Key Points
Practices in early-stage FL are variable and include radiation alone, systemic therapy, CMT, or observation.
Each practice resulted in similar excellent outcomes; randomized trials are required to determine the optimal treatment.
Abstract
Management practices in early-stage (I/II) follicular lymphoma (FL) are variable and include radiation (RT), systemic therapy, or combined modality therapy (CMT). There is a paucity of data regarding maintenance rituximab in this cohort. We conducted an international retrospective study of patients with newly diagnosed early-stage FL staged with positron emission tomography (PET)–computed tomography and bone marrow biopsy. Three hundred sixty-five patients (stage I, n = 221), median age 63 years, treated from 2005-2017 were included, with a median follow-up of 45 months. Management included watchful waiting (WW; n = 85) and active treatment (n = 280). The latter consisted of RT alone (n = 171) or systemic therapy (immunochemotherapy [n = 63] or CMT [n = 46]). Forty-nine systemically treated patients received maintenance rituximab; 72.7% of stage I patients received RT alone, compared to 42.6% with stage II (P < .001). Active therapies yielded comparable overall response rates (P = .87). RT alone and systemic therapy without maintenance rituximab yielded similar progression-free survival (PFS) (hazard ratio [HR], 1.32; 95% confidence interval [CI], 0.77-2.34; P = .96). Maintenance rituximab improved PFS (HR, 0.24; 95% CI, 0.095-0.64; P = .017). The incidence of transformation was lower with systemic therapy compared to RT or WW (HR, 0.20; 95% CI, 0.070-0.61; P = .034). Overall survival was similar among all practices, including WW (P = .40). In the largest comparative assessment of management practices in the modern era, variable practices each resulted in similar excellent outcomes. Randomized studies are required to determine the optimal treatment in early-stage FL.
Visual Abstract
Introduction
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma. Approximately 20% to 25% of patients with FL present with early-stage (I or II) disease.1 A range of treatments is available, and no single standard of care is agreed upon. Practices are highly variable and include watchful waiting (WW), radiation therapy (RT), systemic therapy (ie, immunochemotherapy) alone, or combined modality therapy (CMT) with both systemic therapy and RT. There is a paucity of data regarding maintenance rituximab in this cohort. There are minimal real-world comparative data to guide the management of patients with early-stage FL.
Although the National Comprehensive Cancer Network2 guidelines recommend RT alone for stage I and contiguous stage II disease, registry data indicate the use of RT is patchy and declining.3-5 FL is highly radiosensitive. RT is associated with low toxicity6,7 (particularly with modern dosimetry regimens) and achieves local disease control in >90% of patients.8 Conversely, although early-stage FL appears potentially curable with RT alone, late relapses do occur, particularly outside the radiation field.9-12 This indicates that occult lymphoma is often present at distant sites outside the RT fields and has led others to advocate CMT to provide both local control and eradication of occult disease.13 Further highlighting the lack of a standardized approach, the National LymphoCare study3 found that 30% of stage I patients are managed with a WW approach.
Additional complexity has been introduced by the application of modern staging modalities. 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) has been shown to upstage 23% to 60% of FL cases, thereby avoiding the inappropriate use of RT in this population.14-16 Furthermore, patients rigorously staged with a bone marrow biopsy and imaging (CT or PET-CT) have a superior progression-free survival (PFS) compared with non-rigorously staged patients.3 Consistent with this, a recent multicenter study of RT alone reported that the PFS for stage I/II FL patients in the PET-CT era was better than historical series,10 most likely due to stage migration and more accurate RT planning.
Due to the challenging nature of conducting randomized controlled trials (RCTs) in this setting, only 2 large-scale RCTs have ever been successfully completed. The first was the British National Lymphoma Investigation trial that showed no benefit from adding continuous low-dose chlorambucil to RT.17 It was reported in 1994 before modern staging modalities. The only relevant level 1 evidence available is the Trans-Tasman Radiation Oncology Group (TROG) 99.03 RCT that reported significantly improved PFS for CMT with CVP (cyclophosphamide, vincristine, and prednisolone) or rituximab (R)-CVP compared with RT alone.6 Because of the prolonged accrual period (2000-2012), amendments were introduced to incorporate PET-CT staging and rituximab. PET-CT staged patients had a superior PFS, likely due to stage migration. The addition of rituximab improved PFS by ∼30% (R-CVP + RT vs RT alone).
There remain minimal large-scale comparative data in the PET-CT era to guide therapeutic decision-making. The purpose of this study was to assess the clinical outcomes in a large cohort of early-stage FL patients rigorously staged with PET-CT and bone marrow biopsy in the era of rituximab.
Methods
Patients
We conducted a multicenter retrospective analysis across 13 Australian and 3 Canadian centers for patients with early-stage FL, rigorously staged with PET-CT and bone marrow biopsy. The study protocol was reviewed and approved by each institutional review board. All patients were aged ≥18 years with newly diagnosed stage I or II, grade 1-3A FL. Patients had to be staged with both PET-CT and bone marrow biopsy. Exclusion criteria were stage IE duodenal FL; grade 3B FL; composite, transformed, or a prior diagnosis of lymphoma; or follow-up for <3 months from diagnosis. Data on baseline clinical, laboratory, and radiological features and treatment received were collected at each site. Deidentified data were collated centrally. The site and number of nodal stations involved were captured as per the FL International Prognostic Index (FLIPI) project.1 Bulk was defined as ≥7 cm. Transformation was defined on histological or clinical grounds. Staging was defined according to international lymphoma criteria.18 No specific follow-up regimen was defined, and there was no predefined requirement for imaging after completion of treatment.
Statistical analysis
WW was defined by no initiation of treatment within 6 months of diagnosis. PFS was defined as the time from diagnosis until treatment failure (stable disease or progressive disease), relapse, transformation, or death from any cause. Overall survival (OS) was defined as the time from diagnosis until death from any cause. Time to chemotherapy (TTC) was assessed for WW and RT-alone patients and defined from diagnosis to first chemotherapy. FLIPI scores were calculated for all patients.1 OS following progression of disease with 24 months (POD24) of initial immunochemotherapy was assessed.19 Comparisons of baseline characteristics were performed using the appropriate parametric/nonparametric tests based on the distribution. The Kaplan-Meier method was used to estimate OS, PFS, and TTC, and survival curves were compared using the log-rank test. Hazard ratios (HRs) and associated 95% confidence intervals (CIs) were estimated using Cox proportional hazards regression analysis. Variables that showed different distribution across groups (P < .2) were included in the Cox regression models that used PFS as the dependent variable to identify potential independent prognostic factors.
Results
Patient characteristics
A total of 365 patients (stage I, 221; stage II, 144) diagnosed between 2005 and 2017 fulfilled the eligibility criteria. The median age was 63 years (range, 21-94 years); 57% were male, and B symptoms were present in 6% and bulk in 13% of patients (supplemental Table 1). The median follow-up for living patients was 45 months (range, 3-164 months). Stage II patients had a higher proportion of bulky disease (P < .001) and higher FLIPI scores (P < .001).
Management approaches and baseline characteristics
Baseline characteristics for all patients based on treatment approach are shown in Table 1. Management approaches included WW (n = 85, 23%) and active treatment (n = 280). Patients selected for WW were older compared with active treatment (median [range] 65 years [36-94] vs 60 years [22-85]; P = .005) and had more extranodal involvement (P = .022). There were no other significant differences in baseline characteristics between these patients.
Table 1.
Summary of baseline characteristics for all patients based on treatment approach for early-stage FL
| WW, n = 85 | RT, n = 171 | Systemic therapy,* n = 60 | Systemic + maintenance rituximab, n = 49 | |||||
|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | n/N | % | n/N | % | |
| Age >60 y | 59/85 | 69.4 | 101/170 | 59.4 | 27/60 | 45.0 | 29/48 | 60.4 |
| Male sex | 49/85 | 57.6 | 96/170 | 56.4 | 33/60 | 55.0 | 31/49 | 63.3 |
| ECOG PS ≥2 | 4/84 | 4.7 | 8/171 | 4.7 | 3/60 | 5.0 | 3/49 | 6.1 |
| B symptoms present | 4/85 | 4.7 | 7/167 | 4.2 | 4/60 | 6.7 | 9/49 | 18.4 |
| LoNoDiam >7 cm | 6/56 | 10.7 | 9/132 | 6.8 | 6/41 | 14.6 | 15/42 | 35.7 |
| No. of nodal sites | ||||||||
| 0 | 11/83 | 13.2 | 23/170 | 13.5 | 4/60 | 6.6 | 2/47 | 4.3 |
| 1 | 42/83 | 50.6 | 111/170 | 65.3 | 31/60 | 51.6 | 16/47 | 34.0 |
| 2 | 26/83 | 31.3 | 32/170 | 18.9 | 21/60 | 35.0 | 22/47 | 46.8 |
| 3 | 4/83 | 4.9 | 4/170 | 2.3 | 2/60 | 3.3 | 5/47 | 10.6 |
| 4 | 0/83 | 0 | 0/170 | 0 | 2/60 | 3.3 | 2/47 | 4.3 |
| Extranodal present | 19/85 | 22.3 | 29/171 | 17.0 | 7/60 | 11.7 | 8/47 | 17.0 |
| Stage II | 36/85 | 42.3 | 46/171 | 26.9 | 29/60 | 48.3 | 33/49 | 67.3 |
| Grade 3A | 11/82 | 13.4 | 26/165 | 15.8 | 13/59 | 22.0 | 6/45 | 13.3 |
| B2M >ULN | 9/49 | 18.3 | 19/68 | 27.9 | 7/41 | 17.1 | 7/28 | 25.0 |
| Hb <12 g/dL | 5/84 | 5.9 | 11/169 | 6.5 | 4/59 | 6.8 | 9/49 | 18.4 |
| LDH >ULN | 13/77 | 16.8 | 24/162 | 14.8 | 8/58 | 13.8 | 13/49 | 26.5 |
| FLIPI score | ||||||||
| Low (0-1) | 66/77 | 85.8 | 137/161 | 85.1 | 51/58 | 87.9 | 34/46 | 73.9 |
| Intermediate (2) | 10/77 | 12.9 | 24/161 | 14.9 | 6/58 | 10.4 | 10/46 | 21.7 |
| High (≥3) | 1/77 | 1.3 | 0/161 | 0 | 1/58 | 1.7 | 2/46 | 4.4 |
B2M, β2 microglobulin; ECOG PS, Eastern Cooperative Oncology Group performance status; Hb, hemoglobin; LDH, lactate dehydrogenase; LoNoDiam, largest nodal diameter; ULN, upper limit of normal.
Systemic therapy includes immunochemotherapy and CMT.
Among the 3 actively treated groups (RT alone, n = 171 [47%]; CMT, n = 46 [13%]; and immunochemotherapy only, n = 63 [17%]), several differences were present in baseline variables (supplemental Table 2). Patients treated with RT alone had less bulk, fewer nodal sites, and less B symptoms and more often had stage I disease compared with CMT and immunochemotherapy patients combined (systemic therapy). Patients receiving CMT had fewer B symptoms and lower FLIPI scores than patients receiving immunochemotherapy.
Treatment received
RT was delivered as per local guidelines. No data are available on the use of involved field vs involved site RT or on the protocols used for target volume definition, treatment verification, or quality assurance. For patients treated with RT alone, 75.2% received 24 to 30 Gy and 19% received >30 to 36 Gy (supplemental Table 3A). Doses delivered were comparable between RT alone and CMT patients. Chemotherapy regimens used were R-CVP (26.6%), R-CHOP (46.7%) (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone), bendamustine and rituximab (10.1%), and other (5.5%) (supplemental Table 3B), with no significant PFS difference among them (P = .72; supplemental Figure 1A). Rituximab monotherapy was used in 11.1%. Considering all patients treated with systemic therapy, 106/109 (97.2%) received rituximab. Maintenance rituximab was used in 49 patients (immunochemotherapy, n = 37; CMT, n = 12). No patient in the RT-alone group received maintenance rituximab.
Treatment outcomes
WW vs active treatment
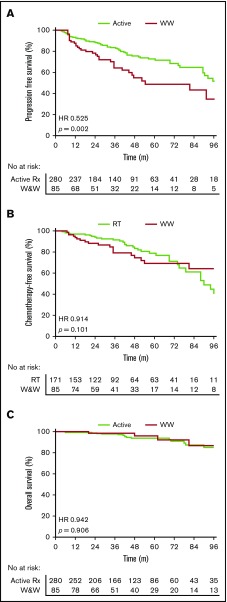
Active treatment was associated with superior PFS compared with WW (HR, 0.52; 95% CI, 0.32-0.85; P = .002) (Figure 1A). There was a marked PFS difference for stage II patients (HR, 0.39; 95% CI, 0.18-0.82; P = .002) but no significant difference for stage I patients (HR, 0.67; 95% CI, 0.35-1.27; P = .17). There was no significant difference in TTC comparing WW and RT-alone patients (HR, 0.91; 95% CI, 0.52-1.62; P = .10) (Figure 1B). OS was similar between the 2 groups (HR, 0.94; 95% CI, 0.34-2.8; P = 0.90) (Figure 1C). Five-year PFS and OS rates for the whole cohort were 66% and 94%, respectively.
Figure 1.
Outcomes with WW vs active treatment in early-stage FL. (A) PFS. (B) TTC. (C) OS.
Active treatment
First-line therapies for actively treated patients yielded comparable overall and complete response rates (overall response rate of 95%, 96%, and 95% for RT, immunochemotherapy, and CMT, respectively; P = .87) (supplemental Table 2). There was a significant difference in PFS between RT alone, immunochemotherapy, and CMT (P = .023) (supplemental Figure 1B) yet no difference in OS (P = .38). There was no difference in PFS between immunochemotherapy and CMT (HR, 1.78; 95% CI, 0.68-4.70; P = .24) (supplemental Figure 1B). Therefore, as both groups received immunochemotherapy, we combined these groups, termed systemic therapy, for subsequent analyses (supplemental Table 2).
Radiotherapy vs systemic therapy vs systemic therapy with maintenance rituximab
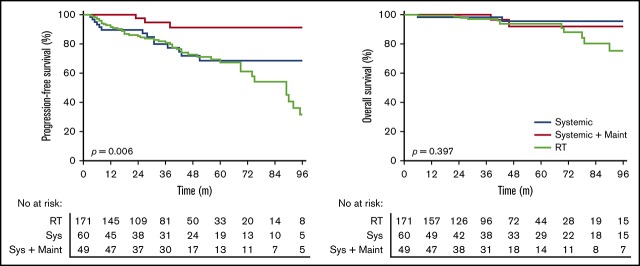
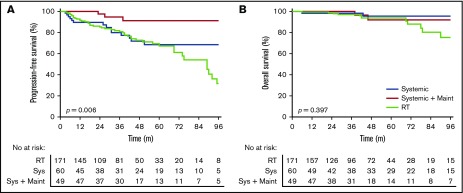
Patients treated with systemic therapy had a similar PFS (HR, 1.32; 95% CI, 0.77-2.34; P = .96) (Figure 2A) and OS (HR, 0.46; 95% CI, 0.17-1.5; P = .21) (Figure 2B) compared with patients treated with RT alone. A similar PFS was seen in stage I (HR, 0.56; 95% CI, 0.28-1.17; P = .18) and II (HR, 0.96; 95% CI, 0.39-2.32; P = .93) patients treated with systemic therapy vs RT alone.
Figure 2.
Outcomes for patients treated with radiotherapy alone, systemic therapy without maintenance rituximab, and systemic therapy with maintenance rituximab. PFS (A) and OS (B).
Systemic therapy with maintenance rituximab (24 months of once every 2 or 3 months treatment) was used in 9% of stage I and 30% of stage II patients. Patients treated with maintenance rituximab more frequently had B symptoms, bulky disease, more nodal sites, stage II disease, and lower hemoglobin compared with patients treated with RT alone or systemic therapy without maintenance rituximab (supplemental Table 4). Patients treated with systemic therapy with maintenance rituximab had a superior PFS compared with systemic therapy without maintenance rituximab (HR, 0.24; 95% CI, 0.095-0.64; P = .017) (Figure 2A) yet there was no difference in OS (HR, 0.89; 95% CI, 0.16-4.90; P = .90) (Figure 2B). A multivariate analysis for actively treated patients that included age, B symptoms, number of nodal sites, bulk, stage, FLIPI, and treatment received (RT alone, systemic therapy without maintenance rituximab, or systemic therapy with maintenance rituximab) demonstrated that only treatment with maintenance rituximab compared with RT alone significantly improved PFS (HR, 0.18; 95% CI, 0.573-59.3; P = .005).
Patterns of relapse and treatment failure
The incidence and patterns of treatment failure differed among treatment groups (Table 2). Relapse was observed in 24.6% of RT-alone patients, 18.3% of systemic therapy patients, and 4.1% of systemic therapy with maintenance rituximab patients (P = .006). Overall, 89% of relapses had distant involvement. The incidence of relapse with in-field involvement or, at the site of original disease for patients receiving systemic therapy, was 4% for RT alone, 8.3% for systemic therapy, and 0% for patients treated with systemic therapy with maintenance rituximab.
Table 2.
Incidence and patterns of treatment failure in patients among treatment approaches in early-stage FL
| RT alone, n = 171 | Systemic therapy,* n = 60 | Systemic therapy + maintenance rituximab, n = 49 | |||||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | n/N | % | P | |
| Total relapses | 42/171 | 24.6 | 11/60 | 18.3 | 2/49 | 4.1 | .006 |
| In-field relapse only | 3/171 | 1.7 | 3/60† | 5.0 | 0/49 | 0 | .254 |
| In-field and distant relapse | 4/171 | 2.3 | 2/60 | 3.3 | 0/49 | 0 | |
| Distant relapse only | 35/171 | 20.4 | 6/60 | 10.0 | 2/49 | 4.1 | |
| Transformation | 11/171 | 6.4 | 2/60 | 3.3 | 0/49 | 0 | .145 |
| Lymphoma-related mortality | 6/171 | 3.5 | 2/60 | 3.3 | 2/49 | 4.1 | .694 |
| All-cause mortality | 12 /171 | 7.0 | 3/60 | 5.0 | 2/49 | 4.1 | .976 |
Systemic therapy includes immunochemotherapy and CMT.
Includes patients treated with systemic therapy only who relapsed at the site of initial disease.
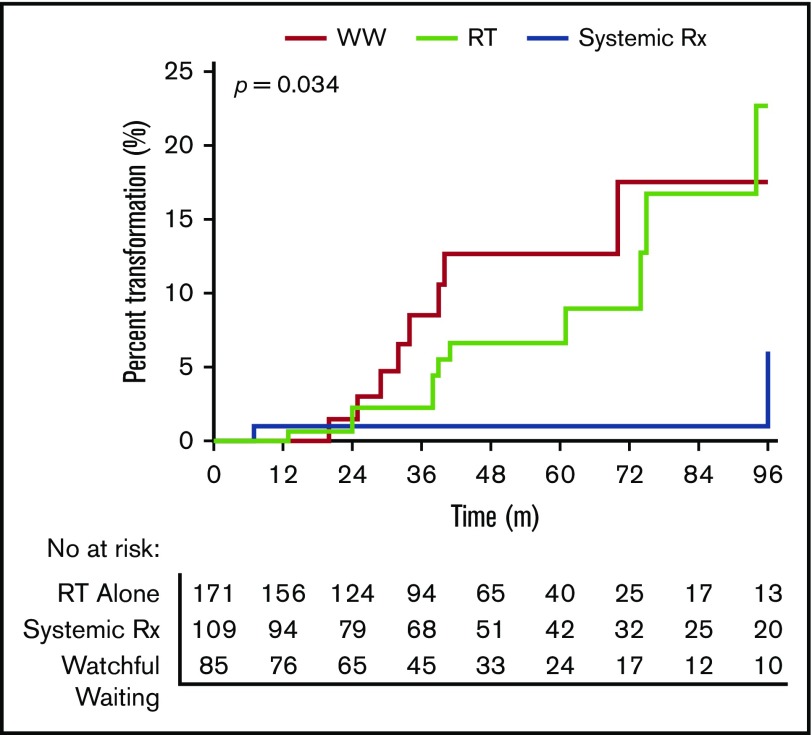
There were 21 transformation events (Figure 3). There was a significantly lower incidence of transformation in patients treated with systemic therapy (n = 2 [1.8%]) than those treated with RT (n = 11 [6.4%]) or WW (n = 8 [9.4%]) (HR, 0.20; 95% CI, 0.070-0.61; P = .034). There were no cases of transformation in patients treated with maintenance rituximab. The risk of transformation was similar between WW and RT-alone patients (P = .50). The median follow-up following transformation was 31 months. Only one patient who transformed died of disease. There were 22 deaths (5.7%) in the cohort (WW, n = 5 [5.8%], 2 lymphoma related and 3 unrelated; RT, n = 12 [7.0%], 6 lymphoma related and 6 unrelated; systemic therapy, n = 3 [5.0%], 2 lymphoma related and 1 unrelated; and systemic therapy with maintenance rituximab, n = 2 [4.1%], lymphoma related).
Figure 3.
Risk of transformation among WW, RT alone, or systemically treated therapy.
Prognostic features
Established pretherapy prognosticators in FL were not effective for identifying high-risk groups (FLIPI, P = .97; FLIPI-2, P = .29). Grade (grade 1-2 vs 3A, P = .94) was not prognostic for PFS in actively treated patients. The early progression of disease, as defined by POD24, did not identify patients with early mortality (P = .89). Clinical variables not associated with PFS on univariate analysis were age >60 years, stage, B symptoms, number of nodal sites, extranodal involvement, and β2 microglobulin. Variables associated with significantly superior PFS were Eastern Cooperative Oncology Group performance status <2 (P = .012) and the absence of bulky disease (P = .032).
Discussion
This international multicenter study of early-stage FL is, to our knowledge, the largest assessment of treatment approaches in the modern era. Only patients rigorously staged with PET-CT and bone marrow biopsy were included. RT alone and systemic therapy without maintenance rituximab produced similar PFS. In univariate and multivariate analysis, systemic therapy with maintenance rituximab was associated with significantly improved PFS compared with RT alone or systemic therapy without maintenance rituximab. Systemic therapy demonstrated a lower rate of transformation compared with RT alone. Our data demonstrate a high 5-year OS rate of 94% for these patients. The variable management practices used each resulted in similar OS. Long-term follow-up will be required to determine if patterns of disease control remain similar among treatments.
RT has traditionally been considered the standard of care for early-stage FL based on single-institution or small studies conducted in the CT era and prior to rituximab that demonstrated effective local disease control,9-12,20,21 with 10-year PFS rates of 40% to 50% in the pre-PET era. In our study, RT alone was given to 47% of patients, demonstrating widespread, but not universal, use. In these patients, results appear comparable to prior reports for response rates,7,8 low in-field recurrence,7,9,10,16 and durability of response.9,20 This highlights the effectiveness of RT for local tumor control. However, ∼23% of patients relapsed outside the field, consistent with a recent report in the PET era.10 The high rate of distant relapses with RT alone suggests the presence of occult disease below the limits of detection of PET-CT.
In contrast to RT alone, systemic therapy represents treatment of potential occult distant disease. Patients receiving systemic therapy without maintenance rituximab had similar PFS compared with patients receiving RT alone. A total of 45% of systemically treated patients received rituximab maintenance, despite this not being included in treatment guidelines. There is a paucity of data regarding maintenance rituximab in early-stage FL. Patients receiving systemic therapy with maintenance rituximab had a superior PFS compared with RT alone. The impact of maintenance rituximab remained significant after multivariable testing. This suggests that effective systemic treatment reduces the risk of relapse from occult distant disease. The benefits of maintenance rituximab occurred despite these patients having more high-risk features than those in the RT alone group. In advanced-stage FL, maintenance rituximab prolonged PFS and time to next treatment, but not OS.22 Although these findings are interesting, our numbers are small, and findings should not be overinterpreted. Large-scale RCTs with long follow-up are required to establish the role of rituximab maintenance in early-stage FL.
The recent TROG 99.03 study demonstrated superior PFS for patients randomized to RT plus R-CVP compared with RT alone.6 The long accrual (2000-2012) and follow-up period (median 9.6 years) enabled the introduction of PET-CT and rituximab to be examined. The randomized nature of the study minimized bias and allowed the significance of these changes to be interpreted. The addition of rituximab to CVP improved 5-year PFS by ∼30% compared with RT alone. PET-CT-staged patients had superior PFS, likely due to stage migration. This provides level 1 evidence that systemic therapy plus RT (CMT) is more effective than RT alone. Recent multicenter prospective phase 2 data also demonstrate that rituximab combined with RT is highly effective and well tolerated.23 In our study, all patients underwent PET-CT staging, and rituximab was almost universally used with systemic therapy.
Reflecting the nature of our study, one weakness is the variety of systemic regimens used, which ranged from R-CVP to rituximab in combination with an anthracycline or bendamustine. RT was combined with systemic therapy in only 42% of systemically treated patients, and the fields used were not standardized. Further studies are required to more fully delineate the role of RT with systemic therapy and the optimal immunochemotherapy backbone.
The concept of WW was developed from RCTs in advanced-stage FL that demonstrated that earlier initiation of therapy did not improve OS compared with delayed initiation.24,25 To our knowledge, a similar RCT in early-stage FL has not been conducted. In early-stage FL, an analysis of 43 patients from Stanford University reported comparable OS to immediate treatment, and with a median follow-up of ∼7 years, 63% of patients had not required chemotherapy.26 Similarly, the LymphoCare study and a French analysis suggested that WW was an acceptable approach.3,27 Conversely, the Surveillance, Epidemiology, and End Results analysis of 6568 patients concluded that OS with RT was superior compared with WW.5
Our data demonstrate that active treatment was associated with superior PFS compared with WW. This is expected, as WW is a management strategy and not a treatment. Conversely, WW patients had a similar TTC compared with RT-alone patients, with ∼70% of WW patients not requiring treatment with immunochemotherapy at 5 years. WW was associated with a similar rate of transformation to RT alone but higher than systemic therapy. WW patients were significantly older. Based on our comparable OS between WW and actively treated patients, WW could be considered as an initial management strategy in early-stage FL; however, long-term follow-up is required to determine if a survival benefit exists favoring active treatment. It must be considered that this was not an intention-to-treat analysis, and patients initially managed with WW but then treated within 6 months would be considered to have received active treatment, leading to immortal time bias.
Our conclusions about the effectiveness of treatments are drawn from retrospective data with the nonrandom allocation of treatment. Unknown confounding variables among patients in treatment groups may have affected results. However, few studies have evaluated management practices in early-stage FL in a comparative approach. Transformation was defined on clinical or histological grounds. There was no predefined requirement for imaging after completion of treatment. Consequently, PFS data are not directly comparable with the TROG 99.03 study, where mandated annual CT scans could detect asymptomatic relapses. Median follow-up was also significantly shorter compared with the TROG 99.03 study (45 months vs 9.6 years), making an estimate of the “cured” population less reliable. Conversely, the natural course of early-stage FL and long follow-up required are barriers to conducting randomized trials. As such, real-world data will provide valuable insights in management.
A number of treatment options are feasible in early-stage FL. Although our data and the TROG 99.03 study demonstrate superior PFS with systemic therapy, these patients have been exposed to the acute and long-term toxicities of chemotherapy. Conversely, grade >2 early and late RT-related toxicity rates are low,6,7 consistent with good tolerability, and RT does not appear to be associated with a long-term increased risk of second cancer compared with the general population.21 Our data and previous reports have not demonstrated that systemic therapy or CMT improves OS compared with RT alone.3,6
Current pre- and posttherapy prognostic markers have been developed exclusively or predominantly in advanced-stage cohorts to identify high-risk patients. In our cohort, FLIPI, FLIPI-2, and POD24 were unable to identify early-stage FL patients experiencing early progression, and grade (1-2 vs 3A) was not prognostic. Integrated molecular biomarkers28-30 have augmented the prognostication of advanced-stage FL and represent an attractive prospect to aid treatment stratification in early-stage FL. Posttherapy PET-CT assessment and minimal residual disease monitoring (eg, cell-free DNA) may aid a risk-stratified approach and should be evaluated in early-stage FL.
In summary, we present the largest real-world comparative assessment of management practices in early-stage FL in the modern era. Our data represents one of the first assessments of outcomes for patients with early-stage FL treated with maintenance rituximab. RT alone became the standard of care prior to the availability of rituximab. Immunochemotherapy is active in early-stage FL, as evidenced by the randomized TROG study that demonstrated that RT followed by systemic therapy was more effective than RT alone. Our data demonstrate that systemic therapy followed by maintenance rituximab was the most effective therapy; however, the retrospective nature of this study limits the ability to make a definitive recommendation regarding therapy. Longer follow-up for our study and the TROG study is required to determine if a PFS benefit for systemic therapy with or without maintenance rituximab translates into improved OS. With available follow-up, all patients, including WW patients, had a similar OS, underscoring the indolent nature of early-stage FL. Taken together, our data and the TROG study challenge the paradigm that RT alone is the standard of care. Randomized trials are required to determine the optimal systemic regimen either with or without RT for early-stage FL.
Supplementary Material
The full-text version of this article contains a data supplement.
Authorship
Contribution: J.W.D.T. and G.H. were responsible for study conception and design; J.W.D.T., G.R., K.C., L.C., D.H., S.B., C.D., J.G., E.S.T., X.T., J.W., J.P., R.C., S.T., S.S., M.N., T.C., C.S.T., E.A., E.H., G.H., R.K., D.T., M.G., A.M.J., K.J.S., D.V., K.M., S.R., W.J., R.K., C.Y.C., and G.H. collected and assembled data; J.W.D.T., M.M., N.M., P.M., M.K.G., and G.H. analyzed and interpreted data; and all authors wrote the manuscript and approved the final version.
Conflict-of-interest disclosure: E.H. has received honoraria/speaker’s fees from Roche, Janssen, Takeda, and Bristol-Myers Squibb; is on the advisory boards of Janssen, Celgene, Merck Sharpe Dohme, and Roche; has received research funding from Bristol-Myers Squibb, Celgene, Merck Sharpe Dohme, and Astra Zeneca; and has received travel expenses from Takeda, Roche, and Janssen. A.M.J. has received honoraria from Janssen; is on the advisory boards of Roche, Janssen, and Merck Sharpe Dohme; and has received travel support from Roche. K.J.S. has received honoraria from Roche, Bristol-Myers Squibb, Merck Sharpe Dohme, Seattle Genetics, Abbvie, and Takeda. D.V. has received honoraria from Roche, Janssen, Lundbeck, Celgene, AstraZeneca, Merck, Gilead, Novartis, and Seattle Genetics. R.K. has received travel support from Roche. C.Y.C. has received speaker’s fees, honoraria, and research funding from, and is on the advisory board of, Roche. P.M. is on the advisory boards of Celgene, Janssen and Amgen, and Pfizer and has received trial support from Janssen. D.T. has received honoraria from Janssen, Novartis, Roche, and Amgen; has received speaker’s fees from Janssen and Roche; is on the boards or advisory committees of Amgen, Janssen, and Roche; and has received research funding from Amgen. The remaining authors declare no competing financial interest.
Correspondence: Greg Hapgood, Princess Alexandra Hospital, 199 Ipswich Rd, Woolloongabba, Brisbane, QLD 4102, Australia; e-mail: greg.hapgood@health.qld.gov.au.
References
- 1.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258-1265. [DOI] [PubMed] [Google Scholar]
- 2.National Clinical Cancer Network. Clinical practice guidelines in oncology: B-cell lymphomas. 2018; version 4. Available at: https://www.nccn.org/. Accessed 11 January 2019.
- 3.Friedberg JW, Byrtek M, Link BK, et al. Effectiveness of first-line management strategies for stage I follicular lymphoma: analysis of the National LymphoCare Study. J Clin Oncol. 2012;30(27):3368-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargo JA, Gill BS, Balasubramani GK, Beriwal S. What is the optimal management of early-stage low-grade follicular lymphoma in the modern era? Cancer. 2015;121(18):3325-3334. [DOI] [PubMed] [Google Scholar]
- 5.Pugh TJ, Ballonoff A, Newman F, Rabinovitch R. Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2010;116(16):3843-3851. [DOI] [PubMed] [Google Scholar]
- 6.MacManus M, Fisher R, Roos D, et al. Randomized trial of systemic therapy after involved-field radiotherapy in patients with early-stage follicular lymphoma: TROG 99.03. J Clin Oncol. 2018;36(29):2918-2925. [DOI] [PubMed] [Google Scholar]
- 7.Hoskin PJ, Kirkwood AA, Popova B, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol. 2014;15(4):457-463. [DOI] [PubMed] [Google Scholar]
- 8.Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100(1):86-92. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BA, Voss N, Woods R, et al. Long-term outcomes for patients with limited stage follicular lymphoma: involved regional radiotherapy versus involved node radiotherapy. Cancer. 2010;116(16):3797-3806. [DOI] [PubMed] [Google Scholar]
- 10.Brady JL, Binkley MS, Hajj C, et al. Definitive radiotherapy for localized follicular lymphoma staged by 18F-FDG PET-CT: a collaborative study by ILROG. Blood. 2019;133(3):237-245. [DOI] [PubMed] [Google Scholar]
- 11.Spry NA, Lamb DS, Vaughan Hudson G, Easterling MJ, MacLennan KA, Jelliffe AM. Localized grade I non-Hodgkin’s lymphoma: results of treatment with radiotherapy alone in 88 patients. Clin Oncol (R Coll Radiol). 1989;1(1):33-38. [DOI] [PubMed] [Google Scholar]
- 12.Barzenje DA, Cvancarova Småstuen M, Liestøl K, et al. Radiotherapy compared to other strategies in the treatment of stage I/II follicular lymphoma: a study of 404 patients with a median follow-up of 15 years. PLoS One. 2015;10(7):e0131158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour JF, Pro B, Fuller LM, et al. Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21(11):2115-2122. [DOI] [PubMed] [Google Scholar]
- 14.Metser U, Dudebout J, Baetz T, et al. [18 F]-FDG PET/CT in the staging and management of indolent lymphoma: a prospective multicenter PET registry study. Cancer. 2017;123(15):2860-2866. [DOI] [PubMed] [Google Scholar]
- 15.Luminari S, Biasoli I, Arcaini L, et al. The use of FDG-PET in the initial staging of 142 patients with follicular lymphoma: a retrospective study from the FOLL05 randomized trial of the Fondazione Italiana Linfomi. Ann Oncol. 2013;24(8):2108-2112. [DOI] [PubMed] [Google Scholar]
- 16.Ng SP, Khor R, Bressel M, et al. Outcome of patients with early-stage follicular lymphoma staged with 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET) and treated with radiotherapy alone. Eur J Nucl Med Mol Imaging. 2019;46(1):80-86. [DOI] [PubMed] [Google Scholar]
- 17.Kelsey SM, Newland AC, Hudson GV, Jelliffe AM. A British National Lymphoma Investigation randomised trial of single agent chlorambucil plus radiotherapy versus radiotherapy alone in low grade, localised non-Hodgkins lymphoma. Med Oncol. 1994;11(1):19-25. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mac Manus MP, Hoppe RT. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J Clin Oncol. 1996;14(4):1282-1290. [DOI] [PubMed] [Google Scholar]
- 21.Guadagnolo BA, Li S, Neuberg D, et al. Long-term outcome and mortality trends in early-stage, Grade 1-2 follicular lymphoma treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64(3):928-934. [DOI] [PubMed] [Google Scholar]
- 22.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42-51. [DOI] [PubMed] [Google Scholar]
- 23.Herfarth K, Borchmann P, Schnaidt S, et al. Rituximab with involved field irradiation for early-stage nodal follicular lymphoma. Results of the MIR study. Hemasphere. 2018;2(6):e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 1997;15(3):1110-1117. [DOI] [PubMed] [Google Scholar]
- 25.Ardeshna KM, Smith P, Norton A, et al. ; British National Lymphoma Investigation . Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003;362(9383):516-522. [DOI] [PubMed] [Google Scholar]
- 26.Advani R, Rosenberg SA, Horning SJ. Stage I and II follicular non-Hodgkin’s lymphoma: long-term follow-up of no initial therapy. J Clin Oncol. 2004;22(8):1454-1459. [DOI] [PubMed] [Google Scholar]
- 27.Michallet AS, Lebras LL, Bauwens DD, et al. Early stage follicular lymphoma: what is the clinical impact of the first-line treatment strategy? J Hematol Oncol. 2013;6(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159-2169. [DOI] [PubMed] [Google Scholar]
- 29.Pastore A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015;16(9):1111-1122. [DOI] [PubMed] [Google Scholar]
- 30.Tobin JWD, Mollee P, Birch S. The tumor microenvironment is independently prognostic of conventional and clinicogenetic risk models in follicular lymphoma [abstract]. Blood. 2017;130(suppl 1). Abstract 728. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.