Abstract
Background:
We quantified CMV antiviral use and hospital length of stay (LOS) associated with CMV infection in a contemporary cohort of conventional (CONV) and CD34-selected (T-cell depleted) HCT recipients managed by preemptive therapy (PET) in a single US center.
Methods
Adults who received first allogeneic HCT at MSKCC from June 2010 through December 2014 were analyzed. Days on PET, number and LOS of readmissions by day 180 post HCT were summarized. Estimated unit value (EUV) was defined as the expected number of PET days for a cohort of 100 HCT with characteristics as the analyzed cohort. Standardized incidence ratio (SIR) was calculated as the ratio of observed outcomes of patients with CMV viremia over the outcomes of patients without CMV viremia.
Results:
Of 318 patients, 88 received CONV and 230 CD34-selected HCT. Rates of CMV viremia were 26.3% for CONV and 41.9% for CD34-selected (P = 0.003). Among pts with viremia 68.2% CONV and 97.9% CD34-selected received PET. EUV for PET was 852 days and 2,821 days for CONV and CD34-selected respectively. The SIR for number of readmission and readmission LOS were 1.7 [95% CI (1.4–2.1)) and 1.2 (1.1–1.3) respectively for CONV and 1.7 (1.3–2.1) and 1.6 (1.5–1.7) respectively for CD34-selected. Overall survival (OS) was similar between patients with and without CMV viremia by HCT type. CMV end-organ disease was associated with lower OS only in CD34-selected HCT (P = 0.0007).
Conclusion:
CMV infection managed by PET requires substantial antiviral utilization and is associated with longer readmission LOS and larger numbers of readmissions particularly among CD34-selected HCT.
Keywords: Cytomegalovirus (CMV) viremia, health outcomes, hematopoietic cell transplant (HCT), T-cell depletion (CD34-selected), preemptive therapy
Introduction
Cytomegalovirus (CMV) viremia occurs in 40–90% of CMV seropositive recipients (R+)1–3 and is associated with increased overall mortality after HCT4. Risk factors for CMV reactivation after HCT are well established and include T-cell depletion, allograft from HLA mismatched or CMV-seronegative donor (D−) and graft versus host disease (GVHD)1,4. Preemptive therapy (PET) has reduced the rates of CMV end-organ disease to less than 5%5 the trade-off being increased use of CMV antivirals with their associated toxicities and potentially prolonged hospital length of stay (LOS). By our institutional algorithm, ex-vivo T-cell depletion by CD34-selection is the first choice for patients with acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). T-cell depletion is an effective strategy for graft versus host disease (GVHD) prophylaxis, alleviating the need for additional pharmacologic immunosuppression6–9. Higher rates of viral infections have been reported in CD34-selected HCT because of delayed immune reconstitution2,10,11. We analyzed a contemporary cohort of CONV and CD34-selected HCT managed preemptively for CMV. Our objectives were to: 1) quantify use of PET and 2) assess the impact of CMV viremia on hospital length of stay (LOS) by Day +180 after HCT.
Methods
Study cohort
Consecutive adults with acute leukemia, chronic leukemia, MDS, myeloproliferative disease, Hodgkin’s disease, and non-Hodgkin’s lymphoma who underwent first CONV or CD34-selected HCT at Memorial Sloan Kettering (MSK) between June 1, 2010 and December 31, 2014 were included in the study. Patients were followed until death, second HCT, or 1 year after HCT whichever occurred first. Data was extracted from medical records and hospital and research databases. The study was reviewed by the MSK Institutional Review Board (IRB) and was granted a waiver of authorization (IRB#16–920).
Graft manipulation and conditioning regimens
Ex vivo CD34-selection was performed by the CliniMACS® CD34+ Reagent System (Miltenyi, Biotec, Gladbach, Germany). All patients received peripheral blood stem cell allografts after myeloablative (CD34-selected) or reduced intensity (CONV) conditioning regimens. Per institutional algorithm, patients with (AML or acute lymphocytic leukemia (ALL) in first complete remission (CR1) and MDS received CD34-selected HCT unless they were deemed ineligible or refused by insurance. In general, eligibility for CD34-selected HSCT included low disease burden if present, or remission; availability of ≥8/10 HLA matched donor; Karnofsky performance status (KPS) ≥70; no active infection or extramedullary disease; and no significant organ dysfunction that would preclude safe administration of myeloablative cytoreductive regimens12. Patients with lymphoma or chronic lymphocytic leukemia (CLL) received conventional HCT after reduced intensity conditioning regimens with low dose total body irradiation (TBI)13 or busulfan and fludarabine., Patients with AML included in the CONV cohort were in remission at the time of HCT.
Supportive care
All patients received acyclovir prophylaxis for herpes simplex virus (HSV) and varicella zoster virus (VZV) per institutional standards of care2. CMV R+ or those with CMV seropositive donors (D+) were routinely monitored by CMV quantitative polymerase chain reaction assays (qPCR) starting on Day (D) +14 post HCT. Initiation of preemptive therapy (PET) was at the discretion of the treating physician. Per institutional standards of care, ≥2 consecutive PCR >500copies/ml in whole blood or >300 IU/ml in plasma for CONV and ≥2 consecutive quantifiable PCR at any value for CD34-selected were the recommended thresholds for initiation of PET. Valganciclovir or ganciclovir [(val)GCV] was the preferred first line therapy. Foscarnet was used preferentially in patients with cytopenias (particularly prior to engraftment) or other contraindications to (val)GCV. Timing for initiation and choice of antiviral was at the discretion of the treating physician. PET continued until > 2 consecutive PCR below the limit of detection for CONV off steroids. CD34-selected recipients on steroids continued maintenance therapy at the discretion of the treating physician.
Laboratory methods
CMV IgG levels were determined using an automated semi-quantitative enzyme-linked fluorescent immunoassay (VIDAS, Biomerieux Inc., NC, USA). qPCR for CMV was performed by the Clinical Microbiology Laboratory at MSK using Roche analyte specific reagent (Roche Diagnostics, IN, USA; prior to March 2013) and the Cobas Ampliprep/Cobas Taqman (CAP/CTM) CMV qPCR in plasma (Roche Molecular Diagnostics, NJ, USA; March 2013 and onward). The lower limit of quantification (LOQ) and linear range (range) were >500–1.0×106 copies/mL for blood and >137–9.1×106 IU/mL for plasma14.
Definitions
CMV viremia was defined as ≥1 CMV qPCR >500 copies/ml for whole blood or >137 IU/mL for plasma. CMV disease was diagnosed using standard definitions15. GVHD scoring was based on consensus guidelines16. PET days were the total number of days on a given antiviral(s) by D +180 after HCT. Length of stay (LOS) was defined as the number of days in the hospital from the day of HCT through D+180 after HCT. Readmission LOS was the number of days in the hospital after discharge from the incident admission for HCT through Day +180 after HCT. Number of readmissions was defined as the number of admissions after the admission for HCT through Day +180 after HCT. “Well-days” were defined as the number of days alive and out of the hospital. The number of well-days was used to account for early mortality that may accounted for shorter hospitalizations17.
Statistical analysis
The incidence for CMV viremia and CMV EOD were estimated by the cumulative incidence analysis,with second HCT, relapse, death, and last follow-up before the event of interest treated as competing risks. Categorical and continuous variables were compared using the chi-square and Mann-Whitney rank- sum tests (Kruskal-Wallis tests), respectively.
The estimated unit value (EUV) provides the number of PET days by Day+180 for a hypothetical cohort of 100 patients based on our observed rates. EUV was calculated as: a×b×c×180 where a: observed cumulative incidence of CMV viremia; b: observed proportion of patients with CMV viremia who received PET; c: number of PET days per 100 patient-days among patients treated with PET18. Standardized incidence ratio (SIR) and 95% confidence intervals (CI) was defined as the observed outcome of interest (number of readmissions, readmission LOS and total LOS) for patients with CMV viremia over the observed outcome for patients without CMV viremia. Patients were followed up through Day +180, relapse, or second HCT, whichever occurred first. CIs were calculated by applied approximation for chi-square percentiles. Overall survival (OS) was estimated using the Kaplan-Meier method. The log- rank test was used for time-to-event analyses. Univariate and multivariate analyses, including logistic regression, Poisson regression and linear regression, were performed for CMV viremia incidence, number of readmission and readmission LOS, respectively. The forward stepwise method was used for model selection. Variables with p values < 0.3 entered the multivariate models and variables with p values < 0.1 stayed in the final models. Statistical analyses were performed with R, version 3.5.1.
Results
Incidence of CMV infection
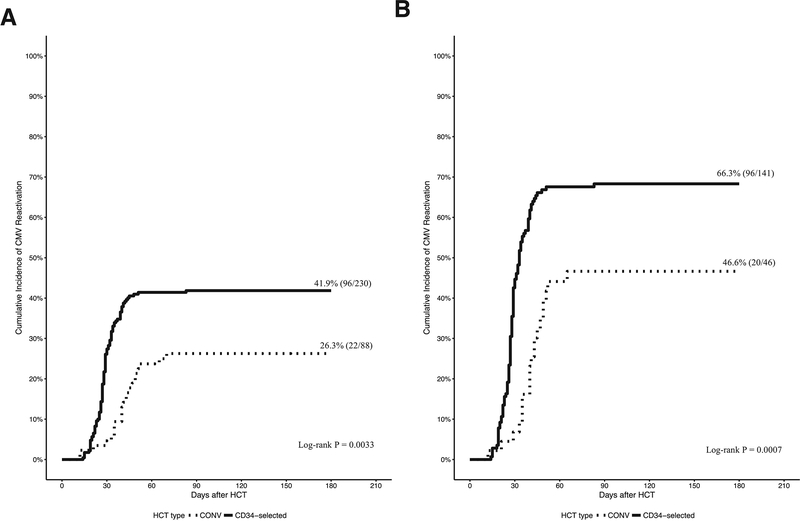
Three hundred eighteen patients, including 88 CONV and 230 CD34-selected HCT recipients were analyzed. Table 1 shows the baseline characteristics by HCT type. The differences in underlying disease, HLA mismatch and conditioning regimen intensity between the 2 groups are inherent to our institutional algorithm. The overall incidence of CMV viremia was (26.3% in CONV and 41.9% in CD34-selected HCT (Figure 1A). Among CMV seropositive recipients the incidence of CMV viremia was 46.6% in CONV and 66.3% in CD34-selected HCT (Figure 1B). The onset of CMV viremia from HCT was median 41 days [interquartile range (IQR) 35–49] and 28 days (IQR: 25–33) for CONV and CD34-selected HCT respectively. CD34-selected had a higher rate (P = 0.0033) and earlier onset of CMV viremia (P < 0.0001) compared with CONV.
Table 1.
Baseline characteristics of the cohort (Total n=318).
| Characteristic | Total (n=318) Number (%) | CONV (n=88) Number (%) | TCD (n=230) Number (%) |
|---|---|---|---|
| Age | |||
| Mean (sd) years | 53.8 (13.0) | 53.4 (12.4) | 53.9 (13.2) |
| Median (IQR) years | 56.2 (46.5–64.6) | 54.7 (47.3–63.3) | 56.9 (45.6–64.8) |
| Sex | |||
| Female | 138 (43.4) | 31 (35.2) | 107 (46.5) |
| Male | 180 (56.6) | 57 (64.8) | 123 (53.5) |
| Recipient (R) and Donor (D) CMV serostatus | |||
| R+/D+ | 108 (34.0) | 31 (35.2) | 77 (33.5) |
| R+/D− | 79 (24.8) | 15 (17.0) | 64 (27.8) |
| R−/D+ | 40 (12.6) | 15 (17.0) | 25 (10.9) |
| R−/D− | 91 (28.6) | 27 (30.7) | 64 (27.8) |
| Underlying disease | |||
| Acute lymphoblastic leukemia | 30 (9.4) | 0 | 30 (13.0) |
| Acute myeloid leukemia | 159 (50.0) | 32 (36.4) | 127 (55.2) |
| Myelodysplastic syndrome | 78 (24.5) | 5 (5.7) | 73 (31.7) |
| Chronic lymphocytic leukemia | 12 (3.8) | 12 (13.6) | 0 |
| Hodgkin’s Disease | 12 (3.8) | 12 (13.6) | 0 |
| Non-Hodgkin’s Lymphoma | 27 (8.5) | 27 (30.7) | 0 |
| Donor type | |||
| Matched related | 114 (35.8) | 39 (44.3) | 75 (32.6) |
| Matched unrelated | 149 (46.9) | 41 (46.6) | 108 (47.0) |
| Mismatched related | 3 (0.9) | 1 (1.1) | 2 (0.9) |
| Mismatched unrelated | 52 (16.4) | 7 (8.0) | 45 (19.6) |
| Conditioning regimen | |||
| Myeloablative | 230 (72.3) | 0 | 230 (100.0) |
| Busulfan/Melphalan/Fludarabine | 153 (48.1) | 0 | 153 (66.5) |
| Clofarabine/Thio-TEPA/Melphalan | 13 (4.1) | 0 | 13 (5.7) |
| TBI/Thio-TEPA/Cyclophosphamide or fludarabine | 64 (20.1) | 0 | 64 (27.8) |
| Reduced intensity | 88 (27.7) | 88 (100.0) | 0 |
| Fludarabine/Busulfan* | 67 (21.1) | 67 (76.1) | 0 |
| Melphalan | 1 (0.3) | 1 (1.1) | 0 |
| TBI/Cyclophosphamide/Fludarabine/Thio- TEPA/ | 20 (6.3) | 20 (22.7) | 0 |
Three patients also received Rituximab.
Figure 1.
Cumulative incidence of CMV viremia.
Figure 1A. Cumulative incidence of CMV viremia in CONV and CD34-selected HCT through Day +180 after HCT. CMV viremia occurred at a median 41 days (IQR 35–49) after CONV and 28 days (25–33) after CD34-selected HCT. Figure 1B. Cumulative incidence of CMV viremia among CMV R+ CONV and CD34-selected HCT through Day +180 after HCT.
To identify predictors for CMV viremia patient and transplant characteristics from Table 1 and acute GVHD (grade 2–4) were examined in univariate logistic models. Female sex, CD34-selection, CMV R+ and CMV D+ were associated with CMV viremia. In multivariate logistic model, CD34-selection and CMV R+ remained significant (Table S2). The incidence of CMV viremia of CD34-selected would be 2.7 times more compared to CONV (95% CI 1.4, 5.5) after adjusting for CMV R or D serostatus and HLA match.
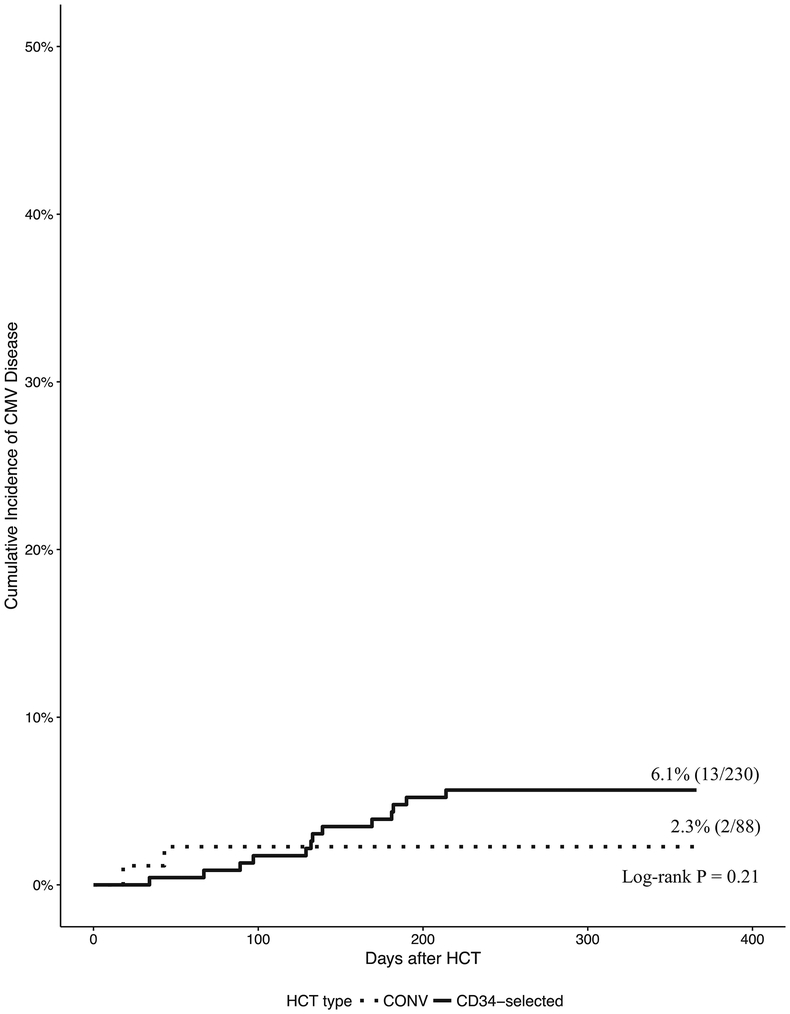
CMV disease
At 1 year, the cumulative incidence of CMV end-organ disease was 2.3% in CONV and 6.1% in CD34- selected HCT (Figure 2). 13/230 CD34-selected HCT developed CMV disease at a median of 134 days (98–182) after HCT compared to 2/88 CONV who developed CMV disease at 19 and 44 days after HCT.
Figure 2.
Cumulative incidence of CMV end-organ disease for CONV and CD34-selected HCT at 1 year after HCT.
Two of 88 CONV developed CMV disease at 19 and 44 days after HCT. Thirteen of 230 CD34- selected HCT developed CMV disease at a median 134 days (98–182) after HCT.
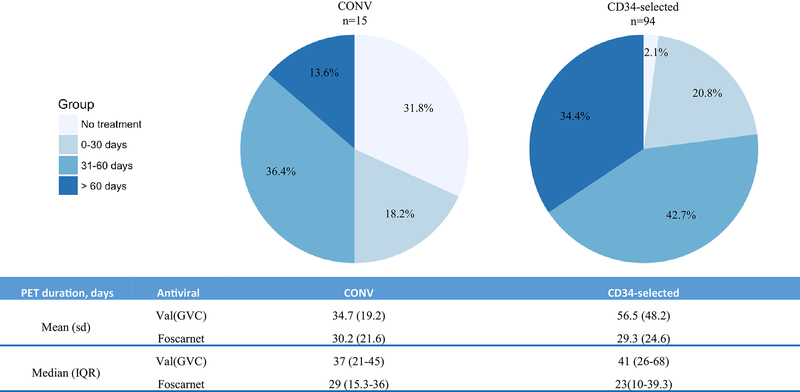
PET utilization
Figure 2 shows the distribution of PET duration by specific cut offs (upper panel) and the number of PET days (lower panel). Mean (standard deviation) and median (interquartile range) of PET days among patients who received PET are shown. A higher proportion of CD34-selected HCT received > 60 days of PET.
Next, we estimated the number of PET days per 100 patient-days and the estimated unit value (EUV) (Table 2). Of 22 CONV with CMV viremia, 15 (68.2%) received PET. Of 96 CD34-selected with CMV viremia, 94 (97.9%) received PET. The EUV for PET was 852 days for CONV and 2,821 days for CD34-selected. The EUV for CD34-selected HCT was 3-fold higher compared with CONV.
Table 2.
Treatment-days among patients received PET through Day +180.
| CONV (n=22) | TCD (n=96) | |
|---|---|---|
| Number (%) of PET among patients with CMV viremia | ||
| Val(GVC) | 15 (68.2) | 87 (90.6) |
| Foscarnet | 6 (27.3) | 40 (41.7) |
| Total | 15 (68.2) | 94 (97.9) |
| Total duration per 100-patient days | ||
| Val(GVC) | 19.6 | 33.1 |
| Foscarnet | 17.5 | 18.1 |
| Total1 | 26.4 | 38.2 |
| Estimated unit value (EUV)2, days | ||
| Val(GVC) | 633 | 2,262 |
| Foscarnet | 226 | 569 |
| Total | 852 | 2,821 |
Adjusted for overlap
EUV provides the number of PET days by Day+180 for a hypothetical cohort of 100 patients based on our observed rates.
Health care utilization
Table 3 shows a comparison of hospitalization metrics by Day +180 among CMV R−, CMV R+ with CMV viremia and CMV R+ without CMV viremia. Next, we compared R+ with viremia with all patients without viremia (including R− and R+ without viremia). Among CD34-selected, patients with CMV viremia had more readmissions, longer readmission LOS and total LOS compared to patients without CMV viremia. In contrast, among CONV the hospitalization metrics were similar between patients with viremia and without viremia. Reasons for readmission were categorized as: related to CMV, viral infection (not CMV), infection (non-viral), GVHD or other. Among CONV, CMV accounted for 9.6% and viral infection (not CMV) for 0% of readmissions. In contrast, GVHD and non-viral infections accounted for 35% of readmissions. Among CD34-selected HCT, CMV accounted for 34.5% and viral infections (non-CMV) for 13% of readmissions while GVHD and non-viral infections combined accounted for 9.8% of all readmissions. Table S1 shows number of readmission, readmission LOS and total LOS by HCT type and CMV viremia by Day +180. We also report the number of “well-days” as a measure of days alive and not hospitalized.
Table 3.
Hospitalization metrics for CMV R−, CMV R+ with CMV viremia and CMV R+ without CMV viremia.
| Median (IQR) | R− | R+ with viremia | R+ without viremia | P-value4 |
|---|---|---|---|---|
| Number of readmission1 | ||||
| CONV | 0 (0–1) | 1 (0–2) | 0 (0–2) | 0.276 |
| TCD | 0 (0–1) | 1 (0–2) | 0 (0–1) | 0.001 |
| Readmission LOS2, days | ||||
| CONV | 0 (0–8.8) | 5 (0–19.3) | 0 (0–12.8) | 0.375 |
| TCD | 0 (0–9) | 8 (0–22) | 0 (0–9) | 0.001 |
| Total LOS3, days | ||||
| CONV | 23 (18.3–34.5) | 32.5 (25.5–61.5) | 31 (20.5–38.3) | 0.090 |
| TCD | 21 (17–32) | 27 (21.8–48.3) | 25 (18–31) | 0.002 |
Number of readmission: Number of readmissions after HCT. Values per 100 patient days are adjusted to 100-patient days of follow up.
Readmission LOS: length of stay from first readmission after HCT to Day+180 or last follow up.
Total LOS: length of stay from HCT infusion to day +180 or last follow up.
P value of Kruskal-Wallis test for comparison of medians of outcomes of interest among group.
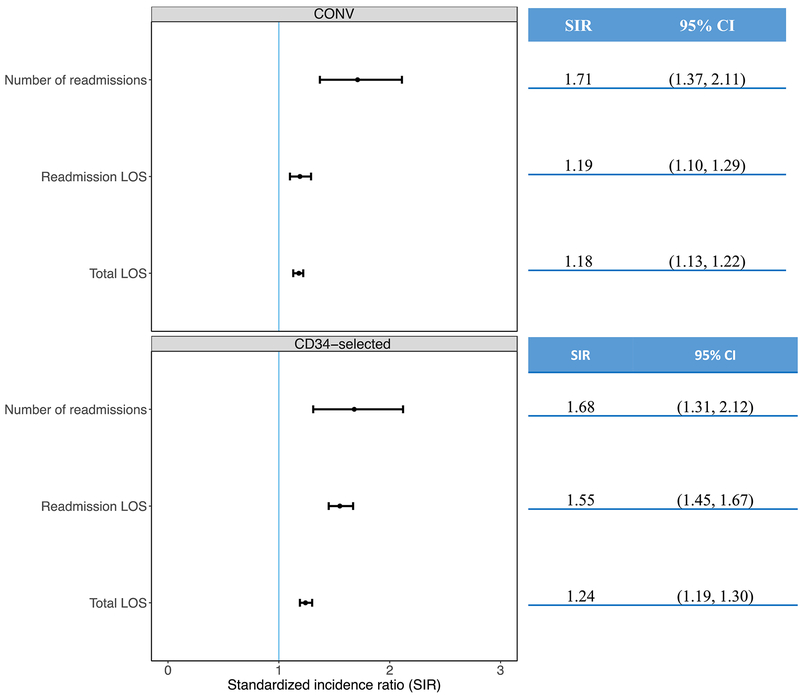
To estimate the impact of CMV on hospitalization metrics we used the standardized incidence ratio (SIR). In CONV, the SIR for overall LOS, readmission LOS, and number of readmissions was increased for patients with CMV viremia compared to patients without CMV viremia (Figure 3, top panel). CONV patients without viremia had similar well-days (days alive and out of the hospital by Day +180) [median150 days (IQR: 78–160)] compared to patients with CMV viremia [median 142 days (IQR: 75–156), P = 0.60].
Figure 3.
PET utilization by Day 180 among CONV and CD34-selected HCT.
Top panel: Percentage of patients with CMV viremia by cutoff for PET duration (no PET, <30 days, 31–60 days and >60 days. Fifty percent of CONV and 10% of CD34 received PET for <30 days. In contrast, 13.8% and 34.4$ of CONV and CD34-selected respectively received PET for >60 days Bottom panel: Mean (Standard deviation) and Median (Interquartile range) Number of antiviral days among patients who received PET.
In CD34-selected, the SIR for overall LOS, readmission LOS and number of readmissions was increased for patients with CMV viremia compared to no viremia (Figure 3, bottom panel). CD34-selected without viremia had more well-days [median155 days (IQR: 138–162)] compared to CD34-selected with CMV viremia [median 145 days (IQR: 116–158), P = 0.0003].
Next, we tried to identify predictors for the number and LOS of readmissions. In univariate Poisson models, CMV viremia, CMV R+, HLA mismatch and presence of GvHD (grade 2–4) were associated with more readmissions. In univariate models CMV viremia and HLA mismatch were associated with longer readmission LOS. The limited sample size and inflated zeros for number or readmissions and the non-Gaussian distribution of readmission LOS precluded multivariate analyses.
Overall Survival
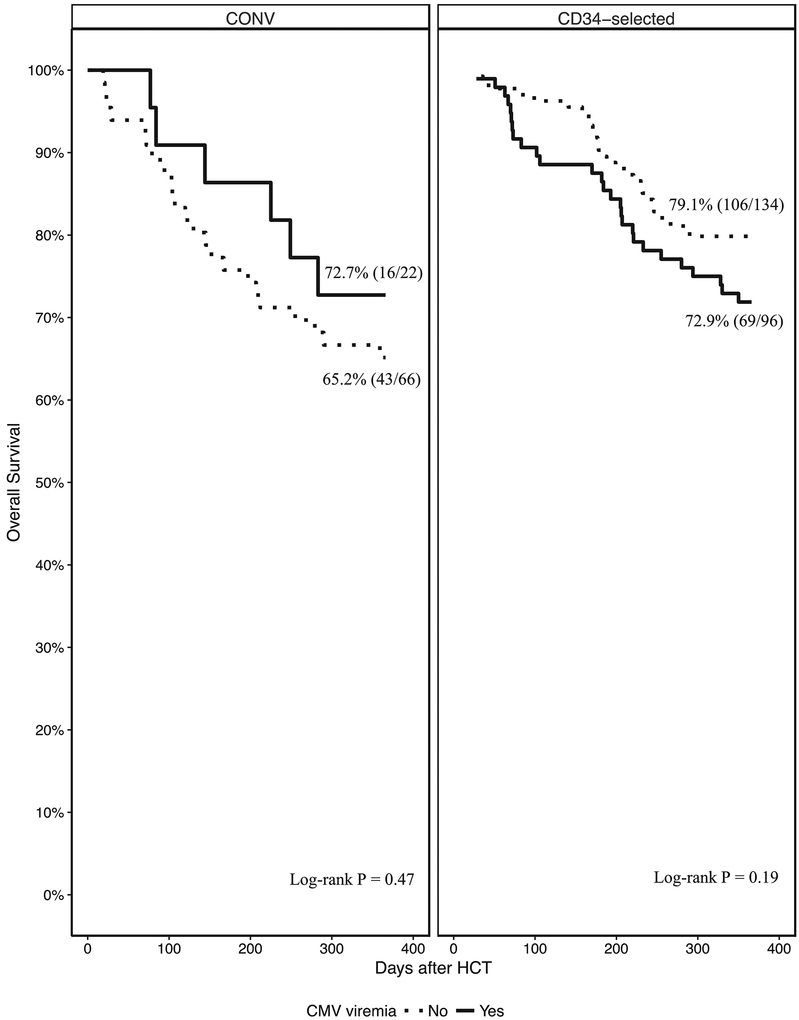
Sixty-eight percent of CONV were alive at 1-year post HCT. OS was 72.7% (16/22) and 65.2% (43/66), for CONV with and without CMV viremia respectively (P = 0.47) (Figure 4). Only 2 CONV developed CMV end-organ disease thus comparison of OS by CMV disease was not performed.
Figure 4.
Standardized incidence ratio (dots) with 95% confidence intervals (whiskers) for total LOS, readmission
LOS and total readmission count by Day 180 after HCT. Top Panel: CONV; Bottom panel: CD34-selected.
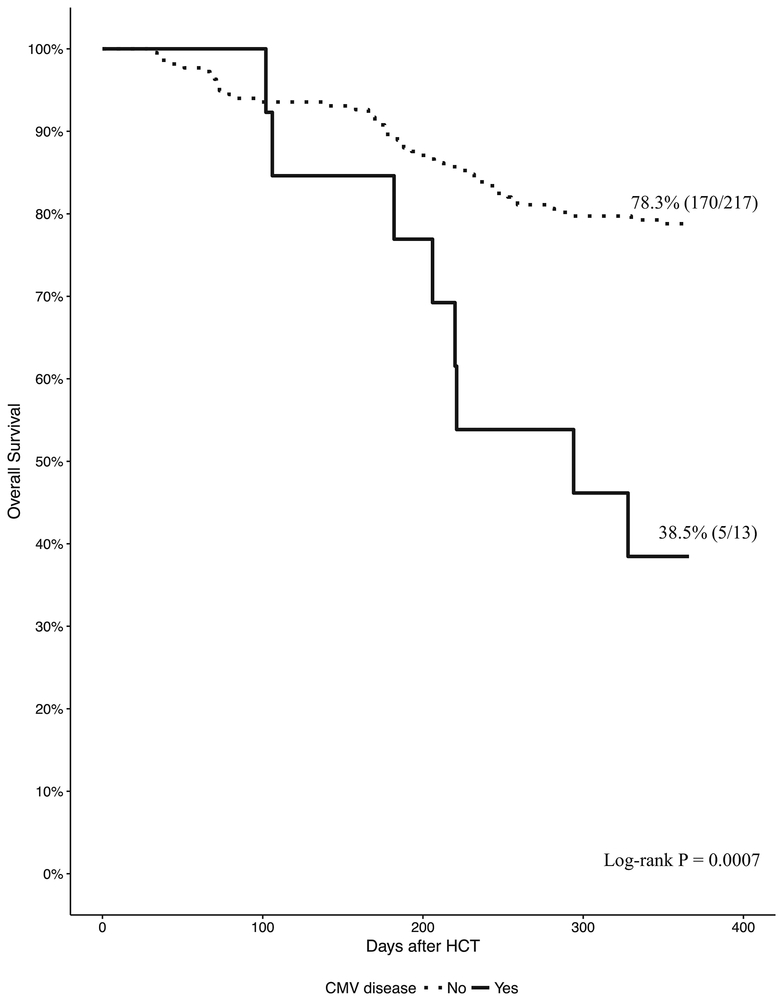
Seventy-seven percent of CD34-selected were alive at 1 year. OS was 72.9% (69/96) and 79.4% (106/134) for CD34-selected HCT with and without CMV viremia respectively (P = 0.19) (Figure 4). Thirteen CD34-selected developed CMV end-organ disease. OS was 38.5% (5/13) and 78.3% (170/217) for CD34-selected with and without CMV end-organ disease respectively (P = 0.0007).
Discussion
The impact of PET on reducing rates of CMV disease and associated mortality in HCT is well defined. In contrast, real world studies quantifying PET and health care resource utilization in HCT managed with the preemptive approach are limited.
We analyzed a contemporary cohort of CONV and CD34-selected HCT from a major US institution. CD34-selection was the preferred HCT type for patients with AML in remission and MDS. Because of the stringent T-cell depletion achieved by CD34-selection, recipients of CD34-selected HCT had higher rates of CMV compared to CONV HCT recipients. The higher incidence of CMV viremia and longer duration of PET in CD34 selected HCT contributed to the observed differences in utilization of PET and health care resources among the 2 HCT types. We have shown that delay of PET initiation in CD34-selected HCT is associated with persistent CMV viremia, antiviral resistance and CMV end-organ disease2,19. Low viral thresholds for initiating PET in high risk HCT (including CD34-selected) are used by other Institutions20. We used the estimated unit value (EUV) to report the expected number of PET days for a cohort of 100 HCT and enable comparison between HCT types. The EUV for PET duration was 3-fold higher in CD34- selected compared with CONV HCT.
Next, we examined health care resource utilization by the presence or absence of CMV viremia. The median LOS for readmissions for patients without CMV viremia and with CMV viremia was similar for CONV HCT. In contrast, among CD34-selected, patients with CMV viremia had significantly longer readmission LOS compared to those without CMV viremia. Importantly, among CD34-selected HCT, approximately one-third of readmissions were for management of CMV. Using standardized incidence ratio, we show increased total LOS, readmission LOS and number of readmissions for both HCT types with CMV viremia. Effective CMV prevention has the potential of reducing LOS and savings from reduced LOS need to be taken into consideration in cost benefit analyses of prophylaxis, specifically in CD34-selected HCT. While economic analyses were beyond the scope of our study, the currently ongoing randomized study PROGRESS II, BMT Clinical Trials Network 1301 (ClinicalTrials.gov Identifier: ) comparing outcomes between CD34-selected and CONV HCT will provide data for such analyses21.
Rates of CMV end-organ disease were similar across HCT types confirming the effectiveness of PET in preventing CMV end-organ disease. Later occurrence of CMV end-organ disease in CD34-selected reflects delayed immune reconstitution. Persistent CMV viremia typically precedes CMV end-organ disease2,19. CMV end-organ disease was associated with decreased survival in CD34-selected HCT. In the pivotal phase 3 study, patients who received letermovir prophylaxis in the first 100 days post HCT had lower rates of CMV infection and a survival advantage through week 24 compared to patients treated preemptively22. Given the late onset of CMV disease in CD34- selected HCT, extended CMV prophylaxis beyond 100 days after HCT or other strategies to restore long term CMV immunity may be warranted23–25.
Our study has several limitations. First, CMV viremia was treated according to the standards of care at our Institution. Because of inherent differences in types and stage of underlying disease between CONV and CD34-selected HCT, the risk for CMV infection is likely different. While our standards for CMV management are accordance with currently published guidelines endorsed by professional societies, variability exists across centers. As a result, our findings may not be applicable to centers with different population mix or when new CMV antivirals and other modalities may become available26. Our methodology may be used to generate center-specific data. Future studies in larger cohorts may enable development of integrative predictive algorithms for PET utilization after controlling for differences in patient characteristics and practice patterns.
In summary, we show differential PET utilization between two different HCT types; CD34-selected had a 3-fold higher PET utilization compared with CONV HCT.CMV infection was associated with more frequent and prolonged readmissions. Among CD34-selected HCT management of CMV was the main reason for readmission (in approximately one third of all readmissions). Our data provides a strong impetus for implementation of effective strategies for the management of CMV infection after HCT
Supplementary Material
Figure 5.
6-month overall survival (OS) at after HCT by CMV viremia.
OS was similar for CONV with and without CMV viremia (P = 0.53) (left panel) and CD34-selected with and without CMV viremia (P = 0.14) (right panel).
Figure 6.
1-year OS survival by CMV end organ disease in CD34-selected HCT
CMV end organ disease was associated with lower OS (P = 0.00069).
Highlights.
CMV viremia was associated with substantial PET use and hospitalizations.
PET utilization was 3-fold higher in CD34-selected compared with conventional HCT.
CMV viremia increased the length of stay and number of readmissions in CD34-selected HCT.
One third of readmissions among CD34-selected HCT were for CMV management.
Acknowledgements
Funding for the study was provided by an investigator sponsored project grant from Merck (G.A.P). Additional funding was provided by the National Cancer Institute, Cancer Center Support Grant P30 CA008748, the National Cancer Institute of the National Institute R25CA020449 (P.N) and National Institutes of Health P01 CA023766 (S.A.G and A.A.J).
Footnotes
Conflict of interest
G.A.P has been an investigator and has received funding and consulting fees from Merck, Chimerix, Shire and Astellas.
Y.T.H has received funding from Merck.
YS: no conflict of interest
MAP has received honoraria from Abbvie, Bellicum, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda. He serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has received research support for clinical trials from Incyte and Miltenyi Biotec.
S.G.: no conflict of interest
AAJ: no conflict of interest
SJK no conflict of interest
DB: no conflict of interest
PN: no conflict of interest
MM: no conflict of interest
References
- 1.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang YT, Neofytos D, Foldi J, et al. Cytomegalovirus Infection after CD34(+)-Selected Hematopoietic Cell Transplantation. Biology of Blood and Marrow Transplantation. 2016;22(8):1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milano F, Pergam SA, Xie H, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011;118(20):5689–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematology. 2016;3(3):E119–E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Camara R CMV in Hematopoietic Stem Cell Transplantation. Mediterr J Hematol Infect Dis. 2016;8(1):e2016031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosuri S, Adrianzen Herrera D, Scordo M, et al. The Impact of Toxicities on First-Year Outcomes after Ex Vivo CD34+-Selected Allogeneic Hematopoietic Cell Transplantation in Adults with Hematologic Malignancies. Biol Blood Marrow Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakubowski AA, Petrlik E, Maloy M, et al. T Cell Depletion as an Alternative Approach for Patients Older than 55 Years Undergoing Allogeneic Stem Cell Transplantation as Curative Therapy for Hematologic Malignancies. Biol Blood Marrow Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplantation. 2015;50(4):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19(6):898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YT, Kim SJ, Lee YJ, et al. Co-Infections by Double-Stranded DNA Viruses after Ex Vivo T Cell-Depleted, CD34+ Selected Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almyroudis NG, Jakubowski A, Jaffe D, et al. Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2007;9(4):286–294. [DOI] [PubMed] [Google Scholar]
- 12.Jakubowski AA, Petrlik E, Maloy M, et al. T Cell Depletion as an Alternative Approach for Patients 55 Years or Older Undergoing Allogeneic Stem Cell Transplantation as Curative Therapy for Hematologic Malignancies. Biol Blood Marrow Transplant. 2017;23(10):1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobbs GS, Kaur N, Hilden P, et al. A novel reduced intensity conditioning regimen for patients with high-risk hematological malignancies undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(7):1010–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babady NE, Cheng C, Cumberbatch E, Stiles J, Papanicolaou G, Tang YW. Monitoring of cytomegalovirus viral loads by two molecular assays in whole-blood and plasma samples from hematopoietic stem cell transplant recipients. J Clin Microbiol. 2015;53(4):1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64(1):87–91. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 17.Ballen KK, Joffe S, Brazauskas R, et al. Hospital Length of Stay in the First 100 Days after Allogeneic Hematopoietic Cell Transplantation for Acute Leukemia in Remission: Comparison among Alternative Graft Sources. Biology of Blood and Marrow Transplantation. 2014;20(11):1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becher Heiko, Winkler Volker. Estimating the standardized incidence ratio (SIR) with incomplete follow-up data. BMC Medical Research Methodology. (2017) 17:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SJ, Foldi J, Papanicolaou G, Huang Y-T. Cytomegalovirus (CMV) Resistance Associated Mutations (RAM) after ex vivo T-Cell Depleted (TCD) Hematopoietic Cell Transplant (HCT). Open Forum Infectious Diseases. 2016;3(suppl_1):2296–2296. [Google Scholar]
- 20.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.BMT CTN Protocol 1301 (PROGRESS II): A Randomized, Multi-Center, Phase III Trial of Calcineurin Inhibitor-Free Intervention for Prevention of Graft-versus Host-Disease 2017.
- 22.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017;377(25):2433–2444. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura R, La Rosa C, Longmate J, et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol. 2016;3(2):e87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Reilly RJ, Koehne G, Hasan AN, Doubrovina E, Prockop S. T-cell depleted allogeneic hematopoietic cell transplants as a platform for adoptive therapy with leukemia selective or virus-specific T-cells. Bone Marrow Transplant. 2015;50 Suppl 2:S43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzannou I, Papadopoulou A, Naik S, et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol. 2017;35(31):3547–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papanicolaou GA, Silveira FP, Langston AA, Pereira MR, Avery RK, Uknis M, Wijatyk A, Wu J, Boeckh M, Marty FM, Villano S. Maribavir for Refractory or Resistant Cytomegalovirus Infections in Hematopoietic-cell or Solid-organ Transplant Recipients: A Randomized, Dose-ranging, Double-blind, Phase 2 Study. Clin Infect Dis. 2018. October 16. doi: 10.1093/cid/ciy706. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.