Abstract
The field of biased agonism has grown substantially in recent years and the μ-opioid receptor has been one of the most intensively studied receptor targets for developing biased agonists. Yet, despite extensive research efforts, the development of analgesics with reduced adverse effects remains a significant challenge. In this review we discuss the evidence to support the prevailing hypothesis that a G protein-biased agonist at the μ-opioid receptor would be an effective analgesic without the accompanying adverse effects associated with conventional μ-opioid agonists. We also assess the current status of established and novel μ-opioid–receptor ligands that are proposed to be biased ligands.
SIGNIFICANCE STATEMENT
The idea that biased agonists at the μ-opioid receptor might provide a therapeutic advantage in terms of producing effective analgesia with fewer adverse effects has driven the design of novel G protein-biased agonists. However, is the desirability of G protein-biased agonists at μ-opioid receptor substantiated by what we know of the physiology and pharmacology of the receptor? Also, do any of the novel biased agonists live up to their initial promise? Here we address these issues by critically examining the evidence that G protein bias really is desirable and also by discussing whether the ligands so far developed are clearly biased in vitro and whether this produces responses in vivo that might be commensurate with such bias.
Introduction
Opioid drugs bring with them blessings and curses, as anyone familiar with the opioid field will know. Drugs such as morphine are among the most important medicines we have for the treatment of acute severe pain. On the other hand, these drugs are associated with addiction as well as deaths owing to overdose. The latter has reached epidemic proportions in the United States (Seth et al., 2018).
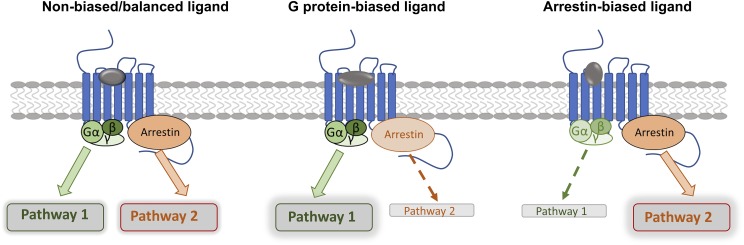
Opioid receptors are G protein–coupled receptors (GPCRs), consisting of μ-, δ-, and κ-opioid–receptor subtypes; in addition, there is the related nociceptin/orphanin FQ peptide (NOP) receptor (Alexander et al., 2013). Although δ-, κ-, and NOP receptors are implicated in mechanisms of analgesia (Günther et al., 2018), it is μ agonist drugs that far and away remain the mainstay of pain treatment. To develop improved analgesics with fewer side effects, newer approaches that still involve μ-opioid receptors are actively being pursued, including the development of allosteric ligands (Burford et al., 2013), bivalent and bifunctional ligands (Li et al., 2007; Günther et al., 2018), and the subject of this review, biased agonists (DeWire et al., 2013; Kelly, 2013). Although an agonist at a GPCR is able to stabilize a range of receptor conformations, biased ligands are thought to stabilize a specific repertoire of receptor conformations that are distinct from that of a nonbiased agonist or an oppositely biased agonist (Kenakin and Morgan, 1989; Urban et al., 2007; Kelly, 2013; Latorraca et al., 2017). Biased ligands will thus generate distinct signaling outputs, or profiles, compared with unbiased or oppositely biased agonists (Fig. 1). It should be noted that bias is always relative, with the bias of test ligands being calculated relative to a standard ligand, usually the endogenous agonist or a well characterized agonist at the receptor (Kenakin et al., 2012). The latter is often assumed to be nonbiased, or balanced, and usually produces efficient activation of the most measured signaling outputs, normally G protein activation and arrestin recruitment.
Fig. 1.
Signaling of biased agonists. In most cases agonists regarded as nonbiased can efficiently activate both G protein- and arrestin-dependent signaling. Relative to this, biased agonists preferentially activate either G protein- or arrestin-dependent signaling as shown in bold arrows.
For the μ-receptor, the idea of biased agonists being desirable therapeutics stems from the observation a number of years ago that in arrestin-3 (also known as β-arrestin 2)-knockout mice, morphine has an increased analgesic effect, perhaps in part because the effect is less liable to tolerance (Bohn et al., 1999). Importantly, in the arrestin-3-knockout mice, the gastrointestinal and respiratory depressant effects of morphine were reported to be reduced (Bohn et al., 2000; Raehal et al., 2005). This led to the key concept—which has dominated the opioid field now for almost 20 years—that the analgesic effects of μ-receptor agonists are G protein-mediated, and the adverse effects (constipation and respiratory depression) as well as tolerance, are mediated in the main by arrestin-3. As a direct consequence, novel G protein-biased agonists have been sought as potential analgesics and are predicted to possess a better adverse effect profile than morphine and other widely prescribed μ-receptor agonists.
Potentially Biased μ Ligands, Past and Present
Drugs biased for either G protein or arrestin signaling have been reported over the past few years, and in the following we briefly summarize the evidence to back up or question such claims.
Herkinorin.
An early candidate for a G protein-biased agonist was herkinorin, a derivative of salvinorin A, the plant-derived hallucinogen. Herkinorin was reported to exhibit substantial G protein bias on the basis of the observation of a lack of arrestin recruitment and internalization in contrast with its ability to induce extracellular signal-regulated kinase phosphorylation (Groer et al., 2007). However, more recently herkinorin was reported to be a full agonist for arrestin recruitment, with efficacy similar to that ofDAMGO (Manglik et al., 2016), calling into question the G protein-biased profile of herkinorin. It should be noted, however, that the above studies used different experimental approaches to investigate arrestin recruitment, which may in part, explain the opposing data. Therefore, whether herkinorin really is a G protein-biased agonist remains to be conclusively determined.
Mitragynine.
Another family of natural compounds suggested to display varying degrees of G protein bias at the μ-receptor is mitragynine and its analogs. These compounds are biosynthesised by Mitragyna speciose, a medicinal plant commonly known as kratom that is used as a stimulant and for its analgesic effects (Yamamoto et al., 1999; Prozialeck et al., 2012). These compounds were inactive in arrestin recruitment assays and were reported to have a favorable in vivo profile, for example, reduced antinociceptive tolerance compared with morphine (Kruegel et al., 2016; Váradi et al., 2016). Although these compounds also act as antagonists at the δ-opioid receptor, which may contribute to the attenuation of some of the adverse effects observed in vivo (Váradi et al., 2016).
Oliceridine.
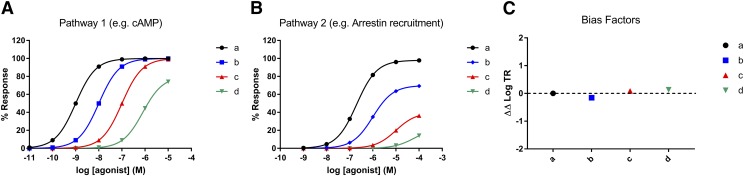
The first important new small molecule to be proposed as a G protein-biased agonist was the Trevena compound TRV130, now known as oliceridine (DeWire et al., 2013). This drug, reported to be a G protein-biased selective μ-receptor agonist, was an effective analgesic in preclinical studies, purportedly having a favorable side-effect profile, inducing less respiratory depression and less constipation than an equianalgesic dose of morphine (DeWire et al., 2013). Yet the data from clinical trials failed to demonstrate significant therapeutic superiority in terms of analgesic efficacy and diminished adverse effects compared with morphine (Stanczyk and Kandasamy, 2018). Following completion of phase 3 trials, the FDA advisory committee flagged significant concerns with regard to the efficacy and safety profile of oliceridine compared with morphine. At present the FDA has declined to approve oliceridine, but Trevena has been granted the opportunity to provide additional preclinical and clinical data to support their oliceridine application (https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndAnalgesicDrugProductsAdvisoryCommittee/UCM622730.pdf). Interestingly, the G protein versus arrestin bias of oliceridine was reported to be only 3-fold relative to morphine, and the statistical significance of any bias was not established (DeWire et al., 2013). The question for this drug going forward is whether it really does offer a favorable in vivo profile. If oliceridine does prove to be advantageous over the established opioids currently in clinical use, then it remains to be determined whether this is really owing to moderate G protein bias, or is instead due to the overall low efficacy of the agonist at the μ-receptor, its pharmacokinetic profile in vivo, or perhaps even a combination of the above. This is a crucial question and one that merits further investigation as other low efficacy agonists at the μ-receptor, not reported as biased agonists, have in some cases also been reported to exhibit a favorable side-effect profile. For example, buprenorphine produces less respiratory depression than higher efficacy agonists, making it reportedly safer than methadone for opioid substitution therapy in opioid addiction (Walsh et al., 1994; Cowan, 2003; Dahan, 2006). Owing to the higher receptor reserve (i.e., more efficient coupling) for G protein activation versus arrestin recruitment, unbiased lower efficacy agonists will often exhibit much weaker arrestin recruitment than G protein activation. This could be, and often is, misinterpreted as G protein bias as opposed to differences in receptor reserve between the two signaling pathways (Fig. 2). However even if technically unbiased, the difference in ability to activate G protein versus arrestin pathways, particularly for lower efficacy agonists, may nevertheless represent an important functional difference with potentially significant in vivo consequences.
Fig. 2.
(A and B) Simulated concentration-response curves to four agonists (a–d) at a GPCR in two different cellular assays measuring coupling efficiency to either pathway 1 or pathway 2. Many would conclude by eye that the red and green agonists are G protein-biased compared with the black agonist. (C) However, when bias factors for the blue, red, and green agonists are quantified by calculating the log ratios of transduction coefficients [∆∆log(τ/KA)], as discussed in the text, none of the agonists are found to be G protein-biased relative to the black agonist, as all of the agonists have values of ∆∆log(τ/KA) approximate to 0, confirming the absence of any bias.
Trevena has also reported another G protein-biased μ-receptor agonist, TRV734, an orally available analog of oliceridine, which is also reported to show a favorable in vivo profile in preclinical and clinical experiments (https://www.trevena.com/pdf/PainWeek2015TRV734Ph1ClinicalStudiesPoster.pdf), but the G protein efficacy of TRV734 is maybe less than that of oliceridine, which again raises the possibility that the improved therapeutic profile arises, in part, from weak efficacy and not bias. Interestingly, at the time of writing, Trevena are also investigating the potential of TRV734 as an opioid maintenance therapy for opioid addiction (https://www.trevena.com/news-details.php?id=217).
PZM21.
In 2016 the ligand PZM21 was reported as a novel structure that was G protein-biased at the μ-receptor that produced analgesia in mice but did not depress respiration (Manglik et al., 2016). However, a subsequent study from our laboratory reported that the bias of PZM21 was marginal at best, although it was able to depress respiration similar to an equi-analgesic dose of morphine (Hill et al., 2018). Furthermore, it was observed that PZM21 also induced analgesic tolerance similar to morphine and that by the fourth day of treatment both PZM21 and morphine were unable to induce analgesia (Hill et al., 2018). These recent data question whether PZM21 could provide any real benefit versus established μ-opioid agonists such as morphine and buprenorphine. Of note, in the original study by Manglik et al., the authors were unable to achieve statistical significance of the bias factor for PZM21, even in the presence of overexpressed GRK2 to facilitate more accurate estimations of arrestin recruitment. As such, no bias factor was reported, suggesting that similar to oliceridine the extent of bias toward G protein signaling is limited, if present at all.
SR-Compounds.
More recently, a series of piperidine-based ligands have been developed, some of which were reported to be markedly G protein-biased. They are analgesic yet are reported to produce relatively little respiratory depression compared with fentanyl or morphine (Schmid et al., 2017). One point of interest regards the pharmacokinetics of these new ligands. Whereas morphine and fentanyl were rapidly absorbed into blood and brain following intraperitoneal administration to mice, the novel ligands were absorbed much more slowly, and the resulting brain concentrations remained high for hours. It will be important to determine whether these distinctive pharmacokinetics play any role in the in vivo effects or lack of effects of these novel ligands, such as whether respiration is affected at later time points than those measured in the study (up to 1 hour). Nevertheless, these novel ligands are the first μ-opioid–receptor agonists reported to possess statistically significant G protein bias, as well as reduced respiratory depression compared with morphine and fentanyl (Schmid et al., 2017).
Endomorphins.
In contrast to the preceding compounds, the endomorphin peptides endomorphin-1 and -2 were reported to be arrestin-biased compared with other μ-opioid agonists (Rivero et al., 2012; Thompson et al., 2015b, 2016; Burgueno et al., 2017). The functional significance of this interesting observation about these peptides has yet to be explored. Indeed the arrestin bias might suggest that endomorphins exert significant respiratory depression versus analgesia, but the limited studies so far do not support this (Zadina et al., 2016).
Fentanyl.
More recently, fentanyl has been described as an arrestin-biased ligand relative to DAMGO, in a comparison of GTPγS assays and arrestin-3 recruitment. It was suggested that the arrestin-biased signaling profile correlated with a narrower therapeutic window owing to a greater propensity to induce respiratory depression (Schmid et al., 2017). However, it should be noted that in the same study, Schmid et al. also found fentanyl to be G protein-biased relative to DAMGO when they compared cAMP signaling to arrestin-3 recruitment (Table 1). Thus the direction of bias was dependent on the cellular assay and receptor species used for quantification (Schmid et al., 2017). Similar discrepancies in the direction of bias for fentanyl were also noted in another study (Burgueno et al., 2017). In contrast, various studies from different laboratories have observed no apparent bias for fentanyl (McPherson et al., 2010; Rivero et al., 2012; DeWire et al., 2013; Winpenny et al., 2016).
TABLE 1.
Reported bias of μ-opioid agonists from a range of published studies
Reported bias along with bias factor. For clarity, with regard to G protein signaling, only data calculated from G protein-activation assays or cAMP signaling are included.
| Ligand | Bias | Bias Factor | Bias Quantification | Assays (receptor species) | Reference Ligand | Reference |
|---|---|---|---|---|---|---|
| Buprenorphine | G protein | 69 | ∆∆log(τ/KA) | cAMP (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | Morphine | Burgueno et al., 2017 |
| DynA 1-6 | G protein | 4.6 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (N.R.) | DAMGO | (Thompson et al., 2015b) |
| Endomorphin-1 | Arrestin; G protein | 0.06; 35.6 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (N.R.); cAMP (hMOPr) vs. Arrestin-2 recruitment (N.R.) | DAMGO | (Thompson et al., 2015b) |
| Arrestin | N.R. | Correlation of operational efficacy (τ) | GTPγS (rMOPr) vs. Arrestin-3 recruitment (N.R.) | N.A. | McPherson et al., 2010 | |
| Arrestin | N.R. | ∆∆log(τ/KA) | Gαi3 BRET (hMOPr) vs. Arrestin-3 recruitment (N.R.) | DAMGO | Thompson et al., 2016 | |
| Endomorphin-2 | Arrestin | N.R. | (βlig) | GTPγS (rMOPr) vs. Arrestin-3 recruitment (N.R) | Leu-enkephalin | Rivero et al., 2012 |
| Arrestin | N.R. | Correlation of operational efficacy (τ) | GTPγS (rMOPr) vs. Arrestin-3 recruitment (N.R.) | N.A. | McPherson et al., 2010 | |
| Arrestin | 0.23 | ∆∆log(τ) | cAMP (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | Morphine | Burgueno et al., 2017 | |
| G protein | N.R. | ∆∆log(τ/KA) | cAMP (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | Pfizer standard 1 | (Hothersall et al., 2017) | |
| Fentanyl | Arrestin; G protein | 0.18; 2.8 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (hMOPr); cAMP (hMOP) vs. Arrestin-3 recruitment (hMOPr) | DAMGO | (Schmid et al., 2017) |
| Arrestin | 0.05; 0.15 | ∆∆log(τ/KA) | GTPγS (mMoPr) v Arrestin-3 recruitment (mMoPr); GTPγS (mMOPr (brain)) v Arrestin-3 recruitment (mMOPRr) | DAMGO | (Schmid et al., 2017) | |
| G protein | 9.1 | ∆∆log(τ/KA) | cAMP (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | Morphine | Burgueno et al., 2017 | |
| Arrestin | 0.37 | ∆∆log(τ) | cAMP (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | Morphine | Burgueno et al., 2017 | |
| Loperamide | G protein | 10.1; 9.4 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (N.R.); cAMP (hMOPr) vs. Arrestin-3 recruitment (N.R.) | DAMGO | (Thompson et al., 2015b) |
| G protein | 18.3; 17.0 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-2 recruitment (N.R); cAMP (hMOPr) vs. Arrestin-2 recruitment (N.R.) | DAMGO | (Thompson et al., 2015b) | |
| Met-enkephalin | G protein | N.R. | ∆∆log(τ/KA) | cAMP (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | Pfizer standard 1 | (Hothersall et al., 2017) |
| Morphine | Arrestin | 0.1 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (N.R.) | DAMGO | (Thompson et al., 2015b) |
| Arrestin | N.R. | ∆∆log(τ/KA) | Gαi1 BRET (hMOPr) vs. Arrestin-3 recruitment (N.R.) | DAMGO | Thompson et al., 2016 | |
| G protein; Arrestin | 1.3; 0.62 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (hMOPr); cAMP (hMOP) vs. Arrestin-3 recruitment (hMOPr) | DAMGO | (Schmid et al., 2017) | |
| G protein | 1.9 | ∆∆log(τ/KA) | GTPγS (mMOPR (brain)) v Arrestin-3 recruitment (mMOPr) | DAMGO | (Schmid et al., 2017) | |
| Sufentanil | Arrestin | 0.16 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | DAMGO | (Schmid et al., 2017) |
| Arrestin | 0.06; 0.14 | ∆∆log(τ/KA) | GTPγS (mMOPr) v Arrestin-3 recruitment (mMOPR); GTPγS (mMOPr (brain)) v Arrestin-3 recruitment (mMOPr) | DAMGO | (Schmid et al., 2017) | |
| α-Neo | G protein | 14.6 | ∆∆log(τ/KA) | cAMP (hMOPr) vs. Arrestin-3 recruitment (N.R.) | DAMGO | (Thompson et al., 2015b) |
| G protein | 8 | ∆∆log(τ/KA) | cAMP (hMOPr) vs. Arrestin-2 recruitment (N.R) | DAMGO | (Thompson et al., 2015b) | |
| G protein | N.R. | ∆∆log(τ/KA) | Gαi2 BRET (hMOPr) vs. Arrestin-3 recruitment (N.R.) | DAMGO | Thompson et al., 2016 | |
| PZM 21 | G protein | N.R. | ∆∆log(τ/KA) | cAMP vs. Arrestin-3 recruitment | N.R. | Manglik et al., 2016 |
| TRV 130 | G protein | 3 | RAi | cAMP (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | Morphine | Dewire et al., 2013 |
| G protein | 4.2 | ∆∆log(τ) | cAMP (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | Morphine | Burgueno et al., 2017 | |
| SR-15098a | G protein | 29; 19 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (hMOPr); cAMP (hMOP) vs. Arrestin-3 recruitment (hMOPr) | DAMGO | (Schmid et al., 2017) |
| G protein | 11; 55 | ∆∆log(τ/KA) | GTPγS (mMOPr) vs. Arrestin-3 recruitment (mMOPr); GTPγS (mMOPr (brain)) vs. Arrestin-3 recruitment (mMOPr) | DAMGO | (Schmid et al., 2017) | |
| SR-15099 | G protein | 47; 27 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (hMOPr); cAMP (hMOP) vs. Arrestin-3 recruitment (hMOPr) | DAMGO | (Schmid et al., 2017) |
| G protein | 12; 55 | ∆∆log(τ/KA) | GTPγS (mMOPr) vs. Arrestin-3 recruitment (mMOPr); GTPγS (mMOPr (brain)) vs. Arrestin-3 recruitment (mMOPr) | DAMGO | (Schmid et al., 2017) | |
| SR-17018 | G protein | 85; 40 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (hMOPr); cAMP (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | DAMGO | (Schmid et al., 2017) |
| G protein | 30; 102 | ∆∆log(τ/KA) | GTPγS (mMOPr) vs. Arrestin-3 recruitment (mMOPr); GTPγS (mMOPr (brain)) vs. Arrestin-3 recruitment (mMOPr) | DAMGO | (Schmid et al., 2017) | |
| (±) SR-11501 | Arrestin | 0.41 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (hMOPr) | DAMGO | (Schmid et al., 2017) |
| Arrestin | 0.12; 0.23 | ∆∆log(τ/KA) | GTPγS (mMOPr) vs. Arrestin-3 recruitment (mMOPr); GTPγS (mMOPr (brain)) vs. Arrestin-3 recruitment (mMOPr) | DAMGO | (Schmid et al., 2017) | |
| (±) SR-14968 | G protein | 36; 5.1 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (hMOPr); cAMP (hMOP) vs. Arrestin-3 recruitment (hMOPr) | DAMGO | (Schmid et al., 2017) |
| G protein | 6.7; 34 | ∆∆log(τ/KA) | GTPγS (mMOPr) vs. Arrestin-3 recruitment (mMOPr); GTPγS (mMOPr (brain)) vs. Arrestin-3 recruitment (mMOPr) | DAMGO | (Schmid et al., 2017) | |
| (±) SR-14969a | G protein | 11; 2.5 | ∆∆log(τ/KA) | GTPγS (hMOPr) vs. Arrestin-3 recruitment (hMOPr); cAMP (hMOP) vs. Arrestin-3 recruitment (hMOPr) | DAMGO | (Schmid et al., 2017) |
| G protein | 2.9; 8.6 | ∆∆log(τ/KA) | GTPγS (mMOPr) vs. Arrestin-3 recruitment (mMOPr); GTPγS (mMOPr (brain)) vs. Arrestin-3 recruitment (mMOPr) | DAMGO | (Schmid et al., 2017) |
N.A. not applicable; N.R, not reported; RAi, intrinsic relative activity.
In summary, this seems to be an opportune time to reassess the role and potential of ligand bias in the therapeutic and side-effect profile of μ-opioid agonist drugs. At the very least, great care should be taken to establish the statistical significance of bias in in vitro assays before making claims about effects being the result of bias in in vivo experimentation.
Future Perspectives for μ-Biased Ligands
Determining Bias Factors.
In addition to the aforementioned drugs, other μ-opioid–receptor agonists have also been reported as either G protein- or arrestin-biased ligands (Table 1), but there appears to be little consistency across studies to date. Potential reasons for these differences include the method used to quantify bias in vitro, the choice of reference ligand, as well as differences in the statistical test used to assess significance. A number of different methods have been described for quantifying biased agonism (Ehlert, 2008; Rajagopal et al., 2011; Kenakin et al., 2012; Onaran et al., 2017), and these different approaches have been comprehensively discussed in a recent review (Kenakin, 2019). For the μ-receptor, the predominant method to determine bias involves the calculation of the log ratios of transduction coefficients [∆∆log(τ/KA)]. In this approach concentration-response data are fitted to a form of the Black-Leff operational model of agonism (Black and Leff, 1983). Within this model, agonism can be quantified by a single computed parameter, the transduction coefficient (τ/KA). The term τ is a composite of agonist efficacy, receptor density, and coupling within the system. The dissociation constant KA represents the functional equilibrium dissociation constant or operational affinity, which is normally different from the affinity of the ligand for the bare receptor determined in radioligand binding experiments. Using this model it is not possible to determine the relative efficacy of agonists, and the two parameters (τ and KA) must be determined as a ratio from the regression across the different curves. To cancel out cell-dependent effects, otherwise known as system bias, the calculated log(τ/KA) values are normalized to a reference ligand generating ∆log(τ/KA) values (Thompson et al., 2016). Finally, to quantify biased agonism across different signaling pathways the ∆log(τ/KA) values for each pathway are expressed as a ratio of one signaling pathway over the other for a given agonist to generate log ratios of transduction coefficients [∆∆log(τ/KA)] (Kenakin et al., 2012).
The approach already described has generated significant amounts of quantitative data related to bias, but, as mentioned in the preceding, at present there is a worrying lack of correlation between studies in the bias factors calculated for the μ-receptor, and even the nature of the bias for a given agonist has been shown to change depending on the signaling output used for bias calculation (e.g., G protein versus cAMP; Table 1). Such data suggest that either the data generation and application of the bias calculation is not being carried out with sufficient rigor, or current methods for bias determination are not fully accounting for confounding factors such as system bias. Indeed a recent comprehensive analysis of current methods to quantify ligand bias suggests that they do not always distinguish system bias from ligand bias (Onaran et al., 2017). For more detailed analysis, readers are referred to more comprehensive reviews (Kelly, 2013; Kenakin and Christopoulos, 2013; Thompson et al., 2015a; Kenakin, 2019).
Experimentally, a potential caveat when analyzing in vitro data for biased signaling is the use of receptor outputs that encompass too much amplification, so that when comparisons are made relative to arrestin recruitment (a signaling output with little or no amplification), a ligand may appear to be biased (as in Fig. 2). It is also often assumed that the extent of amplification is linear across different ligands, but this may not be so. Distinct ligand-dependent conformations of G proteins and arrestins have recently been observed and are suggested to affect the downstream signaling by stabilizing specific effector conformations (Furness et al., 2016; Lee et al., 2016; Nuber et al., 2016). Thus extrapolation of the cAMP signal back to the receptor level, for example, may result in the identification of false positives in terms of biased agonists. Another confounding issue is becoming apparent: In terms of ligand binding and signaling pathway kinetics, in vitro kinetics or kinetic context has been shown to influence the extent and direction of the bias (Lane et al., 2017; Michel and Charlton, 2018).
Biased Agonism versus Partial Agonism.
The majority of G protein-biased μ-receptor ligands described to date have been low-efficacy agonists. This raises the question whether the profile of the ligands investigated thus far can actually be explained by partial agonism rather than actual bias (Fig. 2). For example, it has been argued that oliceridine could be differentiated as a biased agonist owing to the difference in efficacy between analgesia and constipation in comparison with the profiles for morphine and the partial agonist buprenorphine (DeWire et al., 2013). Yet, if the receptor reserve for constipation is less than that for analgesia, then the observed results are to be expected. A recent study (Kuo et al., 2015) looked at seven classic opioid agonists (both full and partial agonists, three of which are widely used clinically) and found that no two had the same profile for producing antinociception, constipation, and respiratory depression such that the rank order of potencies changed between the different in vivo assays. These differences can probably be explained by traditional pharmacological parameters and pharmacokinetics rather than differential bias, sounding a warning about ascribing to bias any slightly different effect of a novel agonist.
G Protein- and Arrestin-Mediated Behaviors In Vivo.
G protein-biased ligands have been widely described as having the potential to improve the therapeutic profile of μ-receptor ligands by functioning as effective analgesics with reduced prevalence of unwanted side effects. However, a review of the literature suggests that the distinction between desired therapeutic effects and adverse effects may not be separated simply into G protein-dependent versus arrestin-dependent effects.
A widely cited view in the opioid field is that respiratory depression is an arrestin-mediated event, an hypothesis whose basis is the observation that morphine-induced respiratory depression is attenuated in arrestin-3 knockout mice (Raehal et al., 2005). However, as mentioned earlier, the proposed G protein-biased agonist PZM21 effectively inhibited respiration (Hill et al., 2018), whereas a very recent study reports that in mice genetically modified to express mutated μ-opioid receptors lacking phosphorylation sites in the COOH-terminus of the receptor (and thus are unable to recruit arrestins), both morphine and fentanyl depress respiration likewise to that seen in wild-type mice (Kliewer et al., 2019). Other studies have implicated G protein-mediated signaling via neuronal potassium channel regulation in opioid-induced respiratory depression. Fentanyl-induced respiratory depression is decreased in G protein-activated inward rectifier potassium channel 2 (GIRK2)–subunit knockout mice (Montandon et al., 2016). Furthermore, local administration of either the broad-spectrum potassium channel blocker barium chloride or the GIRK channel inhibitor Tertiapin-Q into the pre-Bötzinger complex of anesthetized rats attenuated DAMGO-induced respiratory depression (Montandon et al., 2016). In another study, pretreatment with Tertiapin-Q reversed fentanyl-induced respiratory depression in conscious rats (Liang et al., 2018b). Apart from potassium channel modulation, the μ-receptor can also regulate neuronal activity via G protein-mediated regulation of voltage-gated calcium channels (Seward et al., 1991), but the role, if any, of these channels in respiratory depression remains to be investigated. Therefore, there is now compelling evidence to support the involvement of μ-opioid receptor–mediated G protein signaling in respiratory depression.
The role of arrestin-3 in opioid-induced constipation is currently unclear. Morphine acts at opioid receptors both centrally and peripherally to affect gastrointestinal function (Thörn et al., 1996; Imam et al., 2018). An earlier study looking at the involvement of arrestins in opioid-induced side effects reported a reduction in symptoms in the arrestin-3 knockout mice but only in some of the assays used to assess constipation (colonic propulsion, production of fecal boli) but not others (small-intestinal transit), suggesting that multiple signaling pathways contribute to opioid-induced gastrointestinal dysfunction (Raehal et al., 2005). The initial studies of both oliceridine and PZM21 reported less gastrointestinal dysfunction in comparison with morphine in mice (DeWire et al., 2013; Manglik et al., 2016); however, other studies have reported conflicting data on the effect of oliceridine and gastrointestinal dysfunction (Viscusi et al., 2016; Altarifi et al., 2017).
The data from arrestin-3 knockout mice reported that acute desensitization and tolerance to morphine are attenuated in the knockout animals (Bohn et al., 2000), suggesting that G protein-biased μ-receptor ligands may be therapeutically beneficial for maintaining analgesic efficacy in chronic pain states. Studies looking at the chronic administration of reported G protein-biased ligands are currently limited but, again, differences are being noted. For oliceridine, repeated administration over a 3-day period did not produce tolerance to antinociception or gastrointestinal dysfunction, as reported (Altarifi et al., 2017), whereas repeated administration of PZM21 over 4 days was found to induce antinociceptive tolerance but not tolerance to respiratory depressant effects (Hill et al., 2018).
Another major problem with the use of opioid drugs is abuse liability. Interestingly, the rewarding properties of morphine in the conditioned place-preference test were reportedly greater in the arrestin-3 knockout mice (Bohn et al., 2003). Such data actually imply a beneficial role for arrestin-mediated signaling and/or regulation with regard to this particular adverse effect. The majority of studies to date suggest that oliceridine exerts a similar reward profile to that of other clinically used opioids; intracranial self-stimulation studies found oliceridine to have an abuse liability similar to that of morphine (Altarifi et al., 2017), the reinforcing properties of oliceridine were also reportedly similar to that of oxycodone in rats (Austin Zamarripa et al., 2018). Conditioned place preference was observed following oliceridine administration in mice, although this was only apparent at a dose higher than that required for antinociception (Liang et al., 2018). In contrast, Manglik et al. (2016) reported that both oliceridine and PZM21 failed to produce conditioned place preference in mice. Mitragynine pseudoindoxyl was also reported to not induce conditioned place preference in mice (Váradi et al., 2016). Potential explanations for these discrepancies include the use of a single dose and time-point post–drug administration, differences in pharmacokinetics and, in the case of mitragynine pseudoindoxyl, its mixed opioid pharmacology (Negus and Freeman, 2018). Last and importantly, in clinical studies, oliceridine elicited morphine-like subjective effects in humans (Soergel et al., 2014). Such data suggest that G protein-biased ligands will probably not offer superiority in relation to this adverse effect associated with μ-opioid agonist administration.
It seems that the attribution of adverse effects of μ-receptor ligands to arrestin signaling may well represent an oversimplification of the cell signaling pathways (Kliewer et al., 2019). There is evidence to support the role of both G proteins and arrestin signaling in the major side effects associated with the μ-receptor. Some of the differences reported in the literature may be explained in part by differences in the type of behavioral test used, the dosing regimens, or differences between species/strain, but clearly these data highlight the need for a better understanding of the underlying physiology.
Bias at Other Opioid Receptors
Although the understanding and utility of bias at μ-receptors will probably undergo major reassessment over the next year or two, there remains great interest in developing biased ligands at other GPCRs, not least the other members of the opioid family. For the κ-receptor it is suggested that G protein-biased agonists could function as antinociceptive and antipruritic drugs without the dysphoria, sedation, and other side effects typically associated with the κ-receptor (Dogra and Yadav, 2015; Bohn and Aubé, 2017). One such promising G protein-based ligand of the κ-receptor is triazole 1.1; this compound has indeed been described as having analgesic and antipruritic activity but reportedly lacks the sedative and dysphoric properties of nonbiased κ-receptor agonists (Zhou et al., 2013; Brust et al., 2016, 2017). For the δ-receptor, G protein-biased agonists are suggested as potential antihyperalgesics for the treatment of chronic pain states without the associated proconvulsant activity or liability to induce tolerance that has been observed with some δ-receptor ligands (Pradhan et al., 2011; Dripps et al., 2018; Vicente-Sanchez et al., 2018). However, it should be noted that the neuronal signaling pathways (e.g., G protein, arrestin, or other) that mediate the beneficial and adverse effects of κ-receptor and δ-receptor activation have yet to be conclusively defined (Al-Hasani and Bruchas, 2011).
Conclusion
The field of biased agonism has had a significant impact on GPCR drug discovery in recent years. The μ-receptor still affords a promising approach for developing improved novel analgesics, but caution should be exerted when interpreting the in vitro data and during translation to in vivo models (Kenakin, 2018; Michel and Charlton, 2018). The recent issues with oliceridine (https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndAnalgesicDrugProductsAdvisoryCommittee/UCM622730.pdf) highlights the need for new and more strongly biased opioid ligands but overall suggests that a better understanding of the signaling pathways and more rigorous analysis of signaling data may prevent potentially costly drug attrition rates in the future.
Acknowledgments
We thank Graeme Henderson for critically commenting on the draft manuscript.
Abbreviations
- DAMGO
[D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin
- GPCR
G protein-coupled receptor
- GRK2
G protein-coupled receptor kinase 2
- PZM21
1-[(2S)-2-(dimethylamino)-3-(4-hydroxyphenyl)propyl]-3-[(2S)-1-thiophen-3-ylpropan-2-yl]urea
- TRV130
N-[(3-methoxythiophen-2-yl)methyl]-2-(9-pyridin-2-yl-6-oxaspiro[4.5]decan-9-yl)ethanamine
Authorship Contributions
Performed data analysis: Conibear.
Wrote or contributed to the writing of the manuscript: Conibear, Kelly.
Footnotes
This work was supported by the Medical Research Council (Grant. MR/N020669/1).
References
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators (2013) The concise guide to pharmacology 2013/14: G protein-coupled receptors. Br J Pharmacol 170:1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, Bruchas MR. (2011) Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115:1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS. (2017) Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin Zamarripa C, Edwards SR, Qureshi HN, Yi JN, Blough BE, Freeman KB. (2018) The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend 192:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JW, Leff P. (1983) Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220:141–162. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Aubé J. (2017) Seeking (and finding) biased ligands of the kappa opioid receptor. ACS Med Chem Lett 8:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG. (2003) Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci 23:10265–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498. [DOI] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, et al. (2016) Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal 9:ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, et al. (2017) A G protein-biased ligand of the kappa opioid receptor is antinociceptive and antipruritic but does not cause sedation or dysphoria. FASEB J 31. [Google Scholar]
- Burford NT, Clark MJ, Wehrman TS, Gerritz SW, Banks M, O’Connell J, Traynor JR, Alt A. (2013) Discovery of positive allosteric modulators and silent allosteric modulators of the μ-opioid receptor. Proc Natl Acad Sci USA 110:10830–10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgueno J, Pujol M, Monroy X, Roche D, Varela MJ, Merlos M, Giraldo J. (2017) A complementary scale of biased agonism for agonists with differing maximal responses. Sci Rep 7:15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A. (2003) Buprenorphine: new pharmacological aspects. Int J Clin Pract Suppl (133):3–8, NaN–24. [PubMed] [Google Scholar]
- Dahan A. (2006) Opioid-induced respiratory effects: new data on buprenorphine. Palliat Med 20 (Suppl 1):s3–s8. [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, et al. (2013) A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717. [DOI] [PubMed] [Google Scholar]
- Dogra S, Yadav PN. (2015) Biased agonism at kappa opioid receptors: implication in pain and mood disorders. Eur J Pharmacol 763 (Pt B):184–190. [DOI] [PubMed] [Google Scholar]
- Dripps IJ, Boyer BT, Neubig RR, Rice KC, Traynor JR, Jutkiewicz EM. (2018) Role of signalling molecules in behaviours mediated by the δ opioid receptor agonist SNC80. Br J Pharmacol 175:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert FJ. (2008) On the analysis of ligand-directed signaling at G protein-coupled receptors. Naunyn Schmiedebergs Arch Pharmacol 377:549–577. [DOI] [PubMed] [Google Scholar]
- Furness SGB, Liang YL, Nowell CJ, Halls ML, Wookey PJ, Dal Maso E, Inoue A, Christopoulos A, Wootten D, Sexton PM. (2016) Ligand-dependent modulation of G protein conformation alters drug efficacy. Cell 167:739–749.e11. [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. (2007) An opioid agonist that does not induce mu-opioid receptor--arrestin interactions or receptor internalization. Mol Pharmacol 71:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther T, Dasgupta P, Mann A, Miess E, Kliewer A, Fritzwanker S, Steinborn R, Schulz S. (2018) Targeting multiple opioid receptors - improved analgesics with reduced side effects? Br J Pharmacol 175:2857–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, Bailey C, Kelly E, Henderson G. (2018) The novel μ-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175:2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothersall JD, Torella R, Humphreys S, Hooley M, Brown A, McMurray G, Nickolls SA. (2017) Residues W320 and Y328 within the binding site of the μ-opioid receptor influence opiate ligand bias. Neuropharmacology 118:46–58, doi: 10.1016/j.neuropharm.2017.03.007 . [DOI] [PubMed] [Google Scholar]
- Imam MZ, Kuo A, Ghassabian S, Smith MT. (2018) Progress in understanding mechanisms of opioid-induced gastrointestinal adverse effects and respiratory depression. Neuropharmacology 131:238–255. [DOI] [PubMed] [Google Scholar]
- Kelly E. (2013) Efficacy and ligand bias at the μ-opioid receptor. Br J Pharmacol 169:1430–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (2018) Is the quest for signaling bias worth the effort? Mol Pharmacol 93:266–269. [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2019) Biased receptor signaling in drug discovery. Pharmacol Rev 71:267–315. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. (2013) Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov 12:205–216. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Watson C, Muniz-Medina V, Christopoulos A, Novick S. (2012) A simple method for quantifying functional selectivity and agonist bias. ACS Chem Neurosci 3:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP, Morgan PH. (1989) Theoretical effects of single and multiple transducer receptor coupling proteins on estimates of the relative potency of agonists. Mol Pharmacol 35:214–222. [PubMed] [Google Scholar]
- Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, Schulz S. (2019) Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel AC, Gassaway MM, Kapoor A, Váradi A, Majumdar S, Filizola M, Javitch JA, Sames D. (2016) Synthetic and receptor signaling explorations of the Mitragyna alkaloids: mitragynine as an atypical molecular framework for opioid receptor modulators. J Am Chem Soc 138:6754–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A, Wyse BD, Meutermans W, Smith MT. (2015) In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: no two opioids have the same profile. Br J Pharmacol 172:532–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JR, May LT, Parton RG, Sexton PM, Christopoulos A. (2017) A kinetic view of GPCR allostery and biased agonism. Nat Chem Biol 13:929–937. [DOI] [PubMed] [Google Scholar]
- Latorraca NR, Venkatakrishnan AJ, Dror RO. (2017) GPCR dynamics: structures in motion. Chem Rev 117:139–155. [DOI] [PubMed] [Google Scholar]
- Lee MH, Appleton KM, Strungs EG, Kwon JY, Morinelli TA, Peterson YK, Laporte SA, Luttrell LM. (2016) The conformational signature of β-arrestin2 predicts its trafficking and signalling functions. Nature 531:665–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Shiotani K, Miyazaki A, Tsuda Y, Ambo A, Sasaki Y, Jinsmaa Y, Marczak E, Bryant SD, Lazarus LH, et al. (2007) Bifunctional [2′,6′-dimethyl-L-tyrosine1]endomorphin-2 analogues substituted at position 3 with alkylated phenylalanine derivatives yield potent mixed mu-agonist/delta-antagonist and dual mu-agonist/delta-agonist opioid ligands. J Med Chem 50:2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang DY, Li WW, Nwaneshiudu C, Irvine KA, Clark JD. (2018) Pharmacological Characters of Oliceridine, a μ-Opioid Receptor G-Protein-Biased Ligand in Mice. Anesth Analg, doi: 10.1213/ANE.0000000000003662. [DOI] [PubMed] [Google Scholar]
- Liang X, Yong Z, Su R. (2018b) Inhibition of protein kinase A and GIRK channel reverses fentanyl-induced respiratory depression. Neurosci Lett 677:14–18. [DOI] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, et al. (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, et al. (2010) μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol 78:756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Charlton SJ. (2018) Biased agonism in drug discovery-is it too soon to choose a path? Mol Pharmacol 93:259–265. [DOI] [PubMed] [Google Scholar]
- Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, Horner RL. (2016) G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology 124:641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Freeman KB. (2018) Abuse potential of biased mu opioid receptor agonists. Trends Pharmacol Sci 39:916–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber S, Zabel U, Lorenz K, Nuber A, Milligan G, Tobin AB, Lohse MJ, Hoffmann C. (2016) β-Arrestin biosensors reveal a rapid, receptor-dependent activation/deactivation cycle. Nature 531:661–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaran HO, Ambrosio C, Uğur Ö, Madaras Koncz E, Grò MC, Vezzi V, Rajagopal S, Costa T. (2017) Systematic errors in detecting biased agonism: analysis of current methods and development of a new model-free approach. Sci Rep 7:44247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL. (2011) The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 32:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Jivan JK, Andurkar SV. (2012) Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc 112:792–799. [PubMed] [Google Scholar]
- Raehal KM, Walker JKL, Bohn LM. (2005) Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314:1195–1201. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, Lefkowitz RJ. (2011) Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol 80:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero G, Llorente J, McPherson J, Cooke A, Mundell SJ, McArdle CA, Rosethorne EM, Charlton SJ, Krasel C, Bailey CP, et al. (2012) Endomorphin-2: a biased agonist at the μ-opioid receptor. Mol Pharmacol 82:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM. (2017) Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171:1165–1175.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S. (2018) Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. Am J Transplant 18:1556–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seward E, Hammond C, Henderson G. (1991) Mu-opioid-receptor-mediated inhibition of the N-type calcium-channel current. Proc Biol Sci 244:129–135. [DOI] [PubMed] [Google Scholar]
- Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL, Violin JD, Lark MW. (2014) First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol 54:351–357. [DOI] [PubMed] [Google Scholar]
- Stanczyk MA, Kandasamy R. (2018) Biased agonism: the quest for the analgesic holy grail. Pain Rep 3:e650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GL, Kelly E, Christopoulos A, Canals M. (2015a) Novel GPCR paradigms at the μ-opioid receptor. Br J Pharmacol 172:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GL, Lane JR, Coudrat T, Sexton PM, Christopoulos A, Canals M. (2015b) Biased agonism of endogenous opioid peptides at the μ-opioid receptor. Mol Pharmacol 88:335–346. [DOI] [PubMed] [Google Scholar]
- Thompson GL, Lane JR, Coudrat T, Sexton PM, Christopoulos A, Canals M. (2016) Systematic analysis of factors influencing observations of biased agonism at the mu-opioid receptor. Biochem Pharmacol 113:70–87. [DOI] [PubMed] [Google Scholar]
- Thörn SE, Wattwil M, Lindberg G, Säwe J. (1996) Systemic and central effects of morphine on gastroduodenal motility. Acta Anaesthesiol Scand 40:177–186. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13. [DOI] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, et al. (2016) Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with mu agonism and delta antagonism, which do not recruit β-arrestin-2. J Med Chem 59:8381–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Sanchez A, Dripps IJ, Tipton AF, Akbari H, Akbari A, Jutkiewicz EM, Pradhan AA. (2018) Tolerance to high-internalizing δ opioid receptor agonist is critically mediated by arrestin 2. Br J Pharmacol 175:3050–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F. (2016) A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain 157:264–272. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. (1994) Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther 55:569–580. [DOI] [PubMed] [Google Scholar]
- Winpenny D, Clark M, Cawkill D. (2016) Biased ligand quantification in drug discovery: from theory to high throughput screening to identify new biased μ opioid receptor agonists. Br J Pharmacol 173:1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto LT, Horie S, Takayama H, Aimi N, Sakai S, Yano S, Shan J, Pang PK, Ponglux D, Watanabe K. (1999) Opioid receptor agonistic characteristics of mitragynine pseudoindoxyl in comparison with mitragynine derived from Thai medicinal plant Mitragyna speciosa. Gen Pharmacol 33:73–81. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Nilges MR, Morgenweck J, Zhang X, Hackler L, Fasold MB. (2016) Endomorphin analog analgesics with reduced abuse liability, respiratory depression, motor impairment, tolerance, and glial activation relative to morphine. Neuropharmacology 105:215–227. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, et al. (2013) Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem 288:36703–36716. [DOI] [PMC free article] [PubMed] [Google Scholar]