Abstract
Background
This study aimed to investigate a rabbit model of osteochondral regeneration using three-dimensional (3-D) printed polycaprolactone-hydroxyapatite (PCL-HA) scaffolds coated with umbilical cord blood mesenchymal stem cells (UCB-MSCs) and chondrocytes.
Material/Methods
Nine female New Zealand white rabbits were included in the study. The 3-D PCL-HA scaffolds were prepared using fused deposition modeling 3-D printing technology. Seeding cells were prepared by co-culture of rabbit UCB-MSCs and chondrocytes with a ratio of 3: 1. A total of 4×106 cells were seeded on 3-D PCL-HA scaffolds and implanted into rabbits with femoral trochlear defects. After 8 weeks of in vivo implantation, 12 specimens were sampled and examined using histology and scanning electron microscopy (SEM). The International Cartilage Repair Society (ICRS) macroscopic scores and histological results were recorded and compared with those of the unseeded PCL-HA scaffolds.
Results
Mean ICRS scores for the UCB-MSCs and chondrocyte-seeded PCL-HA scaffolds (group A) were significantly higher than the normal unseeded control (NC) PCL-HA scaffold group (group B) (P<0.05). Histology with safranin-O and fast-green staining showed that the UCB chondrocyte-seeded PCL-HA scaffolds significantly promoted bone and cartilage regeneration.
Conclusions
In a rabbit model of osteochondral regeneration using 3-D printed PCL-HA scaffolds, the UCB chondrocyte-seeded PCL-HA scaffold promoted articular cartilage repair when compared with the control or non-seeded PCL-HA scaffolds.
MeSH Keywords: Chondrocytes, Coculture Techniques, Cord Blood Stem Cell Transplantation, Tissue Engineering
Background
Cartilage and subchondral bone tissue are the major components of osteochondral tissue [1]. The joints are important components of human motion, but the ability of the joints for self-repair is limited following damage to the articular cartilage [2]. Damage to both the articular cartilage and subchondral bone often occurs and results in osteoarthritis, joint pain, and joint dysfunction and results in a large healthcare and social burden [3–6]. Current clinical approaches for the treatment of articular osteochondral damage include microfracture surgery, autologous chondrocyte implantation, and cartilage transplantation, but these treatments are limited due to problems with the outcome if tissue repair, limited availability of donors, and donor tissue damage [7].
Recently, osteochondral tissue engineering has developed rapidly, and osteochondral defects can now be repaired by constructing osteochondral tissues in vitro and transplanting them into defective joints in vivo [8–10]. However, approaches using tissue engineering have several limitations [11]. Joint tissue transplants cannot fully match the curvature and smoothness of the joint surface, the mechanical properties of the transplant may not match those of the original tissue, and poor biocompatibility can cause rejection [11]. Osteochondral tissue engineering scaffolds can be constructed using seeded cell and scaffold bioactive substances that can induce chondrocyte differentiation and improve joint repair by active molecules, intra-articular biomechanical stimuli, and in vivo growth factor recruitment [12,13].
Ideally, osteochondral defects should regenerate during treatment. Scaffolds are the main component of osteochondral tissue engineering, and choosing the appropriate scaffold material is essential for the repair of osteochondral defects. Previous studies have reported the use of tissue-engineered scaffolds using structures with different pore shapes, including poly(lactide-co-glycoside) (PLGA) [14–17], polyglycolic acid [18], polyglycolic acid (PGA) and polylactic acid (PLA) [19], and bioceramics [20,21]. However, which of these scaffold materials is best for the repair of osteochondral defects remains to be determined. The biocompatibility and plasticity of polycaprolactone (PCL) make it is one of the most common biodegradable polymers in osteochondral tissue engineering [22–24]. Hydroxyapatite (HA), an inducible biomaterial, which can enhance cell proliferation and differentiation when mixed with PCL [25–28]. HA nanocomposite scaffolds exhibit better surface chemistry and biological synergy when compared with their microcomposite counterparts [29]. Domingos et al. showed that introducing HA into the PCL structure can increase adhesion and human mesenchymal stem cell (MSC) proliferation [30]. In this previous study, a regularly shaped three-dimensional (3-D) scaffold was prepared using a 3-D additive manufacturing method after the PCL and nano-HA were uniformly melted and mixed [30].
Mesenchymal stem cells (MSCs) can be obtained from several sources and are now of great interest due to their potential to differentiate into osteoblasts and chondroblasts [31,32]. Umbilical cord blood (UCB)-MSCs, can be collected noninvasively and ethically, and UCB-MSCs have improved cell activity and proliferation ability, with low immunogenicity [31], and they do not need human leukocyte antigen (HLA) antigen matching [33]. The incidence of posttransplant infection is lower with UCB-MSC transplants when compared with MSCs from other sources, making them an ideal source of seed cells in tissue engineering [32].
Tissue engineering studies have combined 3-D printed scaffolds with living cells to promote tissue repair and regeneration [34,35]. Undifferentiated stem cells can be inoculated onto the scaffold to obtain improved regenerative repair ability compared with scaffolds without cells or scaffolds inoculated with differentiated stem cells [36]. Previous studies have shown that co-cultures of human UCB-MSCs and chondrocytes in vitro at a 3: 1 ratio showed improved activation of human chondrocytes, and UCB-MSCs can differentiate into chondrocytes in vitro [37].
The culture environment and the scaffold material used are equally important in tissue engineering, but few studies have examined the use of combined UCB-MSCs and scaffolds in vivo in osteochondral regeneration. Therefore, this study aimed to investigate a rabbit model of osteochondral regeneration using 3-D printed PCL-HA scaffolds coated with UCB-MSCs and chondrocytes.
Material and Methods
Material and animals
The polycaprolactone (PCL) (Mn 80000) and nano-hydroxyapatite (HA) (particle size <200 nm) were purchased from Sigma-Aldrich (St Louis, MO, USA). Umbilical cord blood mesenchymal stem cells (UCB-MSCs) were purchased from Biotechnology Co., Ltd. (Cyagen, Guangzhou, China).
Rabbits used in the animal model were provided by the Experimental Animal Center of Nanjing Medical University. The use of experimental animals was in accordance with the guidelines of the Ministry of Science and Technology of the Peoples’ Republic of China. The study was approved by the Nanjing Medical University, Nanjing, Jiangsu Province, China (approval no IACUC 160440). Unless otherwise specified, the experimental reagents used in cell culture were purchased from Sigma-Aldrich (St Louis, MO, USA) or Invitrogen (Carlsbad, CA, USA).
Rabbits used in the model of osteochondral regeneration
Nine female New Zealand white rabbits, between 6–7 months of age, weighing approximately 2.5 kg, were obtained from the Animal Laboratory Center of Nanjing Medical University. These rabbits were used to prepare the rabbit model of osteochondral regeneration using three-dimensional (3-D) printed polycaprolactone-hydroxyapatite (PCL-HA) scaffolds coated with umbilical cord blood mesenchymal stem cells (UCB-MSCs) and chondrocytes. Surgical osteochondral defects were created.
Before the experiment, all animals were housed at a room temperature of 25°C, a humidity of 60%, and 12-hour light and dark cycle for one week. All animals had free access to food and water. The Animal Laboratory Committee of Nanjing Medical University (Nanjing, China) approved the use of animals in this study. The animals were treated according to the National Institutes of Health (NIH) guidelines (Bethesda, MD, USA). All animals were examined by veterinarians to assess their health. All procedures were performed under general anesthesia, and following surgery, each rabbit was housed in a single cage.
Cell culture, isolation, and identification
New Zealand white rabbits aged between one to three days were euthanized, and the articular cartilage from the lower extremities was isolated in a sterile surgical environment. The cartilage was sectioned at approximately 1 mm, placed in a sterile centrifuge tube, washed three times with sterile phosphate-buffered saline (PBS), centrifuged at 80×g for 3 minutes, and washed twice with medium without serum. The chondrocytes were isolated by incubation with 2 g/L trypsin for 30 minutes, then digested with 2 g/L of type II collagenase for 8 hours. The cells were counted, then transferred to a 10 cm diameter petri dish at a seeding density of 5×105 cells. Both UCB-MSCs and extracted chondrocytes were cultured in F-12 Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and 100 U/mL of penicillin and streptomycin. Cells were passaged when the cells reached 80% confluence. Third-generation UCB-MSCs and chondrocytes were identified using flow cytometry and then selected for implantation onto the scaffolds.
Design and preparation of the implanted scaffold materials
The PCL (70% by weight) and HA (30% by weight) were thoroughly mixed as raw materials to prepare the 3-D scaffold. The HA powder was placed in a glass vessel and dried in an oven at 120°C for 1 hour. The PCL was melted at 120°C, and the dry HA powder was added to the low-viscosity liquid PCL and thoroughly mixed using a magnetic stirrer at 60 rpm. The composite blend was poured into a biological fused deposition 3-D printer (Fuqifan Electromechanical Technology Co., Ltd., Shanghai, China) through a feed port.
The 3-D printed polycaprolactone and nanohydroxyapatite (PCL-HA) cylindrical 3-D porous tissue-engineering scaffold materials were constructed using computer-aided design (CAD) and fused deposition modeling, as previously described [38]. The scaffold structure was designed using MIMICS 17.0 software (Materialise, Leuven, Belgium). The specific PCL-HA scaffold parameters were a diameter of 5 mm, a thickness of 4 mm, a mesh fill width of 800 μm, a fiber diameter of 200 μm, and a porous structure of 0°, 60°, and 120° [38]. The 3-D printer used a 200 μm print head. The traveling speed was 5 mm/min, and the layer thickness was 200 μm.
After the printer fused the PCL and HA according to the preset parameters, they were extruded and molded in three dimensions (X, Y, and Z). After printing, a 5 mm diameter circular punch was used to smooth the scaffold circumference. Scaffolds were prepared and randomly divided equally into the UCB-MSC-HA scaffold group (group A) and the unseeded normal control (NC)-PCL-HA scaffold group (group B). The structural morphology of the scaffold was observed macroscopically, and its microscopic morphology was observed using scanning electron microscopy (SEM) using a Hitachi S4800 scanning electron microscope (Hitachi, Tokyo, Japan). All scaffolds were sterilized with ethylene oxide and stored in sterile sealed bags before use.
Based on previous studies [37], the UCB-MSCs and rabbit chondrocytes were used in a uniform cell suspension at an optimal ratio of 3: 1. For group A (the UCB-MSC-HA scaffold group), 4×106 cells were cultured in suspension. The inoculated scaffolds were incubated for 3 hours under standard conditions in a humid incubator at 37°C with 5% CO2 to allow the cells to diffuse and adhere to the scaffold fully.
Surgical treatment in the rabbit model
Intramuscular ketamine hydrochloride (15 mg/kg) was used as the general anesthetic for the New Zealand white rabbits. Then, 10 cm of rabbit hair was removed from around the knee joints. The rabbits were placed on their backs on the operating table and their limbs fixed. After routinely disinfecting the surgical with povidone-iodine, the rabbits were covered with sterile drapes. A medial incision was made on the knee, the skin and subcutaneous fascia were incised layer by layer, and the joint capsule was exposed. We then retracted the patellar tendon and quadriceps femoris tendon laterally, exposed the femoral trochlea, and a 5 mm diameter round defect was made on the articular surface of the femoral trochlea to create the osteochondral defect model. The penetration depth was 4 mm, and the defect reached the subchondral bone. The incision was rinsed with normal saline twice to remove residual cartilage, bone chips, and excess blood.
In group A (the UCB-MSC-HA scaffold group) and group B (the NC-PCL-HA scaffold group), the scaffolds filled the defective parts of the knee joints. In the normal knee joint control group, the articular surface of the femoral trochlea was untreated after the joint capsule was incised. After suturing the layers, the incision was disinfected using povidone iodine. Penicillin sodium (20,000 units) was injected intramuscularly, and the rabbits were returned to their cages. After surgery, 20,000 units of penicillin sodium was injected intramuscularly continuously every day for three days. Eight weeks after the surgery, the animals were euthanized by injection with pentobarbital sodium.
Observational indicators
Eight weeks after surgery, specimens were taken from the rabbit knee joints, and their distal femurs were removed for observation and macroscopically scored using the International Cartilage Repair Society (ICRS) macroscopic scoring standards [39]. The ICRS macroscopic evaluation of osteochondral repair is widely used as an indicator to evaluate the osteochondral defect repair in vivo [40,41]. The ICRS macroscopic score was used separately to evaluate the degree of defect repair, integration to the border zone, and macroscopic appearance (Table 1). Two orthopedic surgeons and a pathologist, who were blinded to the study groupings, graded the effects of the defect repair.
Table 1.
ICRS macroscopic evaluation of cartilage repair.
| Cartilage repair assessment ICRS | Points |
|---|---|
| Degree of defect repair | |
| In level with surrounding cartilage | 4 |
| 75% repair of defect depth | 3 |
| 50% repair of defect depth | 2 |
| 25% repair of defect depth | 1 |
| 0% repair of defect depth | 0 |
| Integration of border zone | |
| Complete integration with surrounding cartilage | 4 |
| Demarcating border <1 mm | 3 |
| Three-fourth of graft integrated, one-fourth with a notable border >1 mm | 2 |
| Half of graft integrated with surrounding cartilage, and half with a notable border >1 mm | 1 |
| From no contact to one-fourth cartilage | 0 |
| Macroscopic appearance | |
| Intact smooth surface | 4 |
| Fibrillated surfave | 3 |
| Small, scattered fissures, or cracks | 2 |
| Several, small, or few but large fissures | 1 |
| Total degeneration of grafted area | 0 |
| Overall repair assessment | |
| Grade I: normal | 12 |
| Grade II: nearly normal | 11–8 |
| Grade III: abnormal | 7–4 |
| Grade IV: severely abnormal | 3–1 |
Histology of the joint tissue in the rabbit model
The rabbit joint tissues were fixed with 10% paraformaldehyde, decalcified with 30% formic acid, dehydrated in gradient ethanol, cleared, embedded in paraffin wax, and sectioned at 5 μm onto glass slides. Routine hematoxylin and eosin (H&E) staining was performed (KeyGen Biotech Co. Ltd., Nanjing, China). For the safranin-O and fast-green staining for glycosaminoglycans (GAGs), the tissue sections were routinely dewaxed to water and stained with fresh Weigert’s solution for 5 minutes. The sections were then differentiated for 15 seconds in an acid differentiation solution, incubated in fast-green for 5 minutes, and rinsed with distilled water for 1 minute. The sections were then incubated in the safranin-O stain for 2 minutes, rinsed with distilled water for 1 minute, washed with an alkaline solution for 1 minute and rinsed with distilled water for 1 minute. The tissue sections were finally dehydrated using 95% ethanol and anhydrous ethanol, cleared using dimethylbenzene and mounted in optical resin. Histology was performed following H&E, safranin-O, and fast-green staining using an Olympus X71 light microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data were analyzed using SPSS version 17.0 statistical software (IBM, Chicago, IL, USA). Data were presented as the mean ± standard deviation (SD). The means of the measurement data between groups were compared using one-way analysis of variance (ANOVA). A P-value <0.05 was considered to be statistically significant.
Results
Morphological structure of the three-dimensional (3-D) printed polycaprolactone-hydroxyapatite (PCL-HA) scaffold
The rabbits were divided into group A, the umbilical cord blood mesenchymal stem cells and hydroxyapatite (UCB-MSC-HA) scaffold group, and group B, the normal control polycaprolactone-hydroxyapatite (NC-PCL-HA) scaffold group. Digital photographs were taken of the scaffold samples to observe the appearance and structure of the scaffold (Figure 1A). After the scaffold surface was plated with a conductive adhesive on the sample stage, the scaffold structure was observed using scanning electron microscopy (SEM) (Figure 1B). The SEM image showed that the shape and size of the pores of the scaffold conformed to the theoretical values designed in the manufacturing process, and the scaffolds consisted of interconnected network structures.
Figure 1.
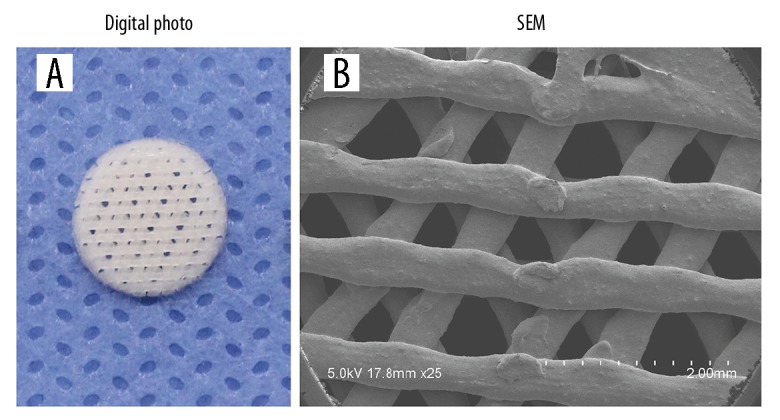
Morphological structure of the three-dimensional (3-D) printed polycaprolactone-hydroxyapatite (PCL-HA) scaffold. (A) Digital photo of the scaffold with a 0°, 60°, and 120° deposition angle. (B) Scanning electron microscopy (SEM) image of the morphology of the three-dimensional (3-D) printed PCL-HA scaffold (top view). Scale bar=2 mm.
Macroscopic appearance of cartilage regeneration
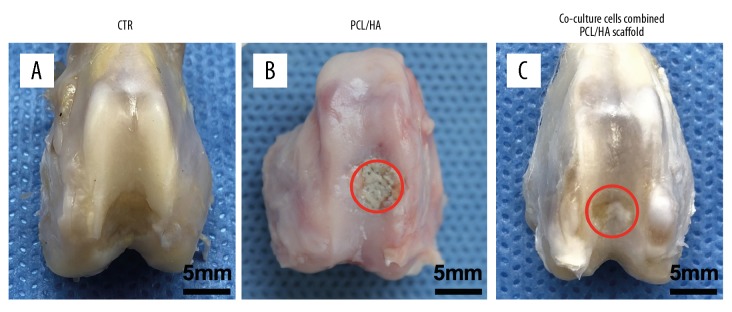
All incisions into the cartilage of the knee joints in the rabbit model underwent healing with the formation of granulation tissue without swelling or edema. No infection was found in any incision. The joint surfaces were observed macroscopically at week 8. The appearance of the normal joint surface (Figure 2A), and the joint surface of the PCL-HA scaffold group (Figure 2B) showed that the articular surface defect was uneven and the defect failed to develop white healing tissue, but pale transparent tissue was visible in the middle of the defect, and the presence of the scaffold was observed. Co-cultured cells combined with the PCL-HA scaffold (Figure 2C) showed articular surface defects filled with opaque white tissue with some irregular tissue plaques on the surface (the red circles in the figure indicate the defects in both groups).
Figure 2.
The joint surfaces were observed macroscopically at week 8. (A) Comparison with the normal articular surface. (B) In the three-dimensional (3-D) printed polycaprolactone-hydroxyapatite (PCL-HA) scaffold group, the articular surface defect was uneven. The defect failed to develop sufficient pink and white tissue, and white transparent tissue was visible in the middle of the defect. The presence of the scaffold is shown. (C) Co-cultured cells, combined with the PCL-HA scaffold, showed articular surface defects filled with opaque white tissue with some irregular tissue plaques on the surface. The red circles in the figure indicate the defects in both groups.
The International Cartilage Repair Society (ICRS) macroscopic scores showed that the composite scaffold with cells showed a significant improvement in osteochondral repair (P<0.001) (Table 2). At 8 weeks, the ICRS macroscopic score of the PCL-HA scaffold combined with co-cultured UCB-MSCs and chondrocytes was significantly greater than that of the unseeded PCL-HA scaffold. Although further studies are needed, these findings showed that 3-D printed PCL-HA scaffolds may have potential in osteochondral tissue engineering based on the ICRS macroscopic scoring of the joint repair. Based on the general appearance, the co-cultured UCB-MSCs and chondrocytes combined with 3-D printed scaffolds showed improved repair of the osteochondral tissue. Therefore, UCB-MSCs and chondrocytes have roles in promoting cartilage formation in vivo. After photographing the morphological appearance, histology was performed.
Table 2.
ICRS macroscopic score results and comparison.
| Group | 8 weeks |
|---|---|
| The PCL/HA scaffold group | 6.64±0.34 |
| The PCL/HA scaffold combined with co-cultured UCB-MSCs and chondrocytes group | 10.42±1.26 |
| P-values | <0.001 |
Histological evaluation of the repaired tissues
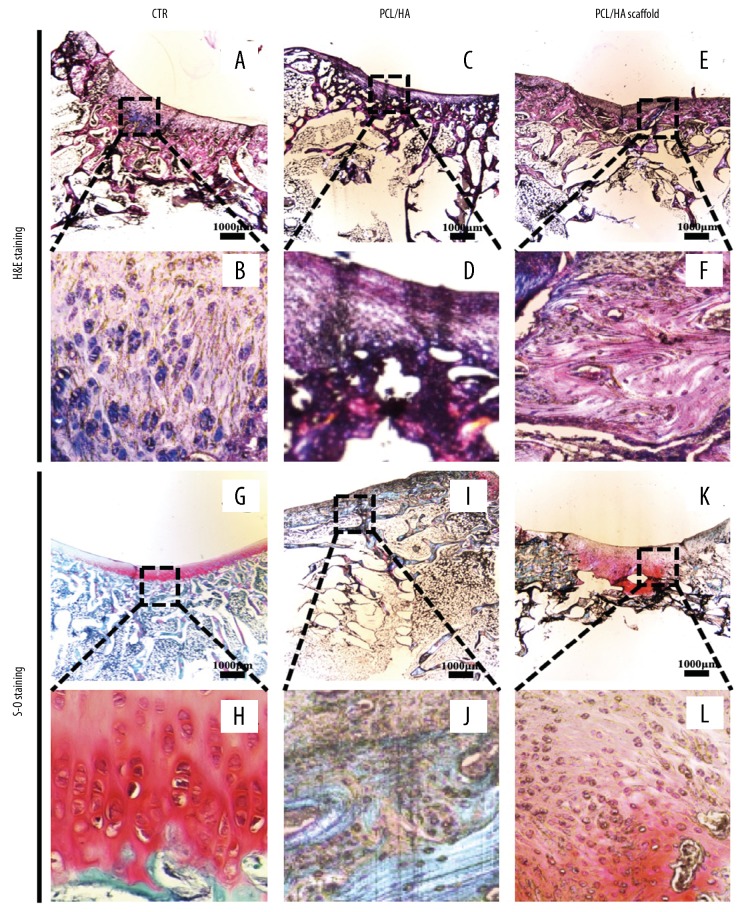
Because PCL is soluble in dimethylbenzene, the scaffolds dissolved during the tissue fixation and processing for histology, and the scaffold morphology was not visible in the tissue sections. Compared with the histology of the normal knee joint (Figure 3A, 3B), the histology of the model supported by the scaffold showed that the repaired tissue penetrated the scaffold and produced new cells and matrix (Figure 3C–3F). Although infiltrating blood vessels and foreign body giant cells were commonly observed, in the lower part of the PCL-HA scaffold, these findings were more significant (Figure 3C, 3D). Round chondrocytes and surrounding matrix staining were visible in the co-cultured cell PCL-HA scaffold group (Figure 3E, 3F). The area of safranin-O staining area of the co-cultured cell PCL-HA scaffold group was significantly greater than that of the control group (Figure 3G, 3H). At 8 weeks, the PCL-HA scaffold group had partial safranin-O staining in the area surrounding the defect, but the defect repair was mainly filled with fibrous and fibrocartilaginous tissue (Figure 3I, 3J). The tissue defect site of the co-cultured cell PCL-HA scaffold group showed positive safranin-O staining and regenerated subchondral bone tissue (Figure 3K, 3L).
Figure 3.
Photomicrographs of the histology of the repaired tissues at week 8. (A, B) Comparison with the normal knee joint. Hematoxylin and eosin (H&E). (C–F) Histology shows that the repaired tissue penetrated the scaffold and produced new cells and matrix. H&E. (C, D) Although infiltrated blood vessels and foreign body giant cells were commonly observed, the lower part of the three-dimensional (3-D) printed polycaprolactone-hydroxyapatite (PCL-HA) scaffold in which the original scaffold was located was more pronounced. (E, F) There were fewer foreign body giant cells in the upper part of the scaffolds in both groups. There were more round cells and surrounding matrix in the co-cultured cell PCL-HA scaffold group. (G, H) The safranin-O staining area of the co-cultured cells in the PCL-HA scaffold group was significantly increased compared with the control group. (I, J) The PCL-HA scaffold group had partial safranin-O staining in the area surrounding the defect, but the defect was mainly filled with fibrous and fibrocartilaginous tissue. (K, L) The tissue defect site of the co-cultured cells in the PCL-HA scaffold group showed positive safranin-O staining and regenerated subchondral tissue.
Discussion
Due to the increase in high-energy injuries to the knee joints caused by aging, sports and traffic accidents, articular damage and defects are increasingly seen in orthopedic clinics [42]. Many types of scaffolds have been developed for the repair of osteochondral defects, including double, triple, and multilayer gradient scaffolds [43]. However, these scaffolds fail to recover or regenerate the biological function of the osteochondral defect, and the junction in each layer of the scaffold is prone to dehiscence [43]. The regenerative tissue may separate from the defect. In this study, bone and cartilage defects were repaired simultaneously using a single-layer scaffold, which showed significant advantages.
There are now several methods available for constructing scaffolds for joint repair and reconstruction. Recently, three-dimensional (3-D) printing tissue engineered scaffolds have attracted attention for their high efficiency in terms of production, low cost, and high precision [44,45]. Rapid prototyping technology based on 3-D printing technology is a current research topic [46]. The 3-D printed scaffold is not limited by defects and complexity and can be customized according to need and made into a tissue engineered construct of precise size and shape [46]. In orthopedics, the osteochondral defect data obtained on computed tomography (CT) scans are often reconstructed by computer-aided design (CAD) and computer-aided manufacturing (CAM) software to design a suitable scaffold [47,48]. Wei and colleagues [49] studied the influence of polycaprolactone-hydroxyapatite (PCL-HA) scaffolds on in vivo repair of osteochondral defects and found that implanting PCL-HA scaffolds promoted integration with host bone, but the cartilage regeneration remained unsatisfactory.
Mesenchymal stem cells (MSCs) are a vital source of seed cells in tissue engineering [50]. MSCs can be obtained from several sources, including the bone marrow (BM), placenta, umbilical cord blood (UCB), and other adult tissues [51]. Compared with bone marrow MSCs, UCB-MSCs possess the multiple differentiation potential of adult stem cells and have become an ideal seed cell [52,53]. Isolated cell culture of UCB-MSCs is relatively easy to perform, and cells with uniform cell phenotypes can be obtained without in vitro enzymes or other substances for digestion and purification, and UCB-MSC isolation is non-invasive and is not associated with ethical issues regarding sourcing [54].
To the best of our knowledge, there have been no previous studies on co-cultured UCB-MSCs and chondrocytes on PCL-HA scaffolds for osteochondral defect regeneration studies. Co-cultured cells combined with the PCL-HA scaffold do not require in vitro induction before implantation or the addition of growth factors. This study showed that no inflammation occurred at the scaffold implantation sites and that the scaffold provided a suitable spatial structure for osteochondral regeneration at the defect site after implantation. Co-cultured cells secrete growth factors and induce recruitment of more stem cells from surrounding tissues. In this study, the co-cultured UCB-MSCs and chondrocytes combined with the PCL-HA scaffold promoted cartilage regeneration and stimulated subchondral bone reconstruction.
This study had several limitations. The number of experimental animals used was small, and significant individual differences between experimental animals could not be excluded. Also, the rabbit model was selected for this study, and further and larger studies are needed. This study involved the use of a rabbit model with an articular osteochondral defect of the femoral trochlea, while clinical osteochondral injuries may occur at other joint sites, and so the results from this study may not be representative of clinical osteochondral knee joint damage. Also, the study period was short, with only an 8-weeks follow-up period to study osteochondral repair. The effects of the PCL-HA scaffolds coated with UCB-MSCs in this rabbit model were evaluated only by macroscopic observation and histology, and no gene expression was studied, and no biomechanical studies of knee joint function were performed. However, in this study, we used the optimal cell ratio in vitro, but differences may exist in vivo, and further optimization is needed. Optimizing the cell ratio in 3-D scaffolds may achieve better functioning of co-cultured UCB-MSCs and chondrocytes on PCL-HA scaffolds.
Conclusions
An optimal regeneration protocol based on scaffolds and seeded cells is critical for the repair of osteochondral defects in vivo. The aim of this study was to investigate a rabbit model of osteochondral regeneration using three-dimensional (3-D) printed polycaprolactone-hydroxyapatite (PCL-HA) scaffolds coated with umbilical cord blood mesenchymal stem cells (UCB-MSCs) and chondrocytes. The findings from this study showed that the UCB chondrocyte-seeded PCL-HA scaffold promoted articular cartilage repair when compared with the control or non-seeded PCL-HA scaffolds. The use of HA improved the physicochemical properties of the scaffold, and the use of co-cultured UCB-MSCs and chondrocytes stimulated regeneration of the rabbit cartilage in vivo, demonstrating that the co-cultured UCB-MSCs and chondrocytes promoted osteochondral formation. UCB-MSCs xenografts can contribute to tissue repair and may provide a viable treatment strategy to repair osteochondral defects. With further improvements in technology, 3-D printed PCL-HA scaffolds combined with co-cultured UCB-MSCs and chondrocytes may be future tissue-engineered constructs to repair osteochondral defects.
Footnotes
Source of support: This study was supported by the Medical Science and Technology Development Foundation, Nanjing Municipality Health Bureau (YKK15132)
Conflict of interest
None.
References
- 1.Du Y, Liu H, Yang Q, et al. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials. 2017;137:37. doi: 10.1016/j.biomaterials.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Mankin HJ. Articular cartilage: Degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 3.Mahboubi M. Mentha spicata as natural analgesia for treatment of pain in osteoarthritis patients. Complement Ther Clin Pract. 2017;26:1–4. doi: 10.1016/j.ctcp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Karim A, Hall AC. Chondrocyte morphology in stiff and soft agarose gels and the influence of fetal calf serum. J Cell Physiol. 2017;232(5):1041–52. doi: 10.1002/jcp.25507. [DOI] [PubMed] [Google Scholar]
- 5.Kraeutler MJ, Kaenkumchorn T, Pascual-Garrido C, et al. Peculiarities in ankle cartilage. Cartilage. 2017;8(1):12. doi: 10.1177/1947603516642572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rongen JJ, Hannink GJ, Tienen TGV, et al. The protective effect of meniscus allograft transplantation on articular cartilage: A systematic review of animal studies. Osteoarthritis Cartilage. 2015;23(8):1242–53. doi: 10.1016/j.joca.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Nukavarapu SP, Dorcemus DL. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol Adv. 2013;31(5):706–21. doi: 10.1016/j.biotechadv.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Sun Y, Cheng X, et al. Repair of osteochondral defects by mosaicplasty and allogeneic BMSCs transplantation. Int J Clin Exp Med. 2015;8(4):6053–59. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ZJ, An RZ, Zhao JY, et al. Repair of articular cartilage defects by tissue-engineered cartilage constructed with adipose-derived stem cells and acellular cartilaginous matrix in rabbits. Genet Mol Res. 2014;13(2):4599–606. doi: 10.4238/2014.June.18.2. [DOI] [PubMed] [Google Scholar]
- 10.Murata D, Tokunaga S, Tamura T, et al. A preliminary study of osteochondral regeneration using a scaffold-free three-dimensional construct of porcine adipose tissue-derived mesenchymal stem cells. J Orthop Surg Res. 2015;10(1):35. doi: 10.1186/s13018-015-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niethammer TR, Holzgruber M, Gülecyüz MF, et al. Matrix based autologous chondrocyte implantation in children and adolescents: A match paired analysis in a follow-up over three years post-operation. Int Orthop. 2017;41(2):343–50. doi: 10.1007/s00264-016-3321-1. [DOI] [PubMed] [Google Scholar]
- 12.Shankar KG, Gostynska N, Montesi M, et al. Investigation of different cross-linking approaches on 3-D gelatin scaffolds for tissue engineering application: A comparative analysis. Int J Biol Macromol. 2017;95:1199–209. doi: 10.1016/j.ijbiomac.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Fan M, Ma Y, Tan H, et al. Covalent and injectable chitosan-chondroitin sulfate hydrogels embedded with chitosan microspheres for drug delivery and tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;71:67–74. doi: 10.1016/j.msec.2016.09.068. [DOI] [PubMed] [Google Scholar]
- 14.Wu G, Cui Y, Wang YT, et al. Repair of cartilage defects in BMSCs via CDMP1 gene transfection. Genet Mol Res. 2014;13(1):291–301. doi: 10.4238/2014.January.17.14. [DOI] [PubMed] [Google Scholar]
- 15.Lu CH, Yeh TS, Yeh CL, et al. Regenerating cartilages by engineered ASCs: Prolonged TGF-β3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol Ther. 2014;22(1):186–95. doi: 10.1038/mt.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K, Zhang Y, Yan S, et al. Repair of an articular cartilage defect using adipose-derived stem cells loaded on a polyelectrolyte complex scaffold based on poly (l-glutamic acid) and chitosan. Acta Biomater. 2013;9(7):7276–88. doi: 10.1016/j.actbio.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Qi Y, Du Y, Li W, et al. Cartilage repair using mesenchymal stem cell (MSC) sheet and MSCs-loaded bilayer PLGA scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1424–33. doi: 10.1007/s00167-012-2256-3. [DOI] [PubMed] [Google Scholar]
- 18.Espinosa M, Vaisman A, Nazal N, et al. Intraarticular administration of dexamethasone after mesenchymal stem cells implantation does not improve significantly the treatment of preestablished full-thickness chondral defect in a rabbit model. Cartilage. 2013;4(2):144–52. doi: 10.1177/1947603512472696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong L, Zhou X, Wu Y, et al. Proteomic analysis profile of engineered articular cartilage with chondrogenic differentiated adipose tissue derived stem cells loaded polyglycolic acid mesh for weight bearing area defect repair. Tissue Eng Part A. 2014;20(4):575–87. doi: 10.1089/ten.tea.2013.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma H, Feng C, Chang J, Wu C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018;79(1):37–59. doi: 10.1016/j.actbio.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Deng C, Li J, et al. 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials. 2019;196:138–50. doi: 10.1016/j.biomaterials.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Patrício T, Domingos M, Gloria A, et al. Characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Procedia CIRP. 2013;5:110–14. [Google Scholar]
- 23.Santis RD, Russo A, Gloria A, et al. Towards the design of 3D fiber-deposited poly(ɛ-caprolactone)/lron-doped hydroxyapatite nanocomposite magnetic scaffolds for bone regeneration. J Biomed Nanotechnol. 2015;11(7):1236–46. doi: 10.1166/jbn.2015.2065. [DOI] [PubMed] [Google Scholar]
- 24.Domingos M, Intranuovo F, Russo T, et al. The first systematic analysis of 3D rapid prototyped poly(ɛ-caprolactone) scaffolds manufactured through BioCell printing: The effect of pore size and geometry on compressive mechanical behaviour and in vitro hMSC viability. Biofabrication. 2013;5(4):045004. doi: 10.1088/1758-5082/5/4/045004. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Liu X, Liu R, et al. Zero-order controlled release of BMP2-derived peptide P24 from the chitosan scaffold by chemical grafting modification technique for promotion of osteogenesis in vitro and enhancement of bone repair in vivo. Theranostics. 2017;7(5):1072–87. doi: 10.7150/thno.18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yijun Y, Shuangshuang R, Yingfang Y, et al. Electrospun fibrous scaffolds with iron-doped hydroxyapatite exhibit osteogenic potential with static magnetic field exposure. J Biomed Nanotechnol. 2017;13(7):835–47. [Google Scholar]
- 27.Yu Y, Ren S, Yao Y, et al. Electrospun fibrous scaffolds with iron-doped hydroxyapatite exhibit osteogenic potential with static magnetic field exposure. J Biomed Nanotechnol. 2017;13(7):835–47. [Google Scholar]
- 28.Dang W, Li T, Li B, et al. A bifunctional scaffold with CuFeSe2 nanocrystals for tumor therapy and bone reconstruction. Biomaterials. 2018;160:92–106. doi: 10.1016/j.biomaterials.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Ronca D, Langella F, Chierchia M, et al. Bone Tissue engineering: 3D PCL-based nanocomposite scaffolds with tailored properties. Procedia CIRP. 2016;49:51–54. [Google Scholar]
- 30.Domingos M, Gloria A, Coelho J, et al. Three-dimensional printed bone scaffolds: The role of nano/micro-hydroxyapatite particles on the adhesion and differentiation of human mesenchymal stem cells. Proc Inst Mech Eng H. 2017;231(6):555–64. doi: 10.1177/0954411916680236. [DOI] [PubMed] [Google Scholar]
- 31.Majore I, Moretti P, Stahl F, et al. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev. 2011;7(1):17–31. doi: 10.1007/s12015-010-9165-y. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Hou KD, Yuan M, et al. Characteristics of mesenchymal stem cells derived from Wharton’s jelly of human umbilical cord and for fabrication of non-scaffold tissue-engineered cartilage. J Biosci Bioeng. 2014;117(2):229–35. doi: 10.1016/j.jbiosc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Arien-Zakay H, Lazarovici P, Nagler A. Tissue regeneration potential in human umbilical cord blood. Best Pract Res Clin Haematol. 2010;23(2):291–303. doi: 10.1016/j.beha.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Caetanoa G, Bártolob P, Domingosb M, et al. Osteogenic differentiation of adipose-derived mesenchymal stem cells into Polycaprolactone (PCL) scaffold. Procedia Eng. 2015;11:59–66. [Google Scholar]
- 35.Yao Q, Wei B, Liu N, et al. Chondrogenic regeneration using bone marrow clots and a porous polycaprolactone-hydroxyapatite scaffold by three-dimensional printing. Tissue Eng Part A. 2015;21(7–8):1388–97. doi: 10.1089/ten.tea.2014.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caetano G, Violante R, Sant Ana AB, et al. Cellularized versus decellularized scaffolds for bone regeneration. Materials Letters. 2016;182(1):318–22. [Google Scholar]
- 37.Zheng P, Ju L, Jiang B, et al. Chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells by co-culture with rabbit chondrocytes. Mol Med Rep. 2013;8(4):1169–82. doi: 10.3892/mmr.2013.1637. [DOI] [PubMed] [Google Scholar]
- 38.Zheng P, Yao Q, Mao F, et al. Adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells in 3-D printed poly-ɛ-caprolactone/hydroxyapatite scaffolds combined with bone marrow clots. Mol Med Rep. 2017;16(4):5078–84. doi: 10.3892/mmr.2017.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Borne MP, Raijmakers NJ, Vanlauwe J, et al. International Cartilage Repair Society (ICRS) and oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15(12):1397–402. doi: 10.1016/j.joca.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Guo X, Park H, Young S, et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6(1):39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caminal M, Moll X, Codina D, et al. Transitory improvement of articular cartilage characteristics after implantation of polylactide: Polyglycolic acid (PLGA) scaffolds seeded with autologous mesenchymal stromal cells in a sheep model of critical-sized chondral defect. Biotechnol Lett. 2014;36(10):2143–53. doi: 10.1007/s10529-014-1585-3. [DOI] [PubMed] [Google Scholar]
- 42.Gudas R, Gudaitė A, Mickevičius T, et al. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: A prospective study with a 3-year follow-up. Arthroscopy. 2013;29(1):89–97. doi: 10.1016/j.arthro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Levingstone TJ, Thompson E, Matsiko A, et al. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 2016;32:149–60. doi: 10.1016/j.actbio.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 44.Luo Y, Lode A, Sonntag F, et al. Well-ordered biphasic calcium phosphate – alginate scaffolds fabricated by multi-channel 3D plotting under mild conditions. J Mater Chem B. 2013;1(33):4088. doi: 10.1039/c3tb20511h. [DOI] [PubMed] [Google Scholar]
- 45.Narayanan LK, Huebner P, Fisher MB, et al. 3-D bioprinting of polylactic acid (PLA) nanofibers-alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater Sci Eng. 2016;2(10):1732–42. doi: 10.1021/acsbiomaterials.6b00196. [DOI] [PubMed] [Google Scholar]
- 46.Yao Q, Wei B, Guo Y, et al. Design, construction and mechanical testing of digital 3D anatomical data-based PCL-HA bone tissue engineering scaffold. J Mater Sci Mater Med. 2015;26(1):5360. doi: 10.1007/s10856-014-5360-8. [DOI] [PubMed] [Google Scholar]
- 47.Kim YS, Shin YS, Park DY, et al. The application of three-dimensional printing in animal model of augmentation rhinoplasty. Ann Biomed Eng. 2015;43(9):2153–62. doi: 10.1007/s10439-015-1261-3. [DOI] [PubMed] [Google Scholar]
- 48.Park CH, Rios HF, Taut AD, et al. Image-based, fiber guiding scaffolds: A platform for regenerating tissue interfaces. Tissue Eng Part C Methods. 2014;20(7):533–42. doi: 10.1089/ten.tec.2013.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei B, Yao Q, Guo Y, et al. Three-dimensional polycaprolactone-hydroxyapatite scaffolds combined with bone marrow cells for cartilage tissue engineering. J Biomater Appl. 2015;30(2):160–70. doi: 10.1177/0885328215575762. [DOI] [PubMed] [Google Scholar]
- 50.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 51.Agrawal P, Pramanik K. Chitosan-poly(vinyl alcohol) nanofibers by free surface electrospinning for tissue engineering applications. Tissue Eng Regen Med. 2016;13(5):485–97. doi: 10.1007/s13770-016-9092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park YB, Ha CW, Lee CH, et al. Restoration of a large osteochondral defect of the knee using a composite of umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel: A case report with a 5-year follow-up. BMC Musculoskelet Disord. 2017;18(1):59. doi: 10.1186/s12891-017-1422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park YB, Song M, Lee CH, et al. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J Orthop Res. 2015;33(11):1580–86. doi: 10.1002/jor.22950. [DOI] [PubMed] [Google Scholar]
- 54.Flynn A, Barry F, O’Brien T. UC blood-derived mesenchymal stromal cells: An overview. Cytotherapy. 2007;9(8):717–26. doi: 10.1080/14653240701584578. [DOI] [PubMed] [Google Scholar]