Abstract
Aggregatibacter actinomycetemcomitans is an oral pathogen that produces the RTX toxin, leukotoxin (LtxA; Leukothera®). A. actinomycetemcomitans is strongly associated with the development of localized aggressive periodontitis. LtxA acts as a virulence factor for A. actinomycetemcomitans to subvert the host immune response by binding to the β2 integrin lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18) on white blood cells (WBCs), causing cell death. In this paper, we reviewed the state of knowledge on LtxA interaction with WBCs and the subsequent mechanisms of induced cell death. Finally, we touched on the potential therapeutic applications of LtxA (trade name Leukothera®) toxin therapy for the treatment of hematological malignancies and immune-mediated diseases.
Keywords: leukotoxin (LtxA), RTX (repeats-in-toxin) toxin, Aggregatibacter actinomycetemcomitans, lymphocyte function-associated antigen-1 (LFA-1), β2 integrins, cell death, oral microbiology, virulence factor, toxin therapy
1. Introduction
Aggregatibacter actinomycetemcomitans is a Gram-negative oral pathogen that is strongly associated with the development of localized aggressive periodontitis [1,2]. A. actinomycetemcomitans belongs to the HACEK group of bacteria that can cause systemic diseases, including endocarditis [3].
In order to colonize, cause disease, and persist in the host, A. actinomycetemcomitans produces an arsenal of virulence factors. These virulence factors include adherence factors, biofilm polysaccharides, lipopolysaccharides (LPS), and toxins [1]. A. actinomycetemcomitans produces two important protein toxins—cytolethal distending toxin (CDT) [4,5] and leukotoxin (LtxA) [6,7]. Both CDT and LtxA play important roles in A. actinomycetemcomitans subversion of the host immune response, but differ in target cell specificity and expression pattern. This review focused on LtxA.
A. actinomycetemcomitans LtxA is a secreted protein of 114 kDa [8]. The amount of LtxA secreted by A. actinomycetemcomitans depends on the size of the leukotoxin promoter region [9]. Minimally leukotoxic 652 strains contain the full-length, 1000 base-pair (bp) promoter, while the highly leukotoxic JP2 strains harbor a 532-bp deletion at the 3’ end of the promoter [9]. It is believed that this 532-bp deletion removes a transcriptional terminator of ltxA expression [10]. The ltx operon (ltxCABD) encodes four genes. ltxC is the first gene in the operon and is required for the post-translational acylation of internal lysine residues necessary for toxin activity [11,12,13]. ltxA is the second gene in the operon and encodes the full-length toxin. The production of LtxA is regulated by a number of factors, including the level of fermentable sugars, pH, and oxygen levels [14,15,16,17]. ltxB and ltxD are the third and fourth genes in the operon and are required for toxin translocation and secretion [13,18,19,20,21]. LtxB localizes to the inner bacterial membrane and interacts with LtxD, which is on the periplasmic side of the inner membrane. LtxB and LtxD interact with a third protein, TdeA (for Toxin and Drug Export) [18,22], on the outer bacterial membrane to form a type I secretion system for LtxA export. Further discussion, including schematic depictions, of the ltx operon [23], regulation of ltxA transcription [10], and regulation of LtxA production [23] can be found in previous publications.
LtxA is a member of the RTX (repeats-in-toxin) toxin family that includes Escherichia coli α-hemolysin (HlyA), Bordetella pertussis adenylate cyclase (CyaA), Mannheimia haemolytica leukotoxin (LktA), Vibrio cholerae Rtx toxin (RtxA), among other Gram-negative pathogens [6,7,24]. A. actinomycetemcomitans LtxA shares approximately 51% amino acid sequence similarity with E. coli HlyA and is 43% identical to M. haemolytica LktA. E. coli HlyA and B. pertussis CyaA intoxicate human leukocytes while M. haemolytica LktA intoxicates bovine leukocytes. Historically, RTX toxins are characterized by their similar structural features and are believed to interact with the plasma membrane of target cells in analogous manners. At the N-terminus, there are conserved amphipathic helices believed to be involved in toxin interaction with host cell membrane and receptor [13,25,26,27]. In the center of the protein are two lysine residues that are covalently modified with fatty acid residues [11,12,28,29]. The repeats domain encompasses the characteristic nonapeptide glycine-rich repeats, containing the consensus sequence GGXG(N/D)DX(L/I/F)X, which is responsible for binding calcium ions, a critical feature for maintaining cytotoxicity [25,30]. LtxA has twelve such repeats [24]. At the C-terminus is a ~100-amino acid domain involved in secretion of the toxin by a type I secretion system. The structural domains of LtxA have been extensively characterized in other studies [31,32].
In this review, we focused on the biology of LtxA interaction with host cell membranes and receptors, the mechanisms by which LtxA intoxicates various subsets of white blood cells and the potential therapeutic applications of LtxA toxin therapy.
2. Interaction of LtxA with White Blood Cells
LtxA has long been known to have a very narrow host range specific for white blood cells (WBCs) of human and Old World primate origin [33,34,35], suggesting that the toxin binds to a specific cell surface receptor. To determine the regions of LtxA responsible for this species and cell type specificity, Lally et al. developed a chimeric toxin and determined that amino acid residues 688–941 are necessary for LtxA to kill target human cells [13]. This amino acid region contains the nonapeptide glycine-rich repeats, as well as 34 residues before and 95 residues after the repeats [13], providing further evidence that this repeated domain is critical for cytotoxicity [25,30]. LtxA contains 12 such repeats [24]. Chimeric LtxA containing only 9 of these repeat regions fails to kill target cells. Thus, these 12 nonapeptide glycine-rich repeat domains are essential for the unique species recognition of LtxA.
2.1. Receptor Independent Interactions with Target Cell Membranes
Prior to interaction with its receptor, lymphocyte function-associated antigen-1 (LFA-1), LtxA may associate with the host cell plasma membrane and induce changes. The earliest observable effects of LtxA on target cells is an increase in cytosolic (Ca2+), followed by a decrease in membrane potential [36]. LtxA can adsorb to cell membranes of toxin-sensitive and toxin-resistant cells [37], but fails to perturb the cell membrane in toxin-resistant cells [36]. LtxA induced Ca2+ mobilization in toxin-sensitive and toxin-resistant cells, suggesting that this occurs in the absence of LFA-1 and is not sufficient for cell lysis [38]. Interestingly, when ltxC is deleted from the ltx operon, there is no increase in cytosolic (Ca2+) in response to LtxA [11,12]. Studies have proposed that initial adsorption of LtxA to the cell membrane occurs via insertion of these fatty acyl chains into the membrane of target cells [12,39]. This supports the model that LtxA-mediated cytotoxicity occurs in two phases and that the fatty acylations on LtxA are required for this initial phase of interaction of membrane interaction [39].
LtxA was believed to be a membrane damaging toxin, and initial morphological studies suggested that LtxA bent target cell membranes and formed pores that disrupted the osmotic equilibrium of the cell [40,41]. However, more recent studies found that while LtxA clustered on target cell surfaces, there were no breaks in the membrane that were consistent with pore formation [40]. LtxA-treated cells demonstrated two membrane defects—the collapse of the microvilli normally present on the outer surface of cells, and the formation of cell surface depressions, followed by lipid-lined cavities [40]. However, the membranes appeared to be continuous, suggesting that LtxA membrane disruptions are produced not by pore formation, but rather by membrane destabilization related to nonlamellar phase formation in a manner independent of LFA-1 [40]. Divalent cations, such as Ca2+, can promote nonlamellar phases [42,43], and interestingly, LtxA elevates cytosolic (Ca2+) in an LFA-1 independent manner [38]. Thus, LtxA-induced Ca2+ mobilization may aid in promoting nonlamellar phase formation and membrane destabilization.
LtxA association with the lipid bilayer results in a structural change in LtxA that generates more α-helical content [44,45]. These distinct structural requirements for LtxA membrane binding and membrane destabilization suggest that they are independent events. This supports the proposed model that the hydrophobic residue-rich portion of LtxA inserts into the membrane, initiating formation of a nonlamellar structure, thus weakening and destabilizing the membrane [32,40,44,45]. Furthermore, LtxA has been shown to associate with the membrane in a conformation where the hydrophobic and C-terminal domains reside outside the cell membrane and the central and repeats domains are inside the membrane. Since the hydrophobic residues were found to reside externally to the membrane, they are not believed to be involved in membrane interactions. Interestingly, the fatty acylations on LtxA were found to be in the liposome, suggesting that these modifications may play a role in membrane interactions and cell association [32], providing a potential function for the post-translational modification of LtxA and perhaps other RTX toxins.
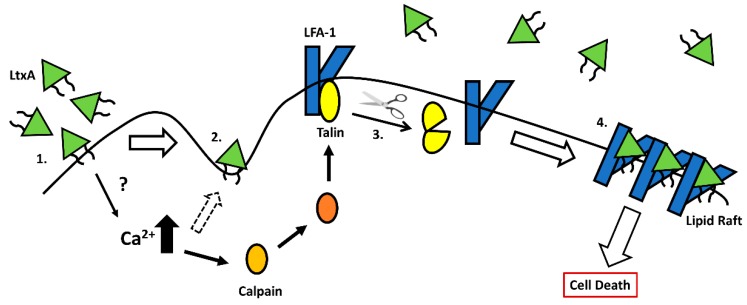
In addition to lipid bilayer destabilization, LtxA is known to almost irreversibly bind to cholesterol [31,38]. LtxA has a strong affinity for cholesterol and contains two cholesterol recognition/amino acid consensus (CRAC) sites—CRAC336 (333LEEYSKR339), which is highly conserved among RTX toxins, and CRAC503 (501VDYLK505), which is unique to LtxA [31]. Both CRAC sites are located within the N-terminal hydrophobic domain of LtxA. Only CRAC336 competitively inhibits LtxA binding and is essential for LtxA cytotoxicity [31,46,47]. The CRAC motifs on LtxA were found both inside and outside the plasma membrane, suggesting that these CRAC motifs are located near the LtxA membrane binding interface [32]. LtxA requires cholesterol for cytotoxicity [31,38]. The lipid raft domain is cholesterol rich and LtxA is proposed to bind cholesterol and aid in the localization and clustering of LFA-1 and LtxA in the lipid raft compartment—a key step in LtxA-mediated cell death [31,38]. LtxA has also been found to bound to cholesterol in non-raft membrane compartments and this interaction may be the cause of the LFA-1-independent increase in intracellular Ca2+ levels [38]. Following this increase in intracellular Ca2+ levels, the calcium d-pendent protease calpain becomes activated [38,48]. Calpain cleaves the cytoskeletal tethering protein, talin, which frees LFA-1, allowing for integrin clustering in the lipid rafts [38,49]. In the cholesterol-rich lipid raft, LtxA then binds to LFA-1 [38]. Figure 1 depicts a proposed model of LtxA interaction with target cell membranes and LFA-1. The nature of the LtxA/LFA-1 interaction is discussed below.
Figure 1.
Model for LtxA association with host cell membranes. 1. LtxA (shown with fatty acyl chains) passively adsorbs onto cell membranes and anchors via the fatty acylation. This is believed to elevate intracellular calcium levels. 2. Association of LtxA with the target cell membranes results in lipid bilayer destabilization, demonstrated by the formation of cell surface depression. 3. The elevated calcium levels activate the calcium-dependent protease calpain. Calpain cleaves talin, a cytoskeletal tethering protein that holds LFA-1 in place in the cell membrane. 4. LFA-1 can cluster in the lipid raft compartment where LtxA interaction with LFA-1 is mediated by the fatty acyl chains at the membrane interface, allowing for the toxin to become partially embedded into the cell membrane. Downstream signaling results in cell death.
2.2. Interaction of LtxA with β2 Intergrins
Lally et al. utilized a series of elegant studies to identify lymphocyte function-associated antigen-1 (LFA-1) as the receptor for LtxA [50]. Upon generation of a monoclonal antibody that inhibited LtxA-mediated cytolysis, affinity column purification pulled out two proteins that had complete homology to CD11a and CD18, the two protein chains that heterodimerize to comprise LFA-1. Passive adsorption of LtxA onto polystyrene beads allowed for co-immunoprecipitation of CD11a and CD18. Additionally, transfection of an expression plasmid containing the genes for CD11a and CD18 into toxin-resistant K562 cells conferred LtxA susceptibility to these cells. More recently, another study reported that LtxA can also bind and utilize Mac-1 (CD11b/CD18) and CR4 (CD11c/CD18) to intoxicate cells with comparable efficiencies [51].
LFA-1 is expressed on all white blood cells while Mac-1 and CR4 are only expressed on cells of myeloid origin and are regulated in their surface expression, unlike LFA-1 [52,53]. The alignment of amino acids sequences of CD11a, CD11b and CD11c reveals sequence identities of 34–35%, suggesting that LtxA may interact with these proteins similarly. While Mac-1 and CR4 have been shown to confer susceptibility to LtxA [51], more studies have characterized LtxA interactions with LFA-1 and thus, the remainder of this discussion will focus on that interaction.
LFA-1 is a key adhesion molecule that aids in leukocyte migration via interaction with the intercellular adhesion molecules (ICAMs) expressed on vascular endothelial cells [54]. LFA-1 exists in three conformational states—a resting state, an intermediate/extended state and an activated state. Resting state LFA-1 is unable to bind ICAM-1. Upon leukocyte activation, LFA-1 changes conformations to the intermediate/extended state which can weakly bind to ICAM-1, allowing for downstream signaling to initiate the full extension to the high affinity, activated form of LFA-1 that allows for tight adhesion and WBC migration to peripheral tissues [55,56]. Typically, interaction of integrins with their ligands results in enhanced survival, differentiation and other immunological events [57,58]. Thus, it is intriguing that LtxA interaction with LFA-1 stimulates cell death to eliminate the most immunologically relevant cells targeting A. actinomycetemcomitans.
Previous studies have sought to characterize the nature of LtxA interaction with LFA-1. It appears that both CD11a and CD18 are required for LtxA to intoxicate cells. Chimeric receptor studies demonstrated that CD18 is the functional receptor for LtxA and confers species-specificity to LtxA [59]. Human cells expressing bovine CD11a/human CD18 were susceptible to LtxA, while human cells expressing human CD11a/bovine CD18 were resistant to the toxin. Furthermore, a 100-amino acid residue region of the cysteine-rich tandem repeats encompassing integrin-epidermal growth-factor-like domains 2, 3, and 4 of the extracellular domain of CD18 are critical for toxin activity against LFA-1 expressing cells [59]. Another study showed that an N-terminal 128 amino acid domain encompassing the β sheets 1 and 2 of the β-propeller domain of CD11a is important for recognition by LtxA [60]. These studies suggest that LtxA comes in contact with both CD11a and CD18; however, it has been hypothesized that CD18 harbors the major toxin binding site due to the ability of LtxA to utilize Mac-1 and CR4 [51]. Studies have also demonstrated that LtxA recognizes CD11a/CD18 through N-linked oligosaccharides on LFA-1, specifically via sialic acid residues, and this glycosylation is required for cytotoxic activity [61,62].
Two recent studies have proposed interesting interactions of LtxA with LFA-1. One proposed that LtxA binds to the extracellular domains of LFA-1 initiating receptor clustering in the lipid raft compartment, allowing the toxin to transverse the membrane where it binds the cytoplasmic domains of CD11a and CD18 with a strong affinity, suggesting that LtxA may exist partially embedded in the cell membrane [63]. It is believed that LtxA membrane association via CRAC motifs allows LtxA to adopt a conformation amenable to membrane insertion and association with both extra- and intra-cellular domains of CD11a and CD18 [32]. Another study proposed that LtxA does not require the cytoplasmic domain of CD18, suggesting downstream integrin signaling is not required for cytotoxicity [64]. Further work is required to resolve the interaction of LtxA with LFA-1 and other β2 integrins.
3. Mechanisms of Action
The sensitivity of WBCs to LtxA is directly correlated to the level of LFA-1 surface expression [35]. Cells which expressed the highest levels of LFA-1 on the cell surface were the most sensitive to LtxA. LtxA preferentially targets the WBCs expressing the active conformation of LFA-1 [65,66]. Thus, LtxA targets the most immunologically relevant cells, allowing A. actinomycetemcomitans to persist, and under the appropriate conditions, causes disease. At high doses, LtxA is believed to destroy cells via pore formation in the cell membrane, a characteristic of RTX toxins, causing necrosis, while at lower doses of LtxA, apoptotic-like mechanisms of cell death eliminate cells [67,68,69]. The mechanisms of LtxA-mediated cell death, especially the events downstream of LFA-1, were poorly understood until recently. The current knowledge of the mechanisms by which LtxA kills various immune cell subsets is described below.
3.1. Monocytes and Macrophages
Initially it was unclear if LtxA initiated apoptosis or necrosis in immune cells in the monocytic lineage. Promyelocytic HL-60 cells treated with LtxA exhibited characteristic features of apoptotic cells, including reductions in cell size, organelle condensation, membrane budding, translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane, and DNA fragmentation [67]. Among leukocytes, monocytes are the most sensitive to LtxA [70] and the kinetics of this process are very rapid [66].
LtxA has been shown to preferentially target CD14+ monocytes that express the purinergic receptor, P2X7R [71]. LtxA is known to increase cytosolic (Ca2+) through a receptor-independent mechanism [38] and interestingly, the inhibition of P2X1R prevents cytosolic (Ca2+) spikes, and P2X7R was found to participate in sustaining this (Ca2+) spike [72]. CD14+ P2X7R expressing cells respond to LtxA with a rapid release of ATP to the extracellular milieu that activates P2X receptors, allowing Ca2+ entry into the cell. LtxA-induced (Ca2+) spikes were at least partially secondary to P2XR activation [72], suggesting that P2XR activation may cause Ca2+ mobilization in response to LtxA. Thus, the activation of P2XR may precede LtxA interaction with LFA-1. Inhibition of P2X1R, P2X4R, and P2X7R prevented LtxA-mediated cell death of the CD14+ cell population, indicating an important role of P2X7R signaling in cell death [71,72]. It is unclear if P2X receptors are important in LtxA-mediated cell death of other immune cell subsets.
Initial studies on LtxA interaction with host cells demonstrated that LFA-1 was the cellular receptor [50]. More recently, studies identified that LtxA was able to kill K562 cells transfected to express Mac-1 (CD11b/CD18) and CR4 (CD11c/CD18) just as efficiently as those expressing LFA-1 (CD11a/CD18) [51]. In support of this, we have preliminary data showing that THP-1 monocytes with CRISPR/Cas9-mediated deletion of CD11a remain sensitive to LtxA, albeit at lower levels than their wild-type counterparts (Vega, BA and Kachlany, SC, unpublished data). LtxA interaction with LFA-1 lead to dephosphorylation and thus, activation of the ubiquitous actin-binding protein, cofilin, which lead to enhanced clustering of LFA-1 on the surface of THP-1 monocytes [73]. Following clustering of LFA-1 in the lipid raft compartment and LtxA binding to LFA-1, the LtxA/LFA-1 complex is internalized via a receptor-mediated endocytosis mechanism [66]. Because cofilin regulates actin dynamics, its activation in response to LtxA may result in increased LFA-1 trafficking and internalization of the LFA-1/LtxA complex.
Once bound to LFA-1, LtxA treatment results in rapid phosphorylation of MAPK p38 and activation of caspase-1 [70,71]. The inhibition of caspase-1 was found to prevent LtxA-mediated cell death in monocytes, suggesting a critical role in the cell death cascade for this protease. The cleavage of caspase-1 and activation of the inflammasome is a precursor to bioprocessing and secretion IL-1β and IL-18, and abundant IL-1 β and IL-18 are produced and released in response to LtxA [70,74,75,76]. This caspase-1 dependent programmed cell death that leads to secretion of IL-1β and IL-18 closely resembles the pro-inflammatory cell death pathway of pyroptosis [77]. However, a separate study demonstrated that while LtxA induced dose-dependent caspase activation in THP-1 monocytes, the inhibition of caspases still led to potent cell death [66], suggesting that LtxA activates another, more prominent cell death pathway in THP-1 monocytes.
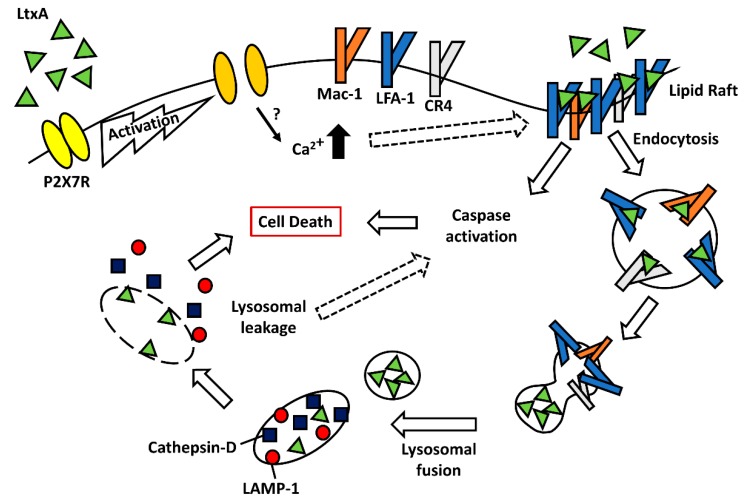
The inhibition of numerous cell death pathways, including necrosis and necroptosis, failed to inhibit LtxA-mediated cytolysis in monocytes. However, pretreatment of cells with the phosphatidylinositol kinase (PI3K) inhibitor 3-MA, was able to significantly inhibit LtxA-mediated killing [66]. 3-MA has been used to inhibit lysosomal mediated cell death pathways, such as autophagy, which lead to investigation into the role of lysosomes in LtxA-mediated cell death in THP-1 cells. Confocal microscopy and flow cytometry revealed LtxA treatment leads to rapid disruption of the lysosomal membrane and acidification of the cytosol [66,78]. LtxA-treated cells exhibited membrane blebbing and altered cellular morphologies prior to lysosomal damage. This can occur in cellular degranulation, which has been proposed as a mechanism of LtxA-mediated cell death [79]. Degranulation requires the presentation of LAMP-1 (CD107a) on the surface of cells [80], which is not observed upon LtxA treatment [66]. The lysosomal aspartyl protease Cathepsin D was detected in cytosolic fractions of LtxA-treated cells and the inhibition of Cathepsin D with pepstatin A significantly inhibited killing of THP-1 cells. Furthermore, after LtxA is internalized into cells, LtxA is shuttled to the lysosomal membrane, where it causes its rupture and release of contents. It is proposed that release of these lysosomal proteases leads to the caspase activation observed. LtxA is the first bacterial toxin shown to localize to the lysosomal compartment where it causes the release of its contents into the cytosol. This lysosomal-mediated cell death pathway appears to occur in monocytes irrespective of which β2 integrin LtxA binds (Vega, BA and Kachlany, SC, unpublished data). Therefore, LtxA induces cell death in monocytes via disruption of lysosomes, causing a release of contents to the cytosol, resulting in its acidification in a manner distinct from degranulation. Figure 2 depicts a proposed model of LtxA-mediated cell death in monocytes and macrophages.
Figure 2.
Model of LtxA-mediated cell death in monocytes. LtxA preferentially targets CD14+ monocytes expressing P2X7R. Upon treatment with LtxA, monocytes rapidly release ATP into the extracellular milieu, activating P2X7R. Activation of P2X7R allows Ca2+ to be mobilized, resulting in the clustering of LFA-1 and other β2 integrins into the lipid raft compartment. LtxA/LFA-1 interaction leads to the activation of caspases. LtxA is internalized in an LFA-1/ β2 integrin-dependent manner where LtxA is shuttled to the lysosome. LtxA ruptures the lysosomal membrane, allowing leakage of lysosomal proteases, such as Cathepsin D, into the cytosol leading to rapid and irreversible cell death.
3.2. Polymorphonuclear Leukocytes
Neutrophils make up the largest population of polymorphonuclear leukocytes (PMNs) in the circulation, while eosinophils, basophils and mast cells are less abundant. As such, most of the mechanistic studies of the effects of LtxA on PMNs have been done primarily in neutrophils.
Electron micrographs of PMNs treated with LtxA revealed rapid morphological changes. Shortly after the addition of LtxA, PMNs demonstrated signs of granule translocation toward the cell surface, the formation and shedding of cell membrane blebs, and karyorrhexis [79,81]. PMNs rapidly release the granule components resistin [82], matrix metalloproteinase 8 [83], myeloperoxidase, lysozyme, β-glucuronidase [84,85], lactoferrin [79], neutrophil elastase [79,86] and other lysosomal enzymes into the extracellular compartment. The release of these granule components was accompanied by a decrease in the number of intracellular granules seen via electron microscopy [79]. Granule release occurred prior to cell lysis, as extracellular lactate dehydrogenase levels remained low. This was intriguing since LtxA lysis of PMNs is believed to occur through pore formation but allows no efflux of cytosolic macromolecules, which may suggest that LtxA does not form pores in PMNs. LtxA treatment increased the levels of CD63 and CD66b (from primary and secondary granules, respectively) on the surface of the cell [79]. Conflicting reports on the involvement of LFA-1 in LtxA-induced PMN degranulation have been published [79,82].
Interestingly, several studies proposed that neutrophils exposed to LtxA can activate and release neutrophil extracellular traps (NETs) in a process called NETosis. NETosis is an innate defense strategy whereby neutrophils release DNA and certain granule components, such as neutrophil elastase, myeloperoxidase and lactoferrin [87,88], to the extracellular space where web-like DNA threads trap bacteria and bacterial products to thwart microbial infections. Neutrophils treated with LtxA quickly demonstrated signs of cell swelling. NET formation was observed after a 3-h incubation with LtxA, suggesting that cell swelling is a slow process, which allows NETosis to proceed to cell death/lysis in a controlled, perhaps distinct process [89,90,91]. Because NETosis is known to cause the release of granule contents and occur prior to neutrophil lysis, it is possible that the degranulation which precedes LtxA-induced neutrophil lysis [79] is due to NETosis. While degranulation is believed to be LFA-1-independent, studies have previously shown LFA-1-dependent NET release [92], which means that degranulation and NETosis could be two separate cell death pathways. Therefore, LtxA interaction with LFA-1 on neutrophils may induce rapid lysis and degranulation in some cells and/or NETosis and slow cell death in others; however, further work is necessary to resolve the mechanism of LtxA intoxication of PMNs. It is important to note that the lack of immortalized neutrophil cell lines and their short in vivo half-life pose a challenge to studying LtxA-mediated cell death in PMNs.
3.3. Lymphocytes
Lymphocytes were originally considered resistant to LtxA-mediated cytotoxicity [85,93]. Exposure of lymphocytes to LtxA resulted in very few cells, showing evidence of cell death using viability stains; however, electron microscopy and flow cytometry revealed cellular alterations indicative of apoptosis [69]. LtxA-induced cell death in lymphocytes occurs much slower than cell death in cells of myeloid origin.
Initial studies identified that upon binding to LFA-1 on lymphocytes, LtxA is not internalized via receptor-mediated endocytosis nor does LtxA cause disruption of the lysosomes [66]. However, a more recent study showed that while LtxA associates with host cell membranes independent of LFA-1, LtxA is at least partially internalized in Jn.9 cells in an LFA-1 dependent manner [63]. Using flow cytometry and fluorescence microscopy, LtxA was found to translocate into the cytosol and remained very close to the plasma membrane. The proximity of LtxA to the cell membrane may explain why previous studies did not observe toxin internalization in lymphocytes. LtxA has strong binding affinities for the membrane proximal region of CD11a and the intermediate domain of CD18 [63]. When bound to these domains of CD11a and CD18, the toxin brings the cytoplasmic domains of CD11a and CD18 closer together, which interferes with LFA-1 activation [63]. Interestingly, LtxA binds CD11a in close proximity to the region that Rap1 GTPase interacts with CD11a. Rap1 GTPase is activated by cytosolic Ca2+ increases and diacylglycerol and binds its effector molecule, RapL, on specific residues within CD11a to stimulate inside-out integrin signaling [94,95]. Thus, LtxA interaction with the membrane proximal region of CD11a could block integrin inside-out signaling. Rap1 and RapL are known to be involved in parts of apoptotic signaling pathways [96,97], and LtxA blockage of canonical integrin signaling could alter signaling pathways, thus allowing cell death signaling to occur through the integrin.
Other studies have identified a critical role for the Fas (CD95) death receptor in LtxA-mediated cell death in T-lymphocytes [98,99]. Fas and CD11a were found to colocalize on the surface of Jurkat E6.1 cells more strongly than CD11a and CD18, the molecules that are known to heterodimerize as LFA-1 [98]. CRISPR/Cas9-mediated deletion of Fas prevented LtxA from intoxicating Jurkat E6.1 cells [99]. Additionally, use of Jurkat A3 cells, which are very sensitive to Fas mediated apoptosis, were more sensitive to LtxA than Jurkat E6.1 cells [98]. These results suggest that Fas plays a major role in LtxA-mediated cell death. While Fas was found to be an early event in LtxA-mediated cell death [99], the potential interplay between LtxA, LFA-1, and Fas has not been deciphered.
The proposal that LtxA does not require the cytoplasmic domain of CD18 [64] or that LtxA interferes with LFA-1 signaling [63] may help explain how LtxA can signal via the Fas-mediated cell death pathway in lymphocytes. It is noteworthy that Fas does not play a role in LtxA-mediated cell death in B-lymphocytes ([100] and Vega, BA and Kachlany, SC, unpublished data). Further work is required to decipher if LtxA utilizes another death receptor in B-lymphocytes.
Upon treatment of lymphocytes with LtxA, classical features of apoptosis, such as decreased cell size, increased cytoplasmic granularity and DNA fragmentation are observed [67]. Downstream of the receptor(s), LtxA kills lymphocytes by a caspase-dependent mechanism [98]. CRISPR/Cas9-mediated deletion of LFA-1 or Fas prevented the activation of caspases in response to LtxA [99]. While LtxA treatment induced cleavage of both caspase-8 and -9, only the inhibition of caspase-8 significantly prevented cell death [98]. This finding was further corroborated using caspase-8 mutant cells that were partially, yet significantly resistant to LtxA-mediated cytolysis. In canonical Fas-mediated apoptosis, the activation of caspase-8 can lead to type I extrinsic apoptosis where caspase-8 directly activates the executioner caspase-3, or type II extrinsic apoptosis where caspase-8 triggers a series of events leading to perturbation of the mitochondria [101]. LtxA treatment of JY B cells resulted in mitochondria distention where cristae were obliterated [101], a phenomenon that is consistent with apoptosis [102,103]. Furthermore, LtxA treatment induced a loss of mitochondrial membrane potential and hyperproduction of reactive oxygen intermediates [68,100]. Fas signaling played a critical role in LtxA-mediated mitochondrial membrane permeabilization in T-lymphocytes [99]. LtxA treatment led to a decrease in ATP levels [100]. Loss of mitochondrial membrane potential led to the translocation of cytochrome c into the cytoplasm and cleavage of caspases-9 and -3. LtxA-mediated caspase-3 activation triggered the terminal events in apoptosis, including cleavage of the DNA repair enzyme poly (ADP-ribose) polymerase (PARP) and DNA fragmentation. Overexpression of anti-apoptotic proteins Bcl-2 and Bcl-xL prevented uncoupling of the electron transport chain from oxidative phosphorylation, cytochrome c release, effector caspase-3 and -7 activation, PARP cleavage, and DNA fragmentation [100].
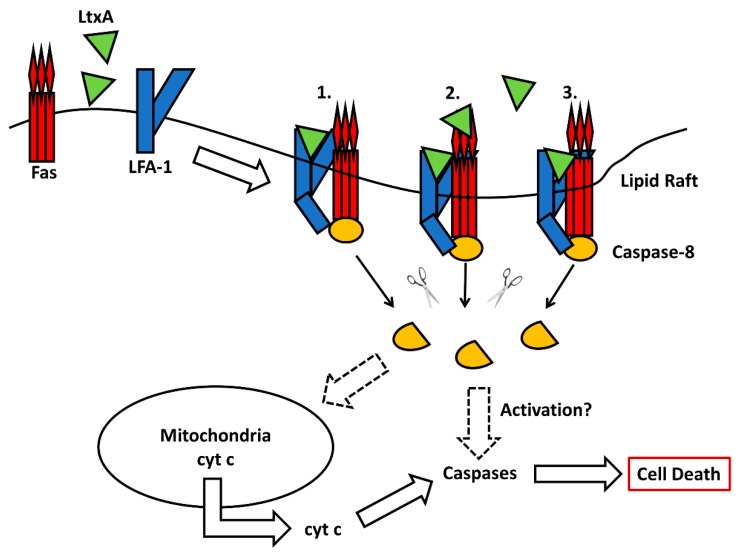
These studies reveal many similarities between the mechanism of LtxA-mediated cell death in lymphocytes to that of canonical Fas-mediated apoptosis. Given the requirement of Fas for cell death [99], the colocalization of Fas and CD11a [98], and the similarities between the cell death signaling pathways, it may be plausible that LtxA interference of LFA-1 activation and signaling leads to apoptotic signaling via the canonical Fas-mediated cell death pathway in T-lymphocytes. These similarities warrant further investigation into the potential involvement of other death receptors in LtxA-mediated cell death in B-lymphocytes. A proposed model of LtxA-mediated cell death in lymphocytes is shown in Figure 3.
Figure 3.
Model of LtxA-mediated cell death in lymphocytes. LtxA induces clustering of LFA-1 in the lipid rafts, where LFA-1 colocalizes strongly with Fas. After association with LFA-1, LtxA partially transverses the cell membrane and interacts with membrane proximal domains of LFA-1, interfering with its activation. The literature supports that LtxA only interacts with LFA-1 1, but it is possible that LtxA interacts with LFA-1 and Fas independently 2. or LtxA simultaneously interacts with both LFA-1 and Fas 3. Faulty activation of LFA-1 may allow the β2 tail to either directly activate caspase-8 or to signal via the Fas-mediated cell death pathway. The activation of caspase-8 can result in perturbation of the mitochondrial membrane, releasing cytochrome c into the cytosol, or further activation of effector caspases. Both pathways culminate in cell death.
4. Potential Therapeutic Applications
Many bacterial toxins have been well studied because of the threats they pose to the host immune system. The natural targeting abilities of several bacterial toxins, such as those from Clostridium botulinum and Corynebacterium diphtheriae have been exploited by clinicians to treat diseases. C. botulinum neurotoxin (BOTOX) and C. diphtheriae diphtheria toxin (ONTAK) have been utilized clinically for the treatment of neuromuscular disorders [104] and T-cell lymphoma [105,106], respectively, among other toxin therapies.
LtxA is currently being investigated as a therapeutic agent (under tradename Leukothera®) for treatment of hematological malignancies and immune-mediated diseases. LFA-1 and other β2 integrins, the receptors for LtxA, are known to be overexpressed on WBCs in leukemias, lymphomas, autoimmune diseases and inflammatory disorders [107,108,109]. Additionally, LFA-1 is known to mediate migration of auto-reactive immune cells to various organs in autoimmune diseases, such as thyroiditis, psoriasis, multiple sclerosis, and rheumatoid arthritis [108]. Because LtxA specifically targets the active conformation of LFA-1 [65,66], LtxA may represent a novel targeted biotherapy for WBC disorders and is being developed as such. Several of the preclinical studies demonstrating the potential therapeutic efficacy of LtxA are described below. A summary of diseases in which β2 integrins play a role, and thus may be treatable with LtxA therapy, is provided in Table 1.
Table 1.
The role of β2 integrins in hematological malignancies and immune-mediated diseases.
| Indication | Integrins | References |
|---|---|---|
| Leukemia | αLβ2 | [107,109,110] |
| Lymphoma | αLβ2 | [107,109,110] |
| Psoriasis | αLβ2, αMβ2, αXβ2 | [111,112] |
| Asthma | αLβ2 | [113,114] |
| HIV | αLβ2 | [65,115,116,117] |
| Alopecia Areata | αLβ2, | [118,119,120,121,122] |
| Systemic Lupus Erythematosus | αLβ2, αMβ2 | [123,124,125,126] |
| Multiple Sclerosis | β2 | [127,128] |
| Crohn’s Disease | αLβ2, αMβ2 | [129,130,131,132] |
| Ulcerative Colitis | αLβ2, αMβ2 | [129,130,131,132] |
| Dry Eye | αLβ2, | [133] |
4.1. Leukemia and Lymphoma
Leukemia and lymphoma are cancers of WBCs that can be either acute or chronic diseases. These diseases are characterized by the uncontrolled proliferation of various subsets of immune cells. Many available standard chemotherapeutic agents are highly cytotoxic and have adverse side effects. LFA-1 is known to be overexpressed and over-activated in leukemias and lymphomas, leading to enhanced migration of diseased cells [107,109,110]. Due to the natural targeted potential of LtxA for these activated leukocytes [35,65,66], the therapeutic applicability of LtxA for the treatment of leukemia and lymphoma was evaluated in several studies.
LtxA has shown preclinical efficacy in SCID mouse xenograft models of human HL-60 myeloid leukemia and human RL B-cell lymphoma [35,98]. Treatment with LtxA resulted in complete tumor regression and enhanced overall survival. Peripheral blood mononuclear cells (PBMCs) from patients with acute monocytic leukemia were shown to be significantly more sensitive to LtxA than PBMCs from healthy controls [35]. Additionally, the expression levels of LFA-1 on the surface of cells correlated with the ability of LtxA to kill these cells—higher levels of LFA-1 lead to more killing by LtxA. Rhesus macaques injected intravenously with LtxA had transient decreases in WBCs post-infusion. LtxA had no effect on RBCs, platelets, hemoglobin, and other blood chemistry values and showed no signs of liver or kidney toxicity [35]. LtxA has also been shown to synergize in vitro with standard chemotherapeutic agents [134], which could allow for combination therapies that result in the administration of lower doses of more cytotoxic drugs, thus diminishing adverse effects. Due to the specificity of LtxA, it poses an advantage over chemotherapeutics because no off-target toxicities have been observed. Thus, LtxA may be an effective and safe novel therapeutic agent for the treatment of leukemia and lymphoma. LtxA is currently in pre-clinical development for the treatment of relapsed and refractory leukemia and lymphoma.
4.2. Psoriasis
Psoriasis is a common chronic skin disease that affects approximately 2–3% of the world’s population [135,136]. Psoriasis is an inflammatory skin disorder that is caused by over-reactive immune cells in the skin inducing rapid, abnormal proliferation and differentiation of epidermal keratinocytes that ultimately manifests on the skin surface as elevated, scaly lesions. Studies have shown that the WBCs involved in the progression of psoriasis have an upregulation and overactivation of LFA-1 [111,112]. While there is no cure for psoriasis, numerous therapeutic options for mild-to-moderate psoriasis, such as topical therapy and phototherapy, are available. Severe disease can be treated with methotrexate, retinoids, cyclosporine, and monoclonal antibodies, but there are known adverse effects [137,138]. LtxA is a much more targeted approach to treating psoriasis because it specifically depletes the immune cells involved in disease progression.
LtxA was shown to be highly effective at treating psoriasis in a human xenograft mouse model [139]. LtxA was highly effective for all parameters measured including epidermal thickness, clinical psoriasis score, and lymphocyte infiltration. Ex vivo studies on PBMCs from psoriasis patients identified that LtxA preferentially targeted CD4+CD25+ T lymphocytes and CD14+CD16+ monocytes in these patients [139]. This subset of T cells [119,140] and monocytes [141] have previously been implicated in the progression of psoriasis. Therefore, LtxA may provide therapeutic benefits and resolution of psoriatic plaques with fewer, if any, adverse side effects.
4.3. Allergic Asthma
Asthma is an inflammatory disease of the airways that affects millions of people worldwide and can cause mortality. Asthma progresses due to infiltration of activated WBCs into the airways, and the release of inflammatory mediators from these cells that subsequently damage the bronchial epithelium [142]. Inflammation in asthma is dependent on the migration of WBCs into the airways. This migration is mediated by LFA-1, which has been shown to be overexpressed on immune cells from asthma patients [113,114]. Investigations into the therapeutic potential of LtxA for treatment of allergic asthma found that PBMCs from asthma patients have a unique population of CD4−CD11ahi cells, the majority of which were CD14+ monocytes, that are preferentially targeted by LtxA [114]. In a mouse model for allergic asthma, LtxA caused resolution of disease, as demonstrated by a decrease in bronchoalveolar lavage fluid WBCs, a reduction in pulmonary inflammation and tissue remodeling, as well as a decrease in proinflammatory cytokines associated with asthma in lung tissue [114]. Thus, LFA-1 may serve as a biomarker in allergic asthma and the use of LtxA to treat this disease could be a useful therapeutic strategy.
4.4. HIV
Human immunodeficiency virus (HIV) infects CD4+ T cells, macrophages, and dendritic cells. HIV infection leads to progressive loss of CD4+ T cells and eventually, the loss of cell-mediated immunity, leading to the development of AIDS. LFA-1 and its ligand, ICAM-1, have been shown to be incorporated into the envelopes of budding HIV-1 virions from activated primary CD4+ T cell [116,117]. HIV-1 virions possessing ICAM-1 are more infectious due to the ability to bind LFA-1 on target cells [143,144]. These molecules play a crucial role in cell–cell transmission of HIV since they are components of the virological synapse [145,146]. The HIV envelope protein gp120 induced the activation of LFA-1 in naïve CD4+ T cells, making these cells more susceptible to HIV infection [65,115]. Virus p24-expressing CD4+ T cells isolated from PBMCs of HIV infected patients expressed higher surface levels of active LFA-1 compared to p24− T cells from the same patients [65]. Ex vivo treatment of HIV-infected PBMCs with LtxA led to a significant reduction in viral DNA burden [65], suggesting that while HIV infection may activate CD4+ T cells, doing so makes them more susceptible to LtxA. Therefore, LtxA may be utilized as a therapeutic strategy to deplete HIV viral reservoirs in infected individuals who are managing the disease with antiretroviral therapy.
4.5. The Role of β2 Integrins in Other Autoimmune and Inflammatory Disorders
Alopecia areata is an autoimmune disorder characterized by damage to hair follicles and subsequent loss of hair [147]. Damage to hair follicles is cause by infiltration of activated, pro-inflammatory WBCs migrating to the follicles. Activated T cells, NK cells, eosinophils and mast cells have all been implicated in the progression of alopecia [118,119,120,121,122]. These immune cell subsets all express LFA-1 and other β2 integrins, thus, LtxA could be a potent and safe anti-inflammatory agent for the treatment of alopecia [148].
Systemic lupus erythematosus is an autoimmune disorder whereby a patient’s own immune cells become hyperactive and begin to attack healthy tissues, producing widespread inflammation [149]. Lupus is characterized by the immune system producing autoantibodies against itself [150]. Autoantibody complexes and immune cells can cause damage to many systems of the body, including joints, kidneys and blood cells. Treatments for lupus typically include immunosuppressive drugs, which can result in a higher frequency of opportunistic infections in these patients. T- and B-lymphocytes from patients with lupus were found to have increased expression of LFA-1 [123,124,125,126]. Additionally, PBMCs isolated from patients with lupus were found to have higher expression levels of Fas [151]. Therefore, LtxA may be a novel therapeutic option for lupus patients that is not immunosuppressive and leads to better health during treatment plans.
Multiple sclerosis is an autoimmune disorder affecting the central nervous system. Autoreactive immune cells destroy the myelin sheath surrounding nerve cells in the brain and spinal cord [152]. This disrupts communication from the brain to the rest of the body. There is no cure for multiple sclerosis and treatment is generally with immunosuppressive agents. LFA-1 was found to be highly expressed on immune cells in the peripheral blood and central nervous system of patients with multiple sclerosis [127,128]. In support of targeting integrins to treat multiple sclerosis, the United States Food & Drug Administration (FDA) approved Tysabri (Natalizumab), a monoclonal antibody that targets the cell adhesion molecule α4-integrin. Tysabri prevents the migration of inflammatory immune cells across the blood-brain barrier. Therefore, LtxA may be a beneficial therapeutic agent for the treatment and/or management of multiple sclerosis by targeting LFA-1 and removing these pro-inflammatory cells from circulation.
Inflammatory bowel disease (IBD) refers to chronic inflammatory disorders of the digestive tract. Ulcerative colitis and Crohn’s disease are the most common forms of IBD. Ulcerative colitis and Crohn’s disease are autoimmune diseases in which the body’s immune cells attack regions of the digestive tract. Ulcerative colitis manifests as ulcers in the lining of the colon and rectum, while Crohn’s disease can affect the small intestine, large intestine, and stomach [153,154,155]. IBD is typically treated with immunosuppressive agents. In patients with ulcerative colitis or Crohn’s disease, leukocytes in the intestinal tissues have been found to have enhanced LFA-1 expression, which could lead to an enhanced migration of autoreactive cells from peripheral blood to intestinal tissues [129,130,131,132]. Therefore, ulcerative colitis and Crohn’s disease may be treatable with LtxA and ongoing studies are investigating this possibility.
Dry eye disease is a common ocular disorder that affects 5–34% of the population [156]. Dry eye disease is believed to be an antigen-specific autoimmune disease associated with inflammation of the lacrimal gland and ocular surface [157,158]. Typically, this results in the inability to produce adequate tears, leading to dryness and eye pain. Although no causative antigen has been identified, the pathophysiology is believed to be caused by antigen-presenting cells priming naïve T cells into CD4+ TH1 and TH17 cells that migrate to the ocular surface [157,158,159,160]. In order for these autoreactive T cells to migrate to the ocular surface, they must have enhanced expression of the activated form of LFA-1 [133,161,162]. The FDA approved Xiidra (Lifitegrast), a small molecule inhibitor of LFA-1, for treatment of dry eye. Xiidra blocks LFA-1 interaction with its natural ligand, ICAM-1 [159,160,163,164], thus diminishing the migration of inflammatory T cells to the ocular surface. Due to the effectiveness of inhibiting LFA-1 and the enhanced expression of LFA-1 in dry eye, LtxA therapy may be beneficial to patients suffering from dry eye disease by eliminating these activated LFA-1-expressing cells.
LtxA may have significant therapeutic potential for the treatment of various autoimmune and inflammatory diseases. LtxA would provide a great benefit over many currently available therapeutics because it is not immunosuppressive and the agent selectively eliminates activated immune cells that contribute to the pathologies in the above-mentioned diseases. However, more investigations are necessary to provide data for proof-of-principle in other autoimmune disorders.
5. Summary and Conclusions
In summary, we described how LtxA initially interacts with target cell membranes in a cholesterol-dependent manner. LtxA is therefore able to interact with LFA-1 or other β2 integrins. LtxA/LFA-1 interaction triggers intracellular signaling pathways, which ultimately leads to cell death. The cell death mechanism varies by immune cell subset. We also discussed preliminary pre-clinical efficacy data supporting the development of LtxA as a novel biologic for the treatment of various hematological malignancies and immune-mediated diseases.
Acknowledgments
We thank the various member of the author’s laboratory who have contributed to studies described here.
Author Contributions
B.A.V., B.A.B., and S.C.K. wrote and revised the manuscript.
Funding
Work in the author’s laboratory has been generously supported by grants from the National Institutes of Health (National Institute of Dental and Craniofacial Research, National Institute of Allergy and Infectious Diseases and National Cancer Institute), New Jersey Commission on Cancer Research, the New Jersey Health Foundation. Brian A. Vega has received support from the National Institute of Allergy and Infectious Diseases grant number T32AI125185.
Conflicts of Interest
The authors declare a conflict of interest. Scott C. Kachlany owns stock in the company (Actinobac Biomed, Inc.) that has licensed the clinical use of leukotoxin. Benjamin A. Belinka Jr. is an employee of Actinobac Biomed, Inc., and owns stock in this company. In addition, Scott C. Kachlany and Brian A. Vega have received consulting fees from Actinobac Biomed, Inc.
Key Contribution
This article provides an update on the current knowledge of mechanisms by which Aggregatibacter actinomycetemcomitans leukotoxin eliminates various subsets of immune cells. Additionally, the article highlights recent advances on the potential applications of LtxA-toxin therapy.
References
- 1.Fine D.H., Kaplan J.B., Kachlany S.C., Schreiner H.C. How we got attached to Actinobacillus actinomycetemcomitans: A model for infectious diseases. Periodontology 2000. 2006;42:114–157. doi: 10.1111/j.1600-0757.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- 2.Slots J., Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: Occurrence and treatment. Periodontology 2000. 1999;20:82–121. doi: 10.1111/j.1600-0757.1999.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 3.Das M., Badley A.D., Cockerill F.R., Steckelberg J.M., Wilson W.R. Infective endocarditis caused by HACEK microorganisms. Annu. Rev. Med. 1997;48:25–33. doi: 10.1146/annurev.med.48.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Shenker B.J., McKay T., Datar S., Miller M., Chowhan R., Demuth D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. (Baltim. Md. 1950) 1999;162:4773–4780. [PubMed] [Google Scholar]
- 5.Shenker B.J., Besack D., McKay T., Pankoski L., Zekavat A., Demuth D.R. Induction of cell cycle arrest in lymphocytes by Actinobacillus actinomycetemcomitans cytolethal distending toxin requires three subunits for maximum activity. J. Immunol. 2005;174:2228–2234. doi: 10.4049/jimmunol.174.4.2228. [DOI] [PubMed] [Google Scholar]
- 6.Kolodrubetz D., Dailey T., Ebersole J., Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect. Immun. (Baltim. Md. 1950) 1989;57:1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lally E.T., Kieba I.R., Demuth D.R., Rosenbloom J., Golub E.E., Taichman N.S., Gibson C.W. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem. Biophys. Res. Commun. 1989;159:256–262. doi: 10.1016/0006-291X(89)92431-5. [DOI] [PubMed] [Google Scholar]
- 8.Kachlany S.C., Fine D.H., Figurski D.H. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect. Immun. 2000;68:6094–6100. doi: 10.1128/IAI.68.11.6094-6100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brogan J.M., Lally E.T., Poulsen K., Kilian M., Demuth D.R. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: Analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 1994;62:501–508. doi: 10.1128/iai.62.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampathkumar V., Velusamy S.K., Godboley D., Fine D.H. Increased leukotoxin production: Characterization of 100 base pairs within the 530 base pair leukotoxin promoter region of Aggregatibacter actinomycetemcomitans. Sci. Rep. 2017;7:1887. doi: 10.1038/s41598-017-01692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balashova N.V., Shah C., Patel J.K., Megalla S., Kachlany S.C. Aggregatibacter actinomycetemcomitans LtxC is required for leukotoxin activity and initial interaction between toxin and host cells. Gene. 2009;443:42–47. doi: 10.1016/j.gene.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Fong K.P., Tang H.Y., Brown A.C., Kieba I.R., Speicher D.W., Boesze-Battaglia K., Lally E.T. Aggregatibacter actinomycetemcomitans leukotoxin is post-translationally modified by addition of either saturated or hydroxylated fatty acyl chains. Mol. Oral Microbiol. 2011;26:262–276. doi: 10.1111/j.2041-1014.2011.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lally E.T., Golub E.E., Kieba I.R. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J. Biol. Chem. 1994;269:31289–31295. [PubMed] [Google Scholar]
- 14.Inoue T., Tanimoto I., Tada T., Ohashi T., Fukui K., Ohta H. Fermentable-sugar-level-dependent regulation of leukotoxin synthesis in a variably toxic strain of Actinobacillus actinomycetemcomitans. Microbiology (Read. Engl.) 2001;147:2749–2756. doi: 10.1099/00221287-147-10-2749. [DOI] [PubMed] [Google Scholar]
- 15.Hritz M., Fisher E., Demuth D.R. Differential regulation of the leukotoxin operon in highly leukotoxic and minimally leukotoxic strains of Actinobacillus actinomycetemcomitans. Infect. Immun. 1996;64:2724–2729. doi: 10.1128/iai.64.7.2724-2729.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolodrubetz D., Phillips L., Jacobs C., Burgum A., Kraig E. Anaerobic regulation of Actinobacillus actinomycetemcomitans leukotoxin transcription is ArcA/FnrA-independent and requires a novel promoter element. Res. Microbiol. 2003;154:645–653. doi: 10.1016/j.resmic.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Ohta H., Miyagi A., Kato K., Fukui K. The relationships between leukotoxin production, growth rate and the bicarbonate concentration in a toxin-production-variable strain of Actinobacillus actinomycetemcomitans. Pt 4Microbiology (Read. Engl.) 1996;142:963–970. doi: 10.1099/00221287-142-4-963. [DOI] [PubMed] [Google Scholar]
- 18.Isaza M.P., Duncan M.S., Kaplan J.B., Kachlany S.C. Screen for leukotoxin mutants in Aggregatibacter actinomycetemcomitans: Genes of the phosphotransferase system are required for leukotoxin biosynthesis. Infect. Immun. 2008;76:3561–3568. doi: 10.1128/IAI.01687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guthmiller J.M., Kolodrubetz D., Kraig E. Mutational analysis of the putative leukotoxin transport genes in Actinobacillus actinomycetemcomitans. Microb. Pathog. 1995;18:307–321. doi: 10.1006/mpat.1995.0028. [DOI] [PubMed] [Google Scholar]
- 20.Guthmiller J.M., Kolodrubetz D., Cagle M.P., Kraig E. Sequence of the lktB gene from Actinobacillus actinomycetemcomitans. Nucleic Acids Res. 1990;18:5291. doi: 10.1093/nar/18.17.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guthmiller J.M., Kraig E., Cagle M.P., Kolodrubetz D. Sequence of the lktD gene from Actinobacillus actinomycetemcomitans. Nucleic Acids Res. 1990;18:5292. doi: 10.1093/nar/18.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crosby J.A., Kachlany S.C. TdeA, a TolC-like protein required for toxin and drug export in Aggregatibacter (Actinobacillus) actinomycetemcomitans. Gene. 2007;388:83–92. doi: 10.1016/j.gene.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kachlany S.C. Aggregatibacter actinomycetemcomitans leukotoxin: From threat to therapy. J. Dent. Res. 2010;89:561–570. doi: 10.1177/0022034510363682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraig E., Dailey T., Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: Homology to the alpha-hemolysin/leukotoxin gene family. Infect. Immun. 1990;58:920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumann U., Wu S., Flaherty K.M., McKay D.B. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: A two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993;12:3357–3364. doi: 10.1002/j.1460-2075.1993.tb06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehm D.F., Welch R.A., Snyder I.S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect. Immun. 1990;58:1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostolaza H., Soloaga A., Goni F.M. The binding of divalent cations to Escherichia coli alpha-haemolysin. Eur. J. Biochem. FEBS. 1995;228:39–44. doi: 10.1111/j.1432-1033.1995.tb20225.x. [DOI] [PubMed] [Google Scholar]
- 28.Stanley P., Koronakis V., Hughes C. Acylation of Escherichia coli hemolysin: A unique protein lipidation mechanism underlying toxin function. Microbiol. Mol. Biol. Rev. MMBR. 1998;62:309–333. doi: 10.1128/mmbr.62.2.309-333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley P., Packman L.C., Koronakis V., Hughes C. Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science (New York N.Y.) 1994;266:1992–1996. doi: 10.1126/science.7801126. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Magraner L., Viguera A.R., Garcia-Pacios M., Garcillan M.P., Arrondo J.L., de la Cruz F., Goni F.M., Ostolaza H. The calcium-binding C-terminal domain of Escherichia coli alpha-hemolysin is a major determinant in the surface-active properties of the protein. J. Biol. Chem. 2007;282:11827–11835. doi: 10.1074/jbc.M700547200. [DOI] [PubMed] [Google Scholar]
- 31.Brown A.C., Balashova N.V., Epand R.M., Epand R.F., Bragin A., Kachlany S.C., Walters M.J., Du Y., Boesze-Battaglia K., Lally E.T. Aggregatibacter actinomycetemcomitans leukotoxin utilizes a cholesterol recognition/amino acid consensus site for membrane association. J. Biol. Chem. 2013;288:23607–23621. doi: 10.1074/jbc.M113.486654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown A.C., Boesze-Battaglia K., Balashova N.V., Mas Gomez N., Speicher K., Tang H.Y., Duszyk M.E., Lally E.T. Membrane localization of the Repeats-in-Toxin (RTX) Leukotoxin (LtxA) produced by Aggregatibacter actinomycetemcomitans. PLoS ONE. 2018;13:e0205871. doi: 10.1371/journal.pone.0205871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiFranco K.M., Kaswala R.H., Patel C., Kasinathan C., Kachlany S.C. Leukotoxin kills rodent WBC by targeting leukocyte function associated antigen 1. Comp. Med. 2013;63:331–337. [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson D.L., Berthold P., Taichman N.S. Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 1988;56:1162–1166. doi: 10.1128/iai.56.5.1162-1166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kachlany S.C., Schwartz A.B., Balashova N.V., Hioe C.E., Tuen M., Le A., Kaur M., Mei Y., Rao J. Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leuk. Res. 2010;34:777–785. doi: 10.1016/j.leukres.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taichman N.S., Iwase M., Lally E.T., Shattil S.J., Cunningham M.E., Korchak H.M. Early changes in cytosolic calcium and membrane potential induced by Actinobacillus actinomycetemcomitans leukotoxin in susceptible and resistant target cells. J. Immunol. (Baltim. Md. 1950) 1991;147:3587–3594. [PubMed] [Google Scholar]
- 37.Iwase M., Lally E.T., Berthold P., Korchak H.M., Taichman N.S. Effects of cations and osmotic protectants on cytolytic activity of Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 1990;58:1782–1788. doi: 10.1128/iai.58.6.1782-1788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fong K.P., Pacheco C.M., Otis L.L., Baranwal S., Kieba I.R., Harrison G., Hersh E.V., Boesze-Battaglia K., Lally E.T. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell. Microbiol. 2006;8:1753–1767. doi: 10.1111/j.1462-5822.2006.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lally E.T., Hill R.B., Kieba I.R., Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999;7:356–361. doi: 10.1016/S0966-842X(99)01530-9. [DOI] [PubMed] [Google Scholar]
- 40.Brown A.C., Boesze-Battaglia K., Du Y., Stefano F.P., Kieba I.R., Epand R.F., Kakalis L., Yeagle P.L., Epand R.M., Lally E.T. Aggregatibacter actinomycetemcomitans leukotoxin cytotoxicity occurs through bilayer destabilization. Cell. Microbiol. 2012;14:869–881. doi: 10.1111/j.1462-5822.2012.01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol. Gen. Genet. MGG. 1988;214:553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- 42.Ortiz A., Aranda F.J., Villalain J., San Martin C., Micol V., Gomez-Fernandez J.C. 1,2-Dioleoylglycerol promotes calcium-induced fusion in phospholipid vesicles. Chem. Phys. Lipids. 1992;62:215–224. doi: 10.1016/0009-3084(92)90058-W. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz A., Killian J.A., Verkleij A.J., Wilschut J. Membrane fusion and the lamellar-to-inverted-hexagonal phase transition in cardiolipin vesicle systems induced by divalent cations. Biophys. J. 1999;77:2003–2014. doi: 10.1016/S0006-3495(99)77041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walters M.J., Brown A.C., Edrington T.C., Baranwal S., Du Y., Lally E.T., Boesze-Battaglia K. Membrane association and destabilization by Aggregatibacter actinomycetemcomitans leukotoxin requires changes in secondary structures. Mol. Oral Microbiol. 2013;28:342–353. doi: 10.1111/omi.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lear J.D., Karakelian D., Furblur U., Lally E.T., Tanaka J.C. Conformational studies of Actinobacillus actinomycetemcomitans leukotoxin: Partial denaturation enhances toxicity. Biochim. Biophys. Acta. 2000;1476:350–362. doi: 10.1016/S0167-4838(99)00241-1. [DOI] [PubMed] [Google Scholar]
- 46.Brown A.C., Koufos E., Balashova N.V., Boesze-Battaglia K., Lally E.T. Inhibition of LtxA toxicity by blocking cholesterol binding with peptides. Mol. Oral Microbiol. 2016;31:94–105. doi: 10.1111/omi.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koufos E., Chang E.H., Rasti E.S., Krueger E., Brown A.C. Use of a Cholesterol Recognition Amino Acid Consensus Peptide To Inhibit Binding of a Bacterial Toxin to Cholesterol. Biochemistry. 2016;55:4787–4797. doi: 10.1021/acs.biochem.6b00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart M.P., McDowall A., Hogg N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J. Cell Biol. 1998;140:699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kucik D.F., Dustin M.L., Miller J.M., Brown E.J. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J. Clin. Investig. 1996;97:2139–2144. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lally E.T., Kieba I.R., Sato A., Green C.L., Rosenbloom J., Korostoff J., Wang J.F., Shenker B.J., Ortlepp S., Robinson M.K., et al. RTX Toxins Recognize a 2 Integrin on the Surface of Human Target Cells. J. Biol. Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 51.Reinholdt J., Poulsen K., Brinkmann C.R., Hoffmann S.V., Stapulionis R., Enghild J.J., Jensen U.B., Boesen T., Vorup-Jensen T. Monodisperse and LPS-free Aggregatibacter actinomycetemcomitans leukotoxin: Interactions with human beta2 integrins and erythrocytes. Biochim. Biophys. Acta. 2013;1834:546–558. doi: 10.1016/j.bbapap.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Miller L.J., Schwarting R., Springer T.A. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J. Immunol. (Baltim. Md. 1950) 1986;137:2891–2900. [PubMed] [Google Scholar]
- 53.Miller L.J., Bainton D.F., Borregaard N., Springer T.A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J. Clin. Investig. 1987;80:535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 55.Hogg N., Smith A., McDowall A., Giles K., Stanley P., Laschinger M., Henderson R. How T cells use LFA-1 to attach and migrate. Immunol. Lett. 2004;92:51–54. doi: 10.1016/j.imlet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Hogg N., Harvey J., Cabanas C., Landis R.C. Control of leukocyte integrin activation. Am. Rev. Respir. Dis. 1993;148:S55–S59. doi: 10.1164/ajrccm/148.6_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- 57.de la Fuente M.T., Casanova B., Moyano J.V., Garcia-Gila M., Sanz L., Garcia-Marco J., Silva A., Garcia-Pardo A. Engagement of alpha4beta1 integrin by fibronectin induces in vitro resistance of B chronic lymphocytic leukemia cells to fludarabine. J. Leukoc. Biol. 2002;71:495–502. [PubMed] [Google Scholar]
- 58.Damiano J.S., Hazlehurst L.A., Dalton W.S. Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia. 2001;15:1232–1239. doi: 10.1038/sj.leu.2402179. [DOI] [PubMed] [Google Scholar]
- 59.Dileepan T., Kachlany S.C., Balashova N.V., Patel J., Maheswaran S.K. Human CD18 is the functional receptor for Aggregatibacter actinomycetemcomitans leukotoxin. Infect. Immun. 2007;75:4851–4856. doi: 10.1128/IAI.00314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kieba I.R., Fong K.P., Tang H.Y., Hoffman K.E., Speicher D.W., Klickstein L.B., Lally E.T. Aggregatibacter actinomycetemcomitans leukotoxin requires beta-sheets 1 and 2 of the human CD11a beta-propeller for cytotoxicity. Cell. Microbiol. 2007;9:2689–2699. doi: 10.1111/j.1462-5822.2007.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morova J., Osicka R., Masin J., Sebo P. RTX cytotoxins recognize beta2 integrin receptors through N-linked oligosaccharides. Proc. Natl. Acad. Sci. USA. 2008;105:5355–5360. doi: 10.1073/pnas.0711400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munksgaard P.S., Skals M., Reinholdt J., Poulsen K., Jensen M.R., Yang C., Leipziger J., Vorup-Jensen T., Praetorius H.A. Sialic acid residues are essential for cell lysis mediated by leukotoxin from Aggregatibacter actinomycetemcomitans. Infect. Immun. 2014;82:2219–2228. doi: 10.1128/IAI.01647-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nygren P., Balashova N., Brown A.C., Kieba I., Dhingra A., Boesze-Battaglia K., Lally E.T. Aggregatibacter actinomycetemcomitans leukotoxin causes activation of LFA-1. Cell. Microbiol. 2019;21:e12967. doi: 10.1111/cmi.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ristow L.C., Tran V., Schwartz K.J., Pankratz L., Mehle A., Sauer J.D., Welch R.A. The Extracellular Domain of the beta2 Integrin beta Subunit (CD18) Is Sufficient for Escherichia coli Hemolysin and Aggregatibacter actinomycetemcomitans Leukotoxin Cytotoxic Activity. mBio. 2019 doi: 10.1128/mBio.01459-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hioe C.E., Tuen M., Vasiliver-Shamis G., Alvarez Y., Prins K.C., Banerjee S., Nadas A., Cho M.W., Dustin M.L., Kachlany S.C. HIV envelope gp120 activates LFA-1 on CD4 T-lymphocytes and increases cell susceptibility to LFA-1-targeting leukotoxin (LtxA) PLoS ONE. 2011;6:e23202. doi: 10.1371/journal.pone.0023202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DiFranco K.M., Gupta A., Galusha L.E., Perez J., Nguyen T.V., Fineza C.D., Kachlany S.C. Leukotoxin (Leukothera(R)) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. J. Biol. Chem. 2012;287:17618–17627. doi: 10.1074/jbc.M111.314674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korostoff J., Wang J.F., Kieba I., Miller M., Shenker B.J., Lally E.T. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect. Immun. 1998;66:4474–4483. doi: 10.1128/iai.66.9.4474-4483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korostoff J., Yamaguchi N., Miller M., Kieba I., Lally E.T. Perturbation of mitochondrial structure and function plays a central role in Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Microb. Pathog. 2000;29:267–278. doi: 10.1006/mpat.2000.0390. [DOI] [PubMed] [Google Scholar]
- 69.Mangan D.F., Taichman N.S., Lally E.T., Wahl S.M. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect. Immun. 1991;59:3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelk P., Johansson A., Claesson R., Hanstrom L., Kalfas S. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 2003;71:4448–4455. doi: 10.1128/IAI.71.8.4448-4455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelk P., Abd H., Claesson R., Sandstrom G., Sjostedt A., Johansson A. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death Dis. 2011;2:e126. doi: 10.1038/cddis.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fagerberg S.K., Jakobsen M.R., Skals M., Praetorius H.A. Inhibition of P2X Receptors Protects Human Monocytes against Damage by Leukotoxin from Aggregatibacter actinomycetemcomitans and alpha-Hemolysin from Escherichia coli. Infect. Immun. 2016;84:3114–3130. doi: 10.1128/IAI.00674-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaur M., Kachlany S.C. Aggregatibacter actinomycetemcomitans leukotoxin (LtxA.; Leukothera) induces cofilin dephosphorylation and actin depolymerization during killing of malignant monocytes. Microbiol. (Read. Engl.) 2014;160:2443–2452. doi: 10.1099/mic.0.082347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelk P., Claesson R., Chen C., Sjostedt A., Johansson A. IL-1beta secretion induced by Aggregatibacter (Actinobacillus) actinomycetemcomitans is mainly caused by the leukotoxin. Int. J. Med. Microbiol. IJMM. 2008;298:529–541. doi: 10.1016/j.ijmm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Kelk P., Claesson R., Hanstrom L., Lerner U.H., Kalfas S., Johansson A. Abundant secretion of bioactive interleukin-1beta by human macrophages induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 2005;73:453–458. doi: 10.1128/IAI.73.1.453-458.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belibasakis G.N., Johansson A. Aggregatibacter actinomycetemcomitans targets NLRP3 and NLRP6 inflammasome expression in human mononuclear leukocytes. Cytokine. 2012;59:124–130. doi: 10.1016/j.cyto.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Cookson B.T., Brennan M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 78.Balashova N., Dhingra A., Boesze-Battaglia K., Lally E.T. Aggregatibacter actinomycetemcomitans leukotoxin induces cytosol acidification in LFA-1 expressing immune cells. Mol. Oral Microbiol. 2016;31:106–114. doi: 10.1111/omi.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johansson A., Claesson R., Hanstrom L., Sandstrom G., Kalfas S. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J. Periodontal Res. 2000;35:85–92. doi: 10.1034/j.1600-0765.2000.035002085.x. [DOI] [PubMed] [Google Scholar]
- 80.Aktas E., Kucuksezer U.C., Bilgic S., Erten G., Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009;254:149–154. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Johansson A., Sandstrom G., Claesson R., Hanstrom L., Kalfas S. Anaerobic neutrophil-dependent killing of Actinobacillus actinomycetemcomitans in relation to the bacterial leukotoxicity. Eur. J. Oral Sci. 2000;108:136–146. doi: 10.1034/j.1600-0722.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- 82.Furugen R., Hayashida H., Yoshii Y., Saito T. Neutrophil-derived resistin release induced by Aggregatibacter actinomycetemcomitans. FEMS Microbiol. Lett. 2011;321:175–182. doi: 10.1111/j.1574-6968.2011.02334.x. [DOI] [PubMed] [Google Scholar]
- 83.Claesson R., Johansson A., Belibasakis G., Hanstrom L., Kalfas S. Release and activation of matrix metalloproteinase 8 from human neutrophils triggered by the leukotoxin of Actinobacillus actinomycetemcomitans. J. Periodontal Res. 2002;37:353–359. doi: 10.1034/j.1600-0765.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 84.Taichman N.S., Dean R.T., Sanderson C.J. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect. Immun. 1980;28:258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsai C.C., McArthur W.P., Baehni P.C., Hammond B.F., Taichman N.S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect. Immun. 1979;25:427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hiyoshi T., Domon H., Maekawa T., Nagai K., Tamura H., Takahashi N., Yonezawa D., Miyoshi T., Yoshida A., Tabeta K., et al. Aggregatibacter actinomycetemcomitans induces detachment and death of human gingival epithelial cells and fibroblasts via elastase release following leukotoxin-dependent neutrophil lysis. Microbiol. Immunol. 2019;63:100–110. doi: 10.1111/1348-0421.12672. [DOI] [PubMed] [Google Scholar]
- 87.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science (New York N.Y.) 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 88.Urban C.F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., Brinkmann V., Jungblut P.R., Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jorch S.K., Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017;23:279–289. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 90.Hirschfeld J., Roberts H.M., Chapple I.L., Parcina M., Jepsen S., Johansson A., Claesson R. Effects of Aggregatibacter actinomycetemcomitans leukotoxin on neutrophil migration and extracellular trap formation. J. Oral Microbiol. 2016;8:33070. doi: 10.3402/jom.v8.33070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Konig M.F., Abusleme L., Reinholdt J., Palmer R.J., Teles R.P., Sampson K., Rosen A., Nigrovic P.A., Sokolove J., Giles J.T., et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016;8:369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McDonald B., Urrutia R., Yipp B.G., Jenne C.N., Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 93.Baehni P., Tsai C.C., McArthur W.P., Hammond B.F., Taichman N.S. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect. Immun. 1979;24:233–243. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katagiri K., Maeda A., Shimonaka M., Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 95.Shimonaka M., Katagiri K., Nakayama T., Fujita N., Tsuruo T., Yoshie O., Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J. Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang B., Sun H., Li W., Zhu C., Jian B., Hou W., Wang H., Yuan J., Yao B. Expression of Rap1 during germ cell development in the rat and its functional implications in 2-methoxyacetic acid-induced spermatocyte apoptosis. Urology. 2013;81:696.e1–698.e8. doi: 10.1016/j.urology.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 97.Liu G., Shi Z., Jiao S., Zhang Z., Wang W., Chen C., Hao Q., Zhang M., Feng M., Xu L., et al. Structure of MST2 SARAH domain provides insights into its interaction with RAPL. J. Struct. Biol. 2014;185:366–374. doi: 10.1016/j.jsb.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 98.DiFranco K.M., Johnson-Farley N., Bertino J.R., Elson D., Vega B.A., Belinka B.A., Jr., Kachlany S.C. LFA-1-targeting Leukotoxin (LtxA.; Leukothera(R)) causes lymphoma tumor regression in a humanized mouse model and requires caspase-8 and Fas to kill malignant lymphocytes. Leuk. Res. 2015;39:649–656. doi: 10.1016/j.leukres.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vega B.A., Schober L.T., Kim T., Belinka B.A., Jr., Kachlany S.C. Aggregatibacter actinomycetemcomitans Leukotoxin (LtxA) Requires Death Receptor Fas, in Addition to LFA-1, To Trigger Cell Death in T Lymphocytes. Infect. Immun. 2019;87:e00309-19. doi: 10.1128/IAI.00309-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamaguchi N., Kieba I.R., Korostoff J., Howard P.S., Shenker B.J., Lally E.T. Maintenance of oxidative phosphorylation protects cells from Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Cell. Microbiol. 2001;3:811–823. doi: 10.1046/j.1462-5822.2001.00161.x. [DOI] [PubMed] [Google Scholar]
- 101.Scaffidi C., Fulda S., Srinivasan A., Friesen C., Li F., Tomaselli K.J., Debatin K.M., Krammer P.H., Peter M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petit P.X., Lecoeur H., Zorn E., Dauguet C., Mignotte B., Gougeon M.L. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J. Cell Biol. 1995;130:157–167. doi: 10.1083/jcb.130.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Petit P.X., Goubern M., Diolez P., Susin S.A., Zamzami N., Kroemer G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: The impact of irreversible permeability transition. FEBS Lett. 1998;426:111–116. doi: 10.1016/S0014-5793(98)00318-4. [DOI] [PubMed] [Google Scholar]
- 104.Jankovic J. Botulinum toxin in clinical practice. J. Neurol. Neurosurg. Psychiatry. 2004;75:951–957. doi: 10.1136/jnnp.2003.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duvic M., Kuzel T.M., Olsen E.A., Martin A.G., Foss F.M., Kim Y.H., Heald P.W., Bacha P., Nichols J., Liepa A. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ONTAK) Clin. Lymphoma. 2002;2:222–228. doi: 10.3816/CLM.2002.n.003. [DOI] [PubMed] [Google Scholar]
- 106.Olsen E., Duvic M., Frankel A., Kim Y., Martin A., Vonderheid E., Jegasothy B., Wood G., Gordon M., Heald P., et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 107.Bechter O.E., Eisterer W., Dirnhofer S., Pall G., Kuhr T., Stauder R., Thaler J. Expression of LFA-1 identifies different prognostic subgroups in patients with advanced follicle center lymphoma (FCL) Leuk. Res. 1999;23:483–488. doi: 10.1016/S0145-2126(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 108.Desgrosellier J.S., Cheresh D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Inghirami G., Wieczorek R., Zhu B.Y., Silber R., Dalla-Favera R., Knowles D.M. Differential expression of LFA-1 molecules in non-Hodgkin’s lymphoma and lymphoid leukemia. Blood. 1988;72:1431–1434. [PubMed] [Google Scholar]
- 110.Horst E., Radaszkiewicz T., Hooftman-den Otter A., Pieters R., van Dongen J.J., Meijer C.J., Pals S.T. Expression of the leucocyte integrin LFA-1 (CD11a/CD18) and its ligand ICAM-1 (CD54) in lymphoid malignancies is related to lineage derivation and stage of differentiation but not to tumor grade. Leukemia. 1991;5:848–853. [PubMed] [Google Scholar]
- 111.de Boer O.J., Wakelkamp I.M., Pals S.T., Claessen N., Bos J.D., Das P.K. Increased expression of adhesion receptors in both lesional and non-lesional psoriatic skin. Arch. Dermatol. Res. 1994;286:304–311. doi: 10.1007/BF00402220. [DOI] [PubMed] [Google Scholar]
- 112.McGregor J.M., Barker J.N., Ross E.L., MacDonald D.M. Epidermal dendritic cells in psoriasis possess a phenotype associated with antigen presentation: In situ expression of beta 2-integrins. J. Am. Acad. Dermatol. 1992;27:383–388. doi: 10.1016/0190-9622(92)70203-R. [DOI] [PubMed] [Google Scholar]
- 113.Lantero S., Alessandri G., Spallarossa D., Scarso L., Rossi G.A. LFA-1 expression by blood eosinophils is increased in atopic asthmatic children and is involved in eosinophil locomotion. Eur. Respir. J. 1998;12:1094–1098. doi: 10.1183/09031936.98.12051094. [DOI] [PubMed] [Google Scholar]
- 114.Gupta A., Espinosa V., Galusha L.E., Rahimian V., Miro K.L., Rivera-Medina A., Kasinathan C., Capitle E., Aguila H.A., Kachlany S.C. Expression and targeting of lymphocyte function-associated antigen 1 (LFA-1) on white blood cells for treatment of allergic asthma. J. Leukoc. Biol. 2014;97:439–446. doi: 10.1189/jlb.5HI0414-196R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arthos J., Cicala C., Martinelli E., Macleod K., Van Ryk D., Wei D., Xiao Z., Veenstra T.D., Conrad T.P., Lempicki R.A., et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 116.Bastiani L., Laal S., Kim M., Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J. Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Capobianchi M.R., Fais S., Castilletti C., Gentile M., Ameglio F., Dianzani F. A simple and reliable method to detect cell membrane proteins on infectious human immunodeficiency virus type 1 particles. J. Infect. Dis. 1994;169:886–889. doi: 10.1093/infdis/169.4.886. [DOI] [PubMed] [Google Scholar]
- 118.Elston D.M., McCollough M.L., Bergfeld W.F., Liranzo M.O., Heibel M. Eosinophils in fibrous tracts and near hair bulbs: A helpful diagnostic feature of alopecia areata. J. Am. Acad. Dermatol. 1997;37:101–106. doi: 10.1016/S0190-9622(97)70219-6. [DOI] [PubMed] [Google Scholar]
- 119.Gilhar A., David M., Ullmann Y., Berkutski T., Kalish R.S. T-lymphocyte dependence of psoriatic pathology in human psoriatic skin grafted to SCID mice. J. Investig. Dermatol. 1997;109:283–288. doi: 10.1111/1523-1747.ep12335758. [DOI] [PubMed] [Google Scholar]
- 120.Ito T., Tokura Y. The role of cytokines and chemokines in the T-cell-mediated autoimmune process in alopecia areata. Exp. Dermatol. 2014;23:787–791. doi: 10.1111/exd.12489. [DOI] [PubMed] [Google Scholar]
- 121.Santos Z., Avci P., Hamblin M.R. Drug discovery for alopecia: Gone today, hair tomorrow. Expert Opin. Drug Discov. 2015;10:269–292. doi: 10.1517/17460441.2015.1009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wasserman D., Guzman-Sanchez D.A., Scott K., McMichael A. Alopecia areata. Int. J. Dermatol. 2007;46:121–131. doi: 10.1111/j.1365-4632.2007.03193.x. [DOI] [PubMed] [Google Scholar]
- 123.Pallis M., Robson D.K., Haskard D.O., Powell R.J. Distribution of cell adhesion molecules in skeletal muscle from patients with systemic lupus erythematosus. Ann. Rheum. Dis. 1993;52:667–671. doi: 10.1136/ard.52.9.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takeuchi T., Amano K., Sekine H., Koide J., Abe T. Upregulated expression and function of integrin adhesive receptors in systemic lupus erythematosus patients with vasculitis. J. Clin. Investig. 1993;92:3008–3016. doi: 10.1172/JCI116924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takasaki Y., Abe K., Tokano Y., Hashimoto H. The expression of LFA-1, ICAM-1, CD80 and CD86 molecules in lupus patients: Implication for immunotherapy. Intern. Med. (Tokyo Jpn.) 1999;38:175–177. doi: 10.2169/internalmedicine.38.175. [DOI] [PubMed] [Google Scholar]
- 126.Kaneko H., Tokano Y., Hashimoto H., Hirose S. The expression of lymphocyte function associated antigen-1, intercellular adhesion molecule-1 on peripheral blood lymphocytes in patients with systemic lupus erythematosus. Nihon Rinsho Men’eki Gakkai Kaishi Jpn. J. Clin. Immunol. 1996;19:60–68. doi: 10.2177/jsci.19.60. [DOI] [PubMed] [Google Scholar]
- 127.Elovaara I., Ukkonen M., Leppakynnas M., Lehtimaki T., Luomala M., Peltola J., Dastidar P. Adhesion molecules in multiple sclerosis: Relation to subtypes of disease and methylprednisolone therapy. Arch. Neurol. 2000;57:546–551. doi: 10.1001/archneur.57.4.546. [DOI] [PubMed] [Google Scholar]
- 128.Lou J., Chofflon M., Juillard C., Donati Y., Mili N., Siegrist C.A., Grau G.E. Brain microvascular endothelial cells and leukocytes derived from patients with multiple sclerosis exhibit increased adhesion capacity. Neuroreport. 1997;8:629–633. doi: 10.1097/00001756-199702100-00010. [DOI] [PubMed] [Google Scholar]
- 129.Bernstein C.N., Sargent M., Rector E. Alteration in expression of beta 2 integrins on lamina propria lymphocytes in ulcerative colitis and Crohn’s disease. Clin. Immunol. (Orlando Fla.) 2002;104:67–72. doi: 10.1006/clim.2002.5223. [DOI] [PubMed] [Google Scholar]
- 130.Vainer B., Nielsen O.H., Horn T. Comparative studies of the colonic in situ expression of intercellular adhesion molecules (ICAM-1, -2, and -3), beta2 integrins (LFA-1, Mac-1, and p150,95), and PECAM-1 in ulcerative colitis and Crohn’s disease. Am. J. Surg. Pathol. 2000;24:1115–1124. doi: 10.1097/00000478-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 131.Palmen M.J., Dijkstra C.D., van der Ende M.B., Pena A.S., van Rees E.P. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin. Exp. Immunol. 1995;101:351–356. doi: 10.1111/j.1365-2249.1995.tb08363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pavlick K.P., Ostanin D.V., Furr K.L., Laroux F.S., Brown C.M., Gray L., Kevil C.G., Grisham M.B. Role of T-cell-associated lymphocyte function-associated antigen-1 in the pathogenesis of experimental colitis. Int. Immunol. 2006;18:389–398. doi: 10.1093/intimm/dxh378. [DOI] [PubMed] [Google Scholar]
- 133.Takahashi M., Mimura Y., Hayashi Y. Role of the ICAM-1/LFA-1 pathway during the development of autoimmune dacryoadenitis in an animal model for Sjogren’s syndrome. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 1996;64:269–274. doi: 10.1159/000164058. [DOI] [PubMed] [Google Scholar]
- 134.Gupta A., Le A., Belinka B.A., Kachlany S.C. In vitro synergism between LFA-1 targeting leukotoxin (Leukothera) and standard chemotherapeutic agents in leukemia cells. Leuk. Res. 2011;35:1498–1505. doi: 10.1016/j.leukres.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 135.Nestle F.O., Kaplan D.H., Barker J. Psoriasis. N. Engl. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 136.Valdimarsson H., Thorleifsdottir R.H., Sigurdardottir S.L., Gudjonsson J.E., Johnston A. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494–501. doi: 10.1016/j.it.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 137.Kunz M. Current treatment of psoriasis with biologics. Curr. Drug Discov. Technol. 2009;6:231–240. doi: 10.2174/157016309789869092. [DOI] [PubMed] [Google Scholar]
- 138.Poulin Y., Langley R.G., Teixeira H.D., Martel M.J., Cheung S. Biologics in the treatment of psoriasis: Clinical and economic overview. J. Cutan. Med. Surg. 2009;13(Suppl. S2):S49–S57. doi: 10.2310/7750.2009.00021. [DOI] [PubMed] [Google Scholar]
- 139.Stenderup K., Rosada C., Dam T.N., Salerno E., Belinka B.A., Kachlany S.C. Resolution of psoriasis by a leukocyte-targeting bacterial protein in a humanized mouse model. J. Investig. Dermatol. 2011;131:2033–2039. doi: 10.1038/jid.2011.161. [DOI] [PubMed] [Google Scholar]
- 140.Wrone-Smith T., Nickoloff B.J. Dermal injection of immunocytes induces psoriasis. J. Clin. Investig. 1996;98:1878–1887. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chiu Y.G., Shao T., Feng C., Mensah K.A., Thullen M., Schwarz E.M., Ritchlin C.T. CD16 (FcRgammaIII) as a potential marker of osteoclast precursors in psoriatic arthritis. Arthritis Res. Ther. 2010;12:R14. doi: 10.1186/ar2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kudo M., Ishigatsubo Y., Aoki I. Pathology of asthma. Front. Microbiol. 2013;4:263. doi: 10.3389/fmicb.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rizzuto C.D., Sodroski J.G. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fortin J.F., Cantin R., Lamontagne G., Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jolly C., Mitar I., Sattentau Q.J. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J. Virol. 2007;81:13916–13921. doi: 10.1128/JVI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jolly C., Kashefi K., Hollinshead M., Sattentau Q.J. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hordinsky M.K. Overview of alopecia areata. J. Investig. Dermatol. Symp. Process. 2013;16:S13–S15. doi: 10.1038/jidsymp.2013.4. [DOI] [PubMed] [Google Scholar]
- 148.Kachlany S.C. Mechanisms of LtxA (Leukotoxin), a Potent New Anti-Inflammatory Agent for the Treatment of Alopecia Areata. J. Investig. Dermatol. Symp. Process. 2015;17:19–22. doi: 10.1038/jidsymp.2015.34. [DOI] [PubMed] [Google Scholar]
- 149.Kiriakidou M., Cotton D., Taichman D., Williams S. Systemic lupus erythematosus. Ann. Intern. Med. 2013;159:ITC4-1. doi: 10.7326/0003-4819-159-7-201310010-01004. [DOI] [PubMed] [Google Scholar]
- 150.Rahman A., Isenberg D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 151.Lorenz H.M., Grunke M., Hieronymus T., Herrmann M., Kuhnel A., Manger B., Kalden J.R. In vitro apoptosis and expression of apoptosis-related molecules in lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arthritis Rheum. 1997;40:306–317. doi: 10.1002/art.1780400216. [DOI] [PubMed] [Google Scholar]
- 152.Nakahara J., Maeda M., Aiso S., Suzuki N. Current concepts in multiple sclerosis: Autoimmunity versus oligodendrogliopathy. Clin. Rev. Allergy Immunol. 2012;42:26–34. doi: 10.1007/s12016-011-8287-6. [DOI] [PubMed] [Google Scholar]
- 153.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 154.Baumgart D.C., Carding S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet (Lond. Engl.) 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 155.Baumgart D.C., Sandborn W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet (Lond. Engl.) 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 156.Messmer E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 2015;112:71–81. doi: 10.3238/arztebl.2015.0071. quiz 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stern M.E., Schaumburg C.S., Dana R., Calonge M., Niederkorn J.Y., Pflugfelder S.C. Autoimmunity at the ocular surface: Pathogenesis and regulation. Mucosal Immunol. 2010;3:425–442. doi: 10.1038/mi.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stern M.E., Schaumburg C.S., Pflugfelder S.C. Dry eye as a mucosal autoimmune disease. Int. Rev. Immunol. 2013;32:19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Perez V.L., Pflugfelder S.C., Zhang S., Shojaei A., Haque R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul. Surf. 2016;14:207–215. doi: 10.1016/j.jtos.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 160.Pflugfelder S.C., Stern M., Zhang S., Shojaei A. LFA-1/ICAM-1 Interaction as a Therapeutic Target in Dry Eye Disease. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2017;33:5–12. doi: 10.1089/jop.2016.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hogg N., Laschinger M., Giles K., McDowall A. T-cell integrins: More than just sticking points. J. Cell Sci. 2003;116:4695–4705. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- 162.Smith A., Stanley P., Jones K., Svensson L., McDowall A., Hogg N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol. Rev. 2007;218:135–146. doi: 10.1111/j.1600-065X.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 163.Tauber J., Karpecki P., Latkany R., Luchs J., Martel J., Sall K., Raychaudhuri A., Smith V., Semba C.P. Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study. Ophthalmology. 2015;122:2423–2431. doi: 10.1016/j.ophtha.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 164.Murphy C.J., Bentley E., Miller P.E., McIntyre K., Leatherberry G., Dubielzig R., Giuliano E., Moore C.P., Phillips T.E., Smith P.B., et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Investig. Ophthalmol. Vis. Sci. 2011;52:3174–3180. doi: 10.1167/iovs.09-5078. [DOI] [PubMed] [Google Scholar]