Abstract
Background:
Bladder cancer recurrence following cystectomy remains a significant cause of bladder cancer-specific mortality. Residual cancer cells (RCCs) contribute to cancer recurrence due either to tumor spillage or undetectable pre-existing micrometastatic tumor clones. We sought to detect and quantify RCCs in pelvic washing using ultra-deep targeted sequencing (UTS) and compare the levels of RCCs with clinical variables and cancer recurrence.
Methods:
17 patients underwent robotic-assisted radical cystectomy (RARC) with primary tumor specimen available. All tumors had negative surgical margins. Pelvic washes and blood were collected intra-operatively: before RARC, after RARC, after pelvic lymph node dissection (PLND), and in the suction fluid collected during the procedure. A two-step sequencing, including whole-exome sequencing (WES) followed by UTS (>50,000X), was used to quantify RCCs in each sample. Eight patients were excluded due to sample quality issues. The final analysis cohort included nine patients. RCC level was quantified for each sample as the relative cancer cell fraction (RCCF), and compared between different time points. The peak RCCF (pRCCF) of each patient was correlated with clinical and pathological variables.
Results:
RCCs were detected in approximately half of the pelvic washing specimens during or after RARC, but not before it. Higher levels of RCCs were associated with aggressive variant histology and cancer recurrence. Verifying the feasibility of using RCCs as a novel biomarker for recurrence requires larger cohorts.
Conclusions:
Detection of RCCs in intra-operative peritoneal washes of bladder cancer patients undergoing radical cystectomy may represent a robust biomarker of tumor aggressiveness and metastatic potential.
Keywords: bladder cancer, residual cancer cell, recurrence, spillage, micrometastatic tumor clones, Robot-assisted cystectomy, urothelial, next-generation sequencing, pneumoperitoneum
Introduction
Robot-assisted radical cystectomy (RARC) was implemented to improve peri-operative outcomes without jeopardizing oncological efficacy [1]. Irrespective of the surgical approach, approximately 18% of RARC patients develop local recurrence after radical cystectomy, the majority within the first two years after surgery [2]. Recurrence of urothelial carcinoma has been reported in abdominal wound-/port-sites, suprapubic tube sites, in the pelvic cavity (resection bed), and on the psoas muscle [3–5].
The exact pathogenesis of bladder cancer recurrence has not been fully elucidated. Even after radical cystectomy with a negative surgical margin, the recurrent rates remained high and were correlated with the disease stage/aggressiveness of the primary tumor [6, 7], suggesting the existence of residual cancer cells (RCCs) such as pre-existing micrometastatic tumor foci as indicated by the patterns of T cell infiltration and tumor-associated antigen in non-neoplastic tissues around cancer[8], or tumor cells spilled during the surgical procedure[9]. During RARC, pneumo-peritoneum, possibly through inhibition of local peritoneal immune response by CO2, or solely by the fluctuation in pressure, may contribute to tumor dissemination [10, 11]. There is a pressing need for an accurate methodology for detection, quantification of RCCs and stratification of their relative risks, whether caused by tumor spillage during surgery and/or pre-exist micrometastatic clones.
In the current study, we investigated using high-throughput sequencing for the detection of RCCs as the potential source of recurrent bladder cancer based on the presence in RCCs of conserved, tumor-specific mutations that were identified in the primary tumor. The ultra-sensitive detection of rare residual disease, of circulating tumor cells (CTCs) or of cell-free DNA (cfDNA), has been advanced dramatically by these new sequencing technologies [12]. Because of their rarity, identification and quantification of RCCs requires not only extremely high coverage but also computational algorithms that accurately distinguish RCCs from background errors due to artifact [13]. Here, we developed this methodology for identification of RCCs in intra-operative pelvic washes using patient-specific (primary tumor-specific) somatic mutations (Figure 1a-b), and to explore the potential association of RCCs with cancer recurrence and other clinical variables.
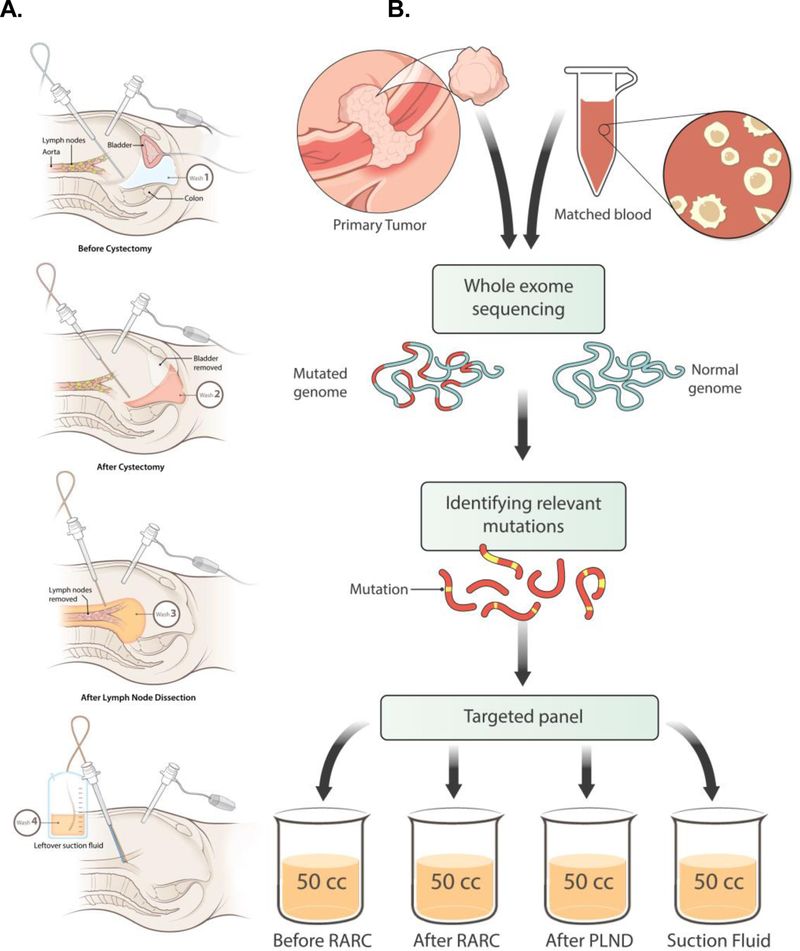
Figure 1.
A. Multiple samples were collected during the process of RARC; B. Sample Processing for NGS-based analysis involving WES and TAS.
Methods
Patient selection
40 patients who underwent RARC and PLND by a single surgeon (K. A. G) at Roswell Park Comprehensive Cancer Center were enrolled under IRB protocols I-258714 and I-160009. The technique of RARC and intra-corporeal ileal conduit was previously reported [14, 15]. All patients were evaluated and followed-up in compliance with the National Comprehensive Cancer Network guidelines.
Sample collection
Intra-operative pelvic irrigations (washes) were collected at: Tf1-before-RARC, Tf2-after-RARC, Tf3-after-PLND, Tf4-suction (Figure 1a, c) and detailed in supplemental methods. An additional wash of Tf5-filter was collected in Pt30. Peripheral blood samples were collected at: before-RARC, after-RARC and after-PLND.
Quantification of residual cancer cells
Whole-exome sequencing (WES) was performed to identify patient-specific somatic mutations. Ultra-deep targeted sequencing (UTS) was performed to sequence selected mutations (12–17/patient) in all specimens (Figure 1b). The detailed descriptions of experimental methods for WES and UTS, and analytic procedures for somatic mutation identification, and RCCF calculation are included in the Supplemental Methods.
For each patient, the highest RCCF observed in pelvic washes at any one of the three time points: before-RARC, after-RARC, after-PLND, or from the suction fluid, was considered the peak RCCF (pRCCF), which was compared with clinical and pathological variables, including tumor stage, lymph node status, positive surgical margin, histology, as well as cancer recurrence, to determine their association.
Results
Study cohort:
40 consecutive patients underwent RARC were initially enrolled. Before the current NGS-based study, we first investigated pelvic washes with conventional methods, such as cytology with immunohistochemistry, and mRNA markers, but were unable to reliably detect RCCs due to limited sensitivity (data not shown). The current study included the 17 patients for which the primary tumor samples were available, which was required for identifying patient-specific mutations (Table 1). All patients received a multidisciplinary consult for neoadjuvant chemotherapy; 6 of 17 patients received it. All patients received intracorporeal ileal conduits. No inadvertent entry into the bladder was observed, and both ureters and the urethra were clipped before removal in all cases. Other than one squamous cell carcinoma (SCC, in Pt#14), all other 16 patients had a primary histology of high-grade urothelial carcinoma (hgUC), including four patients with variant histology such as plasmacytoid, squamous, or glandular differentiation. All tumors had negative surgical margins.
Table 1.
Clinical information of the 17 patients and availability of NGS data
| Histology |
Neoadjuvant Chemotherapy |
Followup (mos) |
Next Generation Sequencing |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt No. |
Pathology Stage |
Primary High Grade Urothelial Ca |
Secondary | Total | Postop Recurrence |
Batch No. |
12+ High- VAF mutations |
Ultradeep Targeted Sequencing |
Ultradeep Targeted Sequencing Quality Assurance |
|
| 10 | TaN1 | Yes | Plasma cytoid | Yes | 28 | 2 | 1 | Yes | Yes | Pass |
| 11 | T2bN0 | Yes | - | No | 24 | No | 2 | No | - | - |
| 13 | T1N0 | Yes | - | Yes | 16 | No | 1 | No | - | - |
| 14 | T4bN0 | No+ | - | No | 28 | 3 | 2 | Yes | Yes | Pass |
| 15 | T2bN0 | Yes | Squamouse + plasma cytoid | No | 27 | 9 | 2 | Yes | Yes | Pass |
| 16 | T4N1 | Yes | Squamous | No | 27 | 16 | 2 | Yes | Yes | Pass |
| 20 | T3aN0 | Yes | - | No | 24 | No | 2 | Yes | Yes | Pass |
| 22 | T1N0 | Yes | - | No | 11 | No | 3 | No | ||
| 24 | T3N2 | Yes | - | Yes | 6 | 2 | 3 | Yes | Yes | Pass |
| 28 | T3aN0 | Yes | - | No | 21 | 9 | 3 | Yes | Yes | Pass |
| 30 | T3bN0 | Yes | Glanduolar differentiation | No | 19 | No‡ | 3 | Yes | Yes | Pass |
| 31 | TisN2 | Yes | - | Yes | 17 | No | 3 | No | - | - |
| 32 | T3bN0 | Yes | - | No | 11 | No | 3 | Yes | Yes | Fail |
| 33 | T3aN0 | Yes | - | No | 18 | No | 3 | Yes | Yes | Pass |
| 40 | T0N0 | Yes | - | No | 18 | No | 3 | No | - | - |
| 41 | T0N1 | Yes | - | Yes | 17 | 17 | 3 | No | - | - |
| 42 | TisN0 | Yes | - | Yes | 16 | No | 3 | No | - | - |
No positive margins
Whole exome sequencing was done in all patients.
Squamous cell carcinoma.
Renal carcinoma 3.6 months postoperatively.
Identification of somatic mutations:
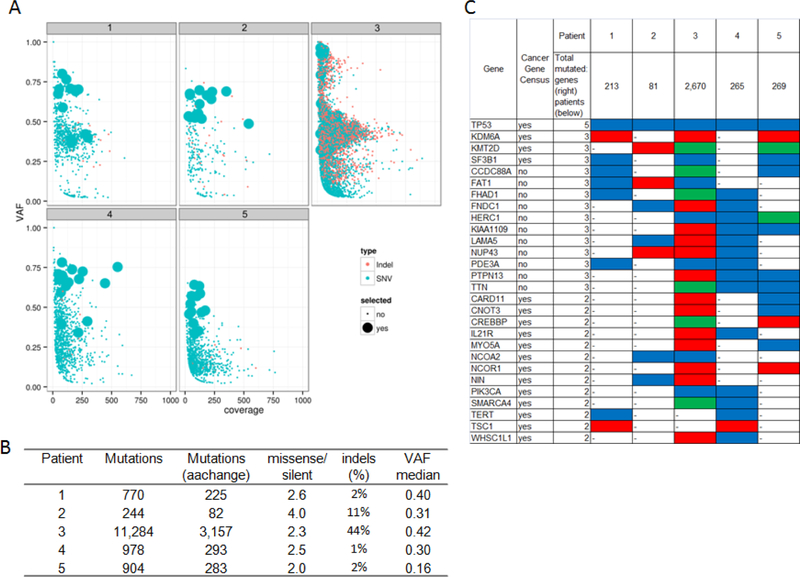
WES was performed on the primary tumor and the matched blood DNAs (germline DNA) for all 17 patients. The WES mapping summary, coverage statistics, and mutational landscape are detailed in the Supplemental Results. Several patients were found to have few somatic mutations, and most of the identified mutations had low variant allele fraction (VAFs) (Figure S1), indicating possible low tumor purity, or significant heterogeneity, or tumorigenesis processes driven by other alterations such as copy number or structural variations. Since the identification of RCCs by targeted sequencing would rely on the tracing of specific mutations identified by WES that are clonal rather than subclonal, for qualified patients at least 12 high-VAF mutations (defined as >=0.3) were identified, which excluded seven patients from further analyses (Figure 2a). The remaining 10 tumors included one SCC and nine hgUCs; four of the hgUCs had variant histology. TP53 was mutated in all five cases with more aggressive histology (SCC or hgUCs with variant histology), but only in 3 of the 5 of the remaining tumors. Mutations in several genes exhibited a potential pattern of being group-specific in the current cohort, including PIK3CA (3/6 in recurrent only), KRAS and MYCN (both 2/4 in non-recurrent only) (Figure 2b).
Figure 2. Primary tumor mutation profiles and selection of mutations for targeted sequencing.
A. Distribution of VAF and coverage of somatic mutations found by WES in each patient;
B. Summary statistics of mutational profile by patient;
C. Frequently mutated genes with highlight in Cancer Gene Census genes12.
Quantification of residual cancer cells:
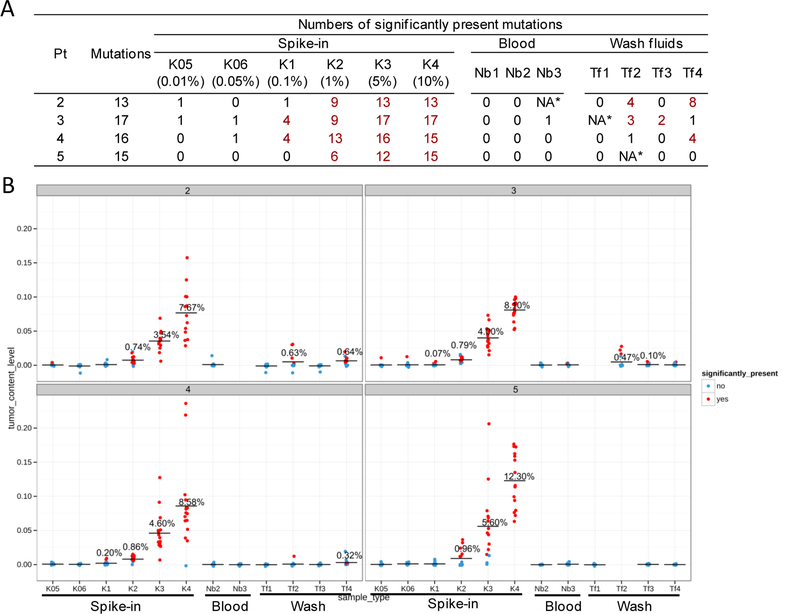
The design and statistics of UTS panels are summarized in Figure 1b-c. For each patient, 12–17 high-VAF mutations (>30% in WES) were selected (Table S2) as markers of the presence of RCCs, and were sequenced to a minimum of 50,000X coverage in the primary tumor (n=1), blood (n=2–3), pelvic washes (n=3–4) and spike-in samples (n=4 or 6). Using the first patient (Pt10) as an example for illustration, 12 mutations were selected as markers and sequenced in all samples, including the spike-in (n=4), primary tumor (n=1), blood (n=3) and pelvic washes (n=3). The VAFs were elevated markedly to varying levels in the spike-in samples, the VAFs in all three blood samples of Pt10 were at baseline, and VAFs were elevated in one pelvic washing sample (after-RARC) (Figure S4). After calculation of RCCF, the spike-in samples demonstrated a high level of accuracy compared to the designed spike-in percentage: 10.11% vs 10% in K4, 4.86% vs 5% in K3, 0.89% vs 1% in K2, and 0.08% vs 0.1% in K1 (Figure 3a). The RCCF in that after-RARC pelvic washing sample was 0.27%. One patient (Pt32) was excluded due to possible sample contamination identified with spike-in controls: in the three spike-in controls with the highest mixing ratios (10%, 5% and 1%), the relative errors were 5–10 folds higher in Pt32 than any other patient (Figure S5). The final analysis cohort included all remaining nine patients.
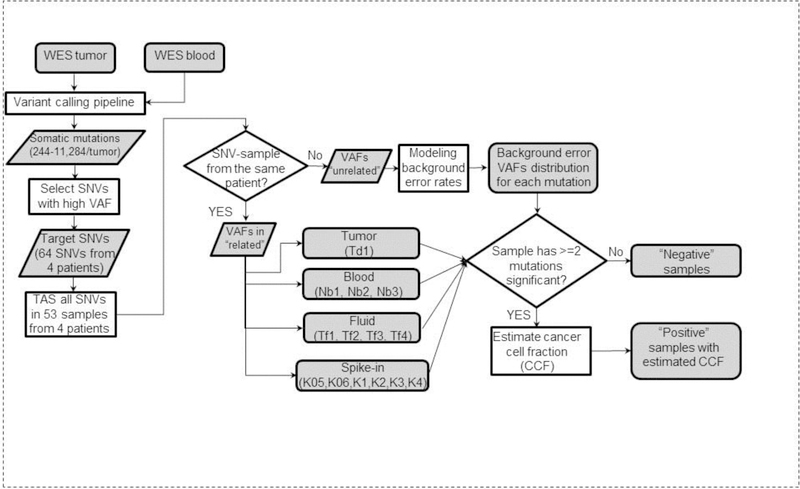
Figure 3. Strategy for identifying tumor-positive samples and estimation of CCF.
WES was first performed to identify somatic mutations. Then based on a list of selected mutations from all four patients, a customized panel was designed and sequenced in all samples from these four patients. The results were used to build background error model of each mutation, and then identify mutations with significantly elevated VAFs to be considered as detected. Any sample with at least two mutations detected was considered as tumor-positive.
Pattern of residual cancer cells:
The levels of residual cancer cells, as measured by RCCFs, in blood and pelvic washes for all patients in the final analysis cohort are listed in Figure 3b. Almost all blood samples (16/17) after-RARC and after-PLND were negative for RCCs except for one sample (Pt30 after-PLND), where RCCs were present at an extremely low level (RCCF=0.02%). All available pelvic washes before RARC were negative for RCCs (0/7). After RARC, most pelvic washes (7/8) contained detectable RCCs, with RCCFs ranging from 0.02–0.63%. After PLND, 3 of 9 pelvic washes were positive for RCCs. The suction fluid was positive in 4/9 patients, including one patient (Pt16) who had no other RCC-positive wash. Altogether, about half (14/26) of the pelvic washes collected during-or after-surgery were positive, compared with 0/7 washes collected before-RARC. A significantly higher portion of after-RARC washes (7/8) were positive than after-PLND washes (3/9, p<0.05). Most patients (8/9) had at least one tumor-positive washing, with the peak RCCF (pRCCF), defined as the highest observed RCCF among all pelvic washes, ranging from 0.07–0.64%.
Association of pRCCF with clinical variables:
To determine if a correlation existed between the detected pRCCF and clinical and histopathologic variables, pRCCF was compared with: tumor stage, histology, lymph node status, surgical margin and recurrence (Figure 4a). A striking association was found between the pRCCF and tumor histology: when ranked by the pRCCFs, the five patients with the highest pRCCFs were exclusively associated with aggressive histologies: the highest pRCCF of 0.64%, was found in Pt14 who was the only patient with a primary histology of SCC, which was more aggressive than hgUC; among the remaining eight hgUC patients, the four with the highest pRCCFs all demonstrated aggressive variant histology, which was not found in the remaining four hgUC with lower pRCCFs. Overall, pRCCFs were significantly higher (p<0.01, Wilcoxon) in patients with aggressive histology (SCC, or hgUC with variant histology, median=0.47) than in patients with hgUC without variant histology (median=0.12).
Figure 4. Identification of tumor-positive samples and estimation of CCF by TAS.
A. Numbers of total selected mutations and detected mutations in every sample of the four patients. A minimum of two detected mutations was required for any sample to be considered as tumor positive (highlighted by red font). NA*: sample whose DNA yield was insufficient to be sequenced.
B. CCFs calculated by the VAF of individual mutations. Red dots represent the mutations whose VAF were significantly higher than background error rates and thus considered as detected. The horizontal bars indicate the mean CCF of the sample (averaged across all mutations in that sample). For samples that were found to be tumor positive (at least two mutations detected), the exact values of mean CCF is displayed above the horizontal bar.
Despite the small sample size, the current results suggest a potential association between the pRCCF and recurrence status: the majority of patients (6/7) with high-pRCCF (>0.1%) developed recurrence within 2–16 months after RARC (median = 5.5 months), while none of the low-or negative-pRCCF patients (0/2) developed a recurrence during a follow-up period between 18 and 24 months. The only high-pRCCF patient who did not have a recurrence (Pt30) had a renal mass 3.6 months after surgery which was later diagnosed as a primary renal cell carcinoma and, therefore, considered as an independent primary tumor. This patient was the only patient with a positive blood sample (after PLND), and all three washes were positive for RCCs. For this patient, we tested an additional sample from an air exhaust filter of the peritoneal cavity, and found it was also positive with a low RCCF of 0.04% (data not shown). Remarkably, two higher grade patients (Pt33 and Pt20: T3a) but with low-or negative-pRCCF did not have a recurrence, whereas, two lower stage patients (Pt10: Ta and Pt15: T2b) with high-pRCCFs experienced recurrence. Among the three patients (Pt28, Pt33 and Pt20) with the exact same stage and histology (T3aN0 and hgUC with no secondary histology), only one patient with high-pRCCF (Pt28) developed a later recurrence. Additionally, no correlation was found between pRCCF and the length of time from cystectomy to recurrence (Figure 4b).
Discussion
Despite the recent advances in neoadjuvant chemotherapy and the aggressive management of bladder cancer, more than half of the patients with muscle-invasive bladder cancer experience recurrence with a significant adverse impact on survival [2, 16]. Prior studies showed that recurrence was associated with disease-related variables and surgery-related factors [17]. Breach of oncologic surgical principles and laparoscopy-related factors also have been implicated in local disease recurrence [10, 18]. However, recent studies failed to show any association between the approach to RC and recurrence [19, 20], suggesting recurrence was associated with disease-related rather than technical (laparoscopy-related) factors [16].
The lack of identification of RCCs by conventional methods in patients that later presented with recurrence suggested RCCs are either extremely low in abundance or already disseminated to distant sites by the time of surgery. In this study using ultra-deep sequencing, RCCs were detectable in the pelvic washes collected after-RARC and after-PLND, but not before-RARC. All patients had negative surgical margins, and most had negative lymph nodes. These findings suggest that even in patients who were “cleared” by conventional markers, RCCs may still be detectable using more sensitive methods.
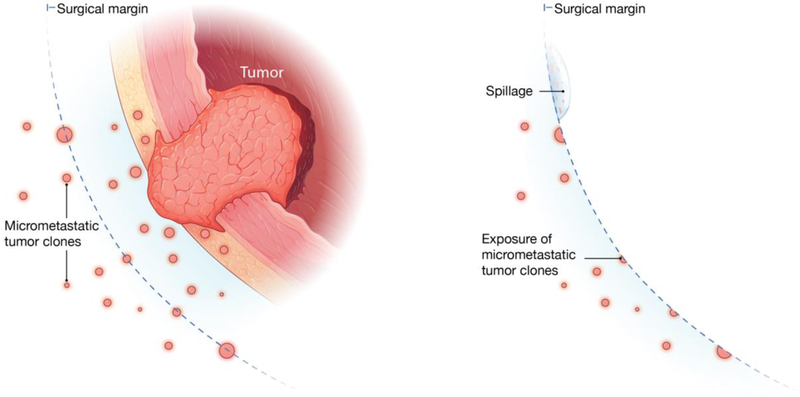
The essential question is the source of RCCs: do they represent “synchronous”, or pre-existing, metastases, or iatrogenic dissmeination due to surgical failure that could give rise to “metachronous” metastases. Between the two hypotheses of accidental tumor spillage versus pre-existing micrometastases (Figure 5), our current results suggest that the association observed between high pRCCF and aggressive histology may indicate that the levels of pRCCFs may be determined by the biology of the tumor, rather than random spillage. The fact that RCCs were undetectible before-RARC may suggest the micrometastases were “sequestered” beneath the intact peritoneum, but became accessible after the disruption of peritoneum and exposure of the underneath tissue structures during surgery (Figure S6). No conclusions can be made due to the limited sample size. Furthermore, unlike previous reports of increased numbers of circulating tumor cells in blood after surgery presumably caused by spillage [21], this study found almost all blood samples after-RARC and after-PLND were negative for RCCs, suggesting tumor cells were not shed into the circulation.
Figure 5. Two hypothesized mechanisms for residual tumor cells.
a, Before surgery. b, After surgery
Compared with conventional markers for identification of residual cancer, such as positive surgical margin or lymph node, the current method based on ultra-deep sequencing has the potential of being used to develop a new concept of “molecular margin free” in helping identifying patients at high risk of disease progression in bladder and other cancers with high recurrence rate after surgery [22]. In this study, pN stage and variant histology had good accuracy, but poor sensitivity, for predicting recurrences as 6/9 and 4/9 patients did not have positive lymph nodes or aggressive histology, respectively. Further, in all nine patients, the surgical margins were negative and, therefore, did not provide any predictive value. Potential patient stratification using pRCCF could be especially useful in these cases without any indication of residual cancer by conventional markers..
The current study is considered a proof-of-principle due to the small sample size. Future studies on larger numbers of patients including a more complete coverage of different subtype, stage and histology will provide a more comprehensive understanding about biological mechanisms responsible for RCCs under different clinical conditions, and confirm RCCs’ relation with disease recurrence. The current cutoff of a pRCCF > 0.1% for patients being considered as high-pRCCF might also need to be optimized with additional clinical variables in larger patient cohorts. The design of targeted sequencing was based on high-VAF mutations, which are more likely to be clonal rather than subclonal mutations. Althought this strategy reduces the chance of potential false-negative due to tumor heterogeneity, it is also more difficult to apply the technique in tumors with low purity or low mutation burden. Lastly, the current detection method, based on whole-exome sequencing and ultra-deep targeted sequencing, was optimized for maximum sensitivity but requires a long turn-around time and high cost, which would need to be improved for potential future clinical utility. The identified patterns of RCCs suggest they are unlikely to be caused by random tumor spillage during surgery, but probably indicate the pre-existence of infiltrating micrometastatic tumor clones clearly reflect the level of aggressiveness of the tumor. These findings, if confirmed by larger future studies, will pave the road for development of novel approaches using the measurement of RCCs as highly sensitive biomarkers for predicting recurrence. Such prognostic information might stratify paients that would benefit from intra-peritoneal chemotherapy, similar to ovarian, gastric and colorectal malignancies [23–25].
Conclusion
This proof-of-principle study based on a limited patient cohort demonstrated the feasibility of using ultra-deep sequencing to achieve accurate quantification of RCCs, and revealed a potential association with histology and cancer recurrence.
Supplementary Material
Acknowledgments
Source of Funding: Roswell Park Alliance Foundation and Friends of Urology. This work was supported by National Cancer Institute (NCI) grant P30CA016056.
References
- [1].Raza SJ, Wilson T, Peabody JO, Wiklund P, Scherr DS, Al-Daghmin A, et al. Long-term oncologic outcomes following robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol 2015;68:721–8. [DOI] [PubMed] [Google Scholar]
- [2].Sonpavde G, Khan MM, Lerner SP, Svatek RS, Novara G, Karakiewicz PI, et al. Disease-free survival at 2 or 3 years correlates with 5-year overall survival of patients undergoing radical cystectomy for muscle invasive bladder cancer. J Urol 2011;185:456–61. [DOI] [PubMed] [Google Scholar]
- [3].Breul J, Block T, Breidenbach H, Hartung R. Implantation metastasis after a suprapubic catheter in a case of bladder cancer. Eur Urol 1992;22:86–8. [DOI] [PubMed] [Google Scholar]
- [4].Chakravarti A, Day DW, MacDermott S. Extravesical transitional cell carcinoma as a result of implantation after perforation of the bladder. BJU Int 2000;85:1150–1. [DOI] [PubMed] [Google Scholar]
- [5].Nabi G, Dogra PN, Pradeep H. Psoas abscess-like metastasis from transitional cell carcinoma of urinary bladder. Indian J Cancer. 2002;39:78–80. [PubMed] [Google Scholar]
- [6].Dotan ZA, Kavanagh K, Yossepowitch O, Kaag M, Olgac S, Donat M, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol 2007;178:2308–12; discussion 13. [DOI] [PubMed] [Google Scholar]
- [7].Christodouleas JP, Baumann BC, He J, Hwang WT, Tucker KN, Bekelman JE, et al. Optimizing bladder cancer locoregional failure risk stratification after radical cystectomy using SWOG 8710. Cancer. 2014;120:1272–80. [DOI] [PubMed] [Google Scholar]
- [8].Parodi A, Traverso P, Kalli F, Conteduca G, Tardito S, Curto M, et al. Residual tumor micro-foci and overwhelming regulatory T lymphocyte infiltration are the causes of bladder cancer recurrence. Oncotarget. 2016;7:6424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoglund M. Bladder cancer, a two phased disease? Semin Cancer Biol 2007;17:225–32. [DOI] [PubMed] [Google Scholar]
- [10].Hewett PJ, Thomas WM, King G, Eaton M. Intraperitoneal cell movement during abdominal carbon dioxide insufflation and laparoscopy. An in vivo model. Dis Colon Rectum 1996;39:S62–6. [DOI] [PubMed] [Google Scholar]
- [11].Jacobi CA, Wenger FA, Ordemann J, Gutt C, Sabat R, Muller JM. Experimental study of the effect of intra-abdominal pressure during laparoscopy on tumour growth and port site metastasis. Br J Surg 1998;85:1419–22. [DOI] [PubMed] [Google Scholar]
- [12].Alix-Panabieres C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov 2016;6:479–91. [DOI] [PubMed] [Google Scholar]
- [13].Arbeithuber B, Makova KD, Tiemann-Boege I. Artifactual mutations resulting from DNA lesions limit detection levels in ultrasensitive sequencing applications. DNA Res 2016;23:547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Poch MA, Raza J, Nyquist J, Guru KA. Tips and tricks to robot-assisted radical cystectomy and intracorporeal diversion. Current opinion in urology. 2013;23:65–71. [DOI] [PubMed] [Google Scholar]
- [15].Azzouni FS, Din R, Rehman S, Khan A, Shi Y, Stegemann A, et al. The first 100 consecutive, robot-assisted, intracorporeal ileal conduits: evolution of technique and 90-day outcomes. Eur Urol 2013;63:637–43. [DOI] [PubMed] [Google Scholar]
- [16].Hussein AA, Saar M, May PR, Wijburg CJ, Richstone L, Wagner A, et al. Early Oncologic Failure after Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J Urol 2017;197:1427–36. [DOI] [PubMed] [Google Scholar]
- [17].Huguet J. Follow-up after radical cystectomy based on patterns of tumour recurrence and its risk factors. Actas Urol Esp 2013;37:376–82. [DOI] [PubMed] [Google Scholar]
- [18].Abaza R, Keck RW, Selman SH. Intraperitoneal chemotherapy for the prevention of transitional cell carcinoma implantation. J Urol 2006;175:2317–22. [DOI] [PubMed] [Google Scholar]
- [19].Nguyen DP, Al Hussein Al Awamlh B, Wu X, O’Malley P, Inoyatov IM, Ayangbesan A, et al. Recurrence patterns after open and robot-assisted radical cystectomy for bladder cancer. Eur Urol 2015;68:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pourmalek F, Abdi H, Black PC. Re: Nguyen Daniel P., Bashir Al Hussein Al Awamlh, Xian Wu, et al. Recurrence Patterns After Open and Robot-assisted Radical Cystectomy for Bladder Cancer. Eur Urol 2015;68:399–405. Eur Urol. 2016;69:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Engilbertsson H, Aaltonen KE, Bjornsson S, Kristmundsson T, Patschan O, Ryden L, et al. Transurethral bladder tumor resection can cause seeding of cancer cells into the bloodstream. J Urol 2015;193:53–7. [DOI] [PubMed] [Google Scholar]
- [22].Hockel M, Dornhofer N. The hydra phenomenon of cancer: why tumors recur locally after microscopically complete resection. Cancer Res 2005;65:2997–3002. [DOI] [PubMed] [Google Scholar]
- [23].Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev 2016:CD005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kang LY, Mok KT, Liu SI, Tsai CC, Wang BW, Chen IS, et al. Intraoperative hyperthermic intraperitoneal chemotherapy as adjuvant chemotherapy for advanced gastric cancer patients with serosal invasion. J Chin Med Assoc 2013;76:425–31. [DOI] [PubMed] [Google Scholar]
- [25].Mohamed F, Cecil T, Moran B, Sugarbaker P. A new standard of care for the management of peritoneal surface malignancy. Curr Oncol 2011;18:e84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.