Abstract
Plasma human immunodeficiency virus type 1 (HIV-1) RNA levels in women are lower early in untreated HIV-1 infection compared with those in men, but women have higher T-cell activation and faster disease progression when adjusted for viral load. It is not known whether these sex differences persist during effective antiretroviral therapy (ART), or whether they would be relevant for the evaluation and implementation of HIV-1 cure strategies. We prospectively enrolled a cohort of reproductive-aged women and matched men on suppressive ART and measured markers of HIV-1 persistence, residual virus activity, and immune activation. The frequency of CD4+ T cells harboring HIV-1 DNA was comparable between the sexes, but there was higher cell-associated HIV-1 RNA, higher plasma HIV-1 (single copy assay), and higher T-cell activation and PD-1 expression in men compared with women. These sex-related differences in immune phenotype and HIV-1 persistence on ART have significant implications for the design and measurement of curative interventions.
Keywords: sex differences, HIV-1, cure, reservoir, immune activation
Biological sex modulates immune-mediated protection from pathogens and autoimmunity, leading to differences in the acquisition and pathogenesis of multiple infections and the efficacy of vaccines and to a female predominance of some autoimmune diseases [1, 2]. Sex differences have been specifically observed in human immunodeficiency virus type 1 (HIV-1) viral dynamics and immune responses [3, 4]. Immunologic sexual dimorphisms are driven by multiple factors including behavioral and socioeconomic dynamics, but also by biological features: genetic differences derived from the chromosomal complement [5, 6], sex-specific epigenetic profiles [7, 8], and the influence of sex hormones [9, 10].
Higher plasma HIV-1 RNA levels in men vs women are evident proximal to seroconversion [11–17]. Importantly, despite this lower level of HIV-1 RNA in women, disease progression rates were comparable in both sexes in the pretreatment era [17]. Viral load (VL) differences attenuate as disease progresses, but there is evidence for sex-driven effects on plasma HIV-1 RNA [18–20]. The mechanisms governing the interaction between HIV-1 RNA levels and disease progression may include differences in T-cell subset distribution and available targets [21], along with hormonal modulation/sex-specific differences in immune responses [22–24] and direct inhibition of viral transcription by estrogen [25, 26].
Immune activation is also discordant by sex. Women have higher T-cell activation for a given level of plasma viremia [27], higher levels of interferon-α production by plasmacytoid dendritic cells (pDCs) after Toll-like receptor 7 stimulation [22, 27], and higher expression of interferon-stimulated genes for a given level of HIV-1 RNA [28]. Taken together, the data demonstrate that the immune response to HIV-1 is sex-specific.
There are few data regarding sex differences in the HIV-1 virus reservoir size and activity, or in cellular immunophenotypes after the initiation of antiretroviral therapy (ART), which is highly efficacious in both men and women [29]. Two cross-sectional studies of ART-treated individuals have reported that women were more likely to have lower levels of HIV-1 DNA than men [30, 31], and others reported sex differences in soluble inflammatory markers linked to non-AIDS morbidity [32–34]. However, no study has prospectively and systematically assessed sex differences in immune phenotype and HIV-1 reservoir, and women are underrepresented in clinical trials relevant to HIV-1 cure [35].
To address this knowledge gap, we prospectively enrolled a cohort of well-matched, HIV-1–infected men and women with ART-suppressed viremia to measure the frequency, activity, and inducibility of latently infected cells and cellular immune activation. We identified substantial differences between men and women that should inform the design and interpretation of clinical trials testing HIV-1 curative interventions.
METHODS
Study Design and Participants
Study participants were enrolled through the University of California, San Francisco (UCSF) SCOPE OPTIONS cohort and the San Francisco General Hospital Positive Health Practice (“Ward 86”) HIV Clinic between March 2014 and April 2015. All participants provided informed consent, and the study was approved by the UCSF institutional review board. Women and men on suppressive ART were prospectively enrolled matched 1:1 by duration of viral suppression (1 to <3 years, 3–10 years, >10 years), absolute CD4+ T-cell count (if <500 cells/μL within 100 cells, or >500 cells/μL) and nadir CD4+ T-cell count (<200, 201–350, >350 cells/μL). Early start of therapy ≤6 months after infection with continuous suppression, viremic control (defined as a majority of plasma VLs <10000 copies), age, race, and total duration of ART were considered and balanced when possible. Elite controllers (untreated VL <400 copies) and participants on systemic hormonal therapy were excluded. Hepatitis B virus, hepatitis C virus, and cytomegalovirus (CMV) coinfection data, demographics, ART, menstrual status, and reproductive history were collected. Peripheral blood mononuclear cells (PBMCs) and plasma were stored for analysis; a subset (n = 6 of each sex) was returned for leukapheresis.
Virologic Measures
Total and integrated HIV-1 DNA was quantified relative to CD3 copy number in isolated CD4+ T cells as previously described [36]. In brief, a long terminal repeat (LTR) primer (U3-R junction with a lambda phage heel sequence) was used in combination with an LTR/Gag primer (total) or 2 Alu-specific primers (integrated) in a preamplification polymerase chain reaction (PCR). Subsequent real-time PCR amplification used a primer to the added lambda phage sequence with an LTR U5 primer and an internal probe for both total and integrated DNA [36]. Cell-associated unspliced HIV RNA was quantified [37, 38] and multiply spliced HIV RNA was measured using the identical seminested PCR with different primers (Supplementary Methods). Single-copy assay (SCA) of viremia was quantified from 7 mL of plasma with PCR targeting a conserved, untranslated HIV-1 gag RNA sequence (HMMC gag assay; see Supplementary Methods [39]). Inducible HIV-1 RNA was measured using the tat/rev induced limiting dilution assay (TILDA) [40]; in brief, purified CD4+ T cells are stimulated with phorbol myristate acetate (100 ng/mL)/ionomycin (1 μg/mL), serially diluted, and lysed, and multiply spliced HIV RNA is measured with a 2-step reverse-transcription PCR assay. Maximum likelihood estimates based on the number of positive wells are used to determine the frequency of cells harboring inducible HIV-1. A modified version of the envelope detection by induced transcription-based sequencing (EDITS) assay [26] was used to quantify spreading viral infections. Purified CD4+ T cells were stimulated with concanavalin A ± 1 μM raltegravir (to block spreading infection) and absence or presence of 300 pg/mL 17β-estradiol. New infection was assessed at 9 days as the increase in HIV RNA–positive cells in the absence of raltegravir (Supplementary Methods).
Immunologic Measures
Cryopreserved PBMCs were batch processed and stained with panels for T-cell activation and exhaustion, monocyte, natural killer (NK), and dendritic cell phenotyping. Estrogen receptor 1 (ESR-1) expression was assessed with intracellular staining after incubation with and without 17β-estradiol. Gating strategies and antibody panels available in the Supplementary Methods and Supplementary Tables 9–11.
Hormone Levels
Plasma levels of progesterone and 17β-estradiol were determined in batch by liquid chromatography–mass spectrometry (Brigham and Women’s Hospital Research Assay Core).
Statistical Analysis
Virologic outcomes were assessed using negative binomial regression to generate estimates of the effect of female sex on the outcome variable, including a measure of input as the exposure variable (eg, 18S RNA for HIV-1 RNA measures, plasma volume for HMMC gag measures) as previously described [41]. This method accounts for the lower precision of measures at the lower limits of detection and with low inputs. Multivariate models were built by stepwise addition of predictor variables, with sex forced as a covariate, until no remaining unselected candidate predictor had P < .05 when added to the current model. Pretreatment maximum VL was excluded from models of residual viremia, as it may be on the causal pathway of sex’s influence on residual viremia [42]. Mixed-effects negative binomial regression was used to assess the fold-effect of sex on the ratios of HMMC gag and HIV-1 RNA measures to the integrated HIV DNA measure, also as previously described [41]. TILDA values were compared by maximum likelihood estimation on the data from all individual experimental wells. For plotting purposes only, one person with no positive wells was given a TILDA value of 2. To estimate the effect of female sex on the TILDA/integrated HIV ratio, we performed customized maximum likelihood modeling of the well-by-well TILDA results together with the detailed integrated HIV data. For TILDA, we used the standard single-hit likelihood calculations for limiting dilution assays, and for integrated HIV we used a negative binomial model with constant dispersion and with the input to the assay (CD3) as the exposure. The model included normally distributed random effects that modeled between-person variation in log(TILDA) and log(TILDA:integrated HIV ratio). EDITS data from a single sequencing chip were assessed for differences in the frequency of infected cells by unpaired t test with Welch correction.
Virologic and immunologic parameters were assessed for associations using Spearman rank correlation in the overall cohort and within each sex. P values for differences in correlations between men and women were calculated using the Fisher z transformation (http://vassarstats.net/rdiff.html). Immune subsets were compared between sexes by Mann–Whitney testing.
Nominal P values are reported without adjustment for multiple testing; adjustment requires that results expected to biologically co-vary (eg, inverse variations in T-cell subsets) detract from each other, when they should be reinforcing [43–45]. We present the full dataset, including exploratory findings, indicating where the unadjusted P value was <.05.
RESULTS
Cohort Characteristics
Demographic and clinical features of the participants (26 women and 26 men) are shown in Table 1. Maximum pretreatment VL was not matched, and the median value in women was 0.13 log lower than in men (P = .14, Mann–Whitney test). Active hepatitis C virus infection and injection drug use (P > .5, Fisher exact test) and rates of viremic controllers (23% men, 35% women; P = .54, Fisher exact test) were balanced between the groups. The CMV-seropositive rate was higher among men than women (100% in men vs 81% in women; P = .05, Fisher exact test). Seventy-three percent of the women reported regular menstrual cycles, and all had detectable 17β-estradiol and progesterone levels (Supplementary Table 1). Of patients with amenorrhea, 2 had history of ovary-sparing hysterectomy and 2 had a history of intrauterine device placement (>6 months prior to study enrollment). Three additional women reported irregular menses; in 2 of these women, the hormone levels and clinical assessment suggested an anovulatory cycle at the time of sampling (Supplementary Table 2).
Table 1.
Demographic and Clinical Characteristics of the Cohort
| Cohort Characteristics | Men | Women |
|---|---|---|
| Age, y, median (IQR) | 43 (33–48) | 41 (35–48) |
| CD4 nadir cells/μL, median (IQR) | 270 (131–442) | 214 (111–317) |
| CD4 at sampling cells/μL, median (IQR) | 646 (544–825) | 677 (530–861) |
| Duration of infection, y, median (IQR) | 7 (4.0–11.5) | 8 (4.8–14.3) |
| Duration of viral suppression, y, median (IQR) | 3.3 (2.1–6.7) | 2.8 (1.8–4.3) |
| Max pretreatment VL, median (IQR) | 4.74 (4.4–5.4) | 4.61 (3.8–5.2) |
| Controller (majority of pretreatment VL <10000) | 6 (23) | 9 (35) |
| CMV positive | 26 (100) | 21 (81) |
| Active HCV infection | 2 (7.7) | 1 (3.8) |
| IDU | 3 (12) | 5 (19) |
| Timing of ART initiationa | ||
| Early, continuous | 1 (4) | 1 (4) |
| Late | 20 (77) | 20 (77) |
| Early and interrupted or unknown | 5 (19) | 5 (19) |
| ART regimen | ||
| PI | 3 (12) | 9 (35) |
| NNRTI | 12 (46) | 11 (42) |
| INSTI | 9 (35) | 6 (23) |
| PI/INSTI | 1 (4) | 0 |
| NNRTI/INSTI | 1 (4) | 0 |
| Race/ethnicity | ||
| White | 9 (35) | 8 (31) |
| Black | 7 (27) | 6 (23) |
| Hispanic | 4 (15) | 4 (15) |
| Asian | 2 (8) | 3 (12) |
| Native American | 1 (4) | 0 |
| Mixed/multiracial/other | 3 (11) | 5 (19) |
| History of sex with male partner(s) | 24 (92) | 26 (100) |
Data are presented as No. (%) unless otherwise indicated. Observations were available for all subjects (26 women and 26 men) with the exception of maximum pretreatment VL; this value was missing in 5 observations, all from the female subjects.
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; HCV, hepatitis C virus; IDU, injection drug use; IQR, interquartile range; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; VL, viral load.
aDefinitions for timing of ART initiation: early is continuous is therapy initiated ≤6 months from estimated date of infection with continuous suppression; late is therapy initiated >6 months after estimated date of infection; early, interrupted, or unknown includes participants with unknown timing of therapy initiation and those who started within 6 months of infection but had interruptions with viral rebound after that point.
Frequencies of CD4+ T Cells Harboring HIV-1 DNA Between Sexes
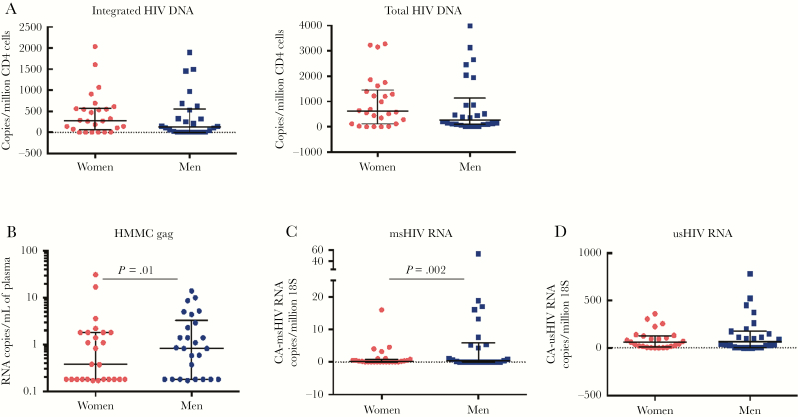
Isolated CD4+ T cells were analyzed for integrated and total HIV-1 DNA. Integrated HIV and total HIV were highly correlated with each other in the overall cohort and within each sex (Supplementary Table 3). Integrated HIV DNA correlated with peak pretreatment viremia overall (r = 0.48, P = .001) and within each sex (women: r = 0.63, P = .002; men: r = 0.46, P = .018), and with nadir CD4+ T-cell count and proximal pretreatment viral load, with similar relationships for total HIV DNA (Supplementary Table 3). HIV DNA content of CD4+ T cells was similar between men and women (Figure 1A, Table 2); women had an estimated a 1.39-fold higher level of integrated HIV DNA, but with a wide 95% confidence interval (95% CI, .57–3.37; P = .47), with similar estimates for total HIV DNA (1.38-fold increase in women [95% CI, .67–2.84]; P = .39). Models incorporating additional clinical characteristics also estimated similarly modest sex differences, not reaching statistical significance.
Figure 1.
Comparison of virologic markers by sex. A, Integrated and total DNA measured in isolated CD4 cells were comparable between men and women. B, Low-level viremia measured by single-copy assay was lower in women than in men. C, Multiply spliced human immunodeficiency virus (HIV) RNA was lower in women. D, There was no statistically significant difference in the level of unspliced HIV RNA. For all values, median and interquartile range are shown; statistical comparisons with negative binomial regression. Abbreviations: CA-HIV, cell-associated-HIV; HIV, human immunodeficiency virus; msHIV, multiply spliced human immunodeficiency virus; usHIV, unspliced human immunodeficiency virus.
Table 2.
Effect of Female Sex on Virologic Measures
| HIV-1 Reservoir Measure | Female Fold-Effect | (95% CI) | P Value |
|---|---|---|---|
| Integrated HIV DNA | 1.39 | (.57–3.37) | .47 |
| Total HIV DNA | 1.38 | (.67–2.74) | .39 |
| SCA (HMMC gag) | 1.02 | (.38–2.72) | .974 |
| SCA (HMMC gag)a | 0.23 | (.08–.72) | .011 |
| SCA (HMMC gag):integrated HIV DNA ratio | 0.43 | (.20–.91) | .027 |
| Cell-associated msHIV RNA | 0.16 | (.05–.51) | .002 |
| Cell-associated msHIV RNAb | 0.25 | (.09–.71) | .009 |
| Cell-associated msHIV RNA:integrated HIV DNA ratio | 0.29 | (.13–.64) | .002 |
| Cell-associated unspliced HIV RNA | 0.65 | (.29–1.43) | .280 |
| Cell-associated unspliced HIV RNAc | 0.68 | (.35–1.32) | .253 |
| Cell-associated unspliced HIV RNA:integrated HIV DNA ratio | 0.52 | (.25–1.07) | .08 |
Negative binomial regression in univariate and multivariate models to assess the quantitative influence of female sex on virologic measures.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; msHIV, multiply spliced human immunodeficiency virus; SCA, single-copy assay.
aAdjusted for duration of suppression and treatment interruptions.
bAdjusted for CD4 cell count nadir and controller phenotype.
cAdjusted for maximum pretreatment viral load, CD4 cell count nadir, race, early treatment initiation, and controller phenotype.
HIV-1 Reservoir Activity in Women Compared to Men
All participants were suppressed to <75 copies of HIV-1 RNA/mL plasma by clinical assays. Women had a 77% lower level of residual plasma viremia by HMMC gag in a multivariate model controlling for years of viral suppression and number of treatment interruptions (fold-effect, 0.23 [95% CI, .08–.72]; P = .011; Figure 1B, Table 2, Supplementary Table 4). The ratio of plasma viremia to integrated HIV DNA averaged 57% lower in women compared with men in a univariate model (fold-effect, 0.43 [95% CI, .20–.91]; P = .027).
We measured cell-associated HIV RNA from CD4+ T cells, finding a 6-fold lower level of multiply spliced HIV RNA in women (negative binomial regression, fold-effect, 0.16 [95% CI, .05–.51]; P = .002) (Figure 1C, Table 2, Supplementary Table 4). In a multivariate model adjusting for nadir CD4 and controller phenotype (both associated with multiply spliced HIV RNA at P < .05), there was a 4-fold lower level of multiply spliced HIV RNA in women (fold-effect, 0.25 [95% CI, .09–.71]; P = .009). The ratio of multiply spliced HIV RNA to integrated HIV DNA was 3.4-fold lower in women (fold-effect, 0.29 [95% CI, .13–.64]; P = .002). Univariate negative binomial regression estimated 35% lower level of unspliced HIV RNA in women, but with a wide 95% CI (fold-effect, 0.65 [95% CI, .29–1.43]; P = .28) (Figure 1D, Table 2, Supplementary Table 4). A multivariate model (early ART, log maximum pretreatment plasma HIV-1 RNA, CD4 nadir, controller phenotype, race) estimated a similar fold-change, again without achieving statistical significance (fold- effect, 0.68 [95% CI, .35–1.32]; P = .25). Sex comparison of the ratio of unspliced HIV RNA to integrated HIV DNA level was similar (fold-effect, 0.52 [95% CI, .25–1.07]; P = .08). Taken together, despite similar measures of HIV DNA, women had less measurable virus activity than men by both the SCA for plasma viremia and the level of multiply spliced HIV RNA in CD4+ T cells.
Subset Analysis of Ex Vivo Reservoir Induction
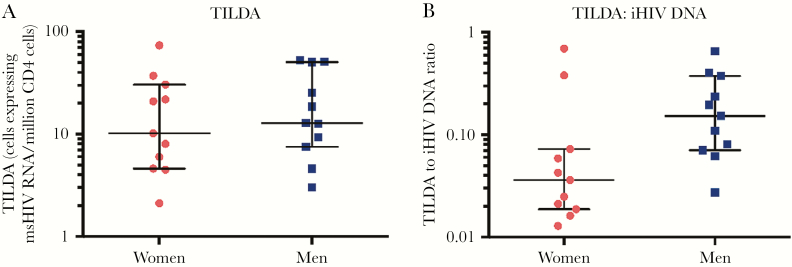
In a subset of subjects (11 men and 11 women), ex vivo induction of spliced tat/rev transcripts after T-cell activation was measured by TILDA [40]. TILDA values did not differ between men and women in this subgroup; female status fold-effect was 0.81 (maximum likelihood estimate; 95% CI, .33–2.0; P = .63; Figure 2A). We then compared the ratio of TILDA:integrated HIV DNA values, using customized maximum likelihood modeling of the well-by-well TILDA results. In this subset, women were estimated to have approximately 2-fold lower ratio of inducible HIV-1 RNA relative to the integrated HIV DNA levels, but again with wide CIs (female sex fold-effect, 0.45 [95% CI, .16–1.21]; P = .11; Figure 2B).
Figure 2.
Sex comparison of the inducible reservoir as measured by the tat/rev induced limiting dilution assay (TILDA) in isolated resting CD4+ T cells. A, Comparison of TILDA values between men and women did not show a statistically significant difference. B, When normalized to integrated human immunodeficiency virus (HIV) DNA levels, female subjects had generally lower ratios of TILDA to integrated HIV DNA, although this did not achieve statistical significance. Median and interquartile range is shown for all values; statistical comparison maximum likelihood estimates for TILDA comparison and with a customized maximum likelihood model for the ratio. Abbreviations: iHIV, integrated human immunodeficiency virus; msHIV, multiply spliced human immunodeficiency virus; TILDA, tat/rev induced limiting dilution assay.
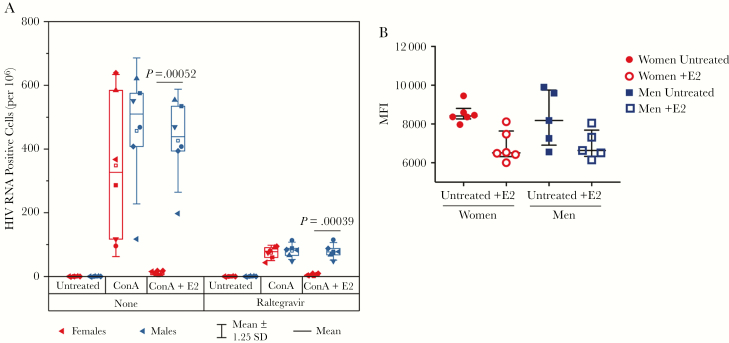
As a complementary method to evaluate provirus activation, CD4+ T cells from a subset of men (n = 6) and women (n = 6) who underwent leukapheresis were evaluated for inducible replication competent virus using a modified EDITS assay [26] (Figure 3A). Purified CD4+ memory cells were stimulated for 9 days to induce HIV transcription in the presence or absence of raltegravir and the number of HIV RNA+ cells was quantified by EDITS [26]. Raltegravir blocked viral spread: 74 vs 80 RNA+ cells per million for women and men, respectively. These values are similar to those obtained from overnight induction by T cell receptor activation [26], but not statistically different between the sexes. In the absence of raltegravir, there was spreading infection with 348 RNA+ cells per million for women and 457 RNA+ cells per million for men. Addition of 300 pg/mL 17β-estradiol blocked both HIV RNA induction (P = .00039) and spreading infection (P = .00052) in women compared with men, consistent with our earlier observation that ESR-1 can act as a repressor of HIV transcription [26]. ESR-1 protein expression was similar by flow cytometry on CD4 cells from men and women (Figure 3B).
Figure 3.
Estrogen blocks human immunodeficiency virus (HIV) RNA transcription and spreading infection. A, Isolated CD4+ T cells from 6 male (blue symbols) and 6 female (red symbols) donors were stimulated with concanavalin A and cultured in the presence or absence of 1 μM raltegravir with and without 300 pg/mL 17β-estradiol. The number of HIV RNA–positive cells per million was quantified via envelope detection by induced transcription-based sequencing after 9 days of culture. Open symbols, median; horizontal line, mean; box plots, interquartile range; whiskers, ±1.25 standard deviations. Statistical comparisons were made with unpaired t test with Welch correction, and significant values are indicated on the graph. B, Peripheral blood mononuclear cells from men (n = 5) and women (n = 6) were cultured with and without 300 pg/mL 17β-estradiol (denoted as +E2 in the figure), and estrogen receptor 1 expression was measured in CD4+ T cells with intracellular staining and quantified by geometric mean fluorescence intensity. There was no statistically significant difference in untreated cells or after culture with 17β-estradiol (median and interquartile ranges are shown, Mann–Whitney statistics). Abbreviations: ConA, concanavalin A; HIV, human immunodeficiency virus; MFI, mean fluorescence intensity; SD, standard deviation.
Levels of Cellular Immune Activation and PD-1 Expression in Women Compared to Men
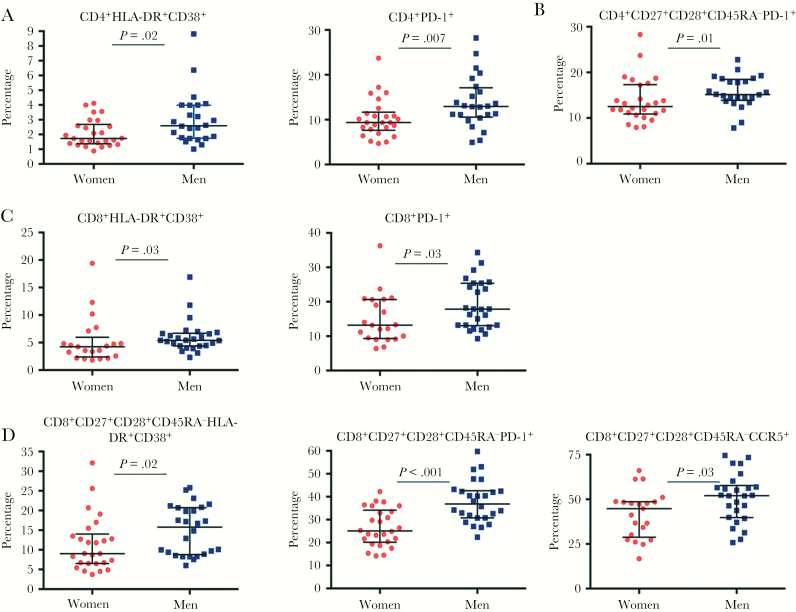
A prespecified primary analysis of immunophenotypes compared T-cell activation, antigen experience/exhaustion, and CCR5 expression on bulk and memory T cells. Activation defined by HLA-DR/CD38 coexpression and antigen experience/exhaustion indicated by PD-1 expression were higher in men in total and memory CD4+ and CD8+ T cells (Figure 4). CCR5 expression percentage was higher only on bulk CD8+ T cells. T-cell comparisons were reanalyzed excluding CMV-seronegative individuals (n = 5, all women); the majority remained statistically significant (Supplementary Table 5). In contrast, the distribution of the innate immune populations of NK cells, monocytes, myeloid dendritic cells (mDCs), and pDCs was not statistically different by sex (Supplementary Table 6).
Figure 4.
Comparison of activation and PD-1 expression in T cells. A, Frequency of total CD4+ T cells coexpressing HLA-DR and CD38, total CD4+ T cells expressing PD-1, and memory CD4+ T cells expressing PD-1 (B) are higher in men than in women. C, Total CD8+ T cells also showed a higher frequency of HLA-DR and CD38 coexpression and PD-1 expression. D, A greater frequency of memory CD8+ T cells coexpressed HLA-DR and CD38 or individually expressed PD-1 or CCR5 in men compared with women. For all values, median and interquartile ranges and comparisons with Mann–Whitney statistics are shown.
Sex Specificity of Relationships Between T-Cell and Viral Parameters
All subsets of T cells, measures of activation, differentiation, and exhaustion were assessed for associations with virologic measures by Spearman rank correlation. Multiple correlations between CD4+ and CD8+ T-cell subset percentages (Supplementary Tables 7 and 8) and measures of viral activity were observed in the overall cohort. This finding is in marked contrast to the lack of identified associations at a P < .05 level between any of the innate immune subsets/activation markers and virologic measures (Supplementary Table 6).
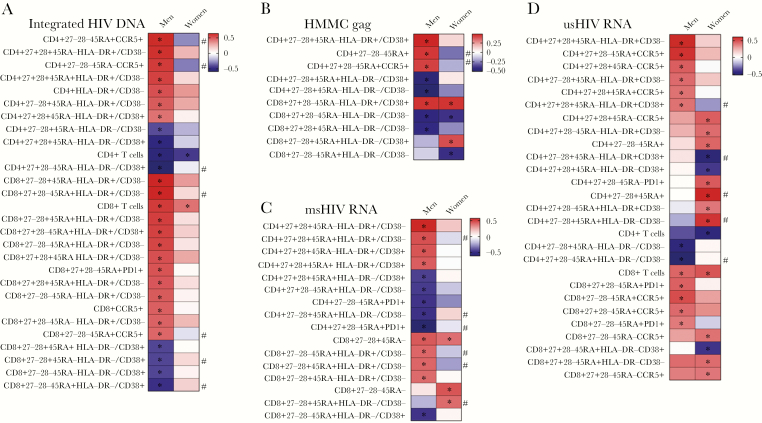
Exploratory analysis revealed distinct relationships between T-cell parameters and virologic measures when men and women were considered separately. Again, there were no statistically significant relationships between innate cell phenotypes and virologic measures. For integrated HIV DNA, only bulk CD4+ and CD8+ T-cell percentage correlated in women, whereas multiple correlations were seen between integrated HIV DNA and T-cell subsets in men (Figure 5A); correlation differences were different at P < .05 for several subsets. Excluding the CMV-seronegative women yielded largely consistent results, with a few unique associations (Supplementary Figure 1).
Figure 5.
Associations between T-cell parameters and virologic measures by sex. A, Correlation analysis for T-cell parameters and integrated human immunodeficiency virus (HIV) stratified by sex. Box color indicates the strength of the Spearman correlation based on ρ value (red indicates a strong positive correlation and blue indicates a strong inverse correlation). *P < .05. #Statistically significant difference in correlation values between men and women. Similar information is shown for Spearman correlation analysis for T-cell parameters and HMMC gag single-copy assay (B), for T-cell parameters and multiply spliced HIV RNA (C), and for T-cell parameters and unspliced HIV RNA (D). Abbreviations: HMMC, xxx; msHIV, multiply spliced human immunodeficiency virus; usHIV, unspliced human immunodeficiency virus.
Of note, the CD4+CD27+CD28+CD45RA–HLA-DR+CD38– subset correlated with all virologic measures in the overall cohort (Supplementary Table 7). The activated transitional memory CD8+CD27+CD28–CD45RA–HLA-DR+CD38+population was positively correlated with the SCA values in all analyses (Spearman ρ, overall cohort: r = 0.47, P = .0005; men: r = 0.46, P = .02; women: r = 0.46, P = .02; women with CMV seronegatives excluded: r = 0.45, P = .04) (Supplementary Table 8, Figure 5B).
Within women, we analyzed the relationship of circulating levels of 17β-estradiol and progesterone to immune and virologic parameters. This analysis identified only an association between these hormones and the circulating percentage of mDCs (17β-estradiol: r = 0.42, P = .036; progesterone: r = 0.55, P = .005). Overall, associations between T-cell parameters and virologic measures displayed sex specificity.
DISCUSSION
In one of the first studies to systematically examine sex differences among HIV-infected individuals on ART, using a matched prospectively enrolled cohort of men and women, we identify key sex differences in residual virus activity and cellular immune activation. These observations suggest that virologic and immunologic outcomes after curative interventions may differ by sex, requiring careful attention to enrolling an adequate number of women in cure studies and to reporting sex-delineated outcomes.
In contrast to prior studies [30, 31], the HIV-1 DNA levels in men and women in this cohort were comparable. The prior studies measured HIV-1 DNA levels in PBMCs [30, 31], while we measured HIV-1 DNA in CD4+ T cells; sex variation in lymphocyte percentages [46] may explain the discrepant findings. Alternatively, our prospective design and matching on CD4+ nadir and other characteristics may have balanced HIV-1 DNA. We further isolated the influence of sex from confounders using multivariate models with relevant characteristics, strengthening our conclusions. Peak pretreatment plasma HIV-1 RNA in women was slightly lower (difference in median 0.13 log, P = .14), consistent with prior studies reporting lower (0.13–0.35 log) viral loads in women [18] and suggesting that our cohort is representative of a typical pattern of pathogenesis. Although our efforts to match the groups may have attenuated the effect of sex by controlling for factors along the causal pathway, these clinically comparable groups highlight the direct role of biological sex in reservoir dynamics.
Despite no substantial differences in HIV-1 DNA levels, we observed lower cell-associated multiply spliced and plasma HIV-1 RNA levels in women. These results are consistent with prior work demonstrating lower per cell HIV RNA production in women in untreated HIV infection [19]. One possible mechanism would be a sex difference in the quality of the DNA reservoir. Whether the higher induction of type 1 interferons in women [27, 28, 47] can amplify hypermutation machinery increasing the proportion of defective HIV genomes in women is unknown. Sequencing of HIV-1 proviruses is warranted to test this hypothesis.
Alternatively, in vivo exposure to estrogen is a potential mechanism for the observed differences in ex vivo measures of virus activity. Estrogen represses HIV-1 transcription in latency models and patient cells [26] and in vitro infection systems [25], indicating a direct role for hormones in mediating sex differences. In a limited sample, we estimated that there was no substantial difference in the short-term TILDA measure of inducible HIV-1 RNA with no estrogen in the system, but with a wide CI, precluding a strong conclusion. Using a modified EDITS assay, we demonstrated that HIV induction and replication in a spreading viral infection was potently blocked by estrogen in samples from women. This reinforces our prior observations of a direct role for the estrogen receptor in maintenance of latency [26]. The precise mechanisms for sex differential responses to estrogen remain undefined, although we did not observe differences in the level of intracellular ESR-1 expression between men and women. We did not observe relationships between virologic measures and plasma 17β-estradiol at single timepoints; however, a longitudinal approach might better address the impact of the contemporaneous hormone levels on virus activity.
There were higher levels of immune activation and PD-1 expression in men along with the higher levels of cell-associated multiply spliced HIV RNA and plasma HIV-1 RNA. PD-1 and HLA-DR/CD38 expression levels in men could be driven by higher levels of stochastic expression of HIV-1 RNA. Alternatively, higher levels of T-cell activation in men (driven by unmeasured confounders or sex-dependent immunologic pathways) could lead to more nonspecific release of inflammatory cytokines that may increase HIV-1 transcriptional activity. It is notable that this sex difference in T-cell activation under ART is in contrast to findings during untreated HIV-1 infection, when women have higher levels of T-cell activation for a given level of viremia [27]. Sex differences in PD-1 expression also bear further investigation; in the oncology literature, female sex may be a predictor of response to checkpoint inhibitor therapy [48].
The exploratory analysis of correlations between immune parameters and virologic outcomes highlighted a few points. The association of the CD8+CD27+CD28–CD45RA–HLA-DR+CD38+ population with SCA values across the cohort and in each sex is notable; this population is an intermediate in the differentiation pathway to an effector memory cell with both cytokine secretion and cytotoxic capacity [49]. Further studies are necessary to define if the positive correlation with low-level residual plasma viremia reveals a direct response to virus or if it reflects a higher state of global activation within the host driven by non-HIV factors. Also notable was the positive correlation between the central/effector memory CD4+CD27+CD28+CD45RA–HLA-DR+CD38– and all virologic measures. Indeed, there are multiple viral associations with HLA-DR+CD38– populations in both CD4+ and CD8+ T cells. These observations suggest a role for cells with high proliferative capacity [50] or a proliferative milieu of cytokines and growth factors in reservoir maintenance and dynamics, an association that should be explored.
Finally, the exploratory sex-stratified immune correlation analysis suggests that there may be distinct relationships between T-cell activation and viral parameters in men and women. The mechanisms and clinical significance of these differences are unclear, but given the focus on immune correlates, sex differences are important whether they are solely biomarkers or connoting mechanism. These differences should be considered in small clinical trials with limited enrollment of women where results may be diluted or skewed by sex imbalance. Further, sex differences can be exploited to determine pathways governing residual virus activity.
Our study has a relatively small sample size and some of the associations are exploratory, but despite these limitations we were able to identify important sex-based differences. We found that women and men differ in the level of residual virus activity during clinically suppressive ART. A variety of immunological and hormonal mechanisms contribute to this effect. These sex-based differences in HIV reservoir dynamics indicate that sex and hormonal status must be regarded as key parameters in the design and analysis of clinical trials examining HIV-1 eradication strategies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all of the study participants; Joy Madamba, Sophie Lyons, and Mollie Hudson for their efforts in participant recruitment; and Nikitha Nair (HIV Molecular Monitoring Core, AIDS and Cancer Virus Program, Frederick National Laboratory for Cancer Research) for her assistance with the single-copy plasma virus analysis.
Author contributions. The study was designed and planned by E. P. S., S. G. D., R. J., P. B., M. G., and J. K. Cohort design, reproductive health questionnaire development, and recruitment were done by E. P. S., M. G., M. C., and R. H. Experimental studies were performed by E. P. S., A. L., A. P., J. M., C. B., V. G., A. E., C. D., R. G., J. L., and A. S. Reagents were contributed by M. A. and G. A. Primary data analysis was done by E. P. S., A. L., A. P., J. M., C. B., V. G., A. E., R. G., C. D., J. K., J. L., S. R. L., N. C., and P. B. Statistical analysis was done by P. B., and E. P. S. wrote the manuscript with editing and input from all of the authors.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the amfAR Research Consortium on HIV Eradication (grant numbers 108842-55-RGRL to E. P. S., S. G. D., N. C., and M. G. and 108841-55-RGRL to J. K.). E. P. S. is supported by a grant from the National Institute of Allergy and Infectious Diseases doi.org/10.13039/100000060 (award number K08AI116344) with additional support for this work from a Johns Hopkins University Catalyst Award. This work was also supported by the Delaney AIDS Research Enterprise (grant number AI096109); the National Institute of Allergy and Infectious Diseases (grant number K24 AI069994); the University of California, San Francisco (UCSF)/Gladstone Institute of Virology and Immunology Center for AIDS Research (CFAR) (grant number P30 AI027763); the Case Western Reserve University/University Hospitals CFAR (grant number P30 AI36219); the NIH/National Center for Research Resources UCSF Clinical and Translational Science Institute (grant number UL 1 TR000004); and the CFAR Network of Integrated Systems (grant number R24 AI067039). This work was also supported in part with federal funds from the National Cancer Institute, NIH (contract number HHSN261200800001E to R. J. G. and J. D. L.). S. R. L. is a National Health and Medical Research Council of Australia practitioner fellow.
Potential conflicts of interest. R. J. is an employee of amfAR and participated in study design and manuscript preparation. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, March 2017.
References
- 1. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38. [DOI] [PubMed] [Google Scholar]
- 2. Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol 2014; 35:97–104. [DOI] [PubMed] [Google Scholar]
- 3. Gianella S, Tsibris A, Barr L, Godfrey C. Barriers to a cure for HIV in women. J Int AIDS Soc 2016; 19:20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep 2018; 15:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dillon S, Aggarwal R, Harding JW, et al. . Klinefelter’s syndrome (47,XXY) among men with systemic lupus erythematosus. Acta Paediatr 2011; 100:819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scofield RH, Bruner GR, Namjou B, et al. . Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum 2008; 58:2511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hewagama A, Gorelik G, Patel D, et al. . Overexpression of X-linked genes in T cells from women with lupus. J Autoimmun 2013; 41:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sawalha AH, Wang L, Nadig A, et al. . Sex-specific differences in the relationship between genetic susceptibility, T cell DNA demethylation and lupus flare severity. J Autoimmun 2012; 38:J216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol 2015; 6:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol 2015; 15:217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anastos K, Gange SJ, Lau B, et al. . Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr 2000; 24:218–26. [DOI] [PubMed] [Google Scholar]
- 12. Evans JS, Nims T, Cooley J, et al. . Serum levels of virus burden in early-stage human immunodeficiency virus type 1 disease in women. J Infect Dis 1997; 175:795–800. [DOI] [PubMed] [Google Scholar]
- 13. Farzadegan H, Hoover DR, Astemborski J, et al. . Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998; 352:1510–4. [DOI] [PubMed] [Google Scholar]
- 14. Katzenstein DA, Hammer SM, Hughes MD, et al. . The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. AIDS Clinical Trials Group Study 175 Virology Study Team. N Engl J Med 1996; 335:1091–8. [DOI] [PubMed] [Google Scholar]
- 15. Lyles CM, Dorrucci M, Vlahov D, et al. . Longitudinal human immunodeficiency virus type 1 load in the Italian Seroconversion Study: correlates and temporal trends of virus load. J Infect Dis 1999; 180:1018–24. [DOI] [PubMed] [Google Scholar]
- 16. Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis 1999; 180:666–72. [DOI] [PubMed] [Google Scholar]
- 17. Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001; 344:720–5. [DOI] [PubMed] [Google Scholar]
- 18. Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels?Clin Infect Dis 2002; 35:313–22. [DOI] [PubMed] [Google Scholar]
- 19. Meditz AL, Folkvord JM, Lyle NH, et al. . CCR5 expression is reduced in lymph nodes of HIV type 1-infected women, compared with men, but does not mediate sex-based differences in viral loads. J Infect Dis 2014; 209:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Napravnik S, Poole C, Thomas JC, Eron JJ Jr. Gender difference in HIV RNA levels: a meta-analysis of published studies. J Acquir Immune Defic Syndr 2002; 31:11–9. [DOI] [PubMed] [Google Scholar]
- 21. Lee S, Kim J, Jang B, et al. . Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. J Immunol 2010; 185:756–62. [DOI] [PubMed] [Google Scholar]
- 22. Seillet C, Laffont S, Trémollières F, et al. . The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 2012; 119:454–64. [DOI] [PubMed] [Google Scholar]
- 23. Seillet C, Rouquié N, Foulon E, et al. . Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor α. J Immunol 2013; 190:5459–70. [DOI] [PubMed] [Google Scholar]
- 24. Zhang MA, Rego D, Moshkova M, et al. . Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci U S A 2012; 109:9505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Szotek EL, Narasipura SD, Al-Harthi L. 17β-Estradiol inhibits HIV-1 by inducing a complex formation between β-catenin and estrogen receptor α on the HIV promoter to suppress HIV transcription. Virology 2013; 443:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Das B, Dobrowolski C, Luttge B, et al. . Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 2018; 115:E7795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meier A, Chang JJ, Chan ES, et al. . Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009; 15:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang JJ, Woods M, Lindsay RJ, et al. . Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. J Infect Dis 2013; 208:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicastri E, Leone S, Angeletti C, et al. . Sex issues in HIV-1-infected persons during highly active antiretroviral therapy: a systematic review. J Antimicrob Chemother 2007; 60:724–32. [DOI] [PubMed] [Google Scholar]
- 30. Cuzin L, Pugliese P, Sauné K, et al. . Dat’AIDS Study Group Levels of intracellular HIV-DNA in patients with suppressive antiretroviral therapy. AIDS 2015; 29:1665–71. [DOI] [PubMed] [Google Scholar]
- 31. Fourati S, Flandre P, Calin R, et al. . Factors associated with a low HIV reservoir in patients with prolonged suppressive antiretroviral therapy. J Antimicrob Chemother 2014; 69:753–6. [DOI] [PubMed] [Google Scholar]
- 32. Krebs SJ, Slike BM, Sithinamsuwan P, et al. . SEARCH 011 Study Team Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV-infected individuals. AIDS 2016; 30:1533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li JZ, Arnold KB, Lo J, et al. . Differential levels of soluble inflammatory markers by human immunodeficiency virus controller status and demographics. Open Forum Infect Dis 2015; 2:ofu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mathad JS, Gupte N, Balagopal A, et al. . New Work Concept Sheet 319 and AIDS Clinical Trials Group A5175 (PEARLS) Study Teams Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr 2016; 73:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnston RE, Heitzeg MM. Sex, age, race and intervention type in clinical studies of HIV cure: a systematic review. AIDS Res Hum Retroviruses 2015; 31:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vandergeeten C, Fromentin R, Merlini E, et al. . Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J Virol 2014; 88:12385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elliott JH, McMahon JH, Chang CC, et al. . Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2015; 2:e520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elliott JH, Wightman F, Solomon A, et al. . Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 2014; 10:e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Somsouk M, Dunham RM, Cohen M, et al. . The immunologic effects of mesalamine in treated HIV-infected individuals with incomplete CD4+ T cell recovery: a randomized crossover trial. PLoS One 2014; 9:e116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Procopio FA, Fromentin R, Kulpa DA, et al. . A novel assay to measure the magnitude of the inducible viral reservoir in HIV-infected individuals. EBioMedicine 2015; 2:874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khoury G, Anderson JL, Fromentin R, et al. . Persistence of integrated HIV DNA in CXCR3 + CCR6 + memory CD4+ T cells in HIV-infected individuals on antiretroviral therapy. AIDS 2016; 30:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riddler SA, Aga E, Bosch RJ, et al. . ACTG A5276s Protocol Team Continued slow decay of the residual plasma viremia level in HIV-1-infected adults receiving long-term antiretroviral therapy. J Infect Dis 2016; 213:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bacchetti P. Peer review of statistics in medical research: the other problem. BMJ 2002; 324:1271–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perneger TV. What’s wrong with Bonferroni adjustments. BMJ 1998; 316:1236–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43–6. [PubMed] [Google Scholar]
- 46. Chen Y, Zhang Y, Zhao G, et al. . Difference in leukocyte composition between women before and after menopausal age, and distinct sexual dimorphism. PLoS One 2016; 11:e0162953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jansen R, Batista S, Brooks AI, et al. . Sex differences in the human peripheral blood transcriptome. BMC Genomics 2014; 15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nosrati A, Tsai KK, Goldinger SM, et al. . Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer 2017; 116:1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype. J Immunol 2002; 168:5538–50. [DOI] [PubMed] [Google Scholar]
- 50. Hua S, Lécuroux C, Sáez-Cirión A, et al. . Potential role for HIV-specific CD38-/HLA-DR+ CD8+ T cells in viral suppression and cytotoxicity in HIV controllers. PLoS One 2014; 9:e101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.