Abstract
Fewer randomized clinical trials (RCTs) are conducted for chronic or recurrent pain in pediatric populations compared with adult populations; thus data to support treatment efficacy in children are limited. This article evaluates design features and reporting practices of RCTs for chronic and recurrent pain that are likely unique to, or particularly important in a pediatric population in order to promote improvements in the evidence base for pediatric pain treatments. Areas covered include outcome measure selection and reporting and reporting of adverse events (AEs) and challenges to recruitment and retention. A search of PubMed and EMBASE identified primary publications describing RCTs of treatments for select chronic and recurrent pain conditions in children or adolescents published between 2000 and 2017. Only 49% of articles identified a primary outcome measure. The primary outcome measure assessed pain intensity in 38% of the trials, specifically measure by verbal rating scale (13%), faces pain scale (11%), visual analogue scale (9%), or numeric rating scale (5%). All of the CONSORT harms reporting recommendations were fulfilled by fewer than 50% of the articles. Discussions of recruitment challenges occurred in 64% of articles that enrolled fewer than 90% of their target sample. However, discussions regarding retention challenges only occurred in 14% of trials in which withdrawal rates were greater than 10%. The goal of this article is to promote comprehensive reporting of pediatric pain RCTs in order to improve the design of future trials, facilitate conduction of systematic reviews and meta-analyses, and better inform clinical practice.
1. Introduction
Chronic and recurrent pain are common in the pediatric population, with median prevalences ranging from 11–38% reported in the literature depending on the type of pain and criteria used to define chronic and recurrent pain.25 However, a paucity of clinical trials is available that provide adequate evidence to inform pediatric pain management and evidence-based treatment guidelines.13 Many of the current pediatric pain treatment recommendations, therefore, rely on efficacy data extrapolated from studies that included only adults.10 Thus, evidence-based treatment guidelines do not exist for most available pain therapies or for pain conditions prevalent in children and adolescents (e.g., sickle cell disease pain).
One result of the dearth of clear evidence and evidence-based guidelines is that chronic pain remains untreated, undertreated, or potentially inappropriately treated in pediatric patients.20 Several possible mechanisms link poorly treated pain in children and adolescents to chronic pain problems in adults. Potential mechanisms include individual temperament 11 and factors related to pain responsiveness,41 changes in peripheral neural development and resulting sensitization related to injury or illness,33 and central sensitization (i.e., increased responsiveness of nociceptive neurons in the central nervous system to normal or subthreshold afferent input).1,39 Unmitigated pain and its comorbidities in childhood can lead to dysfunctional lifestyle patterns as adults.40 This has important public health implications given that chronic pain, if untreated, can lead to physical disability, as well as the development of comorbid problems such as impaired sleep, anxiety, depression, school absenteeism, and significant patient and family distress.8 Consequently, a failure to adequately address pediatric pain may have significant consequences with regards to the future health of our population and the societal health care burden.
Given the short- and long-term consequences of chronic pediatric pain, high quality research is needed to identify effective treatments. In this systematic review, we investigated outcome measures and the quality of their reporting as well as reporting of adverse events and insights into recruitment and retention challenges in RCTs for select chronic and recurrent pain conditions. We focused on design and reporting issues that are likely different from adult populations or particularly challenging in pediatric pain populations. For example, outcome measures are likely to be different for pediatric populations, reporting of AEs are particularly important for vulnerable populations like children, and recruitment and retention may be especially challenging when considering both the parents’ and children’s perspectives and schedules. We included all types of interventions as both pharmacologic and non-pharmacologic interventions are common in pediatric pain. The results of this review are intended to encourage improvement in in the reporting of these important aspects of pediatric pain trials to allow critical evaluation of the results, inclusion of data in meta-analyses, and successful completion of future trials. In turn, these practices may improve the development of valid, evidence-based treatment guidelines.
2. Methods
2.1. Article selection
We systematically evaluated reports of RCTs of select chronic or recurrent pediatric pain conditions published between January 2000 and December 2017. Our choice of select chronic and recurrent pain conditions was based on those that was determined to be relatively common and clinically challenging based on qualitative literature reviews and the opinions of the co-authors with clinical and research expertise in pediatric pain. A search of PubMed and EMBASE was conducted by a medical librarian for articles published in the peer-review journals that reported RCTs of analgesic treatments for these conditions in children (see Appendix 1 for the complete search strategy). Two reviewers (MRC, RH) independently screened the search results and excluded any articles that did not meet the following criteria: (1) included pain patients who were less than 18 years of age (or 25 years of age if the article specifically stated including young adults or adolescents); (2) investigated headache/migraine, abdominal pain/irritable bowel syndrome, musculoskeletal pain (including arthritis, fibromyalgia, and other musculoskeletal pain), or complex regional pain syndrome; trials that included more than a single pain condition were not included (e.g., a trial that enrolled some patients with back pain and others with abdominal pain were excluded) to make it possible to conduct analyses of associations between major categories of pain etiology and study methodologic characteristics; (3) pain was the major focus of the article; (4) evaluated pharmacologic, psychological (e.g., biofeedback, relaxation training), or physical (e.g., massage, physical therapy) treatments that were aimed at the child (rather than guardians); (4) had longer than a single-day follow-up, and (5) were randomized. We excluded trials of post-surgical pain and procedural pain. We also excluded trials in which the treatment targeted the cause of the pain or co-occurring symptoms rather than interventions with a putative analgesic mechanism (e.g., agents treating reflux or constipation in abdominal pain). Finally, we excluded trials in which safety or feasibility was the primary outcome.
2.2. Data extraction
A comprehensive coding manual was developed to guide the extraction of details related to recruitment and retention, type of intervention, treatment dosing, outcome assessment, type of control, number of study visits, outcome measures, and CONSORT harms 22 based on methods described previously15,18,35 (Appendix 2). The complete coding manual was pre-tested and modified by 4 coders (JSG, MRC, RAK, SMS) in 5 rounds of coding using articles that did not meet inclusion criteria for this review. The same articles served as training articles. Each article was coded by 2 independent authors (from among JYC, MRC, NW, XY) all of whom coded a set of the included articles in random order. The 2 coders’ results for each article were then compared and inconsistencies were adjudicated through discussions between JSG and JYC.
2.3. Statistical analysis
Descriptive statistics were used to compare reporting quality between articles published between (1) pharmacologic and non-pharmacologic interventions; and (2) between pain condition categories (migraine/headache; IBS/Abdominal pain; musculoskeletal pain (including arthritis, fibromyalgia, and other musculoskeletal pain). Note, we included fibromyalgia in the musculoskeletal pain group because that is where it is categorized in the ACTTION – APS Pain Taxonomy. We pre-specified that an absolute difference of at least 10 percentage points between groups (e.g., the percentage of trials that specified a primary outcome measure in pharmacologic vs. non-pharmacologic trials) would be considered meaningful. Given the difficulty with interpreting multiple comparisons due to possibly elevated type 1 error rates, statistical tests were not conducted. To investigate possible variables associated with retention, we used Wilcoxon tests with the following independent variables: trial design (i.e., cross-over vs. parallel group), type of intervention (i.e., pharmacologic vs. non-pharmacologic), requirement of travel to study sites (i.e., yes vs. no), number of study visits (≥ 5 or < 5). For these analyses, a p-value < 0.5 was considered statistically significant. All analyses were conducted using JMP Pro Version 12.
3. Results
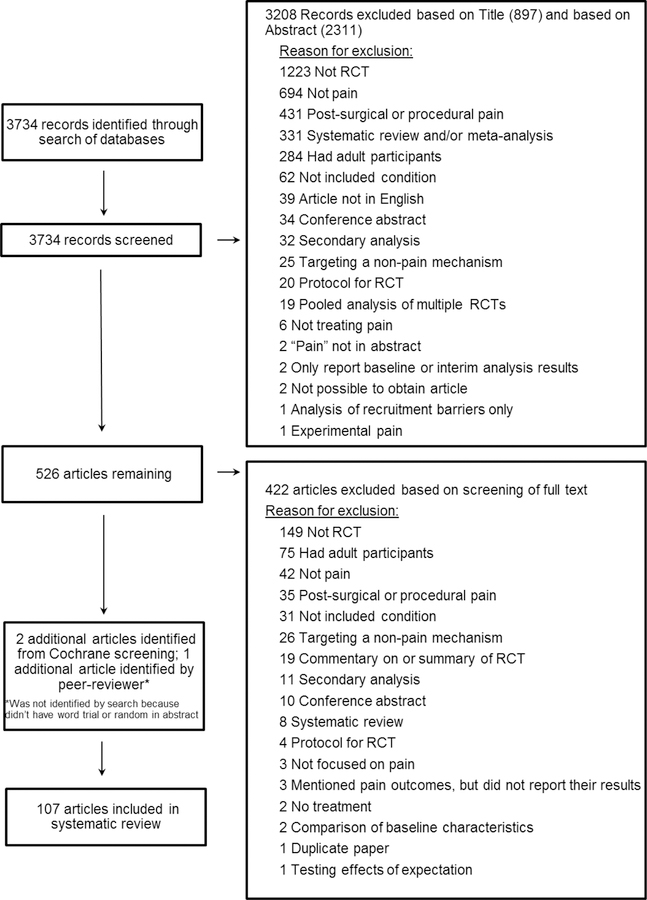
In total, we identified 3,734 unique search hits. We eliminated 897 articles based on the title and another 2,311 articles after reviewing the abstract, resulting in 526 articles for which the full text was screened for inclusion. Of the 526 articles, 422 were excluded, leaving 104 articles. An additional 2 articles were identified by examining 14 Cochrane reviews that were identified on the Cochrane Library website using our search terms and 1 additional article was identified by a peer-reviewer. Thus 107 articles were included in the review (Figure 1, Appendix 3).
Figure 1.
PRISMA diagram
3.1. Coder discrepancies:
A total of 6,295 items were coded for the 107 articles. There were 1,345 discrepancies (21%). Discrepancies were adjudicated by JSG and JYC.
3.2. Article characteristics
Headache/migraine was the most common pain condition in the sample (n=60, 56%) and over half of those studies examined migraine. The second most common pain type was abdominal pain (n=24, 22%). Fifty percent of the studies investigated pharmacologic interventions. A non-active control group (i.e., placebo or waitlist) was included in 59 (55%) studies. Two active treatments were compared in 17 (16%) studies. Forty-eight (45%) trials were double-blinded. The remainder used some combination of assessor and/or patient blinding or blinding was not clearly reported. Twenty-four (22%) studies involved daily administration of the intervention.). Ninety-five (89%) studies required travel to the study site for evaluations (i.e., not including locations that the participants usually frequent (e.g., school or inpatient hospital)). Of the studies that clearly reported the required number of study visits, 53 (71%) required 5 or fewer visits to the study site (Table 1).
Table 1.
Trial demographics
| Characteristic | Total Sample N= 107 (100) |
Pharmacologic N= 54 (50) |
Non-pharmacologic N= 53 (50) |
|---|---|---|---|
| Article, N (% of column) or Median [IQR] | |||
| Type of pain | |||
| Headache/migraine | 60 (56) | 40 (74) | 20 (38) |
| Abdominal/IBS | 24 (22) | 10 (19) | 14 (26) |
| Arthritis | 9 (8) | 1 (2) | 8 (15) |
| Musculoskeletal | 7 (7) | 0 (0) | 7 (13) |
| Fibromyalgia | 5 (5) | 2 (4) | 3 (6) |
| CRPS | 2 (2) | 1 (2) | 1 (2) |
| Trial design | |||
| Parallel | 93 (87) | 41 (76) | 52 (98) |
| Cross-over | 14 (13) | 13 (24) | 1 (2) |
| Sample size* | 63 [36–118] | 100 [42–155] | 50 [36–77] |
| Type of comparison group | |||
| Placebo | 49 (46) | 40 (74) | 9 (17) |
| Two treatments compared | 17 (16) | 10 (19) | 7 (13) |
| Wait list | 10 (9) | 0 (0) | 10 (19) |
| Standard of care (defined by the protocol) | 8 (7) | 0 (0) | 8 (15) |
| Education | 8 (7) | 0 (0) | 8 (15) |
| Standard of care (preference of the treating physician – not standardized) | 5 (5) | 0 (0) | 5 (9) |
| Active control | 4 (4) | 2 (4) | 2 (4) |
| Other (e.g., two different frequencies of sessions of physical therapy) | 6 (6) | 2 (4) | 4 (8) |
| Sponsor | |||
| Industry | 21 (20) | 20 (37) | 1 (2) |
| Not industry (e.g., government or professional organization) | 53 (50) | 14 (26) | 39 (74) |
| No external funding | 5 (5) | 3 (6) | 2 (4) |
| Not reported | 28 (26) | 17 (31) | 11 (21) |
| Study blinding | |||
| Double | 48 (45) | 46 (85) | 2 (4) |
| Assessor only blinded | 18 (17) | 1 (2) | 17 (32) |
| Unclear for assessor (patient could not be blinded) | 12 (11) | 0 (0) | 12 (23) |
| Unclear | 10 (9) | 3 (6) | 7 (13) |
| None | 10 (9) | 2 (4) | 8 (15) |
| Patient blinded | 2 (2) | 1 (2) | 1 (2) |
| Patient and assessor blinded | 2 (2) | 0 (0) | 2 (4) |
| Other | 5 (5) | 1 (2) | 4 (8) |
| Site characteristics | |||
| Single site | 53 (50) | 23 (43) | 30 (57) |
| Multi-site | 47 (44) | 30 (56) | 17 (32) |
| # sites unclear | 7 (7) | 1 (2) | 6 (11) |
| Duration of study follow-up | |||
| <2 months | 20 (19) | 18 (33) | 2 (4) |
| 2–6 months | 47 (44) | 23 (43) | 24 (45) |
| >6 months | 34 (32) | 7 (13) | 27 (51) |
| Unclear | 6 (6) | 6 (11) | 0 (0) |
IBS= Irritable Bowel Syndrome, CRPS= Complex Regional Pain Syndrome, TENS =Transcutaneous Electric Stimulation
A higher percentage of non-pharmacologic trials had a parallel group design (98% non-pharmacologic vs. 76% pharmacologic). Education (15% vs. 0%), waitlist (19% vs. 0%), and standard of care (25% vs. 0%) comparison groups were more common in non-pharmacologic trials, whereas placebo controls were more common in pharmacologic trials (74% vs. 17%). Double-blinding was more common in pharmacologic trials (85% vs. 4%); assessor-only blinding was more common in non-pharmacologic (32% vs. 2%) trials. More pharmacologic trials reported industry sponsorship (37% vs. 2%) (Table 1).
Pharmacologic interventions were most commonly evaluated in headache trials (67% headache vs. 42% abdominal vs. 14% musculoskeletal), while psychological interventions were investigated most commonly in abdominal pain trials (42% abdominal vs. 23% headache vs. 19% musculoskeletal), and exercise interventions were most common in musculoskeletal pain trials (52% musculoskeletal vs. 8% abdominal vs. 2% headache). Headache trials were slightly larger than trials studying other pain conditions (median sample size 73 (headache) vs. 67 (abdominal) vs. 47 (musculoskeletal). Double-blinding was more common in headache trials (58% headache vs. 38% abdominal vs. 14% musculoskeletal); assessor-only blinding was more common in musculoskeletal pain trials (43% abdominal vs. 8% headache vs. 13% abdominal). Follow-up duration was similar between pain condition categories with the exception of the fact that a higher percentage of abdominal pain trials had a follow-up of less than 2 months (38% abdominal vs. 17% headache vs. 0% musculoskeletal) (Supplemental Table 1).
3.3. Reporting
Fifty-two (49%) studies identified 1 primary outcome measure (POM) or multiple POMs for which multiplicity was sufficiently handled statistically, resulting in 55 POMs being identified in the included articles. Twenty-one (38%) of those POMs used a pain intensity rating scale, for example FACES pain scale (FACES), numerical rating scale (NRS), verbal rating scale (VRS), or visual analog scale (VAS). Other primary outcome measures are summarized in Table 2. Forty-four (80%) POMs were patient-reported outcomes (Table 2). A POM was identified more frequently in pharmacologic vs. non-pharmacologic trials (57% vs. 40%).Musculoskeletal pain trials identified a POM more frequently than headache and abdominal pain trials (62% musculoskeletal vs. 50% headache vs. 33% abdominal). See Supplemental Table 2 for the POMs reported by pain condition category.
Table 2.
Primary outcome measures (POMs) and pain-associated outcome domains
| All intervention types N=107 |
Pharmacologic N=54 |
Non-pharmacologic N=53 |
|
|---|---|---|---|
| Articles, N (% of column) | |||
| Articles that reported 1 or multiple POMs with adjustment for multiplicity | 52 (49) | 31 (57) | 21 (40) |
| POMs reported | 55* | 31 | 24* |
| Headache frequency | 8 (15) | 5 (16) | 3 (13) |
| Pain intensity - VRS | 7 (13) | 7 (23) | 0 (0) |
| Number of headache/migraine days | 6 (11) | 4 (13) | 2 (8) |
| Faces Pain Scale (FACES) | 6 (11) | 5 (16) | 1 (4) |
| Pain intensity - VAS | 5 (9) | 2 (6) | 3 (13) |
| Pain intensity - NRS | 3 (5) | 2 (6) | 1 (4) |
| Quality of life measure (i.e., Peds QL, Juvenile Arthritis Quality of Life Questionnaire) | 3 (5) | 1 (3) | 2 (8) |
| Pain composite** | 3 (5) | 0 (0) | 3 (13) |
| Measure of function (i.e., Functional Disability Inventory, Foot Function Index) | 3 (5) | 0 (0) | 3 (13) |
| Pain-free at 2hrs after medication initiation | 1 (2) | 1 (3) | 0 (0) |
| Other measure | 10 (18) | 4 (13) | 6 (25) |
| POM assessor | |||
| POM assessed by patient | 44 (80) | 24 (77) | 20 (83) |
| POM assessed by parent | 3 (5) | 2 (6) | 1 (4) |
| POM assessed by patient and parent separately | 2 (4) | 0 (0) | 2 (8) |
| POM assessed by patient and parent together | 2 (4) | 1 (3) | 1 (4) |
| POM assessed by either patient or parent, depending on the age of patient | 1 (2) | 1 (3) | 0 (0) |
| Unclear | 3 (5) | 3 (10) | 0 (0) |
| Studies that reported non-primary outcome measures | 103 (96) | 51 (94) | 52 (98) |
| Non-primary, pain or pain-related domains assessed | |||
| Pain intensity (VAS, NRS, Likert, FACES) | 65 (63) | 26 (51) | 39 (75) |
| Use of “as needed” medication for pain | 29 (28) | 18 (35) | 11 (21) |
| Number of painful episodes | 29 (28) | 20 (39) | 9 (17) |
| Quality of life (e.g., PedsMIDAS, FDI, PedsQL, CHQ, RMDQ, or mobility) | 27 (26) | 6 (12) | 21 (40) |
| Headache diary, frequency/duration of pain episodes or pain-free days | 23 (22) | 10 (20) | 13 (25) |
| Anxiety, depression (e.g., CDI, STAIC), and/or stress | 20 (19) | 5 (10) | 15 (29) |
| Function and/or physical activity | 19 (18) | 5 (10) | 14 (27) |
| Days of missed school/activities | 18 (17) | 5 (10) | 13 (25) |
| Presence of headache-related symptoms (nausea, vomiting, photophobia, or phonophobia) | 18 (17) | 18 (35) | 0 (0) |
| Disability | 18 (17) | 8 (35) | 10 (19) |
| Sleep | 10 (10) | 7 (14) | 3 (6) |
| Healthcare utilization (length of hospitalization, #ER visits) | 5 (5) | 0 (0) | 5 (10) |
| Pain catastrophizing and/or pain coping | 5 (5) | 0 (0) | 5 (10) |
| Pain composite* | 5 (5) | 0 (0) | 5 (10) |
| Somatization | 5 (5) | 2 (4) | 3 (6) |
| Satisfaction with treatment | 5 (5) | 1 (2) | 4 (8) |
CDI= Clinical Depression Index, CHQ= Child’s Health Questionnaire, EMG = electromyography, ER = emergency room, FDI= Functional Disability Index, FACES= Faces Pain Scale, NRS = numeric rating scale, PedsMIDAS= Pediatric Migraine Disability Assessment, PedsQL= Pediatric Quality of Life Scale, RMDQ= Roland Morris Disability Questionnaire, STAIC = State-Trait-Anxiety Inventory for Children, VAS= visual analog scale, VRS= verbal rating scale
Three added to the denominator for analyses reporting the percentage of (1) different types of POMs, (2) those that were reportedly validated and (3) with different types of assessors to account for one article that identified multiple primary outcome measures and adjusted for multiplicity
A pain composite is any composite measure consisting of a combination of pain intensity, pain duration, pain frequency, pain interference, or quality of pain
One hundred and three (96%) studies reported non-primary outcome measures (i.e., secondary outcome measures or all measures from trials in which no POM was defined). A measure of pain intensity (e.g., FACES, NRS, VAS, VRS) was included as a non-primary outcome measure in 65 (63%) of studies. Other common non-primary outcome measures included use of “as needed” pain medication (n=29, 28%) and quality of life measures (n=27, 26%) (Table 2). More trials of non-pharmacologic treatments than pharmacologic treatments included measures of quality of life (40% vs 12%), depression, anxiety, or stress (29% vs. 10%), number of days of missed school (25% vs. 10%), pain catastrophizing and/or pain coping (10% vs. 0%), pain intensity (75% vs. 51%), function and/or physical activity (27% vs. 10%), healthcare utilization (10% vs. 0%), and pain composite (10% vs. 0%). More trials of pharmacologic treatments than non-pharmacologic treatments included measures of number of painful episodes (39% vs. 17%), use of “as needed” medication for pain (35% vs. 21%), presence of headache-related symptoms (35% vs. 0%), and disability (35% vs. 19%). (Table 2).
Fifty-two (79%) of the articles that reported a sample size calculation reported that the trial randomized at least 90% of their target sample size. Explanations for failure to meet enrollment goals were discussed in 9 (64%) of the 14 articles in which less than 90% of the planned sample size was achieved. Explanations included: (1) manufacturer-related problems with availability of the study drug,7 (2) recurrent abdominal pain observed less frequently in winter,31 (3) interim analysis resulted in early stopping due to early evidence for efficacy (n=2),6,27 (4) medication associated with suicidal risks,32 (5) strict inclusion and exclusion criteria,28 (6) funding ended before enrollment goal reached,37 and (7) under-recognition of fibromyalgia (n=2).2,3
The percentage of participants who completed the trial was reported in 89 (83%) studies. Within those studies, the median withdrawal rate was 14% (IQR=8%-22%). The cross-over study design was the only design characteristic found to be associated with increased withdrawal rates (p = 0.043, median withdrawal rate: 20% in cross-over trials vs. 13% in parallel trials). Of the 57 articles that reported that greater than 10% of participants withdrew from the study, only 8 (14%) discussed retention challenges. Example explanations included: (1) disappointment of allocation to the control group,26 and (2) the methodology used, including long treatment periods and complicated study visit schedules (n=2).2,19
3.4. Fulfillment of CONSORT harms reporting recommendations.
Table 3 summarizes the fulfillment of the CONSORT recommendations for reporting adverse events (AEs) or “harms”.5 The most commonly fulfilled harms recommendation (i.e., reporting the absolute risk of AEs per group in the Results section) was only fulfilled by 47% of the articles (Table 3). Reporting of all 10 harms recommendations was significantly higher in pharmacologic than non-pharmacologic trials (Table 3). Headache trials more commonly met harms reporting requirements (Supplemental Table 3).
Table 3.
Satisfaction of CONSORT adverse event reporting recommendations
| CONSORT AE reporting recommendations | All intervention types N=107 |
Pharmacologic N=54 |
Non-pharmacologic N=53 |
|---|---|---|---|
| Articles, N (% of column) | |||
| 1) Title or abstract: If the study collected data on harms and benefits, the title or abstract should so state. | 39 (36) | 35 (65) | 4 (8) |
| 2) Introduction: If the trial addresses both harms and benefits, the introduction should so state. | 21 (20) | 20 (37) | 1 (2) |
| 3) Methods: List addressed adverse events with definitions for each (with attention, when relevant, to grading, expected versus unexpected events, reference to standardized and validated definitions, and description of new definitions). | 13 (12) | 10 (19) | 3 (6) |
| 4) Methods: Clarify how harms-related information was collected (mode of data collection, timing, attribution methods, intensity of ascertainment, and harms-related monitoring and stopping rules, if pertinent). | 42 (39) | 33 (61) | 9 (17) |
| 5) Methods: Describe plans for presenting and analyzing information on harms (including coding, handling of recurrent events, specification of timing issues, handling of continuous measures, and any statistical analyses). | 10 (9) | 8 (15) | 2 (4) |
| 6) Results: Describe for each arm the participant withdrawals that are due to harms and their experiences with the allocated treatment. | 43 (40) | 34 (63) | 9 (17) |
| 7) Results: Provide the denominators for analyses on harms. | 38 (36) | 30 (56) | 8 (15) |
| 8) Results: Present the absolute risk per arm and per adverse event type, grade, and seriousness and present appropriate metrics for recurrent events, continuous variables, and scale variables, whenever pertinent. | 50 (47) | 38 (70) | 12 (23) |
| 9) Results: Describe any subgroup analyses and exploratory analyses for harms. | 9 (8) | 9 (17) | 0 (0) |
| 10) Discussion: Provide a balanced discussion of benefits and harms with emphasis o3).n study limitations, generalizability, and other sources of information on harms. | 37 (35) | 32 (59) | 5 (9) |
| CONSORT: Consolidated Standards of Reporting Trials | |||
4. Discussion
In 2003, Eccleston and Malleson noted that there was a lack of data on treating chronic pain in children and adolescents.13 Our review suggests that over the past 8 years investigators have begun to address this concern considering the number of RCTs published per year in our sample increased approximately 2.4 times in the second half of the publication years included (i.e. 2008 – 2017) when compared to the first half (i.e., 2000 – 2007). However, increasing the quantity of data available is not sufficient for addressing the challenges of treating pediatric pain. Instead, it is important to improve the quality and impact of those data, both of which are influenced by a variety of factors, for example, specification of primary outcome measures, reporting of adverse events, and optimizing recruitment and retention.
Proper reporting is integral to a reader’s ability to critically assess and determine the strength and validity of the study results, to compare the results with other studies, and to include the results in meta-analyses and systematic reviews. For example, pre-specification of a POM and how it will be analyzed is essential to ensure that the reported analyses do not selectively represent the most favorable outcomes obtained after conducting multiple analyses, a practice that increases false positive rates.17 If a POM is not defined and reported, then readers cannot accurately evaluate the statistical validity of the results. This rate of specification of a POM was 49% in the later period. This percentage is considerably lower than the 62% and 63% that was found in reviews of pharmacologic17 and non-pharmacologic12 predominantly adult, analgesic RCTs published in 3 major pain journals between 2006 and 2013.17 This difference could be due to the fact that we included all journals, rather than 3 major pain journals, in the analysis of pediatric pain trials or a difference between analgesic trials in adults vs. pediatrics. Regardless, the relatively low level of reporting of POMs in recent pediatric pain trials is a major concern that should spur improvements in clinical trial design and reporting in order to increase the quality, integrity, and impact of trial results.
As with any clinical trial, outcome measures used in pediatric pain trials should be validated for use in the condition and specific population of interest.38 No validated pain measure is universally accepted for pediatric pain trials, although some pain outcome measures have been validated for segments of the pediatric population. The most common primary and non-primary outcome measures used in the trials that we examined were pain intensity scales (i.e., FACES, NRS, VAS, or VRS). Even after 2011, when the PedIMMPACT 29 recommendations had been published for 3 years, the percentage of articles reporting outcome measures related to quality of life, mental health, and disability were included in at most one-third of publications. More trials of non-pharmacologic treatments than pharmacologic treatments included measures of quality of life and depression, anxiety, stress, of function or physical activity suggesting that the objectives of non-pharmacologic trials may be more in line with the PedIMMPACT recommendations than pharmacologic trials.
AE reporting is important in all trials, but particularly in pediatric trials as side effects are often a barrier to treatment in this population due to both clinician and parental apprehension. More thorough reporting of AEs is necessary given that the most commonly fulfilled harms recommendation (i.e., reporting the absolute risk of AEs per group in the Results section) was only fulfilled by 50% of articles, even among articles published after 2007, which is 3 years after the CONSORT harms recommendations were published in 2004.22 We also observed a higher rate of reporting for all harms recommendations in pharmacologic than non-pharmacologic trials (Table 3). This is consistent with increased rates of harms reporting between pharmacologic vs. non-pharmacologic trials in reviews of trials that included predominantly adults.21,34 This difference could be due to the perception that non-pharmacologic treatments are safer and therefore do not require thorough AE reporting or due to the regulatory review that many pharmacologic trials undergo. Improvements in harms reporting in pediatric pain trials, particularly those of non-pharmacologic interventions are necessary in order to fully evaluate the safety of treatments in this vulnerable population.
Recruitment has historically been cited as a significant barrier to conducting pediatric pain trials and a challenge to clinicians attempting to execute appropriately-powered studies.4,10 Potential barriers to pediatric trials include parental apprehension regarding enrolling children in an RCT in which they may receive a medication with unknown treatment benefit, potential side effects, or the possibility of being placed in the control group without the investigational treatment.23 The inconvenience and financial burden of study participation have also been implicated as a cause of low recruitment rates in pediatric trials.9,10 The fact that almost 79% of the studies met at least 90% of their recruitment goal is encouraging, given these potential barriers to recruitment. It is important to note, however, that the degree to which trials met their recruitment goal was only evaluable in approximately 62% of the trials that reported a sample size calculation and the number of participants who were randomized in the trial. Furthermore, we did not evaluate the suppositions made for the sample size calculations and it is possible that investigators used assumptions for their sample size calculations that produced a feasible recruitment goal, but that may represent an overly optimistic effect size. Importantly, 64% of the articles that reported enrolling less than 90% of their planned sample size discussed reasons for recruitment challenges, which can help inform efforts to improve recruitment in future trials.
The median withdrawal rate in this sample of pediatric pain trials (14%) was similar to that found in a systematic review for RCTs of pharmacologic trials for predominantly adults published between 2006 and 2012 in 3 major pain journals (17%).15 Although this fairly high retention rate is encouraging, only 14% of articles in which more than 10% of participants withdrew discussed potential barriers to retention. Increased frequency of qualitative reporting regarding retention is encouraged to provide guidance for planning future trials. For example, the authors of 1 article suggested that the good retention rate in their study was influenced by strong relationships created through in-office therapy, an element often viewed as a barrier to treatment due to inconvenience.24 This information could affect how future investigators weight the advantages and limitations of in-office therapy visits in future trials. Publishing of similar accounts could inform trial protocols going forward, improving retention and thus the quality of data in clinical trials.
This review is limited in that we evaluated publications of pediatric pain trials in the peer-reviewed literature, and therefore the summary of outcome measures may not fully reflect the actual trial protocols and execution. Even when a primary outcome measure is identified, it does not ensure that the primary outcome measure reported is the one that was pre-specified in the protocol or that multiple analyses were not performed prior to choosing a “primary” outcome measure on a post-hoc basis. We did not evaluate these issues in this study because we did not believe that these would be markedly different in pediatric vs. adult trials, which we have investigated in the past.16,36 This review is limited to select chronic and recurrent pain conditions, and these results may not generalize to trials of other pediatric chronic and recurrent pain conditions, trials that include heterogeneous chronic pain conditions, or trials of procedural or post-surgical pain. Our choice of select chronic and recurrent pediatric pain conditions was based on qualitative literature reviews and the opinions of the co-authors with expertise in pediatric pain regarding which conditions are common and clinically challenging; other experts may have chosen different pain conditions. Although it would have been interesting to examine whether the overall risk of bias in pediatric pain RCTs has changed over time, we did not include this outcome in our review as we were focused on design and reporting characteristics that are particularly challenging or unique for pediatrics. We analyzed what percentage of trials discussed challenges for recruitment and retention if less than 90% of the planned sample size was enrolled or less than 90% of randomized participants were retained. The cut-off of 90% was chosen arbitrarily. The protocol for this systematic review was not registered before we executed the review.
Conclusions
This systematic review of chronic and recurrent pediatric pain trials demonstrates an increase in the rate of published pediatric pain trials over the last 2 decades. Our review demonstrates inadequacies in reporting quality for RCTS of key features specifically important to pediatric populations. Table 4 provides recommendations that address these shortcomings and that have the potential to optimize reporting. We hope that the information provided by this review will draw attention to these deficiencies and promote continued efforts towards improving the quality of both the design and publication of future pediatric clinical pain trials to meet the needs of clinical practice.
Table 4.
Reporting recommendations for trials of therapies for pediatric chronic and recurrent pain
| Category | Recommendations |
|---|---|
| Eligibility | • When available, use consensus definitions (e.g., AAPT) to define pediatric pain conditions for eligibility criteria. • When no consensus definitions are available clearly articulate the inclusion and exclusion criteria used to diagnoses the included condition. |
| Recruitment and retention and adherence | • Consider use of multiple sites to promote proper power and clearly indicate the number of sites used for recruitment per recommendations.30 • Clearly report methods for obtaining informed consent and assent from patients and adolescents, respectively. • Clearly report the enrollment goal • Clearly report the number of patients screened and enrolled • Clearly report the number of participants randomized and who complete the trial • Discuss and recruitment/retention challenges/or contributions to success in the primary publication • If space constraints prohibit this reporting, consider using supplemental files • Clearly report efforts used to enhance adherence, including engagement of parents or guardians. |
| Outcome measures | • Clearly report the primary outcome measure and whether it was pre-specified • Clearly indicate who completed the outcome measure (i.e., parent, guardian, clinician) • Include references to publications that have evaluated the psychometric properties of the outcome measures in pediatric populations • Consider assessing and reporting secondary outcome measures recommended by the PedsIMMPACT recommendations in primary publications |
| Analyses | • Report any sub-analyses by developmentally appropriate age stratifications based on recommendations by the pediatric issue in the ACCTION guide to clinical trials.45 |
| Adverse events | • Adhere to the CONSORT harms recommendations for pharmacologic and non-pharmacologic trials • Consider additional harms reporting guidelines (e.g., ACTTION)35 |
These reporting recommendations focus on reporting of design characteristics that are of particular importance in pediatric pain populations and should be considered in conjunction with comprehensive reporting guidelines for chronic pain trials.14
Supplementary Material
Perspective.
This review of chronic and recurrent pediatric pain trials demonstrates inadequacies in reporting quality of key features specifically important to pediatric populations. It provides recommendations that address these shortcomings in order to promote continued efforts towards improving the quality of the design and publication of future pediatric clinical pain trials.
Acknowledgements
We thank Linda Hasman, MSLS for conducting the PubMed search. This article was reviewed and approved by the Executive Committee of the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership with the United States Food and Drug Administration.
Disclosures: Financial support for this project was provided by the ACTTION public-private partnership, which has received research contracts, grants, or other revenue from the FDA, multiple pharmaceutical and device companies, philanthropy, and other sources. The views expressed in this article are those of the authors and no official endorsement by the Food and Drug Administration (FDA) or the pharmaceutical and device companies that provided unrestricted grants to support the activities of the ACTTION public-private partnership should be inferred.
Footnotes
The authors have no conflicts of interest to disclose related to the subject matter of this article.
References
- 1.Adams LM, Turk DC. Psychosocial factors and central sensitivity syndromes. Curr Rheumatol Rev 11:96–108, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold LM, Bateman L, Palmer RH, Lin Y. Preliminary experience using milnacipran in patients with juvenile fibromyalgia: lessons from a clinical trial program. Pediatric Rheumatology Online Journal 13:27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold LM, Schikler KN, Bateman L, Khan T, Pauer L, Bhadra-Brown P, Clair A, Chew ML, Scavone J, Pregabalin Adolescent Fibromyalgia Study G. Safety and efficacy of pregabalin in adolescents with fibromyalgia: a randomized, double-blind, placebo-controlled trial and a 6-month open-label extension study. Pediatr Rheumatol Online Journal 14:46, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berde CB, Walco GA, Krane EJ, Anand KJ, Aranda JV, Craig KD, Dampier CD, Finkel JC, Grabois M, Johnston C, Lantos J, Lebel A, Maxwell LG, McGrath P, Oberlander TF, Schanberg LE, Stevens B, Taddio A, von Baeyer CL, Yaster M, Zempsky WT. Pediatric analgesic clinical trial designs, measures, and extrapolation: report of an FDA scientific workshop. Pediatrics 129:354–64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain 41:139–150, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Borusiak P, Biedermann H, Bosserhoff S, Opp J. Lack of efficacy of manual therapy in children and adolescents with suspected cervicogenic headache: results of a prospective, randomized, placebo-controlled, and blinded trial. Headache 50:224–230, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Brousseau DC, Duffy SJ, Anderson AC, Linakis JG. Treatment of pediatric migraine headaches: a randomized, double-blind trial of prochlorperazine versus ketorolac. Annals of Emergency Medicine 43:256–262, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bursch B, Walco GA, Zeltzer L. Clinical assessment and management of chronic pain and pain-associated disability syndrome. Journal of Developmental and Behavioral Pediatrics 19:45–53, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Caldwell PH, Butow PN, Craig JC. Pediatricians’ attitudes toward randomized controlled trials involving children. Journal of Pediatrics 141:798–803, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet 364:803–811, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Conte PM, Walco GA, Kimura Y. Temperament and stress response in children with juvenile primary fibromyalgia syndrome. Arthritis and rheumatism 48:2923–2930, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Dworkin JD, McKeown A, Farrar JT, Gilron I, Hunsinger M, Kerns RD, McDermott MP, Rappaport BA, Turk DC, Dworkin RH, Gewandter JS. Deficiencies in reporting of statistical methodology in recent randomized trials of nonpharmacologic pain treatments: ACTTION systematic review. J Clin Epidemiol 72:56–65, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Eccleston C, Malleson P. Managing chronic pain in children and adolescents. We need to address the embarrassing lack of data for this common problem. BMJ 326:1408–1409, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewandter JS, Eisenach JC, Gross RA, Jensen MP, Keefe FJ, Lee DA, Turk DC. Checklist for the preparation and review of pain clinical trial publications: a pain-specific supplement to CONSORT. Pain Reports e621:1–8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gewandter JS, McDermott MP, McKeown A, Smith SM, Williams MR, Hunsinger M, Farrar J, Turk DC, Dworkin RH. Reporting of missing data and methods used to accommodate them in recent analgesic clinical trials: ACTTION systematic review and recommendations. Pain 155:1871–7, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Gewandter JS, McKeown A, McDermott MP, Dworkin JD, Smith SM, Gross RA, Hunsinger M, Lin AH, Rappaport BA, Rice AS, Rowbotham MC, Williams MR, Turk DC, Dworkin RH. Data interpretation in analgesic clinical trials with statistically nonsignificant primary analyses: an ACTTION systematic review. J Pain 16:3–10, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Gewandter JS, Smith SM, McKeown A, Burke LB, Hertz SH, Hunsinger M, Katz NP, Lin AH, McDermott MP, Rappaport BA, Williams MR, Turk DC, Dworkin RH. Reporting of primary analyses and multiplicity adjustment in recent analgesic clinical trials: ACTTION systematic review and recommendations. Pain 155:461–6, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Gewandter JS, Smith SM, McKeown A, Edwards K, Narula A, Pawlowski JR, Rothstein D, Desjardins PJ, Dworkin SF, Gross RA, Ohrbach R, Rappaport BA, Sessle BJ, Turk DC, Dworkin RH. Reporting of adverse events and statistical details of efficacy estimates in randomized clinical trials of pain in temporomandibular disorders: Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks systematic review. JADA 146:246–54 e6, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Gherpelli JL, Esposito SB. A prospective randomized double blind placebo controlled crossover study of fluoxetine efficacy in the prophylaxis of chronic daily headache in children and adolescents. Arquivos de Neuro-psiquiatria 63:559–563, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Goodman JE, McGrath PJ. The epidemiology of pain in children and adolescents: a review. Pain 46:247–264, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Hunsinger M, Smith SM, Rothstein D, McKeown A, Parkhurst M, Hertz S, Katz NP, Lin AH, McDermott MP, Rappaport BA, Turk DC, Dworkin RH. Adverse event reporting in nonpharmacologic, noninterventional pain clinical trials: ACTTION systematic review. Pain 155:2253–62, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Ioannidis JP, Evans SJ, Gotzsche PC, O’Neill RT, Altman DG, Schulz K, Moher D. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 141:781–8, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Joseph PD, Craig JC, Caldwell PH. Clinical trials in children. British Journal of Clinical Pharmacology 79:357–369, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, Passo MH, Schikler KN, Hashkes PJ, Spalding S, Lynch-Jordan AM, Banez G, Richards MM, Lovell DJ. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum 64:297–305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain 152:2729–38, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Korterink JJ, Ockeloen LE, Hilbink M, Benninga MA, Deckers-Kocken JM. Yoga Therapy for Abdominal Pain-Related Functional Gastrointestinal Disorders in Children: A Randomized Controlled Trial. Journal of Pediatric Gastroenterology and Nutrition 63:481–487, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Lanzi G, D’Arrigo S, Termine C, Rossi M, Ferrari-Ginevra O, Mongelli A, Millul A, Beghi E. The effectiveness of hospitalization in the treatment of paediatric idiopathic headache patients. Psychopathology 40:1–7, 2007 [DOI] [PubMed] [Google Scholar]
- 28.MacLennan SC, Wade FM, Forrest KM, Ratanayake PD, Fagan E, Antony J. High-dose riboflavin for migraine prophylaxis in children: a double-blind, randomized, placebo-controlled trial. Journal of Child Neurology 23:1300–1304, 2008 [DOI] [PubMed] [Google Scholar]
- 29.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain 9:771–83, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Palermo TM, Kashikar-Zuck S, Friedrichsdorf SJ, Powers SW. Special considerations in conducting clinical trials of chronic pain management interventions in childtren and adolescents and their families. Pain Reports. ACTTION Spcial Issu on Clinical Trials of Pain Treatments. e659, 2018 [DOI] [PMC free article] [PubMed]
- 31.Robins PM, Smith SM, Glutting JJ, Bishop CT. A randomized controlled trial of a cognitive-behavioral family intervention for pediatric recurrent abdominal pain. Journal of Pediatric Psychology 30:397–408, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Saps M, Youssef N, Miranda A, Nurko S, Hyman P, Cocjin J, Di Lorenzo C. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 137:1261–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwaller F, Fitzgerald M. The consequences of pain in early life: injury-induced plasticity in developing pain pathways. European Journal of Neuroscience 39:344–352, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, Chang RD, Pereira A, Shah N, Gilron I, Katz NP, Lin AH, McDermott MP, Rappaport BA, Rowbotham MC, Sampaio C, Turk DC, Dworkin RH. Adherence to CONSORT harms-reporting recommendations in publications of recent analgesic clinical trials: an ACTTION systematic review. Pain 153:2415–21, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Wang AT, Katz NP, McDermott MP, Burke LB, Coplan P, Gilron I, Hertz SH, Lin AH, Rappaport BA, Rowbotham MC, Sampaio C, Sweeney M, Turk DC, Dworkin RH. Adverse event assessment, analysis, and reporting in recent published analgesic clinical trials: ACTTION systematic review and recommendations. Pain 154:997–1008, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Smith SM, Wang AT, Pereira A, Chang RD, McKeown A, Greene K, Rowbotham MC, Burke LB, Coplan P, Gilron I, Hertz SH, Katz NP, Lin AH, McDermott MP, Papadopoulos EJ, Rappaport BA, Sweeney M, Turk DC, Dworkin RH. Discrepancies between registered and published primary outcome specifications in analgesic trials: ACTTION systematic review and recommendations. Pain 154:2769–74, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Stevens J, Hayes J, Pakalnis A. A randomized trial of telephone-based motivational interviewing for adolescent chronic headache with medication overuse. Cephalalgia 34:446–454, 2014 [DOI] [PubMed] [Google Scholar]
- 38.U.S. Health and Human Services FDA. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Accessed [05–08-2018] http://www.fda.gov/downloads/Drugs/…/Guidances/UCM193282.pdf 2009 [DOI] [PMC free article] [PubMed]
- 39.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 152:S2–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeltzer LK, Anderson CT, Schechter NL. Pediatric pain: current status and new directions. Current Problems in Pediatrics 20:409–486, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Zouikr I, Bartholomeusz MD, Hodgson DM. Early life programming of pain: focus on neuroimmune to endocrine communication. Journal of Translational Medicine 14:123, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.