Abstract
Background
Although both hepatic fibrosis progression and hepatitis C virus (HCV) contribute to hepatocellular carcinoma (HCC) development, early detection of HCC remains challenging. Therefore, we evaluated clinical markers of fibrosis in HCV patients to improve early HCC diagnosis.
Material/Methods
Our retrospective study included 711 chronic HCV patients: 249 HCC patients and 462 non-HCC patients. To investigate the predictive ability of non-invasive scores for diagnosing HCC development, we compared 4 blood indices: fibrosis index based on 4 factors (FIB-4), aspartate aminotransferase-to-platelet count ratio index (APRI), aspartate aminotransferase-to-alanine aminotransferase ratio (AAR), and gamma-glutamyl transpeptidase-to-platelet count ratio (GPR).
Results
HCC patients had significantly higher scores for all fibrosis indices compared to chronic HCV patients without HCC. Moreover, the diagnostic performance of FIB-4 (area under curve, AUC: 0.961) was superior to that of APRI, AAR, and GPR (AUC: 0.636, 0.746, and 0.661, respectively) for prediction of HCC. FIB-4 also out-performed other indices in the prediction of cirrhotic cases, with an AUC of 0.775 compared to other scores, which ranged from an AUC of 0.597 to 0.671.
Conclusions
Together, these results suggest that FIB-4 is an appropriate diagnostic indicator of liver cirrhosis and HCC in chronic HCV patients in China.
MeSH Keywords: Carcinoma, Hepatocellular; Hepatitis C; Liver Cirrhosis
Background
Hepatocellular carcinoma (HCC) is the fifth most common type of cancer among men and the ninth most common type of cancer among women, making it a global health concern [1,2]. Infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) and excessive alcohol consumption are major factors that contribute to developing HCC [3,4]. There is increasing evidence that non-alcoholic fatty liver diseases (NAFLDs) are strongly associated with the incidence of HCC [5]. Moreover, chronic HCV infection causes approximately 25% of HCC cases worldwide [4]. In fact, half of the increased HCC prevalence over the past 20 years is attributed to HCV [6,7].
HCC risk is closely linked with liver fibrosis progression in patients with chronic HCV infection. Fortunately, liver fibrosis can be evaluated and predicted by several non-invasive blood scores such as the aspartate transaminase (AST)-to-platelet ratio index (APRI), the fibrosis index based on 4 factors (FIB-4), the gamma-glutamyl transpeptidase (GGT)-to-platelet ratio (GPR), and the AST-to- alanine aminotransferase (ALT) ratio (AAR) [8,9].
In addition to liver fibrosis progression, aging is another factor associated with an increased risk of HCC development [10]. Among the fibrosis scores, the FIB-4 incorporates routinely available clinical data including age, AST, platelet count (PLT), and ALT level [11,12] according to Sterling’s formula [9] as follows:
Because the FIB-4 considers both liver fibrosis and age, it may be capable of predicting the risk of HCC development [13].
Here, we assessed the performance of 4 non-invasive measures of inflammation and fibrosis for HCC diagnosis in 711 Chinese chronic HCV patients.
Material and Methods
Patient selection
This was a cross-sectional study of patients with chronic HCV infection (CHC) to assess non-invasive inflammatory and fibrosis indices for HCC diagnostic utility. We included 818 patients with CHC who had been hospitalized at the First Hospital of Jilin University in China from January 2010 to June 2016 and had complete medical data available for the study. CHC was diagnosed based on the serum presence of anti-HCV antibodies and HCV RNA for 6 months or longer. Of the CHC patients, 249 had HCC (cases) and 569 had CHC only. After matching sex distribution to the HCC group, 462 patients with CHC only were included as control patients.
History or evidence of the following conditions resulted in exclusion during patient recruitment: human immunodeficiency virus infection, HBV infection, other hepatitis infections, and any form of cancer or liver disease, such as non-alcoholic fatty liver disease or alcoholic liver disease.
Diagnosis of compensated liver cirrhosis and HCC
Liver cirrhosis diagnoses were confirmed by liver biopsy or a combination of clinical, biochemical, and radiological findings. All compensated liver cirrhosis cases in this study were liver cirrhosis with Child-Pugh class A disease [14,15].
HCC diagnoses were confirmed by: (1) biopsy; (2) computerized tomography and magnetic resonance imaging followed by a portal venous phase washout scan to observe arterial hypervascularization within a lesion; or (3) positive imaging associated with alpha-fetoprotein levels >400 ng/mL.
The Independent Institutional Review Board of the First Hospital of Jilin University approved the study protocol. All participants gave written informed consent before study enrollment.
FIB-4, APRI scores, AAR, and GPR
FIB-4 was calculated as follows [11]:
APRI was calculated as follows [16]:
The AST upper limit was 40 IU/L and ranged from 7 to 40 IU/L.
AAR was calculated as follows:
GPR was calculated as follows:
Study variables
The demographic and clinical data analyzed here included sex; age; and the presence of diabetes mellitus (DM), gallstones, and liver cirrhosis. The following biochemical parameters were also examined: PLT, AST, ALT, gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total bilirubin (TBiL), albumin (ALB), cholinesterase (CHE), FIB-4, APRI, AAR, and GPR.
Statistical analysis
Continuous variables were assessed for normal distribution, compared by independent-samples t tests, and are described using the median and the 25th and 75th percentiles. Categorical variables were compared for significance using chi-square tests and are defined as numbers and percentages. The performances of the FIB-4, APRI, GPR, and AAR in the diagnosis of compensated liver cirrhosis and HCC were determined by receiver operating characteristic (ROC) curves and the areas under the ROC (AUROC) curves [17]. The AUROCs were then compared by Z tests using MedCalc Statistical Software version 16.1 (MedCalc Software bvba, Ostend, Belgium). Maximization of the sum of the sensitivity and specificity or optimization at a minimum of 95% specificity were used to determine the predictive diagnostic cutoffs. Sensitivity, specificity, predictive diagnostic cut-offs, and the percentage of correctly classified cases were used to assess diagnostic accuracy. Adjustment for potential confounding variables was accomplished by multivariate logistic regression. The adjusted odds ratios (AORs) and 95% confidence intervals (CIs) were calculated, and all comparisons were made using 2-tailed tests, as appropriate, using SPSS, version 13.0 (SPSS, Inc., Chicago, IL, USA). The threshold for statistical significance in all comparisons was P<0.05.
Results
Baseline patient characteristics
Table 1 summarizes the demographics and clinical features of the study participants. The diagnostic records obtained for each participant indicated that 462 patients had only CHC and 249 patients had HCC. The HCC patient group was composed of 45.8% males, and the mean age was 62.00 (58.00, 67.00) years. CHC-only patients were defined as the control group and were sex distribution-matched to the HCC group. The CHC-only group was composed of 47.4% males, and the mean age was 59.00 (54.00, 66.00) years. The presence of liver cirrhosis and gallstones was significantly higher in HCC patients compared to CHC-only patients (27.7% vs. 18.0%, P=0.002; 79.5% vs. 18.4%, P<0.001, respectively). Of note, DM presence did not significantly differ between HCC and CHC-only groups.
Table 1.
Demographic and clinical characteristics of cases and controls.
| Variable | HCC N=249 | CHC-only N=462 | P |
|---|---|---|---|
| Male, N (%) | 114 (45.8) | 219 (47.4) | 0.680 |
| Age (years) | 62.00 (58.00, 67.00) | 59.00 (54.00, 66.00) | 0.003 |
| Diabetes, N (%) | 50 (20.1) | 77 (16.7) | 0.257 |
| Gallstone, N (%) | 69 (27.7) | 83 (18.0) | 0.002 |
| Liver cirrhosis, N (%) | 198 (79.5) | 85 (18.4) | <0.001 |
| PLT (109/L) | 90.00 (60.00, 131.50) | 131.50 (92.75, 176.00) | <0.001 |
| AST (IU/L) | 68.00 (39.60, 108.50) | 55.00 (33.00, 92.50) | 0.001 |
| ALT (IU/L) | 46.00 (28.40, 76.20) | 62.00 (30.00, 117.25) | 0.001 |
| GGT (IU/L) | 72.20 (37.40, 148.70) | 53.00 (25.00, 105.50) | <0.001 |
| ALP (IU/L) | 108.70 (79.00, 147.35) | 83.75 (67.00, 113.60) | <0.001 |
| TBiL (μmol/L) | 24.20 (17.45, 46.70) | 16.85 (12.10, 23.93) | <0.001 |
| ALB (g/L) | 32.80 (27.40, 37.85) | 37.60 (34.38, 40.80) | <0.001 |
| CHE (IU/L) | 3560.00 (2048.00, 5294.50) | 6464.50 (4463.75, 7827.75) | <0.001 |
| PT | 12.70 (11.50, 14.40) | 11.50 (10.70, 12.50) | <0.001 |
| AAR | 1.43 (1.05, 1.96) | 0.91 (0.69, 1.27) | <0.001 |
| APRI | 1.94 (0.94, 3.78) | 1.13 (0.56, 2.29) | <0.001 |
| FIB4 | 6.66 (4.06, 11.86) | 0.83 (0.56, 1.48) | <0.001 |
| GPR | 0.85 (0.38, 1.81) | 0.45 (0.20, 0.96) | <0.001 |
Continuous variables are expressed as median (25th, 75th percentiles). AST – aspartate aminotransferase; ALT – alanine aminotransferase; ALP – alkaline phosphatase; GGT – gamma-glutamyl transpeptidase; TBiL – total bilirubin; ALB – albumin; CHE – cholinesterase; PT – prothrombin time; HCC – hepatocellular carcinoma; CHC – chronic hepatitis C; PLT – platelet count; APRI – AST-to-PLT ratio index; GPR – GGT to PLT ratio; AAR – AST to ALT ratio; FIB-4 – fibrosis index based on four factors
HCC patients demonstrated higher levels of AST, GGT, ALP, TBiL, and PT than CHC-only patients. All inflammatory factors, including FIB-4, APRI, AAR, and GPR, were higher in the HCC group compared to the CHC-only group. In contrast, PLT, ALT, ALB, and CHE levels were lower in HCC patients than in CHC-only patients.
Univariate analysis of variables associated with HCC in CHC patients
Age (P<0.001), liver cirrhosis (P<0.001), low PLT values (P<0.001), high AST values (P=0.001), low ALT values (P=0.001), high GGT levels (P<0.001), high ALP levels (P<0.001), high TBiL levels (P<0.001), and low ALB levels (P<0.001) all contributed to HCC (Table 2). Therefore, these factors were analyzed in multivariate analyses and corrected for potential confounding variables as appropriate. Multivariate analyses revealed that the independent factors associated with HCC in CHC patients were age (>60 years, P=0.014), liver cirrhosis (P<0.001), high AST values (P=0.016), low ALT values (P=0.004), and low ALB levels (P=0.021).
Table 2.
Univariate and multivariate analyses of variables associated with HCV-related HCC.
| Variable | HCC N=249 | CHC-only N=462 | P# | AOR (95% CI)* | P** |
|---|---|---|---|---|---|
| Sex | 0.680 | – | – | ||
| Female, N (%) | 135 (54.2) | 243 (52.6) | |||
| Male, N (%) | 114 (45.8) | 219 (47.4) | |||
| Age | <0.001 | 1.644 (1.104–2.448) | 0.014 | ||
| ≤60 | 101 (40.6) | 256 (55.4) | |||
| >60 | 148 (59.4) | 206 (44.6) | |||
| Liver cirrhosis | <0.001 | 12.188 (8.062–18.425) | <0.001 | ||
| No, N (%) | 51 (20.5) | 377 (81.6) | |||
| Yes, N (%) | 198 (79.5) | 85 (18.4) | |||
| PLT (109/L) | 90.00 (60.00, 131.50) | 131.50 (92.75, 176.00) | <0.001 | – | – |
| AST (IU/L) | 68.00 (39.60, 108.50) | 55.00 (33.00, 92.50) | 0.001 | 1.006 (1.001–1.011) | 0.016 |
| ALT (IU/L) | 46.00 (28.40, 76.20) | 62.00 (30.00, 117.25) | 0.001 | 0.993 (0.988–0.998) | 0.004 |
| GGT (IU/L) | 72.20 (37.40, 148.70) | 53.00 (25.00, 105.50) | <0.001 | – | – |
| ALP (IU/L) | 108.70 (79.00, 147.35) | 83.75 (67.00, 113.60) | <0.001 | – | – |
| TBiL (μmol/L) | 24.20 (17.45, 46.70) | 16.85 (12.10, 23.93) | <0.001 | – | – |
| ALB (g/L) | 32.80 (27.40, 37.85) | 37.60 (34.38, 40.80) | <0.001 | 0.965 (0.935–0.995) | 0.021 |
HCV – hepatitis C virus; HCC – hepatocellular carcinoma; AOR – adjusted odds ratio; CI – confidence interval; PLT – platelet count; AST – aspartate aminotransferase; ALT – alanine aminotransferase; GGT – gamma-glutamyl transpeptidase; ALP – alkaline phosphatase; TBiL – total bilirubin; ALB – albumin.
P value for univariate analysis;
Adjusted for gender, age, liver cirrhosis, ALB, TBiL, AST, ALT, GGT, ALP, and PLT;
P value for multivariate analysis.
Interestingly, multivariate analyses did not show significant differences in PLT, GGT, ALP, or TBiL levels between HCC and CHC-only patients.
Diagnostic performance of non-invasive blood tests for compensated liver cirrhosis and HCC
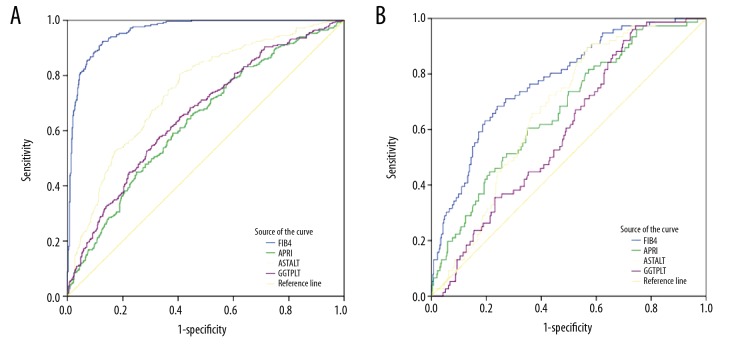
Using the estimated AUROC to predict HCC, we found that the performance of FIB-4 (AUROC=0.961, 95% CI 0.948–0.974, P<0.01) was better than GPR (AUROC=0.661, 95% CI 0.619–0.702, P<0.01), APRI (AUROC=0.636, 95% CI 0.593–0.678, P<0.01), and AAR (AUROC=0.746, 95% CI 0.709–0.783, P<0.01) (Table 3, Figure 1A).
Table 3.
Diagnostic performance of serum models for HCC and compensated liver cirrhosis in CHC patients.
| Variables | Liver cancer | Compensated liver cirrhosis | ||
|---|---|---|---|---|
| AUROC | (95% CI) | AUROC | (95% CI) | |
| FIB-4 | 0.961 | 0.948–0.974 | 0.775 | 0.720–0.831 |
| AAR | 0.746 | 0.709–0.783 | 0.671 | 0.614–0.728 |
| GPR | 0.661 | 0.619–0.702 | 0.597 | 0.535–0.659 |
| APRI | 0.636 | 0.593–0.678 | 0.667 | 0.603–0.732 |
| Comparison of AUROC | ||||
| FIB-4 and APRI | P<0.01 | P<0.05 | ||
| FIB-4 and GPR | P<0.01 | P<0.01 | ||
| FIB-4 and AAR | P<0.01 | P<0.05 | ||
| FIB-4 and AAR | P<0.01 | P<0.05 | ||
| APRI and AAR | P>0.05 | P>0.05 | ||
| APRI and GPR | P>0.05 | P>0.05 | ||
| AAR and GPR | P<0.05 | P>0.05 | ||
HCC – hepatocellular carcinoma; CHC – chronic hepatitis C; GPR – gamma-glutamyl transpeptidase-to-platelet ratio index; AAR – aspartate aminotransferase to alanine aminotransferase ratio; APRI – aspartate transaminase-to-platelet ratio index; FIB-4 – fibrosis index based on four factors; AUROC – area under the receiver operating characteristic curve; CI – confidence interval.
Figure 1.
ROC curves of fibrosis index based on 4 factors (FIB-4), aspartate transaminase-to-platelet ratio index (APRI), gamma-glutamyl transpeptidase-to-platelet ratio (GPR), and aspartate transaminase-to-alanine aminotransferase ratio (AAR) for diagnosing liver cirrhosis (B) and hepatocellular carcinoma (A).
We further estimated the AUROC to predict compensated liver cirrhosis (CLC) in CHC patients. We excluded 249 HCC patients and 31 liver cirrhosis patients with Child-Pugh class B or C disease from the analysis. We used the FIB-4, APRI, GPR, and AAR indices to evaluate the remaining CHC patients for compensated liver cirrhosis (Figure 1B). FIB-4 performance (AUROC=0.775, 95% CI 0.720–0.831) was superior to APRI (AUROC=0.667, 95% CI 0.603–0.732, P<0.05), GPR (AUROC=0.597, 95% CI 0.535–0.659, P<0.01), and AAR (AUROC=0.671, 95% CI 0.614–0.728, P<0.05).
Non-invasive diagnostic test cutoffs for compensated liver cirrhosis and HCC prediction
The optimal cutoff value, sensitivity, specificity, PPV, NPV, and percentage of correctly classified cases of compensated liver cirrhosis and HCC, respectively, for each non-invasive blood test are listed in Table 4. Maximization of the sensitivity and specificity sum revealed that the optimal FIB-4 cutoffs were 1.24 and 2.18 for compensated liver cirrhosis (CLC) and HCC diagnosis, respectively. For CLC diagnosis, we found that the sensitivity was 68.4% and the specificity was 76.1%. The percentage correctly classified was 74.7%. Additionally, the sensitivity and specificity of HCC diagnosis were 92.4% and 87.2%, respectively. The percentage correctly classified was 89.0%. Overall, the FIB-4 was found to be highly reliable for diagnosis of significant CLC and HCC.
Table 4.
Diagnostic thresholds of serum models for compensated liver cirrhosis and HCC.
| Disease | Cut-off* | Se(%) | Sp(%) | PPV | NPV | Correctly Classified (%) | |
|---|---|---|---|---|---|---|---|
| CLC | AAR | 0.79 | 89.5 | 43.4 | 24.9 | 94.9 | 50.6 |
| APRI | 1.40 | 60.5 | 65.1 | 27.1 | 88.5 | 64.3 | |
| FIB4 | 1.24 | 68.4 | 76.1 | 38.0 | 91.8 | 74.7 | |
| GPR | 0.17 | 97.4 | 25.6 | 21.8 | 97.8 | 38.1 | |
| HCC | AAR | 1.00 | 80.3 | 59.5 | 51.7 | 81.8 | 65.4 |
| APRI | 1.32 | 64.3 | 56.7 | 44.4 | 74.6 | 59.4 | |
| FIB4 | 2.18 | 92.4 | 87.2 | 79.6 | 95.5 | 89.0 | |
| GPR | 0.69 | 57.8 | 66.7 | 39.5 | 78.0 | 49.4 |
FIB-4 – fibrosis index based on four factors; GPR – gamma-glutamyl transpeptidase-to-platelet ratio index; AAR – aspartate aminotransferase to alanine aminotransferase ratio; APRI – aspartate transaminase-to-platelet ratio index; HCC – hepatocellular carcinoma; CLC – compensated liver cirrhosis; Se – sensitivity; Sp – specificity; NPV – negative predictive value; PPV – positive predictive value.
Cut-offs were established by maximizing the sum of sensitivity and specificity.
Discussion
Here, we demonstrate that FIB-4 was able to predict HCC development in CHC patients. At a cutoff of 2.18, FIB-4 predicted HCC with 92.4% sensitivity and 87.2% specificity. Moreover, HCC was correctly classified in 89.0% of cases, with an AUROC of 0.961, which suggests that FIB-4 has superior diagnostic abilities compared to other non-invasive liver fibrosis indices.
Liver fibrosis and cirrhosis have been previously shown to be associated with HCC, and FIB-4 is known to be a good predictor of cirrhosis and fibrosis. Together, these findings and our present results (the association of higher AST and older age with HCC development) indicate that FIB-4 is a promising diagnostic tool for HCC development.
FIB-4 and liver cirrhosis have been correlated with HCC incidence in previous studies [18–22]. In addition to predicting liver fibrosis, FIB-4 has been measured in HCV patients and can define groups at high risk for HCC [8]. FIB-4 also showed modest diagnostic ability for predicting liver fibrosis in chronic HBV patients in a recent meta-analysis [23]. Further, FIB-4 is a valuable measure in HCC screening, as demonstrated in a study of HBsAg carriers in which high FIB-4 scores in HBsAg carriers were associated with elevated HCC risk [24]. High FIB-4 scores also predict HCC in people with moderate or heavy alcohol consumption [25] and in HIV patients [22].
In comparison to FIB-4, other diagnostic tools have reported superior diagnostic capabilities for liver fibrosis and HCC [26,27]. For example, transient elastography is routinely used to diagnose liver fibrosis, with high accuracy. Nevertheless, FIB-4 has several diagnostic advantages over transient elastography. Transient elastography measurements are sometimes impossible to obtain in severely obese patients [28], and measurement reproducibility is reduced in patients with steatosis, higher body mass indexes, and lower levels of liver fibrosis [29]. Moreover, ultrasonography of liver elasticity is not universally available. In contrast, FIB-4 is calculated based on variables that are ascertainable from a general blood test and can therefore be measured in most patients. The only variables necessary to calculate FIB-4 are age, AST, ALT, and PLT. Moreover, because measurement of these variables is routinely performed during examination of liver disease patients, FIB-4 can be calculated without additional blood collection or cost.
In addition to FIB-4, we also assessed the diagnostic ability of AAR, APRI, and GPR indices. The AAR is a validated diagnostic measure that is used for assessment of liver fibrosis [30,31]. In fact, the AAR and liver cirrhosis have been shown to be significantly correlated [32]. Specifically, an AAR greater than 1.0 is suggestive of cirrhosis in non-alcoholic liver disease patients. However, in the present study, AAR did not show high diagnostic ability for HCC prediction [33]. This observation is consistent with a recent study showing that the AAR had reduced diagnostic ability to detect fibrosis (AUROC=0.66) in comparison to APRI (AUROC=0.79) and FIB-4 (AUROC=0.81) [34]. In addition, Kim et al. recorded a similar AAR AUROC of 0.64 [35].
Although the APRI is considered inferior to the FIB-4 for the detection of overall and advanced (F3–F4) fibrosis [36], it is an important marker for the prediction of long-term outcomes in patients with HCV infection who have received interferon therapy [19]. In addition, the GRP performed adequately in the detection of liver fibrosis in some previous studies [37]. It is not surprising, however, that the APRI and the GPR were poor predictors of HCC in our study, with AUROCs of 0.636 and 0.661, respectively. Consistent with our results, Mobarak et al. found a similar AUROC for the APRI [13]. The poor performance of the APRI in the prediction of HCC might be a reflection of the complex nature of HCC, which involves many factors in addition to fibrosis progression.
The limitations of our study include its retrospective nature and the lack of detailed information on HCV RNA levels, HCV types, and history of HCV therapy. Further investigation is necessary to define the association between inflammatory markers and to develop a uniform diagnostic method for HCV patients. Secondly, the study design may have introduced bias, potentially resulting in underestimation of the sensitivity and overestimation of the specificity of the non-invasive diagnostic indices [38].
Conclusions
The FIB-4 successfully identified patients with CHC who were at high risk of liver cirrhosis and HCC development. The FIB-4 is useful for non-invasive detection of liver cirrhosis and HCC in at-risk patients because of its ease of application and reproducibility.
Abbreviations
- HCV
hepatitis C virus
- CHC
chronic hepatitis C
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ALP
alkaline phosphatase
- GGT
gamma-glutamyl transpeptidase
- TBiL
total bilirubin
- ALB
albumin
- CHE
cholinesterase
- PT
prothrombin time
- AOR
adjusted odds ratios
- CI
confidence intervals
- FIB-4
fibrosis index based on 4 factors
- APRI
aspartate transaminase-to-platelet ratio index
- AAR
aspartate transaminase-to- alanine aminotransferase ratio
- PLT
platelet count
Footnotes
Source of support: This study was supported by the Science and Technology Development Program of Jilin Province under grant no. 20190103079JH and the Youth Development Foundation of the First Hospital of Jilin University under grant no. JDYY102019004
Data availability statement
The data used to support the findings of this study were supplied by Pujun Gao under license and so cannot be made freely available. Requests for access to these data should be made to Pujun Gao (gpj0411@163.com).
Conflicts of interest
None.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hsu YC, Ho HJ, Wu MS, et al. Postoperative peg-interferon plus ribavirin is associated with reduced recurrence of hepatitis C virus-related hepatocellular carcinoma. Hepatology (Baltimore, Md) 2013;58:150–57. doi: 10.1002/hep.26300. [DOI] [PubMed] [Google Scholar]
- 3.Parikh S, Hyman D. Hepatocellular cancer: A guide for the internist. Am J Med. 2007;120:194–202. doi: 10.1016/j.amjmed.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217–28. doi: 10.3748/wjg.v20.i28.9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–30. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 7.Hassan MM, Frome A, Patt YZ, El-Serag HB. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002;35:266–69. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Tamaki N, Kurosaki M, Matsuda S, et al. Non-invasive prediction of hepatocellular carcinoma development using serum fibrosis marker in chronic hepatitis C patients. J Gastroenterol. 2014;49:1495–503. doi: 10.1007/s00535-013-0914-y. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Li W, Huang Y, Chen L. The gamma-glutamyl transpeptidase-to-platelet ratio predicts liver fibrosis and cirrhosis in HBeAg-positive chronic HBV infection patients with high HBV DNA and normal or mildly elevated alanine transaminase levels in China. J Viral Hepat. 2016;23:912–19. doi: 10.1111/jvh.12563. [DOI] [PubMed] [Google Scholar]
- 10.Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology (Baltimore, Md) 2010;52:518–27. doi: 10.1002/hep.23691. [DOI] [PubMed] [Google Scholar]
- 11.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, Md) 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 12.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology (Baltimore, Md) 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 13.Mobarak L, Omran D, Nabeel MM, Zakaria Z. Fibro markers for prediction of hepatocellular carcinoma in Egyptian patients with chronic liver disease. J Med Virol. 2017;89:1062–68. doi: 10.1002/jmv.24720. [DOI] [PubMed] [Google Scholar]
- 14.Wang CJ, Wu JP, Zhou WQ, et al. The C-reactive protein/albumin ratio as a predictor of mortality in patients with HBV-related decompensated cirrhosis. Clin Lab. 2019;65(8) doi: 10.7754/Clin.Lab.2019.190215. [DOI] [PubMed] [Google Scholar]
- 15.Tarantino G, Citro V, Esposito P, et al. Blood ammonia levels in liver cirrhosis: A clue for the presence of portosystemic collateral veins. BMC Gastroenterol. 2009;9:21. doi: 10.1186/1471-230X-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology (Baltimore, Md) 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 17.Abdollahi M, Pouri A, Ghojazadeh M, et al. Non-invasive serum fibrosis markers: A study in chronic hepatitis. Bioimpacts. 2015;5:17–23. doi: 10.15171/bi.2015.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuzaki R, Tateishi R, Yoshida H, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology (Baltimore, Md) 2009;49:1954–61. doi: 10.1002/hep.22870. [DOI] [PubMed] [Google Scholar]
- 19.Yu ML, Lin SM, Lee CM, et al. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology (Baltimore, Md) 2006;44:1086–97. doi: 10.1002/hep.21363. [DOI] [PubMed] [Google Scholar]
- 20.Nunes D, Fleming C, Offner G, et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346–53. doi: 10.1038/ajg.2009.746. [DOI] [PubMed] [Google Scholar]
- 21.Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970–79. 9.e1–3. doi: 10.1053/j.gastro.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 22.Park LS, Tate JP, Justice AC, et al. FIB-4 index is associated with hepatocellular carcinoma risk in HIV-infected patients. Cancer Epidemiol Biomarkers Prev. 2011;20:2512–17. doi: 10.1158/1055-9965.EPI-11-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase-to-platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: A systemic review and meta-analysis. Hepatology (Baltimore, Md) 2015;61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 24.Suh B, Park S, Shin DW, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology (Baltimore, Md) 2015;61:1261–68. doi: 10.1002/hep.27654. [DOI] [PubMed] [Google Scholar]
- 25.Suh B, Yun JM, Park S, et al. Prediction of future hepatocellular carcinoma incidence in moderate to heavy alcohol drinkers with the FIB-4 liver fibrosis index. Cancer. 2015;121:3818–25. doi: 10.1002/cncr.29577. [DOI] [PubMed] [Google Scholar]
- 26.Zarski JP, Sturm N, Guechot J, et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: The ANRS HCEP-23 study. J Hepatol. 2012;56:55–62. doi: 10.1016/j.jhep.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Poynard T, Ngo Y, Perazzo H, et al. Prognostic value of liver fibrosis biomarkers: A meta-analysis. Gastroenterol Hepatol. 2011;7:445–54. [PMC free article] [PubMed] [Google Scholar]
- 28.Castera L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology (Baltimore, Md) 2010;51:828–35. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 29.Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–73. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 31.Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44:1249–53. doi: 10.1023/a:1026609231094. [DOI] [PubMed] [Google Scholar]
- 32.Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734–39. doi: 10.1016/s0016-5085(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 33.Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology (Baltimore, Md) 2005;41:1376–82. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- 34.Amorim TG, Staub GJ, Lazzarotto C, et al. Validation and comparison of simple noninvasive models for the prediction of liver fibrosis in chronic hepatitis C. Ann Hepatol. 2012;11:855–61. [PubMed] [Google Scholar]
- 35.Kim SM, Sohn JH, Kim TY, et al. [Comparison of various noninvasive serum markers of liver fibrosis in chronic viral liver disease]. Korean J Hepatol. 2009;15:454–63. doi: 10.3350/kjhep.2009.15.4.454. [in Korean] [DOI] [PubMed] [Google Scholar]
- 36.de Oliveira AC, El-Bacha I, Vianna MV, Parise ER. Utility and limitations of APRI and FIB4 to predict staging in a cohort of nonselected outpatients with hepatitis C. Ann Hepatol. 2016;15:326–32. doi: 10.5604/16652681.1198801. [DOI] [PubMed] [Google Scholar]
- 37.Park YE, Kim BK, Park JY, et al. Gamma-glutamyl transpeptidase-to-platelet ratio is an independent predictor of hepatitis B virus-related liver cancer. J Gastroenterol Hepatol. 2017;32:1221–29. doi: 10.1111/jgh.13653. [DOI] [PubMed] [Google Scholar]
- 38.Choi BC. Sensitivity and specificity of a single diagnostic test in the presence of work-up bias. J Clin Epidemiol. 1992;45:581–86. doi: 10.1016/0895-4356(92)90129-b. [DOI] [PubMed] [Google Scholar]