Abstract
The recognition of discernible anatomical regularities that appear to self-organize during development makes apparent the modular organization of the cerebral cortex. The metabolic cost engendered in sustaining interneuronal communications has emphasized the viability of short connections among neighboring neurons. This pattern of connectivity establishes a microcircuit which is repeated in parallel throughout the cerebral cortex. This canonical circuit is contained within the smallest module of information processing of the cerebral cortex; one which Vernon Mountcastle called the minicolumn. Plasticity within the brain is accounted, in part, by the presence of weak linkages that allow minicolumns to process information from a variety of sources and to quickly adapt to environmental exigencies without a need for genetic change. Recent research suggests that interlaminar correlated firing between minicolumns during the decision phase of target selection provides for the emergence of some executive functions. Bottlenecks of information processing within this modular minicolumnar organization may account for a variety of mental disorders observed in neurodevelopmental conditions.
Keywords: cerebral cortex, minicolumns, system theory, module, connectivity
A canonical circuit links cells in modular arrangements throughout the cerebral cortex. The repetitive nature of this construct allows for a simplified design enabled by limited resources. Weak linkages between units provide for versatility in adapting to environmental exigencies.
INTRODUCTION
To some extent our understanding of the brain has been dependent on our ability to establish representational maps that communicate spatial information accurately and reproducibly. Early topographic localization schemes for the cerebral cortex were bottom-up approaches that clustered together microscopic units into larger regions based on characteristics of their somatic morphology, pigment distribution, pathological susceptibility, or myelin architecture. The successful use of defined anatomical characteristics and consequent uniformity of the different parcellation schemes across brains of a given species suggests the presence of discrete scalable modules (Diez et al., 2015; Fischi and Sereno, 2018). These units or modules lessen the evolutionary cost entailed in the creation of customized regions responsive to ever-changing environmental exigencies. In the end, the presence of homologous modules across different species attests to the evolutionary preference for standardization rather than customization, all at the expense of performance (Kuratani, 2009).
The Nobel laureate Francois Jacob once argued that evolution had “tinkered” with developmental biology. Evolution’s desultory approach explained why, despite the influence of natural selection, there were many homologies, as well as imperfections, across organisms (Jacob, 1977). Indeed, growth and differentiation across different organisms appears constrained by similar genes within the evo-devo gene toolkit. From this perspective, the brain is an agglomeration of structural improvisations piled one on top of another through millions of years of evolution with each layer providing for added complexity and a potential bottleneck for information processing (textbox 1) (Linden, 2008; Blazek et al., 2011). Simply stated, “It must be borne in mind that evolution is a tendentious, almost bureaucratic process. Once something novel has been invented, it’s generally retained and not thrown away. The new is built upon the old, but does not replace it” (Freitas, 2008). Some complex functions and behaviors are therefore novel outcomes of a system not originally intended for them. (Casanova and Tillquist, 2008). The imperfect disposition1 of brain parts is only counterbalanced by the presence and versatility of modules whose weak linkages provide for adaptive phenotypic variations (Kirschner an Gerhart, 2006).
Three Brains in One?
Sometime in the 1960’s, Paul MacLean formulated the idea of a triune brain (triune meaning three in one or constituting a trinity in unity). The idea served as a preamble to Francois Jacob’s conception of evolution as a tinkerer rather than an inventor (for a review see MacLean, 1990). This model argues for the chronological stratification of forebrain layers or complexes: the reptilian, paleomammalian (limbic system), and neomammalian. The reptilian complex, arising some 300 million years ago, was the name MacLean used for the basal ganglia, brainstem and cerebellum. MacLean believed that these structures were preeminent in reptiles and birds and helped regulate basic life support functions. A later period of expansion added the limbic system which provided for our emotions and motivational systems. This layer arose some 150-200 million years ago with the emergence of the first mammals. The neomammalian complex (neocortex) and attendant higher-order brain functions was the most recent addition, as it began developing some 30 million years ago.
Over the years, many elements of MacLean’s model have been revised and justly criticized. Among many criticisms two of them are incisive: the volumetric preeminence of the basal ganglia in sauropsids (reptiles and birds) is no longer upheld, and pallial homologues are found in the brains of non-mammals. The Triune model, although discredited in comparative anatomy, is presently used in the field of evolutionary psychology as a way of explaining human nature as the result of adaptations to our ancestral environments.
Weak linkages explain how different parts of a system are coupled so that changes in one of its modules or compartments do not seriously affect other parts of the system. In addition, weak linkages promote the combination of modules depending on prevailing environmental exigencies. The benefits entailed by having modules with weak linkages, in the midst of an otherwise inefficient system, would make them a conserved property.
The brain according to Kitano (2007), is a modular, weakly linked system that exhibits a clear tradeoff between robustness and fragility. It is therefore unsurprising that Brodal in his classic textbook “Neurological Anatomy in Relation to Clinical Medicine” made the observation that the nervous system is composed of a multitude of minor units, each with its particular structural organization, specific with respect to its finer intrinsic organization as well as with its connections with other units (Brodal, 1981). According to Szentàgothai, the basic reason for looking at the organization of the brain in terms of modules is that it offers a framework for the functional interpretation of structural data (Szentàgothai, 1975).
THE NEURON
The concept that the nervous system is made up of discrete cellular elements each recapitulating the holistic properties of the brain was a generalization of Matthias Schleiden and Theodor Schwann’s cell theory as applied to the nervous system. Within this framework, the presence of neurons as discrete cellular elements and units of information processing arose from the pioneering work of Santiago Ramón y Cajal. According to Cajal, neurons were polarized in such a way that impulses were conducted cellulipetally along dendrites and cellulifugally along axons (Bertucchi, 1999). In order to comply with the polarization of the neuron, each cell was compartmentalized both functionally and anatomically by a membrane possessing: 1) a receptive component for input, 2) an impulse-initiating component, and 3) a transmitter-releasing component (Brown, 2001).
The initial assessments of dynamic polarization were based solely on histological features and disregarded contemporaneous findings by Sherrington showing that neuronal conduction was reversible (Bertucchi, 1999). Indeed, exceptions to the “generalized neuron” have posed a frequent inconvenience to neuroscientists. Axons may bear a receptive surface and dendrites have voltage-gated ion channels that allow them to generate action potentials. Some interneurons and dopaminergic cells grow their axons from a dendrite while others may lack either dendrites or axons. Sometimes synapses connect a dendrite to another dendrite or an axon to another axon. Dendrites can transmit signals from the cell body and release via exocytosis a variety of neurotransmitters (Ludwig, et al., 2016). Furthermore, the role of the neuron as the sole unit of information processing has been questioned given the fact that glial networks provide a functional syncytium for electrical and chemical signaling (Froes et al., 1999). Neuronal differences have thus engendered a plethora of classifications for neurons based on their location, shape, size, released neurotransmitter(s), and function. In this regard, the plurality of neurons illustrates differences in kind rather than degree.
Neurons are dependent on the actions of other neurons. According to Brown (2001): “A reflex arc of just two neurons is an abstraction, useful for discussion, which does not exist in nature. Even in systems such as the monosynaptic myotatic reflex in mammals, in which there is one set of afferent and one set of efferent neurons, many neurons are involved.” Indeed, “No matter how complicated a single neuron may be, it cannot play a role in the processing of information without interacting with other neurons” (Shepherd and Koch, 1998). This interdependence of neurons is contrary to the definition of a module (vide infra) as a self-contained unit. Moreover, evidence from cell cultures suggests that this interdependence is crucial for neuronal differentiation.
Primary neuronal cultures can be manipulated to induce morphological changes characteristic of cell differentiation. A cell culture is initiated when a number of cells are seeded into a flask or plate. Dispersed postmitotic cells extend processes and synapse with one another. Once neurons differentiate they rarely divide. The survival of these cells and their maturation process (e.g., synapse formation, dendrite morphology) is heavily dependent on their seeding density (Biffi et al. 2013). In the case of neuronal cultures, the required seeding density varies between 80 and 300 cells/mm2 (Sellstrom et al., 1999).
Neuronal cultures recapitulate in vitro the phenomenon of neuroplasticity, especially during the time period encumbered by brain development. The long-term survival of neurons depends on their making contact with other neurons. Indeed, a mouse that lacks an essential protein for neurotransmitter release, has a wiring plan that looks normal. However, in the days following the stage for synapse formation there is massive cell loss (Verhage et al., 2000). Depriving neurons of their connections dooms any attempts at survival. This vital interdependence makes it difficult to provide an operational definition of a neuron as a module of information processing. In defining the term neuron as a phenomenon (i.e., information processing unit) it would be necessary to repeat the term (i.e., neuron) in the framework of the explanation. Such an argument lends itself to an infinite regress.
SYSTEMS AND MODULES
A system is a grouping of items arranged into a unified whole so as to perform a common goal. A modular design reduces the complexity of a system by subdividing the same into smaller parts. This organizational scheme provides for the integration of different tasks while simultaneously allowing for the functional independence of its parts. Modules self-organize into complex systems under a variety of factors rather than a predefined blueprint (Stam et al., 2010). In biological systems, self-organization allows for environmental and physical factors to play a role in molding the final product. After examining the cytoarchitectural organization of the cerebral cortex, Arbib and Erdi (2000) concluded that, “modular architectonics may be seen as a pattern resulting from the dynamics of self-organization rather than being completely laid down in the genome.”
In the cerebral cortex, connectivity helps define the scale and spatial boundaries of self-organizing modules (Batuev and Babmindra, 1993; Clune et al., 2013). Elements of a module are held in tightly interconnected groups or clusters (Mounier et al., 2010). The underlying organizational scheme follows the principle of an economy of wiring where neurons performing a particular function, and in need of communication with each other, do so more efficiently if they are held close together (Shipp, 2007). By way of contrast, connectivity between modules is reduced or looser (figure 1a,b) (Girvan and Newman, 2002). This property of modules is known as a community structure or clustering (Girvan and Newman, 2002). The identification of connectivity patterns indicative of modular assemblies provides a frame of reference that facilitates our understanding of the power, strength, redundancy, and scalability of a system.
Figure 1a,b. Multiple Subpial Transection (MST): A Disconnection Syndrome?
a. Frank Morrell, MD (1926-1977) was the A. Watson Armour III and Sarah Armour Presidential Professor of Neurological Sciences at Rush-Prebyterian-St. Luke’s Medical Center in Chicago. Taken from Tovar-Spinoza and Rutka (2009) Textbook of Stereotactic and Functional Neurosurgery, Springer.
b. Multiple Subpial Transections consists of linear and parallel cuts 5 mm apart across the region defined as the epileptogenic zone. Taken from Tovar-Spinoza and Rutka (2009) Textbook of Stereotactic and Functional Neurosurgery, Springer.
Biological systems often impose boundary conditions on its modules. These boundaries can be thought as constraints to guide a particular system down a path of operation. In the cerebral cortex, modules of information processing are arranged in a vertical disposition so as to transverse the cortex from white matter to pial surface. Early experiments by Sperry and associates (1955) showed that subpial slicing of the cat’s visual cortex did not impair the function (i.e., vision) of modules in the intervened area. Other experiments indicated that the less extensive horizontal, or between modules connectivity, was critical for the development of seizures (Smith, 1998). Morell hypothesized that subpial transection would therefore disrupt the spread of an ictal discharge without affecting the critical function performed by modules in that given area (Morrell and Hanbery, 1969; Morrell et al., 1999). Multiple subpial transections is now a routine surgical intervention for cases where resective techniques would lead to undesirable loss of function, e.g., preservation of language in Landau-Kleffner syndrome.
Circuits in which components maintain links with their immediate neighbors require shorter and fewer projections. In biological systems, selective pressures have clustered these connections in a small world network; a topology that optimizes connectedness while minimizing wiring costs (Sporns and Honey, 2006; Mounier et al., 2010). In this framework, those nodes that exhibit more frequent connections with each other serve as hubs of activity for the whole system (Sporns et al., 2007; Bullmore and Sporns, 2009). This modular arrangement and its defining pattern of connectivity, generalized throughout the cerebral cortex, results in an arrangement consistent with modeled network activity patterns (Muller-Linow et al., 2008; a critical review is provided by Hilgetag and Goulas, 2016).
The clustering of neuronal connections into specific patterns at multiple levels of organization is a defining feature of the mammalian brain (Callaway, 2002; Bassett and Bullmore, 2006; for a review see Buxhoeveden and Casanova, 2005a). Axonal studies have shown that connectivity in these clusters is primarily local (intracolumnar) in nature and may be established early during brain development (Douglas and Martin, 1998). Ventricular injections of enhanced green fluorescent protein (EGFP)-expressing retroviruses (labeling ontogenetic clones of neurons) combined with multiple electrode whole-cell recording (to study synapse formation) illustrate an increased propensity towards establishing connections among neighboring sister cells during corticogenesis (Yu et al., 2009). The transient electrical coupling between radially aligned sister excitatory neurons regulates the subsequent formation of specific chemical synapses in the neocortex and establishes the basic microcircuit of the cerebral cortex (Yu et al., 2009, 2012).
MINICOLUMNS AND MINICOLUMNOPATHIES
Neuroanatomists of the 19th century described the salience of vertically arranged anatomical elements that seemingly offered an organizational principle to the cerebral cortex. Theodor Meynert(1833-1892) described the presence of intracortical fibers forming pencil-like bundles or small vertical aggregates of corticofugal and corticopetal fibers (Santee, 1907). The introduction of the oxide of chromium as a mordant (method of Weigert) in 1882 allowed for a detailed analysis of Meynert’s bundles (Lorrain Smith et al., 1909). Codifying the length, number and caliber of these radiations provided a means for parcellating the cerebral cortex and thus helped establish the nascent field of myeloarchitectonics (Geyer and Turner, 2013).
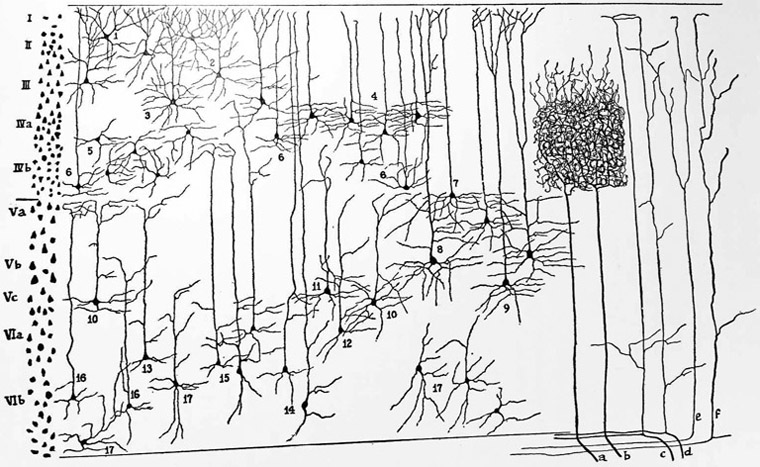
Comments on the vertical disposition of neuronal cell bodies permeated the early history of neuroscience. According to Santiago Ramón y Cajal (1852-1934), “In the vertical or radial direction, the cells are much less separated, often forming radial series that are evident in the second layer, being very accentuated from the fourth layer onward. At certain points, pyramids are so close that one could say that they are found in contact longitudinally” (Cajal, 1899). In many of these early cytoarchitectural studies, a two-dimensional microscopic examination of a gyrus often provided for slanted cuts and distortions in the disposition of cells. The end result was an effacement to any apparent columnarity. In an attempt to solve the mapmaker’s problem of flattening the curved surfaces of the brain (Schwartz et al., 1989), Constantin von Economo (1876-1931) and Georg Koskinas (1885-1975) dissected each gyrus and sulcus perpendicular to their axes. In their influential textbook and atlas on cytoarchitectonics, they devoted one chapter to the anatomical arrangement of cells which they called Radii for their vertical disposition (von Economo and Koskinas, 1925). Later on, Rafael Lorente de Nó (1902-1990) was the first person to propose these vertical arrangements as elementary operative units (figure 2) (Lorente de Nó, 1938). However, the first explicit statement mentioning a modular organization of the neuropil and its functional significance came from Scheibel and Scheibel (1958) when they discussed the architecture of the reticular formation as a series of disc-shaped modules stacked perpendicularly to the brainstem axis. A decade later, the same investigators proposed a similar organization for the intermediate region and ventral horn of the spinal cord (Scheibel and Scheibel, 1969; see also Szentàgothai and Réthelyi, 1973).
Figure 2. Lorente de Nó.
According to Lorente de Nó, “It is possible now to reach a comprehensive view of the organization of the cortex. The small strip reproduced on the left is the vertical section of a cylinder having a specific afferent fiber like a as axis. All of the elements of the cortex are represented in it, and therefore it may be called an elementary unit, in which, theoretically, the whole process of the transmission of impulses from the afferent fiber to the efferent axon may be accomplished” (Lorente de Nó, 1938; p. 290). Within the chains of neurons, de Nó differentiated between short and long links depending on whether they connected cells of the same or different layers. The long links varied little in different mammals, but the short links increased progressively in number from mouse to man. Cajal, who was de Nó’s mentor, thought that short links were the “anatomical expression of the delicacy of function of the brain of man” (Lorente de Nó, 1938; p. 294). Figure reproduced from Lorente de Nó (1938), Physiology of the Nervous System, Oxford University Press.
Over the years different cellular features have been used to study the vertical organization of the cortex, their correspondence to each other, and to help define the scale and spatial boundaries of minicolumns. These anatomical features include: apical dendritic and myelinated axon bundles, pyramidal cells, and the translaminar axon bundles of double bouquet cells (DeFelipe, 2005; Innocenti and Vercelli, 2010). The existence of these radial elements makes the minicolumn a useful anatomical element for comparing cortical homologies across species (Buxhoeveden and Casanova, 2005a) (textbox 2).
Minicolumnar Variability.
Many aspects of minicolumnar morphometry and their intrinsic composition vary between species (Casanova, 2008; Spocter et al., 2015). Dendritic bundles are found in mice, rats, cats, rabbits and primates but not in turtles (Innocenti and Vercelli, 2010). Double bouquet cells are present in primates but not in rodents, lagomorphs, or artiodactyls (Yáñez et al., 2005). These anatomical differences are to be considered when extrapolating findings from animal models to human research.
The minicolumn is a vertical aggregate of cells spanning laminae II-VI of the neocortex (Buxhoeveden and Casanova, 2002a, 2002b). Its core cells are derived from the germinal matrix surrounding the ventricles. The germinal matrix provides a nest for stem cells that is divided into columnar arrangements by glial septa. Early on, these cellular columns are comprised of 3 to 5 cells but later expand to as many as 12. The germinal matrix begins dividing before the 6th week of gestation (Rakic and Kornack, 2001). The initial divisions are symmetrical, resulting in copies of each other, and preventing the original pool of cells from being depleted. The wave of symmetrical divisions is followed by another one of asymmetric divisions wherein two daughter cells acquire different fates: one being a copy of the original stem cell and another one following a restricted path of differentiation into either neurons, astrocytes or oligodendrocytes (Casanova and Trippe, 2006; Casanova et al., 2009). Daughter cells then pursue a tandem gliophilic migration into the cerebral plate where neuroblasts acquire a radial inside-out configuration. In this fashion, each neuroblast migrates past earlier arrivals and detach themselves from their radial scaffold in successively more superficial locations. The close apposition of these cells early during brain development gives rise to transitory gap junctions, the possibility of Hebbian learning, and the formation of an ontogenetic cortical microcircuit (Peinado et al., 1993a; 1993b) (vide supra, “transient electrical coupling between radially aligned sister excitatory neurons”).
Although the minicolumn is a modular structure, the term itself does not imply uniformity in terms of cellular composition or morphology (for a review see Casanova, 2008; Spocter et al., 2015). Variability in minicolumnar width is evident throughout the cortex. The core compartment of the minicolumn, comprised of pyramidal cells and their projections, appears to be largely conserved across certain species (e.g., humans, chimpanzees, macaques) (Buxhoeveden and Casanova, 2005b; Casanova et al., 2009). These pyramidal cells are morphological stable and tethered to the first lamina by their terminal dendrites. Minicolumnar variability, prominent in humans as compared to other species, is primarily evident in the peripheral compartment of minicolumns (Casanova et al., 2009). Differences in minicolumnar morphology among species may be directly related to various interneuron subtypes located within the peripheral space of minicolumns (Casanova et al., 2009b; Raghanti et al., 2010; Spocter et al., 2015). In contrast to pyramidal cells, interneurons lay free of attachments to the first lamina and are capable of modifying their distribution as well as their dendritic and axonal profiles in response to cortical activity (Marin-Padilla, 2011). The disposition of interneurons so as to embrace the core compartment of the minicolumn has been depicted as a shower curtain of inhibition (Szentàgothai, 1975; Szentàgothai and Arbib, 1975; Mountcastle, 1998).
Neuronal migration has been categorized into either radial or tangential depending on the axis that the migratory cells acquire in regards to the pial surface of the neural tube as they make their way to the cortical plate. Neuroblasts from the ventricular zone generally migrate radially to the cortical plate where they will mature into pyramidal cells. Interneurons, the majority from the ganglionic eminences, follow a tangentially oriented path to enter the cortex. However, radial and tangential migration are not exclusionary processes. Some neuroblasts switch between radial and tangential migration, change the shape of their somas, and often make pit stops on their way to the cortex (Hatanaka et al., 2016).
Inhibitory neurons make their initial presence in the lower cortical layers and follow an ascending maturation along pyramidal cell strata with whom they will establish functional contacts (Marin-Padilla, 2011). The distance travelled by the tangentially migrating interneurons is much longer than that of the radially migrating pyramidal neurons. In this way, tangential migration allows future interneurons to achieve destinations far removed from their site of generation. The confluence of the tangential and radial migratory streams results in a series of distinct excitatory-inhibitory functional systems characterized by dyads of pyramidal cells and subtypes of interneurons (e.g., pyramidal-basket cell, pyramidal-double bouquet cells) (Marin-Padilla, 2011; Wong et al., 2018).
The finely choreographed maneuvering between excitatory and inhibitory neurons during corticogenesis is disturbed in some neurodevelopmental conditions. Dissonance between the radial and tangential migration is a diagnostic feature of focal cortical dysplasias; itself, the most common cause of medically refractory seizures in the pediatric population (Blumcke et al., 2011). Dysplasias are abnormalities of growth and differentiation. It is therefore unsurprising that minicolumnopathies serve as a signpost of neuronal migrational disorders (especially in the presence of coexisting heterotopias, accumulation of neurons within the gray/white matter junction, and/or increased cellularity within the molecular layer). It is important to note that minicolumnar abnormalities may occur independently of variations in laminar architecture (Manger et al., 2015). These disorders, or minicolumnopathies, are characterized by shared clinical features (Table 1) and an abnormal developmental trajectory established during gestation.
Table 1.
Defining Characteristics of Minicolumnopathies (Casanova, 2005)
| Comorbidities |
| Gender differences |
| Complex genetic and epigenetic influences |
| Language impairment |
| Alterations of cerebral dominance |
| Frontal lobe syndrome |
| Abnormalities on EEG, especially its high (gamma) frequency bandwidth |
| Change in prevalence through different generations |
The presence of minicolumnar abnormalities in autism is suggestive of a neuronal migration disorder. The findings have proven to be of significance for their explanatory and predictive powers (e.g., changes in the gray white matter ratio, gamma frequencies, excitatory/inhibitory bias) as well as having given rise to a potential therapy aimed at core pathological features of the condition (i.e., transcranial magnetic stimulation) (Sokhadze et al., 2009; Casanova 2014a, 2014b; Casanova et al., 2015). Other neurodevelopmental disorders that share a minicolumnar pathology include dyslexia, Rett syndrome, Down syndrome, and schizophrenia (Buxhoeveden et al., 2002; Casanova, 2007; Casanova et al., 2002, 2003).
Neurodevelopmental disorders, characterized by minicolumnpathies, are linked to evolutionary principles and constraints. Encephalization has proceeded, in part, through changes in the regulation of cortical neurogenesis (Nomura, et al., 2013; Hofman, 2014). Many neurodevelopmental conditions may be complex traits as a consequence of encephalization (Casanova and Tillquist,2004, 2008). Large brains may facilitate the accumulation of genetic variation (Lipp and Wolfer, 2003), the latter a mandatory step for rapid evolution to occur (Lipp, 1989). Novel changes introduced into a receptive brain (e.g., a large brain with modular arrangements and weak linkages) may be easily appropriated by natural selection. This may help explain why cognitive impairment is more commonly seen in conditions related to smaller rather than larger brains (e.g., microcephaly vs. macrocephaly). This model also suggests the accumulation of selectively neutral or slightly deleterious genetic variations in the general population (Lipp and Wolfer, 2003). In the end, encephalization in our species has been made possible through underlying changes in our connectome. Indeed, the amount of white matter accrued with encephalization has increased disproportionally relative to brain size and neocortical volume (Hofman, 2001). As we approach an asymptote to our cortical expansion, pathology related to the complexity of our brains may likely be expressed as a multifactorial organ failure (Ringo, 1991; Hofman, 2001, 2014).
GENETICS AND EVOLUTION OF THE PRIMATE MINICOLUMN
The primate lineage has experienced a rapid evolution at the anatomic and genetic levels. At the anatomical level, body size has ranged dramatically over the last 75 million years: from the first squirrel-sized proto-primates in the early Paleocene to the extinct ape, Gigantopithecus, that existed throughout much of the Pleistocene epoch, weighing over half a ton and standing 9 feet tall (Steiper and Young, 2006). Similar to the dramatic range in body size, relative encephalization has also varied dramatically across extant primates. This suggests that allometric relationships between brain size and body mass have been relaxed in this lineage compared to other mammals (Boddy et al., 2012). Encephalization, largely a result of neocortical enlargement, is primarily due to the expansion of the radial glia population and a consequent increase in numbers of minicolumns (Casanova et al., 2009). Increases in encephalization are especially prominent in the hominin line, beginning with the human ancestor, Homo erectus (Rightmire, 2004).
Although the exome has remained relatively stable, primates have experienced rapid evolution of the regulome. This has been in part due to the insertion and retention of primate-specific Alu retrotransposons, which have been preferentially retained within gene-rich regions of the genome (Kazazaian, 2011). Alu’s have been exapted for the purpose of:
Splice sites, polyadenylation sites, enhancers, and other transcriptional regulators (Häsler and Strub, 2006; Su et al., 2014).
Circular RNA (circRNA), which function as “microRNA sponges” (Qu et al., 2015).
Nuclear hormone response elements (HRE) for the hormones: estrogen, thyroid, and retinoic acid (Babich et al., 1999)
Exonization, forming the majority of human-specific exons (Zhang and Chasin, 2006).
Alu’s, along with other transposable elements, are enriched within the introns of autism risk genes, suggesting they are prime targets for mutation events relevant to the condition (Casanova et al., 2017; Turner et al., 2016). They are also enriched at the breakpoints of copy number variations (CNV) and therefore may be partly responsible for the events that underlie the human-specific gain/loss of enhancers in certain sets of CNS genes as compared to chimpanzee (Sen et al., 2006; McLean et al., 2010; Gu et al., 2016). Interestingly, CNV events are also overrepresented in both autism and schizophrenia, suggesting Alu-derived instability may promote some of these occurrences (Sebat et al., 2007; International Schizophrenia Consortium, 2008).
Most importantly, Alu’s are enriched and well-conserved within introns of CNS and developmental regulatory (DevReg) genes, suggesting they have played integral roles in primate-specific encephalization and minicolumnar expansion (although evidence is currently circumstantial and in need of further research) (Lippman et al., 2004; Polak and Domany, 2006; Casanova et al., 2017). Some of the CNS and many of the DevReg genes in question are expressed within the brain during symmetric division of radial glia, which implies that evolutionary changes to the forces that regulate their expression likewise alter radial glial number and thus minicolumn expansion and overall brain size (Buxhoeveden and Casanova, 2005b; Medina et al., 2005; Richardson et al., 2014; Pollen et al., 2015; Spocter et al., 2015). Alu elements are therefore prime candidates for effecting genetic changes within a relatively short period of time, in contrast to the more constant rate of point mutations that are the basis of the “molecular clock” (Shankar et al., 2004; Oei et al., 2004; Drake et al., 1998).
Although primates are not entirely unique as to the extent of their encephalization, as a clade they have experienced dramatic changes, since the dawn of th Paleocene, within a relatively short period of time. This suggests not only the influence of natural selection but a series of genetic events (in this case, the Alu repeats) that supply a convenient means for such evolution via the addition of extensive regulatory material (Oliver and Greene, 2011). The fact that these retrotransposons are primate specific, were once highly proliferative, and remain strongly conserved across species (particularly within CNS, DevReg, and autism risk genes) is a testament to their potential importance in primate brain evolution and minicolumnar expansion.
FUNCTIONALITY OF THE MINICOLUMN
The physiological basis of the minicolumns has been established by multiple techniques including microelectrodes, 2DG metabolic labelling, evoked potentials, nerve regeneration and two-photon imaging of calcium fluxes (Mountcastle, 1957, 1978, 1997; Kaas et al., 1981; Favorov and Whitsel, 1988a, b; Favorov and Diamond, 1990; Lee and Whitsel, 1992; Shamma et al., 1993; Tommerdahl et al., 1993; Favorov and Kelly, 1994a, b; Sugimoto et al., 1997; Ohki et al., 2005). Using recording electrodes to impale cortical neurons Mountcastle was the first person to investigate the functionality of vertically arranged cellular assemblies within the somatosensory cortices of cats and monkeys (Macaca mulatta) (Mountcastle, 1957; Powell and Mountcastle, 1959) (Table 2). This organization was confirmed in experiments using slanted penetrations which recorded changes in modality as the microlectrodes transversed different layers within adjoining regions (Mountcastle, 1957, 1998).
Table 2.
Mountcastle’s Instrinsic Function of Cortical Microcircuits (Mountcastle, 1998, p. 287)
| Thresholding: a nonlinear relation between the level of presynaptic input and cortical neuronal discharge. |
| Amplification of inputs: as in the example of when a single impulse in a single myelinated fiber of a peripheral nerve in an attending human suffices to evoke a conscious perception. |
| Derivative function: cortical operations tend to accentuate and amplify transient inputs, adapt to constant ones. |
| Feature convergence: the creation of a neural representation of a complex feature or set of features by combining signals of two or more simpler ones. |
| The distribution function: some areas receive the neural signals of certain simpler features of sensory stimuli and distributed then separately to other cortical areas. Areas 3b and V1 in addition to having other functions, serve as distribution centers. |
| Coincidence detection: by convergence of excitation, linking together two events that occur closely in time. |
| Synchronization and coherence of activity in the different nodes of distributed system. |
| Pattern generation: creation of spatial and temporal patterns in output signals that are not present in inputs (e.g., induced rhythms). |
Receptive field mapping studies have strongly supported Mountcastle’s original findings. These studies have found that receptive fields of neurons within a narrow space (approximately 50 microns in diameter) are similar in regards to shape, size and position on the skin. These receptive fields vary significantly from those of neurons located in adjacent minicolumns (note: on average receptive fields of adjacent minicolumns overlap by 22% in cat and 28% in macaque monkey) (Favorov and Whitsel, 1988a,b; Favorov and Diamond, 1990). Similar results have been reported for the primary auditory and visual cortex of cats and monkeys (Abeles and Goldstein, 1970; Hubel and Wiesel, 1974).
In recent years the use of conformal multielectrode recording arrays have characterized the role of interlaminar circuitry in minicolumns of the prefrontal cortex during task-related target selection in nonhuman primates (Opris et al., 2012). Activation of minicolumns with the encoded interlaminar correlated firing sequences resulted in enhanced performance on tasks requiring specific information according to context (Opris et al., 2013). These experiments demonstrate the role of minicolumnar processing during an executive function tasks and suggest future applications in the field of cognitive prosthetics.
It is unsurprising that the minicolumn is an evolutionary improvisation that has been conserved in all examined species of the mammalian clade as well distantly related species such as mice, rats, rabbits and cats (using pyramidal cell arrays for homologous brain regions, see Buxhoeveden and Casanova, 2005b; Spocter et al., 2015). The implementation of a canonical circuit in parallel arrays throughout the neocortex (figure 3) allows for brain development to occur within a manageable time frame. Otherwise, improvising different circuits in disparate brain regions would have taxed our genetic blueprint abilities and amplified possible mistakes when connecting individual elements. In addition, the modular architecture and weak linkages of minicolumns provides for cross-modal plasticity and the ability of the brain to adaptively reorganize itself (textbox 3); as an example, sensory cortices of congenitally deprived individuals are functionally reallocated to process information from remaining senses. A person who is born deaf has his/her auditory cortex coopted to process visual information. The expanded visual cortex, in this case, confers the individual with superior peripheral vision and motion detection (Bola et al., 2016). The attendant plasticity conferred by weak linkages helps explain the genesis of synesthesias, splinter skills and savant abilities in some minicolumnopathies.
Figure 3.
A canonical circuit for the neocortex: Thalamic relay cells mainly from synapse in the middle layers of the cortex, but they also form synapses with neurons in all six cortical layers, including the tufts of pyramidal cells in layer 1. In all layers the excitatory (red) and inhibitory (blue) neurons form recurrent connections with like cells within the same layer (dashed lines) and with other cell types (continuous lines). Layer 4 in some primary sensory cortical areas contain a specialist excitatory cell type, the spiny stellate cell (A), which projects to pyramidal cells and inhibitory cells in layer 4 and other layers. The superficial layer pyramidal cells (B) connect locally and project to other areas of cortex. Inhibitory neurons (C) are found in all layers (only one representative is shown here), and they constitute about 15% of the neurons in the neocortex. The deep layer pyramidal cells (D) also connect recurrently locally and project to subcortical nuclei in the thalamus, midbrain, and spinal cord. Figure taken from Douglas and Martin, 2010. Handbook of Brain Microcircuits, Oxford University Press, ch. 2, p.16.
The Cerebral Cortex as an Analog Computational Device.
In the 1970s, Otto Creutzfeldt proposed that minicolumns worked through the implementation of a common algorithm that was modified by different patterns of transcortical, subcortical, and local connections to suit the specific requirements of each neocortical area (Creutzfeldt, 1977). The analogy was made to an electrical outlet where wiring connections to and from a power plant are standardized in order to produce a predetermined voltage and frequency. The versatility of the electrical outlet resides in its ability to power different devices, e.g. TV, radio, microwave oven. In the cortex the versatility of the minicolumn is engendered by weak linkages. The ability of the minicolumn to process inputs from different parts of the nervous system allows a genetically determined blueprint of parallel circuits to be responsive to environmental exigencies.
We would like to conclude by acknowledging the legacy of our friend and advisor Vernon Mountcastle (1918-2015). His early studies in the 1950’s demonstrated the columnar organization of the cortex. Later on, in the 1960’s and 1970’s, Mountcastle coordinated the implementation of neurophysiological and psychophysical recordings in the same subjects. The combined approach made possible the understanding of information encoding and processing in single cells. His rigorous study designs, quantitating stimulus-response relationships, set the standard for subsequent research in behavioral neurophysiology that continues till present. The anatomy and physiology of minicolumns now forms a foundation to our knowledge in the neurosciences. In the last few decades, minicolumns have come to be used as defining criteria in neuropathology, have led to the implementation of clinical trials in neurodevelopmental disorders, and have given us an inkling as to the mechanistic emergence of higher cognitive functions.
ACKNOWLEDGMENT
The authors would like to recognize the efforts of Veronica Martinez Cerdeño and Stephen Noctor in organizing the meeting on Cortical Evolution 2018 during which this work was presented.
Footnotes
Throughout evolutionary history more recent levels of anatomical complexity have not resulted from a redesign of the whole brain. This has provided for the preservation of brain regions that are anatomical and functional remnants of our evolutionary past. As an example, the midbrain visual center automatically provides information about objects in our surrounding without the same reaching conscious (cortical) awareness (i.e., blindsight abilities). Higher layers of anatomical complexity have thus been added to earlier layers without either establishing connections between them nor integrating their functions (Linden, 2007).
Contributor Information
Manuel F. Casanova, SmartState Endowed Chair in Childhood Neurotherapeutics, University of South Carolina School of Medicine Greenville, Greenville Health System.
Emily L. Casanova, University of South Carolina School of Medicine Greenville, Greenville Health System.
REFERENCES
- Abeles M, Goldstein MH Jr. Functional architecture in cat primary auditory cortex: columnar organization and organization according to depth. J Neurophysiology 33:172–187, 1970. [DOI] [PubMed] [Google Scholar]
- Arbib MA, Erdi P. Precis of neural organization: structure, function, and dynamics. Behav Brain Sci 23:513–571, 2000. [DOI] [PubMed] [Google Scholar]
- Babich V, Aksenov N, Alexeenko V, Oei SL, Buchlow G, Tomilin N. Association of some potential hormone response elements in human genes with the Alu family repeats. Gene 239:341–349, 1999. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist 12:512–523, 2006. [DOI] [PubMed] [Google Scholar]
- Batuev AS, Babmindra VP. Modular organization of the cerebral cortex. Biofizika [PubMed] [Google Scholar]
- Bertucchi G Some aspects of the history of the law of dynamic polarization of the neuron. From William James to Sherington, from Cajal and van Gehuchten to Golgi. J Hist Neurosci 8(2): 191–201, 1999. [DOI] [PubMed] [Google Scholar]
- Biffi E, Regalia G, Menegon A, Ferrigno G, Pedrocchi A. The influence of neuronal density and maturation on network activity of hippocampal cell cultures: a methodological study. PLOS One. Published December 27, 2013. 10.1371/journal.pone.0083899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazek V, Bruzek J, Casanova MF. Plausible mechanisms for brain structural and size changes in human evolution. Coll Antropol 35(3):949–55, 2011. [PubMed] [Google Scholar]
- Blumcke I, Thom M, Aronica E, et al. The clinic-pathological spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the Diagnostic Methods Commission. Epilepsia 52(1):158–174, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy AM, McGowen MR, Sherwood CC, Grossman LI, Goodman M, Wildman DE. Comparative analysis of encephalization in mammals reveals relaxed constraints on anthropoid primate and cetacean brain scaling. J Evol Biol 25:981–994, 2012. [DOI] [PubMed] [Google Scholar]
- Brodal A, Neurological Anatomy in Relation for Clinical Medicine. Oxford University press, Oxford, UK, 3rd ed, 1981. [Google Scholar]
- Brown AG. Nerve Cells and Nervous Systems: n Introduction to Neuroscience 2nd ed., London, UK, Springer, 2001. [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198, 2009. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden D, Casanova MF. The minicolumns hypothesis in neuroscience. Brain 125:935–951, 2002a. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden D, Casanova MF. The minicolumns and evolution of the brain: a review. Brain Behav Evol 60:125–151, 2002b. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden D, Fobbs A, Roy E, Casanova M. Quantitative comparison of radial cell columns in children with Down’s syndrome and controls. J Intellect Disabil Res 46(Pt 1): 76–81, 2002. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Casanova MF. The cell column in comparative anatomy Nova Science Publishers, Inc, New York, ch. 5, pp. 93–116, 2005. [Google Scholar]
- Buxhoeveden DP, Casanova MF. Encephalization, minicolumns, and hominid evolution Nova Science Publishers, Inc, New York, ch 6, pp. 117–136, 2005. [Google Scholar]
- Cajal SR. Estudios sobre la corteza humana. II Estructura de la corteza motriz del hombre y mamiferos superiors. Revista Trimestral Micrográfica 4:117–200, 1899. [Google Scholar]
- Callaway EM. Cell type specificity of local cortical connections. J Neurocytol 31(3-5):231–237, 2002. [DOI] [PubMed] [Google Scholar]
- Calvin WH. How Brains Think: Evolving Intelligence, Then and Now. Basic Books, New York, New York, 1996. [Google Scholar]
- Calvin WH. How Brains Think: Evolving Intelligence, Then and Now. Basic Books, 15th ed, 1997. [Google Scholar]
- Casanova EL, Switala AE, Casanova MF. The feature landscape of autism risk genes indicates their enrichment in developmental regulation. International Meeting for Autism Research (IMFAR), San Francisco, California, May 11, 2017. [Google Scholar]
- Casanova MF. An apologia for a paradigm shift in neurosciences In Casanova Manuel (ed.) Neocortical Modularity and the Cell Minicolumn. NOVA Scientific Publishers, Inc: New York, ch. III, pp. 33–56, 2005. [Google Scholar]
- Casanova MF. Schizophrenia seen as a deficit in the modulation of cortical minicolumns by monoaminergic systems. Int Rev Psychiatry 19(4):361–72, 2007. [DOI] [PubMed] [Google Scholar]
- Casanova MF. The significance of minicolumnar size variability in autism: a perspective from comparative anatomy In Zimmerman A (ed.) Autism Current Theories and Evidence, Current Clinical Neurology, The Human Press, Inc., ch. 16, pp. 349–360, 2008. [Google Scholar]
- Casanova MF. Autism as a sequence: from heterochronic germinal cell divisions to abnormalities of cell migration and cortical dysplasias. Med Hypothesis 83(1):32–8, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF. The neuropathology of autism In Volkmar Fred, Pelphrey Kevin, Paul Rhea, Rogers Sally (eds.). Handbook of Autism and Pervasive Developmental Disorders 4th edition, ch. 21 Pp. 497–531, 2014b. [Google Scholar]
- Casanova MF, Buxhoeveden DP, Cohen M, Switala AE, Roy EL. Minicolumnar pathology in dyslexia. Ann Neurol 52(1):108–10, 2002. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. J Child Neurol 17(9):692–5, 2002. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Switala A, Roy E. Rett syndrome as a minicolumnopathy. Clin Neuropathol 22(4):163–8, 2003. [PubMed] [Google Scholar]
- Casanova MF, Tillquist CL. Genetic complexity, evolution, and autism. Pediatrics 113(5): 2004. http://pediatrics.aappublications.org/content/113/5/e472.comments [Google Scholar]
- Casanova MF, Tillquist CR. Encephalization, emergent properties and psychiatry: a minicolumnar perspective. Neuroscientist 14(1):101–118. 2008. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Trippe J. Regulatory mechanisms of cortical laminar development. Brain Res Rev 51:72–84, 2006. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Vanbogaert E, Narahari P, Trippe J. Minicolumnar width: comparison between supragranular and infragranular layers. J Neurosci Methods 184:19–24, 2009. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Trippe II, Tillquist C, Switala AE. Morphometric variability of minicolumns in the striate cortex of Homo sapiens, Mcacaca mulatta, and Pan troglodytes. J Anat 214:226–234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Sokhadze E, Opris I, Wang Y, Lo X. Autism spectrum disorders: linking neuropathological findings to treatment with transcranial magnetic stimulation. Acta Paediatrica 104(4):346–55, 2015. [DOI] [PubMed] [Google Scholar]
- Clune J, Mouret J-B, Lipson H. The evolutionary origins of modularity. Proc Biol Sci 280(1755):20122863, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J Reflections on the structure of the cortical minicolumns In Casanova MF (ed.) Neocortical Modularity and the Cell Minicolumn. Nova Science Publisher, ch. 4, pp. 57–92, 2005. [Google Scholar]
- Diez I, Bonifazi P, Escudero I, Mateos B, Muñoz MA, Stramaglia S, Cortex JM. A novel brain partition highlights the modular skeleton shared by structure and function. Sci Rep 5:10532, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KAC. Neocortex In Shepherd GM (ed.) The Synaptic Organization of the Brain 4th ed., Oxford University Press, New York, pp. 459–509, 1998. [Google Scholar]
- Douglas RJ, Martin KAC. Neuronal circuits of the neocortex. Ann Rev Neurosci 27:419–451, 2004. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KAC. Canonical cortical circuits In Shepherd GM and Grillner S (eds) Handbook of Brain Microcircuits, Oxford University Press, New York, p. 15–21, 2010. [Google Scholar]
- Drake JW, Charlesworth B, Charlesworth D, Crow JF. Rates of spontaneous mutation. Genetics 148: 1667–1686, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorov OV, Whitsel BL. Spatial organization of the peripheral input to area 1 cell columns. I. The detection of ‘segregates’. Brain Res 1988; 472: 25–42. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Whitsel BL. Spatial organization of the peripheral input to area 1 cell columns. II. The forelimb representation achieved by a mosaic of segregates. Brain Res 1988; 472: 43–56. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Diamond ME. Demonstration of discrete place—defined columns-segregates-in the cat SI. J Comp Neurol 1990; 298: 97–112. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Kelly DG. Minicolumnar organization within somatosensory cortical segregates. I. Development of afferent connections. Cereb Cortex 1994; 4: 408–27. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Kelly DG. Minicolumnar organization within somatosensory cortical segregates. II. Emergent functional properties. Cereb Cortex 1994; 4: 428–42. [DOI] [PubMed] [Google Scholar]
- Fischi B, Sereno MI. Microstructural parcellation of the human brain. Neuroimage. 2018. February 26 pii: S1053-8119(18)30036–3. doi: 10.1016/j.neuroimage.2018.01.036. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- RA Freitas Jr Xenology: An introduction to the scientific study of extraterrestrial life, intelligence, and civilization First edition, Xenology Research Institute, Sacramento, CA, 2008. http://www.xenology.info/Xeno.htm [Google Scholar]
- Froes MM, Correia HP, Garcia-Abreu J, Spray DC, Campos de Carvalho AC, Moura Neto V. Gap-junction coupling between neurons and astrocytes in primary central nervous system cultures. PNAS (13):7541–7547, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S, Turner R. Microstructural parcellation of the human cerebral cortex Springer, New York, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvan M, Newman ME. Community structure in social and biological networks. Proc Natl Acad Sci USA 99(12):7821–6, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Jin K, Crabbe MJC, Zhang Y, Liu X, Huang Y, et al. Enrichment analysis of Alu elements with different spatial chromatin proximity in the human genome. Protein Cell 7: 250–266, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häsler J, Strub K. Alu elements as regulators of gene expression. Nucleic Acids Res 34:5491–5497, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y, Zhu Y, Torigoe M, Kita Y, Murakami F. From migration to settlement: the pathways, migration modes and dynamics of neurons in the developing brain. Proc Jpn Acad B Phys Biol Sci 92(1):1–19, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag CC, Goulas A. Is the brain really a small-world network? Brain Struct Funct 221:2361–2366, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA. Brain evolution in hominds: are we at the end of the road? In Falk Dean and Gibson Kathleen (eds.) Evolutionary Anatomy of the primate cerebral cortex. Cambridge University Press, New York, ch.6, pp. 113–127, 2001. [Google Scholar]
- Hofman MA. Evolution of the brain: when bigger is better. Front Neuroanat 8:15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Computational Neurology 158:267–294, 1974. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Vercelli A. Dendritic bundles, minicolumns, columns and cortical output units. Front Neuroanat 4:11, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 455:237, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F Evolution and Tinkering. Science 196:1161–1166, 1977. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Nelson RJ, Sur M, Merzenich MM. Organization of somatosensory cortex in primates In: Schmitt FO, Worden FG, Adelman G, Dennis SG, editors. The organization of the cerebral cortex. Cambridge (MA: ): MIT Press; 1981. p. 237–61. [Google Scholar]
- Kazazaian HH Jr. Mobile DNA: Finding Treasure in Junk. FT Press: New Jersey, 2011. [Google Scholar]
- Kim DD, Kim TT, Walsh T, Kobayashi Y, Matise TC, Buyske S, et al. Widespread RNA editing of embedded Alu elements in the human transcriptome. Genome Res 14:1719–1725, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner MW, Gerhart JC. The plausibility of Life: Resolving Darwin’s dilemma. Yale University Press, 2006. [Google Scholar]
- Kitano H In search of Achille’s heel: Robustness in biological sciences In Tokoro Mario and Mogi Ken (eds) Creativity and the Brain, World Scientific Publishing Company, Singapore, ch. 3, pp. 41–54, 2007. [Google Scholar]
- Kuratani S Modularity, comparative embryology and evo-devo: Developmental dissection of evolving body plans. Developmental Biology 332(1):61–69, 2009. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Whitsel BL. Mechanisms underlying somatosensory cortical dynamics: 1. In vivo studies. Cereb Cortex 1992; 2: 81–106. [DOI] [PubMed] [Google Scholar]
- Linden DJ. The accidental mind: how brain evolution has given us love, memory, dreams, and God. Belknap Press, 2008. [DOI] [PubMed] [Google Scholar]
- Lipp H-P. Non-mental aspects of encephalization: the brain as a playground of mammalian evolution. Hum Evol 4:45–53, 1989. [Google Scholar]
- Lipp H-P, Wolfer DP. Big brains for bad genes: nonmental correlates of encephalization. Evolutionary Anthropology Suppl 1: 126–131, 2003. [Google Scholar]
- Lippman Z, Gendrel AV, Black M, Vaughn MW, Dedhia N, McCombie WR, et al. Role of transposable elements in heterochromatin and epigenetic control. Nature 430: 471, 2004. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R Architectonics and structure of the cerebral cortex In Fulton JF (ed.) Physiology of the Nervou System. Oxford University Press, New York, 291–330, 1938. [Google Scholar]
- Lorrain Smith J, Mair W, Thorpe JF. An investigation of the principles underlying Weigert’s method of staining medullated nerve. With a note on the staining of fats by potassium dichromate and haematoxylin. J Pathol 13(1):14–27, 1909. [Google Scholar]
- Ludwig M, Apps D, Menzies J, Patel JC, Rice ME. Dendritic release of neurotransmitters. Compr Physiol 7(1):235–252, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. The Triune Brain in Evolution: Role in Paleocerebral Functions. Springer, New York, 1990. [DOI] [PubMed] [Google Scholar]
- McLean CY, Reno PL, Pollen AA, Bassan AI, Capellini TD, Guenther C, et al. Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature 471:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger P, Patzke N, Gravett N, Medger K, Kaswera C, Gilissen E, Bennett NC. Unusual cortical lamination patterns in the sengis (elephant schrews) fo not appear to influence the presence of cortical minicolumns Casanova MF and Opris I (eds.) Recent Advances on the Modular Ogranization of the Cerebral Cortex. Springer, New York, ch. 6, pp. 81–96, 2015. [Google Scholar]
- Marin-Padilla M The Human Brain. Springer, New York, 2011. [Google Scholar]
- Medina L, Brox A, Legaz I, García-López M, Puelles L. Expression patterns of developmental regulatory genes show comparable divisions in the telencephalon of Xenopus and mouse: Insights into the evolution of the forebrain. Brain Res Bull 66: 297–302, 2005. [DOI] [PubMed] [Google Scholar]
- Morrell F, Hanbery JW. A new surgical technique for the treatment of focal cortical epilepsy. Electroencephalography Clin Neurophysiol 26:120, 1969. [PubMed] [Google Scholar]
- Morrell F, Kanner AM, de Toledo-Morrell L, Hoeppner T, Whistler WW. Multiple subpial transection. Adv Neurol 81:259–270, 1999. [PubMed] [Google Scholar]
- Mounier D, Lambiotte R, Bullmore ET. Modular and hierarchical modular organization of brain networks. Front Neurosci 4:200, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol 1957; 20: 408–34. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. An organizing principle for cerebral function: the unit module and the distributed system In: Edelman GM, Mountcastle VB, editors. The mindful brain: cortical organization and the group-selective theory of higher brain function. Cambridge (MA: ): MIT Press; 1978. p. 7–51. [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. [Review]. Brain, 1997; 120: 701–22. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Perceptual Neuroscience: The Cerebral Cortex Harvard University Press, Cambridge, MA, 1998. [Google Scholar]
- Muller-Linow M, Hilgetag CC, Hutt MT. Organization of excitable dynamics in hierarchical biological networks. PLoS Comput Biol 4(9):e1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Gotoh H, Ono K. Changes in the regulation of cortical neurogenesis contribute to encephalization during amniote brain evolution. Nature Communications article number: 2206(2013). [DOI] [PubMed] [Google Scholar]
- Oei S-L, Babich VS, Kazakov VI, Usmanova NM, Kropotov AV, Tomilin NV. Clusters of regulatory signals for RNA polymerase II transcription associated with Alu family repeats and CpG islands in human promoters. Genomics 83: 873–882, 2004. [DOI] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC (2005). Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Green WK. Mobile DNA and the TE-thrust hypothesis: supporting evidence from the primates. Mobile DNA 2:8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Hampson RE, Gerhardt GA, Berger TW, Deadwyler SA. Columnar processing in primate pFC: evidence for executive control microcircuits. J. Cogn. Neurosci 24, 2334–2347, 2012. doi: 10.1162/jocn_a_00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Santos L, Gerhardt GA, Song D, Berger TW, Hampson RE, et al. Prefrontal cortical microcircuits bind perception to executive control. Sci. Rep. 3:2285, 2013. doi: 10.1038/srep02285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. Gap junctional communication and the development of local circuits in neocortex. Cereb Cortex 3:488–498, 1993a. [DOI] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. Extensive dy coupling between rat neocortical neurons during the period of circuit formation. Neuron 10:103–114, 1003b. [DOI] [PubMed] [Google Scholar]
- Polak P, Domany E. Alu elements contain many binding sites for transcription factors and may play a role in regulation of developmental processes. BMC Genom 7: 133, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, et al. Molecular identity of human outer radial glia during cortical development. Cell 163: 55–67, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TPS, Mountcastle VB. Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: a comparison of findings obtained in a single unit analysis with cytoarchitecture. Bull J Hopkins Hosp 105:133–162, 1959. [PubMed] [Google Scholar]
- Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, et al. Circular RNA: a new star of noncoding RNAs. Cancer Lett 365:141–148, 2015. [DOI] [PubMed] [Google Scholar]
- Rakic P, Kornack DR. Neocortical expansion and elaboration during primate evolution: a view from neuroembryology In: Falk D, Gibson KR (editors) Evolutionary anatomy of the primate cerebral cortex. Cambridge University press, New York, p. 30–56, 2001. [Google Scholar]
- Richardson L, Venkataraman S, Stevenson P, Yang Y, Moss J, Graham L, Burton N, et al. EMAGE mouse embryo spatial gene expression database: (2014 update). Nucl Acids Res 42: D835–D844, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rightmire GP. Brain size and encephalization in early to mid-Pleistocene Homo. Am J Phys Anthropol 124:109–123, 2004. [DOI] [PubMed] [Google Scholar]
- Ringo JL. Neuronal interconnection as a function of brain size. Brain, Behavior & Evolution 38:1–6, 1991. [DOI] [PubMed] [Google Scholar]
- Santee HE. Anatomy of the brain and spinal cord with special reference to mechanism and function for students and practitioners P. Blakiston’s Son and Co, Philadelphia, fourth edition, 1907. [Google Scholar]
- Scheiber ME, Scgeiberl AB. Structural substrates for integrative patterns in the brain stem reticular core In: Reticular Formation of the Brain (Henry Ford Hospital International Symposium, Detroit, Michigan, March 14-16, 1957), Jasper HH, Proctor LD, Knighton RS, Noshay WC, and Costello RT, eds., Boston, Mass: Little, Brown, and Co., pp-31–68. [Google Scholar]
- Scheibelr ME, Scheibel AB. Terminal patterns in cat spinal cord. III. Primary afferent collaterals. Brain Res 13:417–443, 1969. [DOI] [PubMed] [Google Scholar]
- Schwartz EL, Shaw A, Wolfson E. A numerical solution to the generalized mapmaker’s problem: flattening nonconvex polyhedral surfaces. IEEE Trans Pattern Anal Mach Intel 11:1005–1008, 1989. [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science 316:445–449, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellstrom A, Jacobsson S. Culturing CNS neurons: a practical approach to cultured embryonic chick neurons In Haynes LW ed. The Neuron in Tissue Culture, Chichester, UK: John Wiley and Sons, p 327–338, 1999. [Google Scholar]
- Sen SK, Han K, Wang J, Lee J, Wang H, Callinan PA, et al. Human genomic deletions mediated by recombination between Alu elements. Am J Med Genet 79:41–53, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamma SA, Fleshman JW, Wiser PR, Versnel H. Organization of response areas in ferret primary auditory cortex. J Neurophysiol 1993; 69: 367–83. [DOI] [PubMed] [Google Scholar]
- Shankar R, Grover D, Brahmachari SK, Mukerji M. Evolution and distribution of RNA polymerase II regulatory sites from RNA polymerase III dependent mobile Alu elements. BMC Evol Biol 4: 37, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Koch C. Introduction to synaptic circuits In Shepherd GM (ed.), Synaptic Organization of the Brain 4th ed. Oxford University Press: New York, New York, p.1–36, 1998. [Google Scholar]
- Shipp S. Structure and function of the cerebral cortex. Current Biology 17(12):p R443–R449, 2007. [DOI] [PubMed] [Google Scholar]
- Smith MC. Multiple subpial transection in patients with extratemporal epilepsy. Epilepsia 39(Suppl. 4):581–589, 1998. [DOI] [PubMed] [Google Scholar]
- Sokhadze EM, El-Baz A, Baruth J, Mathai G, Sears L, Casanova MF. Effects of low frequency repetitive transcranial magnetic stimulation (rTMS) on gamma frequency oscillations and event-related potentials during processing of illusory figures in autism. J Autism Dev Disord 39(4):619–34, 2009. [DOI] [PubMed] [Google Scholar]
- Sperry RW, Miner N, Myers RE. Visual pattern perception following subpial slicing and tantalum wire implantations in visual cortex. J Comp Physiol Psychol 48:50–8, 1955. [DOI] [PubMed] [Google Scholar]
- Spocter MA, Raghanti MA, Butti C, Hoff PR, Sherwood CC. The minicolumns in comparative context Casanova MF and Opris I (eds) Recent Advances on the Modular Organization of the Cortex. Springer, New York, ch 5, pp. 63–80, 2015. [Google Scholar]
- Sporns O, Honey CJ. Small worlds inside big brains. Proc Natl Acad Sci 103:19219–19220, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, et al. Identification and classification of hubs in brain networks. PLoS ONE 10:e1049, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Hillebrand A, Wang H, Van Mieghem P. Emergence of modular structure in a large-scale brain network with interactions between dynamics and connectivity. Front Comput Neurosci 4:133, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiper ES, Young NM. Primate molecular divergence dates. Mol Phylogenet Evol 2006; 41: 384–394. [DOI] [PubMed] [Google Scholar]
- Su M, Han D, Boyd-Kirkup J, Yu X, Han J-D. Evolution of Alu elements towards enhancers. Cell Reports 7:376–385, 2014. [DOI] [PubMed] [Google Scholar]
- Sugimoto S, Sakurada M, Horikawa J, Taniguchi I. The columnar and layer-specific response properties of neurons in the primary auditory cortex of Mongolian gerbils. Hear Res 1997; 112: 175–85. [DOI] [PubMed] [Google Scholar]
- Szentàgothai J, Réthelyi M. Cyto- and neuropil architecture of the spinal cord. In: New Developments in Electromyography an Clinical Neurophysiology Vol 3 Human Reflexes, Pathophysiology of Motor Systems, Methodology of Human Reflexes. Desmedt JE ed. Basel: S. Karger, pp. 20–37. [Google Scholar]
- Szentàgothai J The “module-concept” in cerebral cortex architecture. Brain Res 95(2-3):475–96, 1975. [DOI] [PubMed] [Google Scholar]
- Szentàgothai J, Arbib MA. Conceptual models of neural organization. MIT Press, Cambridge, MA, 1975. [PubMed] [Google Scholar]
- Tommerdahl M, Favorov O, Whitsel BL, Nakhle B, Gonchar YA. Minicolumnar activation patterns in cat and monkey S1 cortex. Cereb Cortex 1993; 3: 399–411. [DOI] [PubMed] [Google Scholar]
- Tovar-Spinoza ZS, Rutka JT. Subpial transection In: Lozano AM, Gildenberg PL, Tasker PL (eds.) Textbook of stereotactic and Functional Neurosurgery, Springer, Berlin, Heidelberg. [Google Scholar]
- Turner TN, Hormozdiari F, Duyzend MH, McClymont SA, Hook PW, Iossifov I, et al. Genome sequencing of autism-affected families reveals disruption of putative noncoding regulatory DNA. Am J Hum Genet 98:58–74, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maiia AS, Plomp JJ, Heeroma AB, Vermeer H, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287:864–869, 2000. [DOI] [PubMed] [Google Scholar]
- Von Economo C, Koskinas G N. Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Wien: Springer, 1925. [Google Scholar]
- Wong FK, Bercsenyi K, Sreenivasan V, Portalés A, Fernández-Otero M, Marín O. Pyramidal cell regulation of interneuron survival sculpts cortical networks. Nature 557(7707):668–673, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez IB, Muñoz A, Contreras J, Gonzalez J, Rodriguez-Veiga E, DeFelipe J. Double bouquet cell in the human cerebral cortex and a comparison with other mammals. J Comp Neurol 486:344–60, 2005. [DOI] [PubMed] [Google Scholar]
- Yu YC, Bultje RS, Wang X, Shi SH. Specific synapse develop preferentially among sister excitatory neurons in the neocortex. Nature 458(7237):501–4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YC, He S, Chen S, et al. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature 486(7401):113–7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Chasin LH. Comparison of multiple vertebrate genomes reveals the birth and evolution of human exons. PNAS 103:13427–13432, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]