Abstract
Background
This study aimed to evaluate an autologous bone mesenchymal stem cell (MSC)-derived extracellular matrix (ECM) scaffold in two animal models of cartilage repair.
Material/Methods
A rabbit model (n=16) and a minipig model (n=8) of cartilage repair were created with cartilage defects of the knee joints treated with bone marrow stimulation (BMS). In the ECM group, autologous bone MSC-derived ECM scaffolds were implanted into the cartilage defects after bone marrow stimulation. In the BMS group, the cartilage defects were treated by bone marrow stimulation only. The renewal capacity of bone MSCs was measured with a colony-forming unit fibroblast (CFU-F) in vitro assay. The extent of cartilage repair was as-sessed at 6 months after surgery.
Results
In the rabbit model, the macroscopic appearance of the exudate of the healing wounds in the ECM group showed less fibrosis, and the histology showed more evenly distributed chondrocytes compared with the BMS group. The CFU-F assay showed that the number of bone MSCs in the ECM group was approximately was twice that of the BMS group. In the minipig model, the macroscopic appearance and magnetic resonance imaging (MRI) findings of the ECM group were improved when compared with the BMS group. The repaired tissue in ECM group had similar histological characteristics and biochemical content to normal hyaline cartilage.
Conclusions
In two animal models of knee joint cartilage repair, the use of an ECM scaffold increased the number of bone MSCs and improved the extent of cartilage repair.
MeSH Keywords: Autoantigens; Bone Marrow; Cartilage, Articular; Swine, Miniature; Tissue Scaffolds
Background
Bone marrow stimulation is a widely used technique for the treatment of cartilage defects. Currently, studies have shown that bone marrow stimulation can stimulate bone mesenchymal stem cells (MSCs) to migrate to cartilage defects where they can differentiate into chondrocytes [1–3]. However, several clinical and animal studies have reported that fibrocartilage that consists of type I collagen is mainly found in tissue repair after bone marrow stimulation [4]. This finding may be caused by the limited locally available bone MSCs as well as insufficient chondrogenic stimulation at the site of the cartilage defect [5,6].
Currently, there have been several studies that have focused on improving the therapeutic approach to cartilage repair [7,8]. In our previous studies, an autologous bone MSC-derived extracellular matrix (ECM) scaffold was developed and implanted into the osteochondral defect of a rabbit model after bone marrow stimulation for cartilage regeneration [9,10]. As previously reported, the use of an autologous bone MSC-derived ECM scaffold could enhance the regeneration of hyaline cartilage [9,10].
In previous studies on cartilage regeneration, the rabbit model has been popular, due to the ease of handling, the requirement for small cages, and low costs of these animal studies [11–13]. However, a limitation of the rabbit model of osteochondral defect is the comparatively thin articular cartilage in this model, which has a mean cartilage thickness of 440 μm, and represents only about 10% of the total thickness defect [12]. Also, the endogenous healing potential, joint dimensions, loading conditions, and mechanics of the rabbit model differ from those in humans [13]. Therefore, additional studies in larger animal models, such as the minipig, dog, goat, and horse, to further evaluate the feasibility of these models are required before preclinical evaluation [6]. However, it is uncertain how effective the autologous bone MSC-derived ECM scaffolds might be in case of insufficient amounts of bone MSCs and the uncertain environment of articular joints. The autologous bone MSC-derived ECM scaffolds may be of benefit for the repair of cartilage defects following bone MSC, in addition to serving only as a physical barrier.
Therefore, this study aimed to evaluate an autologous bone MSC-derived ECM scaffold in two animal models of cartilage repair.
Material and Methods
The local Institutional Animal Experiment Committee of Nanjing Medical University approved the use of rabbits and minipigs in the current study. All experimental procedures were performed under the US National Institutes of Health (NIH) guidelines. Sixteen rabbits and eight minipigs were used in this study. The general health status of all animals was evaluated by veterinary examination. The age of the rabbits ranged from 4–6 months with a mean age of 5.2 months, and their weight ranged from 2.5–4 kg with a mean range of 3.5 kg. The age of the minipigs ranged from 10–16 months with a mean age of 13 months, and their weight ranged from 34–55 kg with a mean weight of 46 kg.
Preparation of the autologous bone MSC-derived ECM scaffold
The preparation of autologous bone mesenchymal stem cell (MSC)-derived extracellular matrix (ECM) scaffolds were as previously described [10]. Briefly, autologous bone marrow was harvested from the iliac crest of rabbits and minipigs. The bone MSCs were isolated using density gradient centrifugation and expanded in monolayer culture. To stimulate ECM deposition, 50 μg/ml l-ascorbic acid was added to the culture medium after cells reached 80% confluence. Four weeks later, the ECM membrane was carefully separated from culture dish and freeze-dried at −70°C under 1 Pa for 48 h. The mechanical strength of samples was improved by crosslinking with 50 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and 50 mM N-hydroxysuccinimide. The samples were washed and freeze-dried again. Finally, the autologous bone MSC-derived ECM scaffold (6 mm in diameter, 2 mm in thickness) was prepared by a biopsy punch and a razor blade and sterilized by ethylene oxide gas.
Cartilage defect model and bone marrow stimulation
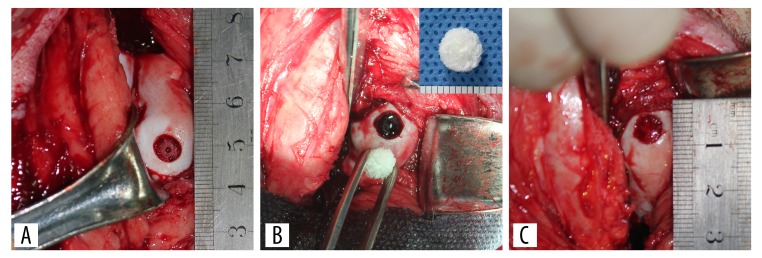
Surgery was performed under sterile conditions, and general anesthesia was performed with ketamine hydrochloride (15 mg/kg) [14]. A medial parapatellar arthrotomy was performed on both knees of each animal. Osteochondral defects (5 mm in diameter, 2 mm in depth) in the trochlear groove of rabbit model and articular cartilage defects (6 mm in diameter, 2 mm in depth) in the medial femoral condyle of minipig model were performed using biopsy punches [13]. The defects in the left knees were treated with bone marrow stimulation (Figure 1A). The autologous bone MSC-derived ECM scaffolds were implanted into the defects by press-fitting in the ECM group (Figure 1B, 1C). We checked whether the scaffold was maintained in the defect after several episodes of passive flexion and extension. The defects in the right knees were treated with bone marrow stimulation only (BMS group).
Figure 1.
The procedure for implantation of autologous bone mesenchymal stem cell (MSC)-derived extracellular matrix (ECM) scaffolds after bone marrow stimulation. The articular cartilage defect was created in the weight-bearing area of the medial femoral condyle in the minipig model (A). After the defect was made, it was treated with bone marrow stimulation using an 18-gauge needle (A), followed by implantation of the autologous bone MSC-derived ECM scaffold by press-fitting (B, C).
The effect of the autologous bone MSC-derived ECM scaffold on tissue repair
The knees of 8 rabbits were opened using forceps and gross observation was performed at 6 hours, 3 days, and 7 days after surgery, respectively. All rabbits were euthanized after 7 days of surgery and the specimens were evaluated for cell morphology and distribution by histology using hematoxylin and eosin (H&E) staining.
The colony-forming unit fibroblast (CFU-F) assay of the exudate of the healing wound
After 6 hours of surgery, the other eight rabbits were euthanized for the CFU-F assay. Briefly, mononuclear cells were released from the exudate of the healing wound of each group by incubation in 0.1% collagenase (Gibco, Rockville, MD, USA) in Dulbecco’s modified Eagle’s medium (DMEM) for 2 hours at 37°C under 5% CO2. Total mononuclear cell was resuspended and cultured in DMEM, which contained 10% fetal bovine serum (FBS), 100 unit/mL penicillin and 100 μg/mL streptomycin and plated on six-well plates. Non-adherent cells were removed after 48 hours. One week later, crystal violet staining was used for the colony-forming unit fibroblast (CFU-F) assay and a cell colony >50 cells was counted as a CFU-F.
Magnetic resonance imaging (MRI) of repaired cartilage in the minipig model
Postoperatively, eight minipigs were returned to their cages and were allowed immediate full weight-bearing. Six months after surgery, magnetic resonance imaging (MRI) of all minipigs was obtained using a 3.0T MR system (Magnetom Trio, Siemens, Erlangen, Germany) with an eight-channel transmit receiver extremity coil. Before MRI scanning, all animals were anesthetized with ketamine hydrochloride (15 mg/kg). The MRI protocol included the following sequences for T1-weighted imaging (T1WI), repetition time (TR)/echo time (TE) 765/19 milliseconds (ms), field of view (FOV) 20×20 cm, matrix 320×320, and slice thickness 1.5 mm For T2-weighted imaging (T2WI), TR/TE was 4590/42 ms, FOV 20×20 cm, matrix 320×320, and the slice thickness of 1.5 mm. Proton density-weighted imaging (PDWI). The TR/TE was 3600/20 ms, the FOV was 20×20 cm, matrix 320×320, and the slice thickness was 1.5 mm. The total scan time was about 5 minutes. After MRI scanning, the MR imaging quality was checked, and no moving artifact was found.
Gross observation and histological evaluation of repaired cartilage in minipig model
All minipigs were euthanized after MRI examination. All peri-articular soft tissues were removed from the distal femurs. Each sample was imaged by a high-resolution camera (Canon, Tokyo, Japan). The macroscopic evaluation of repaired tissue was conducted. The repaired tissue was longitudinally divided into two parts for histological assessment and biochemical analysis.
For histological evaluation, all samples were fixed in 4% neutral buffered formalin at room temperature for 24 hours, followed by decalcification with 5% nitric acid for 48 hours and then washed with running water overnight. The samples were embedded in paraffin wax, sectioned at 3 μm, followed by staining with hematoxylin and eosin (H&E), Safranin O, and Masson’s trichrome [9]. The extent of the repair of the cartilage was evaluated using the International Cartilage Research Society (ICRS) grading scale [9]. To minimize the subjective bias, each sample was independently evaluated by three investigators.
Chemical analyses of repaired cartilage in the minipig model
The biochemical measurements were performed as previously described [10]. Normal appearing hyaline cartilage was examined as a positive control. All samples were dried at 37°C for 48 hours to remove the moisture, then digested with a papain solution at 60°C for 24 hours. The supernatant of samples was used for biochemical measurement. The total DNA content was examined by the Quit-iT dsDNA kit (Invitrogen, Eugene, OR, USA). The salmon testes DNA (Sigma-Aldrich, St. Louis, MO, USA) was used for creating a standard curve. The glycosaminoglycan (GAG) content was examined using the dimethylmethylene blue colorimetric assay. The chondroitin sulfate from shark cartilage (Sigma-Aldrich, St. Louis, MO, USA) was used for generating the standard curve.
Statistical analysis
All statistical analysis was performed using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). All data were expressed as the mean ± standard deviation (SD). The Wilcoxon signed-rank test compared the ICRS scores between the BMS group and the ECM group. One-way analysis of variance (ANOVA) followed by a post hoc test was used to compare the differences in DNA content and GAG content between the MSC group, the BMS group, and the positive control group. A P-value <0.05 was considered to be statistically significant.
Results
The effect of the autologous bone mesenchymal stem cell (MSC)-derived extracellular matrix (ECM) scaffolds on tissue repair
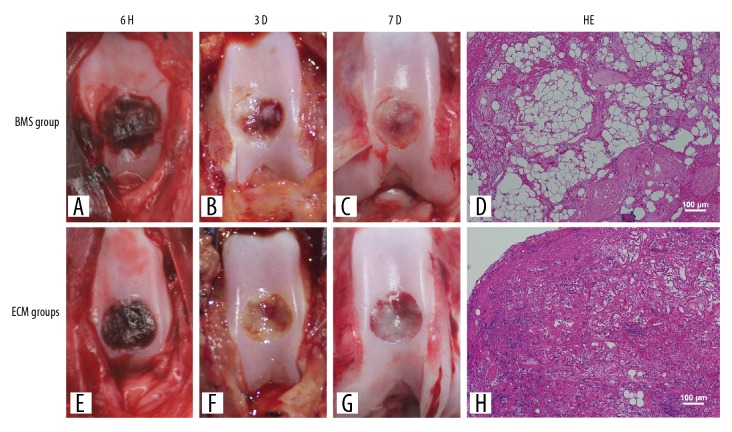
In the forceps test and gross observation, at 3 days after surgery, the exudate of the healing wounds in the BMS group was soft and partially coagulated (Figure 2A–2C), while those in the ECM group were stable and a more solid, and were found to show fibrosis. The surface of the exudate of the healing wound disappeared, and the defects were covered with gray-white fibrous tissue (Figure 2E–2G).
Figure 2.
Macroscopic appearance and histology of the area of exudate of the healing cartilage defect in the autologous bone mesenchymal stem cell (MSC)-derived extracellular matrix (ECM) scaffolds after bone marrow stimulation. The macroscopic appearance showed that the exudate of the healing wound in the bone marrow stimulation (BMS) group (A–C) did not organize well, whereas those in the extracellular matrix (ECM) group (E–G) are undergoing fibrosis. Histology shows that more chondrocytes were evenly distributed in the ECM group (H). The cells show good adhesion to the wall of the scaffold when compared with the BMS group (D).
Histology showed that no significant inflammation and immune response were found in both groups after 7 days. Compared with the BMS group (Figure 2D), more chondrocytes were evenly distributed in the ECM group, the cells adhered to the wall of the scaffold (Figure 2H). However, more red blood cell and macrophages accumulated in the fibrous tissue of the BMS group (Figure 2D).
The effect of autologous bone MSC-derived ECM scaffolds on the number of bone MSCs in the cartilage repair
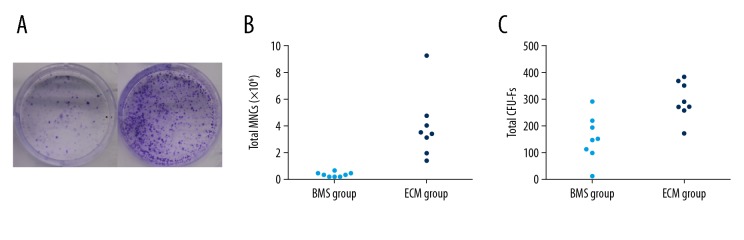
The difference in the number of bone MSCs in the exudate of the healing wound in the BMS and the ECM groups were examined by the colony-forming unit fibroblast (CFU-F) assay (Figure 3A). The total number of mononuclear cells were significantly different between the BMS group and the ECM group (0.37±0.188 and 3.953±2.403, P<0.05) (Figure 3B). The total CFU-F of bone MSCs was approximately two-fold higher in the BMS group (295.5±69.226) than in the MSC group (154.875±83.687, P<0.05) (Figure 3C).
Figure 3.
The effect of the autologous bone mesenchymal stem cell (MSC)-derived extracellular matrix (ECM) scaffolds on the number of bone MSCs in the healing cartilage. The colony-forming unit fibroblast (CFU-F) assay was performed to examine the total number of bone mesenchymal stem cells (MSCs) in the area of healing in the bone marrow stimulation (BMS) group and the extracellular matrix (ECM) group (A). The total number of mononuclear cells in the joint showed a significant difference between the MSC group and the BMS group (B). The total CFU-F of bone MSCs was approximately two-fold higher in the ECM group compared with the BMS group (C).
Macroscopic evaluation in the minipig model
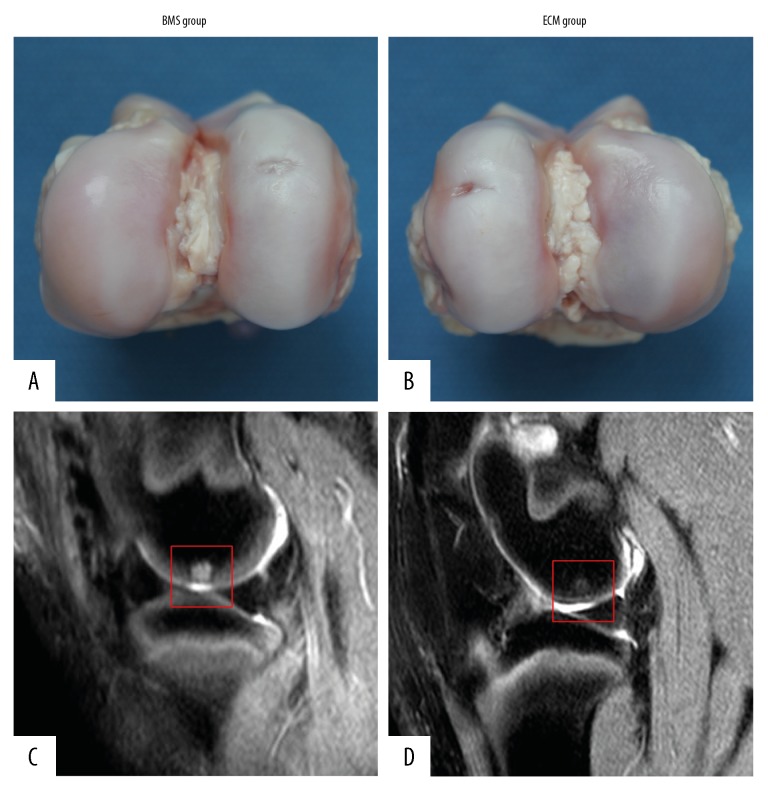
The macroscopic evaluation showed that the microfracture holes in the BMS group disappeared, and the defect was almost completely covered with repaired tissue. However, the repaired tissue appeared thin and visibly irregular, which was different from the surrounding normal hyaline cartilage (Figure 4A).
Figure 4.
Macroscopic appearance and magnetic resonance imaging (MRI) of the repaired tissue in the knee joint of the bone marrow stimulation (BMS) group and the extracellular matrix (ECM) group. The repaired cartilage tissue in the extracellular matrix (ECM) group (B) showed a thickness, appearance, and color that were more similar to normal cartilage at 6 months after surgery when compared with the bone marrow stimulation (BMS) group (A). On magnetic resonance imaging (MRI), the cartilage defect in the BMS group (red square) was still present, and the subchondral signal intensity was very high (C). However, the cartilage defect in the ECM group was almost filled with repaired tissue (red square), which had similar signal intensity on MRI to normal cartilage, and the subchondral signal intensity was much lower (D).
The cartilage defect in the ECM group was almost completely covered with repaired tissue. The thickness, appearance, and the color of the repaired tissue were similar to those of normal hyaline cartilage. Also, the repaired tissue appeared to have good integration with the surrounding tissue (Figure 4B).
Magnetic resonance imaging (MRI) of the knee joint in the minipig model
The cartilage defect still existed, and the subchondral signal intensity was very high in the BMS group (Figure 4C). However, the cartilage defect in the ECM group was almost filled with repaired tissue and had a similar signal intensity to the normal cartilage (Figure 4D). Also, the subchondral signal intensity was much lower (Figure 4D).
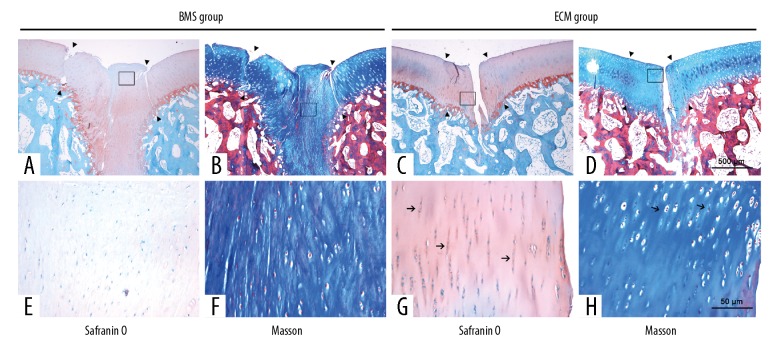
Histological evaluation in the minipig model
Safranin-O (Figure 5A, 5E) and Masson’s trichrome staining (Figure 5B, 5F) showed that the cartilage defects in the BMS group were partially covered with fibrous tissue. A clear demarcation between the repaired tissue and surrounding tissue was observed. A large number of rounded cells were observed in the repaired tissue, which was fibrous, poorly organized, and weakly metachromatic stained. Growth lines were almost absent in the defect. In contrast, intense metachromatic staining and chondrocyte cells clusters were found in the transitional and radial zones of the surrounding hyaline cartilage.
Figure 5.
Photomicrographs of the histology show the Safranin-O and Masson’s trichrome staining of the repaired cartilage of the bone marrow stimulation (BMS) group and the extracellular matrix (ECM) group at 6 months after surgery. Safranin-O staining showed that the repaired tissue in the bone marrow stimulation (BMS) group (A, E) showed poor metachromatic staining and was different from the normal surrounding cartilage and showed clear demarcation (arrowhead). However, the repaired tissue in the extracellular matrix (ECM) group (C, G) showed good integration with the surrounding cartilage (arrowhead), and the intensity of metachromatic staining resembled that of normal cartilage. The chondrocytes formed mature lacunae and were perpendicularly aligned (arrow). Masson’s trichrome staining showed that the defects were partially filled with fibrous tissue in the BMS group (B, F), with large numbers of rounded cells embedded in a fibrous and organized ECM. In contrast, the repaired tissue in the ECM group (D, H) showed several clusters of chondrocyte-like cells (arrow). Growth plates were observed in some zones. (A–D) Magnification ×10. (E–H) Magnification ×400.
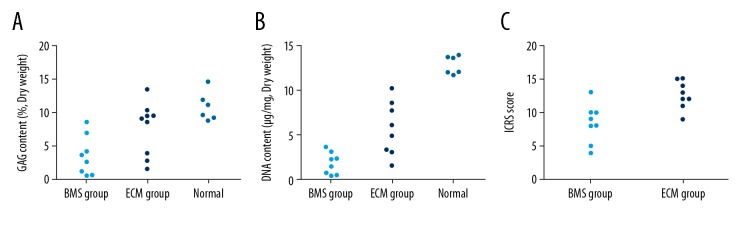
In the ECM group (Figure 5C, 5D, 5G, 5H), the repaired tissue almost completely covered the defect and appeared to have good integration with the surrounding tissue. The repaired tissue had a similar thickness to the surrounding hyaline cartilage and contained chondrocyte-like cells. Numerous clusters of chondrocyte-like cells were observed in the transitional and radial zones of the repaired tissue. The metachromatic staining of these areas was intense. Tidemarks could be observed in some zones, although they did not appear to be completely restored. The International Cartilage Repair Society (ICRS) scores of the ECM group (12.63±2.07) was significantly higher than that of the BMS group (8.38±2.88) at six months after surgery (P=0.012) (Figure 6C)
Figure 6.
The glycosaminoglycan (GAG), DNA, and International Cartilage Repair Society (ICRS) scores of repaired tissue of the bone marrow stimulation (BMS) group and the extracellular matrix (ECM) group at 6 months after surgery. The glycosaminoglycan (GAG) content of the extracellular matrix (ECM) group is not significantly different from that of normal hyaline cartilage (A), and the DNA content in this group was more similar to that of normal hyaline cartilage (B). The International Cartilage Repair Society (ICRS) scores of the ECM group were significantly higher than that of the bone marrow stimulation (BMS) group (C).
Biochemical assays
The glycosaminoglycan (GAG) content in the ECM group was 7.38±4.16%, and significantly higher than that of the BMS group (3.55±2.91%) at 6 months after surgery (P<0.05). Moreover, the difference of the GAG content between the repaired tissue in ECM group and normal hyaline cartilage did not reach a statistical difference (10.89±2.14%; P>0.05) (Figure 6A).
The measured values of DNA content in repaired tissues were 1.87±0.57 μg/mg and 1.28±0.35 μg/mg in BMS group and ECM group, respectively, at 6 months after surgery (P>0.05) (Figure 6B). Compared with the BMS group, the ECM group had more similar DNA content to normal cartilage (0.84±0.12 μg/mg).
Discussion
Several previous studies have shown that bone mesenchymal stem cells (MSCs) have high chondrogenic differentiation potential. The human adult bone MSCs derived from the subchondral plate are similar to the cells derived from bone marrow aspirates [15]. However, histological analysis from human biopsies and animal models have shown that normal hyaline cartilage could not be restored by traditional treatments, which may result in the formation of fibrocartilage or a hybrid tissue containing variable amounts of type II collagen [16]. One of the most important reasons is the insufficient amounts of bone MSCs retained in the exudate of the healing wound formed by mesenchymal stem cell treatment [17].
The number of bone MSCs is important for cartilage regeneration, especially in the early stage. Therefore, the current stem cell treatment approach needs to be improved [5,18]. Truong et al. reported that intravenous administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) could increase the number of bone MSCs in the exudate in the healing wound formed by MSCs and improve the therapeutic outcome of cartilage regeneration [15]. This previous in vitro study showed that the number of bone MSCs in the exudate of the healing wound varied depending on whether autologous bone MSC-derived extracellular matrix (ECM) scaffold was implanted after bone marrow stimulation treatment [15]. Compared with the BMS group, the ECM group showed more bone MSCs after bone marrow stimulation, which may be due to the large number of mononuclear cells and the high colony-forming unit fibroblast (CFU-F) rate. We considered that the autologous bone MSC-derived ECM scaffold of the ECM group may serve as a physical container and that the scaffold had similar characteristics of a sponge structure inserted into the cartilage defect to absorb bone MSCs from the bone marrow.
In the present study, within seven days after bone marrow stimulation treatment, bone MSCs had migrated into the cartilage defect. The continuous macroscopic evaluation showed that autologous bone MSC-derived ECM scaffold implantation could host the cells within and stabilize tissue repair after bone marrow stimulation treatment. Also, uniformly distributed and highly interconnected porous structure of the scaffold was a benefit for cell proliferation and differentiation [10]. The use of autologous bone MSC-derived ECM scaffold after bone marrow stimulation could maintain a higher cell concentration and provide a greater biological environment in the early stage. Furthermore, proliferation was closely associated with chondrogenic differentiation, and that stimulation of MSC aggregation could improve chondrogenesis [19]. Therefore, the bone MSCs within the autologous bone MSC-derived ECM scaffold could start proliferating vigorously in the cartilage defect, then effectively differentiate into chondrocytes.
One important finding in our study was that the repaired tissue in ECM group had similar biochemical content and characteristics to normal hyaline cartilage. Further, good integration with the surrounding cartilage and almost intact tidemarks were also observed in the ECM group. The ECM derived from autologous bone MSCs is the main component of the autologous bone MSC-derived ECM scaffold. The MSC-derived ECM can enhance the in vitro expansion and chondrogenic potential of bone MSCs and inhibit oxidative stress-induced stem cells senescence [20]. We considered that the potential efficacy of autologous bone MSC-derived ECM scaffolds was initially attributed to autologous ECM components. On the other hand, our previous study showed that several growth factors, including β-FGF and TGF were continuously released during the degradation of the autologous bone MSC-derived ECM scaffold [21]. It was plausible that the autologous bone MSC-derived ECM scaffolds could efficiently promote proliferation and chondrogenesis of stem cells during scaffold degradation [22].
Several biological biocompatible scaffolds have been introduced for cartilage repair. A few reports have supported that the combination of these scaffolds has been shown to improve the cartilage repair after bone marrow stimulation [17]. However, most of the currently used biological scaffold is derived from allogeneic tissue. There are potential risks of inflammation, pathogen transmission, immunological reactions, and ethical issues in the use of these scaffolds [23,24]. These problems can be overcome by use of autologous scaffolds, with more safety and efficacy, and could therefore improve the extent of cartilage repair [25,26]. Also, in contrast to the rabbit model, the large joint dimensions, upright and fully extended stifle joint, cartilage defect in a weight-bearing area, and the thickness of articular cartilage in the minipig model could more closely imitate those of humans [27]. Therefore, the results of our in vivo study may indicate that the autologous bone MSC-derived ECM scaffolds are potentially feasible for clinical application. We considered that both rabbit and minipig model could illustrate the course of bone MSCs retention in the exudate of the healing wound formed by bone marrow stimulation in vitro [28]. However, the rabbit models are easy to handle and cost less than the minipig model. Therefore, the rabbit models were used for our in vitro study.
There are some limitations of our present study. First, the follow-up period was limited to 6 months, and so further long-term studies are needed to evaluate the long-term outcome of this potentially novel treatment approach [16]. Also, in terms of postoperative pain, external fixation, which is commonly used in most clinical treatments for cartilage regeneration was not used in the present study. The sample size was relatively small, and the findings from the present study should be regarded as preliminary.
Conclusions
This study showed, that in two animal models of cartilage repair, using an autologous bone mesenchymal stem cell (MSC)-derived extracellular matrix (ECM) scaffold could increase the number of bone MSCs in the exudate of the healing wound formed by bone marrow stimulation and improve the extent of cartilage repair and restoration. The combination of the autologous bone MSC-derived ECM scaffold and bone marrow stimulation may be a practical therapeutic approach for the repair of cartilage degeneration in clinical practice but requires further studies.
Footnotes
Source of support: This study was funded by the National Nature Science Foundation of China (No. 81702205, 81702148, and 81771985) and the Nature Science Foundation of Jiangsu Province (No. BK20170141, and BK20170139)
Conflict of interest
None.
References
- 1.Furukawa T, Eyre DR, Koide S, et al. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62(1):79–89. [PubMed] [Google Scholar]
- 2.Bae DK, Yoon KH, Song SJ, et al. Cartilage healing after microfracture in osteoarthritic knees. Arthroscopy. 2006;22(4):367–74. doi: 10.1016/j.arthro.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Gobbi A, Nunag P, Malinowski K, et al. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13(3):213–21. doi: 10.1007/s00167-004-0499-3. [DOI] [PubMed] [Google Scholar]
- 4.Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455–64. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Min BH, Choi WH, Lee YS, et al. Effect of different bone marrow stimulation techniques (BSTs) on MSCs mobilization. J Orthop Res. 2013;31(11):1814–19. doi: 10.1002/jor.22380. [DOI] [PubMed] [Google Scholar]
- 6.Milano G, Passino ES, Deriu L, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: An experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18(7):971–80. doi: 10.1016/j.joca.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Amin HD, Brady MA, St-Pierre JP, et al. Stimulation of chondrogenic differentiation of adult human bone marrow-derived stromal cells by a moderate-strength static magnetic field. Tissue Eng Part A. 2014;20(11–12):1612–20. doi: 10.1089/ten.tea.2013.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin LH, Choi BH, Kim YJ, et al. Implantation of bone marrow-derived buffy coat can supplement bone marrow stimulation for articular cartilage repair. Osteoarthritis Cartilage. 2011;19(12):1440–48. doi: 10.1016/j.joca.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Tang C, Jin C, Du X, et al. An autologous bone marrow mesenchymal stem cell-derived extracellular matrix scaffold applied with bone marrow stimulation for cartilage repair. Tissue Eng Part A. 2014;20(17–18):2455–62. doi: 10.1089/ten.tea.2013.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang C, Xu Y, Jin C, et al. Feasibility of autologous bone marrow mesenchymal stem cell-derived extracellular matrix scaffold for cartilage tissue engineering. Artif Organs. 2013;37(12):E179–90. doi: 10.1111/aor.12130. [DOI] [PubMed] [Google Scholar]
- 11.Lam J, Lu S, Lee EJ, et al. Osteochondral defect repair using bilayered hydrogels encapsulating both chondrogenically and osteogenically pre-differentiated mesenchymal stem cells in a rabbit model. Osteoarthritis Cartilage. 2014;22(9):1291–300. doi: 10.1016/j.joca.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasanen T, Messner K. Regional variations of indentation stiffness and thickness of normal rabbit knee articular cartilage. J Biomed Mater Res. 1996;31(4):519–24. doi: 10.1002/(SICI)1097-4636(199608)31:4<519::AID-JBM12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 13.Gotterbarm T, Breusch SJ, Schneider U, et al. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: Retrospective analysis of 180 defects. Lab Anim. 2008;42(1):71–82. doi: 10.1258/la.2007.06029e. [DOI] [PubMed] [Google Scholar]
- 14.Jin CZ, Cho JH, Choi BH, et al. The maturity of tissue-engineered cartilage in vitro affects the repairability for osteochondral defect. Tissue Eng Part A. 2011;17(23–24):3057–65. doi: 10.1089/ten.tea.2010.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truong MD, Chung JY, Kim YJ, et al. Histomorphochemical comparison of microfracture as a first-line and a salvage procedure: Is microfracture still a viable option for knee cartilage repair in a salvage situation. J Orthop Res. 2014;32(6):802–10. doi: 10.1002/jor.22592. [DOI] [PubMed] [Google Scholar]
- 16.Mithoefer K, McAdams T, Williams RJ, et al. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: An evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–63. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 17.Mascarenhas R, Saltzman BM, Fortier LA, et al. Role of platelet-rich plasma in articular cartilage injury and disease. J Knee Surg. 2015;28(1):3–10. doi: 10.1055/s-0034-1384672. [DOI] [PubMed] [Google Scholar]
- 18.Benthien JP, Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): Method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1316–19. doi: 10.1007/s00167-010-1356-1. [DOI] [PubMed] [Google Scholar]
- 19.Dexheimer V, Frank S, Richter W. Proliferation as a requirement for in vitro chondrogenesis of human mesenchymal stem cells. Stem Cells Dev. 2012;21(12):2160–69. doi: 10.1089/scd.2011.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pei M, Zhang Y, Li J, et al. Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev. 2013;22(6):889–900. doi: 10.1089/scd.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Xu GY, Tang C, et al. Preparation and characterization of bone marrow mesenchymal stem cell-derived extracellular matrix scaffolds. J Biomed Mater Res B Appl Biomater. 2015;103(3):670–78. doi: 10.1002/jbm.b.33231. [DOI] [PubMed] [Google Scholar]
- 22.Chun SY, Lim GJ, Kwon TG, et al. Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials. 2007;28(29):4251–56. doi: 10.1016/j.biomaterials.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Schussler O, Shen M, Shen L, et al. Effect of human immunoglobulins on the immunogenicity of porcine bioprostheses. Ann Thorac Surg. 2001;71(5 Suppl):S396–400. doi: 10.1016/s0003-4975(01)02523-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim HL, Do JY, Cho HJ, et al. Dura mater graft-associated Creutzfeldt-Jakob disease: The first case in Korea. J Korean Med Sci. 2011;26(11):1515–17. doi: 10.3346/jkms.2011.26.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu H, Hoshiba T, Kawazoe N, et al. Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials. 2011;32(10):2489–99. doi: 10.1016/j.biomaterials.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Zeitouni S, Krause U, Clough BH, et al. Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci Transl Med. 2012;4(132):132ra55. doi: 10.1126/scitranslmed.3003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang CH, Kuo TF, Lin FH, et al. Tissue engineering-based cartilage repair with mesenchymal stem cells in a porcine model. J Orthop Res. 2011;29(12):1874–80. doi: 10.1002/jor.21461. [DOI] [PubMed] [Google Scholar]
- 28.Li TZ, Jin CZ, Choi BH, et al. Using cartilage extracellular matrix (CECM) membrane to enhance the reparability of the bone marrow stimulation technique for articular cartilage defect in canine model. Advanced Functional Materials. 2012;22(20):4292–300. [Google Scholar]