Abstract
Background
Celecoxib has shown anti-tumor activities against several types of cancer. Although the majority of research focuses on its mechanism via cyclooxygenase-2 (COX-2) enzyme inhibition, we identified a distinct mechanism behind celecoxib anti-cancer abilities.
Material/Methods
We treated hepatocellular carcinoma (HCC) Huh-7 cells and tumor xenograft mice models with celecoxib to test its effects on the tumor. Using gene chip method to identify the differential expressed genes after celecoxib treatment and using pathway enrichment analysis to predict the potential pathways for further study. We transfected cells with lentiviral shRNA to detect the effect of RNA binding gene partner of NOB1 (PNO1) on tumor growth in vitro and in vivo. Further we performed western blot to detect the effect of PNO1 on the protein kinase B (AKT) pathway.
Results
Celecoxib inhibited HCC cell growth in vitro and in vivo, and gene chip and pathway enrichment analysis revealed that PNO1 may be the potential target of celecoxib in HCC cells. Celecoxib significantly reduced levels of PNO1 in tumor tissue. Knockdown of PNO1 remarkably suppressed tumor growth and metastasis in vitro and in vivo. Disruption of PNO1 expression significantly reduced protein kinase B (AKT)/rapamycin (mTOR) signaling, indicating that this pathway may be involved in PNO1-mediated tumorigenic activity.
Conclusions
Celecoxib may exert its anti-tumor activity by inhibiting PNO1, and that AKT/mTOR signaling helps mediate the oncogenic effects of PNO1. This work offers the first evidence for a role of PNO1 as an HCC oncogene, which may open new avenues for prevention and treatment of HCC.
MeSH Keywords: Carcinoma, Hepatocellular; Cell Growth Processes; Cyclooxygenase 2 Inhibitors
Background
Liver cancer is still one of the most frequent malignancies worldwide and is associated with high mortality. Hepatocellular carcinoma (HCC) is one of the most prevalent types of liver cancer. Incidence of HCC has increased sharply worldwide in recent years, reaching almost 850 000 new cases per year [1–3]. Although HCC patients enjoy slightly longer survival as a result of advances in diagnosis and therapy [4], overall prognosis remains poor. The long-term survival of HCC patients depends strongly on early diagnosis and treatment [5], but this is usually not the case: most cases of HCC are diagnosed when the disease is already advanced or has metastasized. Therefore, a better knowledge of the molecular mechanisms underlying HCC tumor growth and metastasis becomes an urgent need.
Cyclooxygenase-2 (COX-2) is widely expressed in various cancers types and it promotes cancer progression and cancer cell resistance to chemo- and radiotherapy [6]. Celecoxib is one of the members of the COX-2 selective nonsteroidal anti-inflammatory drug (NSAID) family. Celecoxib is extensively used to treat pain and inflammation in cases of osteoarthritis [7], rheumatoid arthritis [8], and ankylosing spondylitis [9], as well as acute pain [10] in adults. It is a potent first-line NSAID analgesic recommended by the World Health Organization for cancer pain management. Celecoxib may help slow the progression of liver, lung, breast, and prostate tumors [11–13]. Most research has focused on how celecoxib exerts anti-cancer effects by inhibiting COX-2. We were interested in whether celecoxib might exert its therapeutic effects through other pathways as well.
Here we examined whether the drug acts through partner of NOB1 homolog (PNO1), and our gene chip data indicated that PNO1 may be the potential target of celecoxib. So far, little is known about its biological function. PNO1 is widely expressed in liver, lung, spleen, and kidney. The present study shows that knockdown of PNO1 remarkably reduces HCC cells proliferation and metastasis, similarly to celecoxib administration. This suggests an important role for PNO1 in HCC. We further showed that celecoxib-induced inhibition of HCC cell growth in vitro and in vivo was associated with significantly lower PNO1 levels in tumor tissue. This work demonstrates for the first time that PNO1 may be an HCC oncogene, identifying a novel potential mechanism behind the anti-cancer activity of celecoxib.
Material and Methods
Cell cultures and treatment
Commercial human HCC Huh-7 cells were obtained from Shanghai Cell Biology Institute (Shangai, China). Short hairpin RNA (shRNA) targeting the human PNO1 gene (AAAGGTACCGTTAGATGGTCTCCC) and negative control shRNA (GCCCTGCACCTACTGCACTAA) were produced by GeneChem Technology (Shangai, China). Huh-7 cells were cultured in RPMI 1640 complete medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco, MA, USA). Cells were cultured at 37°C in humidified air with 5% CO2. Cells were transfected with PNO1-shRNA or negative control shRNA using lipofectamine 2000 (Invitrogen, CA, USA) at room temperature. Huh-7 cells were infected with virus-containing medium for 24 hours and stable expression clones were selected with 2 μg/mL puromycin (Invitrogen, CA, USA) for 7 days. And the untransfected cells were used as control. The transfection efficiency was confirmed by real-time polymerase chain reaction (RT-PCR) and western blot.
Cell proliferation assays
Cells were grown in 96-well plates and treated for 48 hours with various concentrations of celecoxib (0.01, 0.1, 1, 10, 20, 50, and 100 μM) (Pfizer, NY, USA). Then 10 μL/well of Cell Counting Kit-8 solution (CCK-8, Invitrogen, USA) was added to the plates, which were incubated for 40 minutes at 37°C. Viable cells were counted and the effect of celecoxib was assessed as described [14].
Adhesion assay
Plates with 96 wells were precoated with 100 μL of laminin-1. Cells were treated with phosphate-buffered saline (PBS) or celecoxib at 37°C for 2 hours. Plates were washed with PBS and stained with 100 μL of 10% crystal violet for 20 minutes. After washing again with PBS, 100 μL of 10% acetic acid was added to solubilize the dye and then kept the plate in room temperature for 5 minutes for staining, and the plates were scanned on a FLUOstar OMEGA plate reader (BMG Labtech, NC, USA) as described [13].
Invasion assay
The ChemoTx invasion kit (Neuro Probe, Gaithersburg, USA) was used to assess invasion as described [13]. Cells were cultured in serum-free medium for 24 hours, then re-suspended in Gey’s Balanced Salt Solution containing 1% bovine serum albumin (BSA) and various concentrations of celecoxib. The framed filter membrane was carefully fitted onto the top of the plate, which was incubated for 4 hours at 37°C to allow cell invasion into the lower compartment. Cells were stained with MTT (5 mg/mL in DMSO, 3 μL) at 37°C for 1 hour. The liquid was aspirated, and the wells washed with PBS. Absorbance was measured at 595 nm using a FLUOstar OMEGA plate reader.
Gene chip analysis after celecoxib treatment
After Huh-7 cells treating with celecoxib at 20 μM for 48 hours, total RNA was extracted from cells using TRIzol (Invitrogen, CA, USA). Amplified RNA (aRNA) was prepared using GeneChip 3′IVT Express Kit (Affymetrix, CA, USA). Then we purified the aRNA and hybridized the fragmented aRNA with microarray probes. After the hybridization, the chip was washed and dyed, and finally scanned by GeneChip Scanner 3000 (Affymetrix, CA, USA). And the heatmap and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were analyzed by software of KEGG Mapper 4.0 (Kyoto, Japan).
High-content screening (HCS) for cell proliferation analysis
Cells transfected with PNO1-shRNA lentivirus or negative vector were seeded in a 96-well plate at a density of 2000 cells/well (100 uL per well) and were cultured at 37°C, 5% CO2. And after 24 hours, Celigo system (Thermo, CA, USA) provided quantitative analysis for each individual well every day during the next 5 days procedures.
Colony formation assay
HCC Huh-7 cells were transfected with PNO1-shRNA or negative control shRNA, plated in wells plate (1000 cells/well), then cultured for 14 days at 37°C. The medium was removed, and cells were carefully rinsed with PBS. A mixture of 6.0% glutaraldehyde and 0.5% crystal violet (2 to 3 mL/well) was added, followed by incubation for 30 minutes at room temperature for staining. The glutaraldehyde crystal violet mixture was carefully removed, and cells were rinsed with distilled water. Plates with colonies were left to dry in normal air at room temperature. Colonies were counted under a stereomicroscope with an automatic counting “colony counter pen” (Thomas Scientific, NJ, USA).
Animal experiments
This study was performed in accordance with the recommendations of the Medical Ethics Committee of Guangxi Zhuang Autonomous Region, China. The protocol was approved by the Medical Ethics Committee of Guangxi Medical University Cancer Hospital (certificate LW2019018). An appropriate number of animals were used in the study.
BALB/c athymic (nude) mice (4 to 6 weeks, 16±2 g) were obtained from Guangxi Medical University (Nanning, Guangxi, China). All mice were housed in a specific pathogen-free room with free access to food and water. The protocol was approved by the Committee of Animal Management and Use of the Guangxi Medical University. Mice were divided into 2 treatment groups (each with 8 animals): placebo group, which received an oral saline solution; and a celecoxib group, which received 30 mg/kg/day orally. 5×106 HCC Huh-7 cells were subcutaneously injected into the mouse to establish the xenograft model. Animals were assessed daily for tumorigenesis, and tumor diameter >3 mm by day 7 was counted as tumor formation. Animal euthanasia was conducted by administration of intravenous anesthetic.
A second experiment was performed in 4-week-old nude mice that were randomly divided into 3 groups (each with 8 animals), which were left untreated or injected subcutaneously with Huh-7 cells transfected with PNO1-shRNA or negative control shRNA. Mice were monitored for tumor formation as described above, then euthanized after 6 weeks. Tumors and lung tissues were harvested for further analysis. Tumor volume was calculated using the formula [15]: π/4×width×height×length.
Hematoxylin and eosin staining
At 6 weeks after HCC Huh-7 cells had been injected into mice, lung tissue samples were harvested, fixed with 4% paraformaldehyde, and stained with hematoxylin and eosin as described [16]. The sample slices were assessed by a pathologist who was blinded to experimental conditions.
Quantitative RT-PCR
In order to test the expression of PNO1 in tumors from different mice, RNA was extracted from tumor tissue using TRIzol (Invitrogen, CA, USA). RNA samples were reverse-transcribed into cDNA using a reverse transcription kit (Toyobo, Osaka, Japan). Quantitative real-time PCR was performed [15–17] on an ABI PRISM 7500 sequence detection system (Applied Biosystems, CA, USA) using the following primers for PNO1:
5′-TGTTAAACCCCTAAAGGGAGACC-3′(forward),
5′-CCTTGTCCGTGTCACATTCTCT-3′(reverse),
and the following primers for GAPDH as endogenous control:
5′-GGCACAGTCAAGGCTGAGAATG-3′ (forward) and
5′-ATGGTGGTGAAGACGCCAGTA-3′ (reverse).
The PCR conditions consisted of a 10 minutes step at 95°C, 40 cycles at 95°C for 15 seconds each and 1 minute at 65°C. Relative expression of PNO1 was calculated using the formula: 2−ΔΔCt (ΔΔCt=ΔCt of the treated group−ΔCt of the control group).
Western blot analysis
Tumor samples were solubilized in radioimmunoprecipitation assay (RIPA) buffer for 30 minutes at 4°C with shaking for protein extraction and the protein was quantitated by the BCA protein assay (Invitrogen, CA, USA). Equal amounts of protein (40 μg) from the different groups were subjected to electrophoresis on a 10% sodium dodecyl sulphate (SDS) polyacrylamide gel, then transferred onto nitrocellulose membranes. The membranes were blocked with 5% BSA for 2 hours, then incubated at 4°C overnight with primary antibodies PNO1 (1: 800, catalog numbers: ab163419) (Abcam, Cambridge, United Kingdom) or AKT, mTOR, COX2 (1: 1000, Cell Signaling Technology, MA, USA) and GADPH (1: 2000, Cell Signaling Technology, MA, USA). GAPDH was used as the loading control. Membranes were washed 3 times with PBS (5 minutes each), then incubated with horseradish peroxidase-conjugated mouse anti-rabbit secondary antibody (1: 10000, catalog numbers: sc2357) (Santa Cruz Biotechnology, CA, USA). Membranes were visualized by chemiluminescence and using Image Lab software 4.1 (Bio-Rad, CA, USA) for densitometry analysis.
Statistical analysis
All data were presented as mean±standard deviation (SD) and analyzed using SPSS 18.0 (IBM, Chicago, IL, USA). Differences between 2 groups were assessed for significance using Student’s t-test. Differences between multiple experimental groups were compared using one-way analysis of variance, followed by the least significant difference (LSD) method. P < 0.05 was defined as statistical significance.
Results
Effect of celecoxib on HCC cells in vitro and in vivo
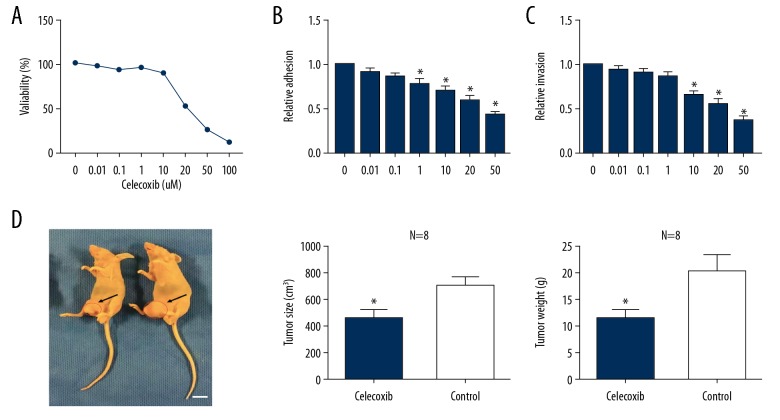
The effects of celecoxib on Huh-7 HCC cell proliferation were assessed using the CCK-8 assay. Celecoxib suppressed tumor growth in a dose-dependent manner after 48-hour exposure, with a mean half maximal inhibitory concentration (IC50) of 20 μM (Figure 1A). Laminin adhesion assays showed that 48-hour treatment with celecoxib at doses between 1 μM and 50 μM decreased cellular adhesion by 18% to 50% (P<0.05; Figure 1B). ChemoTx invasion assays showed that 48-hour treatment with celecoxib at 10 to 50 μM decreased invasive ability by 30% to 60% (P<0.05; Figure 1C). Next, we aimed to confirm these in vitro findings in nude mice bearing HCC xenografts. Celecoxib treatment significantly reduced tumor size (Figure 1D) and tumor weight (Figure 1D) compared to control mice.
Figure 1.
Effect of celecoxib on Huh-7 hepatocellular carcinoma (HCC) cell in vitro and vivo. (A) Cell proliferation assessed by Cell Counting Kit-8 assay after 48 hours treatment with celecoxib (0.01 to 100 μM). (B) Cell adhesion assessed by laminin-1 assay after 48 hours treatment with celecoxib (0.1 to 50 μM). (C) Cell invasion determined by ChemoTx kit after 48 hours treatment with celecoxib (0.1 to 50 μM). * P<0.05 between celecoxib-treated and non-treated cells. Xenograft-bearing mice were euthanized, and the tumors were obtained on day 42. (D) Mice tumor size and weight after celecoxib treatment (scale approximately 1 cm).
Celecoxib significantly downregulated PNO1 in vitro
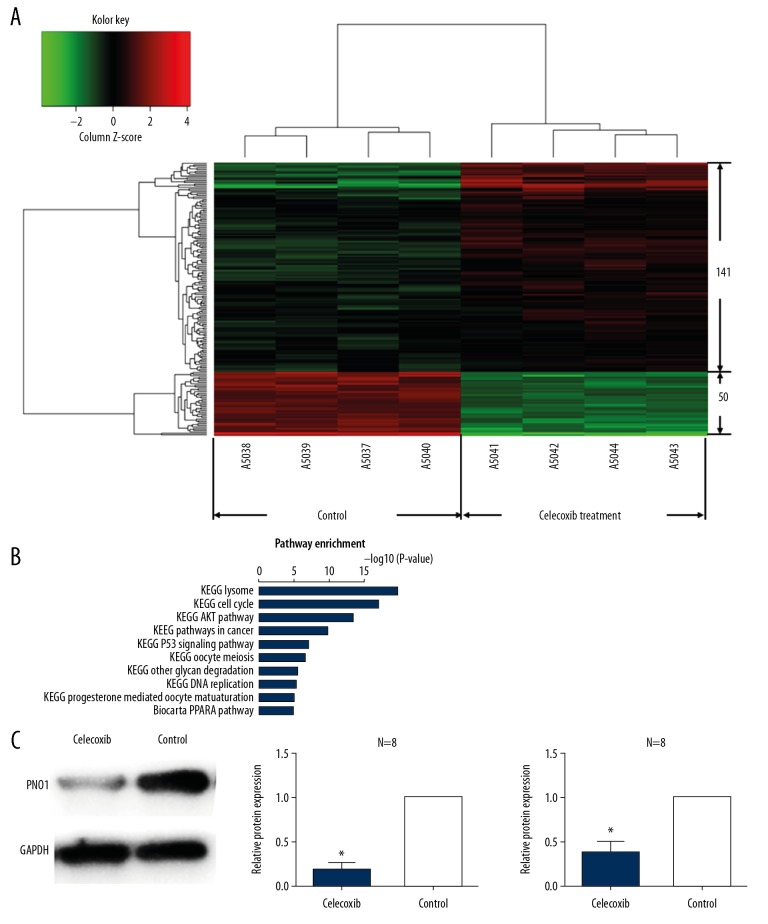
Our data showed that celecoxib can significantly inhibit HCC cell proliferation. But which gene involve in its mechanism remain a mystery. In order to investigate the potential candidate genes involving in HCC cell inhibition mechanism, we used gene chip method to analysis the differentially expressed genes after HCC cells treating by celecoxib. Our result showed that there were 50 genes downregulated and 141 genes upregulated after celecoxib treatment as the heatmap showed (Figure 2A). Table 1 shows the top 30 downregulated genes after celecoxib treating. And PNO1 showed 5.41-fold change downregulated compare to the control group (P<0.01). The data suggested that celecoxib show strong negative regulation effect on PNO1. Therefore, we pick up PNO1 for further experiment. And the therapeutic effects of celecoxib were associated with substantial PNO1 downregulation in tumor tissue, both at the protein level and mRNA level (Figure 2C).
Figure 2.
Differentially expressed genes after celecoxib treatment and KEGG pathways analysis. (A) Heat-map of all the differentially expressed genes after celecoxib treatment, there were 50 downregulated genes and 141 upregulated genes. (B) The P-values and names of the top 10 over-represented KEGG pathways, calculated on the basis of all the differentially expressed genes after the treatment of celecoxib. (C) Expression of PNO1 after celecoxib treatment analyzed by western blot. * P<0.05 between celecoxib-treated and non-treated animals. KEGG – Kyoto Encyclopedia of Genes and Genomes; PNO1 – RNA binding gene partner of NOB1.
Table 1.
The top 30 down-regulated genes list after celecoxib treatment.
| Gene name | Fold change | Regulation | P-value |
|---|---|---|---|
| PNO1 | 5.41 | Down | 1.175E-06 |
| RHAG | 2.17 | Down | 0.00013489 |
| TFPI2 | 2.12 | Down | 0.0002269 |
| IL32 | 2.09 | Down | 0.00038079 |
| ALPP | 2.05 | Down | 0.00038241 |
| AP1M2 | 2.02 | Down | 7.1885E-06 |
| ZNFX1-AS1 | 2.01 | Down | 8.2326E-05 |
| ANXA8L1 | 1.96 | Down | 0.00077881 |
| ANXA8L2 | 1.96 | Down | 0.00077881 |
| ANXA8 | 1.96 | Down | 0.00077881 |
| EFEMP1 | 1.94 | Down | 0.0001551 |
| ZDHHC11 | 1.86 | Down | 0.00019652 |
| SRP19 | 1.83 | Down | 0.000701 |
| CYR61 | 1.81 | Down | 0.00064606 |
| OLFML1 | 1.80 | Down | 0.00083506 |
| DKK1 | 1.76 | Down | 0.00013597 |
| ADM | 1.73 | Down | 0.0002931 |
| CTSL2 | 1.73 | Down | 0.00052096 |
| F3 | 1.71 | Down | 0.00108547 |
| C4BPB | 1.66 | Down | 0.00046583 |
| C1orf106 | 1.66 | Down | 0.00039219 |
| KIAA0368 | 1.63 | Down | 0.00119009 |
| ANTXR1 | 1.62 | Down | 9.0461E-05 |
| HUWE1 | 1.62 | Down | 0.00018888 |
| C3orf23 | 1.59 | Down | 0.00027176 |
| FAM46B | 1.58 | Down | 0.00099215 |
| KIF20A | 1.57 | Down | 3.0571E-05 |
| RPS26 | 1.57 | Down | 0.00056348 |
| CAV1 | 1.57 | Down | 0.00027822 |
| RPSA | 1.56 | Down | 0.00024262 |
PNO1 knockdown inhibited cell proliferation in vitro and reduced tumor growth in xenograft mice
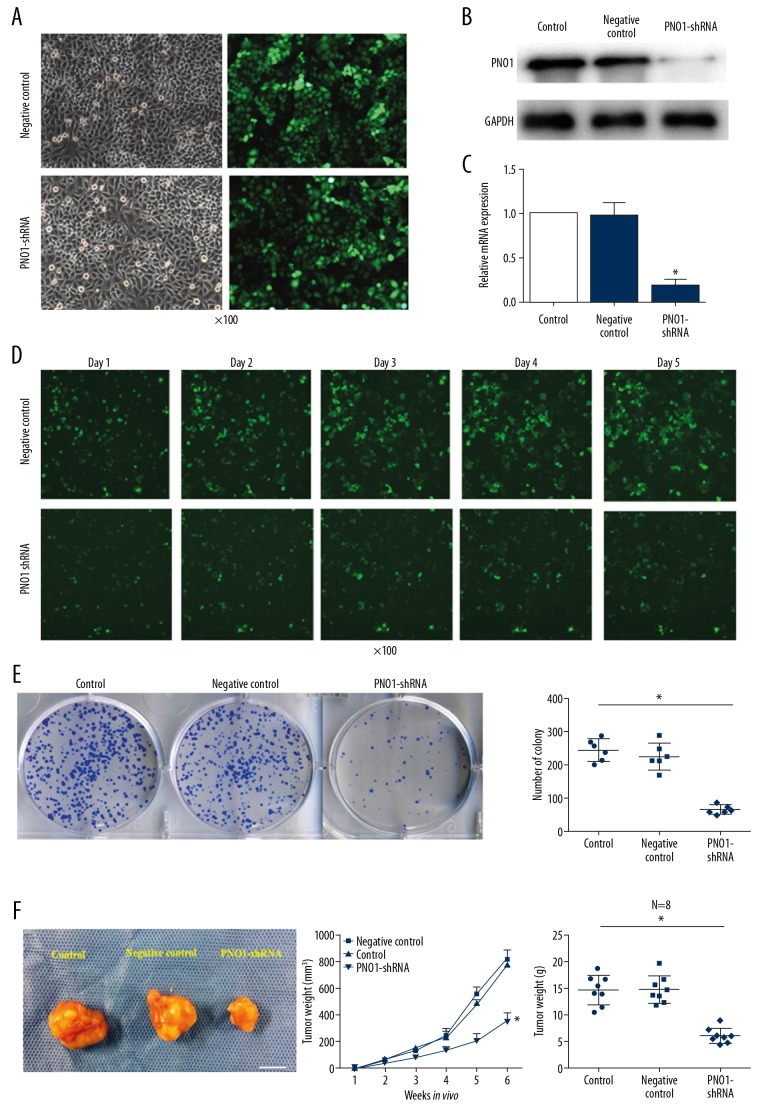
Since PNO1 is the top downregulated gene after celecoxib treatment, which prone us to investigate its effect on HCC. After 48-hours of transfection of PNO1-shRNA into Huh-7 cells, stable fluorescence signal was observed (Figure 3A). And the transfection of PNO1-shRNA into cells were confirmed by western blot and PCR as illustrated in the Figure 3B and 3C. Using High-Content Screening assay, we found that cell proliferation was significantly inhibited in cells bearing PNO1-shRNA compare with the negative control (Figure 3D). What was more, PNO1 knockdown significantly decreased the number of cell colonies (Figures 3E). To evaluate whether PNO1 can affect tumor growth in vivo, we established a xenograft model in which PNO1 was knocked down using lentivirus-expressed shRNA. Our data showed that, cells bearing PNO1-shRNA that injected into the mice formed significantly smaller tumors than cells transduced with a negative control lentivirus (Figure 3F). These data indicated that PNO1 may have a positive effect on the HCC cell proliferation.
Figure 3.
Effect of PNO1 on tumor growth in vitro and in vivo. (A) The green fluorescence signal of Huh-7 cells transfected with PNO1-shRNA and negative-shRNA lentivirus respectively. (B, C) PNO1 knockdown was confirmed by western blot and PCR. (B) protein level after transfection with PNO1-shRNA or negative-control shRNA. (C) PNO1 mRNA level after transfection with PNO1-shRNA or negative-control shRNA. (D) High-Content Screening assay to analysis cell proliferation after Huh-7 cells transfected with PNO1-shRNA and negative-shRNA lentivirus. (E) Cell colony formation after PNO1 depletion. (F) Huh-7 cells were transfected with PNO1-shRNA or negative-control shRNA (control) and were injected into nude mice. After 6 weeks, tumor tissues were obtained from xenograft-bearing mice (scale approximately 1 cm). * P<0.05 between negative control or control. PNO1 – RNA binding gene partner of NOB1; PCR – polymerase chain reaction.
Silencing of PNO1 greatly attenuated pulmonary metastasis
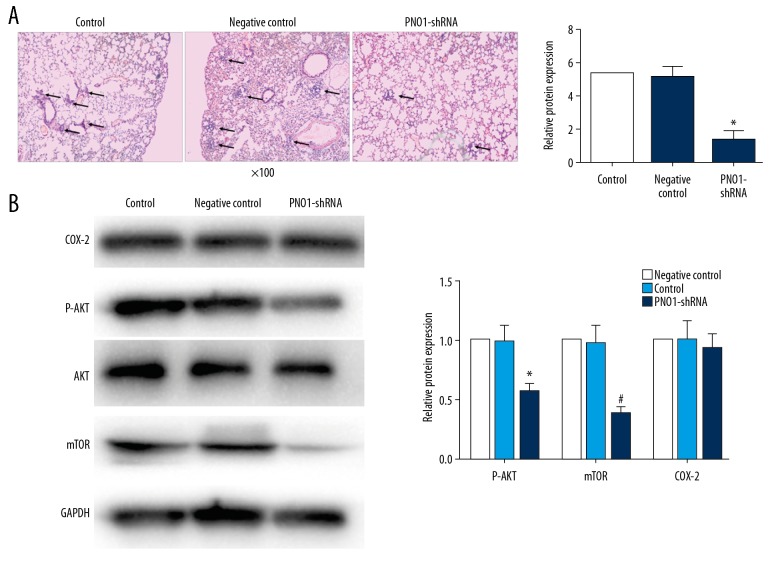
We have learned that PNO1 may promote HCC cell growth and invasive ability, next we examined whether PNO1 can influence HCC metastasis. Animals bearing PNO1-knockdown xenografts were sacrificed and lung tissue was stained by hematoxylin and eosin. And the data showed that PNO1 knockdown remarkably decreased pulmonary metastasis (Figure 4A).
Figure 4.
Effects of PNO1 knockdown on lung metastasis and AKT/mTOR signaling. (A) Hematoxylin/eosin staining showed that PNO1 knockdown significantly decreased the number of metastatic nodules in the lung (arrow) compared with the negative control and the control mice (image magnification 100×). (B) Levels of P-AKT, mTOR and COX-2 detected by western blot in tumor tissues 6 weeks after PNO1 knockdown. * P<0.05 between negative control or control. PNO1 – RNA binding gene partner of NOB1; AKT – protein kinase B; mTOR – mammalian target of rapamycin; COX-2 – cyclooxygenase-2.
PNO1 may exert oncogenic effects via the AKT/mTOR pathway
In order to have a better understanding of the differentially expressed genes after celecoxib treatment obtained from gene chip data. We perform pathway enrichment analysis to dig the potential pathway involving in the effect of celecoxib on HCC cells. According to the gene information of all pathways in KEGG, the differential genes were enriched and analyzed. After sorting according to P value, we selected the top 10 to display (Figure 2B). AKT pathway is the one of the most common pathways in tumor study and rank number 3 in the pathway enrichment analysis, so we picked up AKT pathway for further study. And our data showed that PNO1 knockdown significantly decreased AKT phosphorylation and mTOR, however, knockdown did not alter COX2 expression. These data suggesting that AKT activity may require PNO1, and COX2 is not the downstream target of PNO1 (Figure 4B).
Discussion
Celecoxib is a first-line NSAID and an effective inhibitor of COX-2 with demonstrated anti-cancer activities in many different types of cancer cells and animal models [18–22]. Celecoxib has shown efficacy against HCC in an animal models [23], and we confirmed those results in a xenograft tumor mice model which different from the rat orthotopic Novikoff hepatoma model that treat with epirubicin and celecoxib in the previous study [23]. We found that these therapeutic effects were associated with strong downregulation of PNO1 and downstream AKT/mTOR signaling. Leaving celecoxib aside, we then showed that PNO1 knockdown on its own significantly reduced tumor volume, tumor weight, and metastasis to lungs. Our experiments establish novel potential targets of celecoxib in HCC, and they provide the first evidence implicating PNO1 in HCC.
Celecoxib appears to inhibit multiple oncogenic targets and pathways depending on the cancer, including proliferation [24], apoptosis [25], angiogenesis [26], invasion [27], and tumor-induced immune suppression. Some of these pathways are dependent on COX-2, while others are independent of the enzyme [13,28]. In our present study, we found that COX-2 belongs to one of the 50 downregulation genes in the gene chip raw data, but it did not in the top 30 downregulation genes, so we did not list COX-2 in Table 1 and ignore its effect on the present cell model. Through our gene chip data analysis, we pick up PNO1 for the potential candidate target for the mechanism of action of celecoxib in HCC. And our results are consistent with the idea that celecoxib exerts its anticancer activities at least partly by downregulating PNO1. At the same time, PNO1 knockdown did not alter COX-2 expression in our experiments, suggesting that COX-2 is not a downstream target of PNO1 in HCC.
We provide here the first evidence that PNO1 can modulate a classical signaling pathways involved in tumor growth, in this case the AKT/mTOR pathway. We found that PNO1 knockdown remarkably reduced AKT phosphorylation and mTOR in HCC tissues. Further studies should examine whether PNO1 may contribute to other types of cancer through the AKT/mTOR pathway. Previous work has provided few details into the role of PNO1 in healthy or diseased cells. Our finding of a link to AKT/mTOR signaling suggests the potential for pleiotropic effects, since this signaling pathway regulates many processes, including metabolism, proliferation, cell survival, growth and angiogenesis, both in physiological and pathological conditions [29,30].
Our study presents several limitations because we did not address about the disease. Do not repeat the experiments about whether PNO1 acts through a COX-2 independent mechanism in carcinogenesis. In our present study, the data suggests that COX-2 is not the downstream of PNO1. And future studies should focus on whether PNO1 is the downstream of COX-2. At the present study we just have investigate one of the down-regulated genes after celecoxib treatment, and how about the up-regulated genes need further investigation. Aling Shen et al. [31] revealed that PNO1 expression was higher in colorectal cancer tissue than in noncancerous tissue. How about the PNO1 expression in HCC patient still unknown and should be discussed in the further studies.
Despite these limitations, our results suggest that celecoxib inhibits HCC cell growth via a PNO1-dependent mechanism, and they implicate PNO1 in HCC for the first time. PNO1 knockdown significantly reduced tumor proliferation and metastasis. Our results identify several new directions for further research into how HCC progresses and how it may be detected earlier and treated more effectively.
Conclusions
Celecoxib may exert its anti-tumor activity by inhibiting PNO1, and that AKT/mTOR signaling helps mediate the oncogenic effects of PNO1. This work offers the first evidence for a role of PNO1 as an HCC oncogene, which may open new avenues for prevention and treatment of HCC
Abbreviations
- AKT
protein kinase B
- BSA
bovine serum albumin
- HCC
hepatocellular carcinoma
- mTOR
mammalian target of rapamycin
- NSAID
nonsteroidal anti-inflammatory drug
- PNO1
RNA binding gene partner of NOB1
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (81670079), the International Communication of Guangxi Medical University Graduate Education (2017), the Basic Ability Improvement Project for Young and Middle-Aged Teachers of Guangxi Zhuang Autonomous Region 2017 (2017KY0087), Guangxi Science and Technology Development Projects (2013BC26260), and Guangxi Key R&D Program (AB16380205)
References
- 1.Laursen L. A preventable cancer. Nature. 2014;516:S2–3. doi: 10.1038/516S2a. [DOI] [PubMed] [Google Scholar]
- 2.Balogh J, Victor D, 3rd, Asham EH, et al. Hepatocellular carcinoma: A review. J Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 4.Lu LC, Shao YY, Chan SY, et al. Clinical characteristics of advanced hepatocellular carcinoma patients with prolonged survival in the era of anti-angiogenic targeted-therapy. Anticancer Res. 2014;34:1047–52. [PubMed] [Google Scholar]
- 5.Akamatsu N, Cillo U, Cucchetti A, et al. Surgery and hepatocellular carcinoma. Liver Cancer. 2016;6:44–50. doi: 10.1159/000449344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashemi Goradel N, Najafi M, Salehi E, et al. Cyclooxygenase-2 in cancer: A review. J Cell Physiol. 2019;234:5683–99. doi: 10.1002/jcp.27411. [DOI] [PubMed] [Google Scholar]
- 7.Hochberg MC, Martel-Pelletier J, Monfort J, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: A multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75:37–44. doi: 10.1136/annrheumdis-2014-206792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garner SE, Fidan D, Frankish RR, et al. WITHDRAWN: Celecoxib for rheumatoid arthritis. Cochrane Database Syst Rev. 2017;6:CD003831. doi: 10.1002/14651858.CD003831.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kristensen LE, Jakobsen AK, Askling J, et al. Safety of etoricoxib, celecoxib, and nonselective nonsteroidal antiinflammatory drugs in ankylosing spondylitis and other spondyloarthritis patients: A Swedish national population-based cohort study. Arthritis Care Res (Hoboken) 2015;67:1137–49. doi: 10.1002/acr.22555. [DOI] [PubMed] [Google Scholar]
- 10.Onda A, Ogoshi A, Itoh M, et al. Comparison of the effects of treatment with celecoxib, loxoprofen, and acetaminophen on postoperative acute pain after arthroscopic knee surgery: A randomized, parallel-group trial. J Orthop Sci. 2016;21:172–77. doi: 10.1016/j.jos.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Vosooghi M, Amini M. The discovery and development of cyclooxygenase-2 inhibitors as potential anticancer therapies. Expert Opin Drug Discov. 2014;9:255–67. doi: 10.1517/17460441.2014.883377. [DOI] [PubMed] [Google Scholar]
- 12.Stasinopoulos I, Shah T, Penet MF, et al. COX-2 in cancer: Gordian knot or Achilles heel? Front Pharmacol. 2013;4:34. doi: 10.3389/fphar.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suri A, Sheng X, Schuler KM, et al. The effect of celecoxib on tumor growth in ovarian cancer cells and a genetically engineered mouse model of serous ovarian cancer. Oncotarget. 2016;7:39582–94. doi: 10.18632/oncotarget.8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morisaki T, Umebayashi M, Kiyota A, et al. Combining celecoxib with sorafenib synergistically inhibits hepatocellular carcinoma cells in vitro. Anticancer Res. 2013;33:1387–95. [PubMed] [Google Scholar]
- 15.Aikawa T, Whipple CA, Lopez ME, et al. Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells. J Clin Invest. 2008;118:89–99. doi: 10.1172/JCI32412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Dai H, Zhu L, et al. Microvesicles packaging IL-1beta and TNF-alpha enhance lung inflammatory response to mechanical ventilation in part by induction of cofilin signaling. Int Immunopharmacol. 2018;63:74–83. doi: 10.1016/j.intimp.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, Zhang S, Huan X, et al. L Down-regulation of TRAF4 targeting RSK4 inhibits proliferation, invasion and metastasis in breast cancer xenografts. Biochem Biophys Res Commun. 2018;500:810–16. doi: 10.1016/j.bbrc.2018.04.164. [DOI] [PubMed] [Google Scholar]
- 18.Reckamp KL, Koczywas M, Cristea MC, et al. Randomized phase 2 trial of erlotinib in combination with high-dose celecoxib or placebo in patients with advanced non-small cell lung cancer. Cancer. 2015;121:3298–306. doi: 10.1002/cncr.29480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alamdarsaravi M, Ghajar A, Noorbala AA, et al. Efficacy and safety of celecoxib monotherapy for mild to moderate depression in patients with colorectal cancer: A randomized double-blind, placebo controlled trial. Psychiatry Res. 2017;255:59–65. doi: 10.1016/j.psychres.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard R, Rodriguez-Enriquez S, Pacheco-Velazquez SC, et al. Celecoxib inhibits mitochondrial O2 consumption, promoting ROS dependent death of murine and human metastatic cancer cells via the apoptotic signalling pathway. Biochem Pharmacol. 2018;154:318–34. doi: 10.1016/j.bcp.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Elzoghby AO, Mostafa SK, Helmy MW, et al. Multi-reservoir phospholipid shell encapsulating protamine nanocapsules for co-delivery of letrozole and celecoxib in breast cancer therapy. Pharm Res. 2017;34:1956–69. doi: 10.1007/s11095-017-2207-2. [DOI] [PubMed] [Google Scholar]
- 22.Flamiatos JF, Beer TM, Graff JN, et al. Cyclooxygenase-2 (COX-2) inhibition for prostate cancer chemoprevention: double-blind randomised study of pre-prostatectomy celecoxib or placebo. BJU Int. 2017;119:709–16. doi: 10.1111/bju.13612. [DOI] [PubMed] [Google Scholar]
- 23.Chu TH, Chan HH, Hu TH, et al. Celecoxib enhances the therapeutic efficacy of epirubicin for Novikoff hepatoma in rats. Cancer Med. 2018;7:2567–80. doi: 10.1002/cam4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riva B, De Dominici M, Gnemmi I, et al. Celecoxib inhibits proliferation and survival of chronic myelogeous leukemia (CML) cells via AMPK-dependent regulation of beta-catenin and mTORC1/2. Oncotarget. 2016;7:81555–70. doi: 10.18632/oncotarget.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toriyama S, Horinaka M, Yasuda S, et al. A histone deacetylase inhibitor, OBP-801, and celecoxib synergistically inhibit the cell growth with apoptosis via a DR5-dependent pathway in bladder cancer cells. Mol Cancer Ther. 2016;15:2066–75. doi: 10.1158/1535-7163.MCT-16-0010. [DOI] [PubMed] [Google Scholar]
- 26.Gao JH, Wen SL, Feng S, et al. Celecoxib and octreotide synergistically ameliorate portal hypertension via inhibition of angiogenesis in cirrhotic rats. Angiogenesis. 2016;19:501–11. doi: 10.1007/s10456-016-9522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behr CA, Hesketh AJ, Barlow M, et al. Celecoxib inhibits Ewing sarcoma cell migration via actin modulation. J Surg Res. 2015;198:424–33. doi: 10.1016/j.jss.2015.03.085. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Q, Guo J, Wang G, et al. Suppression of immune regulatory cells with combined therapy of celecoxib and sunitinib in renal cell carcinoma. Oncotarget. 2017;8:1668–77. doi: 10.18632/oncotarget.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manning BD, Toker A. AKT/PKB signaling: Navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lien EC, Lyssiotis CA, Juvekar A, et al. Glutathione biosynthesis is a metabolic vulnerability in PI(3)K/Akt-driven breast cancer. Nat Cell Biol. 2016;18:572–78. doi: 10.1038/ncb3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen A, Chen Y, Liu L, et al. EBF1-mediated upregulation of ribosome assembly factor PNO1 contributes to cancer progression by negatively regulating the p53 signaling pathway. Cancer Res. 2019;79:2257–70. doi: 10.1158/0008-5472.CAN-18-3238. [DOI] [PubMed] [Google Scholar]