Abstract
Brain damage after hypoxia-ischemia (HI) occurs in an age-dependent manner. Neuroprotective strategies assumed to be effective in the adult might have deleterious effects in the immature brain. In order to create effective therapies, the complex pathophysiology of HI in developing brain requires exploring new mechanisms. Critical determinants of neuronal survival after HI are the extent of vascular dysfunction, inflammation, and oxidative stress, followed later by tissue repair. The key enzyme of these processes in human body is arginase (ARG) that acts via bioavailability of nitric oxide, and synthesis of polyamines and proline. ARG is expressed throughout the brain in different cells, however, little is known about the effect of ARG in pathophysiological states of brain, especially hypoxia-ischemia. Here, we summarize the role of ARG during neurodevelopment, as well as in various brain pathologies.
Keywords: arginase, hypoxia-ischemia, neuroinflammation, neonatal brain
Introduction
Hypoxic-ischemic (HI) brain injury accounts for a significant proportion of mortality and long term disability in children, affecting 0.7–1.2 million infants annually [1]. While progress in respiratory and intensive care technology has greatly improved survival rates, the incidence of motor and cognitive disorders linked to perinatal and early postnatal brain injury has actually increased in the last two decades [2]. Despite the significant socio-economic burden of neonatal hypoxia-ischemia (HI), currently there are very few preventative and/or protective therapies available for patients who have suffered brain injury from HI with only one treatment licensed for use, hypothermia. The etiology of HI is complex, with a clear initial primary insult phase followed by a more delayed, secondary injury phase that can continue for long periods of time. While the initial, primary insult contributes to neuronal cell death, the degree of secondary injury induced by inflammation and oxidative stress is perhaps the more critical determinant of neuronal survival. Many factors contribute to cell death in this extended injury phase [3] and given its duration, this phase can easily be considered the most viable time period to initiate effective therapy.
Arginases (ARG) are enzymes expressed throughout the brain in two known isoforms and have conventionally been studied in their role as the penultimate step in the urea cycle [4]. However, more recent evidence reveals the breadth of different roles that ARG play, particularly in various disease states in both beneficial and detrimental ways [5]. Given that ARG are among the most acutely upregulated enzymes in ischemic injury models (cardiac [6], liver [7], retina[8]) and are key regulatory enzymes of many inflammatory states, elucidating the molecular mechanisms of the ARG pathway will enable the development of more effective therapies to improve outcome. We speculate that it is ultimately the complex interplay between the individual characteristics of the ARG isoenzymes, their interactions with other co-regulators, and their temporal expression patterns that determine the specific effects of ARG, particularly in the CNS after HI injury.
1. Arginase
1.1. ARG isoforms and expression
ARG is expressed in two isoforms, arginase-1 (ARG-1) and 2 (ARG-2) that differ in their genetic coding, subcellular localization, tissue distribution, immunological cross-reactivity, molecular regulation and function [9,10]. ARG-1 is a cytosolic homotrimer enzyme with a 35 kDa subunit [9] encoded by the arg1 gene located on chromosome 6q23 [11]. It is constitutively and abundantly expressed in the liver and some ARG-1 expression has also been reported in several extra-hepatic tissues such as brain, stomach, pancreas, and lung [10,12]. The second isoform, ARG-2, is a 40 kDa mitochondrial enzyme encoded by the arg2 gene located on chromosome 14q24.1–24.3 [13]. Unlike ARG-1, ARG-2 is confined mainly to the kidney, brain, prostate, intestine, and the pancreas [10,12–14]. Both isoforms of ARG share a similar structure, with more than 50 % homology of their amino acid residues with 100 % homology in the areas critical for their L-arginine metabolizing function [13–15], suggesting an overlap in their metabolizing functions. The individual functional impact of these isoforms however is highly specific to cell and organ types.
1.2. ARG functions
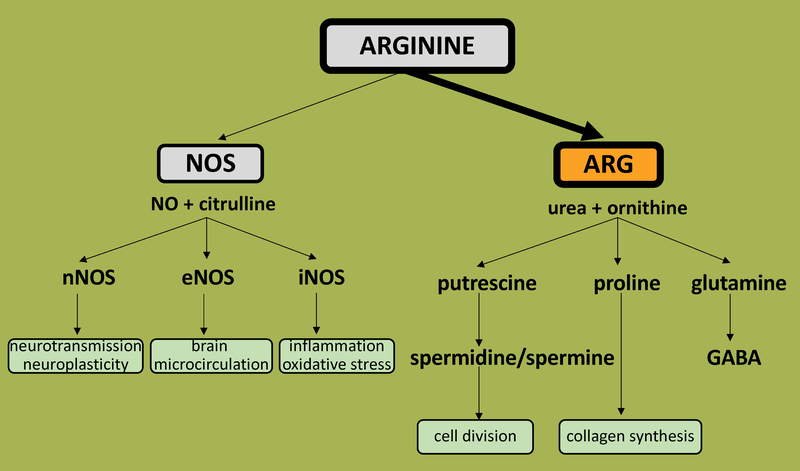
ARG is a metalloenzyme involved in the urea cycle, a series of biochemical reactions that produce urea from ammonia for excretion. Specifically, it is involved in the penultimate step which is the hydrolysis of the semi-essential amino acid L-arginine to form L- ornithine and urea [4]. The breakdown of L-arginine has important regulatory roles in formation of active biomolecules that include nitric oxide (NO), citrulline, polyamines, and proline, among others (Fig. 1).
Figure 1: Schematic diagram of relationship between ARG and NO-synthase isoforms in brain.
NOS- nitric oxide synthase, ARG- arginase, eNOS- endothelial NO-synthase, iNOS-inducible NO-synthase, nNOS- neuronal NO-synthase, ARG-arginase, NO-nitric oxide, GABA-gamma-aminobuthytic acid
The primary function of ARG-1 is detoxification by removing excessive nitrogen produced from amino acid metabolism through the hepatic urea cycle [4]. ARG-1 knockout mice exhibit severe symptoms of hyperammonemia and do not survive past P10-P14 [16] while patients with genetic mutation-induced ARG-1 deficiency demonstrate hyperammonemia, and symptoms that mostly involve nervous system like progressive spastic paraparesis, epileptic seizures and psychomotor and growth retardation [17]. On the other hand, ARG-1 overexpression or increase in ARG activity have been associated with various complex disease states, implicating its role as a key regulatory enzyme in inflammation [18] and repair [19]: pulmonary disease [20], diabetes [21], carcinogenesis [22], hypoxic-ischemic and reperfusion injury in different organs [23–28] as well as cardiovascular disease [23,29].
While the overall role of ARG-2 has remained unknown for some time, more recent studies have begun to uncover its role in vascular endothelial damage via oxidative stress [30–32] and mitochondrial dysfunction [33]. Identified as the predominant isozyme in human and mouse endothelial cells [34,35], ARG-2 is implicated in the regulation of endothelial senescence [30,35] as well as function in many disease states [34,36,37]. Specifically, ARG-2 hydrolyzes L-arginine in vascular endothelial cells, limiting its availability for the generation of NO via eNOS resulting in vascular endothelial dysfunction [38], oxidative stress and enhanced expression of endothelial inflammatory adhesion molecules [30]. Loss of both genes for ARG-2 in ARG-2 knockout mice leads to systemic hypertension phenotype with blunted response to vasoconstriction [39] highlighting a prominent role for ARG-2 in endothelial dysfunction.
The discovery of ARG-1 and ARG-2 expression in the brain has raised the question of whether complete urea cycle occurs in brain and what its role might be. Previous conceptions claimed that the complete urea cycle may not occur in the brain, however many recent reports from Alzheimer’s and Huntington disease studies have now established the presence of elevated urea levels and urea cycle genes in the brain [40,41]. These findings have garnered much interest in the role of ARG in the brain and studies have started focusing on the regulation of ARG and other elements in specific disease states and how modulation of members of the urea cycle may affect these disease states. The data on the role of ARG after brain HI is limited, and some is conflicting. However, all recent studies point out that ARG responds to HI conditions in brain and may play pivotal role in neural injury, especially via NO-pathway and repair via ARG effect on polyamine synthesis.
1.2.1. ARG and the NO pathway
NO is a well-known signaling molecule of great interest, particularly in the CNS. It is produced by NO-synthase (NOS), an enzyme with many cellular isoforms that regulate its expression and function based on cellular location. Neuronal nNOS (or NOS-1) and endothelial eNOS (or NOS-3) are commonly associated with the nanomolar levels of NO production that mediate intracellular signaling processes or vascular homeostasis respectively [42]. Inducible NOS (iNOS/NOS-2), produces high levels of NO in the micromolar range and plays an important role in tissue inflammation and host defenses [43]. The substrate for NOS activity and NO formation is L-arginine and consequently, NOS function is reciprocally regulated by ARG via substrate depletion [42]. Interestingly, although the affinity of L-arginine is much higher for purified NOS (Km ~ 2–20 μM) than for ARG (Km ~ 1–5 mM), as the maximum activity of ARG is more than 1,000 times that of NOS, similar rates of substrate utilization occur at physiologic L-arginine concentrations [15]. Several findings suggest that the limitation of NO production by ARG, and therefore its physiologic function, is critically dependent on the particular combination of ARG isoform with NOS isoform. Upregulation of either ARG-1 or ARG-2 is effective in limiting NO production by iNOS but considering the rate of L-arginine utilization by nNOS is much lower than that for iNOS a similar outcome cannot be generalized for nNOS [42]. The decrease in nitrite production by nNOS correlates with an increase of cytosolic ARG-1 activity but not with mitochondrial ARG-2 activity [42]. For eNOS however, the overexpression of ARG-2 is able to inhibit NO production, although the effect is smaller than that of transgene ARG-1 [44], highlighting the specificity of enzyme function based on isoform and location. Interestingly, the intermediate in NO synthesis, Nω-hydroxy-L-arginine, as well as ARG product L- ornithine decrease ARG activity, suggesting the presence of multiple factor-regulation of the ARG-NOS interactions and metabolic pathways [20].
1.2.2. ARG and the polyamine and proline pathways
ARG effects on polyamine or proline synthesis are dependent on the subcellular co-localization of the ARG isoforms with the enzymes of the polyamine or proline pathways. The co-localization of ARG-1 with ornithine decarboxylase in the cytosol directs ornithine, a product of ARG metabolism, towards polyamine synthesis [44]. In contrast, when ARG-2 is co-localized in mitochondria with ornithine aminotransferase, ornithine preferentially forms proline and glutamate [44]. Considering ornithine is a precursor for neurotransmitter synthesis of both glutamate and GABA [45], cellular localization and activities of these 2 isoforms could play major roles in mediating neurotransmitter levels and subsequent responses to injury processes. However, in ARG-1−/− mice, while there is a mild decrease in net brain glutamine compared to littermate controls, GABA levels are unchanged [46] suggesting additional regulatory mechanisms, rather than ARG-1 alone. Polyamines (namely putrescine, spermidine and spermine) are intracellular and interact with nucleotides and phospholipids and consequently are involved in many functions related to cell survival, proliferation, maturation, and neurite growth, among other functions [47,48]. Polyamines could also exert harmful effects, such as neuronal damage from excitotoxicity via overactivation of NMDA-receptors [49]. Therefore, depending on the context, ARG may have beneficial or detrimental role via modulation of excitotoxicity or tissue repair.
2. ARG pathway in brain
2.1. ARG localization in brain
Within the CNS, the two isoforms of ARG differ in their regional, cellular, and subcellular expression patterns. ARG-1 and ARG-2 are both expressed throughout the brain of chickens [50] and rodents [12], with ARG-1 expressed at higher levels than ARG-2 [12]. In rat, expression of ARG-1 and ARG-2 was detected in the cortex, hippocampus, thalamus, basal ganglia, cerebellum, brainstem, and spinal cord, among other substructures [10,12,51–53]. Within these regions, ARG has been noted to be localized to variable cellular subtypes. In hippocampus, ARG expression is found in both excitatory and inhibitory neurons [51] and in the cerebellum, expression was noted in basket, stellate, Golgi cells and Purkinje cells [51,52]. Although some initial studies suggested ARG expression is confined to neurons [12], further studies clarified both isoforms to be expressed in glia, including oligodendrocytes and microglia [10,51,53]; and astrocytes [54]. It remains to be elucidated which cells represent the major sources of ARG in HI conditions of the brain and what changes occur in terms of ARG activity and expression as the injury evolves.
2.2. ARG spatiotemporal changes in brain with age
Damage in the neonatal brain after HI is both region- and cell- specific [3] and involves development-specific processes markedly different from an adult brain [55]. Therefore, understanding spatiotemporal changes of ARG in development is critical.
Both ARG expression as well as activity undergo spatiotemporal changes with age. ARG-1 expression in murine brain, and in ganglion cells of the peripheral nervous system have been noticed as early as embryonic life; positive staining is seen from E13 to P1, peaking from E15 to E17 [56]. Expression is evident in the cervical, thoracic and lumbar dorsal root ganglia, confined to sensory neuron cell bodies from E13 to P1, in the vagal nucleus, as well as in the medulla at E17 [56]. Relatively high expression of ARG-1 was found in microglial cells early in the postnatal period (P3), which diminished by 70 % at P21, and by more than 90 % at 12 months [57]. This pattern paralleled that of microglial iNOS, suggesting a temporal link between two enzymes that utilize L-arginine, though the details of these interactions in microglial development are yet to be elucidated [57]. ARG-2 expression on the other hand, is undetectable at the earlier ages (E13-P1) [56] but evident in adulthood [12,53]. Similarly, in chicks, ARG expression peaks in brain and retina shortly after the chicks hatch and then slowly declines by adulthood [50].
ARG activity appears to change in association with changes in brain molecular structure and function. In rats, gross ARG brain activity is highest in fetal and neonatal brains and then decreases by fourfold by adulthood [58]. In young adult rats, ARG activity is highest in the postrhinal cortex and in subregions of hippocampus, particularly in the dentate gyrus, followed by CA2/3, then followed by CA 1. Lower ARG activity has been found in the temporal cortex, and lowest in the entorhinal and perirhinal cortices [59,60]. There are no significant differences in ARG activity or expression in prefrontal cortex in young compared to aged rats [61], however ARG activity in the postrhinal cortex is decreased [59,60]. These findings conflict somewhat with another study by the same group that found a significant increase in ARG activity only in the perirhinal cortex of aged rats [62]. Similar findings of increases in ARG activity was described in an animal model comparing the activities of ARG in young 1 month old with 14 months old mice [63]. Activity does not, however, appear to correlate with expression levels, as highest expression of ARG-1 is noted in postrhinal and perirhinal cortices and significantly lower ARG-1 expression is found in entorhinal cortex, dorsal hippocampus, and temporal cortex [60]. ARG-2 expression, in contrast, does not vary by region. As the mice age, there is no change in expression of either isoform ARG-1 or ARG-2 noted across hippocampal regions [60], however there is a clear temporal pattern for hippocampal ARG activity, which decreases with age in CA1 and CA2/3, but not the dentate gyrus [60].
These studies draw attention to the fact that spatiotemporal and age-dependent variations in ARG expression and activity clearly exist in the brain and are perhaps indicative of the differential regulation of each of these regions during development and conceivably, post injury.
2.3. ARG role in neurodevelopment
While several studies have now established the importance of ARG and its downstream products in embryogenesis and placental development, few have specifically investigated the role of ARG in early neurodevelopment.
ARG activity is noted to be highest during the periods of highest protein and polyamine synthesis [58,64,65], suggesting a pivotal role for this enzyme starting early in development. ARG knockout animals exhibit decreased dendritic complexity, intrinsic excitability, synapse number, and functional synaptic deficits consistent with the anatomical changes observed with an unexpected gradation of abnormalities based on whether this is a single-copy or double-copy loss of Arg1. Gene therapy with Arg1 at neonatal stages rescues nearly all of these abnormalities [66]. Intriguingly however, neural stem cells isolated from the germinal zones of ARG-1 knockout embryos were capable of differentiating into neurons, oligodendrocytes, and astrocytes [67]. They appeared to mature more rapidly than wild type and heterozygous stem cells, elaborating longer, with more complex neurites, and more frequently expressing mature neuronal markers, such as β3-tubulin and neurofilament [67]. It is possible that a compensatory upregulation of agmatinase and spermine synthase in ARG-1 knockout neural stem cells may upregulate alternative polyamine synthesis pathways and intermediate substrates, circumventing the loss of ARG-1 to continue polyamine-dependent proliferation [67]. Together, it appears that while alternative pathways of polyamine synthesis may enable anatomical differentiation and maturation of the neurons in states of ARG deficiency, ARG is still necessary for the formation of the dendritic network and synaptic connections.
In early neurons, the strict temporal control of neuronal ARG expression appears to have developmental consequences for axon growth. It has been shown in cultured dorsal root ganglion (DRG) neurons, [47] that ARG levels are initially high in young DRG neurons, which correlates with myelin-induced promotion of axon growth early in development. However, with a predictably timed drop in ARG activity at P4, the effect of myelin rapidly switches to inhibit axon growth [47]. It was suggested that neurotrophin signaling elevates cAMP levels, activating transcription of ARG-1, which then indirectly increases levels of polyamines leading to axon growth by overcoming the inhibitory signals of myelin [47]. When cAMP spontaneously drops at age P4, this molecular pathway is downregulated and inhibitory signaling from myelin prevents further axon elaboration [47]. ARG-1 is thus thought to play a fundamental role in the promotion of axon growth, by improving their ability to overcome actions of inhibitory factors such as myelin-associated glycoprotein (MAG) which inhibits axon growth in late postnatal neuronal development and also after CNS injury [47]. Overexpression of ARG-1 or the addition of polyamines both enhanced axon growth in cultured cerebellar neurons that were grown on myelin [47], while inhibitors of ARG and ornithine decarboxylase blocked axon growth, presumably by preventing the conversion of arginine to ornithine or ornithine to polyamines, respectively [47]. ARG clearly has a critical role in neurodevelopment, neurogenesis and axonal repair mechanisms. As such, understanding the developmental patterns and modulation of this pathway following injury and especially after HI are critical first steps towards developing effective therapeutic strategies.
3. ARG response after brain injury
3.1. ARG changes in expression and activity
To date, three phases of injury progression have been described after neonatal brain HI: the acute primary energy failure due to the HI insult; a secondary subacute phase, which is a consequence of reoxygenation and reperfusion and, finally, a tertiary chronic phase in which previous events can get worse and inflammation becomes chronic [68]. ARG appears to respond to all phases by changes in its activity and expression.
In a rat model of anoxia-hypoxia ARG expression, while unchanged during periods of anoxia, increased significantly during the reperfusion phase 5 days after the insult [69]. Similarly, Quirie et al., [70], using a rat photothrombotic model of brain ischemia observed that while ARG-2 isoform was not modified by the injury, ARG-1 expression increased at the injury site on day 8, peaking on day 15. They also noted a 29 % decrease in ARG-1 activity compared to controls, postulated to be due to possible delayed expression of endogenous ARG inhibitors. Another similar report (Hamzei Taj et al.) showed ARG-1 expression to be strongest within the first week after middle cerebral artery occlusion (MCAO), decreasing by the second week [71]. Another study observed that higher ARG-1 activity still persists on day 90 after injury suggesting ARG-1 remains elevated for a longer period of time [26].
In terms of spatiotemporal expression after an ischemic insult, increased ARG-1 expression localized to activated microglia within the lesion core, whereas the neuronal ARG-1 expression was strongest in cortical neurons located in the non-affected hemisphere. A smaller degree of the ARG-1 expression was found also in astrocytes sporadically located within the glial scar [71]. In a photothrombotic model of ischemia, ARG-1 was higher in activated macrophages, neurons and astrocytes in the area of the lesion [70]. Interestingly, ARG-1 expression in astrocytes followed a similar pattern to that of brain derived-neurotrophic factor (BDNF), indicating a possible role of ARG in BDNF regulation, neuronal survival, growth and neuroplasticity [70,72]
It appears that the observed differences in ARG localization, expression and activity in response to brain HI are age and animal model- dependent, making it vital to extrapolate findings in a similarly appropriate manner to ensure accurate understanding (Tab.1). This understanding of the dynamics of ARG in response to brain HI insults and its role in the pathophysiology of the injury at particular time points has important implications for appropriate therapeutic targeting of ARG pathway. In the immediate phase of injury, it may be the influence of ARG on the NO pathway that plays a significant role. While the effects of ARG inhibition might be detrimental by increasing the availability of L-arginine for iNOS and nNOS as previously stated, it is possible that it is also beneficial via improvement in cerebral perfusion and attenuation of vascular oxidant stress via the eNOS pathway. We postulate that inhibition of ARG in the acute phase of brain HI may decrease neurotoxicity via decreasing polyamine synthesis. However, ARG inhibition might not be favorable during the later stages of injury, since polyamines and proline participate in tissue repair.
3.2. ARG regulation in HI environment
HI creates a molecular environment of vascular dysregulation, oxidant stress, inflammation, and excitotoxicity that lead to progressive damage over a long period of time [3]. This injury is a critical determinant of permanent neuronal loss and poor clinical neurological outcomes. Various mechanisms activated in response to neonatal brain HI alter the ARG metabolic pathway.
3.2.1. Hypoxia
The hypoxic environment induces both ARG expression and activity [73]. ARG-2 is stimulated by HIF-2α [74], and HIF-2α is one of the predominant adaptive responders to acute brain hypoxia in the oxygen deprived environment after stroke [75]. Hypoxic upregulation of ARG-2 expression has been shown to also occur via AMPKα1-signaling [76], micro RNAs [77], PI3K-Akt pathway [78], direct induction of the ARG-2 promoter over ARG-1 [79], and activation of the extracellular signal-regulated kinase or epidermal growth factor receptor (EGFR) tyrosine kinase which stimulate both ARG-1 mRNA and ARG2 mRNA expression [80–82]. Interestingly however, ARG-2 mRNA was suppressed > 50% by O2 deprivation [83], suggesting that the exact influence of hypoxia on ARG in the brain remains to be clarified further.
3.2.2. Ischemia and reperfusion injury
ARG is upregulated during ischemia-reperfusion and whether this is protective or detrimental is organ-dependent. ARG-1 is one of the most abundant and fastest upregulated genes [6,84] with increased activity [85–87] following ischemia-reperfusion injury in models of myocardial infarction. This upregulation has been shown to be largely detrimental, mostly likely via decreasing NO levels [87] and bioavailability [88]. The transcription factor forkhead box O4 (FoxO4) [89] and TNF-α [90] have both been implicated in mediating this upregulation. In retinal ischemia-reperfusion injury models, ARG-2 deletion leads to significantly reduced glial activation, reactive oxygen species formation and cell death by necroptosis leading to decreased ganglion cell loss, microvascular degeneration and preserved retinal morphology [8]. Similarly, in the small intestine [91] and liver [92], ARG blockade been shown to increase L-arginine bioavailability resulting in less injury in the latter following ischemia-reperfusion. Essentially, in reperfusion models of organs other than the brain, ARG upregulation following injury appears to have detrimental effects. While data from the brain is limited, a recent study has shown that deletion of ARG-2 in cerebral ischemia has detrimental effects with higher infarction volumes, excitotoxic damage, as well decreased cerebral blood flow and higher neurologic deficit scores [24], clearly highlighting organ-specific roles for this enzyme and its isoforms.
4. ARG and molecular mechanisms of the brain HI
The source of ARG in tissue after HI injury varies. ARG may be released from damaged tissue cells, newly expressed in cells at the injury site or originate from cells that migrate into the lesion, like macrophages or microglia. In a model of a spinal cord injury and autoimmune encephalitis, ARG-1 is expressed exclusively in infiltrating myeloid cells and not microglia [93]. Zarruk et al. observed a similar pattern, where macrophages upregulate the expression of ARG-1 mRNA and ARG protein as a response to brain ischemia to a greater extent compared to microglia [94]. The definitive sources of ARG after HI remain to be established. Different types of brain injury must be taken into consideration. For example, brain cells exposed to hypoxic injury may have different expression profiles compared to cells exposed to both hypoxia and ischemia.
4.1. ARG and neuroinflammation
Neuroinflammation is a critical aspect of injury and post-injury mechanisms in the brain and it is conceivable that regulators of neuroinflammation may have important roles in mediating both injury and repair processes. Many factors involved in neuroinflammation have been shown to regulate ARG, highlighting a pivotal role for ARG in these processes.
Selected inflammatory cytokines vary in their ability to stimulate ARG. IL-13 and IL-4 induce ARG-1 overexpression via cAMP and activation of JAK/STAT6 pathways [95,96], while IL-13 induces expression of ARG-2 by its STAT3- but not STAT-6 mediated effect on IL-13Rα2 [97]. IL-10, on the other hand however, stimulates ARG-1 minimally [98]. While the stimulatory effects of interleukins have been well established, data on the effects of IFN-γ are inconclusive. Multiple studies have shown that IF-γ inhibits activity of both ARGs [99], ARG-1 [98] and ARG-2 [100] although at least one report is contradictory [101]. Interestingly, in a murine model of autoimmune encephalitis ARG inhibition with 2(S)-amino-6 -boronohexanoic acid (ABH) leads to decreased production of IFN-γ [102]. Regulation of ARG via cytokines appears to be more complex process where suppressors of cytokine signaling (SOCs) play a role via negative feedback inhibition on the JAK/STAT signaling pathway [103]. SOCs1 and SOCs3 are both expressed in microglia [103] and regulate microglia activation [104] and macrophage polarization. Interestingly, this regulation is associated via changes in ARG/iNOS ratio; SOCs1 leads to high ARG:iNOS ratio and M2 activation [105]. Knockdown of SOCs1 on the other hand, decreases M2-induced ARG-1 expression and activity but also reciprocally enhances iNOS activity, SOCs3 expression associated with M1 activation [105], and suppression of ARG-1 activity [106]. Considering microglia are one of the first responders of injury in the neonatal brain and have been implicated to have in both beneficial as well as detrimental effects, these findings are critical to the understanding of post-injury processes.
In addition to these, the prostaglandins PGE1, PGE2 and PGE3 [107] and TGFβ1 [108] have also been shown to modulate ARG expression.
4.2. ARG and excitotoxicity
ARG2−/− knockout mice have been shown to have increased injury and neurologic impairment caused by MCAO and excitotoxic injury likely due to L-arginine being re-directed to the excitotoxicity-induced NOS pathway, leading to downstream oxidative stress [24]. Interestingly however, it appears that polyamines, produced from the ARG metabolite ornithine, may exacerbate excitotoxicity by directly binding NMDA receptors [49]. Intraocular NMDA injections upregulate ARG-1 which presumably leads to increased ornithine, and polyamine production and both pharmacologic blockade of polyamine synthesis, as well as competitive blockade of polyamine binding sites on NMDA receptors, reduce excitotoxic retinal ganglion cell death [49].
A significant component of neuronal injury after a neonatal brain HI, results from excitotoxicity that stems from the pathologic release of excess glutamate. As studies have begun to elaborate the downstream signaling pathways that lead from glutamate receptor binding to cell death, most of the damage is thought to result from oxidative stress induced by NOS upregulation and increased production of NO. As NOS and ARG share the substrate L-arginine, many models seem to suggest that shunting of L-arginine through the NOS pathway is neurotoxic, while alterations that would lead to increased ARG utilization of L-arginine might be neuroprotective. Competition between these two pathways may require a more delicate balance, however, as the polyamines that are produced downstream of ARG metabolites may themselves be neurotoxic [54]. Existing studies have just begun to aid our understanding of ARG metabolism in excitotoxic injury, but future studies will be necessary to understand exactly how to harness these enzymatic pathways to maximize neuroprotection and minimize neuronal death.
4.3. ARG and apoptosis
Depletion of L-arginine by ARG results in neuronal starvation and impaired neuronal survival, inducing apoptosis and subsequent neuronal death [109]. In the same study, inhibition of ARG-1 with difluoromethylornithine (DFMO) reversed abnormal L-arginine utilization, suggesting a causal link between ARG activity and apoptotic death. In conflict with this report however, overexpression of ARG-1 can prevent neuronal death, potentially via suppression of NO production and subsequent peroxynitrate production combined with an increase in polyamine synthesis [47]. ARG has also been shown to promote cell survival via intracellular depletion of L-arginine leading to activation of the serine/threonine kinase GCN2 to phosphorylate the translational initiation factor 2 alpha associated with cell survival [110,111].
4.4. ARG and oxidative stress
The neonatal brain is selectively vulnerable to oxidative stress [112]. ARG and oxidative stress have a reciprocal relationship. ARG activity is stimulated by free radicals and activated ARG decreases bioavailability of NOS substrate, L-arginine, further increasing oxidative stress [113–115]. Hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) lead to increased ARG activity and expression via protein kinase C activation and subsequent RhoA/Rho associated kinase activation [115] and this mechanism likely also occurs in microglial cells after injury [116]. In addition to this, ARG contributes to depletion of substrate L-arginine for all three NOS isoforms and NOS uncoupled from its substrate generates superoxide and/or H2O2, a feature characteristic for all NOS isoforms [117]. The specific downstream effects of substrate depletion may vary depending on the NOS isoform; while eNOS uncoupling leads to radical formation resulting in endothelial damage, deprivation of L-arginine from iNOS is associated with a neuroprotective role for ARG, given improvement in oxidative and nitrosative stress [117]. The effects of substrate depletion may also differ with timing after injury. For example, during reperfusion the uncoupled iNOS is a source of free radicals and can contribute to significant reperfusion injury [117]. The contribution of L-arginine availability for NOS to radical formation is NOS-isoform dependent. While increased L-arginine availability for nNOS decreases the rate and the amount of total radical production, in eNOS it is only the rate that is affected [118]. Besides impaired NO formation, ARG also decreases production of L-citrulline, which has been shown to have antioxidative properties [119], improve neuronal survival as well as attenuate eNOS and nNOS expression after ischemic injury [120], restore NO levels, increase eNOS and suppress iNOS after glutamate toxicity [121]. Treatment with L-arginine on the other hand, increased iNOS [121]. All these findings very fine-tuned roles for ARG in mediating oxidative stress and downstream injury mechanisms, highlighting the need for careful consideration of its effects specifically on the NOS pathway.
4.5. ARG and blood brain barrier (BBB) regulation
The disruption of the BBB, impaired cerebral autoregulation, and local changes in perfusion are the vascular pathophysiological features of hypoxic-ischemic and reperfusion injury of the brain. The role of ARG in vascular regulation and endothelial senescence has been extensively studied in several peripheral organs and it is likely that at least some of these effects could be extrapolated to the brain. As ARG-1 and ARG-2 have both been detected in cerebral microvasculature, it is assumed that ARG affects cerebral microcirculation, BBB integrity, as well as endothelial function (reviewed in [31]). The primary isoform in endothelial cells is ARG-2 [38], which, as discussed earlier, interacts with eNOS to regulate production of NO [122]. Decreasing L-arginine availability for eNOS by ARG leads to eNOS uncoupling [123] which promotes oxidant stress. Upregulation of ARG leads to induction of an endothelial senescence phenotype, eNOS-uncoupling, elevated adhesion molecule expression, and enhanced monocyte-endothelial cell interaction. While ARG-2 exhibits these effects through S6K1-signaling [30], ARG-1 does not [124]. Multiple vascular factors act as ARG inducers at the site of vascular injury. For example, release of transforming growth factor beta [125], or thrombin in endothelial cells which modulate endothelial ARG gene expression through AP-1 promoter sequence-bound transcription factors c-Jun and ATF-2 [126]. Besides effects on local microcirculation, ARG stimulated by brain injury may impact the systemic vasculature as well. ARG-1 expression increases in mesenteric arteries in a rat model of TBI and supplementation with L-arginine or ARG inhibition with nor-NOHA reversed the ARG effects [127]. However, clinical significance of systemic changes in ARG expression after brain injury is unknown.
5. Clinical applications
5.1. ARG as a biomarker
Both ARG-1 gene expression and serum activity have been shown to increase in adult patients with acute ischemic stroke and more importantly, correlate with infarct volume and severity of the stroke [128], suggesting a considerable role for ARG as a biomarker after stroke. Interestingly, ARG gene expression and serum protein levels also correlate with increases in peripheral neutrophil counts [128] which has been associated with poor neurological outcomes in asphyxiated newborns [129]. The relationship between ARG expression and neutrophils might be different in newborns however, since neutrophils show different patterns of recruitment after stroke and migrate to the injury site at later time points compared to adults [130]. The utility of ARG as a biomarker is possible, however it needs to be examined further in validation cohorts.
5.2. ARG as a therapy
Given all the evidence thus far, the potential role of multiple steps of the ARG pathway in brain injury may represent a promising therapeutic target of neonatal brain HI. Manipulation of the ARG pathway at certain time points after the hypoxic insult may provide neuroprotection and potentially also enhance neurorestoration. To do this however, besides establishing the appropriate time window for ARG inhibition, it is also important to characterize the effects of inhibition of the specific isoform of this enzyme. For example, in macrophage subpopulations, selective inhibition of ARG-1 in profibrotic M2 macrophages might lead to overexpression of the M1 inflammatory phenotype, which conversely expresses the ARG-2 isoform predominantly and in turn, aggravates iNOS-mediated inflammatory effects. On the other hand, inhibition of ARG-2 may enhance the profibrotic/repair M2 phenotype with potential deleterious effects on vessels and other organs [131].
Different synthetic inhibitors of ARG have been synthetized, including boronic acid analogs, N-hydroxide-L-arginine, N-hydroxy-nor-L-arginine, and newer (R)-2-amino-6-borono-2-(2(piperidin-1yl)ethyl) hexanoic acid [131]. Currently, although there are approximately 27 ARG inhibitors patented and studied [132], studies have highlighted the lack of specificity in most of these inhibitors. While some insight into effects of ARG inhibition might come from knockout models, results need to be evaluated carefully owing to unknown compensatory pathways that might mask the ARG effects. Of course, that the life span of ARG-1 knockout mice is limited also presents a considerable disadvantage. Despite this however, ARG inhibitors have already been the subject of clinical trials showing promising effects in patients with cardiovascular diseases (coronary artery disease, pulmonary hypertension, atherosclerosis, heart failure, vascular dysfunction in diabetes mellitus, hypertension) [133–135].
The role of ARG inhibition after brain HI is unclear. While there are several studies describing benefits of ARG inhibition, some of the studies, as discussed above, report the role of ARG as a neuroprotective enzyme. Comparing the effects of ARG inhibition is limited by the number of studies which are focused on different brain pathologies and the variety of time points for intervention.
5.2.1. The benefits of ARG activation
Increasing ARG activation/expression seems to be effective largely via regulation of microglial polarization. Early treatment with micro-RNA-124, small non-coding RNA molecules involved in post-transcriptional regulation of gene expression, has been shown to increase expression of ARG-1 in immune cells with polarization of macrophages/microglia towards the anti-inflammatory M2-phenotype associated with increased neuronal survival [71]. In mice subjected to traumatic brain injury, administration of IL-2C increased expression of ARG-1, among other genes, in the anti-inflammatory M2-microglia [136]. Similar effect has been observed in treatment with atorvastatin that increases M2-polarization and associated ARG-1 expression [137]. Considering that M2-microglia have been shown to elicit beneficial effects in the injured brain [138], these findings suggest modulating ARG expression after HI could have beneficial effects especially via microglial polarization amongst others. ARG catalytic function may be supported by manganese (Mn) administration as shown in studies with a mouse model of Huntington disease, where Mn supplementation increased ARG-2 activity [139].
5.2.2. The benefits of ARG inhibition
On the contrary, many studies have shown ARG inhibition following injury to be beneficial. In the brain, treatment with clomethiazole, associated with a decrease in ARG activity was shown to be neuroprotective [140]. ARG inhibition was also shown to decrease disease symptoms and accelerate recovery in a mouse model of autoimmune encephalitis [102] and reduce injury volume in TBI [141] and MCAO [142], while ARG deletion significantly improved cerebral blood flow following TBI [143]. Among commonly used medications with high safety profiles that decrease ARG activity belongs caffeine that acts via adenosine, a competitive ARG inhibitor [144,145]. As discussed earlier, it is likely that ARG has very specific effects based on age, timing, organ as well as isoform and the conflicting nature of findings relating to ARG-mediated neuroprotection likely indicate the importance of careful extrapolation.
Conclusion
The ARG pathway is a complex metabolic pathway that is affected by multiple factors, and it appears that the ultimate effects do not occur in isolation, but rather as the culmination of complex interactions that might be species, organ system, and cell specific. Currently, there are no specific inhibitors to study the effects on isoforms separately, and a knockout mouse shows a decreased life-span. Gender differences and gene polymorphisms may play a role. Despite these disadvantages, a better understanding of ARG effects on HI brain injury in a newborn represents an attractive opportunity for new neuroprotective therapies.
Table 1:
Summary of the studies describing the ARG pathway after stroke, hypoxia and TBI
| Author | Animal model | Timeline for measurement of changes in ARG pathway post injury | Age | Observed Effect(s) |
|---|---|---|---|---|
| Ahmad et al., 201624 | Male C57BL/6 wildtype and Arg-2 knockout mice; MCAO occlusion | Day 7 | Adult | Higher infarction volumes and neurologic deficits; Excitotoxic damage. |
| Hamzei et al, 201671 | Male C57BL/6 wildtype mice; MCAO occlusion | Day 6 and 14 | Adult | ARG-1 expression highest 1st week; Number of Arg-1+ microglia/macrophages correlated with neuronal protection and with functional improvement after microRNA-124 treatment. |
| Barakat et al, 2018142 | Male Wistar wildtype rats; MCAO occlusion | -not stated- | Adult | Increased ARG-1 and ARG-2 expression inhibited by L-citrulline and L-ornithine treatment; Reduction of infarct size and brain edema. |
| Clarkson et al, 200425 | Male Wistar wildtype rat pups; MCAO occlusion | Day 7 | Pups | Significant decrease in infarction size and ARG activity after spermine supplementation. |
| Clarkson et al, 200526 | Male Wister wildtype rat pups; MCAO occlusion followed by hypoxia | Day 3 and Day 90 | Pups | Increased ARG activity after HI on day 3, persistent increase on day 90 after HI. Decreased ARG activity after clomethiazole; Neuroprotection. |
| Gao et al., 2017136 | Male C57BL/6 wildtype mice; TBI; controlled cortical impact (CCI) | Day 3 | Adult | Treatment with IL-2C significantly increased the number of ARG-1+ cells around the lesion area. |
| Xu et al, 2017137 | C57BL/6 wildtype mice; TBI model: CCI | Day 1 and day 3 | Adult | Increased ARG-1 expression in M2 -microglia after atorvastatin therapy. |
| Bitner et al., 2010143 | C57Bl6/J mice and ARG-2 knockout mice; TBI model: CCI | 15 min and 1hr | Adult | The absence of ARG-2 significantly improves CBF recovery after trauma. |
| Swamy et al, 201069 | Male Sprague Dawley wildtype rats exposed to anoxia for 4–5min | Day 5. | Adult | Increased ARG expression; activity increased in reperfusion phase. |
| Quirie et al, 201370 | Wistar wildtype rats; photothrombotic stroke model | Days 1, 8, 15 and 30 | Adult | Increased ARG-1 expression on day 8; ARG-2 unchanged; activity highest on day 8. |
6.1. Acknowledgement
Special thanks to Donna Ferriero, Praneeti Pathipati, Sandrijn van Schaik and Jeff Fineman for their help and support.
6.4. Funding Sources
This study is funded by R35- 5R35NS097299; UCSF Pediatric Critical Care Division and Thrasher Research Fund P0530684.
Footnotes
6. Statements
6.2. Statement of Ethics
The authors have no ethical conflicts to disclose.
References
- 1.Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team: 4 million neonatal deaths: when? Where? Why? Lancet 2005. January;365:891–900. [DOI] [PubMed] [Google Scholar]
- 2.Millar LJ, Shi L, Hoerder-Suabedissen A, Molnár Z: Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front Cell Neurosci 2017. May 8;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferriero DM: Neonatal Brain Injury. N Engl J Med 2004. November 4;351:1985–1995. [DOI] [PubMed] [Google Scholar]
- 4.Meijer AJ, Lamers WH, Chamuleau RA: Nitrogen metabolism and ornithine cycle function. Physiol Rev 1990. July;70:701–748. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB: Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol Rev 2018. April;98:641–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harpster MH, Bandyopadhyay S, Thomas DP, Ivanov PS, Keele JA, Pineguina N, et al. : Earliest changes in the left ventricular transcriptome post-myocardial infarction. Mamm Genome 2006. July 14;17:701–715. [DOI] [PubMed] [Google Scholar]
- 7.Jeyabalan G, Klune JR, Nakao A, Martik N, Wu G, Tsung A, et al. : Arginase blockade protects against hepatic damage in warm ischemia-reperfusion. Nitric Oxide 2008. August;19:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shosha E, Xu Z, Yokota H, Saul A, Rojas M, Caldwell RW, et al. : Arginase 2 promotes neurovascular degeneration during ischemia/reperfusion injury. Cell Death Dis 2016;7:e2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ash DE: Structure and function of arginases. J Nutr 2004. October 1;134:2760S–2764S; discussion 2765S–2767S. [DOI] [PubMed] [Google Scholar]
- 10.Choi S, Park C, Ahn M, Lee JH, Shin T: Immunohistochemical study of arginase 1 and 2 in various tissues of rats. Acta Histochem 2012. September;114:487–494. [DOI] [PubMed] [Google Scholar]
- 11.Sparkes RS, Dizikes GJ, Klisak I, Grody WW, Mohandas T, Heinzmann C, et al. : The gene for human liver arginase (ARG1) is assigned to chromosome band 6q23. Am J Hum Genet 1986; [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Iyer RK, Kern RM, Rodriguez WI, Grody WW, Cederbaum SD: Expression of arginase isozymes in mouse brain. J Neurosci Res 2001. November 1;66:406–422. [DOI] [PubMed] [Google Scholar]
- 13.Gotoh T, Araki M, Mori M: Chromosomal localization of the human arginase II gene and tissue distribution of its mRNA. Biochem Biophys Res Commun 1997; DOI: 10.1006/bbrc.1997.6473 [DOI] [PubMed] [Google Scholar]
- 14.Vockley JG, Jenkinson CP, Shukla H, Kern RM, Grody WW, Cederbaum SD: Cloning and characterization of the human type II arginase gene. Genomics 1996; DOI: 10.1006/geno.1996.0606 [DOI] [PubMed] [Google Scholar]
- 15.Morris SM, Kepka-Lenhart D, Chen LC: Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. [Internet]. Am J Physiol 1998. November [cited 2018 Aug 9];275:E740–7. [DOI] [PubMed] [Google Scholar]
- 16.Iyer RK, Yoo PK, Kern RM, Rozengurt N, Tsoa R, O’Brien WE, et al. : Mouse model for human arginase deficiency. [Internet]. Mol Cell Biol 2002. July [cited 2018 Aug 13];22:4491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlune A, vom Dahl S, Häussinger D, Ensenauer R, Mayatepek E: Hyperargininemia due to arginase I deficiency: the original patients and their natural history, and a review of the literature. Amino Acids 2015. September 27;47:1751–1762. [DOI] [PubMed] [Google Scholar]
- 18.Munder M: Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol 2009. October;158:638–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange PS, Langley B, Lu P, Ratan RR: Novel Roles for Arginase in Cell Survival, Regeneration, and Translation in the Central Nervous System. J Nutr 2004. October 1;134:2812S–2817S. [DOI] [PubMed] [Google Scholar]
- 20.Maarsingh H, Pera T, Meurs H: Arginase and pulmonary diseases. Naunyn Schmiedebergs Arch Pharmacol 2008. August;378:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pernow J, Jung C: The Emerging Role of Arginase in Endothelial Dysfunction in Diabetes. [Internet]. Curr Vasc Pharmacol 2016. [cited 2018 Aug 13];14:155–62. [DOI] [PubMed] [Google Scholar]
- 22.Korrer MJ, Routes JM: Possible Role of Arginase-1 in Concomitant Tumor Immunity. PLoS One 2014. March 10;9:e91370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlüter K-D, Schulz R, Schreckenberg R: Arginase induction and activation during ischemia and reperfusion and functional consequences for the heart. Front Physiol 2015;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad AS, Shah ZA, Doré S: Protective Role of Arginase II in Cerebral Ischemia and Excitotoxicity. [Internet]. J Neurol Neurosci 2016. [cited 2018 Aug 13];7 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27308186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CLARKSON AN, LIU H, PEARSON L, KAPOOR M, HARRISON JC, SAMMUT IA, et al. : Neuroprotective effects of spermine following hypoxic-ischemic-induced brain damage: A mechanistic study. FASEB J 2004. July 7;18:1114–1116. [DOI] [PubMed] [Google Scholar]
- 26.Clarkson AN, Liu H, Rahman R, Jackson DM, Appleton I, Kerr DS: Clomethiazole: mechanisms underlying lasting neuroprotection after hypoxia-ischemia. FASEB J 2005. June 4;24:1–24. [DOI] [PubMed] [Google Scholar]
- 27.Nijboer CHA, Van Velthoven C: Cerebral and Hepatic Inflammatory Response after Neonatal Hypoxia-Ischemia in Newborn Rats 2016; DOI: 10.1159/000346685 [DOI] [PubMed] [Google Scholar]
- 28.Narayanan SP, Suwanpradid J, Saul A, Xu Z, Still A, Caldwell RW, et al. : Arginase 2 Deletion Reduces Neuro-Glial Injury and Improves Retinal Function in a Model of Retinopathy of Prematurity. PLoS One 2011. July 21;6:e22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Ming X-F: Functions of Arginase Isoforms in Macrophage Inflammatory Responses: Impact on Cardiovascular Diseases and Metabolic Disorders. Front Immunol 2014. October 27;5:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yepuri G, Velagapudi S, Xiong Y, Rajapakse AG, Montani J-P, Ming X-F, et al. : Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell 2012. December;11:1005–1016. [DOI] [PubMed] [Google Scholar]
- 31.Lucas R, Fulton D, Caldwell RW, Romero MJ: Arginase in the vascular endothelium: friend or foe? Front Immunol 2014;5:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu C, Yu Y, Montani J-P, Ming X-F, Yang Z: Arginase-I enhances vascular endothelial inflammation and senescence through eNOS-uncoupling. BMC Res Notes 2017. December 2;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo B-H, Yi B-G, Jeong M-S, Kwon S-H, Hoe K-L, Kwon Y-G, et al. : Arginase II inhibition prevents interleukin-8 production through regulation of p38 MAPK phosphorylation activated by loss of mitochondrial membrane potential in nLDL-stimulated hAoSMCs. Exp Mol Med 2018. February 2;50:e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ming X-F, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, et al. : Thrombin Stimulates Human Endothelial Arginase Enzymatic Activity via RhoA/ROCK Pathway: Implications for Atherosclerotic Endothelial Dysfunction. Circulation 2004. December 14;110:3708–3714. [DOI] [PubMed] [Google Scholar]
- 35.Scalera F, Closs EI, Flick E, Martens-Lobenhoffer J, Boissel JP, Lendeckel U, et al. : Paradoxical effect of l-arginine: Acceleration of endothelial cell senescence. Biochem Biophys Res Commun 2009. September 4;386:650–655. [DOI] [PubMed] [Google Scholar]
- 36.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, et al. : Diabetes-induced Coronary Vascular Dysfunction Involves Increased Arginase Activity. Circ Res 2008. January 4;102:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, et al. : Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol 2009. October;107:1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim HK, Lim HK, Ryoo S, Benjo A, Shuleri K, Miriel V, et al. : Mitochondrial arginase II constrains endothelial NOS-3 activity. Am J Physiol Circ Physiol 2007. December;293:H3317–H3324. [DOI] [PubMed] [Google Scholar]
- 39.Huynh NN, Andrews KL, Head GA, Khong SML, Mayorov DN, Murphy AJ, et al. : Arginase II knockout mouse displays a hypertensive phenotype despite a decreased vasoconstrictory profile. Hypertens (Dallas, Tex 1979) 2009. August 1;54:294–301. [DOI] [PubMed] [Google Scholar]
- 40.Handley RR, Reid SJ, Brauning R, Maclean P, Mears ER, Fourie I, et al. : Brain urea increase is an early Huntington’s disease pathogenic event observed in a prodromal transgenic sheep model and HD cases. Proc Natl Acad Sci 2017. December 26;114:E11293–E11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Begley P, Church SJ, Patassini S, Hollywood KA, Jüllig M, et al. : Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: Snapshot of a pervasive metabolic disorder. Biochim Biophys Acta - Mol Basis Dis 2016. June;1862:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Que LG, George SE, Gotoh T, Mori M, Huang Y-CT: Effects of Arginase Isoforms on NO Production by nNOS. Nitric Oxide 2002. February;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogdan C: Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol 2015. March;36:161–178. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Meininger CJ, Hawker JR, Haynes TE, Kepka-Lenhart D, Mistry SK, et al. : Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Metab 2001. January;280:E75–E82. [DOI] [PubMed] [Google Scholar]
- 45.Shank RP, LeM. Campbell: Ornithine as a precursor of glutamate and GABA: Uptake and metabolism by neuronal and glial enriched cellular material. J Neurosci Res 1983;9:47–57. [DOI] [PubMed] [Google Scholar]
- 46.Lee EK, Hu C, Bhargava R, Ponnusamy R, Park H, Novicoff S, et al. : AAV-based gene therapy prevents neuropathology and results in normal cognitive development in the hyperargininemic mouse. Gene Ther 2013. August 7;20:785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT: Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. [Internet]. Neuron 2002. August 15 [cited 2018 Aug 14];35:711–9. [DOI] [PubMed] [Google Scholar]
- 48.Malaterre J, Strambi C, Aouane A, Strambi A, Rougon G, Cayre M: A novel role for polyamines in adult neurogenesis in rodent brain. Eur J Neurosci 2004. July;20:317–30. [DOI] [PubMed] [Google Scholar]
- 49.Pernet V, Bourgeois P, Di Polo A: A role for polyamines in retinal ganglion cell excitotoxic death. J Neurochem 2007. November;103:1481–1490. [DOI] [PubMed] [Google Scholar]
- 50.Grillo MA, Fossa T, Dianzani U: Arginase, ornithine decarboxylase and Sadenosylmethionine decarboxylase in chicken brain and retina. Int J Biochem 1983;15:1081–4. [DOI] [PubMed] [Google Scholar]
- 51.Peters D, Berger J, Langnaese K, Derst C, Madai VI, Krauss M, et al. : Arginase and Arginine Decarboxylase – Where Do the Putative Gate Keepers of Polyamine Synthesis Reside in Rat Brain? PLoS One 2013. June 19;8:e66735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura H, Saheki T, Nakagawa S: Differential cellular localization of enzymes of Larginine metabolism in the rat brain. [Internet]. Brain Res 1990. October 15 [cited 2018 Aug 14];530:108–12. [DOI] [PubMed] [Google Scholar]
- 53.Braissant O, Gotoh T, Loup M, Mori M, Bachmann C: L-arginine uptake, the citrulline-NO cycle and arginase II in the rat brain: an in situ hybridization study. [Internet]. Brain Res Mol Brain Res 1999. July 5 [cited 2018 Aug 14];70:231–41. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Ryu H, Ferrante RJ, Morris SM, Ratan RR: Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci 2003. April 15;100:4843–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vexler ZS, Yenari M a: Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci 2009. January;31:378–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu H, Iyer RK, Yoo PK, Kern RM, Grody WW, Cederbaum SD: Arginase expression in mouse embryonic development. Mech Dev 2002. July;115:151–5. [DOI] [PubMed] [Google Scholar]
- 57.Crain JM, Nikodemova M, Watters JJ: Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res 2013. September;91:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konarska L, Tomaszewski L: Studies on L-Arginase in Developing Rat Small Instestine, Brain, and Kidney I. Ontogenic Evolution of Arginase lsoenzymes [Internet]. 1986, [cited 2018 Aug 14].Available from: https://ac.els-cdn.com/0885450586900708/1-s2.0-0885450586900708-main.pdf?_tid=e34693d7-f8fe-4d1c-b108a81413de7d93&acdnat=1534288254_5f5a85f1d872969292e95c6913c06927 [DOI] [PubMed] [Google Scholar]
- 59.Liu P, Smith PF, Appleton I, Darlington CL, Bilkey DK: Regional variations and age-related changes in nitric oxide synthase and arginase in the sub-regions of the hippocampus. Neuroscience 2003;119:679–87. [DOI] [PubMed] [Google Scholar]
- 60.Liu P, Smith PF, Appleton I, Darlington CL, Bilkey DK: Nitric oxide synthase and arginase in the rat hippocampus and the entorhinal, perirhinal, postrhinal, and temporal cortices: Regional variations and age-related changes. Hippocampus 2003. January 1;13:859–867. [DOI] [PubMed] [Google Scholar]
- 61.Liu P, Smith P., Appleton I, Darlington C, Bilkey D: Age-related changes in nitric oxide synthase and arginase in the rat prefrontal cortex. Neurobiol Aging 2004. April;25:547–552. [DOI] [PubMed] [Google Scholar]
- 62.Liu P, Smith PF, Appleton I, Darlington CL, Bilkey DK: Potential involvement of NOS and arginase in age-related behavioural impairments. Exp Gerontol 2004. August;39:1207–22. [DOI] [PubMed] [Google Scholar]
- 63.Badaut J, Copin J-C, Fukuda AM, Gasche Y, Schaller K, da Silva RF: Increase of arginase activity in old apolipoprotein-E deficient mice under Western diet associated with changes in neurovascular unit. J Neuroinflammation 2012. December 18;9:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.SHIMIZU H, KAKIMOTO Y, SANO I: Changes in Concentration of Polyamines in the Developing Mouse Brain. Nature 1965. September 11;207:1196–1197. [DOI] [PubMed] [Google Scholar]
- 65.Murthy MRV, Rappoport DA: Biochemistry of the developing rat brain VI. Preparation and properties of ribosomes. Biochim Biophys Acta - Nucleic Acids Protein Synth 1965. January 11;95:132–145. [DOI] [PubMed] [Google Scholar]
- 66.Cantero G, Liu X-B, Mervis RF, Lazaro MT, Cederbaum SD, Golshani P, et al. : Rescue of the Functional Alterations of Motor Cortical Circuits in Arginase Deficiency by Neonatal Gene Therapy. J Neurosci 2016. June 22;36:6680–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Becker-Catania SG, Gregory TL, Yang Y, Gau C-L, de Vellis J, Cederbaum SD, et al. : Loss of arginase I results in increased proliferation of neural stem cells. J Neurosci Res 2006. September;84:735–46. [DOI] [PubMed] [Google Scholar]
- 68.Arteaga O, Álvarez A, Revuelta M, Santaolalla F, Urtasun A, Hilario E: Role of Antioxidants in Neonatal Hypoxic–Ischemic Brain Injury: New Therapeutic Approaches. Int J Mol Sci 2017. January 28;18:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swamy M, Salleh MJM, Sirajudeen KNS, Yusof WRW, Chandran G: NITRIC OXIDE (NO), CITRULLINE - NO CYCLE ENZYMES, GLUTAMINE SYNTHETASE AND OXIDATIVE STRESS IN ANOXIA (HYPOBARIC HYPOXIA) AND REPERFUSION IN RAT BRAIN. Int J Med Sci 2010;147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quirié A, Demougeot C, Bertrand N, Mossiat C, Garnier P, Marie C, et al. : Effect of stroke on arginase expression and localization in the rat brain. Eur J Neurosci 2013. April;37:1193–1202. [DOI] [PubMed] [Google Scholar]
- 71.Hamzei Taj S, Kho W, Riou A, Wiedermann D, Hoehn M: MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 2016. June;91:151–165. [DOI] [PubMed] [Google Scholar]
- 72.Bathina S, Das UN: Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 2015. Dec 10;11:1164–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prieto CP, Krause BJ, Quezada C, San Martin R, Sobrevia L, Casanello P: Hypoxia-reduced nitric oxide synthase activity is partially explained by higher arginase-2 activity and cellular redistribution in human umbilical vein endothelium. Placenta 2011. December;32:932–940. [DOI] [PubMed] [Google Scholar]
- 74.Cowburn AS, Crosby A, Macias D, Branco C, Colaço RDDR, Southwood M, et al. : HIF2α–arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci 2016. August 2;113:8801–8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martín-Aragón Baudel MAS, Rae MT, Darlison MG, Poole AV., Fraser JA: Preferential activation of HIF-2α adaptive signalling in neuronal-like cells in response to acute hypoxia. PLoS One 2017. October 2;12:e0185664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue J, Nelin LD, Chen B: Hypoxia induces arginase II expression and increases viable human pulmonary artery smooth muscle cell numbers via AMPKα 1 signaling. Am J Physiol Cell Mol Physiol 2017. April 1;312:L568–L578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin Y, Jin Y, Chen B, Tipple TE, Nelin LD: Arginase II is a target of miR-17–5p and regulates miR-17–5p expression in human pulmonary artery smooth muscle cells. Am J Physiol Cell Mol Physiol 2014. July 15;307:L197–L204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen B, Xue J, Meng X, Slutzky JL, Calvert AE, Chicoine LG: Resveratrol prevents hypoxia-induced arginase II expression and proliferation of human pulmonary artery smooth muscle cells via Akt-dependent signaling. Am J Physiol Cell Mol Physiol 2014. August 15;307:L317–L325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pandey D, Nomura Y, Rossberg MC, Hori D, Bhatta A, Keceli G, et al. : Hypoxia Triggers SENP1 (Sentrin-Specific Protease 1) Modulation of KLF15 (Kruppel-Like Factor 15) and Transcriptional Regulation of Arg2 (Arginase 2) in Pulmonary EndotheliumHighlights. Arterioscler Thromb Vasc Biol 2018. April;38:913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD: Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Physiol Cell Mol Physiol 2010. April;298:L600–L606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White HA, Jin Y, Chicoine LG, Chen B, Liu Y, Nelin LD: Hypoxic proliferation requires EGFR-mediated ERK activation in human pulmonary microvascular endothelial cells. Am J Physiol Cell Mol Physiol 2017. May 1;312:L649–L656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nelin LD, Chicoine LG, Reber KM, English BK, Young TL, Liu Y: Cytokine-induced endothelial arginase expression is dependent on epidermal growth factor receptor. Am J Respir Cell Mol Biol 2005. October;33:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Louis CA, Reichner JS, Henry WL, Mastrofrancesco B, Gotoh T, Mori M, et al. : Distinct arginase isoforms expressed in primary and transformed macrophages: regulation by oxygen tension. [Internet]. Am J Physiol 1998. March [cited 2018 Aug 15];274:R775–82. [DOI] [PubMed] [Google Scholar]
- 84.Zimmermann M, Beer L, Ullrich R, Lukovic D, Simader E, Traxler D, et al. : Analysis of region specific gene expression patterns in the heart and systemic responses after experimental myocardial ischemia. Oncotarget 2017. September 5;8:60809–60825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grönros J, Kiss A, Palmér M, Jung C, Berkowitz D, Pernow J: Arginase inhibition improves coronary microvascular function and reduces infarct size following ischaemia-reperfusion in a rat model. Acta Physiol 2013. June;208:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonon AT, Jung C, Katz A, Westerblad H, Shemyakin A, Sjöquist P-O, et al. : Local Arginase Inhibition during Early Reperfusion Mediates Cardioprotection via Increased Nitric Oxide Production. PLoS One 2012. July 31;7:e42038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.HEIN TW, ZHANG C, WANG W, CHANG C-I, THENGCHAISRI N, KUO L: Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J 2003. December 16;17:2328–2330. [DOI] [PubMed] [Google Scholar]
- 88.Jung C, Gonon AT, Sjoquist P-O, Lundberg JO, Pernow J: Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res 2010. January 1;85:147–154. [DOI] [PubMed] [Google Scholar]
- 89.Zhu M, Goetsch SC, Wang Z, Luo R, Hill JA, Schneider J, et al. : FoxO4 Promotes Early Inflammatory Response Upon Myocardial Infarction via Endothelial Arg1Novelty and Significance. Circ Res 2015. November 6;117:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, et al. : TNF- Contributes to Endothelial Dysfunction by Upregulating Arginase in Ischemia/Reperfusion Injury. Arterioscler Thromb Vasc Biol 2007. June 1;27:1269–1275. [DOI] [PubMed] [Google Scholar]
- 91.Filiz S, Enis U, Mustafa I, Aysegul C, Elvan B: Protective Effect of Mesna on Intestinal Ischemia-reperfusion Injury by Nitric Oxide and Arginase in an Experimental Rat Model. Int J Pharmacol 2017. October 15;13:1038–1046. [Google Scholar]
- 92.Jeyabalan G, Tsung A, Klune J, Billiar T, Geller D: Arginase blockade as a strategy to mitigate liver ischemia-reperfusion (I/R) injury. J Am Coll Surg 2006. September 1;203:S89–S90. [Google Scholar]
- 93.Greenhalgh AD, Passos dos Santos R, Zarruk JG, Salmon CK, Kroner A, David S: Arginase-1 is expressed exclusively by infiltrating myeloid cells in CNS injury and disease. Brain Behav Immun 2016. August;56:61–67. [DOI] [PubMed] [Google Scholar]
- 94.Zarruk JG, Greenhalgh AD, David S: Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Exp Neurol 2018. March;301:120–132. [DOI] [PubMed] [Google Scholar]
- 95.Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, et al. : Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest 2003. June 15;111:1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei LH, Jacobs AT, Morris SM, Ignarro LJ: IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am J Physiol Physiol 2000. July;279:C248–C256. [DOI] [PubMed] [Google Scholar]
- 97.Cho W-K, Lee C-M, Kang M-J, Huang Y, Giordano FJ, Lee PJ, et al. : IL-13 receptor α 2 - arginase 2 pathway mediates IL-13-induced pulmonary hypertension. Am J Physiol Cell Mol Physiol 2013. January 15;304:L112–L124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, et al. : Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. [Internet]. J Immunol 2001. December 1 [cited 2018 Aug 20];167:6533–44. [DOI] [PubMed] [Google Scholar]
- 99.Modolell M, Corraliza IM, Link F, Soler G, Eichmann K: Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH 1 and TH 2 cytokines. Eur J Immunol 1995. April;25:1101–1104. [DOI] [PubMed] [Google Scholar]
- 100.Wang WW, Jenkinson CP, Griscavage JM, Kern RM, Arabolos NS, Byrns RE, et al. : Coinduction of Arginase and Nitric Oxide Synthase in Murine Macrophages Activated by Lipopolysaccharide. Biochem Biophys Res Commun 1995. May 25;210:1009–1016. [DOI] [PubMed] [Google Scholar]
- 101.Liscovsky MV, Ranocchia RP, Gorlino CV, Alignani DO, Morón G, Maletto BA, et al. : Interferon-gamma priming is involved in the activation of arginase by oligodeoxinucleotides containing CpG motifs in murine macrophages. Immunology 2009. September;128:e159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu L, Hilliard B, Carmody RJ, Tsabary G, Shin H, Christianson DW, et al. : Arginase and autoimmune inflammation in the central nervous system. [Internet]. Immunology 2003. September [cited 2018 Aug 20];110:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baker BJ, Akhtar LN, Benveniste EN: SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol 2009. August;30:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qin H, Yeh W-I, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, et al. : Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci 2012. March 27;109:5004–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Whyte CS, Bishop ET, Rückerl D, Gaspar-Pereira S, Barker RN, Allen JE, et al. : Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J Leukoc Biol 2011. November;90:845–54. [DOI] [PubMed] [Google Scholar]
- 106.Schmok E, Abad Dar M, Behrends J, Erdmann H, Rückerl D, Endermann T, et al. : Suppressor of Cytokine Signaling 3 in Macrophages Prevents Exacerbated Interleukin-6Dependent Arginase-1 Activity and Early Permissiveness to Experimental Tuberculosis. Front Immunol 2017. November 10;8:1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bansal V, Syres K, Harbrecht BG, Ochoa JB: Effect of prostoglandins in arginase I expression. J Surg Res 2003. October 1;114:288. [Google Scholar]
- 108.Zhou X, Spittau B, Krieglstein K: TGFβ signalling plays an important role in IL4-induced alternative activation of microglia. J Neuroinflammation 2012. December 4;9:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kan MJ, Lee JE, Wilson JG, Everhart AL, Brown CM, Hoofnagle AN, et al. : Arginine Deprivation and Immune Suppression in a Mouse Model of Alzheimer’s Disease. J Neurosci 2015. April 15;35:5969–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Estévez AG, Sahawneh MA, Lange PS, Bae N, Egea M, Ratan RR: Arginase 1 regulation of nitric oxide production is key to survival of trophic factor-deprived motor neurons. J Neurosci 2006. August 16;26:8512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sattlegger E, Swanson MJ, Ashcraft EA, Jennings JL, Fekete RA, Link AJ, et al. : YIH1 Is an Actin-binding Protein That Inhibits Protein Kinase GCN2 and Impairs General Amino Acid Control When Overexpressed. J Biol Chem 2004. July 16;279:29952–29962. [DOI] [PubMed] [Google Scholar]
- 112.Ferriero DM: Oxidant mechanisms in neonatal hypoxia-ischemia. [Internet]. Dev Neurosci 2001. January;23:198–202. [DOI] [PubMed] [Google Scholar]
- 113.Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, et al. : Upregulation of Arginase by H2O2 Impairs Endothelium-Dependent Nitric Oxide-Mediated Dilation of Coronary Arterioles. Arterioscler Thromb Vasc Biol 2006. June 15;26:2035–2042. [DOI] [PubMed] [Google Scholar]
- 114.Sankaralingam S, Xu H, Davidge ST: Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc Res 2010. January 1;85:194–203. [DOI] [PubMed] [Google Scholar]
- 115.Chandra S, Romero M, Shatanawi A, Alkilany A, Caldwell R, Caldwell R: Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol 2012. January;165:506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pathipati P, Müller S, Jiang X, Ferriero D: Phenotype and Secretory Responses to Oxidative Stress in Microglia. Dev Neurosci 2013;35:241–254. [DOI] [PubMed] [Google Scholar]
- 117.Berka V, Liu W, Wu G, Tsai A-L: Comparison of oxygen-induced radical intermediates in iNOS oxygenase domain with those from nNOS and eNOS. J Inorg Biochem 2014. October;139:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berka V, Wang L-H, Tsai A-L: Oxygen-Induced Radical Intermediates in the nNOS Oxygenase Domain Regulated by l -Arginine, Tetrahydrobiopterin, and Thiol †. Biochemistry 2008. January 8;47:405–420. [DOI] [PubMed] [Google Scholar]
- 119.Marquet-de Rougé P, Clamagirand C, Facchinetti P, Rose C, Sargueil F, Guihenneuc-Jouyaux C, et al. : Citrulline diet supplementation improves specific age-related raft changes in wild-type rodent hippocampus. Age (Dordr) 2013. October;35:1589–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yabuki Y, Shioda N, Yamamoto Y, Shigano M, Kumagai K, Morita M, et al. : Oral l-Citrulline administration improves memory deficits following transient brain ischemia through cerebrovascular protection. Brain Res 2013. July 3;1520:157–167. [DOI] [PubMed] [Google Scholar]
- 121.Lee K-E, Kang Y-S: l -Citrulline restores nitric oxide level and cellular uptake at the brain capillary endothelial cell line (TR-BBB cells) with glutamate cytotoxicity. Microvasc Res 2018. November 2;120:29–35. [DOI] [PubMed] [Google Scholar]
- 122.Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, et al. : Oxidized Low-Density Lipoprotein-Dependent Endothelial Arginase II Activation Contributes to Impaired Nitric Oxide Signaling. Circ Res 2006. September 28;99:951–960. [DOI] [PubMed] [Google Scholar]
- 123.Shin WS, Berkowitz DE, Ryoo SW: Increased arginase II activity contributes to endothelial dysfunction through endothelial nitric oxide synthase uncoupling in aged mice. Exp Mol Med 2012. October 31;44:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu C, Yu Y, Montani J-P, Ming X-F, Yang Z: Arginase-I enhances vascular endothelial inflammation and senescence through eNOS-uncoupling. BMC Res Notes 2017. December 2;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang J, George SC: Enhanced Arginase Activity By TGF-Beta 2 Impacts Nitric Oxide Production In Lung Epithelial Cells By Limiting L-Arginine Availability [Internet]; in : B28. OXIDATIVE STRESS, SIGNAL TRANSDUCTION AND MECHANISMS OF LUNG INJURY. American Thoracic Society, 2010, pp A2699–A2699. [Google Scholar]
- 126.Zhu W, Chandrasekharan UM, Bandyopadhyay S, Morris SM, DiCorleto PE, Kashyap VS, et al. : Thrombin induces endothelial arginase through AP-1 activation. Am J Physiol Cell Physiol 2010. April;298:C952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Villalba N, Sackheim AM, Nunez IA, Hill-Eubanks DC, Nelson MT, Wellman GC, et al. : Traumatic Brain Injury Causes Endothelial Dysfunction in the Systemic Microcirculation through Arginase-1–Dependent Uncoupling of Endothelial Nitric Oxide Synthase. J Neurotrauma 2017. January 1;34:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Petrone AB, O’Connell GC, Regier MD, Chantler PD, Simpkins JW, Barr TL: The Role of Arginase 1 in Post-Stroke Immunosuppression and Ischemic Stroke Severity. Transl Stroke Res 2016. April 30;7:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morkos AA, Hopper AO, Deming DD, Yellon SM, Wycliffe N, Ashwal S, et al. : Elevated total peripheral leukocyte count may identify risk for neurological disability in asphyxiated term neonates. J Perinatol 2007. June 19;27:365–370. [DOI] [PubMed] [Google Scholar]
- 130.Povroznik JM, Engler-Chiurazzi EB, Nanavati T, Pergami P: Absolute lymphocyte and neutrophil counts in neonatal ischemic brain injury. SAGE Open Med 2018. January 19;6:205031211775261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steppan J, Nyhan D, Berkowitz DE: Development of novel arginase inhibitors for therapy of endothelial dysfunction. Front Immunol 2013. September 17;4:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ivanenkov YA, Chufarova NV: Small-molecule arginase inhibitors. Pharm Pat Anal 2014. January;3:65–85. [DOI] [PubMed] [Google Scholar]
- 133.Wu G, Morris SM: Arginine metabolism: nitric oxide and beyond. [Internet]. Biochem J 1998. November 15 [cited 2018 Aug 13];336 ( Pt 1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zaiman A, Fijalkowska I, Hassoun PM, Tuder RM: One hundred years of research in the pathogenesis of pulmonary hypertension. Am J Respir Cell Mol Biol 2005. November;33:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vadivel A, Aschner JL, Rey-Parra GJ, Magarik J, Zeng H, Summar M, et al. : L-Citrulline Attenuates Arrested Alveolar Growth and Pulmonary Hypertension in Oxygen-Induced Lung Injury in Newborn Rats. Pediatr Res 2010. December;68:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao W, Li F, Zhou Z, Xu X, Wu Y, Zhou S, et al. : IL-2/Anti-IL-2 Complex Attenuates Inflammation and BBB Disruption in Mice Subjected to Traumatic Brain Injury. Front Neurol 2017. June 30;8:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu X, Gao W, Cheng S, Yin D, Li F, Wu Y, et al. : Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation 2017. August 23;14:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ziemka-Nalecz M, Jaworska J, Zalewska T: Insights Into the Neuroinflammatory Responses After Neonatal Hypoxia-Ischemia. J Neuropathol Exp Neurol 2017. August 1;76:644–654. [DOI] [PubMed] [Google Scholar]
- 139.Bichell TJV, Wegrzynowicz M, Tipps KG, Bradley EM, Uhouse MA, Bryan M, et al. : Reduced bioavailable manganese causes striatal urea cycle pathology in Huntington’s disease mouse model. Biochim Biophys Acta - Mol Basis Dis 2017. June;1863:1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clarkson AN, Liu H, Rahman R, Jackson DM, Appleton I, Kerr DS: Clomethiazole: mechanisms underlying lasting neuroprotection following hypoxia-ischemia. FASEB J 2005. June 4;19:1036–1038. [DOI] [PubMed] [Google Scholar]
- 141.Cherian L, Sheiko T, Bryan R, Craigen W, Goodman CRC: Inhibition of arginase with N-hydroxy-nor-L-arginine (NOHA) reduces histological damage after cortical impact injury. J Neurotrauma 2009;26:96. [Google Scholar]
- 142.Barakat W, Fahmy A, Askar M, El-Kannishy S: Effectiveness of arginase inhibitors against experimentally induced stroke. Naunyn Schmiedebergs Arch Pharmacol 2018. June 29;391:603–612. [DOI] [PubMed] [Google Scholar]
- 143.Bitner BR, Brink DC, Mathew LC, Pautler RG, Robertson CS: Impact of arginase II on CBF in experimental cortical impact injury in mice using MRI. J Cereb Blood Flow Metab 2010. June 7;30:1105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ofluoglu E, Pasaoglu H, Pasaoglu A: The effects of caffeine on L-arginine metabolism in the brain of rats. Neurochem Res 2009. March 10;34:395–9. [DOI] [PubMed] [Google Scholar]
- 145.Nikolic J, Bjelakovic G, Stojanovic I: Effect of caffeine on metabolism of L-arginine in the brain. [Internet]. Mol Cell Biochem 2003. February [cited 2018 Aug 26];244:125–8. [PubMed] [Google Scholar]