moreover, in drought sensitive tomato the augmentation of Si improved the proline and gamma-aminobutyric acid (GABA) levels. Enhancement of GABA reduced the level of glutathione oxidized glutathione (GSSG) to oxidized glutathione (GSH) ratio and maintains equilibrium in redox homeostasis [23]. Over expression of Lsi1 into the rice under cold stress efficiently improved several physiological processes [24]. Freezing tolerance of Si studied in Vitis vinefera showed that foliar application was more effective than the soil. Freezing stresswas overcome by Si in improving PSII, carotenoid, and membrane integrity [25]. Freezing resistance could be attributed to the higher antioxidant capacity in the Si treatment of Pistaciavera [26]. Application of Si reduced the cadmium translocation from root to shoot in Nicotiana tobacum. Reduction of Cd uptake could contribute for lowering the potential health risks of Cd contamination [27]. The activities of enzymatic and non-enzymatic antioxidants activities were highly modulated in wheat under various abiotic stress conditions such as drought, salt, and Cd stress [28]. Wu et al. [29] reported that Si decreased the Cd uptake by modulating the root endodermal suberin development in wheat. It is correlated with higher expression of Cd efflux-related gene Triticum aestivum transmembrane 20 (TaTM20). Si efficiently regulated the sulfur deficiency and osmotic stress on barley by modulating the sulfur transporter(HsST1) and abscisic acid (ABA) metabolism related genes such as glyceraldehydes-3-phosphate dehydrogenase (GAPDH), cyclophilin (CYC), and ADP-Ribosylation factor (ADP-RF1) [30]. Similarly, Si treated Poaannua seedlings displayed more tolerance against Cd toxicity [31]. Influence of Si under osmotic stress on starch metabolism, glycolytic, and TCA pathways has been reported in Hordeum vulgare [32]. Likewise, supplementation of Si in the in vitro culture medium ameliorated hyperhydricity. Recently, Manivannan et al. [33] reported that inclusion of potassium silicate (K2SiO3) and calcium silicate (CaSiO3) on different concentrations along with 1.0 mgL−1 of 6-benzyladenine and 0.5 mgL−1 of indole-3-acetic acid induced shoot proliferation of carnation. Modulation in the expression of SOD, GPX, CAT and protection of stomatal damage under Si treatment could be important key factors to recover the hyperhydricity. In addition to that up regulated proteins in Si treatment was categorized into ribosomal binding, oxido-reduction, hormone/cell signaling, metal/ion binding, defense, and photosynthesis [10]. In Rosa hybrida, Si nutrition and salinity stress altered the expression of proteins associated with various physiological and developmental process and vital metabolism [20]. In detail, the supplementation of Si regulated the levels of proteins involved in the redox homeostasis, transcription and translation, lipid metabolism, signaling, carbohydrate metabolism, metal ion binding and transportation, cell wall synthesis, and terpene metabolism. Overall, Si mediated stress amelioration is a complex mechanism involving a cascade of physiological and metabolic reactions which need a deeper insight to unravel the exact molecular strategy implemented by Si to impart stress resistance in plants.
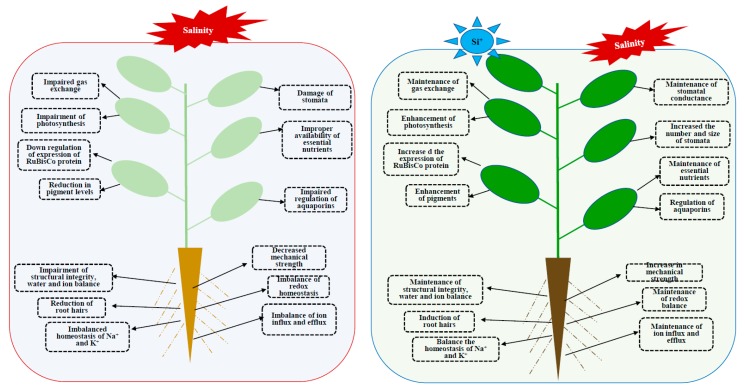
Taken together, salinity causes deleterious effect to the plant growth and development whereas Si supplementation showed improved tolerance against salt stress. Therefore, the present endeavor reports key mechanisms on the role of Si in overcoming salt stress such as restriction of Na+/Cl− uptake via root, improvement of photosynthetic process, maintenance of redox homeostasis, and effective management of essential elements. The overall schematic illustration of Si mediated enhancement son plant under salinity conditions have been illustrated in Figure 1.
Figure 1.
Schematic representation of damages caused by salinity stress and the Si mediated mitigation of salinity in plants.
2. Mechanism to Restrict Na+/Cl− Uptake via Root
2.1. Structural Modifications Imparted by Si to Combat Salt Stress
In roots, Si deposition on the inner tangential and radial cell walls enhances the mechanical strength of the plant [34]. However, the silicification of cells in most cases resulted in the restriction of cell elongation. The biomass enhancement by Si supplementation in rice imparted mechanical hardness to the tissues and aided in the maintenance of optimal water balance [35]. Recent report by Soundararajan et al. ([20]) has illustrated the structural integrity maintenance of root cell wall by Si in salt stressed R. hybrida. According to the report, Si supplementation resulted in the induction of root hairs could have supported for an effective mineral nutrient absorbance from the hydroponics medium [20]. Moreover, the higher occurrence of root hairs aids in the uptake of water and helps to combat the salinity, drought, and heavy metal stress [36]. Further in-depth studies are necessary to identify the molecular mechanism behind the Si mediated induction of root hairs and morphological modifications in root structure.
2.2. Aquaporin in Mineral Uptake under Silicon Nutrition and Stressed Condition
Under salinity stress, the primary tolerance have been achieved by maintaining the lower intracellular Na+ concentration either by increasing the Na+ efflux or decreasing the influx of Na+ ion [37]. Generally, Na+ enters the plant system passively through roots by non-selective cation channels and Na+ transporters like high affinity K+ transporters (HKTs) [38]. Previous studies have identified the Si-mediated selective ion uptake and management of Na+/K+ ion channels. In sugarcane, the addition of Si hindered the uptake of Na+ but increased the accumulation of K+ [39]. Similar activities of Si have been reported in roses [20], aloe [40], and zinnia [19]. Apart from the above mentioned mechanism, Si have also been reported to regulate the levels of both macro and micro nutrients under salt stress [36].
Plant transporters in root play a vital role in the uptake of minerals from the soil or hydroponics medium. The uptake of minerals and water are highly inter-related since both involve transporters. Aquaporin are important transporters that regulate the uptake and transportation of water and minerals across the cell membranes [41]. Plant aquaporin belongs to membrane intrinsic proteins (MIP) family of transmembrane proteins. Based on the phylogenetic distribution, subcellular localization, substrate selection, length of the sequence, and function the aquaporin are classified into subfamilies such as plasma membrane intrinsic proteins (PIP), tonoplast intrinsic proteins (TIP), nodulin26-like intrinsic proteins (NIP), small basic intrinsic proteins (SIP), uncharacterized intrinsic proteins (XIP), and hybrid intrinsic proteins (HIP) [42]. Apart from water uptake, aquaporins are involved in the facilitation of mineral transportation. Previous studies have demonstrated the participation of aquaporin in the regulation of solute transports such as urea, ammonia, hydrogen peroxide, lactic acid, and silica acid [43]. The substrate selectivity of an aquaporin is primarily depend on the following factors, occurrence of NPA motifs for the exclusion of H+ and a filter consisting of an aromatic/arginine region in the pore area [44].
Various isoforms of NIP subfamily such as NIP1 is highly permeable to water, whereas NIP2 aids in the transportation of metalloids and Si, andNIP3 function as boric acid transporter [43]. Si influx and efflux transporters such as Lsi1 and Lsi2 belonging to NIP subfamily have been identified by Ma et al. [44] in rice. These transporters are one of the major factors that determine the transportation, distribution, and accumulation of Si. During salinity stress, in the presence of Si the cellular water balance has been regulated by PIP subfamily. On the other hand, NIP subfamily involved in the uptake of Si [45]. Si regulated the PIP aquaporin expression and restored the hydraulic conductance in roots of Sorghum under short term salt stress [46,47]. Moreover, in plants the deficiency of nutrients under stressed condition correlated with the decrease in water uptake. This process illustrates the vital role of aquaporin in nutrient management. For instance, the significant role of aquaporin in the homeostasis of nutrients include the provision of support for the passive movement of nutrients along with water and channeling the apoplastic/symplastic water flow within tissues [48]. In addition, Martines-Ballesta et al. [49] reported the nutrient balance by the synergistic regulation of aquaporins along with ATPase and Ca-ATPase. Numerous factors influence the activity of aquaporin such as levels of abscisic acid and calcium, free radicals and hormones like ethylene [50,51]. Among these factors, even a small variation in the free radical contents affect the aquaporins [52]. Higher H2O2 levels generated by salinity stress prevented the activity of aquaporin by obstructing the oxidant gating, post-translational modification like phosphorylation, and aquaporin re-localization [52]. However, the molecular rationale behind the aquaporin interactions and mineral nutrients under stressful environment are still under study. Several researches have been progressed towards deciphering the impacts of Si nutrition to plants upon stressed conditions however only a few studies have dealt with the molecular aspects of Si mediated gene regulations in plants [53,54]. In Sorghum, the inclusion of Si resulted in the improvement of water uptake by enhancing the function of aquaporin by the up-regulation of aquaporin genes such as SbPIP1;6, SbPIP2;2, and SbPIP2;6 in root [47]. According to Gao et al. [55], Si increase the aquaporin related gene expression which in turn attenuate the lethal effects of Na+ ion upon high salinity condition.
It has been evident that the presence of Si increased root growth and balances the homeostasis of Na+ and K+ ratio. The substantial enhancement of genes associated with aquaporin by Si might lead to the improvement of water status in plants under stressful environment. Therefore, amendment of Si develops the restoration of water status and mineral ion balance which could promote the reclamation of plants from ionic and osmotic stresses.
3. Leaf Physiology and Photosynthesis
Photosynthesis is the primary metabolism of plant that provides energy for the growth and development. It can be easily affected by any kind of stress including salinity. Altered photosynthesis disrupts the carbon availability. Improper photosynthetic process leads to accumulation of ROS. Degradation of chlorophyll affects the overall photosynthetic process. Oxidative stress causes the damage in cellular organelles. In severe cases, leaf scorch and curling of leaves results in plant death. Prevention of photosynthetic damage is one of the vital steps to overcome the salinity stress [56,57].
3.1. Improved Photosynthetic Efficiency
Decline in plat metabolism and biomass under salinity stress was correlated with the decreased photosynthesis rate [58]. However, previous reports showed that exogenous treatment of Si improved the photosynthetic gas exchange process on several plants including tomato [59,60], sorghum [61], maize [62], tobacco [63], and pumpkin [64]. Exogenous application of Si in low levels (0.8 and 1.6mM) significantly enhanced the photosynthetic rate and water-use efficiency in Maize [62]. According to Zhu and Gong [65] Si restricts the Na+ accumulation and improved K+ uptake as well as maintenance of osmotic balance helps to enhance photosynthetic process under salt and drought condition. Ability of Si to increase the photosynthetic pigment, chlorophyll concentrations, and protect the photochemical apparatus in saline condition was reported in Spartina densiflora [66], Abelmoschus esculentus [67], and Capsicum annuum [7]. Furthermore, Manivannan et al. [7] described that the abundance of carbon fixation and photosynthetic-related proteins were higher in Si supplemented NaCl treatment. In addition, Muneer et al. [16] investigated the chloroplast proteins of tomato under salinity stress conditions with or without Si. The results showed that total chlorophyll and carotenoid content, net-photosynthesis rate, stomatal conductance, and transpiration were increased in the salt treatments with Si supplementation. Furthermore, addition of Si alleviated the reduction in cytochrome b6/f and the ATP-synthase complex under salt stress. Recently, Soundararajan et al. [68] also showed that Si increased the abundance of photosynthesis and energy metabolism-related proteins in R. hybrida under salt stress. Beneficial effects and higher yield in Si treatment can be correlated with the ability of Si to activate genes/proteins involved in metabolic process [69].
3.2. Stomata and Chloroplast Movement
During stress conditions improper gas exchange and reduction of CO2 accumulation was mostly due to damage/malfunctioning of stomata [70]. However, several researches have showed that Si treatments maintain a higher stomatal conductance and transpiration rate in plants [47,56,62,67]. Parveen and Ashraf [62] reported that application of Si in the rooting medium improved stomatal conductance and leaf sub-stomatal CO2 assimilation rate under saline condition in Sorghum bicolor [56]. Exogenous application of Si improved the stomatal conductance due to the increase in the stomata number and stomata size in salinity stressed okra (Abelmoschus esculentus) plants [67]. Application of Si maintain a higher stomatal conductance due to the improvement of the leaf water content [47]. In Capsicum annuum most of the stomata were closed in NaCl treatment however, stomata remained normal in NaCl with Si [7]. It has been demonstrated that the Si enhanced the expression of the zinc finger protein-160, which controls the movement of stomatal aperture to avoid water loss under saline condition [71].
3.3. Photosystem I and II
Furthermore, the reduction of photosynthesis of plants under salt stress could be due to the damage of the photosynthetic apparatus, resulting in the decrease in PSII efficiency [72]. It has been reported that salinity stress inhibits PSII [73,74]. However, reduction of PS I and II could be alleviated by the application of Si. Muneer et al. [16] showed that PSI and PSII complexes were nearly absent with high salt stress, but Si addition helped the plants to retain the protein complexes in tomato plants. In addition, the expression of PSI-monomer/cytochrome b6f was decreased under salinity stress, while the reduction of PSI-monomer/cytb6f was alleviated when Si supplemented with NaCl to the tomato seedlings. Matios-Naranjo et al. [75] showed that addition of Si under salinity condition improved pigment concentration and PSII efficiency in the halophytic grass Spartina densiflora. Khan et al. [76] reported that Si is a beneficial nutrient to increase the efficiency of photosystem II in maize under salinity stress. Soundararajan et al. [68] showed that decrease in the expression of key proteins in photosynthesis and energy metabolism such as NAD(P)H-quinone oxidoreductase enzyme, which regulates the turnover of the PSII reaction center, were improved upon the addition of Si under NaCl treatment in rose.
Firstly, Si supplementation prevents the degradation of photosynthetic pigments such as chlorophyll and carotenoid. Secondly, damage of photosynthetic apparatus including stomata was avoided in the Si treated NaCl plants. Finally, enhanced expression of PSI, PSII, and other photosynthesis-related proteins were associated with the photosynthesis and physiological improvement.
4. Redox Homeostasis
Usually ROS are generated during the metabolic processes from the organelles such as mitochondria, chloroplast, peroxisomes, glyoxysomes, and plasma membrane. In the controlled level ROS act as signaling molecules [77]. Active involvement of antioxidant enzymes and low molecular antioxidants detoxify the ROS to keep it under controlled level. Under stress conditions, plants experience oxidative stress due to the higher lipid peroxidation rate, thiobarbituric acid reactive substances (TBARS), and enormous generation of ROS. Especially, uncontrolled superoxide anion (O2−) and hydrogen peroxide (H2O2) are able to damage lipids, proteins, and nucleic acids.
Si Mediated Maintenance of Redox Homeostasis under Salinity Stress
Previous studies suggest that to cope up with the salt stress, plants need to maintain the equilibrium between the rates of ROS generation and its scavenging. At low concentration, ROS can act as signaling molecules whereas in higher level ROS damages the cellular components. Efficient antioxidant system was rendered by the Si in the several plants by regulating the SOD, GPX, APX, and CAT. Primarily SOD catalysis the dismutation of superoxide into hydrogen peroxide and oxygen. Later enzymes such as CAT, GPX, and APX detoxify the H2O2 into water [78]. Even though, O2− has the very limited half-life (2μs), reduction of O2− into H2O2 can travel long distance as it has half-life of about 1 ms. Other than above two, •OH molecules also formed during the excessive generation of O2− and H2O2. Formation of •OH radical is mediated by iron in the Haber-Weiss and Fenton reactions. Therefore, uncoupled electron of ROS could be able to react with other metabolites and cross-link with the essential metabolic reactions. These excessive generations affect the normal growth and development of plants. Numerous studies have evidenced the Si mediated maintenance of redox homeostasis [7,8,16,18].
Effect of Si on the maintenance of ROS varied between plant species and treatment. In Salvia under temperature stress CAT level was decreased [8] whereas in wheat the activity of CAT was increased under salt stress [79]. In Brassica napus, Si mitigated the oxidative damage caused by higher H2O2 and TBARS. Methylglyoxal (MG) toxicity aroused due to higher TBARS was inhibited with the induced expression of glyoxalase I (Gly I) and Gly II. Oxidative stress mitigation was associated with the higher activities of antioxidant enzymes such as APX, MDHAR, DHAR, GR, GST, GPX, and CAT [80]. In another study, Si protected the photosynthetic pigments and reduced the oxidative stress by activating antioxidant enzymes, increased ascorbate-glutathione (AsA-GSH) pool, proline, and glyoxalase systems in drought stressed B. napus [81]. Similar result was observed in B. juncea under salt stress with Si treatment alone or in combination with 24-Epibrassinolide (EBL) [82]. Amelioration of salt stress related damages such as leaf scorching and stomatal malfunction in the R. hybrida was correlated with the improved photosynthetic efficiency, reduction in the degradation of photosynthetic pigment, lesser lipid peroxidation rate, accumulation of ROS, and enhanced antioxidant metabolism [20]. Biofortification and prevention of water loss in the salt tolerant and salt sensitive cultivars of rice was observed in Na2SiO3 treatment. Higher activities of ascorbate-glutathione cycle related enzymes such as glutathione reductase (GR), APX, GPX, and GST boosted antioxidant defense mechanism. Effect of Si on salt-sensitivity cultivar was distinct than the salt-tolerant cultivars [83].
Although regulation of antioxidant enzymes and low molecular weight antioxidant are varied between species as well as treatments, Si-mediated redox homeostasis is critical toretain ROS level and essential metabolic process of plants.
5. Essential Elements Management by Si under Salt Stress
The Si mediated alleviation of stress involves the effective managements of essential elements in plant system. Upon salinity stress the proper nutrient channeling is affected and leads to the imbalance in the macro and micro elements. Several studies have reported the positive role of Si in the nutrient management.
Potassium, the monovalent cation with high mobility involved in the maintenance of osmotic strength is essential for the plant growth and yield [84]. In addition, K plays a key role in the synthesis of proteins, photosynthesis, and activities of glycolytic enzymes in plants. Further, the potassium metabolism is essential for the maintenance of carbohydrate synthesis and nitrogen assimilation in plants. Higher levels of Na under salinity stress hinder the acquisition of K and result in the deficiency. The augmentation of Si modulated the competitive uptake between Na and K and improved the intercellular distribution of K in salt stressed wheat [85]. In blueberry, the Si deposition in the lower epidermis of the stomata and enhancement of K has been considered as the vital mode of stress alleviation [86]. Moreover, ABA formed during the salt stress seals the stomata during the increased K+ efflux from the guard cells [87]. The Si-mediated movement of stomata could be associated signal perception, electrochemical gradient, osmotic adjustment, and ion balance [88]. The size and structural similarity between K+ and Na+ leads to the competitive uptake since the K+ transporters lacks discrimination between the K+ and Na+ ions. However, the Si application increased the selectivity between K+ and Na+ ions [89]. Moreover, Si treatment increased the K accumulation in K-deficient condition than the K− sufficient environment [90].
In similar manner with potassium, the element calcium (Ca) is important for the proper maintenance of structural membrane integrity and plays a vital role in the senescence process [91]. Irrespective of the low mobility of Ca, even a slight modulation in the active pools of Ca within the cytoplasm reflects in the major physiological processes. In addition, Ca plays a major role in the cell division and cell elongation processes. In plants, Ca is uptake by apoplast bound to the outer surface of the plasma membrane in exchangeable form. Higher concentration of Na inhibits the absorption of Ca which has been reversed by the addition of Si in rice [92]. Moreover, the synergistic improvement of both K and Ca by Si in the salinity stress condition could facilitate the balance of permeability and selectivity of cell membrane under stressed conditions [93]. In salt stressed barley, Ca deficiency resulted in the higher leakage of solutes from the cells which has been ameliorated by the supplementation of Si [94]. Moreover, the cytosolic Ca is involved in the process of induction of stress-tolerance genes under salinity condition. Thus, the Si mediated enrichment of Ca could assist in cell developmental process particularly under stressed environment.
Nitrogen (N) is an inevitable element and acts as the predominant factor for the plant growth [95]. Under extreme salt condition, the uptake of N is hindered [96]. Deficiency of N reduces photosynthesis and cellular expansion [97]. In rice the salt mediated deficiency of N has been alleviated by the application Si which increased the N level and chlorophyll content [98]. Likewise, phosphorous (P) is vital for the energy metabolism, structural component of nucleic acids, and lipids. High concentration of salt decreased the level of P availability due to the high ionic strength exerted by Na+ and Cl− ions [99]. Whereas the application of Si balanced the P levels under stressed condition, for instance Si channeled the utilization of internal P during the deficient conditions and on the other hand Si inhibits the excess accumulation of P [100]. Apart from the abovementioned macronutrients, the Si also facilitated the management of micronutrients such as boron, iron, zinc, copper, and manganese in diverse crops under salinity stress condition.
As macro and micro nutrients are necessary for the plant metabolism, lesser availability of essential elements inhibits the growth and development. However, restriction of Na+ and subsequently competitive uptake of other ions in Si treatment could support the maintenance of essential elements under salinity condition.
6. Conclusions
Application of Si consists of numerous benefits to plants particularly in the alleviation of salinity stress by improving photosynthesis, redox balance, and nutrient management. Stimulation of root growth and maintenance of cell wall integrity withstands the selective permeability in plants. Meanwhile, prevention of damage of photosynthetic apparatus and higher photosynthetic rate sustain the availability of carbohydrate. Proper redox-homeostasis equilibrium avoids the cross-reaction of excessively generated ROS with other key metabolism. Restriction in Na uptake, counter intake of K, Ca, P, and maintenance of water influx on Si treatment under salt stress are associated with the efficient regulation of essential elements for overall physiological improvement.
Due to its wide merits, the addition of Si as a supplemental nutrient in the contemporary agricultural practices such as soil-less cultivation system has been recognized in several areas. However, in-depth molecular rationales behind the Si mediated stress mitigation have to be addressed in future. Recent advancements in the omics technologies could aid in the understanding of Si biology in various plants particularly under stressed conditions. Further, researches determining the possible mechanisms and ways for innovative incorporation of Si in the culture/growth media for the improvement of plant yield as well as stress tolerance could benefit the cultivation of agricultural and horticultural crops
Acknowledgments
The authors thank anonymous reviewers and editors for providing valuable suggestions that improved the quality of the manuscript.
Author Contributions
B.L. and P.S., collected literatures and wrote the manuscript; P.S. and A.M., organized and edited the manuscript; A.M., proof-read, and finalized the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China [Grant No. 31700624].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hussain M., Ahmad S., Hussain S., Lal R., Ul-Allah S., Nawaz A. Rice in saline soils: Physiology, biochemistry, genetics, and management. In: Sparks D.L., editor. Advances in Agronomy. Volume 148. Academic Press; Cambridge, MA, USA: 2018. pp. 231–287. [Google Scholar]
- 2.Ferchichi S., Hessini K., Dell’Aversana E., D’Amelia L., Woodrow P., Ciarmiello L.F., Fuggi A., Carillo P. Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct. Plant Biol. 2018;45:1096–1109. doi: 10.1071/FP18046. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad R., Hussain S., Anjum M.A., Khalid M.F., Saqib M., Zakir I., Hassan A., Fahad S., Ahmad S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In: Hasanuzzaman S., Hakeem K.R., Nahar K., Alharby H.F., editors. Plant Abiotic Stress Tolerance. Springer; Cham, Switzerland: 2019. pp. 191–205. [Google Scholar]
- 4.Fing D.H., Wang G.Z., Si W.T., Zhou Y., Liu Z., Jia J. Effects of salt stress on photosynthetic pigments and activity of ribulose-1,5-bisphosphate carboxylase/oxygenase in Kalidium foliatum. Russ. J. Plant Physiol. 2018;65:98–103. [Google Scholar]
- 5.Morton M.J., Awlia M., Al-Tamimi N., Saade S., Pailles Y., Negrão S., Tester M. Salt stress under the scalpel–dissecting the genetics of salt tolerance. Plant J. 2019;97:148–163. doi: 10.1111/tpj.14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luyckx M., Hausman J.F., Lutts S., Guerriero G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017;8:411. doi: 10.3389/fpls.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manivannan A., Soundararajan P., Muneer S., Ko C.H., Jeong B.R. Silicon mitigates salinity stress by regulating the physiology, antioxidant enzyme activities, and protein expression in Capsicum annuum ‘Bugwang’. BioMedRes. Int. 2016;2016:3076357. doi: 10.1155/2016/3076357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soundararajan P., Sivanesan I., Jana S., Jeong B.R. Influence of silicon supplementation on the growth and tolerance to high temperature in Salvia splendens. Hort. Environ. Biotechnol. 2014;55:271–279. doi: 10.1007/s13580-014-0023-8. [DOI] [Google Scholar]
- 9.Fauteux F., Chain F., Belzile F., Menzies J.G., Bélanger R.R. The protective role of silicon in the Arabidopsis–powdery mildew pathosystem. Proc. Natl. Acad. Sci. USA. 2006;103:17554–17559. doi: 10.1073/pnas.0606330103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soundararajan P., Manivannan A., Cho Y.S., Jeong B.R. Exogenous supplementation of silicon improved the recovery of hyperhydric shoots in Dianthus caryophyllus L. by stabilizing the physiology and protein expression. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keeping M.G., Kvedaras O.L. Silicon as a plant defence against insect herbivory: Response to Massey, Ennos and Hartley. J. Anim. Ecol. 2008;77:631–633. doi: 10.1111/j.1365-2656.2008.01380.x. [DOI] [PubMed] [Google Scholar]
- 12.Ma J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004;50:11–18. doi: 10.1080/00380768.2004.10408447. [DOI] [Google Scholar]
- 13.Yeo A.R., Flowers S.A., Rao G., Welfare K., Senanayake N., Flowers T.J. Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ. 1999;22:559–565. doi: 10.1046/j.1365-3040.1999.00418.x. [DOI] [Google Scholar]
- 14.Shi Y., Wang Y., Flowers T.J., Gong H. Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. J. Plant Physiol. 2013;170:847–853. doi: 10.1016/j.jplph.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Verma K.K., Liu X.H., Wu K.C., Singh R.K., Song Q.Q., Malviya M.K., Song X.P., Singh P., Verma C.L., Li Y.R. The Impact of Silicon on Photosynthetic and Biochemical Responses of Sugarcane under Different Soil Moisture Levels. Silicon. 2019:1–13. doi: 10.1007/s12633-019-00228-z. [DOI] [Google Scholar]
- 16.Muneer S., Park Y.G., Manivannan A., Soundararajan P., Jeong B.R. Physiological and proteomic analysis in chloroplasts of Solanum lycopersicum L. under silicon efficiency and salinity stress. Int. J. Mol. Sci. 2014;15:21803–21824. doi: 10.3390/ijms151221803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Z., Wei G., Li J., Qian Q., Yu J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.) Int. J. Mol. Sci. 2004;167:527–533. [Google Scholar]
- 18.Soundararajan P., Manivannan A., Park Y.G., Muneer S., Jeong B.R. Silicon alleviates salt stress by modulating antioxidant enzyme activities in Dianthus caryophyllus ‘Tula’. Hort. Environ. Biotechnol. 2015;56:233–239. doi: 10.1007/s13580-015-0111-4. [DOI] [Google Scholar]
- 19.Manivannan A., Soundararajan P., Arum L.S., Ko C.H., Muneer S., Jeong B.R. Silicon-mediated enhancement of physiological and biochemical characteristics of Zinnia elegans ‘Dreamland Yellow’ grown under salinity stress. Hort. Enviro. Biotechnol. 2015;56:721–731. doi: 10.1007/s13580-015-1081-2. [DOI] [Google Scholar]
- 20.Soundararajan P., Manivannan A., Ko C.H., Jeong B.R. Silicon Enhanced Redox Homeostasis and Protein Expression to Mitigate the Salinity Stress in Rosa hybrida ‘Rock Fire’. Plant Growth Regulat. 2018;37:16–34. doi: 10.1007/s00344-017-9705-7. [DOI] [Google Scholar]
- 21.Zama E.F., Reid B.J., Sun G.-X., Yuan H.-Y., Li X.-M., Zhu Y.-G. Silicon (Si) biochar for the mitigation of arsenic (As) bioaccumulation in spinach (Spinacia oleracean) and improvement in the plant growth. J. Clean. Prod. 2018;189:386–395. doi: 10.1016/j.jclepro.2018.04.056. [DOI] [Google Scholar]
- 22.Kumar Dubey A., Kumar N., Ranjan R., Gautam A., Pande V., Sanyal I., Mallick S. Application of glycine reduces arsenic accumulation and toxicity in Oryza sativa L. by reducing the expression of silicon transporter genes. Ecotoxicol. Environ. Saf. 2018;148:410–417. doi: 10.1016/j.ecoenv.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Ali N., Schwarzenberg A., Yvin J.-C., Hosseini S.A. Regulatory role of silicon in mediating differential stress tolerance responses in two contrasting tomato genotypes under osmotic stress. Front. Plant Sci. 2018;9 doi: 10.3389/fpls.2018.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azeem S., Li Z., Zheng H., Lin W., Arafat Y., Zhang Z., Lin X., Lin W. Quantitative proteomics study on Lsi1 in regulation of rice (Oryza sativa L.) cold resistance. Plant Growth Regul. 2016;78:307–323. doi: 10.1007/s10725-015-0094-2. [DOI] [Google Scholar]
- 25.Habibi G. Effects of soil-and foliar-applied silicon on the resistance of grapevine plants to freezing stress. Acta Biol. Szeged. 2015;59:109–117. [Google Scholar]
- 26.Habibi G. Exogenous silicon leads to increased antioxidant capacity in freezing-stressed pistachio leaves. J. Acta Agric. Slov. 2015;105:10. doi: 10.14720/aas.2015.105.1.05. [DOI] [Google Scholar]
- 27.Lu Y., Ma J., Teng Y., He J., Christie P., Zhu L., Ren W., Zhang M., Deng S. Effect of silicon on growth, physiology, and cadmium translocation of Tobacco (Nicotiana tabacum L.) in Cadmium-Contaminated Soil. Pedosphere. 2018;28:680–689. doi: 10.1016/S1002-0160(17)60417-X. [DOI] [Google Scholar]
- 28.Alzahrani Y., Kuşvuran A., Alharby H.F., Kuşvuran S., Rady M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018;154:187–196. doi: 10.1016/j.ecoenv.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 29.Wu J., Mock H.-P., Giehl R.F.H., Pitann B., Mühling K.H. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard. Mater. 2019;364:581–590. doi: 10.1016/j.jhazmat.2018.10.052. [DOI] [PubMed] [Google Scholar]
- 30.Maillard A., Ali N., Schwarzenberg A., Jamois F., Yvin J.-C., Hosseini S.A. Silicon transcriptionally regulates sulfur and ABA metabolism and delays leaf senescence in barley under combined sulfur deficiency and osmotic stress. Environ. Exp. Bot. 2018;155:394–410. doi: 10.1016/j.envexpbot.2018.07.026. [DOI] [Google Scholar]
- 31.Li P., Zhao C.Z., Zhand Y.Q., Wang X.M., Wang J.F., Wang F., Bi Y.R. Silicon enhances the tolerance of Poa annua to cadmium by inhibiting its absorption and oxidative stress. Biol. Plant. 2017;61:741–750. doi: 10.1007/s10535-017-0731-x. [DOI] [Google Scholar]
- 32.Hosseini S.A., Maillard A., Hajirezaei M.R., Ali N., Schwarzenberg A., Jamois F., Yvin J.-C. Induction of Barley Silicon Transporter HvLsi1 and HvLsi2, increased silicon concentration in the shoot and regulated Starch and ABA Homeostasis under Osmotic stress and Concomitant Potassium Deficiency. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manivannan A., Soundararajan P., Cho Y.S., Park J.E., Jeong B.R. Sources of silicon influence photosystem and redox homeostasis-related proteins during the axillary shoot multiplication of Dianthus caryophyllus. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2018;152:704–710. doi: 10.1080/11263504.2017.1320312. [DOI] [Google Scholar]
- 34.Epstein E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- 35.Agarie S., Agata W., Kubota H., Kaufmann P.B. Physiological role of silicon in photosynthesis and dry matter production in rice plants. Jpn. J. Crop Sci. 1992;61:200–206. doi: 10.1626/jcs.61.200. [DOI] [Google Scholar]
- 36.Singh A.K., Kumar R., Pareek A., Sopory S.K., Singla-Pareek S.L. Overexpression of rice CBS domain containing protein improves salinity, oxidative, and heavy metal tolerance in transgenic tobacco. Mol. Biotechnol. 2012;52:205–216. doi: 10.1007/s12033-011-9487-2. [DOI] [PubMed] [Google Scholar]
- 37.Rios J.J., Martínez-Ballesta M.C., Ruiz J.M., Blasco B., Carvajal M. Silicon-mediated improvement in plant salinity tolerance: The role of aquaporins. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumwald E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000;12:431–434. doi: 10.1016/S0955-0674(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 39.Ashraf M., Rahmatullah, Afzal M., Ahmed R., Mujeeb F., Sarwar A., Ali L. Alleviation of detrimental effects of NaCl by silicon nutrition in salt-sensitive and salt-tolerant genotypes of sugarcane (Saccharum officinarum L.) Plant Soil. 2010;326:381–391. doi: 10.1007/s11104-009-0019-9. [DOI] [Google Scholar]
- 40.Garg N., Bhandari P.J.P. Interactive effects of silicon and arbuscular mycorrhiza in modulating ascorbate-glutathione cycle and antioxidant scavenging capacity in differentially salt-tolerant Cicer arietinum L. genotypes subjected to long-term salinity. Protoplasma. 2016;253:1325–1345. doi: 10.1007/s00709-015-0892-4. [DOI] [PubMed] [Google Scholar]
- 41.Maurel C., Boursiac Y., Luu D.-T., Santoni V., Shahzad Z., Verdoucq L. Aquaporins in Plants. Physiol. Rev. 2015;95:1321–1358. doi: 10.1152/physrev.00008.2015. [DOI] [PubMed] [Google Scholar]
- 42.Bienert G.P., Chaumont F. Plant aquaporins: Roles in water homeostasis, nutrition, and signaling processes. In: Geisler M., Venema K., editors. Transporters and Pumps in Plant Signaling. Springer; Berlin, Heidelberg: 2011. pp. 3–36. [Google Scholar]
- 43.Wu B., Beitz E. Aquaporins with selectivity for unconventional permeants. Cell. Mol. Life Sci. 2007;64:2413–2421. doi: 10.1007/s00018-007-7163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J.F., Tamai K., Yamaji N., Mitani N., Konishi S., Katsuhara M., Ishiguro M., Murata Y., Yano M. A silicon transporter in rice. Nature. 2006;440:688. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- 45.Pommerrenig B., Diehn T.A., Bienert G.P. Metalloido-porins: Essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci. 2015;238:212–227. doi: 10.1016/j.plantsci.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Liu P., Yin L., Deng X., Wang S., Tanaka K., Zhang S. Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J. Exp. Bot. 2014;65:4747–4756. doi: 10.1093/jxb/eru220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu P., Yin L., Wang S., Zhang M., Deng X., Zhang S., Tanaka K. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 2015;111:42–51. doi: 10.1016/j.envexpbot.2014.10.006. [DOI] [Google Scholar]
- 48.Chen D., Wang S., Yin L., Deng X. How does silicon mediate plant water uptake and loss under water deficiency? Front. Plant Sci. 2018;9:281. doi: 10.3389/fpls.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martínez-Ballesta M.C., Martínez V., Carvajal M. Aquaporin functionality in relation to H+-ATPase activity in root cells of Capsicum annuum grown under salinity. Physiol. Plant. 2003;117:413–420. doi: 10.1034/j.1399-3054.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 50.Parent B., Hachez C., Redondo E., Simonneau T., Chaumont F., Tardieu F. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: A trans-scale approach. J. Plant Physiol. 2009;149:2000–2012. doi: 10.1104/pp.108.130682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu W., Yuan Q., Wang Y., Cai R., Deng X., Wang J., Zhou S., Chen M., Chen L., Huang C., et al. Overexpression of a Wheat Aquaporin Gene, TaAQP8, enhances salt stress tolerance in Transgenic Tobacco. Plant Cell Physiol. 2012;53:2127–2141. doi: 10.1093/pcp/pcs154. [DOI] [PubMed] [Google Scholar]
- 52.Boursiac Y., Chen S., Luu D.-T., Sorieul M., van den Dries N., Maurel C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005;139:790–805. doi: 10.1104/pp.105.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song A., Li P., Fan F., Li Z., Liang Y. The effect of Silicon on photosynthesis and expression of its relevant genes in rice (Oryza sativa L.) under high-zinc stress. PLoS ONE. 2014;9:e113782. doi: 10.1371/journal.pone.0113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin L., Wang S., Tanaka K., Fujihara S., Itai A., Den X., Zhang S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2016;39:245–258. doi: 10.1111/pce.12521. [DOI] [PubMed] [Google Scholar]
- 55.Gao Z., He X., Zhao B., Zhou C., Liang Y., Ge R., Shen Y., Huang Z. Overexpressing a Putative Aquaporin Gene from Wheat, TaNIP, Enhances Salt Tolerance in Transgenic Arabidopsis. Plant Cell Physiol. 2010;51:767–775. doi: 10.1093/pcp/pcq036. [DOI] [PubMed] [Google Scholar]
- 56.Yin L., Wang S., Li J., Tanaka K., Oka M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol. Plant. 2013;35:3099–3107. doi: 10.1007/s11738-013-1343-5. [DOI] [Google Scholar]
- 57.Hu L., Xiang L., Zhang L., Zhou X., Zou Z., Hu X. The photoprotective role of spermidine in tomato seedlings under salinity-alkalinity stress. PLoS ONE. 2014;9:e110855. doi: 10.1371/journal.pone.0110855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta B., Huang B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014;2014:701596. doi: 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haghighi M., Pessarakli M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013;161:111–117. doi: 10.1016/j.scienta.2013.06.034. [DOI] [Google Scholar]
- 60.Li H., Zhu Y., Hu Y., Han W., Gong H. Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. ActaPhysiol. Plant. 2015;37:71. doi: 10.1007/s11738-015-1818-7. [DOI] [Google Scholar]
- 61.Nabati J., Kafi M., Masoumi A., Mehrjerdi Z. Effect of salinity and silicon application on photosynthetic characteristics of sorghum (Sorghum bicolor L) Int. J. Agric. Sci. 2013;3:483–492. [Google Scholar]
- 62.Parveen N., Ashraf M. Role of silicon in mitigating the adverse effects of salt stress on growth and photosynthetic attributes of two maize (Zea mays L.) cultivars grown hydroponically. Pak. J. Bot. 2010;42:1675–1684. [Google Scholar]
- 63.Hajiboland R., Cheraghvareh L. Influence of Si supplementation on growth and some physiological and biochemical parameters in salt-stressed tobacco (Nicotiana rustica L.) plants. J. Sci. Islamic Repub. Iran. 2014;25:205–217. [Google Scholar]
- 64.Siddiqui M.H., Al-Whaibi M.H., Faisal M., Al Sahli A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014;33:2429–2437. doi: 10.1002/etc.2697. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Y., Gong H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014;34:455–472. doi: 10.1007/s13593-013-0194-1. [DOI] [Google Scholar]
- 66.Mateos-Naranjo E., Andrades-Moreno L., Davy A.J. Silicon alleviates deleterious effects of high salinity on the halophytic grass Spartina densiflora. Plant Physiol. Biochem. 2013;63:115–121. doi: 10.1016/j.plaphy.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 67.Abbas T., Balal R.M., Shahid M.A., Pervez M.A., Ayyub C.M., Aqueel M.A., Javaid M.M. Silicon-induced alleviation of NaCl toxicity in okra (Abelmoschus esculentus) is associated with enhanced photosynthesis, osmoprotectants and antioxidant metabolism. Acta Physiol. Plant. 2015;37:6. doi: 10.1007/s11738-014-1768-5. [DOI] [Google Scholar]
- 68.Soundararajan P., Manivannan A., Ko C.H., Muneer S., Jeong B.R. Leaf Physiological and Proteomic Analysis to Elucidate Silicon Induced Adaptive Response under Salt Stress in Rosa hybrida ‘Rock Fire’. Int. J. Mol. Sci. 2017;18:1768. doi: 10.3390/ijms18081768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soratto R.P., Crusciol C.A.C., Castro G.S.A., Costa C.H.M.D., Ferrari Neto J. Leaf application of silicic acid to white oat and wheat. Rev. Bras. de Ciência do Solo. 2012;36:1538–1544. doi: 10.1590/S0100-06832012000500018. [DOI] [Google Scholar]
- 70.Hetherington A.M., Woodward F.I. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 71.Huang X.Y., Chao D.Y., Gao J.P., Zhu M.Z., Shi M., Lin H.X. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 2009;23:1805–1817. doi: 10.1101/gad.1812409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y., Yu L., Wang L., Guo S. Bottle gourd rootstock-grafting promotes photosynthesis by regulating the stomata and non-stomata performances in leaves of watermelon seedlings under NaCl stress. J. Plant Physiol. 2015;186–187:50–58. doi: 10.1016/j.jplph.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 73.Gorbe E., Calatayud A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012;138:24–35. doi: 10.1016/j.scienta.2012.02.002. [DOI] [Google Scholar]
- 74.Oukarroum A., Bussotti F., Goltsev V., Kalaji H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015;109:80–88. doi: 10.1016/j.envexpbot.2014.08.005. [DOI] [Google Scholar]
- 75.Mateos-Naranjo E., Gallé A., Florez-Sarasa I., Perdomo J.A., Galmés J., Ribas-Carbó M., Flexas J. Assessment of the role of silicon in the Cu-tolerance of the C4 grass Spartina densiflora. J. Plant Physiol. 2015;178:74–83. doi: 10.1016/j.jplph.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Khan W.U.D., Aziz T., Hussain I., Ramzani P.M.A., Reichenauer T.G. Silicon: A beneficial nutrient for maize crop to enhance photochemical efficiency of photosystem II under salt stress. Arch. Agron. Soil Sci. 2017;63:599–611. doi: 10.1080/03650340.2016.1233322. [DOI] [Google Scholar]
- 77.Foyer C.H., Noctor G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiolgia Plant. 2003;119:355–364. doi: 10.1034/j.1399-3054.2003.00223.x. [DOI] [Google Scholar]
- 78.Choudhury S., Panda P., Sahoo L., Panda S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal.Behav. 2013;8:e23681. doi: 10.4161/psb.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang Y., Chen Q., Liu Q., Zhang W., Ding R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.) J. Plant Physiol. 2003;160:1157–1164. doi: 10.1078/0176-1617-01065. [DOI] [PubMed] [Google Scholar]
- 80.Hasanuzzaman M., Nahar K., Rohman M.M., Anee T.I., Huang Y., Fujita M. Exogenous Silicon Protects Brassica napus Plants from Salinity-Induced Oxidative Stress Through the Modulation of AsA-GSH Pathway, Thiol-Dependent Antioxidant Enzymes and Glyoxalase Systems. Gesunde Pflanz. 2018;70:185–194. doi: 10.1007/s10343-018-0430-3. [DOI] [Google Scholar]
- 81.Hasanuzzaman M., Nahar K., Anee T.I., Khan M.I.R., Fujita M. Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. South Afr. J. Bot. 2018;115:50–57. doi: 10.1016/j.sajb.2017.12.006. [DOI] [Google Scholar]
- 82.Siddiqui H., Yusuf M., Faraz A., Faizan M., Sami F., Hayat S. 24-Epibrassinolide supplemented with silicon enhances the photosynthetic efficiency of Brassica juncea under salt stress. South Afr. J. Bot. 2018;118:120–128. doi: 10.1016/j.sajb.2018.07.009. [DOI] [Google Scholar]
- 83.Das P., Manna I., Biswas A.K., Bandyopadhyay M. Exogenous silicon alters ascorbate-glutathione cycle in two salt-stressed indica rice cultivars (MTU 1010 and Nonabokra) Environ. Sci. Pollut. Res. 2018;25:26625–26642. doi: 10.1007/s11356-018-2659-x. [DOI] [PubMed] [Google Scholar]
- 84.Liebersbach H., Steingrobe B., Claassen N. Roots regulate ion transport in the rhizosphere to counteract reduced mobility in dry soil. Plant Soil. 2004;260:79–88. doi: 10.1023/B:PLSO.0000030191.92338.6a. [DOI] [Google Scholar]
- 85.Tuna A.L., Kaya C., Higgs D., Murillo-Amador B., Aydemir S., Girgin A.R. Silicon improves salinity tolerance in wheat plants. Environ. Exp. Bot. 2008;62:10–16. doi: 10.1016/j.envexpbot.2007.06.006. [DOI] [Google Scholar]
- 86.Morikawa C.K., Saigusa M. Mineral composition and accumulation of silicon in tissues of blueberry (Vaccinumcorymbosus cv. Bluecrop) cuttings. Plant Soil. 2004;258:1–8. doi: 10.1023/B:PLSO.0000016489.69114.55. [DOI] [Google Scholar]
- 87.Bies-Etheve N., Gaubier-Comella P., Debures A., Lasserre E., Jobet E., Raynal M., Delseny M., Cooke R. Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis Thaliana. Plant Mol. Biol. 2008;67:107–124. doi: 10.1007/s11103-008-9304-x. [DOI] [PubMed] [Google Scholar]
- 88.Agarie S., Hanaoka N., Ueno O., Miyazaki A., Kubota F., Agata W., Kaufman P.B. Effects of silicon on tolerance to water deficit and heat stress in rice plants (Oryza sativa L.) monitored by electrolyte leakage. Plant Prod. Sci. 1998;1:96–103. doi: 10.1626/pps.1.96. [DOI] [Google Scholar]
- 89.Liang Y., Shen Q., Shen Z., Ma T. Effects of silicon on salinity tolerance of two barley cultivars. J. Plant Nutr. 1996;19:173–183. doi: 10.1080/01904169609365115. [DOI] [Google Scholar]
- 90.Miao B.H., Han X.G., Zhang W.H. The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Ann. Bot. 2010;105:967–973. doi: 10.1093/aob/mcq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lahaye P.A., Epstein E. Calcium and salt tolerance by bean plants. Physiol. Plant. 1971;25:213–218. doi: 10.1111/j.1399-3054.1971.tb01430.x. [DOI] [Google Scholar]
- 92.Ma J.F., Miyake Y., Takahashi E. Silicon as a beneficial element for crop plants. Stud. Plant Sci. 2001;8:17–39. [Google Scholar]
- 93.Song J.Q., Fujiyama H. Ameliorative effect of potassium on rice and tomato subjected to sodium salinization. Soil Sci. Plant Nutr. 1996;42:493–501. doi: 10.1080/00380768.1996.10416318. [DOI] [Google Scholar]
- 94.Liang Y. Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil. 1999;209:217–224. doi: 10.1023/A:1004526604913. [DOI] [Google Scholar]
- 95.Marschner H. Mineral Nutrition of Higher Plants. 2nd ed. Academic Press; San Diego, CA, USA: 1995. [Google Scholar]
- 96.Alva A.K., Syvertsen J.P. Irrigation water salinity effects, soil nutrient distribution, root density and leaf nutrient levels of citrus under drip fertigation. J. Plant Nutr. 1991;14:715–727. doi: 10.1080/01904169109364237. [DOI] [Google Scholar]
- 97.Fallah A. Study of silicon and nitrogen effects on some physiological characters of rice. Int. J. Agric. Crop Sci. 2012;4:238–241. [Google Scholar]
- 98.Ávila F.W., Baliza D.P., Faquin V., Araújo J.L., Ramos S.J. Silicon-nitrogen interaction in rice cultivated under nutrient solution. Rev. Cienc. Agron. 2010;41:184–190. doi: 10.1590/S1806-66902010000200003. [DOI] [Google Scholar]
- 99.Navarro J.M., Botella M.A., Cerdá A., Martinez V. Phosphorus uptake and translocation in salt-stressed melon plants. J. Plant Physiol. 2001;158:375–381. doi: 10.1078/0176-1617-00147. [DOI] [Google Scholar]
- 100.Ma J., Takahashi E. Effect of silicon on the growth and phosphorus uptake of rice. Plant Soil. 1990;126:115–119. doi: 10.1007/BF00041376. [DOI] [Google Scholar]