A new paradigm has emerged for treating infections, which focuses on targeting host pathways that are critical for pathogen invasion, survival, and multiplication. We show that ibrutinib, a ITK/BTK inhibitor, could be host-directed drug for treatment of visceral leishmaniasis.
Keywords: Ibrutinib, Bruton tyrosine kinase (BTK), interleukin 2–inducible kinase (ITK), visceral leishmaniasis
Abstract
Background
New drugs are needed for leishmaniasis because current treatments such as pentavalent antimonials are toxic and require prolonged administration, leading to poor patient compliance. Ibrutinib is an anticancer drug known to modulate T-helper type 1 (Th1)/Th2 responses and has the potential to regulate immunity against infectious disease.
Methods
In this study, we evaluated the efficacy of oral ibrutinib as a host-targeted treatment for visceral leishmaniasis (VL) caused by Leishmania donovani using an experimental mouse model.
Results
We found that oral ibrutinib was significantly more effective than the pentavalent antimonial sodium stibogluconate (70 mg/kg) for the treatment of VL caused by L. donovani. Ibrutinib treatment increased the number of interleukin 4– and interferon γ–producing natural killer T cells in the liver and spleen and enhanced granuloma formation in the liver. Further, ibrutinib treatment reduced the influx of Ly6Chi inflammatory monocytes, which mediate susceptibility to L. donovani. Finally, ibrutinib treatment was associated with the increased production of the cytokines interferon γ, tumor necrosis factor α, interleukin 4, and interleukin 13 in the liver and spleen, which are associated with protection against L. donovani.
Conclusions
Our findings show that oral ibrutinib is highly effective for the treatment of VL caused by L. donovani and mediates its antileishmanial activity by promoting host immunity. Therefore, ibrutinib could be a novel host-targeted drug for the treatment of VL.
Leishmania are obligate intracellular parasites that cause a variety of clinical diseases, including cutaneous leishmaniasis (CL), mucosal leishmaniasis, and visceral leishmaniasis (VL). The World Health Organization classifies leishmaniasis as a neglected tropical disease, with >12 million current infections globally and approximately 2 million new cases annually [1].
VL is the most severe form of Leishmania infection and second-most common fatal parasitic infection after malaria. This infection is caused by Leishmania donovani and Leishmania chagasi and is characterized by dissemination of the parasites to the liver, spleen, and bone marrow [2]. The pentavalent antimonials (sodium stibogluconate) introduced 100 years ago still remain the first-line drug for leishmaniasis, including VL worldwide, despite the fact that these drugs display cardiac, renal, and hepatic toxicity and require daily parenteral administration for at least 20 days [2]. In addition, the emergence of drug-resistant parasites, particularly L. donovani, is rapidly increasing worldwide [3–6]. Owing to these drawbacks, there is a need for development of novel, highly effective and minimally toxic therapeutic agents against VL.
Ibrutinib is an orally bioavailable small-molecule inhibitor of Bruton tyrosine kinase (BTK) and is approved to treat a variety of B-cell malignancies, like chronic lymphocytic leukemia [7, 8]. It is well documented that BTK plays a critical role in B-cell receptor (BCR) signaling, which mediates proliferation of B cells that are known to be involved in the pathogenesis of CL [9] and VL [10]. We had previously found that ibrutinib blocks the activity of interleukin 2–inducible T-cell kinase (ITK), suppresses T-helper type 2 (Th2) cell differentiation and inhibits progression of CL caused by Leishmania major [11]. Collectively, these findings suggest that ibrutinib could block immune mechanisms mediating susceptibility to VL and therefore could be a host-targeted drug for the treatment of this disease.
In the present study, we determined the efficacy of ibrutinib against VL, using a mouse model of L. donovani infection. We show that oral ibrutinib is significantly more effective than the low-dose treatment regimen of the conventional antileishmanial drug sodium stibogluconate against L. donovani. Furthermore, our studies show that ibrutinib exhibits no direct microbicidal activity against Leishmania organisms but mediates its antileishmanial activity by promoting a protective immune response.
MATERIALS AND METHODS
Animals
Female wild-type BALB/c mice aged 6–7 weeks were purchased from Envigo (previously Harlan Laboratories, Indianapolis, IN). All mice were housed at The Ohio State University animal facility according to policies of University Laboratory Animal Resources. All experimental procedures were approved by Institutional Animal Care and Usage Committee (protocol number 2010A0048-R2).
L. donovani Infection, Treatment, and Analysis of Parasite Burdens
All experimental mice were infected by intravenous inoculation of 107L. donovani (LV82) amastigotes isolated from the spleens of previously infected hamsters as described previously [12]. On day 14 after infection, infected mice were randomized into 4 groups. Group 1 mice were treated with 6 mg/kg ibrutinib orally (purchased from Acorn Pharma Tech, Redwood City, CA) dissolved in 0.5% methyl cellulose plus 0.1% sodium dodecyl sulfate/sodium lauryl sulfate (vehicle) [11, 13], group 2 mice were treated with vehicle alone once daily through oral gavage for 2 weeks, group 3 mice were treated with a single dose of 70 mg SbV/kg sodium stibogluconate (SSG; Brawn Laboratories, Haryana, India), and group 4 mice received phosphate-buffered saline (PBS) alone. All animals were euthanized on day 14 after treatment (ie, 28 days after infection), and parasite burdens in the spleen and livers were assessed by calculating Leishman-Donovan units (LDU) as described previously [12].
Histopathologic Analysis and Granuloma Calculations
A portion of the infected livers harvested from euthanized mice were formalin fixed and processed for hematoxylin-eosin staining as described previously [12]. The number and types of granulomas were assessed by a certified pathologist and scored as follows: (1) no cellular response, (2) developing granuloma (with an initial influx of lymphocytes and monocytes, as well as the presence of amastigotes), (3) mature/functional granuloma, (4) parasite-free granuloma (involuting epithelioid granuloma devoid of amastigotes), and (5) free tissue without granulomas. Liver granuloma totals are representative of the average of 5 individual mice from one of the 3 representative studies.
Cytokine Enzyme-Linked Immunosorbent Assays (ELISAs) and Nitric Oxide Levels
Cell suspensions were prepared by gentle teasing of the infected spleens in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% HEPES. The spleen cells were centrifuged, and erythrocytes were lysed by resuspending the cell pellet in ACK lysis buffer. After washing, viable cells were counted by trypan blue exclusion and plated in 96-well tissue culture plates at 5 × 105 cells/well in complete RPMI 1640 medium. Splenocytes were then stimulated with 20 μg/mL L. donovani freeze-thaw antigen (LdAg) for 72 hours, and culture supernatants were collected to measure levels of interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin 4 (IL-4), interleukin 10 (IL-10), interleukin 12 (IL-12), and interleukin 13 (IL-13) by ELISA. All regents for cytokine ELISAs were purchased from Biolegend (San Diego, CA) and/or BD Biosciences (San Jose, CA). The levels of nitric oxide release in the culture supernatants were analyzed by the Greiss assay as described previously [14].
Reverse Transcription–Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was isolated from spleens and livers harvested from mice, by the TRIzol extraction method (purchased from Life Technologies, Carlsbad, CA). Complementary DNAs were prepared using the iScript reverse transcription kit, and RT-PCR analyses were performed with the IQ SYBR green super mix in a CFX 96 RT-PCR cycler (purchased from Bio-Rad, Hercules, CA). Primers were selected from the Primer bank website (available at: http://pga.mgh.harvard.edu/primerbank). Data obtained were normalized by using the housekeeping gene β-actin and are presented as the fold induction over that for uninfected wild-type mice.
Flow Cytometry
To characterize the immune cell populations in the infected livers and spleens, the splenocytes and immune cells isolated from the infected livers by Percoll density gradient centrifugation were analyzed by flow cytometry. Briefly, 1 × 106 cells were incubated with normal mouse serum and then stained with the respective cocktail mixes of fluorescently conjugated antibodies against phenotypic surface markers (CD3, CD4, CD8, Tim3, ST2, DX5, CD19, IFN-γ, IL-4, CD11b, Ly6G, Ly6C, and F4/80, purchased from Biolegend; San Diego). Cells were acquired using a FACS LSR-II flow cytometer (BD Biosciences, San Jose), and data analysis was performed with FlowJo software (TreeStar, Ashland, OR).
Statistical Analysis
All experiments were performed using 6–7-week-old female mice in groups of 5–8 mice. Unpaired Student t tests were used to determine the statistical significance of differences between different experimental and control groups. A P value of < .05 was considered significant.
RESULTS
Oral Ibrutinib Is Effective for the Treatment of VL Caused by L donovani
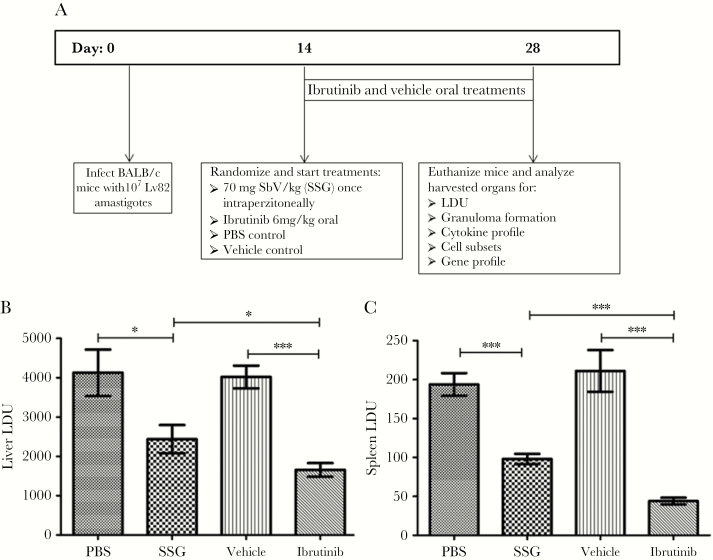
To determine the efficacy of ibrutinib treatment against VL, we analyzed liver and spleen parasite loads in L. donovani–infected mice that were treated with ibrutinib, SSG, vehicle or PBS at day 14 post-treatment. Ibrutinib-treated mice contained significantly fewer parasites in their livers (Figure 1B) and spleens (Figure 1C) compared to other treatment groups (Figure 1). The livers of ibrutinib-treated mice contained 50% fewer parasites compared to the vehicle as well as PBS treated groups (Figure 1B). Similarly, ibrutinib treated mice also showed a significant reduction (75% reduction) in spleen parasitic burdens compared to vehicle treated mice (Figure 1C). As expected, the SSG treatment also reduced parasitic loads in both the livers (Figure 1B) and spleens (Figure 1C) compared to the PBS control group. However, SSG-treated mice still showed significantly more parasites in their livers and spleens compared to the ibrutinib treated group. Together, these results demonstrate the efficacy of oral ibrutinib against VL and show that ibrutinib is more effective than suboptimal SSG in the treatment of L. donovani infection.
Figure 1.
Ibrutinib treatment reduces the parasitic load during Leishmania donovani infection. A, Timeline of the study. Female BALB/C mice aged 6–7 weeks were infected with 107 LV82 Leishmania donovani amastigotes. At 14 days after infection, mice were randomized into 4 groups and then the treatments began. Mice were treated orally with either ibrutinib (6 mg/kg) or vehicle formulations once daily from days 14 to 28 after infection. Other groups were treated with a single dose of 70 mg/kg sodium stibogluconate (SSG) and phosphate-buffered saline (PBS) via intraperitoneal injection. Mice were euthanized at day 28 after infection, and tissues were harvested for analysis. Parasitic burdens in livers (B) and spleens (C) of PBS-, vehicle-, ibrutinib-, and SSG-treated groups, respectively. Data are mean Leishman-Donovan units (LDU) ± standard error of the mean and represent the values of one of the 3 independent experiments, with ≥5 mice/group. *P < .05 and ***P < .001, by the unpaired t test.
Ibrutinib Treatment Enhances Formation of Mature Granulomas in the Liver
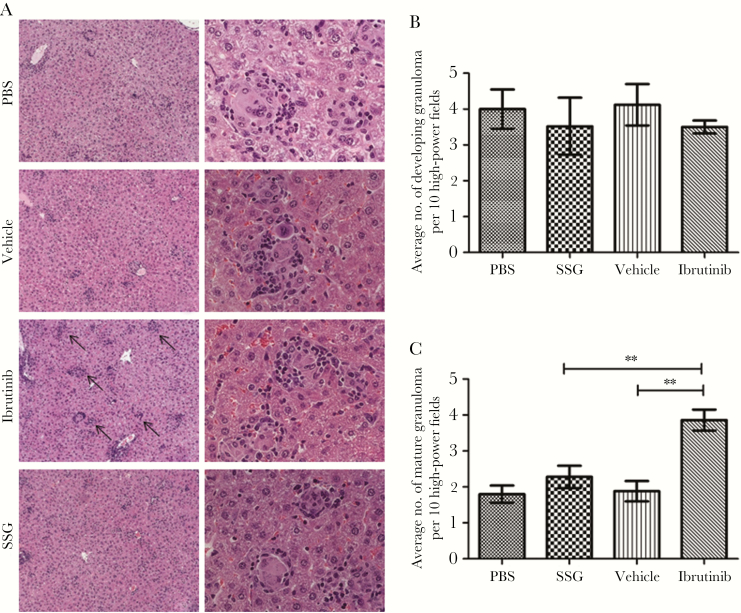
Granuloma formation in the liver is crucial for the resolution of VL, as mature granulomas mediate parasite clearance and ultimately lead to the resolution of L. donovani infection [15, 16]. Therefore, we analyzed the effects of ibrutinib treatment on granuloma formation. We found that L. donovani–infected mice treated with ibrutinib contained significantly more mature granulomas containing few or no parasites in their livers, compared with SSG- and vehicle-treated controls (Figure 2A and 2C). No significant differences were noted in the number of developing granulomas among the groups (Figure 2B). These results suggest that ibrutinib treatment enhances parasite clearance by promoting the formation of mature granulomas.
Figure 2.
Ibrutinib treatment leads to increased numbers of mature hepatic granulomas. All groups of mice were euthanized on day 28 after infection, and livers were harvested and analyzed for formation of granulomas. Liver sections were prepared and stained with hematoxylin-eosin to enumerate granuloma formation. A, Images of hepatic granulomas from all groups at 100× and 400× the original magnification. B, Liver sections were scored for the number of granulomas. Data represent the number of granulomas per 10 high-power fields at 200× the original magnification and are indicated as mean ± standard error of the mean from one of the 3 independent experiments, with ≥5 mice/group. PBS, phosphate-buffered saline; SSG, sodium stibogluconate. **P < .01, by the unpaired t test.
Ibrutinib Treatment Is Associated With Increased Expression of Protective Cytokines in the Liver and Spleen
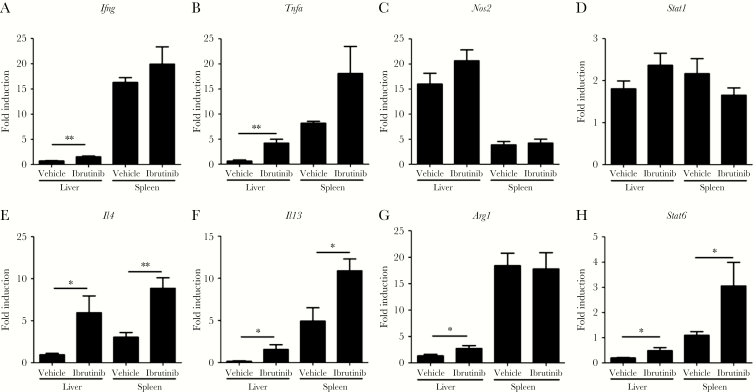
To further determine the mechanism of ibrutinib-meditated antileishmanial activity, we compared cytokine responses in the livers and spleens of L. donovani–infected mice treated with ibrutinib or vehicle. Ibrutinib-treated mice showed a significant increase in Ifng (Figure 3A) and Tnfa (Figure 3B) messenger RNA (mRNA) in their livers as compared to vehicle-treated controls. Furthermore, livers and spleens of ibrutinib-treated mice contained significantly more Il4 (Figure 3E), Il13 (Figure 3F), and Stat6 (Figure 3H) mRNA, compared with vehicle-treated controls. This upregulated expression of both Il4 and Il13 following ibrutinib treatment are in agreement with the increased mature hepatic granulomas (Figure 2C), as well as the documented roles of these cytokines in immunity against L. donovani. No significant differences in Nos2 (Figure 3C) and Stat1 (Figure 3D) mRNA were found between the groups. Taken together, these results demonstrate that ibrutinib treatment enhances protective immunity in the liver and spleen of L. donovani–infected mice.
Figure 3.
Ibrutinib treatment promotes both T-helper type 1 (Th1) and Th2 protective immunity in visceral leishmaniasis. Gene expression in the livers and spleens of vehicle- and ibrutinib-treated groups were analyzed by real-time polymerase chain reaction analysis. Messenger RNA transcripts encoding interferon γ (Ifng; A), tumor necrosis factor α (Tnfa; B), Nos2 (Nos2; C), STAT1 (Stat1; D), interleukin 4 (Il4; E), interleukin 13 (Il13; F), arginase 1 (Arg1; G), and STAT6 (Stat6; H) were determined in the livers and spleens of ibrutinib-treated and vehicle-treated mice infected with Leishmania donovani. Data are fold induction over values for uninfected naive mice from one of the 3 independent experiments, with ≥ 5 mice/group. *P < .05 and **P < .01, by the unpaired t test.
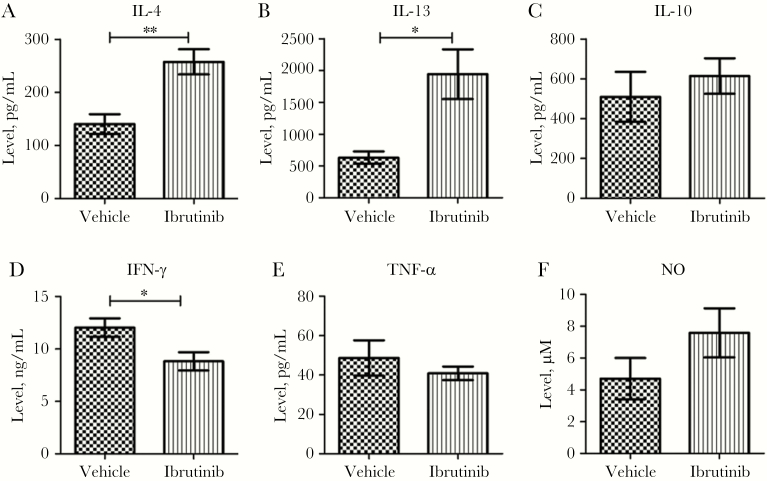
LdAg-Stimulated Spleen Cells from L. donovani-Infected Ibrutinib-Treated Mice Produce Higher Levels of IL-4 and IL-13
To determine whether increased expression of Il4 and Il13 mRNA in the spleen correlates with increased production of these cytokines, spleen cells from ibrutinib- or vehicle-treated mice were stimulated with LdAg for 72 hours, and levels of IL-4 and IL-13 in the culture supernatants were measured by ELISA. Consistent with the higher Il4 and Il13 mRNA levels, splenocytes of the ibrutinib-treated group produced increased levels of IL-4 (Figure 4A) and IL-13 (Figure 4B) in their culture supernatants. Interestingly, we noticed lower levels of IFN-γ (Figure 4D) in the culture supernatants of the ibrutinib-treated group as compared to the vehicle-treated group. No significant difference in the production of IL-10 (Figure 4C), TNF-α (Figure 4E), and NO (Figure 4F) was observed between the groups. No IL-12 was detected in culture supernatants from either group (data not shown).
Figure 4.
Increased production of interleukin 4 (IL-4) and interleukin 13 (IL-13) in splenic cells of ibrutinib-treated mice. Splenocytes were harvested on day 20 after infection, and single-cell suspensions were prepared and restimulated with Leishmania donovani freeze-thaw antigen for 72 hours. Cytokine production was analyzed in the culture supernatants by ELISA. Interleukin 4 (IL-4; A), interleukin 13 (IL-13; B), interleukin 10 (IL-10; C), interferon γ (IFN-γ; D), and tumor necrosis factor α (TNF-α; E) cytokines in antigen restimulated splenocytes of ibrutinib-treated and vehicle-treated mice. F, Levels of nitric oxide (NO) production in splenocyte culture supernatants was measured by the Greiss assay. Data are mean ± standard error of the mean and represent the values of one of the 3 independent experiments, with ≥5 mice/group. *P < .05 and **P < .01, by the unpaired t test.
Ibrutinib Treatment Increases IL-4– and IFN-γ–Producing NK T Cells in the Infected Organs
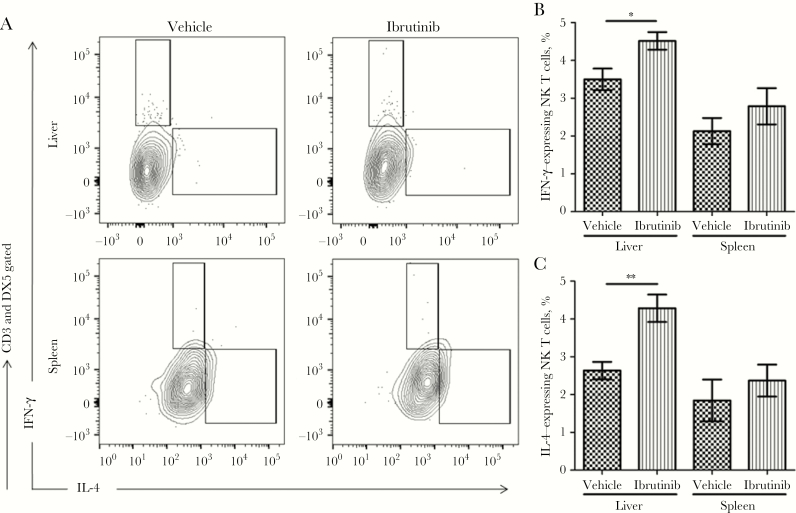
The increased IFN-γ and IL-4 production in the liver following ibrutinib treatment led us to investigate the possible cellular source of these cytokines in the infected organs. It is well documented that CD4+ Th1 cells and CD4+ Th2 cells are the main sources of IFN-γ and IL-4, respectively. Additionally, NK T cells have also been shown to produce both IFN-γ and IL-4 at the early stages of L. donovani infection [17]. Therefore, we investigated whether NK T cells and/or CD4+ T cells are the source of IL-4 and IFN-γ in ibrutinib-treated mice, using flow cytometry. Our data revealed that the frequencies of NK T cells producing IL-4 and IFN-γ in the livers of ibrutinib-treated mice was significantly higher as compared to controls (Figure 5A–C). In addition, the spleens of the ibrutinib-treated mice showed an increased proportion of IL-4–expressing NK T cells and IFN-γ–expressing NK T cells as compared to the control group (Figure 5A–C). However, there were no significant differences in the frequencies of NK T-cell populations in the ibrutinib-treated and control groups. Similarly, no differences were noted in the frequencies of IL-4– or IFN-γ–producing CD4+ T cells between the groups (data not shown).
Figure 5.
Ibrutinib treatment increases interferon γ (IFN-γ) and interleukin 4 (IL-4) production in natural killer (NK) T cells. The livers and spleens of the ibrutinib-treated and vehicle-treated groups were harvested and analyzed for cell populations responsible for the production of IFN-γ and IL-4. A, Intracellular detection of IFN-γ and IL-4 by flow cytometry after 14 days of ibrutinib treatment. Single-cell suspensions were prepared from livers and spleens and stimulated with PMA (20 ng/mL) plus ionomycin (1 μg/mL) in the presence of brefeldin for at least 6 hours. Cells were gated for CD3 and DX5 and then evaluated for IFN-γ and IL-4 production. B, Percentage of IFN-γ–expressing NK T cells among CD3+DX5+ gated cells. C, Percentage of IL-4–expressing NK T cells among CD3+DX5+ gated cells. Data are representative of one of the 3 independent experiments, with 5 mice/group. *P < .05 and **P < .01, by the unpaired t test.
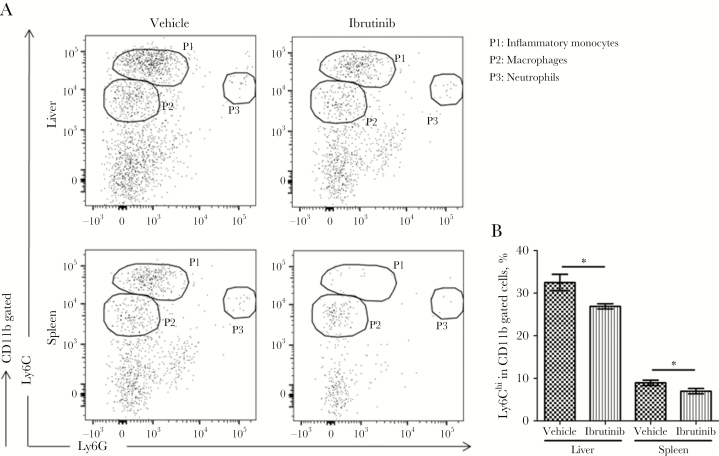
Ibrutinib Treatment Resulted in Reduced Accumulation of Ly6Chi Inflammatory Monocytes
A recent study by our group has shown that Ly6Chi inflammatory monocytes are the preferential target cells for L. donovani and are involved in the pathogenesis of VL [18]. Similarly, macrophages and neutrophils are known host target cells for Leishmania organisms. Because ibrutinib has been shown to modulate the activity of macrophages and monocytes [19], we analyzed Ly6Chi inflammatory monocytes, macrophages, and neutrophils in the livers and spleens of ibrutinib- or vehicle-treated mice by flow cytometry. Mice treated with ibrutinib had a significantly lower proportion of Ly6Chi inflammatory monocytes in the livers and spleens as compared to vehicle-treated mice (Figure 6A and 6B). However, no significant differences were noted in the proportion of macrophages and neutrophils between the groups (data not shown). These results, together with our observations involving NK T cells, suggest that ibrutinib reduces the levels of disease-promoting Ly6Chi inflammatory monocytes while enhancing the number of IFN-γ– and IL-4–producing NK T cells in the organs, which may ultimately lead to the clearance of L. donovani.
Figure 6.
Ibrutinib treatment affects the immune populations important for visceral leishmaniasis exacerbation. Livers and spleens were harvested from ibrutinib-treated and vehicle-treated mice on day 28 after infection and analyzed for myeloid cell populations by flow cytometry. A, Representative dot plots of myeloid cell populations from the livers and spleens of vehicle- and ibrutinib-treated mice. P1 represents the inflammatory monocytes (CD11b+Ly6ChighLY6G−), P2 represents macrophages (CD11b+Ly6Clow LY6G−), and P3 represents neutrophils (CD11b+Ly6C−LY6G+). B, Frequencies of Ly6Chi cells in the total number of CD11b gated cells in the livers and spleens of vehicle- and ibrutinib-treated mice. Data are representative of one of the 3 independent experiments, with 5 mice/group. *P < .05, by the unpaired t test.
DISCUSSION
A previous study by our group had found that the Food and Drug Administration–approved BTK/ITK inhibitor ibrutinib inhibits disease progression of cutaneous L. major infection by promoting a protective immune response [11]. The present study demonstrates that ibrutinib is also effective in the treatment of VL caused by L. donovani and is significantly more effective than a suboptimal dose of the conventional antileishmanial drug SSG in reducing parasitic loads in the livers and spleens. Furthermore, ibrutinib treatment was associated with enhanced formation of mature hepatic granulomas, increased production of protective cytokines by NK T cells, and reduced accumulation of disease-promoting Ly6Chi inflammatory monocytes in the visceral organs. Taken together, these findings indicate that ibrutinib could be a novel host-directed drug for the treatment of VL.
All chemotherapies currently available for leishmaniasis use drugs that mediate their antiparasitic activity by direct cytotoxicity against the parasite. However, these treatments are prolonged and can be toxic, leading to poor patient compliance. In addition, the emergence of drug-resistant parasites continues to be a major problem in VL. Insights into mechanisms of host-pathogen interactions and host immune responses are leading to advancement in the development of novel host-directed therapies for treatment of infections. Such treatments could mediate their therapeutic activity by targeting host pathways necessary for pathogen survival and/or by promoting host mechanisms that mediate pathogen clearance. For example, we previously reported that PI3Kγ mediates the entry of Leishmania mexicana into phagocytic host cells and that blockade of this enzyme significantly reduces parasite burdens and impairs progression of CL [20]. A recent study by our group also found that therapeutic targeting of inflammatory Ly6Chi monocytes during experimental L. donovani infection, using a CCR2 antagonist, leads to a significant reduction in parasite burdens in visceral organs [18]. In the present study, we found that ibrutinib was significantly more effective than the conventional antileishmanial drug SSG in the treatment of VL. Ibrutinib-treated mice contained significantly fewer parasites in their liver and spleen as compared to SSG-treated, vehicle-treated, and PBS-treated groups. Furthermore, ibrutinib treatment was associated with significantly increased transcript levels of Th1-associated IFN-γ and TNF-α and Th2-associated IL-4 and IL-13 in the liver, accompanied by increased production of IL-4 and IL-13 by the splenic cells, compared with vehicle-treated controls.
Interestingly, our findings revealed that the spleen cells from L. donovani–infected mice treated with ibrutinib produced more IL-4 and IL-13. This result differs from our previous observations in CL caused by L. major [11], in which ibrutinib therapy was associated with decreased production of these cytokines from the draining lymph node cells. Although the exact mechanism(s) of diverse cytokine responses induced by ibrutinib in CL versus VL is not clear, these differences could be partly attributed to different effects of ibrutinib on organ-specific immune responses in the spleen versus the draining lymph node. Nonetheless, it is well documented that although IL-4 and IL-13 are susceptibility factors in CL caused by L. major and L. mexicana [21–24], these cytokines are critical for the resolution of L. donovani infection, development of mature hepatic granulomas [24–27], and efficient response to antileishmanial chemotherapy [27, 28]. Indeed, in the present study, infected livers from L. donovani–infected mice treated with ibrutinib contained significantly more mature granulomas, compared with controls. It is also known that ibrutinib promotes M2 macrophage polarization through the IL-4- and STAT6-mediated pathway [19, 29]. Consistent with the above studies, we have also found that the liver and spleens of ibrutinib-treated mice showed higher numbers of Stat6 mRNA transcripts. In addition, the livers of ibrutinib-treated mice showed increased numbers of Arg1 mRNA transcripts, suggesting the induction of M2 macrophage–associated tissue repair mechanisms, which occur after the clearance of infection. Taken together, our findings indicate that ibrutinib mediates its antileishmanial activity in VL by promoting the upregulation of cytokines that mediate protection, as well as by suppressing immune mechanisms of susceptibility.
Several clinical as well and experimental studies have documented that B cells and antibodies play a detrimental role in VL [10, 30]. Indeed, patients with VL display hypergammaglobulinemia due to polyclonal B-cell activation, which correlates with the severity of the disease [31]. In addition, B cells also produce IL-10, which is a known susceptibility factor in VL. Given that ibrutinib targets BTK kinase and inhibits B-cell activation by blocking the BCR activation pathway, we compared B-cell responses in ibrutinib-treated and control mice. Interestingly, no significant differences were noted in the serum levels of immunoglobulin G or the proportion of B-cell populations in the spleen between groups (data not shown). These findings suggest that the therapeutic effect of ibrutinib was not due to suppression of the B-cell response.
NK T cells are known to be potential sources of both IFN-γ and IL-4 production upon stimulation through T-cell receptors [32–34]. In the context of L. donovani, many studies have established the dual and complex role played by invariant NK T cells [17, 35–37]. The role of ITK in the upregulation of NK1.1 and the maturation and development of NK T cells has also been demonstrated previously [38, 39]. Additionally, it has been shown that Itk−/− mice have a defect in NK T-cell number [38, 39]. In contrast, in the present study we did not notice any significant defect in the NK T-cell population by ibrutinib treatment (data not shown). However, ibrutinib treatment significantly enhanced the production of both IFN-γ and IL-4 by NK T cells in L. donovani–infected mice. It has been shown that Itk−/− mice spontaneously express elevated levels of serum immunoglobulin E levels, resulting in the expansion of a specific subset of γδ T cells called γδ NK-T cells [40–42]. In addition, the lack of ITK in γδ NK T cells (Itk−/− γδ NK T cells) causes them to express high levels of IL-4, which is normally expressed in low levels by a mature subset of wild-type NK T cells [40]. Therefore, it is possible that the increased IL-4 and IFN-γ produced by NK T cells in ibrutinib-treated mice could be due to the irreversible blockade of ITK by this drug.
It was previously shown that the presence of monocytes and macrophages, which are the preferential target cells of Leishmania parasites, promotes susceptibility to VL [43, 44]. A recent study from our group also demonstrated that Ly6Chi inflammatory monocytes are preferential targets for L. donovani and showed that these cells accumulate in the liver and spleen during L. donovani infection and exacerbate the disease [18]. Our present study revealed that ibrutinib treatment was associated with a significant reduction in the proportion of Ly6Chi inflammatory monocytes in both the livers and spleens. At this point, it is unclear how the blockade of ITK/BTK could lead to a reduction in inflammatory monocyte accumulation, but ongoing studies in our laboratory have revealed that ibrutinib promotes the differentiation of Ly6Chi inflammatory monocytes to dendritic cells (data not shown). Nonetheless, it is evident that the reduced number of Ly6Chi inflammatory monocytes in the organs of ibrutinib-treated mice could at least partly contribute to disease resolution.
In conclusion, our data show that ibrutinib is safe and therapeutically more effective than the low-dose treatment regimen of conventional antileishmanial SSG in the treatment of VL. Furthermore, our findings indicate that ibrutinib mediates its antileishmanial activity by promoting protective immunity and targeting host pathways that mediate susceptibility. Owing to its effectiveness and low toxicity, ibrutinib could be a novel host-targeted drug for treatment of VL caused by L. donovani.
Presented in part: 6th World Congress on Leishmaniasis, Toledo, Spain, 16–20 May 2017.
Notes
Acknowledgments. We thank the Department of Pathology, The Ohio State University Medical Center, and the Analytical Cytometry Shared Resource, The Ohio State University, for their kind support in allowing us to use the flow cytometry facilities; and Dr Bijay Kumar Jha, for proofreading the manuscript.
Financial support. This work was supported by the Department of Pathology, The Ohio State University.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Leishmaniasis. Accessed 1 August 2018 http://www.who.int/leishmaniasis/en/. [Google Scholar]
- 2. de Freitas EO, Leoratti FM, Freire-de-Lima CG, Morrot A, Feijó DF. The Contribution of Immune Evasive Mechanisms to Parasite Persistence in Visceral Leishmaniasis. Front Immunol 2016; 7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sundar S, More DK, Singh MK, et al. . Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis 2000; 31:1104–7. [DOI] [PubMed] [Google Scholar]
- 4. Lira R, Sundar S, Makharia A, et al. . Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis 1999; 180:564–7. [DOI] [PubMed] [Google Scholar]
- 5. Clementi A, Battaglia G, Floris M, Castellino P, Ronco C, Cruz DN. Renal involvement in leishmaniasis: a review of the literature. NDT Plus 2011; 4:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polonio T, Efferth T. Leishmaniasis: drug resistance and natural products (review). Int J Mol Med 2008; 22:277–86. [PubMed] [Google Scholar]
- 7. Pan Z, Scheerens H, Li SJ, et al. . Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem 2007; 2:58–61. [DOI] [PubMed] [Google Scholar]
- 8. Harrison C. Trial watch: BTK inhibitor shows positive results in B cell malignancies. Nat Rev Drug Discov 2012; 11:96. [DOI] [PubMed] [Google Scholar]
- 9. Hoerauf A, Solbach W, Röllinghoff M, Gessner A. Effect of IL-7 treatment on Leishmania major-infected BALB.Xid mice: enhanced lymphopoiesis with sustained lack of B1 cells and clinical aggravation of disease. Int Immunol 1995; 7:1879–84. [PubMed] [Google Scholar]
- 10. Smelt SC, Cotterell SE, Engwerda CR, Kaye PM. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol 2000; 164:3681–8. [DOI] [PubMed] [Google Scholar]
- 11. Dubovsky JA, Beckwith KA, Natarajan G, et al. . Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013; 122:2539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oghumu S, Gupta G, Snider HM, et al. . STAT4 is critical for immunity but not for antileishmanial activity of antimonials in experimental visceral leishmaniasis. Eur J Immunol 2014; 44:450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A 2015; 112:E966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varikuti S, Oghumu S, Saljoughian N, et al. . Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop 2017; 173:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murray HW. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int J Exp Pathol 2001; 82:249–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray HW. Mononuclear cell recruitment, granuloma assembly, and response to treatment in experimental visceral leishmaniasis: intracellular adhesion molecule 1-dependent and -independent regulation. Infect Immun 2000; 68:6294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Svensson M, Zubairi S, Maroof A, Kazi F, Taniguchi M, Kaye PM. Invariant NKT cells are essential for the regulation of hepatic CXCL10 gene expression during Leishmania donovani infection. Infect Immun 2005; 73:7541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Terrazas C, Varikuti S, Oghumu S, et al. . Ly6Chi inflammatory monocytes promote susceptibility to Leishmania donovani infection. Sci Rep 2017; 7:14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fiorcari S, Maffei R, Audrito V, et al. . Ibrutinib modifies the function of monocyte/macrophage population in chronic lymphocytic leukemia. Oncotarget 2016; 7:65968–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cummings HE, Barbi J, Reville P, et al. . Critical role for phosphoinositide 3-kinase gamma in parasite invasion and disease progression of cutaneous leishmaniasis. Proc Natl Acad Sci U S A 2012; 109:1251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hurdayal R, Brombacher F. The role of IL-4 and IL-13 in cutaneous Leishmaniasis. Immunol Lett 2014; 161:179–83. [DOI] [PubMed] [Google Scholar]
- 22. Kopf M, Brombacher F, Köhler G, et al. . IL-4-deficient Balb/c mice resist infection with Leishmania major. J Exp Med 1996; 184:1127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta G, Oghumu S, Satoskar AR. Mechanisms of immune evasion in leishmaniasis. Adv Appl Microbiol 2013; 82:155–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Satoskar A, Bluethmann H, Alexander J. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect Immun 1995; 63:4894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stäger S, Alexander J, Carter KC, Brombacher F, Kaye PM. Both interleukin-4 (IL-4) and IL-4 receptor alpha signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect Immun 2003; 71:4804–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McFarlane E, Carter KC, McKenzie AN, Kaye PM, Brombacher F, Alexander J. Endogenous IL-13 plays a crucial role in liver granuloma maturation during Leishmania donovani infection, independent of IL-4Rα-responsive macrophages and neutrophils. J Infect Dis 2011; 204:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander J, Carter KC, Al-Fasi N, Satoskar A, Brombacher F. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur J Immunol 2000; 30:2935–43. [DOI] [PubMed] [Google Scholar]
- 28. Murray HW, Flanders KC, Donaldson DD, et al. . Antagonizing deactivating cytokines to enhance host defense and chemotherapy in experimental visceral leishmaniasis. Infect Immun 2005; 73:3903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ní Gabhann J, Hams E, Smith S, et al. . Btk regulates macrophage polarization in response to lipopolysaccharide. PLoS One 2014; 9:e85834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deak E, Jayakumar A, Cho KW, et al. . Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur J Immunol 2010; 40:1355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gardinassi LG, Dotz V, Hipgrave Ederveen A, et al. . Clinical severity of visceral leishmaniasis is associated with changes in immunoglobulin g fc N-glycosylation. MBio 2014; 5:e01844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol 2010; 11:197–206. [DOI] [PubMed] [Google Scholar]
- 33. Leite-de-Moraes MC, Diem S, Michel ML, et al. . Cutting edge: histamine receptor H4 activation positively regulates in vivo IL-4 and IFN-gamma production by invariant NKT cells. J Immunol 2009; 182:1233–6. [DOI] [PubMed] [Google Scholar]
- 34. Choi P, Reiser H. IL-4: role in disease and regulation of production. Clin Exp Immunol 1998; 113:317–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robert-Gangneux F, Drogoul AS, Rostan O, et al. . Invariant NKT cells drive hepatic cytokinic microenvironment favoring efficient granuloma formation and early control of Leishmania donovani infection. PLoS One 2012; 7:e33413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beattie L, Svensson M, Bune A, et al. . Leishmania donovani-induced expression of signal regulatory protein alpha on Kupffer cells enhances hepatic invariant NKT-cell activation. Eur J Immunol 2010; 40:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stanley AC, Zhou Y, Amante FH, et al. . Activation of invariant NKT cells exacerbates experimental visceral leishmaniasis. PLoS Pathog 2008; 4:e1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J Immunol 2002; 169:2397–406. [DOI] [PubMed] [Google Scholar]
- 39. Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. J Immunol 2008; 180:3007–18. [DOI] [PubMed] [Google Scholar]
- 40. Yin CC, Cho OH, Sylvia KE, et al. . The Tec kinase ITK regulates thymic expansion, emigration, and maturation of γδ NKT cells. J Immunol 2013; 190:2659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in gammadeltaT cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci U S A 2009; 106:8308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qi Q, Xia M, Hu J, et al. . Enhanced development of CD4+ gammadelta T cells in the absence of Itk results in elevated IgE production. Blood 2009; 114:564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nandan D, Reiner NE. Leishmania donovani engages in regulatory interference by targeting macrophage protein tyrosine phosphatase SHP-1. Clin Immunol 2005; 114:266–77. [DOI] [PubMed] [Google Scholar]
- 44. Chandra D, Naik S. Leishmania donovani infection down-regulates TLR2-stimulated IL-12p40 and activates IL-10 in cells of macrophage/monocytic lineage by modulating MAPK pathways through a contact-dependent mechanism. Clin Exp Immunol 2008; 154:224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]