Abstract
Background
In China, evidence regarding to the association between platelet to lymphocyte ratio (PLR) and glucocorticoid (GC) resistance in participants with primary newly identified immune thrombocytopenia (ITP) is limited. We aimed to investigate whether PLR is independently linked with GC-resistant ITP.
Material/Methods
We non-selectively and consecutively collected 154 newly diagnosed ITPs. The start enrollment time and the end enrollment time were from March 2013 to June 2017. The independent and dependent variables were PLR measured at diagnosis and GC non-response. Other variables involved in the present work can be summarized as demographic data and factors that were correlated with PLR reported by published studies. Univariate and multivariate binary logistic regression model and sensitivity analysis were used to evaluate the associations between PLR and GC resistance.
Results
After adjusting covariates, PLR level was negatively associated with GC non-response [odds ratio (OR)=0.89, 95% confidence intervals (CI): 0.80 to 0.98], and supported by propensity score matching model (OR=0.74, 95%CI: 0.57 to 0.96]. Nonlinearity of PLR and GC resistance was observed whose inflection point was 5.08 (by 2-piecewise model). The OR and 95%CI on both sides of inflection point were 3.14 (0.81 to 12.21) and 0.81 (0.69 to 0.95), respectively. Subgroup analysis showed no significant differences from subgroups.
Conclusions
Threshold effect on PLR and GC resistance is observed. When PLR is larger than 5.08, a unit increase of PLR is independently associated with 19% reduction of GC resistance.
MeSH Keywords: Glucocorticoids; Platelet Activation; Purpura, Thrombotic Thrombocytopenic
Background
As an autoimmune disorder, the clinical manifestations of immune thrombocytopenia (ITP) are low platelet counts with potentially spontaneous bruising, petechial rash, mucosal bleeding or even life-threatening hemorrhage [1]. Although the current guidelines recommend glucocorticoids (GC) with or without intravenous immunoglobulin (IVIG) as the first-line treatment for ITP, 30% of newly diagnosed ITP patients develop primary GC resistance [2–4]. In the PubMed database there is a paucity with respect to risk factors of GC resistance in adult newly diagnosed ITP patients in China.
Platelet to lymphocyte ratio (PLR) is the ratio of platelets to lymphocytes. Previous literature reported that PLR is an independent risk factor for poor prognosis in gastrointestinal tract, advanced cancer, and female reproductive system tumor [5–8]. Other studies reported that PLR is associated with the incidence of sudden sensorineural hearing loss [9], mood disorders [10], and cardiovascular events [11,12]. To our knowledge, however, there is limited literature study on the relationship between PLR and steroid resistance in Chinese patients with newly diagnosed ITP. Given a series of studies have reported that the platelet count and lymphocytes at baseline are closely related to the clinical features and clinical outcomes of ITP [13–15]. Therefore, we speculate that PLR might be an independent risk factor for GC resistance in newly diagnosed ITP patients.
In this present work, our goal was to explore the correlation between PLR and GC resistance in newly identified ITP.
Material and Materials
Study design
This research is a historical cohort study. The exposure variable is PLR obtained at diagnosis. The outcome variable is GC resistance (dichotomous variable, 1=no response to GC; 0=response).
Data source
We non-selectively and consecutively collected data from all participants with newly identified ITP in the People’s Hospital of Guizhou Province, Guiyang City, China. Data were anonymous and compiled from electronic medical records of hospital. We did not sign the patient consent form because of the nature of historical cohort study, but the hospital institutional review board have approved this study.
Study cohort
A total of 437 patients were included in this cohort. Cohort entry was the date of the first PLR value any time from March 2013 to June 2017. The diagnosis of ITP patients is mainly based on a Chinese expert consensus (version 2012 [16] and 2016). Inclusion criteria was as follows: 1) ITP identified within the past 3 months; 2) non-myelofibrosis or other thrombocytopenic diseases; and 3) without spleen enlargement. Exclusion criteria was as follows: 1) ITP secondary to other diseases; 2) drug-induced thrombocytopenia; 3) participants who were identified as hepatitis B virus, hepatitis C virus or human immunodeficiency virus (HIV) burden; 4) the participants who were pregnant; 5) severe dysfunction of the heart, kidneys, liver, or lungs; 6) received treatment of immunosuppressant within the past 3 months; and 7) received IVIG therapy.
Variables
We collected PLR values at baseline and recorded values as an untransformed continuous variable. Venous blood (2 mL) specimens were collected from patients and then tested by the center laboratory of our institution (automatic hematology analyzer, BM830, Bao Ling Man Sunshine Technology Co., Ltd., China). The reference range of platelet count and absolute lymphocyte count in our institution was accepted as 100 to 300×109/L and 1.1 to 3.2×109/L, respectively.
According to published guideline [16], we defined the final outcome variable as response+complete remission (CR) (Y=0) and no response to GC (Y=1). It was noted that determining no response must meet the following conditions: platelet count should be tested at least twice, and interval could not be less than 1 day. Complete remission (CR) was defined as platelet count above 100×109/L and absence of bleeding after a circle of first-line treatment. Response (R) was defined as platelet count above 30×109/L and at least 2-fold increase of the baseline platelet count and absence of bleeding [4,16]. No response (NR) was defined as platelet count below 30×109/L or 2-fold increase of the baseline platelet count or bleeding.
Covariates involved in this present work can be summarized as demographic data, general information, our prior work, previous literature, and clinical experiences [17–20]. Therefore, the following variables were used to construct the fully-adjusted model: sex, age, height, weight, smoking status, drink habits, Helicobacter pylori burden, comorbidities (type 2 diabetes, cardiovascular disease, hyperuricemia), mean platelet volume (MPV), bleeding symptoms (skin, mucosa, organ), first-line treatment strategy (oral prednisone, high-dose dexamethasone) and mean platelet distribution (MPD) at diagnosis.
First-line treatment strategies
In this study, first-line treatment included oral prednisone, high-dose dexamethasone with or without intravenous immunoglobulin. According to the Chinese expert consensus [16], first-line treatment selection for newly identified ITP participants was based on their platelet count at diagnosis and severity of bleeding symptoms. 1) High-dose dexamethasone: 40 mg/day, orally, days 1–4 and days 15–18. Monitoring patient’s blood pressure and blood glucose during treatment. Simultaneously took proton pump inhibitors to protect gastric mucosa. 2) Oral prednisone: starting dose at 1 mg/kg/day, orally, quickly reduced to the maintenance dose (15 mg/day) after remission, until drug withdrawal.
Statistical analysis and sensitivity analysis
We first observed the distribution of baseline data of participants in different PLR groups (tertile, low, middle, high). We presented continuous variables as mean ± standard deviation (Gaussian distribution) or median (quartile) (skewed distribution). Categorical variables were presented as in percentage. χ2 (categorical variables), One-way ANOVA (normal distribution), or Kruskal-Wallis H (skewed distribution) were employed to test for the differences of baseline data among different PLR groups.
To correctly evaluate the independent association of PLR and GC resistance, we analyzed data according to following principles: 1) the relationship between PLR and GC resistance is linear or nonlinear; 2) confounders controlling and effect modifier clarifying; and 3) the independent effect between PLR and GC resistance. Therefore, univariate and multivariate binary logistic regression model were employed (unadjusted model, model adjusted for demographics and fully adjusted model). Besides, the subgroup analyses were performed using stratified binary logistic regression models. Tests for effect modification by subgroup used interaction terms between subgroup indicators, followed by the likelihood ration test.
Sensitivity analyses were listed as follows. 1) We converted the PLR into a categorical variable according to tertile. The purpose was to verify the results of PLR as a continuous variable and further observe the nonlinear trend. 2) Propensity score matching was used to compensate for differences in baseline characteristics. The specific parameter setting, method and matching balance test of PS matching are shown in Supplementary Tables 1 and 2, and Figure 1. Unlike non-randomized comparative effectiveness researches using exposure variables (whether treated) to calculate propensity scores for the purpose of post-randomization, this study still used the outcome variable (GC-resistance) for computational propensity score, our purpose was mainly to verify whether the results of the fully adjusted model are robust. 3) As a linear model, binary logistic regression was underpowered to address nonlinearity. Therefore, a generalized additive model was employed in our study. The further explanation of nonlinearity was performed by 2-piecewise logistic regression model. We firstly calculated the inflection point using a recursion algorithm, and then evaluated OR on both side of inflection point by 2-piecewise model.
Figure 1.
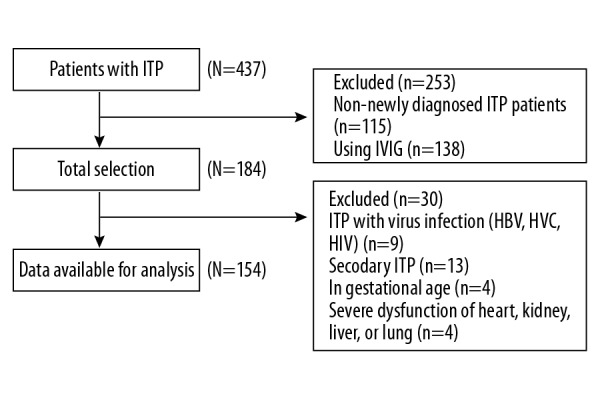
The flowchart of patients’ selection. ITP – immune thrombocytopenia; IVIG – intravenous immunoglobulin; HBV – hepatitis B; HCV – hepatitis C; HIV – human immunodeficiency virus.
The statistical software is packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA). P values less than 0.05 (2-sided) were considered as statistical difference.
Results
The selection of patients
After screening by inclusion and exclusion criteria, a total of 253 participants were excluded due to non-newly diagnosed ITP and IVIG user. Within the remaining 184 participants, 9 cases were seropositive for viral infection, 13 cases were identified as secondary ITP, 4 cases were pregnant, and 4 cases had severe dysfunction of major organs; 154 patients were used for data analysis.
Baseline characteristics of participants
The baseline characteristics of patients were listed in Table 1. The average age of the entire cohort was 41.5±19.4 years, and 64.9% (100 out of 154 patients) were female. Among the 154 selected participants, 14 did not respond to glucocorticoid (14 out of 154 patients 9.09%). According to tertile of PLR, the statistical differences were not detected in all variables except smoking status, high dose dexamethasone, and oral prednisone (P values were 0.003, 0.004, and 0.004, respectively). Compared with low PLR group, fewer current smokers were observed in middle PLR (T2) group and high PLR (T3) group.
Table 1.
Baseline characteristics of participants.
| PLR (tertile) | Low | Middle | High | P-value |
|---|---|---|---|---|
| N | 50 | 52 | 52 | |
| Age (years, mean ±SD) | 44.56±18.95 | 41.04±20.02 | 39.02±19.05 | 0.346 |
| Height (cm, mean ±SD) | 162.34±8.09 | 163.29±9.25 | 162.92±7.89 | 0.849 |
| Weight (kg, mean ±SD) | 59.76±9.76 | 59.71±9.93 | 56.62±8.70 | 0.158 |
| Sex (n, %) | 0.315 | |||
| Male | 20 (40.00%) | 20 (38.46%) | 14 (26.92%) | |
| Female | 30 (60.00%) | 32 (61.54%) | 38 (73.08%) | |
| Smoking (n, %) | 0.003 | |||
| No | 31 (62.00%) | 31 (59.62%) | 44 (84.62%) | |
| Current smoker | 16 (32.00%) | 17 (32.69%) | 2 (3.85%) | |
| Ex-smoker | 3 (6.00%) | 4 (7.69%) | 6 (11.54%) | |
| Alcohol consumption (n, %) | 0.671 | |||
| No | 43 (86.00%) | 42 (80.77%) | 45 (86.54%) | |
| Yes | 7 (14.00%) | 10 (19.23%) | 7 (13.46%) | |
| HP infection (n, %) | 0.941 | |||
| Negative | 35 (70.00%) | 38 (73.08%) | 37 (71.15%) | |
| Positive | 15 (30.00%) | 14 (26.92%) | 15 (28.85%) | |
| Diabetes history (n, %) | 0.198 | |||
| No | 44 (88.00%) | 43 (82.69%) | 48 (94.12%) | |
| Yes | 6 (12.00%) | 9 (17.31%) | 3 (5.88%) | |
| Hyperuricemia (n, %) | 0.149 | |||
| No | 38 (76.00%) | 42 (80.77%) | 47 (90.38%) | |
| Yes | 12 (24.00%) | 10 (19.23%) | 5 (9.62%) | |
| Cardiovascular diseases (n, %) | 0.959 | |||
| No | 43 (86.00%) | 45 (86.54%) | 44 (84.62%) | |
| Yes | 7 (14.00%) | 7 (13.46%) | 8 (15.38%) | |
| Bleeding in skin (n, %) | 0.144 | |||
| No | 39 (78.00%) | 32 (61.54%) | 39 (75.00%) | |
| Yes | 11 (22.00%) | 20 (38.46%) | 13 (25.00%) | |
| Bleeding in mucosa (n, %) | 0.763 | |||
| No | 36 (72.00%) | 35 (67.31%) | 34 (65.38%) | |
| Yes | 14 (28.00%) | 17 (32.69%) | 18 (34.62%) | |
| Bleeding in organ (n, %) | 0.684 | |||
| No | 44 (88.00%) | 48 (92.31%) | 48 (92.31%) | |
| Yes | 6 (12.00%) | 4 (7.69%) | 4 (7.69%) | |
| High-dose dexamethasone (n, %) | 0.004 | |||
| No | 23 (46.00%) | 28 (53.85%) | 40 (76.92%) | |
| Yes | 27 (54.00%) | 24 (46.15%) | 12 (23.08%) | |
| Oral prednisone (n, %) | 0.004 | |||
| No | 27 (54.00%) | 24 (46.15%) | 12 (23.08%) | |
| Yes | 23 (46.00%) | 28 (53.85%) | 40 (76.92%) | |
| Outcome (n, %) | 0.093 | |||
| Response | 42 (84.00%) | 50 (96.15%) | 48 (92.31%) | |
| No response | 8 (16.00%) | 2 (3.85%) | 4 (7.69%) |
PLR – platelet to lymphocyte ratio; MPV – mean platelet volume; MPD – mean platelet distribution width; HP – Helicobacter pylori.
Multivariate analysis
To evaluate the linear relationship between PLR and GC-resistance, and the robustness of our results, we simultaneously showed unadjusted, minimally adjusted and fully adjusted models. They were listed in Table 2. In the crude model, odds ratio (OR) was 0.93, and the range of 95% confidence interval (CI) was 0.85 to 1.02. It could be interpreted that a unit increase of PLR was associated with the 7% reduction of risk of GC-resistance. When we adjusted all covariates, which are presented in Table 1; the OR was 0.89 (95%CI 0.80 to 0.98). This meant that for every 1 unit increase in PLR, the risk of GC resistance was reduced by 11%. We also used the propensity score matching model to evaluate the relationship between PLR and GC resistance. Although the OR magnitude of the PS matching model was slightly different from the fully adjusted model, the OR values were in the same direction (OR=0.74, 95%CI: 0.57 to 0.96). By observing the trend of OR values in different models, we found that although the magnitude of the OR and the range of CIs were slightly different, their direction and range suggested that PLR was a protective factor for GC-resistance. This finding was robustness.
Table 2.
Results of multivariate analysis.
| Exposure | Crude model (n=154) (OR, 95% CI) | Minimally adjusted model | Fully adjusted (n=154) Model (OR, 95% CI) | Propensity score matching model (OR, 95% CI) |
|---|---|---|---|---|
| PLR | 0.93 (0.85 to 1.02) | 0.93 (0.85 to 1.02) | 0.89 (0.80 to 0.98) | 0.74 (0.57 to 0.96) |
| PLR (tertile) | ||||
| Low | Ref | Ref | Ref | – |
| Middle | 0.21 (0.04 to 1.04) | 0.21 (0.04 to 1.04) | 0.06 (0.01 to 0.47) | – |
| High | 0.44 (0.12 to 1.56) | 0.45 (0.12 to 1.64) | 0.25 (0.05 to 1.33) | – |
| P for trend | 0.157 | 0.173 | 0.048 | – |
Crude model: not adjusted for other covariants. Minimally adjusted model: adjusted for sex and age. Fully adjusted model: adjusted for age, sex, weight, height, smoking status, alcohol consumption, HP (Helicobacter pylori) infection, diabetes history, hyperuricemia, cardiovascular diseases, bleeding in skin, bleeding in mucosa, bleeding in organ, treatment protocol (high-dose dexamethasone, oral prednisone). OR – odds ratio; CI – confidence interval; Ref – reference; PLR – platelet to lymphocyte ratio.
In order to verify our findings further and observe the possibility of nonlinearity, we also converted PLR into a categorical variable (by tertile). We observed the potential nonlinear relationships in both of the crude model and fully adjusted model (Table 2). In these 2 models, the change of OR value in different level of PLR (T1 to T3) was non-equidistant. Therefore, we used 2-piecewise regression models to further analyze the curve relationship.
The nonlinearity of PLR and GC resistance addressing
In the present work, the non-linear relationship between PLR and risk of GC-resistance was observed (after adjusting for covariates present in Table 1) by generalized additive model. Therefore, we firstly used recursion algorithm to calculate the inflection point of curve (Figure 2). The value calculated for the inflection point of curve was 5.08. Using 2-piecewise linear regression model, we divided the curve according to the inflection point, and established the binary logistic regression model on the left and right of the inflection point, and then calculated the OR and CIs respectively (Table 3). On the left of the inflection point (PLR <5.08), the OR was 3.14 and the range of 95%CI was 0.81 to 12.21. This meant that at the left of the inflection point (<5.08), the increase in PLR was not associated with GC resistance. On the right of the inflection point, however, the OR was 0.81, and the range of 95%CI was 0.69 to 0.95. The result showed that PLR was negatively associated with GC resistance in patients with primary newly diagnosed ITP unless PLR exceeded 5.08. When the PLR was greater than 5.08, the risk of glucocorticoid resistance was decreased by 19% for every 1 of PLR increase (threshold effect).
Figure 2.
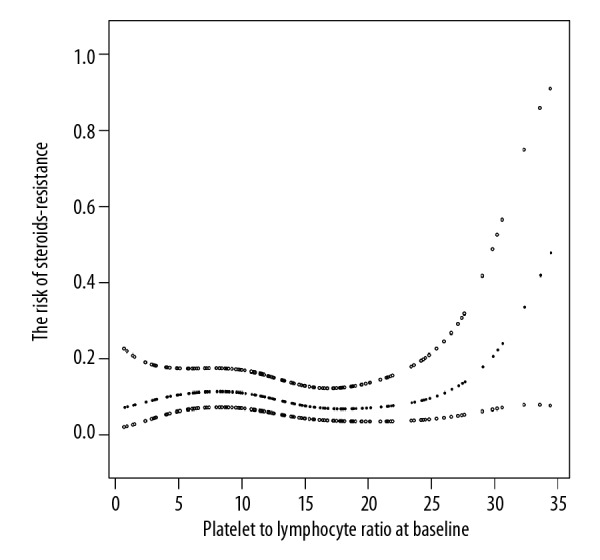
The nonlinearity addressing between PLR and GC resistance. PLR – platelet to lymphocyte ratio; GC – glucocorticoid.
Table 3.
Results of 2-piecewise linear regression model.
| Inflection point of PLR (%) | Effect size (OR) | 95% CI | P value |
|---|---|---|---|
| <5.08 | 3.14 | 0.81 to 12.21 | 0.572 |
| ≥5.08 | 0.81 | 0.69 to 0.95 | 0.022 |
Exposure: PLR Outcome: ITP treatment response status (response=0, no response=1). Adjust strategy: adjusted for age, sex, weight, height, smoking status, alcohol consumption, HP infection, diabetes history, hyperuricemia, cardiovascular diseases, bleeding in skin, bleeding in mucosa, bleeding in organ, treatment protocol (high-dose dexamethasone, oral prednisone). PLR – platelet to lymphocyte ratio; OR – odds ratio; CI – confidence interval; ITP – immune thrombocytopenia; HP – Helicobacter pylori.
The results of subgroup analyses
We listed results of subgroup analysis in Table 4. Subgroup analysis showed that the association of PLR with glucocorticoid resistance remained stable in different subgroups when grouped by gender, smoking status, alcohol consumption, H. pylori infection, diabetes history, hyperuricemia, and clinical bleeding symptoms. All values of P for interaction were larger than 0.05. Therefore, at least in this study, we did not find that the aforementioned variables can modify the relationship between PLR and GC resistance.
Table 4.
Results of subgroup analysis and interaction analysis.
| Number of cases | OR | 95% CI low | 95%CI high | P (interaction) | |
|---|---|---|---|---|---|
| Sex | 0.704 | ||||
| Male | 54 | 0.95 | 0.83 | 1.09 | |
| Female | 100 | 0.92 | 0.82 | 1.04 | |
| Smoking | 0.096 | ||||
| No smoking+ex-smoking | 119 | 0.96 | 0.87 | 1.05 | |
| Current smoking | 35 | 0.75 | 0.54 | 1.02 | |
| Alcohol consumption | 0.384 | ||||
| No | 130 | 0.94 | 0.86 | 1.03 | |
| Yes | 24 | 0.81 | 0.56 | 1.16 | |
| HP infection | 0.639 | ||||
| Negative | 110 | 0.96 | 0.84 | 1.10 | |
| Positive | 44 | 0.92 | 0.83 | 1.03 | |
| Diabetes history | - | ||||
| No | 135 | 1.02 | 0.97 | 1.08 | |
| Yes | 19 | 0.94 | inf | 1.10 | |
| Hyperuricemia | 0.412 | ||||
| No | 127 | 1.02 | 0.96 | 1.09 | |
| Yes | 27 | 0.98 | 0.90 | 1.07 | |
| Cardiovascular diseases | 0.198 | ||||
| No | 132 | 1.03 | 0.98 | 1.11 | |
| Yes | 22 | 0.79 | 0.59 | 1.07 | |
| Bleeding in skin | 0.709 | ||||
| No | 110 | 0.93 | 0.84 | 1.04 | |
| Yes | 44 | 0.90 | 0.76 | 1.06 | |
| Bleeding in mucosa | 0.088 | ||||
| No | 105 | 1.01 | 0.88 | 1.12 | |
| Yes | 49 | 0.90 | 0.76 | 1.06 | |
| Bleeding in organ | - | ||||
| No | 140 | 0.99 | 0.94 | 1.05 | |
| Yes | 14 | 1.04 | Inf | inf | |
| High-dose dexamethasone | 0.960 | ||||
| No | 91 | 0.93 | 0.83 | 1.04 | |
| Yes | 63 | 0.92 | 0.80 | 1.07 | |
| Oral prednisone | 0.960 | ||||
| No | 63 | 0.92 | 0.80 | 1.07 | |
| Yes | 91 | 0.93 | 0.83 | 1.04 | |
| Age | 0.615 | ||||
| <60 years | 120 | 0.95 | 0.85 | 1.05 | |
| ≥60 years | 34 | 0.90 | 0.85 | 1.02 |
The model was adjusted for age, sex, weight, height, smoking status, alcohol consumption, HP infection, diabetes history, hyperuricemia, cardiovascular diseases, MPV, bleeding in skin, bleeding in mucosa, bleeding in organ, treatment protocol (high-dose dexamethasone, oral prednisone, IVIG), MPD. In each case, the model was not adjusted for the stratification variable. Following variables excluded because of <20 obs. in a category: Diabetes history and bleeding in organ. OR – odds ratio; CI – confidence interval; HP – Helicobacter pylori; MPV – mean platelet volume; IVIG – intravenous immunoglobulin; MPD – mean platelet distribution.
Discussion
In this historical cohort study, 154 patients with newly identified ITP were involved. PLR measured at baseline was negatively associated with the risk of GC resistance after adjusting for covariate by multivariate logistic regression. Furthermore, smooth curve fitting by GAM model and piecewise linear fitting (by 2-piecewise logistic regression) found a threshold effect on the relationship between PLR and glucocorticoid resistance. The inflection point was 5.08. On the left side of the inflection point (≤5.08), the link of PLR and glucocorticoid resistance was not observed. But on the right side of the inflection point (≥5.08), for every additional unit of PLR, the risk of glucocorticoid resistance decreased by 19%.
Although the treatment of ITP has made great progress (such as rituximab and thrombopoietin receptor agonists), glucocorticoids remain the first line of treatment for ITP [21]. The second-line drugs recommended in the guidelines include: splenectomy, recombinant human thrombopoietin (rhTPO), eltrombopag, azathioprine, rituximab, and other drugs (cyclosporine, danazol, and vinblastine) [16]. Compared with second-line treatments, GC have the durable side effects and inexpensive price [16,22–26]. Perhaps this is why hematologists were less enthusiastic about the clinical marker discovery of hormone resistance in ITP patients. However, the annual incidence of adult ITP is 5 to 10/10 million [4,16–18], and the incidence of GCs resistance in these patients is 30%. For China’s huge population base, therefore, steroid resistance is a problem that cannot be ignored due to side effects caused by GC therapy (e.g., Cushing features, osteoporosis, and other toxicities) [21]. If we can effectively predict the possible steroid resistance, it will help to improve treatment efficiency, reduce the incidence of treatment-related adverse events, and reduce the cost of treatment.
To the best of our knowledge, this is first time to report that PLR is a protective factors for GC resistance in patients with newly diagnosed ITP. Previous research has reported initial platelet count, lymphocyte count is associated with ITP progression and outcome. Ahmed et al. reported that low baseline lymphocyte count was associated with no response to GC therapy [27]. Similar findings were also reported by Nagata et al. [28]. Additionally, 2 observational studies in children have referred to the association of platelets with ITP, but the results were inconsistent. One of the studies found that persistent ITP had a significantly lower initial platelet count compared with newly diagnosed ITP [29]; another study concluded that initial platelet count was not associated with GC resistance [30]. Therefore, in view of the aforementioned evidence, we used the ratio of the 2 to observe their association with GC resistance. It might be more efficient to either the individual platelet or the lymphocyte counts which is related to GC resistance. In fact, the result we obtained was consistent with previous studies.
Interestingly, we also find the threshold effect between PLR and GC resistance and calculated inflection point. In biomedical research, the association between 2 factors is often nonlinear. Most of these nonlinear correlations appear to be that the association can only be observed within a certain range (threshold effect). In our work, we found the negative association between PLR and GC resistance can only be observed when PLR was larger than 5.08. It was noted that the inflection point in our study was not equivalent to the cut point calculated by the receiver operating characteristic (ROS) curve. The latter was used to distinguish whether or not this event occurs, and the inflection point obtained in this study means that only within a certain range, the negative correlation between PLR and GC resistance can be observed.
Our present work had a number of strengths. Firstly, we handled exposure variable (PLR) as both a continuous variable and a categorical variable, and calculated the effect sizes (OR) through binary logistic regression models. Such an approach can reduce the contingency in the data analysis, enhance the robustness of results, and the trend of OR values of the categorical variable were helpful to observe the non-linear relationship. Secondly, the employed additive model (GAM) is powerful to address the nonlinearity [31]. Thirdly, this research is an observational study; it was inevitably to have potential confounders. Therefore, the use of strict statistical adjustment was helpful to minimize residual confounders. Fourthly, the subgroup analysis made the use of data better and yielded stable conclusion in different subgroups in this study. Finally, in consideration of baseline differences in clinical characteristics between the PLR groups, PS matching analyses were used and further confirmed our initial findings.
There were several limitations to consider in this study. Firstly, we included only those patients who have no history of hepatitis B. This limited the generalizability of our results to individuals with hepatitis B infection. Secondly, we also restricted our study population to exclude pregnant women, which means that our findings were not necessarily applicable to patients in pregnancy. Thirdly, although we performed PS matching to balance baseline differences, it was possible that other confounders were not accounted for in the analyses. Fourthly, since this study was not included in patients receiving IVIG therapy, therefore, our findings cannot be applied to patients who are receiving IVIG therapy. Finally, the present work mainly aimed to clarify the independent association between PLR and GC resistance. Therefore, we did not evaluate the predictive value of PLR. The center of our future work will focus on the establishment of predictive models and incorporate PLR as a potential predictor into the equation.
Conclusions
The association between PLR and GC resistance was non-linear. A threshold effect was observed between the 2 with an inflection point of 5.08. When the PLR was greater than 5.08, the elevation of 1 unit of PLR was associated with 19% reduction of risk of GC resistance.
Supplementary Data
Supplementary Table 1.
Propensity score parameter list.
| The variables used in calculating the propensity score | Age, sex, weight, height, smoking status, alcohol consumption, Helicobacter pylori infection, diabetes history, hyperuricemia, cardiovascular diseases, bleeding in skin, bleeding in mucosa, bleeding in organ, treatment protocol (high-dose dexamethasone, oral prednisone). | |
| Propensity scoring algorithm | Logistic regression model | |
| C-statistical | 0.787 | |
| Matching method | Greedy matching within specified caliper distances | |
| Distance metric | 0.05 | |
| Matching ratio | (no response to treatment) 1: 3 (response to treatment) | |
| Use of replacement | With replacement | |
| Matching sample size | No response to treatment: 9 cases | Total: 36 cases |
| Response to treatment: 27 cases | ||
Supplementary Table 2.
Balanced test of baseline data after matching.
| No response to GC (n=27) | Response to GC (n=9) | Standardized difference absolute value | P-value | |
|---|---|---|---|---|
| Age (year, mean ±SD) | 36.89±20.97 | 39.11±23.19 | 0.1005 | 0.79 |
| Height (cm, mean ±SD) | 163.74±7.13 | 163.11±7.25 | 0.0875 | 0.8207 |
| Weight (kg, mean ±SD) | 59.48±9.22 | 60.44±11.48 | 0.0925 | 0.8 |
| Sex (n, %) | 0 | 1 | ||
| Male | 9 (33.3) | 3 (33.3) | ||
| Female | 18 (66.7) | 6 (66.7) | ||
| Smoking (n, %) | 0.6412 | |||
| No | 21 (77.8) | 7 (77.8) | 0 | |
| Current smoker | 5 (18.5) | 1 (11.1) | 0.2097 | |
| Ex-smoker | 1 (3.7) | 1 (11.1) | 0.2857 | |
| Alcohol consumption (n, %) | 0.1104 | 1 | ||
| No | 23 (85.2) | 8 (88.9) | ||
| Yes | 4 (14.8) | 1 (11.1) | ||
| HP infection (n, %) | 0.0743 | 1 | ||
| Negative | 14 (51.9) | 5 (55.6) | ||
| Positive | 13 (48.1) | 4 (44.4) | ||
| Hyperuricemia (n, %) | 0.4 | 1 | ||
| No | 25 (92.6) | 9 (100) | ||
| Yes | 2 (7.4) | 0 (0) | ||
| Diabetes history (n, %) | 0.1104 | 1 | ||
| No | 23 (85.2) | 8 (88.9) | ||
| Yes | 4 (14.8) | 1 (11.1) | ||
| Cardiovascular diseases (n, %) | 0.4 | 1 | ||
| No | 25 (92.6) | 9 (100) | ||
| Yes | 2 (7.4) | 0 (0) | ||
| Bleeding in skin (n, %) | 0.0743 | 1 | ||
| No | 14 (51.9) | 5 (55.6) | ||
| Yes | 13 (48.1) | 4 (44.4) | ||
| Bleeding in mucosa (n, %) | 0.075 | 1 | ||
| No | 16 (59.3) | 5 (55.6) | ||
| Yes | 11 (40.7) | 4 (44.4) | ||
| Bleeding in organ (n, %) | 0.128 | 1 | ||
| No | 25 (92.6) | 8 (88.9) | ||
| Yes | 2 (7.4) | 1 (11.1) | ||
| High-dose dexamethasone (n, %) | 0.0776 | 1 | ||
| No | 17 (63) | 6 (66.7) | ||
| Yes | 10 (37) | 3 (33.3) | ||
| Oral prednisone (n, %) | 0.0776 | 1 | ||
| No | 10 (37) | 3 (33.3) | ||
| Yes | 17 (63) | 6 (66.7) |
GC – glucocorticoid; PLR – platelet to lymphocyte ratio; SD – standard deviation; HP – Helicobacter pylori.
Footnotes
Source of support: This work was supported by grants from the Guizhou Science and Technology Fund Project ([2013] No 2183)
Conflicts of interest
None.
References
- 1.Stentoft J. ITP: From idiopathic purpura to immune thrombocytopenia and back. Br J Haematol. 2016;175(5):755–56. doi: 10.1111/bjh.14414. [DOI] [PubMed] [Google Scholar]
- 2.Rodeghiero F, Ruggeri M. ITP and international guidelines: What do we know, what do we need? Presse Med. 2014;43(4 Pt 2):e61–67. doi: 10.1016/j.lpm.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):2829–35. doi: 10.1182/blood-2017-03-754119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, Xu M, Qin P, et al. A multicenter randomized open-label study of rituximab plus rhTPO vs. rituximab in corticosteroid-resistant or relapsed ITP. Blood. 2015;125(10):1541–47. doi: 10.1182/blood-2014-06-581868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li B, Zhou P, Liu Y, et al. Platelet-to-lymphocyte ratio in advanced cancer: Review and meta-analysis. Clin Chim Acta. 2018;483:48–56. doi: 10.1016/j.cca.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Rejec A, Butinar J, Gawor J, et al. Evaluation of complete blood count indices (NLR, PLR, MPV/PLT, and PLCRi) in healthy dogs, dogs with periodontitis, and dogs with oropharyngeal tumors as potential biomarkers of systemic inflammatory response. J Vet Dent. 2017;34(4):231–40. doi: 10.1177/0898756417731775. [DOI] [PubMed] [Google Scholar]
- 7.Nora I, Shridhar R, Huston J, et al. The accuracy of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as a marker for gastrointestinal malignancies. J Gastrointest Oncol. 2018;9(5):972–78. doi: 10.21037/jgo.2018.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng F, Tian Y, Liu S, et al. Combination of PLR, MLR, MWR, and tumor size could significantly increase the prognostic value for gastrointestinal stromal tumors. Medicine (Baltimore) 2016;95(14):e3248. doi: 10.1097/MD.0000000000003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Z, Li Z, Xiang H, et al. Prognostic role of haematological indices in sudden sensorineural hearing loss: Review and meta-analysis. Clin Chim Acta. 2018;483:104–11. doi: 10.1016/j.cca.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Mazza MG, Lucchi S, Tringali A, et al. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):229–36. doi: 10.1016/j.pnpbp.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Balta S, Ozturk C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. 2015;26(7):680–81. doi: 10.3109/09537104.2014.979340. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Zhou Y, Ma Y, et al. The prognostic value of the platelet-to-lymphocyte ratio in acute coronary syndrome: A systematic review and meta-analysis. Kardiol Pol. 2017;75(7):666–73. doi: 10.5603/KP.a2017.0068. [DOI] [PubMed] [Google Scholar]
- 13.Tang YT, He P, Li YZ, et al. Diagnostic value of platelet indices and bone marrow megakaryocytic parameters in immune thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2017;28(1):83–90. doi: 10.1097/MBC.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 14.Negash M, Tsegaye A, G/Medhin A. Diagnostic predictive value of platelet indices for discriminating hypo productive versus immune thrombocytopenia purpura in patients attending a tertiary care teaching hospital in Addis Ababa, Ethiopia. BMC Hematol. 2016;16:18. doi: 10.1186/s12878-016-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaspekova SG, Shustova ON, Golubeva NV, et al. Relationships of mean platelet volume and plasma thrombopoietin with glycocalicin levels in thrombocytopenic patients. Acta Haematol. 2015;133(3):295–99. doi: 10.1159/000362531. [DOI] [PubMed] [Google Scholar]
- 16.Thrombosis and Hemostasis group, Hematology Society, Chinese Medical Association. [Consensus of Chinese experts on diagnosis and treatment of adult primary immune thrombocytopenia (version 2012)]. Zhonghua Xue Ye Xue Za Zhi. 2012;33(11):975–77. [in Chinese] [PubMed] [Google Scholar]
- 17.Gumus F, Solak I, Eryilmaz MA. The effects of smoking on neutrophil/lymphocyte, platelet/lymphocyte ratios. Bratisl Lek Listy. 2018;119(2):116–19. doi: 10.4149/BLL_2018_023. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro SR, Covre LP, Stringari LL, et al. Peripheral blood CD4(+)/CD25(+) regulatory T cells in alcoholic patients with Strongyloides stercoralis infection. Parasitol Res. 2017;116(3):1071–74. doi: 10.1007/s00436-016-5355-0. [DOI] [PubMed] [Google Scholar]
- 19.Mertoglu C, Gunay M. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr. 2017;11(Suppl 1):S127–31. doi: 10.1016/j.dsx.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Su P, Hong L, Zhao Y, et al. The association between hyperuricemia and hematological indicators in a Chinese adult population. Medicine (Baltimore) 2016;95(7):e2822. doi: 10.1097/MD.0000000000002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cobra F, Melo MR, Faria CD, et al. Simultaneous evaluation of in vivo glucocorticoid sensitivity and expression of glucocorticoid receptor alpha-isoform in rheumatoid arthritis patients. Arq Bras Endocrinol Metabol. 2009;53(1):24–30. doi: 10.1590/s0004-27302009000100005. [DOI] [PubMed] [Google Scholar]
- 22.Splenectomy in ITP. Blood. 2018;131(11):1264. [Google Scholar]
- 23.Mazza P, Minoia C, Melpignano A, et al. The use of thrombopoietin-receptor agonists (TPO-RAs) in immune thrombocytopenia (ITP): A “real life” retrospective multicenter experience of the Rete Ematologica Pugliese (REP) Ann Hematol. 2016;95(2):239–44. doi: 10.1007/s00277-015-2556-z. [DOI] [PubMed] [Google Scholar]
- 24.Alva ME, Rivera R, Arocho R, et al. Cost per response analysis for thrombopoietin receptor agonists (Tpo-Ras), in the treatment of adult chronic immune thrombocytopenia (ITP) in Mexico. Value Health. 2014;17(7):A530. doi: 10.1016/j.jval.2014.08.1682. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Lopez TJ, Fernandez-Fuertes F, Hernandez-Rivas JA, et al. Efficacy and safety of eltrombopag in persistent and newly diagnosed ITP in clinical practice. Int J Hematol. 2017;106(4):508–16. doi: 10.1007/s12185-017-2275-4. [DOI] [PubMed] [Google Scholar]
- 26.Tripathi AK, Shukla A, Mishra S, et al. Eltrombopag therapy in newly diagnosed steroid non-responsive ITP patients. Int J Hematol. 2014;99(4):413–17. doi: 10.1007/s12185-014-1533-y. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed I, Rajpurkar M, Thomas R, et al. Initial lymphocyte count and the development of persistent/chronic immune thrombocytopenic purpura. Pediatr Blood Cancer. 2010;55(3):508–11. doi: 10.1002/pbc.22570. [DOI] [PubMed] [Google Scholar]
- 28.Nagata A, Sekiguchi N, Kurimoto M, et al. Significance of lymphocyte counts at diagnosis in the management of ITP: the relationship between lymphocyte counts and treatment success in H. pylori-infected patients. Int J Hematol. 2015;101(3):268–72. doi: 10.1007/s12185-015-1737-9. [DOI] [PubMed] [Google Scholar]
- 29.Makis A, Gkoutsias A, Palianopoulos T, et al. Prognostic factors for immune thrombocytopenia outcome in Greek children: A retrospective single-centered analysis. Adv Hematol. 2017;2017 doi: 10.1155/2017/7878605. 7878605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett CM, Neunert C, Grace RF, et al. Predictors of remission in children with newly diagnosed immune thrombocytopenia: Data from the Intercontinental Cooperative ITP Study Group Registry II participants. Pediatr Blood Cancer. 2018;65(1):73–77. doi: 10.1002/pbc.26736. [DOI] [PubMed] [Google Scholar]
- 31.Forest S. Nonlinear regularization operators as derived from the micromorphic approach to gradient elasticity, viscoplasticity and damage. Proc Math Phys Eng Sci. 2016;472(2188):20150755. doi: 10.1098/rspa.2015.0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Propensity score parameter list.
| The variables used in calculating the propensity score | Age, sex, weight, height, smoking status, alcohol consumption, Helicobacter pylori infection, diabetes history, hyperuricemia, cardiovascular diseases, bleeding in skin, bleeding in mucosa, bleeding in organ, treatment protocol (high-dose dexamethasone, oral prednisone). | |
| Propensity scoring algorithm | Logistic regression model | |
| C-statistical | 0.787 | |
| Matching method | Greedy matching within specified caliper distances | |
| Distance metric | 0.05 | |
| Matching ratio | (no response to treatment) 1: 3 (response to treatment) | |
| Use of replacement | With replacement | |
| Matching sample size | No response to treatment: 9 cases | Total: 36 cases |
| Response to treatment: 27 cases | ||
Supplementary Table 2.
Balanced test of baseline data after matching.
| No response to GC (n=27) | Response to GC (n=9) | Standardized difference absolute value | P-value | |
|---|---|---|---|---|
| Age (year, mean ±SD) | 36.89±20.97 | 39.11±23.19 | 0.1005 | 0.79 |
| Height (cm, mean ±SD) | 163.74±7.13 | 163.11±7.25 | 0.0875 | 0.8207 |
| Weight (kg, mean ±SD) | 59.48±9.22 | 60.44±11.48 | 0.0925 | 0.8 |
| Sex (n, %) | 0 | 1 | ||
| Male | 9 (33.3) | 3 (33.3) | ||
| Female | 18 (66.7) | 6 (66.7) | ||
| Smoking (n, %) | 0.6412 | |||
| No | 21 (77.8) | 7 (77.8) | 0 | |
| Current smoker | 5 (18.5) | 1 (11.1) | 0.2097 | |
| Ex-smoker | 1 (3.7) | 1 (11.1) | 0.2857 | |
| Alcohol consumption (n, %) | 0.1104 | 1 | ||
| No | 23 (85.2) | 8 (88.9) | ||
| Yes | 4 (14.8) | 1 (11.1) | ||
| HP infection (n, %) | 0.0743 | 1 | ||
| Negative | 14 (51.9) | 5 (55.6) | ||
| Positive | 13 (48.1) | 4 (44.4) | ||
| Hyperuricemia (n, %) | 0.4 | 1 | ||
| No | 25 (92.6) | 9 (100) | ||
| Yes | 2 (7.4) | 0 (0) | ||
| Diabetes history (n, %) | 0.1104 | 1 | ||
| No | 23 (85.2) | 8 (88.9) | ||
| Yes | 4 (14.8) | 1 (11.1) | ||
| Cardiovascular diseases (n, %) | 0.4 | 1 | ||
| No | 25 (92.6) | 9 (100) | ||
| Yes | 2 (7.4) | 0 (0) | ||
| Bleeding in skin (n, %) | 0.0743 | 1 | ||
| No | 14 (51.9) | 5 (55.6) | ||
| Yes | 13 (48.1) | 4 (44.4) | ||
| Bleeding in mucosa (n, %) | 0.075 | 1 | ||
| No | 16 (59.3) | 5 (55.6) | ||
| Yes | 11 (40.7) | 4 (44.4) | ||
| Bleeding in organ (n, %) | 0.128 | 1 | ||
| No | 25 (92.6) | 8 (88.9) | ||
| Yes | 2 (7.4) | 1 (11.1) | ||
| High-dose dexamethasone (n, %) | 0.0776 | 1 | ||
| No | 17 (63) | 6 (66.7) | ||
| Yes | 10 (37) | 3 (33.3) | ||
| Oral prednisone (n, %) | 0.0776 | 1 | ||
| No | 10 (37) | 3 (33.3) | ||
| Yes | 17 (63) | 6 (66.7) |
GC – glucocorticoid; PLR – platelet to lymphocyte ratio; SD – standard deviation; HP – Helicobacter pylori.


