Abstract
Background/Aims:
This study was carried out to investigate the effect of Bletilla striata polysaccharide (BSP) treating on lipopolysaccharide (LPS)-induced intestinal epithelial barrier disruption in rat intestinal epithelial cell (IEC) line.
Materials and Methods:
LPS was used to stimulate the IEC-18 cells (1 μg/ml), with or without different concentrations of BSP (25, 50 and 100 μg/ml) for 24 h. Transepithelial electrical resistance (TEER) was measured to detect the permeability of cells. Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in the cell supernatant were detected with the enzyme-linked immunosorbent assay (ELISA). Real-time polymerase chain reaction (PCR) was employed to detect the mRNA levels of zonulae occludens (ZO)-1 and occludin. Western blot and immunofluorescence analysis were used for analyzing the expression level and the distribution patterns of ZO-1 and occludin protein.
Results:
After treatment with BSP, the IL-6 and TNF-α levels in the cell supernatant were significantly decreased compared with the experiment group (P < 0.05 or 0.01). The permeability of IEC was decreased in BSP groups when compared with the experiment group (P < 0.05 or 0.01). In addition, compared with the experiment group, treatment with BSP up-regulated mRNA and protein expression levels of ZO-1 and occludin, and kept the ZO-1 and occludin protein intact in IEC-18 cells injured with LPS (P < 0.05 or 0.01).
Conclusion:
BSP has the capacity to protect IEC-18 cells from LPS-induced injury. The mechanisms may be associated with decreasing the inflammatory cytokine levels of IL-6 and TNF-α, and elevating the expression of ZO-1 and occludin, which might serve as a new protective agent for LPS-induced intestinal epithelial barrier disruption.
Keywords: Bletilla striata polysaccharide, IEC-18 cells, occludin, ZO-1
INTRODUCTION
The integrity of intestinal epithelial cells (IEC) is an important part of the intestinal mucosal barrier, which is the first defense to prevent the absorption of the noxious substances, allergens and toxins, while allowing the selective absorption of nutrients.[1] Intercellular tight junction (TJ), adherent junctions (AJs) and desmosomes constitute the integrity of intestinal epithelium layer.[2] A series of critical illnesses, such as liver cirrhosis and shock can lead to increased intestinal permeability.[3,4] Increased intestinal permeability leads to the damage of immune homeostasis and increase of inflammation in the intestine, which worsens or perpetuates critical illness, therefore, protecting the intestinal barrier is an important therapeutic strategy to maintain gastrointestinal health.
Lipopolysaccharide (LPS), which is the main component of a Gram negative bacterial cell wall and released from bacterial cell wall by shedding or through bacterial lysis, has been shown to be a key factor that affects intestinal barrier function.[5] Studies have shown that LPS could activate proinflammatory cytokine such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and also injure the intercellular TJ between epithelial cells.[6] It has been reported that LPS could increase the permeability of IEC, which is correlated with the redistribution of intestinal TJ-associated proteins.[7]
Bletilla striata is a traditional Chinese medicine that has been widely used to treat traumatic bleeding, hemorrhoids, ulcerative carbuncle and many other diseases for thousands of years.[8,9,10] A lot of compounds have been extracted from Bletilla striata, such as phenolic acid, phenanthrene, bibenzyl and polysaccharides.[11] Bletilla striata polysaccharide (BSP) is a major active component among them. It has been shown to have a variety of therapeutic effects of anti-inflammatory, anti-fibrosis nature and induce endothelial cells proliferation and vascular endothelial growth factor expression.[12,13,14]
However, it is still unknown whether BSP could ameliorate LPS-induced intestinal epithelial barrier disruption. In this study, we aimed to investigate the protective effect of BSP on LPS-induced damage in IEC-18 cells and to explore the mechanisms.
MATERIALS AND METHODS
Chemicals and reagents
BSP (≥95% titration) was purchased from Dalian MeiLun Biotechnology Co., Ltd. RPMI Medium 1640 basic and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). LPS was purchased from Sigma (St. Louis, USA). The cell counting kit-8 (CCK-8) was purchased from Dojindo Laboratories (Japan). Rabbit anti-rat ZO-1 and occludin were obtained from ProteinTech Group, Inc. (Wuhan, China), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and horseradish peroxidase (HRP)-labeled secondary antibody were acquired from Wuhan Boster Biotechnology Co., Ltd. (Wuhan, China). The enzyme-linked immunosorbent assay (ELISA) kits of IL-6 and TNF-α were purchased from Elabscience Biotechnology (Wuhan, China), RNAiso Plus, PrimeScript™ RT reagent Kit and SYBR Premix Ex Taq kit were purchased from TaKaRa (Dalian, China).
Cell culture and chemical treatment
The rat IEC-18 cell line was maintained in the RPMI-1640 medium with 10% FBS at 37°C in 5% CO2and 95% humidified air. LPS was used for cellular model establishment. Intervention groups were divided into a control group, an experiment group and BSP (25 μg/ml, 50 μg/ml, and 100 μg/ml) groups. After the cells were passaged in 6-well plates [for real-time polymerase chain reaction (RT-PCR) and western blot] or 96-well plates (for CCK-8 experiments) for 24 h and cultured to 70% density, the supernatants were removed and LPS (1 μg/ml) was used to stimulate the cells excluding the control group. In the last 12 h, BSP (25 μg/ml, 50 μg/ml, and 100 μg/ml) was added into the wells except for the control group and experiment group. After 24 h, the supernatants and cells were harvested.
Cell viability assays
The cell cytotoxicity was assessed by the CCK-8 assay. IEC-18 cells were seeded at a density of 1.0 × 105/ml in 96-well plates for 24 h to promote attachment. After incubation, the medium was replaced with 100 μl fresh medium containing various concentrations of BSP (0 μg/ml, 25 μg/ml, 50 μg/ml, and 100 μg/ml) for 12 h. Following treatment, the cells were incubated in the dark with 10 μl CCK-8 solution for 2 h at 37°C. The absorbance was read at 490 nm on microplate reader.
Transepithelial electrical resistance measurement
IEC-18 cells were cultured in the medium and made into a density of 2.5 × 105 cells/ml single cell suspension. About 1.5 ml 1640 complete medium was added into the lower cell chambers, and 1 ml cell suspension was added into the upper cell chambers, incubated at 37°C, 5% CO2, and saturated humidity overnight. It was made sure that the cells formed a tight layer and no holes were formed by dead cells. In case this condition was not met, the cells were to be re-cultured or re-seeded. According to the experimental groups, upper and lower chamber in an experiment group and BSP groups were replaced with the 1640 complete medium including LPS and incubated at 37°C and 5% CO2in saturated humidity conditions for 24 h. After 12 h, BSP groups were added BSP (25 μg/ml, 50 μg/ml, and 100 μg/ml). The normal group was added complete medium as control. Then the resistance value was detected by the calibrated Millipore Millicell ERS-2 cell resistance meter. The measured resistance value was multiplied by the area of the filter to obtain an absolute value of transepithelial electrical resistance (TEER), expressed as Ωcm2. And the TEER values were measured as follows: TEER = (measured resistance value − blank value) × single cell layer surface area (cm2).
ELISA
The release of proinflammatory cytokine in the IEC-18 cells was determined with the ELISA. Briefly, IEC-18 cells were seeded in 6-well culture plates at 3.0 × 105 cells/well overnight. The cells were exposed to LPS for 24 h. In the last 12 h, BSP at a series of concentrations (25 μg/ml, 50 μg/ml, and 100 μg/ml) were added into the wells. After cell stimulation, cell-free supernatants were collected for the determination of IL-6 and TNF-α. The procedure conformed to the instruction manuals of the relative kits. All samples were tested in duplicate.
Quantitative RT-PCR assays
Total RNA was isolated from IEC-18 cells using RNAiso Plus according to the manufacturer's protocol and kept at −80°C before use. The cDNAs were produced with the PrimeScript™ RT reagent Kit and incubated at 37°C for 15 min and 85°C for 5 s. RT-PCR reactions were performed using a StepOne Plus device (Applied Biosystems) at 95°C for 10 s, followed by 40 cycles of 95°C for 5 s and 60°C for 20 s according to the instructions of the SYBR Premix Ex Taq kit. The data were analyzed by the 2−ΔΔCt method. All the primers were synthesized by the GenScript (Nanjing, China) and the sequences were listed as follows.
ZO-1 forward 5′-GCTCACCAGGGTCAAAATGT-3′,
reverse 5′-GGCTTAAAGCTGGCAGTGTC-3′,
occludin forward 5′-TTACGGCTATGGAGGGTACAC-3′,
reverse 5′-GACGCTGGTAACAAAGATCAC-3′,
GADPH forward 5′-GGAAAGCTGTGGCGTGAT-3′,
Reverse 5′-AAGGTGGAAGAATGGGAGTT-3′.
Western blot analysis
The procedures were carried out according to our past study.[15] For cell lysate preparation, the IEC-18 monolayer cells were washed twice with ice-cold PBS, then lysed in the RIPA buffer with a cocktail of protease inhibitors on ice. Total proteins were extracted from the IEC-18 cells. Total protein concentration was determined with a BCA protein assay kit. Proteins were loaded on the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and then the proteins in the gels were transferred to polyvinylidene difluoride (PVDF) membrane. Membranes were saturated with 5% non-fat milk powder containing PBS for 1 h at room temperature, and then incubated overnight at 4°C with different primary antibodies. The dilutions of the primary antibodies were as follows, ZO-1 1:1000; occludin 1:1000; GAPDH 1:5000. After washing three times in TBST, membranes were incubated with HRP-labeled secondary antibody for 1 h. After further washing for three times with TBST, electrochemiluminescence (ECL) was used to identify immunoreactive bands. The densitometry analysis of the immunoreactive bands was performed using the Fuji Ultrasonic-Doppler velocity profile (UVP) system and the Image J program.
Immunofluorescence staining analysis
The changes of ZO-1 and occludin proteins in IEC-18 cells were determined with immunofluorescence staining. Briefly, the cells on the coverslips were grown to confluence. Then the cells were fixed in 4% paraformaldehyde for 10 min at room temperature after PBS solution was used to wash the cells, followed by the use of PBS with 0.1% Triton-X100 to permeabilize the cells for 20 min and blocked with 5% bovine serum albumin for 30 min. Thereafter, the cells were incubated with ZO-1 antibody (1:200 dilution) and occludin antibody (1:200 dilution) at 4°C overnight. After washing with PBS three times, the cells were incubated with goat anti-rabbit IgG conjugated to FITC (1:200 dilution) for 1 h at room temperature. Confocal microscope was used to capture the fluorescence images.
Statistical analysis
All statistical analyses were performed with the SPSS software version 12.0. The results were expressed as the means ± SD. Comparisons of the measurement data between groups were performed with the Student's t test and one-way analysis of variance (ANOVA) followed by the Tukey's test. P < 0.05 was considered to be statistically significant. Graphs were drawn with the GraphPad Prism software (version 6).
RESULTS
Cytotoxicity of BSP on IEC-18 cells
In this study, we evaluated the effect of BSP at a series of concentrations (0 μg/ml, 25 μg/ml, 50 μg/ml, and 100 μg/ml) on the viability of IEC-18 cells [Figure 1]. As shown in Figure 1a, based on the CCK-8 assay, there were no cytotoxic or inhibitory effects on the growth of IEC-18 cells when pretreatment with a prepared solution of BSP with 100 μg/ml, 50 μg/ml and 25 μg/ml on IEC-18 cells for 12 h. As shown in Figure 1b, the same result was shown for the cell morphology observation.
Figure 1.
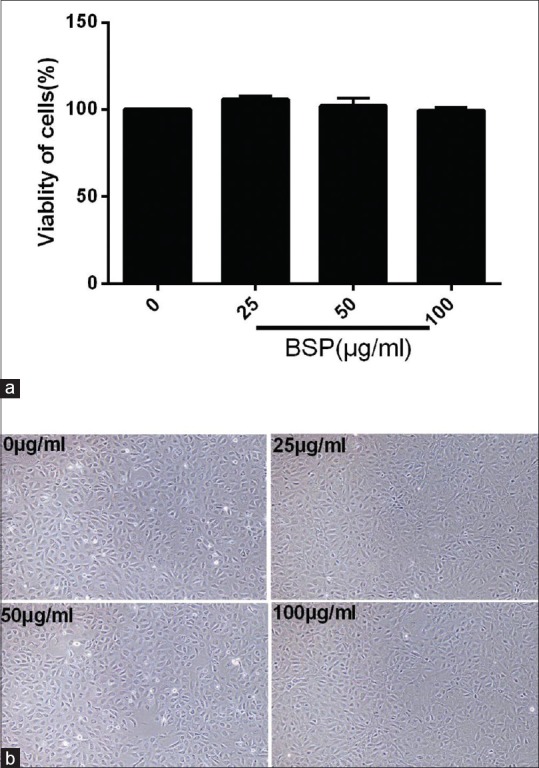
Cytotoxicity of Bletilla striata polysaccharide (BSP) on intestinal epithelial cell (IEC)-18. (a) A cell counting kit-8 assay of IEC-18 cells viability after BSP treatment for 12 h. (b) Cell morphologies of IEC-18 cells after BSP treatment for 12 h. Values are expressed as the means ± SD (n= 3)
Effect of BSP on TEER in LPS stimulated IEC-18 cells
TEER was used to evaluate monolayer permeability, the cell permeability was lower when the TEER became higher. The small chamber film diameter, surface and aperture area were 12 mm, 1.13 cm2, and 0.4 μm, respectively. The background resistance of blank was deducted from each value. As shown in Figure 2, compared with the control group, the TEER value in the experiment group was markedly decreased (P < 0.01). After treatment with different concentrations of BSP, the TEER values were elevated (P < 0.01 or 0.05).
Figure 2.
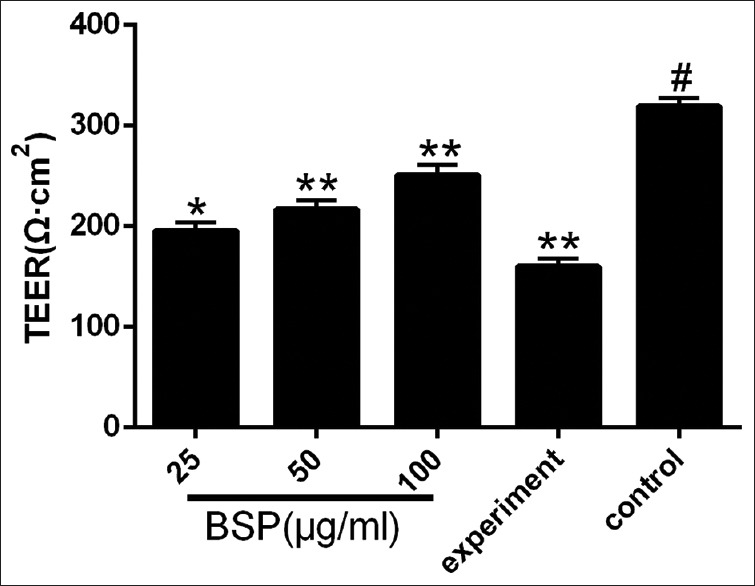
Effect of Bletilla striata polysaccharide on transepithelial electrical resistance in lipopolysaccharide-stimulated intestinal epithelial cell-18 cells. Values are expressed as the means ± SD (n= 3). *P< 0.05 compared with the experiment group; **P< 0.01 compared with the experiment group; #P< 0.01 compared with the control group
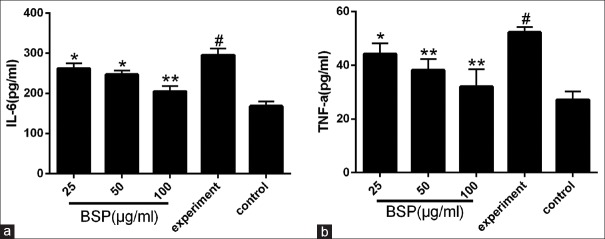
Effect of BSP on inflammatory cytokines expression
As shown in Figure 3a and 3b, stimulation with LPS resulted in significant increases in the production of IL-6 and TNF-α compared with the control group (P < 0.01). However, after treatment with BSP at a series of concentrations, the production of both inflammatory cytokines, IL-6 and TNF-α, were significantly decreased (Figure 3a and 3b, P < 0.05 or 0.01).
Figure 3.
Effect of Bletilla striata polysaccharide on the expression of interleukin-6 (a) and tumor necrosis factor-α (b) in lipopolysaccharide-stimulated intestinal epithelial cell-18 cells. Values are expressed as the means ± SD (n = 3). *P < 0.05 compared with the experiment group; **P< 0.01 compared with the experiment group; #P< 0.01 compared with the control group
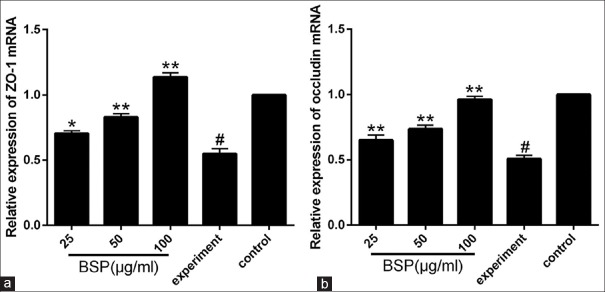
Effect of BSP on the mRNA expression of ZO-1 and occludin
We further determined the effect of BSP (25 μg/ml, 50 μg/ml and 100 μg/ml) on the mRNA levels of ZO-1 and occludin. As shown in Figure 4a and 4b, compared to the control group, the mRNA expression of ZO-1 and occludin in the experiment group were significantly decreased (P < 0.01). However, treatment with BSP elevate the mRNA levels of ZO-1 and occludin compared with the experiment group (P < 0.05 or 0.01).
Figure 4.
Effect of Bletilla striata polysaccharide on mRNA expression of ZO-1 (a) and occludin (b) in lipopolysaccharide-stimulated intestinal epithelial cell-18 cells. Values are expressed as the means ± SD (n= 3). *P< 0.05 compared with the experiment group; **P< 0.01 compared with the experiment group;#P< 0.01 compared with the control group
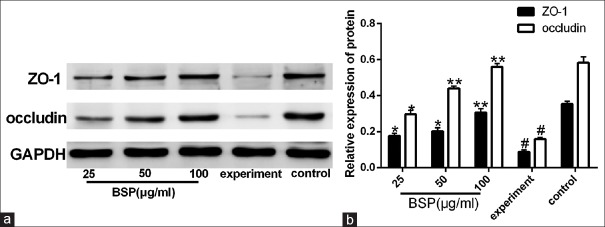
Effect of BSP on the protein expression of ZO-1 and occludin
The quantitative RT-PCR assay shows that BSP can up-regulate the mRNA expression of ZO-1 and occludin in LPS-induced IEC-18 cells and whether BSP could up-regulate the protein expression of ZO-1 and occludin. As shown in Figure 5a and 5b, compared with the control group, the protein expression of ZO-1 and occludin in IEC-18 cells were significantly decreased (P < 0.01). However, after treatment with BSP at different concentrations, the protein expression of ZO-1 and occludin were increased compared to that in the experiment group (P < 0.05 or 0.01). This finding was consistent with the result from qRT-PCR.
Figure 5.
Effect of Bletilla striata polysaccharide on protein (a and b) expression of ZO-1 and occludin in lipopolysaccharide-stimulated intestinal epithelial cell-18 cells. Values are expressed as the means ± SD (n= 3). *P< 0.05 compared with the experiment group; **P< 0.01 compared with the experiment group;#P< 0.01 compared with the control group
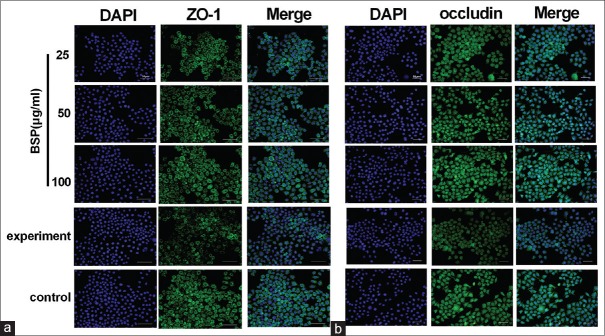
Effect of BSP on the distribution patterns of ZO-1 and occludin
As shown in Figure 6, we found that occludin and ZO-1 protein staining appeared to be reduced and more fragmented in the cell–cell contacts after stimulation with LPS, whereas the distribution patterns for ZO-1 and occludin protein remained unchanged in cells treated with different concentrations of BSP. These results indicated that stimulation with LPS altered the distribution patterns for ZO-1 and occluding protein, and BSP has the capacity to keep ZO-1 and occluding protein intact.
Figure 6.
The immunofluorescence method was used to detect the protein expressions of ZO-1 (a) and occludin (b) in lipopolysaccharide-stimulated intestinal epithelial cell-18 cells. ×400 magnification
DISCUSSION
The injury of intestinal epithelial barrier can cause disturbance of intestinal flora, the overproliferation of Gram negative bacilli and massive production of endotoxin.[16] Excessive endotoxins may get into the systemic circulation and damage the liver. This not only causes damage to the liver, as it cannot completely remove the endotoxin, but also induces the production of inflammatory cytokines, destroy the intestinal mucosal barrier function and lead to the increase of intestinal mucosal permeability.[17]
LPS can cause immune stress by stimulating non-specific immune system and result in the production of proinflammatory cytokines.[18] Increasing evidence has shown that proinflammatory cytokines are important pathophysiological regulators of intestinal epithelial TJ permeability.[19,20] IL-6 and TNF-α are the key proinflammatory cytokines that lead to the loss of the function of intestinal mucosal barrier. Studies have shown that reducing the production of cytokines may enhance the recovery of epithelial barrier function.[21]
In this study, we used LPS at concentration of 1.0 μg/ml to establish the LPS-induced injury IEC-18 cell inflammation model. We found that the production of proinflammatory cytokine, IL-6 and TNF-α, in the experiment group were significantly increased. Treatment with different concentrations of BSP could suppress the proinflammatory cytokine production (IL-6 and TNF-α). Thus, the decrease in proinflammatory cytokines in LPS-induced injury in IEC-18 cells treated with BSP may be an effective method for protecting against intestinal mucosal barrier.
Intestinal mucosal epithelial cells and intercellular connection are the main components of intestinal mechanical barrier. And the intestinal mechanical barrier transmembrane proteins ZO-1 and occludin are important structures that form a TJ, which determine the intestinal tract and the permeability of intestinal barrier.[22] Once the TJ proteins are deleted, reduced, or matated, the permeability of IEC will increase,[23] and bacteria, endotoxins, and other macromolecules would enter the systemic circulation through TJs, leading to local or systemic inflammatory responses.[24] ZO-1 is an important structural and functional marker of TJ in intestinal mucosal epithelium,[25] and was the first TJ protein to be discovered[26] which serves an important role in regulating intestinal mucosal barrier function. Occludin, a four-transmembrane protein, comprising four putative membrane-spanning segments,[27] is a TJ protein that is exclusively localized in epithelial and endothelial cells.[28] Studies have shown that high expression of occludin is correlated with low endothelium permeability.[29] Several studies have shown that LPS could lead to the decrease of ZO-1 and occludin protein expression.[30,31] Therefore, maintaining the integrity of TJ proteins is of great significance.
In this study, it was demonstrated that BSP is able to up-regulate the expression of ZO-1 and occludin at gene and protein level. Meanwhile, BSP could keep intact ZO-1 and occludin protein in IEC-18 cells injured with LPS. TEER is a very important parameter of the epithelial barrier function which indicates changes in the permeability and integrity of the cell monolayer. In this study, we found that TEER in IEC-18 cells was decreased when induced with LPS, however, treatment with different concentrations of BSP could ameliorate the level of TEER.
In conclusion, the results of the present study indicate that BSP can down-regulate the LPS-induced expression of related inflammatory cytokines, IL-6 and TNF-α, and maintain the integrity of intestinal epithelial barrier transmembrane proteins ZO-1 and occludin, which provides us a basis for further study on the protective mechanism of BSP on LPS-induced intestinal epithelial barrier disruption and may be a new way to treat LPS-related intestinal diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This study was supported by the Wuhan Science and Technology Project (2017060201010222) and The Second Batch of Young Middle-aged Talents in Wuhan.
REFERENCES
- 1.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life. 2015;70:631–59. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Zhang R, Wang J, Yu P, Liu Q, Zeng D, et al. Protective effects of baicalin on LPS-induced injury in intestinal epithelial cells and intercellular tight junctions. Can J Physiol Pharmacol. 2015;93:233–7. doi: 10.1139/cjpp-2014-0262. [DOI] [PubMed] [Google Scholar]
- 3.Trebicka J, Krag A, Gansweid S, Appenrodt B, Schiedermaier P, Sauerbruch T, et al. Endotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2011;23:1218–25. doi: 10.1097/MEG.0b013e32834a75dc. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Zhao B, Chen Y, Ma L, Chen EZ, Mao EQ. Biliary tract external drainage protects against intestinal barrier injury in hemorrhagic shock rats. World J Gastroenterol. 2015;21:12800–13. doi: 10.3748/wjg.v21.i45.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem. 2014;83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 6.Hu LN, Fang XY, Liu HL, Gao Z, Wu XF, Sun Y, et al. Protective effects of 18β-glycyrrhetinic acid on LPS-induced injury in intestinal epithelial cells. Chin J Nat Med. 2013;11:24–9. [Google Scholar]
- 7.Bein A, Zilbershtein A, Golosovsky M, Davidov D, Schwartz B. LPS Induces hyper-permeability of intestinal epithelial cells. J Cell Physiol. 2017;232:381–90. doi: 10.1002/jcp.25435. [DOI] [PubMed] [Google Scholar]
- 8.Diao HJ, Li X, Chen JN, Luo Y, Chen X, Dong L, et al. Bletilla striata polysaccharide stimulates inducible nitric oxide synthase and proinflammatory cytokine expression in macrophages. J Biosci Bioeng. 2008;105:85–9. doi: 10.1263/jbb.105.85. [DOI] [PubMed] [Google Scholar]
- 9.Xiang YL, Ye Q, Li WB, Xu WL, Yang HL. Preparation of wet-spun polysaccharide Ebers from Chinese medicinal Bletilla striata. Mater Lett. 2014;117:208–10. [Google Scholar]
- 10.Hossain MM. Therapeutic orchids: Traditional uses and recent advances--an overview. Fitoterapia. 2011;82:102–40. doi: 10.1016/j.fitote.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 11.He X, Wang X, Fang J, Zhao Z, Huang L, Gao H, et al. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J Ethnopharmacol. 2017;195:20–38. doi: 10.1016/j.jep.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Jiang F, Li W, Huang Y, Chen Y, Jin B, Chen N, et al. Antioxidant, antityrosinase and antitumor activity comparison: The potential utilization of fibrous root part of Bletilla striata (Thunb.). Reichb.f. PLoS One. 2013;8:e58004. doi: 10.1371/journal.pone.0058004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Liu D, Chen SJ, Wang Y, Jiang HX, Yin HP. A new glucomannan from Bletilla striata: Structural and anti-fibrosis effects. Fitoterapia. 2014;92:72–8. doi: 10.1016/j.fitote.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Sun J, Luo Y, Xue W, Diao H, Dong L, et al. A polysaccharide isolated from the medicinal herb Bletilla striata induces endothelial cells proliferation and vascular endothelial growth factor expression in vitro. Biotechnol Lett. 2006;28:539–43. doi: 10.1007/s10529-006-0011-x. [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Luo L, Zhu ZD, Zhou X, Wang Y, Xue J, et al. Chlorogenic Acid Inhibits Liver Fibrosis by Blocking the miR-21-Regulated TGF-β1/Smad7 Signaling Pathway in Vitro and in Vivo. Front Pharmacol. 2017;8:929. doi: 10.3389/fphar.2017.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter SR, Zahs A, Palmer JL, Wang L, Ramirez L, Gamelli RL, et al. Intestinal barrier disruption as a cause of mortality in combined radiation and burn injury. Shock. 2013;40:281–9. doi: 10.1097/SHK.0b013e3182a2c5b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song HL, Lv S, Liu P. The roles of tumor necrosis factor-alpha in colon tight junction protein expression and intestinal mucosa structure in a mouse model of acute liver failure. BMC Gastroenterol. 2009;9:70. doi: 10.1186/1471-230X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Chen H, Chen Q, Jiao FZ, Zhang WB, Gong ZJ. The protective mechanism of CAY10683 on intestinal mucosal barrier in acute liver failure through LPS/TLR4/MyD88 pathway. Mediators Inflamm. 2018;13:7859601. doi: 10.1155/2018/7859601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soyka MS, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immun. 2012;130:1087–96. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–71. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Brit J Nutr. 2007;98:S29–35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- 22.Shen ZY, Zhang J, Song HL, Zheng WP. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-α-regulated mechanism. World J Gastroenterol. 2013;19:3583–95. doi: 10.3748/wjg.v19.i23.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1054–64. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–7. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 25.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–38. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–66. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, et al. Mammalian occludin in epithelial cells: Its expression and subcellular distribution. Eur J Cell Biol. 1997;73:222–31. [PubMed] [Google Scholar]
- 29.Li JK, Ge R, Zhao CX, Tang J, Li JG, Li QS. Farrerol regulates occludin expression in hydrogen peroxide-induced EA. hy926 cells by modulating ERK1/2 activity. CNS Neurosci Ther. 2013;19:53–60. [Google Scholar]
- 30.Wang X, Wang S, Li Y, Wang F, Yang X, Yao J. Sulfated Astragalus polysaccharide can regulate the inflammatory reaction induced by LPS in Caco2 cells. Int J Biol Macromol. 2013;60:248–52. doi: 10.1016/j.ijbiomac.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Cao J, Yu DM, Zhang TZ, Zhou J, Chen KY, Ge J, et al. Protective effect of penehyclidine hydrochloride on lipopolysaccharide-induced acute kidney injury in rat. Genet Mol Res. 2015;14:9334–42. doi: 10.4238/2015.August.10.14. [DOI] [PubMed] [Google Scholar]