Abstract
The moment of the fertilization of an egg by a spermatozoon—the point of “sperm success”—is a key milestone in the biology of sexually reproducing species and is a fundamental requirement for offspring production. Fertilization also represents the culmination of a suite of sexually selected processes in both sexes and is commonly used as a landmark to measure reproductive success. Sperm success is heavily dependent upon interactions with other key aspects of male and female biology, with the immune system among the most important. The immune system is vital to maintaining health in both sexes; however, immune reactions can also have antagonistic effects on sperm success. The effects of immunity on sperm success are diverse, and may include trade-offs in the male between investment in the production or protection of sperm, as well as more direct, hostile, immune responses to sperm within the female, and potentially the male, reproductive tract. Here, we review current understanding of where the biology of immunity and sperm meet, and identify the gaps in our knowledge.
1. Introduction
The production of sperm via spermatogenesis is well-understood from many perspectives (e.g., reviewed in Nishimura & L’Hernault, 2017). However, within the male, there is a potential trade-off between the energy demands required for making high-quality sperm and the demands of protecting the soma from infectious disease. Moreover, the female reproductive tract is a potentially hostile environment that contains molecules and cells that protect the female from infection but that may also be harmful to sperm. These defenses include a low pH and reactive oxygen species, an abundance of antimicrobial peptides, scavenging macrophages, and, in vertebrates, antibodies generated by the acquired immune system. Although they may be immunologically protective in intent, these chemical and cellular defenses have the potential to impair sperm viability or function (i.e., sperm success). Here, we discuss the interactions between sperm and the immune system, delineating areas in which function of one can impair function of the other (summarized in Fig. 1). The main themes we consider are (1) energetic trade-offs between investment by males in making sperm or by males and females in maintaining viable sperm versus investment in the immune system; (2) shared genetics, such as where specific genes have pleiotropic sperm- and immune-related functions; (3) the impact of infections in males and females, both somatic and sexually transmitted, on sperm; (4) immune responses directly against the sperm in males and females; and (5) the role of immunity in sperm selection by female choice.
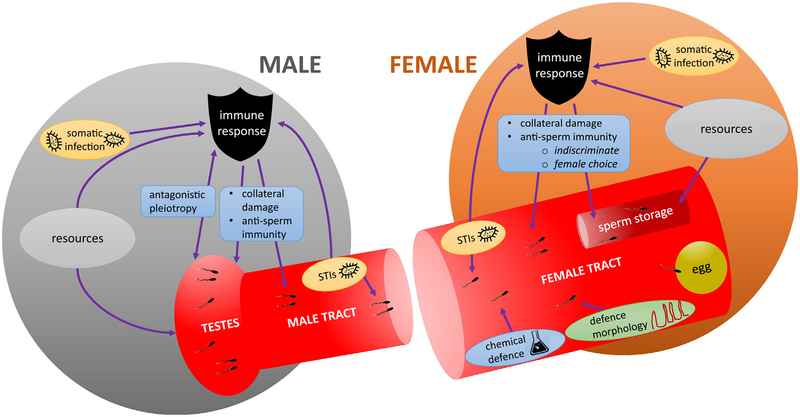
Fig. 1.
Schematic of the interplay between immunity and sperm success in males and females. Resources may trade-off between the immune system and the production of sperm in males, and the protection of sperm in storage in females, leading to sub-maximal trait values. Trade-offs could also come about genetically: genes of shared male immune and sperm-related function could generate antagonistic pleiotropy, preventing simultaneous maximization of both traits. STIs can harm sperm directly, and thus immune defense against STIs could protect sperm. However, immune responses to STIs or somatic infections could also harm sperm as a side-effect of their defensive actions, causing collateral damage to sperm. Immune responses may negatively impact sperm directly, particularly in vertebrates via anti-sperm antibodies, both in females, and in males as an auto-immune response. Female immune responses may indiscriminately harm sperm, which could contribute to infertility, or they may form part of adaptive female choice, selecting out the sperm of disfavored males after mating, but before fertilization. Within the female reproductive tract sperm interact with the chemical and morphological environment—which is likely be designed to inhibit pathogen proliferation, and may help or hinder the sperm—on the way to sperm storage sites and fertilization.
2. Sperm success and the male immune system
In the next sections, we describe how relationships and interactions between sperm and the male immune system can be both harmful (Sections 2.1–2.4) and helpful (Section 2.4) to sperm success (Fig. 1).
2.1. Expensive ejaculates and trade-offs with immunity
Given the relatively small size of sperm cells in most species (among the smallest cells in the body) in comparison to ova (among the largest), the production of sperm was once thought to be energetically cheap (Gee, 1999). However, biologists have since begun to realize that (1) males are typically limited in their ejaculate production rate; (2) females often mate with multiple males, which leads to sperm competition, in which rival ejaculates compete for access to eggs; and (3) sperm competition favours ejaculates with larger numbers of sperm (and potentially more seminal fluid) to increase their probability of winning fertilizations (Birkhead & Pizzari, 2002; Cameron, Day, & Rowe, 2007; Dewsbury, 1982; Parker, 1970; Perry, Sirot, & Wigby, 2013; Wigby & Chapman, 2004). These findings have led to the theory that males should strategically allocate sperm and seminal fluid across different matings in order to maximize their reproductive returns (Cameron et al., 2007; Parker, 1990; Parker et al., 1996). This newer perspective has now gained extensive empirical support from studies across a range of vertebrate and invertebrate species (Garbaczewska, Billeter, & Levine, 2013; Hopkins, Sepil, & Wigby, 2017; Kelly & Jennions, 2011; Sirot, Wolfner, & Wigby, 2011; Wigby et al., 2009). To increase their reproductive success, males (particularly in polyandrous or polygynandrous species) must devote considerable resources into developing large testes and accessory glands, and into using those organs to produce large volumes of sperm and seminal fluid. Together, these factors point toward non-trivial costs of ejaculate production which, in extreme cases, can lead to situations where females become sperm-limited because they fail to receive sufficient sperm from a single mating to fertilize all their eggs (Wedell, Gage, & Parker, 2002).
The fundamental assumption of life-history theory is that resources allocated to one trait will trade-off against those needed for other traits (Zera & Harshman, 2001). Ejaculates are no exception: the cost of producing them is expected to decrease resources available for other important life-history traits. The idea of such trade-offs has received empirical support from studies that suggest that investment in ejaculates compromises traits involved in attracting mates, such as ornaments or weapons (Parker & Pizzari, 2010; Simmons & Emlen, 2006; Simmons, Lüpold, & Fitzpatrick, 2017). Nevertheless, some of the most compelling evidence for trade-offs involving ejaculates comes from negative correlations between ejaculate quality and somatic immunity. The idea of an antagonistic relationship between male sexual and immunological physiology has been widely recognized in evolutionary biology, since Folstad and Karter (1992) suggested the “immunocompetence handicap hypothesis.” In vertebrates, testosterone is vital for the development of male reproductive traits, but it also suppresses immunity. The immunocompetence handicap hypothesis posits that these antagonistic effects could make male sexual characteristics reliable indicators of general male genetic quality or condition—and thus targets for sexually selected female preferences—because high levels of sexual characteristics provide evidence that those males can cope with testosterone-mediated immune suppression. A similar logic can apply to primary male sexual characteristics, whereby high testosterone levels should be associated with large testes and high-quality ejaculates, yet result in reduced immune function. In support of these ideas, negative relationships have been found between the size of the testes and the size of the spleen—which has important immune functions—in bats, Cape ground squirrels Xerus inauris, and Arctic char Salvelinus alpinus (Hosken & O’Shea, 2001; Liljedal, Folstad, & Skarstein, 1999; Manjerovic & Waterman, 2012; Roberts, Buchanan, & Evans, 2004). These data are broadly consistent with a testosterone-mediated ejaculate-immunity trade-off (assuming that testes and spleen size are reliable indicators of general testosterone and immunity levels, respectively). Nevertheless, the data do not uniformly support the concept of costly ejaculates. While a positive relationship between testes size and lice burden was found in the Cape ground squirrel (Manjerovic & Waterman, 2012), nematode infection (also an indication of depressed immunity) was negatively associated with ejaculate quality in arctic char (Liljedal et al., 1999). The complete picture is therefore one in which the consequences of investment in ejaculates for realized resistance to parasites may vary according to the taxa of the host and/or parasite.
The potential for trade-offs in males between immunity and the ejaculate is not limited to vertebrates. Studies on insects, which lack testosterone, also provide evidence in support of the immunocompetence handicap hypothesis. For example, removal of sexual selection in dung fly Scatophaga stercoraria populations, via experimental evolution under enforced monogamy, results in the evolution of decreased testis size, but increased phenoloxidase activity (Hosken, 2001). Since phenoloxidase is an important component of the insect innate immune system, these results are consistent with a testes-immunity trade-off. In field crickets Teleogryllus oceanicus, negative genetic and phenotypic correlations between sperm viability and lysozyme activity (Simmons & Roberts, 2005), and a diet-dependent reduction in viability under immune challenge (Simmons, 2011), lend support to the existence of within-individual trade-offs in resource allocation. Again supporting such trade-offs, in decorated crickets, Gryllodes sigillatus, immune challenged males make smaller spermatophores (which are discrete, sperm-containing ejaculate packages), while inducing elevated spermatophore production experimentally reduces immune capacity (Gershman et al., 2010; Kerr, Gershman, & Sakaluk, 2010). The commonality of negative relationships between measures of immunity and ejaculate quality suggests a widespread trade-off between these traits, with compromised immunity being a cost of producing a high quality, competitive ejaculate. Nevertheless, the molecular-genetic mechanisms that underpin these potential trade-offs remain largely opaque.
2.2. Antagonistic pleiotropy between sperm success and immune capacity
Another reason why sperm success and male immune capacity may show a negative association is because of constraints on fitness maximization imposed by genetic architecture. Antagonistic pleiotropy occurs when a single gene affects multiple traits related to fitness, and some allelic variants promote fitness through one trait while compromising fitness through others. Antagonistic pleiotropy could prevent males from simultaneously reaching individual optima for sperm function and immunity if genes have shared immunity and sperm-related functions. An example of this may be Thioester-containing Protein 1 (TEP1), which contributes to immune defense against malaria parasites in the mosquito Anopheles gambiae (Blandin et al., 2004). The same protein is also involved in the removal of aberrant sperm in the testes, which is important for ensuring male fertility. Remarkably, the allelic variant that promotes male fertility also renders mosquitoes more susceptible to malaria (Pompon & Levashina, 2015). If such phenomena are widespread, sperm-immunity trade-offs could arise through shared genetic function and antagonistic pleiotropy, as opposed to through resource allocation. This particular example may also provide evidence of genetic antagonism between the sexes, because only female A. gambiae are exposed to malaria parasites via blood-feeding, since males do not feed on blood.
2.3. Vertebrate male sperm auto-immunity
Roughly 1000 genes in the mouse genome have been detected as being active primarily in germ cells in the testis (Miyata et al., 2016). The protein products of many of these genes have been detected on the surface of sperm, serving as potential antigens (Tokuhiro et al., 2012). The existence of sperm-specific molecules creates the possibility that, in organisms with acquired immunity (i.e., vertebrates), males could generate antibodies against their own sperm, leading to infertility by targeting their own developing or mature sperm cells. Male vertebrates possess a range of traits to prevent the development of anti-sperm auto-antibodies. These traits include the blood-testis barrier, which prevents the immune system from encountering germ cell-specific antigens that arise during spermatogenesis (Cheng & Mruk, 2012). If this system breaks down, anti-sperm antibodies can be produced, causing immune infertility (Bohring & Krause, 2003). The “sperm protection hypothesis” suggests that immunosuppression by testosterone directly benefits sperm by protecting them from the male’s immune system (Hillgarth, Ramenofsky, & Winfield, 1997). A meta-analysis of human studies lends empirical support to this theory, as immunosuppressive corticosteroid treatments reduce anti-sperm antibodies and improve sperm performance (Skau & Folstad, 2005). More generally, selection may act to balance immunocompetence versus the risk of autoimmunity to sperm and potentially other cells. Such compromises are expected to be particularly acute in vertebrates given their acquired immune systems. Invertebrates lack any highly-specific self/non-self-recognition mechanisms, making it seem unlikely that they would recognize sperm as “foreign.” Nevertheless, invertebrates are subject to the general problem of autoimmunity (Schmid-Hempel, 2005), although it is unclear whether sperm would be a particular autoimmune target.
2.4. Effects of infections in males, including STIs, on sperm success
The previous sections have presented a number of ways in which a male’s immune system could negatively impact sperm success. The male immune system can also promote sperm success by fighting against sperm-damaging sexually transmitted infections (STIs). STIs are relevant because—by their nature—the causative pathogens are often present in the reproductive organs and thus in close proximity to sperm. STIs have been most studied in humans, and in other animals of economic importance, but evidence suggests that they are taxonomically widespread (Knell & Webberley, 2004; Lockhart, Thrall, & Antonovics, 1996). Some STIs seem to be particularly harmful to sperm. These sperm-damaging STIs include viruses present in human semen, including papillomavirus and the human immunodeficiency virus (HIV) (Dejucq & Jégou, 2001; Lai et al., 1997; Le Tortorec & Dejucq-Rainsford, 2010). Urogenital infections in men can decrease male fertility through various mechanisms. Infections may disrupt local immune regulation and thus be associated with the development of auto-antibodies to sperm, such as those described in Section 2.3. Infections may also cause an obstruction preventing the normal passage of the ejaculate, or may disrupt the function of male accessory glands and thus reduce the quality or quantity of seminal fluid. Additionally, pathogens may damage sperm directly, or via inflammation induced by the infection (Schuppe et al., 2017; Wald, 2005). Some researchers have hypothesized that STI-induced infertility in either sex is an adaptive strategy of STIs (Apari, de Sousa, & Müller, 2014). This argument is based on the idea that sperm success, resulting in fertilization (and in mammals, pregnancy), often contributes to the inhibition of female sexual activity for a period of time. Given that sexual activity is required for STIs present in females to reach new hosts, preventing fertilization can elevate sexual activity and thus STI transmission opportunities. Alternatively, if STIs reduce female polyandry—perhaps by reducing overall health—then males could potentially gain fitness from infecting their mates, if the reduction in sperm competition outweighed the various costs of the STI (Knell & Webberley, 2004; Wardlow & Agrawal, 2018). Similarly, if infection with an STI elevates short-term female reproductive output, benefitting the male that delivers the infection, then males can theoretically evolve lower resistance to STIs (Johns, Henshaw, Jennions, & Head, 2019). However, considerably more work is required to establish whether STI-mediated effects on fertility or behavior represent adaptive strategies for pathogens or their hosts, or are simply side-effects of other actions that are under selection.
Deleterious effects of infections on sperm success need not be local to the reproductive organs. Systemic infections are likely to reduce the overall condition of a male and thus diminish the pool of resources available for producing high quality sperm. As mentioned in Section 2.1, immune-challenged decorated cricket males make smaller spermatophores (Kerr et al., 2010), consistent with an immune-mediated reduction in condition that, in turn, impacts sperm. Negative effects of immune system activation on sperm function have also been seen in great tits Parus major (Losdat et al., 2011) and guppies Poecilia reticulata (Devigili et al., 2017), lending further support to the idea. However, a similar test in the house sparrow Passer domesticus, while demonstrating a negative impact of infection on testosterone, failed to detect harm to sperm quality (Needham et al., 2017). Thus, whether somatic infections negatively impact sperm is likely specific to the host or parasite species, or dependent on the strength of infection.
3. Sperm success and the female immune system
In the next sections, we describe how interactions between sperm and the female immune system can potentially be harmful to sperm (Sections 3.1 and 3.2) but also can be important in determining which particular sperm are successful (Section 3.3) (Fig. 1).
3.1. Female reproductive tract immunity effects on sperm success
The organs of the female reproductive tract are subject to being colonized by pathogens and can also provide a pathway into the body cavity, where pathogen proliferation can wreak havoc, causing major health and fitness consequences (Soper, 2010). The female therefore has multiple adaptations to impede the invasion and proliferation of pathogens in the reproductive tract, or their ascent within it. Physical impediments in the female tract include (1) production of a cleansing outward flow of fluid (in mammals, at least; Mullins & Saacke, 1989), (2) secretion of a thick (viscoelastic) mucus (also in mammals, at least; Taherali, Varum, & Basit, 2018, (3) construction of narrow labyrinths of passages (seen in Muro et al., 2016; Suarez, Brockman, & Lefebvre, 1997; Yaniz et al., 2014). Chemical impediments include (4) acidification of the vaginal fluid in mammals to a level that kills bacterial pathogens (O’Hanlon, Moench, & Cone, 2013) and may also damage sperm. Interestingly, this acidity is known to be produced by bacteria resident in the vagina, particularly Lactobacillus spp. (O’Hanlon et al., 2013). (5) Last, and our primary focus, are immunological impediments to pathogen survival. These can include components of the innate immune system (including inflammatory responses, reactive oxygen species, and antimicrobial peptides) that could potentially damage sperm. In addition, because sperm, like pathogens, contain proteins (i.e., antigens) that are foreign to the female, vertebrate females have the potential to develop antibodies to sperm proteins. Potentially, these various types of impediments, presumably evolved for use against pathogens, could block the survival of sperm or their ability to reach the site of fertilization.
Sperm are more adept at circumventing many of these impediments than are most pathogens. First, due to the shape of the sperm and their mechanism of swimming, hydrodynamic interactions of mammalian sperm with the fluid flow in the female tract enable the sperm to orient and swim “upstream” in the tract, toward the site of fertilization (Miki & Clapham, 2013; Tung et al., 2015a, 2015b). Muscle and organ movements in the tract can also facilitate the movement of sperm toward storage sites (in Drosophila; Adams & Wolfner, 2007; Avila & Wolfner, 2009; Mattei et al., 2015; and reviewed in Suarez & Pacey, 2006). The evolution of shapes, energetics, or swimming behaviors that allow individual sperm to move faster also include mechanisms by which sperm can cooperate (reviewed in Pizzari & Foster, 2008; Immler, 2008), and in some cases bundle together and swim faster than single sperm (e.g., hooks on deer mouse Peromyscus maniculatus sperm head; Moore, Dvorakova, Jenkins, & Breed, 2002). Second, mammalian sperm with normal motility and morphology readily swim through the mucus that fills the cervix of estrous females, as demonstrated in cattle and humans (Anilkumar et al., 2001; Katz, Slade, & Nakajima, 1997). This ability is based, at least in part, on physical hydrodynamic interactions between sperm and the viscoelasticity of the cervical mucus (Katz et al., 1997). Further along the tract, mucous secretions have been detected in the lumen of the oviduct (Jansen, 1980; Jansen & Bajpai, 1982; Suarez et al., 1997). Transillumination of mouse oviducts reveals that sperm swim quickly within the lumen, despite the presence of the secretions (Suarez, 1987). Third, again in mammals, there is evidence that some labyrinth-like structures that pose physical impediments to most pathogens actually serve as guides to sperm. For example, the microgrooves that line the cervical canal in cattle can provide privileged pathways through the cervix for sperm. These pathways cannot be accessed by some pathogens that cause STIs, such as trichomoniasis (Tung et al., 2015a). Fourth, while sperm are sensitive to destruction by the acidity of the fluid of the vagina, they are protected by pH buffers in the seminal plasma (Fox, Meldrum, & Watson, 1973; O’Hanlon et al., 2013; Owen & Katz, 2005). Additionally, male-derived seminal proteins can help sperm reach and enter (Drosophila) or bind to (bovine) sperm storage sites (Avila & Wolfner, 2009; Gwathmey, Ignotz, & Suarez, 2003; Gwathmey et al., 2006; Neubaum & Wolfner, 1999; Bloch Qazi & Wolfner, 2003). In mammals, either the sperm leave the vagina rapidly by swimming into the more neutral mucus in the cervical canal (e.g., humans, cattle; Sobrero & Macleod, 1962) or the male bypasses the vagina altogether and deposits semen directly into the cervix or uterus (e.g., rodents, pigs; Bedford & Yanagimachi, 1992; Hunter, 1981). Finally, in some taxa, males provide their own physical protection for sperm: either placing them within the female inside a spermatophore sac, or covering the sperm with a “coat” that is removed later, during storage (Friedländer & Gitay, 1972; Wedell, 1993).
In producing immune responses, the female is faced with a balance between protecting herself against pathogen invasion versus potentially compromising fertilization. The responses of the innate immune system, including inflammatory responses, rapidly generate protection against pathogens and serve as a first line of defense. This system is activated by small molecular motifs conserved in pathogens, such as bacterial lipopolysaccharides and peptidoglycans, and depends on receptors present on immune cells, particularly Toll-like receptors in mammals and peptidoglycan recognition proteins in insects (e.g., reviewed in Dziarski & Gupta, 2006; Kurata, 2014; Sheldon, Owens, & Turner, 2017). Such molecular motifs are not present on the surface of sperm, but bacteria are commonly introduced to the female tract by the male during mating (Cottell et al., 2000; Schulze et al., 2018; Sidaway, 2016) and could trigger an innate immune response. In addition to inflammatory responses triggered by incidentally introduced microbes, cells of the Drosophila female tract, as well as mucosal epithelial cells and neutrophils of the mammalian female tract, produce anti-microbial peptides that can kill some bacteria and viruses (Amjadi et al., 2014). In Drosophila at least, further production of these anti-microbial peptides is induced by mating (Kapelnikov et al., 2008; McGraw, Clark, & Wolfner, 2008), which may serve as a first line of defense for the sperm against microbes that may be present in the female reproductive tract, or introduced during mating. However, recent data have indicated that the vagina, uterus, and oviduct of mammals all contain their own resident, and presumably largely commensal, bacterial biomes. Furthermore, sequencing of 16S rDNA from these microbiomes indicates that the composition of bacterial species in the vagina, uterus, and oviduct differ, despite sharing several species (Chen et al., 2017; Moreno & Franasiak, 2017). The effect of these resident bacteria on immunological activity and fertility is not well understood.
In vertebrates, the female tract can also activate acquired immune responses, which enlist specific antibodies in the defense against pathogens. As in males, females can develop anti-sperm antibodies, but in females the sperm surface proteins in the reproductive tract are non-self and thus clear potential antigens (Tokuhiro et al., 2012). Although sperm-surface antigens could theoretically always provoke an immune response, only 2–3% of women are estimated to produce anti-sperm antibodies (Clark & Schust, 2013). However, in this subset of women anti-sperm antibodies have long been identified as a likely cause of infertility (Dondero et al., 1993; Meaker, 1922). A higher frequency of immunoglobulins IgG and IgA is found on cervicovaginal secretions and sera of women with fertility problems in at least some populations, providing further evidence of their potential role in infertility (Mahdi et al., 2011; Lu, Huang, & Lu, 2008; but see Vujisić et al., 2005). In estrous cervical mucus, anti-sperm antibodies are usually at levels far below those in the bloodstream (Stern et al., 1992). However, mucus samples containing anti-sperm antibodies are known to inhibit movement of sperm (Chiu & Chamley, 2004), suggesting a potential infertility mechanism.
Involvement of acquired immune responses in differential fertilization success is also well established in birds. In domestic fowl, Gallus domesticus, female exposure to sperm leads to an immune response, which can reduce the fertilizing efficiency of inseminations. For example, experimental injection of testicular material into hens depresses the fertility of females for the following 12–67 days (McCartney, 1923). This response to sperm exposure is associated with rising levels of sperm antibodies in blood plasma and specifically in serum levels of sperm agglutinins, which modulate sperm agglutination in vitro (Hosoda, Abe, & Otsuka, 1964; Wentworth & Mellen, 1963; see Haley & Abplanalp, 1970 and Burke, Rieser, & Shoffner, 1971 for similar patterns in turkeys, Meleagris gallopavo, and Japanese quails, Coturnix japonica, respectively). However, it remains unclear whether these same patterns of agglutination occur in vivo, or whether agglutination harms sperm success.
Avian IgY (the equivalent of mammalian IgG) appears to play a particularly important role in female anti-sperm responses in birds. For example, an excess of sperm recovered from the vagina of domestic fowl hens following insemination are bound by IgY compared to sperm recovered from the distal end of the female reproductive tract (the infundibulum), where fertilization occurs, suggesting that IgY may be implicated in targeting sperm (Steele & Wishart, 1992). Interestingly, antibodies bound to sperm are also found in experimentally inseminated hens who had never before mated, suggesting that immunity against other antigens (e.g., pathogens) may have cross-reactivity, causing sperm targeting (Bakst, 2011). Similarly, turkey hens with low fertility expressed IgY on the epithelium of their sperm storage tubules, a site that is typically immunosuppressed (Bakst, 2011; Das, Isobe, & Yoshimura, 2008) and where IgY is not expressed in hens with high fertility (Kirk et al., 1989). In some lines of domestic fowl, female infertility is associated with infiltration of lymphocytes in the sperm storage tubules as well as high numbers of antigen-presenting cells (Ia + cells), and T cells (CD4+, CD8+) (Das, Nagasaka, & Yoshimura, 2005), while in Japanese quails, insemination is followed by a rapid increase of leukocytes (neutrophils and lymphocytes) in the utero-vaginal junction, some of which are in close contact with the inseminated sperm, suggesting that female immune reactions could negatively affect sperm following mating (Higaki et al., 1995).
To avoid the problem of anti-sperm antibodies causing infertility, it seems likely that the innate immune system is especially active as a defensive system in the female tract of mammals, as it is in insects such as Drosophila. The male-derived strategy of moving sperm rapidly out of the vagina, or bypassing the vagina altogether in species such as bedbugs, where males inseminate directly into the body cavity (but see Reinhardt, Naylor, & Siva-Jothy, 2003), is also thought to help evade local female immune responses (Suarez, 2015). Combined with the deposition of large numbers of sperm this strategy could also increase the chance that some sperm will survive those attacks. In some insect species, such as Drosophila pseudoobscura and some lepidopterans, the male introduces two types of sperm into the female: “eusperm,” which are functional for fertilization, and the more numerous “parasperm,” which are thought to act as decoys against harmful molecules in the reproductive tract and perhaps titrate out such molecules (Holman & Snook, 2006, 2008; Swallow & Wilkinson, 2002). Mammals also appear to use a “strength-in-numbers” strategy: large numbers of inseminated sperm reach the mammalian uterus, and from there thousands or tens of thousands pass into the lower oviduct (Overstreet & Cooper, 1978; Suarez et al., 1997). In response to the large numbers of sperm that enter the uterus, an innate immune response eventually builds up in the uterine cavity. Large numbers of neutrophils enter the cavity to trap and kill sperm by extruding DNA extracellular traps (Alghamdi & Foster, 2005; Zambrano et al., 2016) and phagocytosing sperm (Hong et al., 2017). In this way, the neutrophils eventually clear sperm from the uterine cavity, helping to prepare the uterus for implantation (Troedsson et al., 2001).
3.2. Immunity and sperm storage in females
Although sperm-use often begins relatively rapidly after copulation, in many species sperm can be stored for some period before use—typically days or weeks—but in some species up to years (reviewed in Orr & Brennan, 2015; Orr & Zuk, 2012; Schnakenberg, Siegal, & Bloch Qazi, 2012). In particular, females of invertebrate taxa, such as insects, commonly store sperm in specialized organs, such as seminal receptacles and spermathecae, that bud off the main conduit in the female tract, and may provide protected sites for the sperm. However, many female mammals also contain sperm reservoirs, where sperm can be maintained alive prior to ovulation, for up to a few days in most species (up to 5 days in humans; Holt & Fazeli, 2016), or as long as throughout the winter during hibernation in the some bats (Racey et al., 1987; Suarez & Pacey, 2006). In storage reservoirs, sperm speed and numbers would not likely be sufficient to protect sperm against immune responses. Given that the female immune system has the potential to destroy sperm, storage organs must therefore protect sperm from the female’s own immunity, presumably by being areas of low immunological activity (reviewed in Orr & Brennan, 2015). Although mammalian sperm are held in the storage reservoir of many (if not all) species by binding to the mucosal epithelium, and thus are exposed to fluid contents of the female tract, here they seem protected against attack by the innate immune system. In cattle, binding of sperm to oviductal mucosal epithelium in vitro upregulates expression of anti-inflammatory cytokines (TGFB1 and IL10) in the epithelial cells (Yousef et al., 2016) which may help to protect sperm from harmful immune action. Still, much remains to be learned about how the immune system may be repressed in the oviduct during sperm storage and how epithelium binding helps maintain vitality of the sperm (Suarez, 2016).
Several studies on insects provide evidence of interplay between female immunity and sperm in storage. Experiments using the field cricket T. oceanicus provide evidence that experimental bacterial infections in females reduce the viability of sperm in storage (McNamara, van Lieshout, & Simmons, 2014), while in D. melanogaster, stimulation of the immune system using a pathogen mimic (peptidoglycan) results in reduced viability of stored sperm (Radhakrishnan & Fedorka, 2012). Effects of infections on stored sperm viability could arise through one of several mechanisms. First, the impact of infection on stored sperm could represent a resource trade-off in female investment between immune responses versus investment in maintaining the viability of sperm in storage. However, in D. melanogaster, sperm survive well in spermathecae dissected out of the female body. Given that removed organs are cut-off from somatic resources this finding argues against a trade-off (Radhakrishnan & Fedorka, 2012). Second, it may be that the bacteria interact with—and harm—the sperm directly. In vitro studies using bed bug Cimex lectularius sperm show that incubation with environmental microbes damages sperm (Otti, McTighe, & Reinhardt, 2013), raising the potential they may also be able to do so in vivo. Finally, sperm may be harmed as a side-effect of the female’s immune response, for example, if the by-products are especially toxic to sperm (McNamara et al., 2014; Radhakrishnan & Fedorka, 2012). Males may thus act to suppress the female immune system. Evidence in the cricket, Allonemobius socius, is consistent with the idea that the male ejaculate interferes with the female immune system, potentially to help prevent sperm from being harmed (Fedorka & Zuk, 2005). Studies of D. melanogaster have also revealed an increase in the frequency of empty sperm storage organs in females that had mated to males whose genitalia had been dipped in peptidoglycan, which thus might approximate a male infected with an STI (Radhakrishnan & Fedorka, 2012). These data suggest that females eject the sperm of these “infected” males from their storage organs, perhaps to remove dead sperm or avoid further infection, raising the possibility that STI-infected males suffer an additional loss of sperm success via female rejection of their sperm from storage.
Comparable patterns have been seen in other insect species. A negative relationship between sperm storage and immunity occurs in a species with extreme long-term sperm storage: the ant, Atta colombica. Atta queens can live for decades, and it is thought that their reproductive success is at least partly limited by the ability to store sufficient numbers of sperm, which are required to fertilize eggs to produce workers for the colony. In this species, females found with more sperm in storage, and who store the sperm of more males, display a lower encapsulation response (the surrounding of pathogens and parasites with layers of dead melanized hemocytes), which is consistent with costs arising from storing sperm in general, and from encountering multiple male genotypes (Baer, Armitage, & Boomsma, 2006). However, being a correlational result, it is also possible that females who display lower encapsulation responses tend to mate with more males and store more sperm. Either way, the work highlights that sperm storage and immunity are often intimately—and negatively—associated.
3.3. The role of female immunity in post-copulatory sexual selection: Immune-mediated cryptic female choice
The high potential for female immune responses to sperm, as outlined above, presents the opportunity to generate post-copulatory sexual selection. As described earlier, where females mate with more than one male (which is very common; reviewed in Taylor, Price, & Wedell, 2014), the sperm of different males can compete for fertilization opportunities in sperm competition (Parker & Pizzari, 2010; Wigby & Chapman, 2004). However, this situation also creates the conditions necessary for cryptic female choice (CFC) whereby females generate a systematic bias in the paternity share of competing males (Eberhard, 1996; Firman et al., 2017), and there are good reasons to believe that CFC can occur via the female immune response. Here, we briefly discuss: (1) the scope for immune-mediated CFC across taxa, (2) the adaptive significance and functional causes of immune-mediated CFC, (3) underpinning immunological mechanisms, and (4) evolutionary consequences.
The scope for female immunity to drive CFC is strongly dependent on taxon and reproductive system. In external fertilizers, where partner interactions are limited or altogether absent, female immunity is expected to have a limited role in post-copulatory sexual selection, although immune factors on the egg could potentially influence fertilization patterns via differential sperm chemotaxis (Eisenbach & Giojalas, 2006; Evans et al., 2012). Female immunity is expected to be more important in internal fertilizers, particularly those with prolonged female sperm storage and acquired immune responses where responses have the potential to be dynamic and change over time.
Three non-mutually exclusive scenarios could drive immune-mediated CFC. Immune-mediated CFC may represent an adaptive female strategy for (1) her own viability, by neutralizing ejaculates that contain pathogens, or in response to ejaculate traits with harmful effects on females (Morrow & Innocenti, 2012) or (2) increasing the fitness of her offspring, by selecting sires that maximize offspring viability or reproductive success. Alternatively (3) immune-mediated CFC could be a non-adaptive by-product of some other naturally-selected function, e.g., resistance against certain pathogens/parasites may generate an immune predisposition against the sperm of certain male phenotypes. A bias in paternity share is selected for only in the second hypothesis, while it is a non-adaptive consequence in the other two.
One class of genes that has been hypothesized to play a particularly important role in immune-mediated CFC is the Major Histocompatibility Complex (MHC), also known as Human Leukocyte Antigen (HLA) complex in humans. The MHC plays a fundamental role in vertebrate acquired immunity by enabling antigen presentation to T cells and self versus non-self recognition. The extreme polymorphism of the MHC has long attracted evolutionary explanations, including a role for sexual selection (Edwards & Hedrick, 1998). In principle, females may be able to optimize the MHC of their offspring by biasing fertilization in favor of sperm or ejaculates with certain MHC haplotypes (Firman et al., 2017; Milinski, 2006; Ziegler, Kentenich, & Uchanska-Ziegler, 2005). Consistent with this idea, Zheng et al. (2001) found that insemination upregulated MHC class II genes, in certain regions of the female reproductive tract of domestic fowl hens. MHC class II genes code for molecules involved in the recognition and presentation of extracellular antigens by antigen-presenting cells, and thus represent the class of MHC that one would expect to be involved in recognition of sperm antigens. Løvlie et al. (2013) found that female red junglefowl, Gallus gallus, retain more sperm following natural mating by males that are less MHC-similar, suggesting that female fowl select against the sperm of genetically related males to avoid inbreeding, as indicated by previous work (Pizzari, Løvlie, & Cornwallis, 2004). However, further research has failed to find strong evidence that MHC similarity plays an important role in female sperm selection above and beyond inbreeding avoidance (e.g., Collet, 2010). The idea of MHC-based sperm selection finds further empirical support in the externally-fertilizing three-spined stickleback, Gasterosteus aculeatus, where fertilization is biased in favor of combinations of gametic MHC haplotypes that maximize offspring viability (Lenz et al., 2018). However, in whitefish, Coregonus sp., there is no evidence for MHC effects (Wedekind et al., 2004). One possible explanation of the discrepancy between the Lenz et al. (2018) and Wedekind et al. (2004) studies is that the latter considered MHC genotypes of male and female partners, in contrast to variation in MHC haplotypes of individual gametes, which was used in the former study. An effect of individual MHC sperm haplotypes consistent with the results of Lenz et al. (2018) would require MHC haploid expression in mature sperm cells, although evidence for this remains limited. Thus, the importance of MHC in immune-mediated CFC requires considerably more investigation.
Regardless of underpinning mechanisms, patterns of sperm selection via female immunity may have far-reaching evolutionary implications. Males are expected to evolve strategies to escape female immune responses. For example, it is possible that the abundance of seminal fluid proteins with antimicrobial properties detected in the male seminal fluid of different species (Borziak et al., 2016; Dorus, Skerget, & Karr, 2012) may be partly explained by males attempting to lower the microbial load of an ejaculate in order to reduce the risk of triggering female immune reactions. Other strategies may include masking the antigenicity of the sperm surface through components of the glycocalyx, the matrix of hundreds of glycoproteins and glycolipids that encapsulates the entire sperm cell and plays a key role in sperm transport within the female reproductive tract. The sperm glycocalyx is rich in sialic acids, which tend to occupy the outermost part of the sperm surface. In mammals, highly sialylated glycoproteins such as beta defensins can play a key role for protecting sperm against female antibody binding (Yudin et al., 2005) and phagocytosis by macrophages (Ma et al., 2016). Similarly, in domestic fowl, experimental desialylation leaves sperm functionally intact in vitro but prevents them from moving through the female vagina following insemination (Steele & Wishart, 1992). These patterns indicate that sialic decoration of the sperm glycocalyx may have evolved at least partly as a male adaptation to evade anti-sperm female immune responses (Ma et al., 2016).
These tight coevolutionary immune interactions between sperm and female reproductive tract may in turn contribute post-copulatory prezygotic reproductive isolation and ultimately speciation. For example, Ghaderi et al. (2011) recently presented compelling evidence of prezygotic reproductive barriers mediated by female immune responses against the sperm glycocalyx in mammals. Humans lost the ability to produce a type of sialic acid common in other mammals, the N-glycolylneuraminic acid (Neu5Gc), approximately 3 million years ago, and are now capable of producing Neu5Gc-specific antibodies. Female mice immunized to express anti-Neu5Gc antibodies suffer from lower fertility when mated with transgenic Neu5Gc-positive males. Moreover, human anti-Neu5Gc antibodies target Neu5Gc antigens on the sperm of chimpanzees, Pan troglodytes, and kill these sperm. Thus, female immunity against Neu5Gc-carrying sperm may have played an important role in human evolution by creating reproductive isolation in ancestral polymorphic hominin populations (Ghaderi et al., 2011). Female immune responses to sperm have also been advocated to explain patterns of post-copulatory pre-zygotic reproductive isolation in bird populations, such as between Japanese quails and domestic fowl, mediated by female differential sperm agglutination (Haley & Abplanalp, 1970), and between collared flycatchers, Ficedula albicollis, and pied flycatchers, F. hypoleuca, mediated by acquired immunity (Cramer et al., 2016).
Collectively, these empirical results indicate that female anti-sperm antibody responses have the potential to systematically bias the outcome of sperm competition, with potentially far-reaching ramifications for inter-sexual coevolution and speciation. These responses, however, are likely to be complex and temporally dynamic, and to date, the extent of immune-mediated CFC remains to be defined.
4. Conclusions
It is clear that sperm success and immunity are linked in varied ways, from the onset of spermatogenesis in the male, through to the moment of fertilization in the female (in internal fertilizers). However, our review has identified many gaps that remain in our current knowledge. For example, although several lines of evidence suggest that males often face resource trade-offs between investing in immunity versus investing in sperm production the mechanisms underpinning resource trade-offs remain opaque. One potentially fruitful future approach may be to use isotope labeling of molecular components of food, for example, heavy lysine (de Godoy, 2014), and track where those molecules end up. By quantifying the amount of consumed material in testes versus immunoactivity organs, it may be possible to test the idea that resource use by sperm and the immune system is a zero-sum game. A similar approach may help us better understand the costs of sperm storage in females.
Although testosterone has long been invoked as a potential hormonal mediator of the immunity versus sperm trade-off in vertebrates, our understanding in invertebrates lags behind. Juvenile hormone is a broad scale mediator of immunity and reproduction in insects (Schwenke, Lazzaro, & Wolfner, 2016), yet its relevance for direct impacts on sperm remains little studied, and ripe for investigation. Similarly, whereas anti-sperm immunity in both females and males clearly plays a key role in fertility in vertebrates, we know very little about whether immune systems directly impact sperm in invertebrates. Again, this is an area ripe for investigation, with studies at cellular level likely to provide us the biggest advances in knowledge.
Antagonistic pleiotropy, or more broad negative epistatic effects between genes involved in immunity and sperm, remains almost entirely the subject of speculation, with very little data to go on. As our general functional genetic understanding of model organisms improves, we may stumble upon examples, but the field lacks the same investment as has been seen in aging, where 100s of candidates of antagonistic pleiotropy between senescence and early life-benefits have been identified (Austad & Hoffman, 2018). Even a much more modest effort to understand the shared genetics of immunity and male sperm success would provide a considerable new insight into the mechanisms linking immunity and sperm, with potentially large benefits to the fields of disease and reproductive biology.
STIs clearly impact fertility in many cases, often via harmful effects on sperm. A key current question is whether STIs harm sperm as an adaptive strategy, to promote their own transmission, or merely as a side effects of other aspects of their biology. Answering this question will likely require development of mathematical theory (e.g., Johns et al., 2019) as well as careful empirical experimentation, particularly in short-lived lab systems that allow observation of experimental evolution.
It is certain that the immune systems of both the female and male present a risk to sperm, particularly in species with acquired immunity. It is perhaps remarkable that males and females avoid complete destruction of the sperm and remain fertile. Although we have some understanding of how females and males protect sperm from their own immune system, much remains to be discovered about sperm protection and about what makes the mechanisms break down, leading to infertility. The intriguing possibility that immune targeting of sperm could be co-opted by females to discriminate among the sperm of different males has far-reaching ramifications for evolution. Progress in identifying the discrimination among specific sperm phenotypes and genotypes by the female immune system may require novel approaches such as labeling the sperm of individual males, or haploid subsets of sperm, in order to directly visualize their fate within the female immune environment.
Acknowledgments
We thank Ruth Lehmann for the invitation that allowed us to probe this interesting topic together. The literature in this field is large, and we apologize to those authors whose excellent work could not be cited here due to space limitations. We thank the following sources for support: NIH grants R01-HD038921 (M.F.W.) and R01-HD059060 (M.F.W. and AG Clark), a BBSRC fellowship to S.W. (BB/K014544/1), and an industrial LINK award from the BBSRC and Aviagen® to T.P. (BB/L009587/1).
References
- Adams EM, & Wolfner MF (2007). Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. Journal of Insect Physiology, 53, 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi AS, & Foster DN (2005). Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biology of Reproduction, 73(6), 1174–1181. [DOI] [PubMed] [Google Scholar]
- Amjadi F, et al. (2014). Role of the innate immunity in female reproductive tract. Advanced Biomedical Research, 3(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilkumar R, et al. (2001). Correlation between the spermatozoal characteristics and sperm penetration distance in polyacrylamide gel and bovine cervical mucus. Theriogenology, 55(2), 685–691. [DOI] [PubMed] [Google Scholar]
- Apari P, de Sousa JD, & Müller V (2014). Why sexually transmitted infections tend to cause infertility: An evolutionary hypothesis. PLoS Pathogens, 10(8), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN, & Hoffman JM (2018). Is antagonistic pleiotropy ubiquitous in aging biology? Evolution, Medicine, and Public Health, 1, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, & Wolfner MF (2009). Acp36DE is required for uterine conformational changes in mated Drosophila females. Proceedings of the National Academy of Sciences of the United States of America, 106(37), 15796–15800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B, Armitage SAO, & Boomsma JJ (2006). Sperm storage induces an immunity cost in ants. Nature, 441(7095), 872–875. [DOI] [PubMed] [Google Scholar]
- Bakst MR (2011). Physiology and endocrinology symposium: Role of the oviduct in maintaining sustained fertility in hens. Journal of Animal Science, 89(5), 1323–1329. [DOI] [PubMed] [Google Scholar]
- Bedford JM, & Yanagimachi R (1992). Initiation of sperm motility after mating in the rat and hamster. Journal of Andrology, 13(5), 444–449. [PubMed] [Google Scholar]
- Birkhead TR, & Pizzari T (2002). Postcopulatory sexual selection. Nature Reviews Genetics, 3(4), 262–273. [DOI] [PubMed] [Google Scholar]
- Blandin S, et al. (2004). Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell, 116(5), 661–670. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi MCB, & Wolfner MF (2003). An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. The Journal of Experimental Biology, 206, 3521–3528. [DOI] [PubMed] [Google Scholar]
- Bohring C, & Krause W (2003). Immune infertility: Towards a better understanding of sperm (auto)-immunity: The value of proteomic analysis. Human Reproduction, 18(5), 915–924. [DOI] [PubMed] [Google Scholar]
- Borziak K, et al. (2016). The seminal fluid proteome of the polyandrous Red junglefowl offers insights into the molecular basis of fertility, reproductive ageing and domestication. Scientific Reports, 6, 35864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WH, Rieser JW, & Shoffner RN (1971). The effects of isoimmunization with semen, on fertility in the Turkey hen. Poultry Science, 50(6), 1841–1847. [DOI] [PubMed] [Google Scholar]
- Cameron E, Day T, & Rowe L (2007). Sperm competition and the evolution of ejaculate composition. The American Naturalist, 169(6), E158–E172. [DOI] [PubMed] [Google Scholar]
- Chen C, et al. (2017). The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nature Communications, 8(1), 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, & Mruk DD (2012). The blood-testis barrier and its implications for male contraception. Pharmacological Reviews, 64(1), 16–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WW-C, & Chamley LW (2004). Clinical associations and mechanisms of action of antisperm antibodies. Fertility and Sterility, 82(3), 529–535. [DOI] [PubMed] [Google Scholar]
- Clark GF, & Schust DJ (2013). Manifestations of immune tolerance in the human female reproductive tract. Frontiers in Immunology, 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet JMM (2010). The operation of sexual selection in the red junglefowl. Oxford University. D. Phil. Thesis. [Google Scholar]
- Cottell E, et al. (2000). Are seminal fluid microorganisms of significance or merely contaminants? Fertility and Sterility, 74(3), 465–470. [DOI] [PubMed] [Google Scholar]
- Cramer ERA, et al. (2016). Females discriminate against heterospecific sperm in a natural hybrid zone. Evolution, 70(8), 1844–1855. [DOI] [PubMed] [Google Scholar]
- Das SC, Isobe N, & Yoshimura Y (2008). Mechanism of prolonged sperm storage and sperm survivability in hen oviduct: A review. American Journal of Reproductive Immunology, 60(6), 477–481. [DOI] [PubMed] [Google Scholar]
- Das SC, Nagasaka N, & Yoshimura Y (2005). Changes in the localization of antigen presenting cells and T cells in the utero-vaginal junction after repeated artificial insemination in laying hens. Journal of Reproduction and Development, 51(5), 683–687. [DOI] [PubMed] [Google Scholar]
- de Godoy LMF (2014). SILAC yeast: From labeling to comprehensive proteome quantification In Martins-de-Souza D (Ed.), Shotgun proteomics: Methods and protocols (pp. 81–109). New York, NY: Springer New York. [DOI] [PubMed] [Google Scholar]
- Dejucq N, & Jégou B (2001). Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiology and Molecular Biology Reviews, 65(2), 208–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devigili A, et al. (2017). Postcopulatory cost of immune system activation in Poecilia reticulata. Ethology Ecology and Evolution, 29(3), 266–279. [Google Scholar]
- Dewsbury DA (1982). Ejaculate cost and male choice. The American Naturalist, 119, 601–610. [Google Scholar]
- Dondero F, et al. (1993). Immunological infertility in humans. Experimental and Clinical Immunogenetics, 10(2), 65–72. [PubMed] [Google Scholar]
- Dorus S, Skerget S, & Karr TL (2012). Proteomic discovery of diverse immunity molecules in mammalian spermatozoa. Systems Biology in Reproductive Medicine, 58(4), 218–228. [DOI] [PubMed] [Google Scholar]
- Dziarski R, & Gupta D (2006). The peptidoglycan recognition proteins (PGRPs). Genome Biology, 7(8), 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard WG (1996). Female control: Sexual selection by cryptic female choice. Princeton and Chichester: Princeton University Press. [Google Scholar]
- Edwards SV, & Hedrick PW (1998). Evolution and ecology of MHC molecules: From genomics to sexual selection. Trends in Ecology & Evolution, 13(8), 305–311. [DOI] [PubMed] [Google Scholar]
- Eisenbach M, & Giojalas LC (2006). Sperm guidance in mammals—An unpaved road to the egg. Nature Reviews Molecular Cell Biology, 7(4), 276–285. [DOI] [PubMed] [Google Scholar]
- Evans JP, et al. (2012). Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proceedings of the Royal Society B: Biological Sciences, 279(1739), 2855–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorka KM, & Zuk M (2005). Sexual conflict and female immune suppression in the cricket. Allonemobious socius, Journal of Evolutionary Biology, 18, 1515–1522. [DOI] [PubMed] [Google Scholar]
- Firman RC, et al. (2017). Postmating female control: 20 years of cryptic female choice. Trends in Ecology & Evolution, 32(5), 368–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstad I, & Karter AJ (1992). Parasites, bright males, and the immunocompetence handicap. American Naturalist, 139, 603–622. [Google Scholar]
- Fox CA, Meldrum SJ, & Watson BW (1973). Continuous measurement by radio-telemetry of vaginal pH during human coitus. Journal of Reproduction and Fertility, 33(1), 69–75. [DOI] [PubMed] [Google Scholar]
- Friedländer M, & Gitay H (1972). The fate of the normal-anucleated spermatozoa in inseminated females of the silkworm Bombyx mori. Journal of Morphology, 138(1), 121–129. [DOI] [PubMed] [Google Scholar]
- Garbaczewska M, Billeter JC, & Levine JD (2013). Drosophila melanogaster males increase the number of sperm in their ejaculate when perceiving rival males. Journal of Insect Physiology, 59(3), 306–310. [DOI] [PubMed] [Google Scholar]
- Gee H (1999). Size and the single sex cell. Nature. 10.1038/news991125-4. [DOI] [Google Scholar]
- Gershman SN, et al. (2010). Give ‘til it hurts: Trade-offs between immunity and male reproductive effort in the decorated cricket, Gryllodes sigillatus. Journal of Evolutionary Biology, 23(4), 829–839. [DOI] [PubMed] [Google Scholar]
- Ghaderi D, et al. (2011). Sexual selection by female immunity against paternal antigens can fix loss of function alleles. Proceedings of the National Academy of Sciences of the United States of America, 108(43), 17743–17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey TM, Ignotz GG, & Suarez SS (2003). PDC-109 (BSP-A1/A2) promotes bull sperm binding to oviductal epithelium in vitro and may be involved in forming the oviductal sperm reservoir. Biology of Reproduction, 69, 809–815. [DOI] [PubMed] [Google Scholar]
- Gwathmey TM, et al. (2006). Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biology of Reproduction, 75, 501–507. [DOI] [PubMed] [Google Scholar]
- Haley LE, & Abplanalp H (1970). Possible immunological basis for a reduction of fertility in cross-mating fowl with Japanese quail. Journal of Reproduction and Fertility, 23(3), 375–381. [DOI] [PubMed] [Google Scholar]
- Higaki K, et al. (1995). Localization of spermatozoa and leukocytes in vagina and uterovaginal junction after copulation in Japanese quail (Coturnix coturnix japonica). Japanese Poultry Science, 32(6), 387–393. [Google Scholar]
- Hillgarth N, Ramenofsky M, & Winfield J (1997). Testosterone and sexual selection. Behavioral Ecology, 8(1), 108–109. [Google Scholar]
- Holman L, & Snook RR (2006). Spermicide, cryptic female choice and the evolution of sperm form and function. Journal of Evolutionary Biology, 19(5), 1660–1670. [DOI] [PubMed] [Google Scholar]
- Holman L, & Snook RR (2008). A sterile sperm caste protects brother fertile sperm from female-mediated death in Drosophila pseudoobscura. Current Biology, 18, 292–296. [DOI] [PubMed] [Google Scholar]
- Holt WV, & Fazeli A (2016). Sperm storage in the female reproductive tract. Annual Review of Animal Biosciences, 4(1), 291–310. [DOI] [PubMed] [Google Scholar]
- Hong J, et al. (2017). Strong inhibition of neutrophil-sperm interaction in cattle by selective phosphatidylinositol 3-kinase inhibitors. Biology of Reproduction, 97(5), 671–687. [DOI] [PubMed] [Google Scholar]
- Hopkins BR, Sepil I, & Wigby S (2017). Seminal fluid. Current Biology, 27(11), 404–405. [DOI] [PubMed] [Google Scholar]
- Hosken DJ (2001). Sex and death: Microevolutionary trade-offs between reproductive and immune investment in dung flies. Current Biology, 11(10), R379–R380. [DOI] [PubMed] [Google Scholar]
- Hosken DJ, & O’Shea JE (2001). Sperm production and immune function in two Australian bats, Chalinolobus morio and Nyctophilus geoffroyi. Ethology Ecology and Evolution, 13(2), 173–180. [Google Scholar]
- Hosoda T, Abe T, & Otsuka S (1964). Serological studies on the insemination of domestic fowl. Japanese Poultry Science, 1(1), 33–38. [Google Scholar]
- Hunter RH (1981). Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. Journal of Reproduction and Fertility, 63(1), 109–117. [DOI] [PubMed] [Google Scholar]
- Immler S (2008). Sperm competition and sperm cooperation: The potential role of diploid and haploid expression. Reproduction, 135(3), 275–283. [DOI] [PubMed] [Google Scholar]
- Jansen RP (1980). Cyclic changes in the human fallopian tube isthmus and their functional importance. American Journal of Obstetrics and Gynecology, 136(3), 292–308. [DOI] [PubMed] [Google Scholar]
- Jansen RP, & Bajpai VK (1982). Oviduct acid mucus glycoproteins in the estrous rabbit: Ultrastructure and histochemistry. Biology of Reproduction, 26(1), 155–168. [DOI] [PubMed] [Google Scholar]
- Johns S, Henshaw JM, Jennions MD, & Head ML (2019). Males can evolve lower resistance to sexually transmitted infections to infect their mates and thereby increase their own fitness. Evolutionary Ecology, 33(2), 149–172. [Google Scholar]
- Kapelnikov A, et al. (2008). Mating induces an immune response and developmental switch in the Drosophila oviduct. Proceedings of the National Academy of Sciences of the United States of America, 105(37), 13912–13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DF, Slade DA, & Nakajima ST (1997). Analysis of pre-ovulatory changes in cervical mucus hydration and sperm penetrability. Advances in Contraception: The Official Journal of the Society for the Advancement of Contraception, 13(2–3), 143–151. [DOI] [PubMed] [Google Scholar]
- Kelly CD, & Jennions MD (2011). Sexual selection and sperm quantity: Meta-analyses of strategic ejaculation. Biological Reviews of the Cambridge Philosophical Society, 86(4), 863–884. [DOI] [PubMed] [Google Scholar]
- Kerr AM, Gershman SN, & Sakaluk SK (2010). Experimentally induced spermatophore production and immune responses reveal a trade-off in crickets. Behavioral Ecology, 21(3), 647–654. [Google Scholar]
- Kirk TA, et al. (1989). The relationship of infertility to antibody production in the uterovaginal sperm storage tubules of Turkey breeder hens. Theriogenology, 31(5), 955–961. [DOI] [PubMed] [Google Scholar]
- Knell RJ, & Webberley KM (2004). Sexually transmitted diseases of insects: Distribution, evolution, ecology and host behaviour. Biological Reviews of the Cambridge Philosophical Society, 79, 557–581. [DOI] [PubMed] [Google Scholar]
- Kurata S (2014). Peptidoglycan recognition proteins in Drosophila immunity. Developmental and Comparative Immunology, 42(1), 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YM, et al. (1997). The effect of human papillomavirus infection on sperm cell motility. Fertility and Sterility, 67(6), 1152–1155. [DOI] [PubMed] [Google Scholar]
- Le Tortorec A, & Dejucq-Rainsford N (2010). HIV infection of the male genital tract—Consequences for sexual transmission and reproduction. International Journal of Andrology, 33(1), e98–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz TL, et al. (2018). Cryptic haplotype-specific gamete selection yields offspring with optimal MHC immune genes. Evolution, 72(11), 2478–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedal S, Folstad I, & Skarstein F (1999). Secondary sex traits, parasites, immunity and ejaculate quality in the Arctic charr. Proceedings of the Royal Society B: Biological Sciences, 266(1431), 1893–1898. [Google Scholar]
- Lockhart AB, Thrall PH, & Antonovics J (1996). Sexually transmitted diseases in animals: Ecological and evolutionary implications. Biological Reviews, 71(3), 415–471. [DOI] [PubMed] [Google Scholar]
- Losdat S, et al. (2011). Immune activation reduces sperm quality in the great tit. PLoS One, 6(7), e22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løvlie H, et al. (2013). Cryptic female choice favours sperm from major histocompatibility complex-dissimilar males. Proceedings of the Royal Society of London B, 280, 20131296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J-C, Huang Y-F, & Lu N-Q (2008). Antisperm immunity and infertility. Expert Review of Clinical Immunology, 4(1), 113–126. [DOI] [PubMed] [Google Scholar]
- Ma X, et al. (2016). Sialylation facilitates the maturation of mammalian sperm and affects its survival in female uterus. Biology of Reproduction, 94(6), 123 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi BM, et al. (2011). Frequency of antisperm antibodies in infertile women. Journal of Reproduction & Infertility, 12(4), 261. [PMC free article] [PubMed] [Google Scholar]
- Manjerovic MB, & Waterman JM (2012). Immunological sex differences in socially promiscuous African ground squirrels. PLoS One, 7(6), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei AL, et al. (2015). Integrated 3D view of postmating responses by the Drosophila melanogaster female reproductive tract, obtained by micro-computed tomography scanning. Proceedings of the National Academy of Sciences of the United States of America, 112(27), 8475–8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney JL (1923). Studies on the mechanism of sterilization of the female by spermotoxin. American Journal of Physiology-Legacy Content, 63(2), 207–217. [Google Scholar]
- McGraw LA, Clark AG, & Wolfner MF (2008). Post-mating gene expression profiles of female Drosophila melanogaster in response to time and to four male accessory gland proteins. Genetics, 179, 1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara KB, van Lieshout E, & Simmons LW (2014). Females suffer a reduction in the viability of stored sperm following an immune challenge. Journal of Evolutionary Biology, 27(1), 133–140. [DOI] [PubMed] [Google Scholar]
- Meaker SR (1922). Some aspects of the problem of sterility. The Boston Medical and Surgical Journal, 187(15), 535–539. [Google Scholar]
- Miki K, & Clapham DE (2013). Rheotaxis guides mammalian sperm. Current Biology, 23(6), 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinski M (2006). The major histocompatibility complex, sexual selection, and mate choice. Annual Review of Ecology, Evolution, and Systematics, 37, 159–186. [Google Scholar]
- Miyata H, et al. (2016). Genome engineering uncovers 54 evolutionarily conserved and testis-enriched genes that are not required for male fertility in mice. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7704–7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Dvorakova K, Jenkins N, & Breed W (2002). Exceptional sperm cooperation in the wood mouse. Nature, 418(6894), 174–177. [DOI] [PubMed] [Google Scholar]
- Moreno I, & Franasiak JM (2017). Endometrial microbiota—New player in town. Fertility and Sterility, 108(1), 32–39. [DOI] [PubMed] [Google Scholar]
- Morrow EH, & Innocenti P (2012). Female postmating immune responses, immune system evolution and immunogenic males. Biological Reviews, 87(3), 631–638. [DOI] [PubMed] [Google Scholar]
- Mullins KJ, & Saacke RG (1989). Study of the functional anatomy of bovine cervical mucosa with special reference to mucus secretion and sperm transport. The Anatomical Record, 225(2), 106–117. [DOI] [PubMed] [Google Scholar]
- Muro Y, et al. (2016). Behavior of mouse spermatozoa in the female reproductive tract from soon after mating to the beginning of fertilization. Biology of Reproduction, 94(4), 80. [DOI] [PubMed] [Google Scholar]
- Needham KB, et al. (2017). Repeated immune challenges affect testosterone but not sperm quality. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 327(6), 398–406. [DOI] [PubMed] [Google Scholar]
- Neubaum DM, & Wolfner MF (1999). Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics, 153(2), 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, & L’Hernault SW (2017). Spermatogenesis. Current Biology, 27(18), R988–R994. [DOI] [PubMed] [Google Scholar]
- O’Hanlon DE, Moench TR, & Cone RA (2013). Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One, 8(11), e80074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr TJ, & Brennan PLR (2015). Sperm storage: Distinguishing selective processes and evaluating criteria. Trends in Ecology & Evolution, 30(5), 261–272. [DOI] [PubMed] [Google Scholar]
- Orr TJ, & Zuk M (2012). Sperm storage. Current Biology, 22(1), R8–R10. [DOI] [PubMed] [Google Scholar]
- Otti O, McTighe AP, & Reinhardt K (2013). In vitro antimicrobial sperm protection by an ejaculate-like substance. Functional Ecology, 27(1), 219–226. [Google Scholar]
- Overstreet JW, & Cooper GW (1978). Sperm transport in the reproductive tract of the female rabbit: II. The sustained phase of transport. Biology of Reproduction, 19(1), 115–132. [DOI] [PubMed] [Google Scholar]
- Owen DH, & Katz DF (2005). A review of the physical and chemical properties of human semen and the formulation of a semen simulant. Journal of Andrology, 26(4), 459–469. [DOI] [PubMed] [Google Scholar]
- Parker GA (1970). Sperm competition and its evolutionary consequences in the insects. Biological Reviews, 45, 525–567. [Google Scholar]
- Parker GA (1990). Sperm competition games: Raffles and roles. Proceedings of the Royal Society B: Biological Sciences, 242(1304), 120–126. [Google Scholar]
- Parker G. a., & Pizzari T (2010). Sperm competition and ejaculate economics. Biological Reviews of the Cambridge Philosophical Society, 85, 897–934. [DOI] [PubMed] [Google Scholar]
- Parker GA, et al. (1996). Sperm competition games: Individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. London Ser. B, 263, 1291–1297. [Google Scholar]
- Perry JC, Sirot L, & Wigby S (2013). The seminal symphony: How to compose an ejaculate. Trends in Ecology & Evolution, 28(7), 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, & Foster KR (2008). Sperm sociality: Cooperation, altruism, and spite. PLoS Biology. 6(5), e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Løvlie H, & Cornwallis CK (2004). Sex-specific, counteracting responses to inbreeding in a bird. Proceedings Biological sciences/The Royal Society, 271, 2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompon J, & Levashina EA (2015). A new role of the mosquito complement-like Cascade in male fertility in Anopheles gambiae. Schneider DS, Ed. PLoS Biology, 13(9), e1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racey PA, et al. (1987). Sperm-epithelium relationships in relation to the time of insemination in little brown bats (Myotis lucifugus). Journal of Reproduction and Fertility, 80(2), 445–454. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan P, & Fedorka KM (2012). Immune activation decreases sperm viability in both sexes and influences female sperm storage. Proceedings of the Royal Society B: Biological Sciences, 279(1742), 3577–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt K, Naylor R, & Siva-Jothy MT (2003). Reducing a cost of traumatic insemination: Female bedbugs evolve a unique organ. Proceedings of the Biological Sciences, 270, 2371–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ML, Buchanan KL, & Evans MR (2004). Testing the immunocompetence handicap hypothesis: A review of the evidence. Animal Behaviour, 68(2), 227–239. [Google Scholar]
- Schmid-Hempel P (2005). Evolutionary ecology of insect immune defenses. Annual Review of Entomology, 50, 529–551. [DOI] [PubMed] [Google Scholar]
- Schnakenberg SL, Siegal ML, & Bloch Qazi MC (2012). Oh, the places they’ll go: Female sperm storage and sperm precedence in Drosophila melanogaster. Spermatogenesis, 2(3), 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze M, et al. (2018). Detection and characterization of Lactobacillus spp. in the porcine seminal plasma and their influence on boar semen quality. Drevet JR, Ed. PLoS One, 13(9), e0202699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppe H-C, et al. (2017). Urogenital infection as a risk factor for male infertility. Deutsches Aerzteblatt International, 114(19), 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenke RA, Lazzaro BP, & Wolfner MF (2016). Reproduction-immunity trade-offs in insects. Annual Review of Entomology, 61, 239–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon IM, Owens S-E, & Turner ML (2017). Innate immunity and the sensing of infection, damage and danger in the female genital tract. Journal of Reproductive Immunology, 119, 67–73. [DOI] [PubMed] [Google Scholar]
- Sidaway P (2016). Infection: Sexual transmission of gonorrhoea is facilitated by other bacteria. Nature Reviews Urology, 13(10), 562. [DOI] [PubMed] [Google Scholar]
- Simmons LW (2011). Resource allocation trade-off between sperm quality and immunity in the field cricket, Teleogryllus oceanicus. Behavioral Ecology, 23(1), 168–173. [Google Scholar]
- Simmons LW, & Emlen DJ (2006). Evolutionary trade-off between weapons and testes. Proceedings of the National Academy of Sciences of the United States of America, 103(44), 16346–16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons LW, Lüpold S, & Fitzpatrick JL (2017). Evolutionary trade-off between secondary sexual traits and ejaculates. Trends in Ecology & Evolution, 32(12), 964–976. [DOI] [PubMed] [Google Scholar]
- Simmons LW, & Roberts B (2005). Bacterial immunity traded for sperm viability in male crickets. Science, 309(5743), 2031 2031. [DOI] [PubMed] [Google Scholar]
- Sirot LK, Wolfner MF, & Wigby S (2011). Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 108, 9922–9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skau PA, & Folstad I (2005). Does immunity regulate ejaculate quality and fertility in humans? Behavioral Ecology, 16(2), 410–416. [Google Scholar]
- Sobrero AJ, & Macleod J (1962). The immediate postcoital test. Fertility and Sterility, 13, 184–189. [DOI] [PubMed] [Google Scholar]
- Soper DE (2010). Pelvic inflammatory disease. Obstetrics and Gynecology, 116(2 Pt. 1), 419–428. [DOI] [PubMed] [Google Scholar]
- Steele MG, & Wishart GJ (1992). Evidence for a species-specific barrier to sperm transport within the vagina of the chicken hen. Theriogenology, 38(6), 1107–1114. [DOI] [PubMed] [Google Scholar]
- Stern JE, et al. (1992). Antisperm antibodies in women: Variability in antibody levels in serum, mucus, and peritoneal fluid. Fertility and Sterility, 58(5), 950–958. [DOI] [PubMed] [Google Scholar]
- Suarez SS (1987). Sperm transport and motility in the mouse oviduct: Observations in situ. Biology of Reproduction, 36(1), 203–210. [DOI] [PubMed] [Google Scholar]
- Suarez SS (2015). Gamete and zygote transport In Plant T & Zeleznich A (Eds.), Knobil and Neill’s physiology of reproduction (pp. 197–232). Oxford: Elsevier. [Google Scholar]
- Suarez SS (2016). Mammalian sperm interactions with the female reproductive tract. Cell and Tissue Research, 363(1), 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS, Brockman K, & Lefebvre R (1997). Distribution of mucus and sperm in bovine oviducts after artificial insemination: The physical environment of the oviductal sperm reservoir. Biology of Reproduction, 56(2), 447–453. [DOI] [PubMed] [Google Scholar]
- Suarez SS, & Pacey AA (2006). Sperm transport in the female reproductive tract. Human Reproduction Update, 12(1), 23–37. [DOI] [PubMed] [Google Scholar]
- Swallow JG, & Wilkinson GS (2002). The long and short of sperm polymorphisms in insects. Biological Reviews of the Cambridge Philosophical Society, 77(2), 153–182. [DOI] [PubMed] [Google Scholar]
- Taherali F, Varum F, & Basit AW (2018). A slippery slope: On the origin, role and physiology of mucus. Advanced Drug Delivery Reviews, 124, 16–33. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Price TAR, & Wedell N (2014). Polyandry in nature: A global analysis. Trends in Ecology & Evolution, 29(7), 376–383. [DOI] [PubMed] [Google Scholar]
- Tokuhiro K, et al. (2012). Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility. Proceedings of the National Academy of Sciences of the United States of America, 109(10), 3850–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troedsson MH, et al. (2001). Interaction between equine semen and the endometrium: The inflammatory response to semen. Animal Reproduction Science, 68(3–4), 273–278. [DOI] [PubMed] [Google Scholar]
- Tung CK, et al. (2015a). Emergence of upstream swimming via a hydrodynamic transition. Physical Review Letters, 114(10), 108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung CK, et al. (2015b). Microgrooves and fluid flows provide preferential passageways for sperm over pathogen Tritrichomonas foetus. Proceedings of the National Academy of Sciences of the United States of America, 112(17), 5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujisić S, et al. (2005). Antisperm antibodies in semen, sera and follicular fluids of infertile patients: Relation to reproductive outcome after in vitro fertilization. American Journal of Reproductive Immunology, 54(1), 13–20. [DOI] [PubMed] [Google Scholar]
- Wald M (2005). Male infertility: Causes and cures. Sexuality, Reproduction and Menopause, 3(2), 83–87. [Google Scholar]
- Wardlow AM and Agrawal AF, Sexual conflict and Sexually Transmitted Infections (STIs): Coevolution of sexually antagonistic host traits with an STI, The American Naturalist 193 (1), E1–E14. 10.1086/700564. [DOI] [PubMed] [Google Scholar]
- Wedekind C, et al. (2004). MHC-linked susceptibility to a bacterial infection, but no MHC-linked cryptic female choice in whitefish. Journal of Evolutionary Biology, 17(1), 11–18. [DOI] [PubMed] [Google Scholar]
- Wedell N (1993). Spermatophore size in bushcrickets: Comparative evidence for nuptial gifts as a sperm protective device. Evolution, 47(4), 1203–1212. [DOI] [PubMed] [Google Scholar]
- Wedell N, Gage MJGG, & Parker GA (2002). Sperm competition, male prudence and sperm-limited females. Trends in Ecology & Evolution, 17, 313–320. [Google Scholar]
- Wentworth BC, & Mellen WJ (1963). Effects of spermatozoal antibodies and method of insemination on fecundity of white leghorn females In Poultry science. Savoy, IL: Poultry Science Associates Inc; 61874. p. 1317. [Google Scholar]
- Wigby S, & Chapman T (2004). Sperm competition. Current Biology, 14(3), R100–R103. [PubMed] [Google Scholar]
- Wigby S, et al. (2009). Seminal fluid protein allocation and male reproductive success. Current Biology, 19, 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniz JL, et al. (2014). Three-dimensional architecture of the ovine oviductal mucosa. Anatomia, Histologia, Embryologia, 43(5), 331–340. [DOI] [PubMed] [Google Scholar]
- Yousef MS, et al. (2016). Sperm binding to oviduct epithelial cells enhances TGFB1 and IL10 expressions in epithelial cells as well as neutrophils in vitro: Prostaglandin E2 as a main regulator of anti-inflammatory response in the bovine oviduct. PLoS One, 11(9), e0162309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin AI, et al. (2005). Beta-defensin 126 on the cell surface protects sperm from immunorecognition and binding of anti-sperm antibodies. Biology of Reproduction, 73(6), 1243–1252. [DOI] [PubMed] [Google Scholar]
- Zambrano F, et al. (2016). Leukocytes coincubated with human sperm trigger classic neutrophil extracellular traps formation, reducing sperm motility. Fertility and Sterility, 106(5), 1053–1060.e1. [DOI] [PubMed] [Google Scholar]
- Zera AJ, & Harshman LG (2001). The physiology of life history trade-offs in animals. Annual Review of Ecology and Systematics, 32, 95–126. [Google Scholar]
- Zheng WM, et al. (2001). An in situ hybridization study of the effects of artificial insemination on the localization of cells expressing MHC class II mRNA in the chicken oviduct. Reproduction, 122(4), 581–586. [PubMed] [Google Scholar]
- Ziegler A, Kentenich H, & Uchanska-Ziegler B (2005). Female choice and the MHC. Trends in Immunology, 26(9), 496–502. [DOI] [PubMed] [Google Scholar]