Abstract
While the gold standard for inducing mesenchymal stem cell (MSC) chondrogenesis utilizes pellet culture, most tissue engineering strategies for cartilage regeneration encapsulate MSCs as single cells, partially due to the technical challenge to homogeneously encapsulate cell pellets in three-dimensional (3D) hydrogels. It remains unclear whether encapsulating MSCs as single cell suspension or cell aggregates in 3D hydrogels would enhance MSC-based cartilage formation. In this study, we determined that the optimal size of MSC micropellets (μPellets) that can be homogeneously encapsulated in hydrogels with high cell viability is 100 cells/pellet. Using optimized μPellet size, MSCs were encapsulated either as single cell suspension or μPellets in four soft hydrogel formulations with stiffness ranging 3–6 kPa. Regardless of hydrogel formulations, single cell encapsulation resulted in more neocartilage deposition with improved mechanical functions over μPellet encapsulation. For single cell encapsulation, polyethylene glycol (PEG) hydrogels containing chondroitin sulfate led to the most cartilage matrix deposition, with compressive modulus reaching 211 kPa after only 21 days, a range approaching the stiffness of native cartilage. The findings from this study offer valuable insights on guiding optimal method design for MSCs and hydrogel-based cartilage regeneration. The optimized μPellet encapsulation method may be broadly applicable to encapsulate other stem cell types or cancer cells as aggregates in hydrogels.
Impact Statement
While the gold standard for inducing mesenchymal stem cell (MSC) chondrogenesis utilizes pellet culture, it remains unclear whether encapsulating MSCs as cell pellets in three-dimensional hydrogels would enhance MSC-based cartilage formation. In this study, we determined the optimal size of MSC micropellet (μPellet) that can be homogeneously encapsulated in hydrogels with high cell viability. Unexpectedly, single cell encapsulation resulted in more robust new cartilage formation than μPellet encapsulation. Furthermore, tuning hydrogel formulation led to rapid cartilage regeneration with stiffness approaching that of native cartilage. The findings from this study would facilitate clinical translation of MSCs and hydrogel-based therapies for cartilage regeneration with optimized parameters.
Keywords: mesenchymal stem cells, cartilage, cell encapsulation, hydrogels, polymeric scaffolds, pellet encapsulation
Introduction
Cartilage development begins with mesenchymal condensation, which is characterized by rapid mesenchymal stem cell (MSC) proliferation, formation of cell clusters, and differentiation into chondrocytes.1 To mimic the mesenchymal condensation process, researchers have used pellet culture to enhance chondrogenesis of MSCs,2,3 and scaffold-free cell constructs have yielded cartilage with morphology similar to native cartilage.4 Despite the promise of the scaffold-free approach, one challenge is the need for hundreds of million cells per milliliter to fill the defect, which is hard to obtain and difficult for treating defects with clinically relevant dimension.4,5 Furthermore, as cell pellets increase in size, the core of the pellet would suffer from necrosis due to insufficient diffusion.6,7
To overcome the challenge associated with scaffold-free cell pellet strategies, hydrogels have been widely used as three-dimensional (3D) matrices to enhance stem cell-based cartilage repair.8–13 In contrast to using cells alone, encapsulating cells in hydrogels requires significantly fewer cells to fill defects of the same volume and enhances nutrient diffusion. Furthermore, hydrogels can serve as an artificial niche to provide cell–matrix interactions and offer better initial mechanical support.14,15
To mimic the extracellular matrix (ECM) compositions in native cartilage, naturally-derived polymers such as chondroitin sulfate (CS), hyaluronic acid (HA), and heparin sulfate (HS) have been used as 3D hydrogels to support chondrogenesis of MSCs in vitro and in vivo.8–10,16–19 To enhance MSC-based cartilage matrix deposition in 3D, soft hydrogels (0.5–10 kPa) have been shown to be superior than stiff hydrogels (20–100 kPa).10,19–21 However, one challenge of using soft natural polymer-based hydrogels in vivo is premature degradation before sufficient neocartilage production.
One potential solution to increase the stability of ECM-based hydrogels is to mix it with polyethylene glycol (PEG), a synthetic polymer with bioinert background.8,12 Compared to use of natural polymers alone, PEG provides a broader range of tunable biochemical and mechanical properties. Mixed hydrogel compositions that combine PEG with other natural polymers have been shown to support cell-based cartilage regeneration in 3D both in vitro and in vivo.9–11,16
Previous research on using hydrogels to enhance MSC chondrogenesis involves encapsulating single cells in 3D hydrogels, which is convenient but excludes direct cell–cell contact. To enhance cell–cell contact in hydrogels, recent studies have explored encapsulating cell pellets with varying size.22–24 One challenge with pellet encapsulation is the difficulty to yield homogeneous 3D distribution, and the results have been contradictory.
One study showed cartilage formation by large MSC pellets (more than 5,000 cells per pellet) in 3D hydrogels. However, due to the increased weight of large cell pellets, they would rapidly sink down to the bottom of the hydrogels before crosslinking is complete. As such, encapsulating large cell pellets was limited to only fabricated thin hydrogel discs and not suitable for regenerating cartilage with clinically relevant dimension.22,23 Another study encapsulated small micropellets (μPellets), <50 cells per μPellet, and reported no improved cartilage formation.24
Furthermore, previous studies have used a variety of hydrogel materials, and direct comparison is difficult due to varying hydrogel compositions and pellet size. There remains a need to determine the optimal size of cell pellets that can be homogeneously encapsulated in 3D hydrogels and to determine the effects of hydrogel compositions on modulating cartilage formation by MSCs encapsulated as pellets and single cells.
To answer the questions above, here we first determined the optimal size of μPellet that would support homogeneous suspension in 3D hydrogels with high cell viability. μPellets with optimal size were subsequently encapsulated in four hydrogel compositions to evaluate the effects that material composition has on supporting new cartilage formation by single cells or μPellets in 3D. We have chosen to maintain the stiffness of all hydrogels similar between compositions, as previous literature suggests that MSC-based neocartilage deposition is best supported in soft hydrogels within the range of 3–6 kPa.10,20,21
We hypothesized that μPellets would enhance direct cell–cell contact of MSCs in 3D hydrogels, thereby accelerating chondrogenesis and neocartilage formation. To test our hypotheses, μPellets with variable size (100, 250, and 500 cells per pellet) were encapsulated in 3D hydrogels to access cell viability and distribution throughout the hydrogel. Cartilage production by either single cells or μPellets encapsulated in four hydrogel compositions [chondroitin sulfate-methacrylate [CS-MA], CS-MA/poly(ethylene glycol) diacrylate [PEGDA], hyaluronic acid-methacrylate [HA-MA], and HA-MA/PEGDA] was compared using biochemical assays, mechanical testing, and histology.
Materials and Methods
Cell culture
Human bone marrow mesenchymal stem cells (Lonza) were expanded until passage 5 before use. Cells were expanded in growth medium containing high glucose Dulbecco's minimal essential medium (Invitrogen), 10% (v/v) fetal bovine serum (Gibco), 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen), and 10 ng/mL basic fibroblast growth factor (PeproTech).
μPellet formation
MSCs were formed into μPellets of sizes 500, 250, and 100 cells per pellet using AggreWell™ 400 plates (STEMCELL Technologies). Cells were suspended in growth medium at desired concentrations for varying μPellet sizes, pipetted into each AggreWell plate, and centrifuged at 150 g for 5 min. Cells were left in AggreWell plates for 24 h in growth medium to allow μPellets to stabilize. The formed μPellets were gently transferred to a conical tube, centrifuged at 150 g for 5 min, and then resuspended in hydrogels for 3D encapsulation.
Polymer synthesis
CS-MA was synthesized following our previously reported method.10 Briefly, CS sodium salt (Sigma) was reacted with N-hydroxysuccinimide (Sigma) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (Sigma) in a buffer of 2-morpholinoethanesulfonic acid for 5 min. Following incubation, 2-aminoethyl methacrylate was added and mixed for 24 h at room temperature. The final product was dialyzed against water for 4 days, lyophilized, and stored at −20°C until use. HA-MA was synthesized from HA sodium salt (Sigma) following the same protocol. Nuclear magnetic resonance (NMR) confirmed CS-MA product with a degree of methacrylation of 15% and HA-MA with a degree of methacrylation of 13%. PEGDA, molecular weight (MW) = 5,000 g/mol, was purchased from Laysan Bio. PEG, MW = 20,000 g/mol (20K PEG), was purchased from Sigma.
Cell encapsulation and in vitro chondrogenesis
Cell number per gel was maintained constant for single cell or μPellet encapsulation. Cells were suspended at 10 × 106 cells/mL in hydrogel precursor solution containing the desired polymer concentrations (Supplementary Table S1) and 0.05% photoinitiator, lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP).
To achieve homogeneous suspension in 3D, we added 20K PEG at an optimized concentration (18%) to the hydrogel precursor solution to increase viscosity. This prolongs the time of homogeneous suspension of μPellets before crosslinking is complete. This uncrosslinkable PEG was used only to increase viscosity and diffused out after hydrogel was formed. While other density modifiers could be used, such as iodixanol, sucrose, or dextrose, we optimized use of 18% 20K PEG for achieving high cell viability and no change in Young's Modulus.25 LAP was synthesized accordingly by following a previously reported method.26
To induce gelation, cell–hydrogel mixture (50 μL) was pipetted into a cylindrical mold (3 mm in height, 5 mm in diameter) and exposed to ultraviolet light (365 nm, 4 mW·cm−2) for 5 min. The formed cell-laden hydrogels were cultured in chondrogenic medium supplemented with 10 ng/mL recombinant human transforming growth factor beta 3 (TGF-β3; Peprotech) for 21 days at 37°C with 5% CO2 before analyses.
The chondrogenic medium consists of high-glucose Dulbecco's modified Eagle's medium (Gibco) supplemented with 100 nM dexamethasone (Sigma), 50 μg/mL ascorbic-2-phosphate (Sigma), 40 μg/mL proline (Sigma), 5 μg/mL insulin-transferrin-selenium premix (BD Biosciences), 100 μg/mL sodium pyruvate (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). To enhance nutrient diffusion, 24-well plates were placed on a gyratory rocker inside the incubator throughout the culture. Cell viability was assessed at multiple time points (immediately before encapsulation, days 1 and 21) using the LIVE/DEAD™ cell viability assay (Thermo Fisher).
Mechanical testing
Unconfined compression tests were performed with an Instron 5944 materials testing system (Instron Corporation) fitted with a 10 N load cell (Interface, Inc.). Custom-made aluminum compression plates were lined with polytetrafluoroethylene to minimize friction. Acellular hydrogels (n = 3) were incubated in phosphate buffered saline (PBS) for 24 h at room temperature to reach equilibrium before mechanical testing. Cell-laden hydrogels (n = 5) were tested at days 1 and 21. All tests were conducted in PBS solution at room temperature. Before each test, a preload of 1 mN was applied, and the upper plate was lowered at a rate of 1% strain/s to a maximum strain of 30%. The compressive modulus was determined from the curve fit equation for strain between 10% and 20% strain.
Biochemical analyses
Hydrogels were collected for biochemical analyses at days 1 and 21 (n = 5/group). The swelling ratio was calculated as the wet weight divided by the dry weight. Lyophilized samples were digested in papainase solution (Worthington Biochemical) at 60°C for 16 h. DNA content was quantified using the Quant-iT™ PicoGreen™ double stranded DNA (dsDNA) assay (Thermo Fisher). Fold of cell proliferation was calculated as day 21 DNA content divided by day 1 DNA content. Total collagen content was determined by first quantifying hydroxyproline content using acid hydrolysis followed by a reaction with p-dimethylaminobenzaldehyde and chloramine T (Sigma) and then estimating collagen content by assuming 1:7.46 hydroxyproline:collagen mass ratio.11
Histological analyses
Samples were harvested for histological analyses on days 1 and 21 (n = 2/group). Samples were fixed in 4% paraformaldehyde (Sigma) for 30 min, washed in PBS, transferred to optimal cutting temperature compound (Thermo Fisher) for 48 h to fully embedded constructs, and flash frozen in liquid nitrogen. Hydrogels were sectioned at a thickness of 12 μm per slice. To visualize sGAG, samples were stained with Safranin O (Sigma) and counterstained with Fast Green (Thermo Fisher). Cell nuclei were counterstained with Weigert's Hematoxylin solution (Sigma), and samples were mounted with VectaMount (Vector Laboratories).
Immunostaining was performed for type I, type II, and type X collagens. Rabbit polyclonal antibody type I, type II, and type X collagens (Abcam) were diluted at 1:100 and incubated overnight at 4°C. Secondary antibody (Alexa Fluor 488 goat anti-rabbit; Thermo Fisher) was diluted at 1:200 with Hoechst (4 μg/mL) and incubated for 1 h at room temperature. Samples were mounted with the aqueous mounting medium, Fluoromount (Sigma), and images were taken with a Zeiss fluorescence microscope.
Statistical analysis
GraphPad Prism (GraphPad Software) was used to perform statistical analysis. Two-way analysis of variance and multiple pairwise comparisons with the Holm-Sidak test were used to determine statistical significance (p < 0.05). All data are represented as mean ± standard deviation with at least three biological replicates per group.
Results
Optimizing the size of μPellets for encapsulation in 3D hydrogels
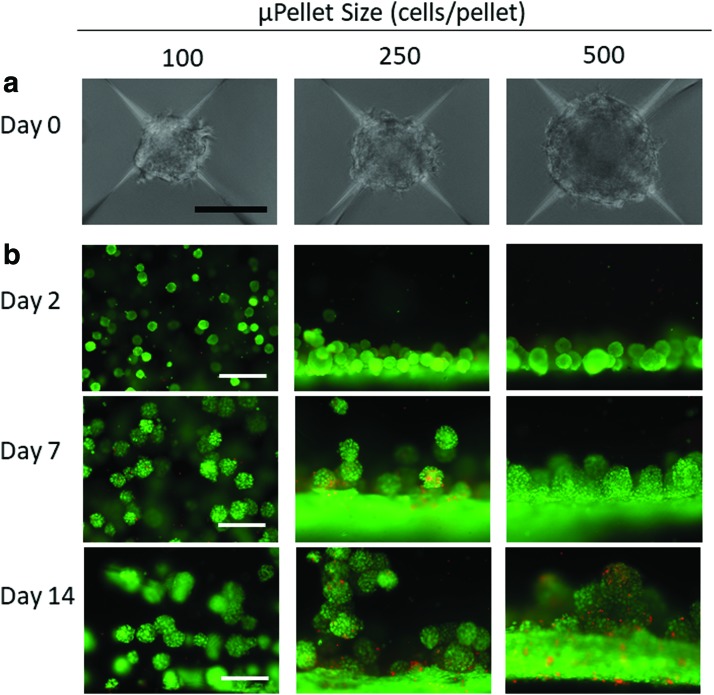
Our method of using AggreWell to form μPellets resulted in high-throughput production of MSC μPellets with uniform size and shape (Fig. 1a). Increasing the number of cells from 100 to 500 cells per μPellet led to increased size of individual μPellets from 100 to 175 μm (Fig. 1a). Using live/dead staining, we observed that only small μPellets formed using 100 cells/pellet supported homogeneous encapsulation and high cell viability after 3D encapsulation. In contrast, μPellets of 250 cells or larger fell to the bottom of the hydrogel precursor solution before gelation was complete, resulting in an inhomogeneous distribution in the z-plane (Fig. 1b).
FIG. 1.
Determining optimal size of μPellet for 3D encapsulation. (a) Brightfield images of μPellets 24 h after formation using AggreWell method. Scale bar = 100 μm. (b) Viability and distribution of MSC μPellet of variable size postencapsulation over time. Small μPellet formed using 100 cells/pellet resulted in homogeneous distribution in 3D hydrogels with good viability throughout 14 days of culture. Green: live cells, Red: dead cells, scale bar = 500 μm. 3D, three-dimensional; μPellet, micropellet; MSC, mesenchymal stem cell. Color images are available online.
We also assessed cell viability over time at multiple points (days 1, 2, 7, and 14) following encapsulation. While pellets with variable sizes showed comparable viability at day 1 (Supplementary Fig. S1), increasing μPellet size from 100 to 500 cells per pellet decreased cell viability (Fig. 1b). Taking together, μPellet with 100 cells/pellet was determined to be the optimal size for encapsulation in 3D hydrogels and was used for all further experiments.
Characterizing single cell versus μPellet encapsulation in four hydrogel compositions
To determine whether the trend of cartilage formation by single cell versus μPellet encapsulation in 3D depended on hydrogel compositions, four hydrogel formulations were assessed, including CS, PEG/CS, HA, and PEG/HA (Supplementary Table S1).
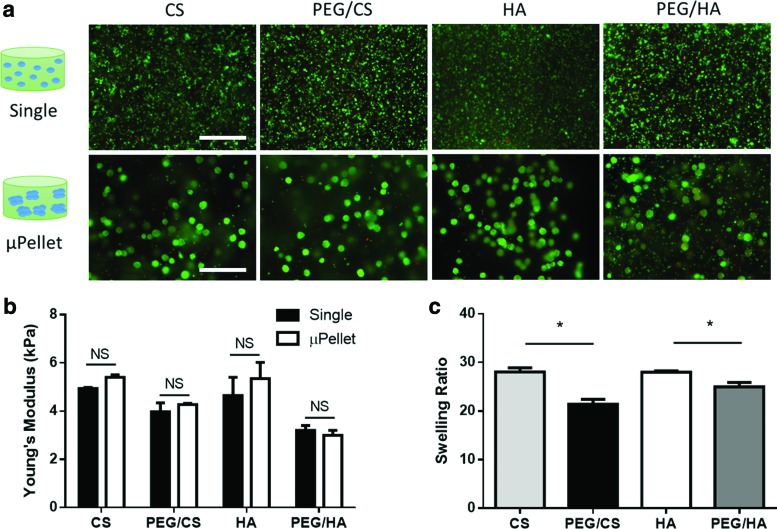
PEG/CS and PEG/HA groups contained equivalent concentrations of PEG and ratios of PEG:GAG. All materials supported high cell viability at day 1 (Fig. 2a). Unconfined compression testing confirmed that Young's Moduli of all hydrogels were between 3 and 6 kPa, and no statistically significant difference was observed between single cell and μPellet encapsulation methods (Fig. 2b). Swelling ratio was measured to assess the effects of PEG incorporation on crosslinking density of hydrogel network. Both PEG/CS and PEG/HA groups exhibited lower swelling ratio compared to CS and HA, respectively (PEG/CS 21.4 vs. CS 28.1, p < 0.05; PEG/HA 25.0 vs. HA 28.0, p < 0.05) (Fig. 2c), suggesting that PEG incorporation increased hydrogel crosslinking density.
FIG. 2.
Characterization of cell viability, mechanical strength, and swelling ratio of four hydrogel compositions used for single cell versus μPellet encapsulation 24 h postencapsulation. (a) All hydrogels supported high cell viability. Green: live cells, Red: dead cells. Scale bar = 500 μm; (b) Young's modulus of all compositions; “NS” = not significant (n = 3); (c) Swelling ratio of acellular hydrogels after incubating for 24 h to reach equilibrium; *p < 0.5 (n = 3). Color images are available online.
Single cell encapsulation enhanced cartilage formation by MSCs in 3D over μPellet encapsulation
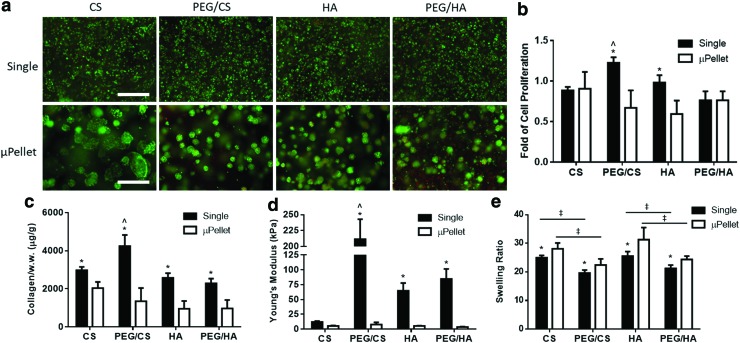
After 21 days of culture in chondrogenic medium, MSCs demonstrated comparable high cell viability in all groups (Fig. 3a). However, cell proliferation, cartilage matrix production, and mechanical properties of the resulting cartilage varied significantly among the groups (Fig. 3b–d). While cell proliferation was similar in CS and PEG/HA hydrogels between single cell versus μPellet encapsulation, significantly higher cell proliferation was observed in PEG/CS and HA hydrogels using single cell encapsulation (Fig. 3b).
FIG. 3.
Single cell encapsulation enhanced MSC proliferation and led to more robust cartilage formation by MSCs than μPellet encapsulation. (a) Live/dead staining of single versus μPellet groups at day 21. Green: live cells, Red: dead cells, scale bar = 500 μm. Quantification of (b) cell proliferation, (c) total collagen production per wet weight, (d) Young's modulus of resulting cartilage after 21 days of culture in chondrogenic medium. (*Denotes significant difference between single cell and μPellet in the same hydrogel composition: p < 0.05, n = 5; ^denotes significant difference compared to PEG/CS single cell encapsulation control: p < 0.05, n = 5). (e) Swelling ratio of cell-laden hydrogels at day 21 showed that PEG incorporation led to decreased swelling ratio (‡p < 0.05, n = 5). CS, chondroitin sulfate; PEG, polyethylene glycol. Color images are available online.
Regardless of hydrogel compositions, single cell encapsulation produced more collagen per wet weight than μPellet encapsulation counterparts (Fig. 3c). Collagen production per cell at day 21 showed that single cell encapsulation enhanced collagen production per cell (Supplementary Fig. S2). A similar trend was observed in the mechanical strength of resulting cartilage, as measured by the Young's Modulus (Fig. 3d). Incorporating PEG into sGAG hydrogel decreased the swelling ratio, and this trend remained for both single cell and μPellet encapsulation (Fig. 3e).
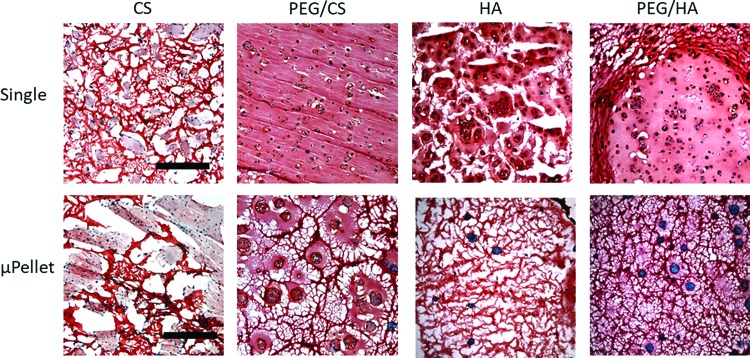
While biochemical assays give a quantitative measurement of newly formed cartilage, histology offers complementary information with regards to distribution of newly formed cartilage. Safranin O staining of sGAG, a key component of cartilage matrix, showed that single cell encapsulation led to more intense cartilage matrix staining regardless of hydrogel compositions (Fig. 4). Since our hydrogels contained sGAG, acellular hydrogels were stained as controls (Supplementary Fig. S3) and compared with cell-containing hydrogels to help determine the cellular contribution of sGAG deposition. Compared to μPellet encapsulation, single cell encapsulation also improved distribution of sGAG produced by MSCs with enhanced interconnectivity.
FIG. 4.
Cartilage formation by single cell versus μPellet encapsulation after 21 days of culture in chondrogenic medium, as shown by Safranin O staining. Single cell encapsulation generally resulted in more cartilage formation than their respective μPellet group in the same hydrogel composition. For single cell encapsulation, PEG/CS resulted in most intense and interconnected cartilage matrix staining. Red: sGAG, Black: nuclei, scale bar = 500 μm. Color images are available online.
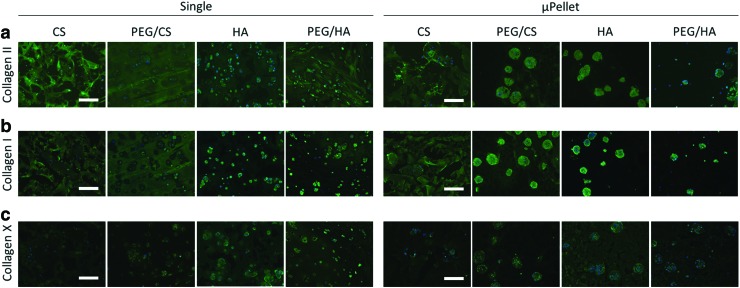
Phenotypic analysis was performed through immunostaining of type II, I, and X collagen (Fig. 5). The results showed that hydrogel composition, not encapsulation method, played a more important role in determining cartilage phenotype. For example, CS hydrogels led to the least amount of type X collagen regardless of single cell or μPellet encapsulation. In contrast, HA hydrogels resulted in the most intense type X collagen, suggesting a hypertrophic cartilage phenotype. Both encapsulation methods resulted in positive type II collagen staining in addition to type I staining, suggesting a more fibroblastic cartilage phenotype.
FIG. 5.
Characterizing the phenotype of cartilage formed by single cell versus μPellet encapsulation in four hydrogel formulations using immunostaining. (a) Type II Collagen; (b) Type I Collagen; and (c) Type X Collagen. Green: respective collagen, Blue: nuclei; scale bar = 200 μm. Color images are available online.
Discussion
In the present study we determined an optimal size of μPellet that supports homogeneous encapsulation in 3D hydrogels, which allows a side-by-side comparison of single cell versus pellet encapsulation for cartilage regeneration. We hypothesized that μPellet encapsulation in 3D would be superior to single cell encapsulation given the important role of cell–cell contact during normal cartilage development. In contrast to our hypothesis, single cell encapsulation led to more robust cartilage matrix deposition in 3D hydrogels with enhanced mechanical functions than μPellet encapsulation (Figs. 3 and 4). This trend is true regardless of hydrogel compositions. When encapsulation method was kept constant (i.e., single cell or μPellet encapsulation), varying hydrogel compositions modulated both the quantity and phenotype of newly formed cartilage (Fig. 5).
Among all tested formulations, single cell encapsulation in PEG/CS hydrogels resulted in the most robust cartilage formation and mechanical properties. Young's Modulus of engineered cartilage from single cells in PEG/CS hydrogels increased from 4 kPa to over 200 kPa after only 21 days of culture, which is approaching the bulk stiffness range of native articular cartilage.27 Mechanical strength of engineered cartilage is dependent not only on the total amount of matrix but also the distribution. The increased amount and interconnectivity of newly deposited cartilage in PEG/CS hydrogels contributed to the significant increase in Young's Modulus of resulting cartilage produced by single cells.
One important technical contribution of this study is determining the optimal size of the μPellets that can be homogeneously encapsulated in 3D hydrogels with high viability over time (Figs. 1 and 2). Using AggreWell plates, we demonstrated that uniform μPellets of varying sizes can be easily formed in a large quantity and scaled up for 3D encapsulation. Our results showed that the optimal number of cells per pellet (100 cells per pellet) for hydrogel encapsulation is one order of magnitude lower than previous report, which used ∼5,000 cells per pellet.22,23 These larger pellets failed to support homogeneous distribution and are not suitable for generating 3D tissues with clinically relevant dimensions.
While a major advantage of using the pellet size of 100 cells is that they are homogeneously distributed and support higher cell viability, that is not the only reason this smaller pellet was chosen. As shown in our Results section, increasing pellet size also led to increased cell death within the pellet after encapsulation. Given that the total number of cells was kept constant within a given volume, using larger pellets would result in fewer pellets in hydrogels, with greater distances between individual pellets. In contrast, single cell encapsulation allows the smallest distance between encapsulated cells, which resulted in substantially more interconnected matrix (Figs. 4 and 5).
In addition, when encapsulated in hydrogels, pellets were much less efficient in degrading the surrounding hydrogel network than single cells. We expect that using larger pellets would further decrease the total amount of hydrogel degradation and further limit neocartilage deposition due to physical constraint of the surrounding hydrogel network.
Regardless of hydrogel formulations, single cell encapsulation was found to be superior to μPellet encapsulation for optimal cartilage formation by MSCs in 3D hydrogels. This unexpected finding may be explained by several factors.
First, encapsulation of cells in 3D hydrogels creates physical restriction around the cells due to the nanoporosity of hydrogel network, which is several orders of magnitude smaller than the micrometer sized cells. Unlike 3D hydrogel encapsulation, free pellets without hydrogels can form cartilage without the need to overcome the physical constraint.28 Since the total number of cells is the same in each hydrogel, the density of μPellets (100 cells/μPellet) per gel is only 1% compared to single cell encapsulation.
As a result, it is more difficult for μPellets to degrade and remodel the surrounding hydrogel niche, thereby limiting the ability of MSCs to proliferate and deposit new matrix. Indeed, our data showed that cell number in the hydrogels all decreased in μPellet groups (Fig. 3b), and the newly deposited matrix was largely limited to peri-μPellet regions in all hydrogel compositions (Figs. 4 and 5). In contrast, single cells encapsulated in optimized PEG/CS hydrogels proliferated over time (Fig. 3b), and newly deposited cartilage matrix was highly interconnected throughout the hydrogel (Figs. 4 and 5).
Second, it is more difficult for nutrients to diffuse into μPellets encapsulated in hydrogels than single cells. While μPellet cell viability was not compromised significantly, diffusion of larger molecules influencing cell proliferation or chondrogenesis may have been limited for MSCs within encapsulated μPellets. This is supported by our data, in which single cell encapsulation resulted in higher total matrix production (Fig. 3b, c), as well as matrix production per cell (Supplementary Fig. S2).
Finally, while μPellets increased the direct cell–cell contact, the distance between each μPellet was much greater than between individual cells when encapsulated as single cells. This may change the autocrine/paracrine signaling among the encapsulated MSCs, thereby influencing the final cartilage formation. Given that single cell encapsulation is much more convenient than μPellets, it is also more desirable for clinical translation of MSC-based therapies for repairing cartilage in situ with clinically relevant dimensions.
While varying hydrogel formulation did not change the trend that single cell encapsulation always led to more cartilage formation than μPellet encapsulation, varying hydrogel compositions significantly impacted the quantity and distribution of newly formed cartilage within each encapsulation method. CS and HA are found in natural cartilage ECM and have been widely used as 3D hydrogels to induce MSC chondrogenesis using standard single cell encapsulation method suspensions.8–10,16
Consistent with previous reports, we showed that CS and HA hydrogels supported chondrogenesis and neocartilage formation, but incorporation of a small amount of PEG to CS substantially enhanced cartilage formation by MSCs (Figs. 3 and 4). This is likely due to the improved balance between the speed of cell-mediated degradation and new matrix production in PEG/CS hydrogels compared to CS hydrogel alone, as shown by previous reports.9,10 When using a low concentration of naturally-derived polymers alone, one potential risk is premature hydrogel degradation by the cells before sufficient new matrix is laid down.8
For single cell encapsulation, PEG/CS hydrogels far exceeded other hydrogel formulations in supporting MSCs to form articular cartilage with improved structures and mechanical functions. Specifically, we observed 20% more cell proliferation (Fig. 3b) and 30% more total collagen deposition (Fig. 3c) in PEG/CS hydrogels encapsulated with single cells compared to other groups. Varying hydrogel formulation also impacted the phenotype of newly formed cartilage regardless of cell encapsulation methods (Fig. 5). CS hydrogels resulted in a more hyaline cartilage phenotype with minimal hypertrophy, as shown by most robust type II collagen and the least type X collagen staining. In contrast, HA-containing hydrogels generally showed more type X collagen, suggesting that HA promoted hypertrophic cartilage phenotype.
It is well documented in previous literature that MSC-based cartilage formation generally produces cartilage that exhibits a fibrocartilage phenotype, as shown by high expression of type I collagen.19,29 Similar to previous literature, in this study we also observed high collagen I expression in all groups, suggesting a fibrocartilage phenotype. One possible way to reduce type I collagen expression is using small interfering RNA (siRNA) or short hairpin RNA (shRNA) delivery to knock down type I collagen, as shown previously.30
Given that cartilage is a weight bearing tissue, the ability to maintain structural integrity while under compression is highly desirable. Most impressively, the Young's Modulus of PEG/CS group reached 211 kPa at day 21, which is approaching the stiffness of native articular cartilage27 and is 17-fold higher compared with the CS group. Improvement in the mechanical properties of engineered cartilage not only depends on the amount of total matrix produced but also the distribution and interconnectivity of newly deposited matrix. While single cells encapsulated in PEG/CS only resulted in ∼50% more collagen than the CS group, we observed a 17-fold increase in Young's Modulus due to substantially enhanced interconnectivity of newly deposited matrix throughout the hydrogels (Fig. 4).
In the present study, we have designed all hydrogel compositions to have comparable stiffness to remove hydrogel stiffness, a confounding factor in modulating differentiation. However, this leads to different concentrations of CS and HA (Supplementary Table S1), which may contribute to the differences observed across different hydrogel formulations. One limitation of the study is that the biochemical and mechanical properties of hydrogels in the present study are not decoupled. However, given that the goal of this study is to compare the effects of varying hydrogel compositions on cartilage formation by MSCs in 3D using two different encapsulation methods, it does not require decoupling niche properties in hydrogel design. Our conclusions remain valid despite the coupled changes in niche properties of the chosen hydrogel formulations.
In summary, in this study we demonstrated that single cell encapsulation in 3D hydrogels is a superior approach to μPellet encapsulation for achieving more robust MSC-based cartilage tissue regeneration. Varying hydrogel compositions can further enhance the structure, mechanical function, and phenotype of newly formed cartilage. Among all the groups tested, we identified single cell encapsulation in PEG/CS hydrogels as the leading formulation that results in rapid deposition of new cartilage with improved distribution throughout the hydrogels, yielding a compressive modulus approaching that of native articular cartilage.
The findings from this study offer valuable insights to facilitate clinical translation of MSCs and hydrogels for cartilage regeneration. Furthermore, while this study focuses on chondrogenesis and cartilage tissue engineering, our μPellet encapsulation method may be easily modified for encapsulating other cell types as aggregates in various 3D hydrogel formulations, such as tumor cells or other stem cells.31–34
Supplementary Material
Acknowledgments
The authors acknowledge NIH R01DE024772 (F.Y.), NSF CAREER award CBET-1351289 (F.Y.), California Institute for Regenerative Medicine Tools and Technologies Award RT3-07804 (F.Y.), the Stanford Bio-X Interdisciplinary Initiative Seed grant (F.Y.), the Stanford Child Health Research Institute Faculty Scholar Award (F.Y.), NSF Graduate Research Fellowship Program (H.R.), and the Stanford Interdisciplinary Graduate Fellowship from the Stanford Bio-X program (H.R.) for support.
Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. DeLise A.M., Fischer L., and Tuan R.S. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8, 309, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238, 265, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Zhang L., Su P., Xu C., Yang J., Yu W., and Huang D. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett 32, 1339, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Hu J.C., and Athanasiou K.A. A self-assembling process in articular cartilage tissue engineering. Tissue Eng 12, 969, 2006 [DOI] [PubMed] [Google Scholar]
- 5. DuRaine G.D., Brown W.E., Hu J.C., and Athanasiou K.A. Emergence of scaffold-free approaches for tissue engineering musculoskeletal cartilages. Ann Biomed Eng 43, 543, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tare R.S., Howard D., Pound J.C., Roach H.I., and Oreffo R.O.C. Tissue engineering strategies for cartilage generation—micromass and three dimensional cultures using human chondrocytes and a continuous cell line. Biochem Biophys Res Commun 333, 609, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Krull R., and Bley T. Filaments in Bioprocesses. New York: Springer, 2015 [Google Scholar]
- 8. Kim I.L., Mauck R.L., and Burdick J.A. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials 32, 8771, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varghese S., Hwang N.S., Canver A.C., Theprungsirikul P., Lin D.W., and Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol 27, 12, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Wang T., Lai J.H., Han L.H., Tong X., and Yang F. Chondrogenic differentiation of adipose-derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness. Tissue Eng Part A 20, 2131, 2014 [DOI] [PubMed] [Google Scholar]
- 11. Lai J.H., Kajiyama G., Smith R.L., Maloney W., and Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep 3, 1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bryant S.J., and Anseth K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res 59, 63, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Huang A.H., Farrell M.J., and Mauck R.L. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech 43, 128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bosnakovski D., Mizuno M., Kim G., Takagi S., Okumura M., and Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 93, 1152, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Tibbitt M.W., and Anseth K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103, 655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang T., Lai J.H., and Yang F. Effects of hydrogel stiffness and extracellular compositions on modulating cartilage regeneration by mixed populations of stem cells and chondrocytes in vivo. Tissue Eng Part A 22, 1348, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen W.-C., Wei Y.-H., Chu I.M., and Yao C.-L. Effect of chondroitin sulphate C on the in vitro and in vivo chondrogenesis of mesenchymal stem cells in crosslinked type II collagen scaffolds. J Tissue Eng Regen Med 7, 665, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Burdick J.A., and Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 23, H41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang T., and Yang F. A comparative study of chondroitin sulfate and heparan sulfate for directing three-dimensional chondrogenesis of mesenchymal stem cells. Stem Cell Res Ther 8, 284, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy C.M., Matsiko A., Haugh M.G., Gleeson J.P., and O'Brien F.J. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen–glycosaminoglycan scaffolds. J Mech Behav Biomed Mater 11, 53, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Toh W.S., Lim T.C., Kurisawa M., and Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials 33, 3835, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Lee S., Lee K., Kim S., and Jung Y. Enhanced cartilaginous tissue formation with a cell aggregate-fibrin-polymer scaffold complex. Polymers 9, 348, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park H., Kim D., and Lee K.Y. Interaction-tailored cell aggregates in alginate hydrogels for enhanced chondrogenic differentiation. J Biomed Mater Res Part A 105, 42, 2017 [DOI] [PubMed] [Google Scholar]
- 24. Potier E., Rivron N.C., Van Blitterswijk C.A., and Ito K. Micro-aggregates do not influence bone marrow stromal cell chondrogenesis. J Tissue Eng Regen Med 10, 1021, 2016 [DOI] [PubMed] [Google Scholar]
- 25. Joshi-Barr S., Karpiak J.V., Ner Y., Wen J.H., Engler A.J., and Almutairi A. Density gradient multilayered polymerization (DGMP): a novel technique for creating multi-compartment, customizable scaffolds for tissue engineering. J Vis Exp 72, 1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fairbanks B.D., Schwartz M.P., Halevi A.E., Nuttelman C.R., Bowman C.N., and Anseth K.S. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater 21, 5005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mansour J.M. Biomechanics of cartilage. In: Outis C.A. (ed). Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, PA: Lippincott Williams and Wilkins, 2003, p.66 [Google Scholar]
- 28. Drury J.L., and Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24, 4337, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Pelttari K., Steck E., and Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury 39, 58, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Zhang F., Yao Y., Zhou R., Su K., Citra F., and Wang D.A. Optimal construction and delivery of dual-functioning lentiviral vectors for type I collagen-suppressed chondrogenesis in synovium-derived mesenchymal stem cells. Pharm Res 28, 1338, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Fagotto F., and Gumbiner B.M. Cell contact-dependent signaling. Dev Biol 180, 445, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Kantak S.S., and Kramer R.H. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem 273, 16953, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Kondo J., Endo H., Okuyama H., et al. . Retaining cell–cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci U S A 108, 6235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang J., Peng R., and Ding J. The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials 31, 2470, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.