Abstract
Kin selection theory predicts that individuals should generally behave less aggressively or more amicably towards relatives than nonkin. However, how individuals treat conspecifics depends on genetic relatedness but also on the ecological context, which influences the benefits and costs of their interactions. In this study, we used microsatellite DNA markers and behavioral tests to examine the influence of kinship and proximity on the social behavior of Mongolian gerbils Meriones unguiculatus living in different social groups, and whether these effects varied with sex and season. We recorded the duration of 4 behavioral categories (investigative, neutral, amicable, and agonistic) during a 10-min pairwise test. We found that genetic relatedness had significant effects on the duration of investigative, neutral, and amicable behavior, but not on agonistic behavior. We also found significant interaction effects of relatedness and distance between burrow systems (i.e., spatial distance) on investigative, neutral, and amicable behavior, which suggests that the effects of kinship on social behavior were restricted by spatial proximity. The interaction effect between sex and relatedness on amicable behavior showed that male gerbils became more intimate with individuals of the same sex that had higher pairwise relatedness than females. Furthermore, both male and female gerbils enhanced their aggression during the food-hoarding season, but the intensity of these changes was significantly higher in females. Overall, our results suggest that the effects of kinship and spatial proximity on social behavior exhibit sexual or seasonal patterns, thereby implying ecological context-dependent responses to out-group individuals in Mongolian gerbils.
Keywords: familiarity, kinship, Meriones unguiculatus, seasonality, social behavior
Kin selection theory predicts that individuals should behave less aggressively (enhanced social tolerance) or more amicably toward closer kin than less related individuals (Hamilton 1964a, 1964b). In species that live in family groups, individuals may simultaneously gain indirect and direct benefits from helping kin. For example, meerkats Suricata suricatta are more likely to perform sentinel behavior when pups join foraging groups (Santema and Clutton-Brock 2013) and the sentinels have a lower predation risk when they are guarding (Clutton-Brock et al. 1999). Moreover, helpers may gain kin selection benefits from raising related offspring, and these offspring might also boost the direct fitness of helpers in the future (Hamilton 1964a, 1964b; Kokko et al. 2001; Ren et al. 2017). Nevertheless, agonistic behaviors commonly occur between relatives due to competition for resources (Hare and Murie 1996; Stockley and Bro-Jorgensen 2011). In many cases, animals exhibit their peak aggression during the breeding season (BS) (e.g., eastern broad-toothed field mouse, Apodemus mystacinus: Vachova and Frynta 2004), and they behave less amicably toward kin during the BS compared with other times of the year (e.g., black-tailed prairie dog, Cynomys ludovicianus: Hoogland 1986). Thus, how individuals treat conspecifics depends on their genetic relatedness, but also on the ecological context, which influences the benefits and costs of cooperative and competitive interactions.
Evidence increasingly indicates that unrelated conspecifics that engage in frequent interactions due to their close spatial proximity may develop “kin-like” behavior (Arnberg et al. 2015; Meshriy et al. 2011; Sanchez and Hudgens 2015). Unrelated individuals may gain immediate shared benefits from mutualism or reciprocity (Clutton-Brock 2002, 2009; Madden et al. 2012). For instance, as the home range overlap increases, there is a higher probability of nest-sharing among unrelated female dusky-footed woodrats Neotoma fuscipes (Innes et al. 2012), thereby suggesting that proximity can lead to social tolerance in this species. In addition, many territorial species are less aggressive toward known neighbors than they are toward strangers (Maciej et al. 2013; Monclús et al. 2014; Temeles 1994), and individuals may save time and energy by averting a costly and unnecessary fight with familiar neighbors (Fisher 1954; O'Connor et al. 2000).
Mongolian gerbils Meriones unguiculatus are geographically widespread in the typical steppe, desert steppe, or desert areas of northern China, Mongolia, and the Trans-Baikal region of Russia (Wilson and Reeder 2005). Mongolian gerbils live in social groups comprising 2–18 individuals throughout the year (Liu et al. 2009), where each group occupies an exclusive territory and all group members share the burrow system (Ågren et al. 1989a). The reproduction and recruitment of Mongolian gerbils occur mainly from March to August (Liu et al. 2007, 2009), and they start to store food from September to October (Ågren et al. 1989a, 1989b). Thus, there are 2 distinct annual life-history stages in Mongolian gerbils: the BS (March to August) and food-hoarding season (FHS) (September to October). Previous studies have shown that the home-range size of a social group increases with the number of male gerbils in the group during the BS, whereas it is positively correlated with the number of female group members during the FHS (Wang et al. 2011a). In addition, field observations and genetic data have demonstrated that social groups are basically family groups (Ågren et al. 1989a, 1989b; Wang et al. 2011b), and that inter-group genetic distances and geographic distances are positively related (Wang et al. 2017). Trespassing by neighbors and chases involving individuals from adjacent groups are frequently observed during the BS (Ågren et al. 1989a, 1989b), and females commonly copulate with neighboring males (Ågren 1984a, 1984b; Ågren et al. 1989a). Consequently, the gerbils that live in different social groups may be kin. These characteristics make the Mongolian gerbil a suitable model species for investigating the influence of kinship and spatial proximity on social behavior, which have not been thoroughly examined in this species.
In this study, we conducted an experiment to test our hypothesis that the social behavioral traits of individuals living in different groups are affected by genetic relatedness and spatial distance (distance between burrow systems) in a natural population of Mongolian gerbils Meriones unguiculatus. We predicted that gerbils may exhibit higher rates of amicable interactions and allocate less time toward exploring genetically and spatially close individuals. We also tested whether these effects on social behavior differed between sexes and seasons. The social associations and home-range overlap frequently occur among individuals from adjacent social groups during the BS (Ågren et al. 1989b; Wang et al. 2011a), and fewer intergroup social connections are established during the FHS than the BS (Deng et al. 2017). Thus, we predicted that the effects of kinship and spatial proximity on social behavior might be stronger during the BS than the FHS. In addition, multiple breeding females can be found at the same time in natural populations of wild gerbils and reproductively active male gerbils mainly defend the territories (Ågren et al. 1989b). Therefore, we predicted that these effects may be stronger in males but not in female gerbils given that females usually encounter relatively lower reproductive competition than males (Clutton-Brock and Huchard 2013b).
Materials and Methods
Study site, trapping, and animal identification
Our studies were conducted at Houhatai (42°23.613′N, 116°06.524′E, altitude 1300 m), which is located about 25 km north of Shangdu, Zhenglan Qi, Inner Mongolia, China. The study site is a typical steppe area. The average monthly temperature ranged from –13.9°C to 21°C and the annual total precipitation was 324.6 mm during 2014.
Our trapping plot comprised a 2-ha (100 m × 200 m) grassland dominated by Leymus chinensis, Artemisia sieversiana, Thalictrum petaloideum, Stellera chamaejasme, Klasea centauroides, and Aster altaicus, which provided food or cover for gerbils. The sympatric small mammals comprised the Daurian ground squirrel Spermophilus dauricus, Daurian pika Ochotona dauurica, and striped dwarf hamster Cricetulus barabensis. The potential predators of Mongolian gerbils were the steppe polecat Mustela eversmanii, corsac fox Vulpes corsac, and some raptors such as the common kestrel Falco tinnunculus and upland buzzard Buteo hemilasius. No livestock grazed on the study site during our study period.
Mark-recapture experiments were conducted from April 29 to October 24 at 2-week intervals in 2014, where each trapping session lasted for 3 consecutive days. We did not trap during the winter to avoid mortality. Mongolian gerbils were live-trapped using wire mesh live traps (28 cm × 13 cm × 10 cm) baited with fresh peanuts. To enhance the likelihood of successful trapping, we used a concentric circle trapping method (Liu et al. 2007). The trap station was arranged in 3 or 4 concentric circles within a burrow system to cover most of the range. In total, around 380 traps were set each time to cover all of the trap stations. The traps were set at 05:00–06:00 h from May to August, and checked every 1–2 h until about 1100 h. The traps were then closed between 11:00 and 15:00 h to avoid trap mortality due to the heat, and trapping was resumed at 16:00 h and continued until 19:00 h. Trapping started between 06:30 and 07:30 h, and continued until 17:30 h during September and October. We also checked the traps every 1–2 h during this period. Gerbils were active during the trapping periods employed in this study (Liu et al. 2007).
All of the captured gerbils were toe clipped when initially captured to allow permanent identification. The clipped toes were preserved in 95% ethanol for subsequent genetic analyses. All of the burrow systems were marked and their coordinates were recorded with a tape. In the field, a typical Mongolian gerbil burrow system comprises entrance holes, feeding chambers, nest chambers, and tunnels (Scheibler et al. 2006). The core area of the burrow system usually has about 10–20 entrances holes in the ground, which form a burrow entrance cluster (Ågren et al. 1989a). There are areas with few or no entrances between the burrow systems, so it is easy to determine the distribution of the burrow systems based on the burrow entrance clusters (Ågren et al. 1989a, Wang et al. 2011a). Therefore, coordinates were used to calculate the distance between each 2 burrow systems according to the Pythagorean Theorem, where the distance from the center point of the core area of the burrow system was the average distance between all of the burrows in one social group and those of another social group. The distance between burrow systems was used as an index of the spatial distance between Mongolian gerbils living in different social groups.
The following data were recorded for each captured gerbil: location, sex, body mass, and reproductive condition (male: testes scrotal or abdominal, ventral scent glands invisible, clear contour or large visible pores surrounded by secreted substance; female: vulva closed or open, pregnant, lactating) (Liu et al. 2007). Age was estimated based on body mass and the developmental stage of the ventral sebaceous gland (juvenile: <30 g and with no sign of ventral gland; subadult: 30–50 g, unless they had a ventral active gland wider than 4.2 mm; adult: >50 g) (Ågren et al. 1989a; Liu et al. 2007). We treated gerbils captured in the same burrow system in 2 consecutive trapping sessions as members of the same social group (Ågren et al. 1989a). After recording the data, all of the gerbils were taken to a tent beside the plot and only adults from different groups were selected for behavioral tests. The trapping and handling procedures for the Mongolian gerbils were approved by the Institutional Animal Use and Care Committee of the Institute of Zoology, Chinese Academy of Sciences (Ethical Inspection License No: IOZ13047).
Procedures in behavioral tests
To test the social behavior in pairwise encounters, we used a neutral arena (Olivier and Dalen 1982), which has been employed widely in rodent behavioral studies (Klatt et al. 2015; Shen et al. 2015; Zhang et al. 2001). Adult gerbils (>50 g) of the same sex captured from different burrow systems were selected for pairwise encounters in behavioral tests and they were matched randomly. Social behavior was tested by staging paired encounters in a rectangular neutral arena (42.5 cm × 31 cm × 19 cm), where each dyad was tested only once during a specific season in our study. The arena was divided into 2 equal compartments using a removable opaque partition. Two individuals were placed on either side of the partition and allowed to acclimatize to the novel environment for 5 min. The barrier was then removed and interactions were observed for a 10-min period. A digital voice recorder was used to record any behavior, which was observed continuously by a specific observer. We terminated the tests immediately if one of the following occurred: 1) continuous fighting physically for almost 1 min at a time; or 2) an actual injury occurred.
We measured the durations of the following 4 categories of behavior observed during each 10-min test in the arena: 1) investigative behavior, including sniffing the nose, body, and anal zone of the other gerbil; 2) neutral behavior where the gerbils remained more than 5 cm apart without exhibiting any agonistic behavior and they ignored each other; 3) amicable behavior defined as the pair of gerbils located less than 5 cm apart and they exhibited affiliative behaviors such as remaining side by side, one over the other, or grooming; and 4) agonistic behavior such as upright boxing, defense, and wrestling. All of these behaviors have been observed in natural and captive populations of Mongolian gerbils (Hurtado-Parrado et al. 2015). The arena was cleaned thoroughly with 75% ethanol between tests to remove any odors from the previously tested individuals. At the end of each trial, all of the gerbils were released back into the burrow systems from which they were captured. Gerbils were housed individually in plastic cages (30 cm × 15 cm × 20 cm) during the behavioral tests and adequate food was provided. Animals were kept in the tent for less than 3 h.
Pairwise relatedness based on microsatellite markers
DNA was extracted from tissues using a TIANamp Genomic DNA Kit (TianGen Biotech Company Ltd, Beijing, China). Nine microsatellite loci (Mungµ1, Mungµ2, Mungµ3, Mungµ4, Mungµ5, Mungµ6, Mungµ7, Mungµ8, and Mungµ9) developed for Mongolian gerbils were used to estimate relatedness (Neumann et al. 2001). Polymerase chain reaction (PCR) was conducted in a 10-µL reaction mixture containing 0.5 ng genomic DNA, 5 µL Premix Taq (TianGen Biotech Company Ltd), and 0.6 µM of the forward (fluorescently labeled with 5#-TAMARA, HEX, or FAM) and reverse primers. PCR was run under the following conditions: initial denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 94°C for 40 s, annealing at a specific temperature (Ta) for 45 s, and extension at 72°C for 1 min, with a final extension at 72°C for 5 min. The specific annealing temperature (Ta) for each locus was specified by Neumann et al. (2001).
Successful PCR amplification was verified by examining samples on agarose gels. Amplified fragments were electrophoresed on an ABI 3730 XL capillary sequencer (Applied Biosystems, Foster City, CA) and the allele size was determined with GENEMAPPER 4.1 software (Applied Biosystems).
Hardy–Weinberg equilibrium tests were conducted with GenePop 4.3 (Rousset 2008). Micro-checker 2.0 (Van Oosterhout et al. 2004) was used to test for the probability of null alleles. We calculated genetic diversity metrics comprising the observed heterozygosity (Ho), expected heterozygosity (He), number of effective alleles (Na), and inbreeding coefficient (FIS) using GenAlEx 6.501 (Peakall and Smouse 2012). The pairwise genetic relatedness was estimated between test pairs using GenAlEx 6.501 (Peakall and Smouse 2012) with Lynch and Ritland’s estimator (Lynch and Ritland 1999). The polymorphic information content (PIC) and discriminatory power (DP) of loci were estimated using Cervus 3.0.7 (Kalinowski et al. 2007).
Statistical analysis
The behavioral data were examined using Shapiro–Wilk test to determine their normality and the results did not indicate normal distributions (P < 0.001). Thus, we transformed the data using the Box-Cox method (Gurka et al. 2006) in the MASS package (Ripley et al. 2018). We used linear mixed effects (LME) models to determine whether the durations of 4 social behaviors were affected by the pairwise relatedness, spatial distance, sex, and season. Relatedness, distance, sex, and season were treated as fixed effects, and pairwise encounter and month as random effects. The results were expressed as the mean ± standard error (SE). P < 0.05 was considered to indicate a statistically significant difference. LME models were analyzed using the lme4 package (Bates et al. 2013). All statistical analyses were performed with R software (R Core Team 2016).
Results
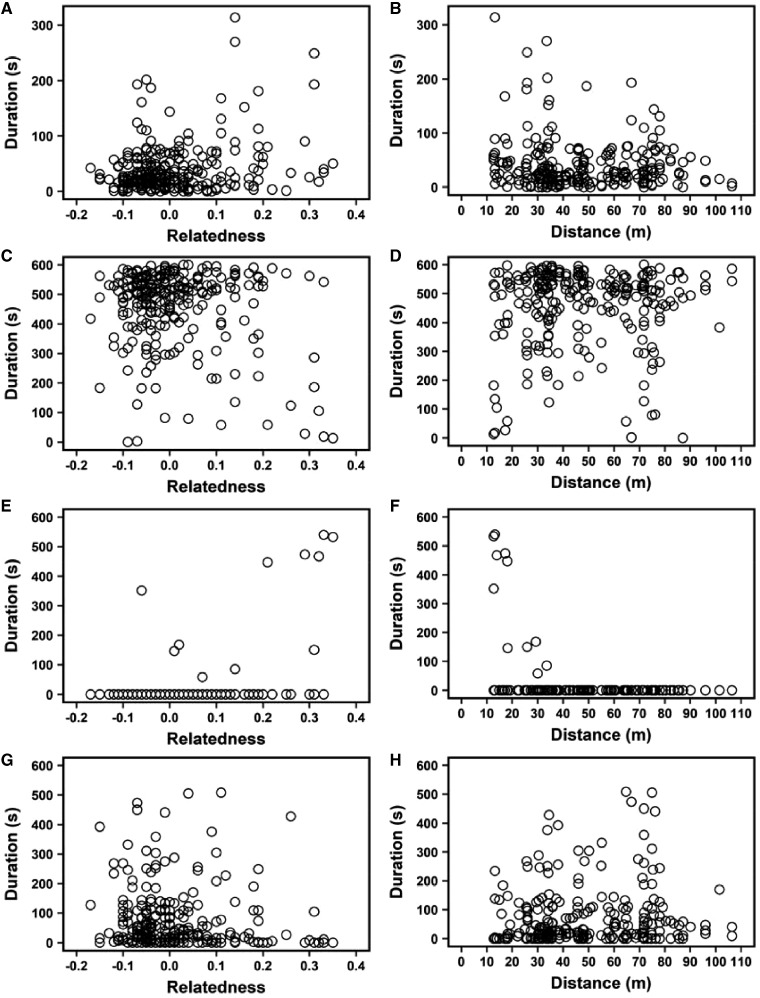
In total, 253 gerbils (137 females and 116 males) from 24 burrow systems were identified during the study period. We captured gerbils 1371 times during the BS and 609 times during the FHS. The distance between 2 burrow systems ranged from 11.6 m to 147.0 m with an average of 66.1 ± 1.6 m. We conducted 249 pairwise behavior tests and about 77.1% (192/249) pairwise encounters were observed aggression. Initially, the gerbils in the pairwise encounters generally sniffed each other (i.e., investigative behavior) or kept away and ignored each other (i.e., neutral behavior). After several investigative and neutral behaviors, the encounters became amicable or agonistic. Amicable and agonistic behaviors did not occur within 10 min in some tests (Table 1 , Figure 1 ). The relatedness between pairs of individuals was relatively low (Table 1).
Table 1.
Pairwise relatedness (Lynch and Ritland’s genetic relatedness coefficients) and the duration (seconds) of 4 behaviors in encounters during the breeding season (BS) and food-hoarding season (FHS)
| Sex | Season | Relatedness | Investigative behavior | Neutral behavior | Amicable behavior | Agonistic behavior | Sample size (No. of paired encounters) |
|---|---|---|---|---|---|---|---|
| Female | BS | 0.019 ± 0.011 | 33.75 ± 2.89 | 496.66 ± 11.46 | 23.76 ± 10.35 | 34.20 ± 4.97 | 92 |
| Female | FHS | 0.028 ± 0.018 | 62.51 ± 12.46 | 396.68 ± 26.09 | 17.12 ± 11.99 | 114.51 ± 21.81 | 41 |
| Male | BS | −0.005 ± 0.010 | 36.35 ± 4.27 | 462.17 ± 12.96 | 6.81 ± 6.10 | 81.42 ± 11.07 | 78 |
| Male | FHS | 0.017 ± 0.009 | 44.18 ± 6.88 | 432.95 ± 22.86 | 0.00 ± 0.00 | 110.29 ± 22.02 | 38 |
Data represent the mean ± SE.
Figure 1.
Relationships between the duration (seconds) of social behavior and pairwise relatedness (Lynch and Ritland’s genetic relatedness coefficients) and spatial distance (distance between burrow systems) in Mongolian gerbils (N = 209): (A, B) investigative behavior, (C, D) neutral behavior, (E, F) amicable behavior, and (G, H) agonistic behavior.
Genetic analysis
The Mungµ8 allele was not amplified by PCR and it exhibited relatively low polymorphism, so we excluded it from subsequent analyses. Mungµ3 deviated from Hardy-Weinberg equilibrium after sequential Bonferroni corrections for multiple comparisons (P < 0.01). Thus, we removed this locus from any further estimations of relatedness. The number of alleles per locus ranged from 8 to 11, whereas the mean He = 0.783 ± 0.020 and mean Ho = 0.789 ± 0.030. The mean PIC was 0.756 ± 0.022, the mean DP for 7 loci was 0.927 ± 0.014, and mean FIS was –0.211 ± 0.020 (Table 2 ). We detected no evidence of null alleles.
Table 2.
Characteristics of microsatellite loci used to estimate relatedness in Mongolian gerbils
| Locus | Allele size (base pairs) | N a | H o | H e | PIC | DP | FIS |
|---|---|---|---|---|---|---|---|
| Mungµ1 | 212–232 | 10 | 0.720 | 0.764 | 0.734 | 0.917 | −0.223 |
| Mungµ2 | 107–129 | 10 | 0.829 | 0.848 | 0.822 | 0.959 | −0.194 |
| Mungµ3 | 125–149 | 9 | 0.683 | 0.688 | 0.683 | – | −0.199 |
| Mungµ4 | 204–220 | 8 | 0.646 | 0.659 | 0.646 | 0.847 | −0.128 |
| Mungµ5 | 161–185 | 11 | 0.805 | 0.808 | 0.780 | 0.936 | −0.261 |
| Mungµ6 | 113–131 | 10 | 0.829 | 0.802 | 0.770 | 0.933 | −0.230 |
| Mungµ7 | 162–184 | 11 | 0.927 | 0.856 | 0.833 | 0.962 | −0.145 |
| Mungµ9 | 119–205 | 9 | 0.793 | 0.812 | 0.780 | 0.941 | −0.311 |
Na, number of alleles; Ho, observed heterozygosity; He, expected heterozygosity; PIC, polymorphic information content; DP, discriminatory power; FIS, inbreeding coefficient.
Effects of relatedness and spatial distance on behavioral traits
The pairwise relatedness had significant effects on investigative, neutral, and amicable behavior. In particular, the durations of investigative behavior (t = 2.869, P = 0.0046) and amicable behavior (t = 5.432, P < 0.0001) increased significantly, whereas the duration of neutral behavior (t = –2.734, P = 0.0071) decreased significantly with increasing relatedness (Figure 1, Table 3 ). We also found a significant negative relationship between the durations of amicable behavior and spatial distance (t = –2.562, P = 0.0111, Table 3), and amicable behavior only occurred between individuals with a spatial distance of less than 40 m (Figure 1f). In addition, the interaction between relatedness and distance had significant negative effects on investigative behavior (t = –2.022, P = 0.0475) and amicable behavior (t = –7.505, P < 0.0001), but positive effects on neutral behavior (t = 4.764, P < 0.0001, Table 3). However, pairwise relatedness (P = 0.1248) or spatial distance (P = 0.5687) had no significant effects on the intensity of agonistic behavior (Figure 1g-h, Table 3).
Table 3.
LME models for testing the effects of pairwise relatedness (Lynch and Ritland’s genetic relatedness coefficients) and spatial distance (distance between burrow systems), sex, seasonality, and their interaction on the duration of social behaviors in Mongolian gerbils
| Behavioral category | Estimate | SE | t | P |
|---|---|---|---|---|
| Investigative behavior | ||||
| Sex ¦ Male | −0.9020 | 5.7218 | −0.158 | 0.8750 |
| Seasonality ¦ FHS | 18.7815 | 9.2196 | 2.037 | 0.0718 |
| Distance | −0.1565 | 0.1363 | −1.148 | 0.2523 |
| Relatedness | 83.0134 | 28.9383 | 2.869 | 0.0046 † |
| Sex×seasonality | −22.1472 | 11.9224 | −1.858 | 0.0654 |
| Sex×distance | −0.0271 | 0.2682 | −0.101 | 0.9197 |
| Sex×relatedness | −18.4826 | 60.3405 | −0.306 | 0.7595 |
| Seasonality×distance | −0.5388 | 0.2845 | −1.894 | 0.0603 |
| Seasonality×relatedness | 201.9876 | 59.4921 | 3.395 | 0.0007 † |
| Distance×relatedness | −2.8057 | 1.3875 | −2.022 | 0.0475 † |
| Neutral behavior | ||||
| Sex ¦ Male | −12.6218 | 16.9520 | −0.745 | 0.4577 |
| Seasonality ¦ FHS | −69.3533 | 28.1718 | −2.462 | 0.0385 † |
| Distance | 0.3481 | 0.4028 | 0.864 | 0.3962 |
| Relatedness | −232.7818 | 85.1354 | −2.734 | 0.0071 † |
| Sex×seasonality | 83.9565 | 34.8727 | −2.408 | 0.0188 † |
| Sex×distance | 0.1029 | 0.7908 | 0.130 | 0.8967 |
| Sex×relatedness | 224.6669 | 176.3941 | 1.274 | 0.2035 |
| Seasonality×distance | −0.4918 | 0.8408 | −0.585 | 0.5717 |
| Seasonality×relatedness | −358.4613 | 175.9891 | −2.037 | 0.0425 † |
| Distance×relatedness | 18.5908 | 3.9025 | 4.764 | < 0.0001 † |
| Amicable behavior | ||||
| Sex ¦ Male | −8.8841 | 10.0254 | −0.886 | 0.3760 |
| Seasonality ¦ FHS | −2.7259 | 6.9205 | −0.394 | 0.6945 |
| Distance | −0.5665 | 0.2211 | −2.562 | 0.0111 † |
| Relatedness | 251.6024 | 46.3225 | 5.432 | < 0.0001 † |
| Sex×seasonality | −5.7055 | 13.8146 | −0.413 | 0.6797 |
| Sex×distance | 0.5058 | 0.4339 | 1.166 | 0.2444 |
| Sex×relatedness | −209.8939 | 90.9768 | −2.307 | 0.0235 † |
| Seasonality×distance | 0.0862 | 0.3388 | 0.255 | 0.7992 |
| Seasonality×relatedness | 7.5315 | 71.0561 | 0.106 | 0.9158 |
| Distance×relatedness | −14.3321 | 1.9096 | −7.505 | < 0.0001 † |
| Agonistic behavior | ||||
| Sex ¦ Male | 26.8136 | 12.7975 | 2.097 | 0.0379 † |
| Seasonality ¦ FHS | 59.4638 | 18.1132 | 3.283 | 0.0123 † |
| Distance | 0.1795 | 0.3046 | 0.589 | 0.5687 |
| Relatedness | −100.1879 | 64.8515 | −1.545 | 0.1248 |
| Sex×seasonality | −56.0029 | 26.8553 | −2.085 | 0.0410 † |
| Sex×distance | −0.1014 | 0.6008 | −0.169 | 0.8664 |
| Sex×relatedness | −129.0281 | 135.2281 | −0.954 | 0.3405 |
| Seasonality×distance | 1.1558 | 0.6306 | 1.833 | 0.0734 |
| Seasonality×relatedness | 142.8407 | 136.6413 | 1.045 | 0.2973 |
| Distance×relatedness | 1.3495 | 3.1414 | 0.430 | 0.6706 |
†Significant P-values are indicated in bold. FHS, food-hoarding season.
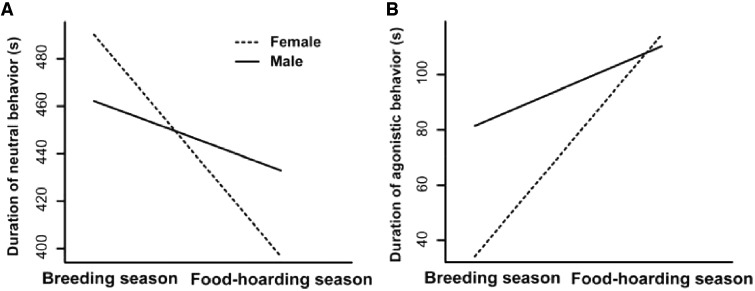
The effects of relatedness and spatial distance on social behavior exhibited no consistent patterns in different sexes or seasons. The interaction between relatedness and sex had significant negative effects on amicable behavior (t = –2.307, P = 0.0235, Table 3). The interaction between relatedness and seasonality had significant positive effects on investigative behavior (t = 3.395, P = 0.0007), but negative effects on neutral behavior (t = –2.037, P = 0.0425, Table 3). In addition, there were significant negative interaction effects of sex and seasonality on neutral (t = –2.408, P = 0.0188) and agonistic behavior (t = –2.085, P = 0.0410, Table 3, Figure 2 ).
Figure 2.
Interaction effects between sex and seasonality on the duration (seconds) of (A) neutral behavior and (B) agonistic behavior.
Discussion
As expected, the social behavior in staged encounters between Mongolian gerbils was influenced by relatedness, spatial distance, and their interactions. Gerbils spent significantly more time sniffing related individuals and behaved more amicably with increasing pairwise genetic relatedness. Thus, gerbils were more indifferent to each other as the pairwise relatedness decreased. However, the significant interaction effects between relatedness and spatial distance on investigative, neutral, and amicable behavior indicate that the effects of kinship on social behavior were constrained by space. In addition, we found that amicable behavior occurred only between individuals with a spatial distance of less than 40 m, which is approximately the active range of Mongolian gerbils (Ågren et al. 1989a). Furthermore, amicable behavior was exhibited by both close relatives and distantly related gerbils. These results imply that familiarity may play a key role in social associations of Mongolian gerbils. It should be noted that a higher level of familiarity does not necessarily indicate closer spatial distance, but it implies a closer social distance based on frequent interactions. For example, female banner-tailed kangaroo rats Dipodomys spectabilis avoid inbreeding via the development of familiarity based on prior associations rather than by using spatial cues, even if males live in familiar spatial locations (Waser et al. 2012). Thus, the relatively low inbreeding coefficient in gerbils may be related to a similar behavioral strategy for inbreeding avoidance, although females commonly copulate with neighboring males (Ågren 1984a, 1984b).
However, there were no significant effects of relatedness or spatial distance on agonistic behavior. We observed that 192 out of 249 pairs of encounters resulted in aggression, thereby confirming previous direct observations that most intergroup interactions between gerbils are aggressive (Ågren et al. 1989a). Many studies have demonstrated that high relatedness increases the incidence of amicable behavior among relatives such as supportive behavior (Smith et al. 2003) and cooperation (Langergraber et al. 2007), and it can reduce the intensity of aggression (Dobson et al. 2012). However, we found no evidence that agonistic intergroup interactions between gerbils changed with variations in the genetic or spatial distance. Scheibler et al. (2004) also reported that the intensities of inter- and intra-family aggression in Mongolian gerbils were generally similar. This suggests that the effects of relatedness and familiarity on aggression may be limited in this species, which indicates a high level of competition for food or mate resources between intergroup individuals.
We also found that the effects of relatedness and spatial distance on social behavior exhibited sexual or seasonal patterns. Analyses of the LME models showed that there were interaction effects between relatedness and seasonality on investigative and neutral behavior, thereby suggesting that the effects of kinship on investigative behavior are stronger during the FHS but stronger on neutral behavior during the BS. The negative interaction effects between sex and relatedness on amicable behavior suggest that male gerbils are less intimate with individuals of the same sex than females when the pairwise relatedness is low, but the intensity of the increase in intimacy was higher for males as their relatedness increased. These findings support the idea that individuals may alter their behavioral strategies to adapt to variations in the ecological context (Montiglio et al. 2016; Owen et al. 2017).
Our results showed that both male and female gerbils were highly aggressive during the FHS. The food-hoarding period is known to be a crucial period for non-hibernating northern small mammals (Kuhn and Vander Wall 2008; Morrison et al. 2009). Our previous field study showed that fewer intergroup social connections were established during the FHS than the BS (Deng et al. 2017). Other previous studies of Mongolian gerbils have demonstrated that the home range size is positively related to group size (Ågren et al. 1989a; Wang et al. 2011a), and the home ranges overlap less during the FHS than the BS (Wang et al. 2011a), thereby demonstrating that food defense is an important function of territorial behavior (Ågren et al. 1989a; Ebensperger 2001). Furthermore, food availability is a determinant of winter survival and of the social groups or population sizes for Mongolian gerbils in the following spring (Liu et al. 2011). Similarly, highly aggressive behavior during the defense of territories is also found in American red squirrels Tamiasciurus hudsonicus, which are highly aggressive during both the breeding and non-BS while defending food stored in their individual territories (Boonstra et al. 2008). Therefore, we consider that the high levels of aggression between intergroup gerbils during the FHS associated with an increase in territoriality were caused by food resource competition.
Nevertheless, we found significant negative interaction effects between sex and seasonality on agonistic behavior, which indicates that although Mongolian gerbils exhibited enhanced aggression during the FHS, the intensities of the changes differed significantly between the sexes. Female gerbils exhibited significantly lower aggression during the BS than the FHS, whereas the intensity of aggression in males did not differ significantly between the 2 seasons. Moreover, female gerbils exhibited significantly lower aggression than males during the BS. In most groups of animals, the intensity of aggression is lower in females than males, and the secondary sexual characteristics are generally less developed (Darwin 1871), which suggests that females usually experience relatively lower reproductive competition than males (Clutton-Brock and Huchard 2013b). Females primarily fight to defend access to resources such as food and shelter according to Bateman’s principle (Arseneau-Robar et al. 2017; Bateman 1948). In Mongolian gerbils, males have opportunities to copulate with visiting or resident females by defending an undisturbed area, but they are probably unable to prevent their female partners from leaving their territories to solicit copulations in adjacent territories (Ågren et al. 1989a). In our study, males were more aggressive against out-group individuals of the same sex than females during the BS, which probably reflects the paternity benefits of repelling intruding male gerbils. This finding supports the suggestion that intergroup encounters may have very different fitness impacts on males and females, and that responses to intruders may reflect differences in the benefit and cost trade-offs between the sexes (Mares et al. 2012; Nichols et al. 2015).
A second reason why agonistic interactions are less frequent and intense in females than males during the BS is that the risks associated with escalated conflict are usually higher for females than males (Cant and Young 2013; Clutton-Brock and Huchard 2013a). For example, a fatal injury to a female may also lead to increased mortality for any dependent offspring (e.g., ring-tailed lemurs, Lemur catta: Jolly et al. 2000). In addition, even when the risk of injury is relatively low, there may be an energy trade-off between chasing and pup feeding (Mares et al. 2012). Thus, lactating females may rarely engage in aggressive interactions (e.g., chacma baboons, Papio ursinus) (Huchard and Cowlishaw 2011).
In conclusion, our results demonstrate that the behavioral traits of Mongolian gerbils that live in different social groups are affected by kinship, spatial proximity, and their interaction. Furthermore, we showed that the effects of kinship and spatial proximity on social behavior exhibited sexual or seasonal patterns, thereby indicating the occurrence of context-dependent responses to out-group individuals in Mongolian gerbils. Further studies are required to investigate the social association patterns and to quantify their costs and benefits in order to advance our understanding of behavioral interactions in social rodents.
Acknowledgements
We are grateful to all the members of the Animal Physiological Ecology Group for helpful discussions. We also thank Mr Bin Wu, Plant Protection Station of Taipusiqi, for help with the field work. We are grateful to Dr Michael Cant for his help revising the manuscript. This study was financially support from the National Natural Science Foundation of China (No. 31372211) for W.L. and from the Chinese Academy of Sciences (KSCX2-EW-N-005) for D-H.W.
References
- Ågren G, 1984a. Alternative mating strategies in the Mongolian gerbil. Behav 91:229–243. [Google Scholar]
- Ågren G, 1984b. Incest avoidance and bonding between siblings in gerbils. Behav Ecol Sociobiol 14:161–169. [Google Scholar]
- Ågren G, Zhou Q, Zhong W, 1989a. Ecology and social-behavior of Mongolian gerbils Meriones unguiculatus at Xilinhot, Inner-Mongolia, China. Anim Behav 37:11–27. [Google Scholar]
- Ågren G, Zhou Q, Zhong W, 1989b. Territoriality, cooperation and resource priority-hoarding in the Mongolian gerbil Meriones unguiculatus. Anim Behav 37:28–32. [Google Scholar]
- Arnberg NN, Shizuka D, Chaine AS, Lyon BE, 2015. Social network structure in wintering golden-crowned sparrows is not correlated with kinship. Mol Ecol 24:5034–5044. [DOI] [PubMed] [Google Scholar]
- Arseneau-Robar TJM, Taucher AL, Schnider AB, van Schaik CP, Willems EP, 2017. Intra- and interindividual differences in the costs and benefits of intergroup aggression in female vervet monkeys. Anim Behav 123:129–137. [Google Scholar]
- Bateman AJ, 1948. Intra-sexual selection in Drosophila. Hered 2:349–368. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, 2013. Linear Mixed-Effect Models Using S4 Classes. R package. [Google Scholar]
- Boonstra R, Lane JE, Boutin S, Bradley A, Desantis L et al. , 2008. Plasma DHEA levels in wild, territorial red squirrels: seasonal variation and effect of ACTH. Gen Comp Endocr 158:61–67. [DOI] [PubMed] [Google Scholar]
- Cant MA, Young AJ, 2013. Resolving social conflict among females without overt aggression. Philos T R Soc B 368:20130076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T, 2002. Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296:69–72. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T, 2009. Cooperation between non-kin in animal societies. Nature 462:51–57. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T, Huchard E, 2013a. Social competition and its consequences in female mammals. J Zool 289:151–171. [Google Scholar]
- Clutton-Brock TH, Huchard E, 2013b. Social competition and selection in males and females. Philos Trans R Soc Lond B Biol Sci 368:20130074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, O'Riain MJ, Brotherton PNM, Gaynor D, Kansky R et al. , 1999. Selfish sentinels in cooperative mammals. Science 284:1640–1644. [DOI] [PubMed] [Google Scholar]
- Darwin C, 1871. The Descent of Man, and Selection in Relation to Sex. London: John Murray. [Google Scholar]
- Deng K, Liu W, Wang DH, 2017. Inter-group associations in Mongolian gerbils: quantitative evidence from social network analysis. Integr Zool 12:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson FS, Viblanc VA, Arnaud CM, Murie JO, 2012. Kin selection in Columbian ground squirrels: direct and indirect fitness benefits. Mol Ecol 21:524–531. [DOI] [PubMed] [Google Scholar]
- Ebensperger LA, 2001. A review of the evolutionary causes of rodent group-living. Acta Theriol 46:115–144. [Google Scholar]
- Fisher J, 1954. Evolution and bird sociality. In: Huxley J, Hardy A, Ford E, editors. Evolution as a Process. London: George Allen and Unwin; 71–83. [Google Scholar]
- Gurka M, Edwards LJ, Muller KE, Kupper LL, 2006. Extending the Box-Cox transformation to the linear mixed model. J Roy Stat Soc a Sta 169:273–288. [Google Scholar]
- Hamilton WD, 1964a. The genetical evolution of social behaviour. I J Theor Biol 7:1–16. [DOI] [PubMed] [Google Scholar]
- Hamilton WD, 1964b. The genetical evolution of social behaviour. II. J Theor Biol 7:17–52. [DOI] [PubMed] [Google Scholar]
- Hare JF, Murie JO, 1996. Ground squirrel sociality and the quest for the ‘holy grail’: does kinship influence behavioral discrimination by juvenile Columbian ground squirrels? Behav Ecol 7:76–81. [Google Scholar]
- Hoogland JL, 1986. Nepotism in prairie dogs Cynomys ludovicianus varies with competition but not with kinship. Anim Behav 34:263–270. [Google Scholar]
- Huchard E, Cowlishaw G, 2011. Female-female aggression around mating: an extra cost of sociality in a multimale primate society. Behav Ecol 22:1003–1011. [Google Scholar]
- Hurtado-Parrado C, Gonzalez CH, Moreno LM, Gonzalez CA, Arias M et al. , 2015. Catalogue of the behaviour of Meriones unguiculatus f. dom. (Mongolian gerbil) and wild conspecies, in captivity and under natural conditions, based on a systematic literature review. J Ethol 33:65–86. [Google Scholar]
- Innes RJ, McEachern MB, Van Vuren DH, Eadie JM, Kelt DA et al. , 2012. Genetic relatedness and spatial associations of dusky-footed woodrats Neotoma fuscipes. J Mammal 93:439–446. [Google Scholar]
- Jolly A, Caless S, Cavigelli S, Gould L, Pereira ME et al. , 2000. Infant killing, wounding and predation in Eulemur and Lemur. Int J Primatol 21:21–40. [Google Scholar]
- Kalinowski ST, Taper ML, Marshall TC, 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106. [DOI] [PubMed] [Google Scholar]
- Klatt BJ, Getz LL, McGuire B, 2015. Interspecific interactions and habitat use by prairie voles Microtus ochrogaster and meadow voles M. pennsylvanicus. Am Midl Nat 173:241–252. [Google Scholar]
- Kokko H, Johnstone RA, Clutton-Brock TH, 2001. The evolution of cooperative breeding through group augmentation. P Roy Soc B-Biol Sci 268:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KM, Vander Wall SB, 2008. Linking summer foraging to winter survival in yellow pine chipmunks Tamias amoenus. Oecologia 157:349–360. [DOI] [PubMed] [Google Scholar]
- Langergraber KE, Mitani JC, Vigilant L, 2007. The limited impact of kinship on cooperation in wild chimpanzees. P Natl Acad Sci USA 104:7786–7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wan X, Zhong W, 2007. Population dynamics of the Mongolian gerbils: seasonal patterns and interactions among density, reproduction and climate. J Arid Environ 68:383–397. [Google Scholar]
- Liu W, Wang GM, Wan XR, Zhong WQ, 2011. Winter food availability limits winter survival of Mongolian gerbils Meriones unguiculatus. Acta Theriol 56:219–227. [Google Scholar]
- Liu W, Wang GM, Wang YN, Zhong WQ, Wan XR, 2009. Population ecology of wild Mongolian gerbils Meriones unguiculatus. J Mammal 90:832–840. [Google Scholar]
- Lynch M, Ritland K, 1999. Estimation of pairwise relatedness with molecular markers. Genetics 152:1753–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciej P, Patzelt A, Ndao I, Hammerschmidt K, Fischer J, 2013. Social monitoring in a multilevel society: a playback study with male Guinea baboons. Behav Ecol Sociobiol 67:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden JR, Nielsen JF, Clutton-Brock TH, 2012. Do networks of social interactions reflect patterns of kinship? Curr Zool 58:319–328. [Google Scholar]
- Mares R, Young AJ, Clutton-Brock TH, 2012. Individual contributions to territory defence in a cooperative breeder: weighing up the benefits and costs. Proc Biol Sci 279:3989–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshriy MG, Randall JA, Parra L, 2011. Kinship associations of a solitary rodent Dipodomys ingens at fluctuating population densities. Anim Behav 82:643–650. [Google Scholar]
- Monclús R, Saavedra I, de Miguel J, 2014. Context-dependent responses to neighbours and strangers in wild European rabbits Oryctolagus cuniculus. Behav Process 106:17–21. [DOI] [PubMed] [Google Scholar]
- Montiglio PO, Wey TW, Chang AT, Fogarty S, Sih A, 2016. Correlational selection on personality and social plasticity: morphology and social context determine behavioural effects on mating success. J Anim Ecol 86:213–226. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Pelchat G, Donahue A, Hik DS, 2009. Influence of food hoarding behavior on the over-winter survival of pikas in strongly seasonal environments. Oecologia 159:107–116. [DOI] [PubMed] [Google Scholar]
- Neumann K, Maak S, Stuermer IW, von Lengerken G, Gattermann R, 2001. Low microsatellite variation in laboratory gerbils. J Hered 92:71–74. [DOI] [PubMed] [Google Scholar]
- Nichols HJ, Cant MA, Sanderson JL, 2015. Adjustment of costly extra-group paternity according to inbreeding risk in a cooperative mammal. Behav Ecol 26:1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KI, Metcalfe NB, Taylor AC, 2000. Familiarity influences body darkening in territorial disputes between juvenile salmon. Anim Behav 59:1095–1101. [DOI] [PubMed] [Google Scholar]
- Olivier B, Dalen DV, 1982. Social behaviour in rats and mice: an ethologically based model for differentiating psychoactive drugs. Aggressive Behav 8:163–168. [Google Scholar]
- Owen MA, Swaisgood RR, Blumstein DT, 2017. Contextual influences on animal decision-making: significance for behavior-based wildlife conservation and management. Integr Zool 12:32–48. [DOI] [PubMed] [Google Scholar]
- Peakall R, Smouse PE, 2012. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research: an update Bioinformatics 28:2537–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2016. R: A Language and Environment for Statistical Computing. Available from: www.R-project.org (accessed 2 July 2018).
- Ripley B, Venables B, Bates D, Hornik K, Gebhardt A et al. , 2018. MASS: support functions and datasets for Venables and Ripley's “Modern Applied Statistics with S”. Available from: https://cran.r-project.org/web/packages/MASS/index.html (accessed 1 November 2018). [Google Scholar]
- Rousset F, 2008. GENEPOP'007: a complete re–implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour 8:103–106. [DOI] [PubMed] [Google Scholar]
- Ren Y, Huang K, Guo ST, Pan RL, Derek DW et al. , 2017. Kinship promotes affiliative behaviors in a monkey. Curr Zool 64:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JN, Hudgens BR, 2015. Interactions between density, home range behaviors, and contact rates in the Channel Island fox Urocyon littoralis. Ecol Evol 5:2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santema P, Clutton-Brock T, 2013. Meerkat helpers increase sentinel behaviour and bipedal vigilance in the presence of pups. Anim Behav 85:655–661. [Google Scholar]
- Scheibler E, Weinandy R, Gattermann R, 2004. Social categories in families of Mongolian gerbils. Physiol Behav 81:455–464. [DOI] [PubMed] [Google Scholar]
- Scheibler E, Liu W, Weinandy R, Gattermann R, 2006. Burrow systems of the Mongolian gerbil (Meriones unguiculatus Milne Edwards, 1867). Mamm Biol 71:178–182. [Google Scholar]
- Shen W, Zhang X-Y, Liu D-Z, Wang D-H, 2015. Hormones orchestrated pre-and post-copulatory sexual traits in male Mongolian gerbils. Physiol Behav 143:90–96. [DOI] [PubMed] [Google Scholar]
- Smith K, Alberts SC, Altmann J, 2003. Wild female baboons bias their social behaviour towards paternal half-sisters. Proc Biol Sci 270:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P, Bro-Jorgensen J, 2011. Female competition and its evolutionary consequences in mammals. Biol Rev 86:341–366. [DOI] [PubMed] [Google Scholar]
- Temeles EJ, 1994. The role of neighbours in territorial systems: when are they ‘dear enemies’? Anim Behav 47:339–350. [Google Scholar]
- Vachova H, Frynta D, 2004. Social interactions in Apodemus mystacinus: an autumnal increase of aggression at the onset of breeding. Israel J Zool 50:301–310. [Google Scholar]
- Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P, 2004. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538. [Google Scholar]
- Wang G, Liu W, Wang Y, Wan X, Zhong W, 2017. Restricted dispersal determines fine-scale spatial genetic structure of Mongolian gerbils. Curr Zool 63:687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu W, Wang G, Wan X, Zhong W, 2011a. Home-range sizes of social groups of Mongolian gerbils Meriones unguiculatus. J Arid Environ 75:132–137. [Google Scholar]
- Wang YN, Liu W, Wang GM, Zhong WQ, Wan XR, 2011b. Genetic consequences of group living in Mongolian gerbils. J Hered 102:554–561. [DOI] [PubMed] [Google Scholar]
- Waser PM, Berning ML, Pfeifer A, 2012. Mechanisms of kin discrimination inferred from pedigrees and the spatial distribution of mates. Mol Ecol 21:554–561. [DOI] [PubMed] [Google Scholar]
- Wilson DE, Reeder DM, 2005. Mammal Species of the World. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- Zhang JX, Zhang ZB, Wang ZW, 2001. Scent, social status, and reproductive condition in rat-like hamsters Cricetulus triton. Physiol Behav 74:415–420. [DOI] [PubMed] [Google Scholar]