Abstract
Sperm cryopreservation is routinely used in reproductive medicine, livestock production and wildlife management. Its effect on offspring performance is often assumed to be negligible, but this still remains to be confirmed in well-controlled within-subject experiments. We use a vertebrate model that allows us to experimentally separate parental and environmental effects to test whether sperm cryopreservation influences offspring phenotype under stress and non-stress conditions, and whether such effects are male-specific. Wild brown trout (Salmo trutta) were stripped for their gametes, and a portion of each male's milt was cryopreserved. Then, 960 eggs were simultaneously fertilized with either non-cryopreserved or frozen-thawed semen and raised singly in the presence or absence of a pathogen. We found no significant effects of cryopreservation on fertilization rates, and no effects on growth, survival nor pathogen resistance during the embryo stage. However, fertilization by cryopreserved sperm led to significantly reduced larval growth after hatching. Males varied in genetic quality as determined from offspring performance, but effects of cryopreservation on larval growth were not male-specific. We conclude that cryopreservation causes a reduction in offspring growth that is easily overlooked because it only manifests itself at later developmental stages, when many other factors affect growth and survival too.
Keywords: sperm cryopreservation, vertebrate, good genes, infection, assisted reproductive technology, fish
1. Introduction
Sperm cryopreservation is an important routine in livestock production, human medicine, research and conservation biology [1–6]. It is arguably always harmful to spermatozoa, affecting their motility, various aspects of cellular integrity and often DNA integrity [7,8]. However, its widespread use in many taxa, including humans, suggests that effects of sperm cryopreservation on offspring performance are usually assumed to be negligible, even if most studies in this context focus on embryogenesis and studies on potential effects later in life are comparatively rare. Kopeika et al. [8] concluded from their review that, with regard to possible long-term effects of sperm cryopreservation, ‘there are still insufficient data available on the potential impact … on future offspring’ (p. 218).
There are two possibilities as to how sperm cryopreservation could affect offspring performance. First, cryopreservation may induce artificial selection on spermatozoa as not all of them survive the freezing and thawing procedures [9]. Such selection could then positively or negatively affect mean offspring phenotypes [10,11]. Second, sperm cryopreservation could influence offspring development by affecting the genetic and epigenetic information that is transmitted to the zygote [7,8]. Such genetic effects are more likely to be expressed later in life than in early embryos, because maternal effects on offspring performance dominate at early embryonic stages, while paternal effects become more important with increasing age of the embryo [12].
Testing for long-term effects of sperm cryopreservation on offspring performance is challenging in most taxa, especially in mammals. If such effects are small compared with the usual parental effects on offspring phenotypes [13], maternal and paternal effects on the zygote must be experimentally controlled in order to detect effects of cryopreservation. This is difficult in species with only few offspring. Parental effects must also be experimentally controlled for if they could be confounding, for example, if donors of cryopreserved sperm differ systematically from donors of non-cryopreserved sperm. Such systematic differences may often exist in livestock production and are especially likely in human medicine where sperm cryopreservation is used to treat couples that suffer from infertility or to store gametes from donors before they undergo a medical treatment that could affect gametogenesis [5,14]. Moreover, cryopreserved sperm are often used in the context of fertility treatments that may include, for example, intracytoplasmic sperm injection. Such treatments can induce considerable stress to the zygote [15] and need to be experimentally separated from potential effects of sperm cryopreservation to learn more about possible effects of the latter. Finally, if sperm cryopreservation affects, for example, later maternal–fetal communication [16,17], differential maternal investment [18] could potentially modify the immediate effects that sperm cryopreservation could have on offspring.
Given the difficulties in determining the long-term effects of sperm cryopreservation on progeny, it may not be surprising that studies on the subject often reach contradictory findings, especially if based on non-experimental data collected on humans [19,20]. Studies on mice (Mus musculus) led to conflicting results, too. On the one hand, different types of cryopreserved sperm (i.e. using different protocols) could be used to produce seemingly normal offspring [21,22]. On the other hand, Fernández-Gonzalez et al. [23] found that mice produced with cryopreserved sperm often have shorter lifespans and higher risk of developing tumours or behavioural syndromes. However, effects of cryopreservation could have been confounded with effects of intracytoplasmic sperm injection in their study. In fish, no effect of sperm cryopreservation on offspring performance could be found in some species [24–29], while significant effects on several developmental traits were reported in others [30–33]. In the latter case, effects of cryopreservation on potential indicators of offspring fitness were sometimes negative [30,31] and sometimes positive [32], and sometimes results were mixed [33]. Most discrepancies among these studies may be explained by non-sufficient controls of potentially confounding parental and/or environmental effects. In some cases, possible effects of cryopreservation on embryo viability also need to be disentangled from variation in fertilization success [34].
Because main effects of sperm cryopreservation on offspring performance are still debated, it may not be surprising that little is known about possible interactions between cryopreservation and other factors. It is, for example, possible that effects of sperm cryopreservation on offspring performance vary among males or men, which could then potentially explain some of the contradictory findings in the literature. Analogously, it is possible that interactions exist between effects caused by fertilization by cryopreserved sperm and effects caused by the environment of the developing offspring. Such interactions could, for example, include environmental stress amplifying the effects of sperm cryopreservation on offspring performance. They may even reveal cryptic genetic variation [35].
An ideal model for testing the effects of cryopreservation on offspring viability would be a species with large clutch sizes (to allow for within-subject comparisons while controlling for family effects), external fertilization and no parental care (to control for potential differential investment). Cryopreserved and non-cryopreserved sperm of many males would have to be tested in within-subject comparisons and simultaneously on the same maternal background to test for potential male-specific effects of sperm cryopreservation, and the resulting embryos would have to be raised in various conditions to test for possible interactions between cryopreservation and the environment of the developing offspring. Salmonid fish fulfil these requirements as they are external fertilizers with large clutch sizes and show no parental care. We chose the brown trout (Salmo trutta) and sampled wild populations to test the effect of sperm cryopreservation on offspring performance for different males. Experimental protocols have been developed and successfully used in this species to separate parental from environmental effects on different measures of offspring performance [36–39], and a large number of independent replicates that allow detecting small effect sizes are possible. Moreover, highly effective sperm cryopreservation protocols have been developed and successfully tested on this species [40,41].
Here, we test experimentally whether sperm cryopreservation influences offspring phenotype under non-stress conditions and under ecologically relevant stress conditions. We also test whether sperm donors vary in the genetic quality as determined by indicators of offspring performance, and whether genetic quality covaries with the changes that sperm cryopreservation may impose on offspring phenotypes.
2. Methods
(a). Gametes collection and sperm cryopreservation
In total, 40 males and 10 females were caught by electrofishing on their spawning ground from three tributaries (Müsche, Gürbe and Kiese) of the River Aare [42]. These tributaries differ in their ecology and host populations that are genetically and morphologically distinct [42]. Fish were kept in a hatchery until collection of the gametes. The milt of the males was stripped drop by drop into large Petri dishes (145 × 20 mm, Greiner Bio-one, Germany). Milt from drops that seemed not contaminated by urine or faeces was then transferred into 2 ml mini-tubes and stored on ice until further use. Eggs of each female were stripped into individual plastic containers and then distributed to 8 Petri dishes (60 × 15 mm; Greiner Bio-one, Germany) where they were fertilized with either cryopreserved or non-cryopreserved sperm (see detailed description below).
Sperm was cryopreserved using the protocol of Ciereszko et al. [40] that renders fertilization success similar to that of non-cryopreserved milt even at very low sperm to egg ratios (down to 110 000 : 1 [41], suggesting minimal alteration of milt quality). Five hundred microlitres of milt were diluted on ice at a 1 : 5 ratio in a solution of 10% methanol and 0.15 M glucose in a 2 ml microtube. Then, two 0.5 ml cryostraws (MTG Technologies, Germany) were filled with 500 µl diluted milt and sealed. After 10 min of equilibration on ice, the straws were placed on a floating rack at 1.5 cm above the surface of liquid nitrogen during 15 min. Straws were then plunged into liquid nitrogen until used for fertilization.
(b). Fertilization and incubation of embryos
The experiment was performed in 10 breeding blocks of 1 female crossed with 4 males each (i.e. 4 half-sib families per breeding block, 40 families in total). Electronic supplementary material, figure S1A illustrates such a breeding block and the treatments that were applied first to the sperm and later to the embryos. Within each breeding block, in total, 96 eggs per female were equally distributed to 8 Petri dishes (i.e. 12 eggs per Petri dish). Cryopreserved or non-cryopreserved milt of one of the four males each was then added to these eggs, so that each male × female combination was produced once with cryopreserved sperm and once with non-cryopreserved sperm.
To thaw the cryopreserved milt, straws were removed from liquid nitrogen, plunged for 30 s in water at 25°C, then put on ice for at least 1 min. The content of each straw was disposed around the assigned eggs in the Petri dishes, carefully avoiding direct contact with the eggs. For the controls, 83 µl (corresponding to the absolute volume of milt in a straw) of non-cryopreserved milt was disposed analogously around the assigned egg samples. Both non-cryopreserved sperm and frozen-thawed sperm were activated on average 33 min post-stripping (range 24–52 min) by adding 6 ml of Actifish solution (IMV Technologies, France) to each Petri dish (500 µl per egg) and gently moving the Petri dish to enhance mixing of gametes. After 5 min, 5 ml of standardized water (294 mg l−1 CaCl2 · 2H2O, 123.25 mg l−1 MgSO4 · 7H2O, 64.75 mg l−1 NaHCO3, 5.75 mg l−1 KCl) [43] was added to each Petri dish. The eggs were then allowed to harden for 2 h.
After hardening, the freshly fertilized eggs were transferred to a climate chamber where they were washed following the protocol of von Siebenthal et al. [44]. After washing, they were singly distributed into 24-well plates (Greiner Bio-one, Germany) filled with 1.8 ml per well of autoclaved standardized water (see electronic supplementary material, figure S1B for the distribution scheme). Plates were stored at 6.5°C under a 12 h light cycle (no water exchange). Fertilization success was assessed at 14 days post-fertilization (dpf). Eggs were considered fertilized if the spinal cord of a developing embryo was visible. The calculation of embryo mortality was based on fertilized eggs.
(c). Pathogen culture and inoculation
At 35 dpf, frozen and dried Aeromonas salmonicida isolated from brown trout (DSM 21281, DSMZ, Germany) were rehydrated with 5 ml TSB (tryptic soy broth, Fluka, Switzerland) following the instructions of the provider. The suspension was used to inoculate four flasks containing each 100 ml of TSB. Flasks were incubated at 22°C for 24 h until exponential growth phase was attained. Cultures were washed and counted using a Helber counting chamber as described in Clark et al. [45]. Bacteria were diluted in standardized water and 1% TSB, such that adding 200 µl to individual wells of the 24-well plates (at 36 dpf) would result in a concentration of 106 cfu ml−1 in each well (that each contained one embryo only). The sham-treated embryos received 200 µl of standardized water only. Half of the eggs of each combination of male × female × sperm treatment were exposed to bacteria, the other half was sham exposed (i.e. the treatments were full-factorial within each breeding block; see electronic supplementary material, figure S1).
(d). Measurements of embryo traits
Embryos were daily monitored to record mortality and time until hatching. On the day of hatching, larvae were transferred to 12-well plates (BD Biosciences, USA) filled with 3 ml of standardized water per well. Plates were then photographed from below under standardized conditions in a custom-made photo box with a Canon 70D (50 mm, f/3.2, 1/400 s, RAW format) or at 600 dpi with a scanner (Epson Perfection V37) for larvae measurements. Larvae were photographed again 14 days after hatching. The standard length of each larvae was measured in ImageJ [46] (electronic supplementary material, figure S2) by two different experimenters who both were naive with regard to the treatments. The respective measurements were highly correlated (length at hatching, n = 604, r2 = 0.89; length 14 days later, n = 547, r2 = 0.95). Means of both measures were used for further analyses. Yolk sac volume was calculated as in Jensen et al. [47] based on the minor and major axis of the vitellus section. Individual larval growth was calculated as the larval length at 14 days post-hatching minus the length at hatching. Yolk sac consumption was determined as yolk sac volume at hatching minus yolk sac volume 14 days later. Some measurements could not be taken because of larval mortality, low photo quality or accidents during handling (see legend in electronic supplementary material, figure S2). Sample sizes are therefore given in the respective figures.
(e). Effect of cryopreservation on sperm motility
In order to investigate potential effects of the cryopreservation protocol on sperm characteristics, 15 further brown trout males were sampled from the same populations. The same protocol as before was used for gametes collection and cryopreservation. Fresh sperm was diluted at 10% in Storfish (IMV Technologies, France). Frozen sperm was thawed as described above. A sample of both fresh and frozen-thawed sperm were activated with Actifish in a final dilution ratio of 1 : 500. Then, 2 µl of activated semen was analysed in a 4-well chamber slide (Leja, IMV Technologies, France) on a cooling stage set at 6.5°C. Motility of fresh and cryopreserved sperm was analysed with the CASA Qualisperm software (Biophos AG, Switzerland). Motility (percentage of motile cells and fast moving (greater than 100 µm s−1) cells), concentration (number of cells ml−1) and average path velocity (VAP in µm s−1) were recorded under a phase contrast microscope at ×20 magnification 20 s after activation by the software based on two consecutive measures whose difference was not allowed to exceed 10% for concentration and 15% for motility traits.
(f). Statistical analyses
The paired Student t-tests were used to analyse the effect of cryopreservation on sperm characteristics. Mortality and fertilization success were analysed as binomial variables in generalized linear mixed effect models, whereas incubation time, length at hatching, yolk sac volume at hatching and larval growth were analysed as continuous variables in linear mixed effect models. Mixed models were performed with the lme4 package [48] in Rstudio [49]. Pathogen treatment (control or exposure to bacteria) and fertilization method (cryopreserved or non-cryopreserved milt) were entered as fixed effects. For the analyses on larval growth, length at hatching was also entered as a fixed effect to control for the initial variation in hatching length. Male and female (i.e. breeding block) identity were entered as random effects. For some variables, some of the 160 experimental cells (family × pathogen treatment × fertilization method) were empty due to missing values. The respective family was then excluded from the corresponding comparison. The significance of an effect was tested by comparing a model including or lacking the latter with the reference model. The relative quality of models was estimated by the Akaike information criterion (AIC), and likelihood ratio tests (LRTs) were used to assess differences in goodness of fit between models.
3. Results
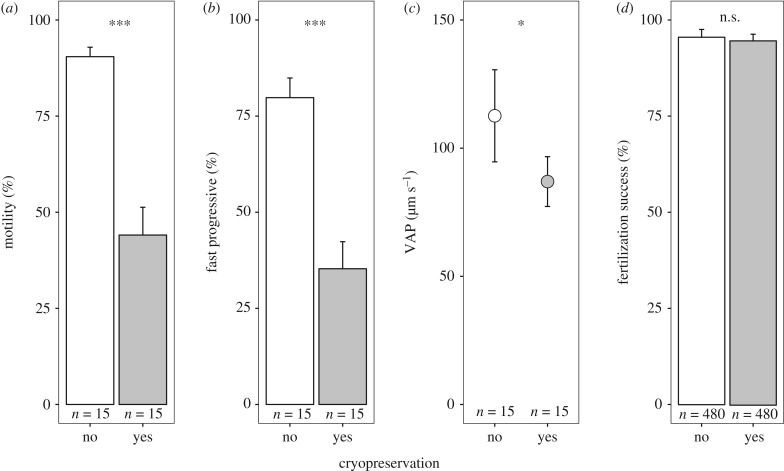
All measures of sperm velocity and motility were negatively affected by the cryopreservation. The proportion of motile spermatozoa, fast spermatozoa and the average path velocity were reduced after cryopreservation (figure 1a–c). Fertilization success was overall very high (figure 1d), and the apparent difference between the non-cryopreserved (95.2 ± 1.9%) and the cryopreserved treatment (94.4 ± 2%) was not significant (table 1a), and not affected by male identity nor breeding block (non-significant interaction terms in table 1a).
Figure 1.
Effect of cryopreservation on sperm quality indicators: (a) motility (paired t-test: t14 = 12.1, p < 0.001), (b) percentage of fast progressive sperms (t14 = 9.7, p < 0.001) and (c) the average path velocity VAP (t14 = 2.3, p = 0.04). (d) The fertilization success of the 40 males used in the breeding experiment, based on 2 × 12 eggs per male (see table 1a for statistics). Plots show means and 95% confidence intervals for non-cryopreserved sperm (empty bars or symbol) and cryopreserved sperm (filled bars or symbol). Asterisks indicate the levels of significance (***p < 0.001; *p < 0.05; ‘n.s.’, not significant).
Table 1.
Treatment effects on embryo traits. LRTs comparing generalized linear mixed-effects models on (a) fertilization success and (b) embryo mortality, and (c) linear mixed-effects models on hatching time. Models including or lacking the term of interest were compared with the reference model in italics. Significant p-values are highlighted with bold type. Fixed effects: p, pathogen treatment; c, cryopreservation; random effects: m, male; b, breeding block.
| model terms | effect tested | AIC | d.f. | χ2 | p-value |
|---|---|---|---|---|---|
| (a) fertilization success | |||||
| c + m + b | 261 | 4 | |||
| c + m | b | 261 | 3 | 2.1 | 0.14 |
| c + b | m | 321 | 3 | 62.5 | <0.001 |
| m + b | c | 259 | 3 | 0.8 | 0.37 |
| c + c|m + b | c × m | 264 | 6 | 0.3 | 0.87 |
| c + m + c|b | c × b | 263 | 6 | 0.4 | 0.83 |
| (b) embryo mortality | |||||
| p + c + m + b | 625 | 5 | |||
| p + c + m | b | 657 | 4 | 34.4 | <0.001 |
| p + c + b | m | 623 | 4 | 0.5 | 0.48 |
| p + c + m + b | c | 623 | 4 | 0.1 | 0.75 |
| c + m + b | p | 734 | 4 | 111.6 | <0.001 |
| p + c + m + c|f | c × b | 629 | 7 | 0.1 | 0.97 |
| p + c + m + p|b | p × b | 613 | 7 | 15.6 | <0.001 |
| p + c + c|m + t | c × m | 628 | 7 | 0.2 | 0.90 |
| p + c + p|m + b | p × m | 624 | 7 | 4.8 | 0.09 |
| p + c + p|c + m + b | p × c | 625 | 6 | 1.5 | 0.21 |
| (c) hatching time | |||||
| p + c + m + b | 2900 | 6 | |||
| p + c + b | b | 2906 | 5 | 7.9 | 0.005 |
| p + c + m | m | 2918 | 5 | 19.2 | <0.001 |
| p + m + b | c | 2904 | 5 | 5.6 | 0.02 |
| c + m + b | p | 2931 | 5 | 32.8 | <0.001 |
| p + c + m + c|b | c × b | 2904 | 8 | 0 | 1.0 |
| p + c + m + p|b | p × b | 2894 | 8 | 10.0 | 0.007 |
| p + c + c|m + b | c × m | 2917 | 8 | 0 | 1.0 |
| p + c + p|m + b | p × m | 2891 | 8 | 13.7 | <0.001 |
| p + c + p|c + m + b | p × c | 2902 | 7 | 0.2 | 0.68 |
Breeding blocks by themselves had no significant effects on fertilization success (table 1a), but affected all other measured traits (tables 1 and 2; electronic supplementary material, table S1). Male identity had a significant effect on fertilization success (table 1a,c), hatching time (table 1c), length at hatching (table 2a) and on larval growth (table 2c) when taken in consideration with larval size at hatching (l × m interaction in table 2c; electronic supplementary material, figure S6), but not on the other traits measured. Importantly, larvae of parental sib groups that hatched later tend to be smaller than the ones that hatched earlier, i.e. male identity had a significant effect on embryo growth (electronic supplementary material, figure S3).
Table 2.
Treatment effects on larval traits. LRTs comparing linear mixed-effects models on (a) length at hatching, (b) yolk sac volume at hatching and (c) larval growth. Models including or lacking the term of interest were compared with the reference model in italics. Significant p-values are highlighted with bold type. Fixed effects: p, pathogen treatment; c, cryopreservation; l, length at hatching; random effects: m, male; b, breeding block.
| model terms | effect tested | AIC | d.f. | χ2 | p-value |
|---|---|---|---|---|---|
| (a) length at hatching | |||||
| p + c + m + b | 821 | 6 | |||
| p + c + m | b | 851 | 5 | 32.1 | <0.001 |
| p + c + b | m | 828 | 5 | 9.4 | 0.002 |
| p+ m + b | c | 819 | 5 | 0.2 | 0.61 |
| c + m + b | p | 866 | 5 | 47.0 | <0.001 |
| p + c + m + c|b | c × b | 820 | 8 | 4.8 | 0.09 |
| p + c + m + p|b | p × b | 823 | 8 | 2.2 | 0.34 |
| p + c + c|m + b | c × m | 824 | 8 | 0.5 | 0.79 |
| p + c + p|m + b | p × m | 832 | 8 | 0 | 1.0 |
| p + c + p|c + m + b | p × c | 821 | 7 | 2.2 | 0.14 |
| (b) yolk volume at hatching | |||||
| p + c + m + b | 4273 | 6 | |||
| p + c + m | b | 4362 | 5 | 90.8 | <0.001 |
| p + c + b | m | 4271 | 5 | 0 | 1.0 |
| p + m + b | c | 4272 | 5 | 0.8 | 0.36 |
| c + m + b | p | 4272 | 5 | 1.0 | 0.33 |
| p + c + m + c|b | c × b | 4273 | 8 | 4.0 | 0.13 |
| p + c + m + p|b | p × b | 4276 | 8 | 1.4 | 0.49 |
| p + c + c|m + b | c × m | 4275 | 8 | 2.0 | 0.37 |
| p + c + p|m + b | p × m | 4277 | 8 | 0.2 | 0.91 |
| p + c + p|c + m + b | p × c | 4275 | 7 | 0.1 | 0.72 |
| (c) larval growth | |||||
| l + p + c + m + b | 488 | 7 | |||
| l + p + c + m | b | 492 | 6 | 6.0 | 0.01 |
| l + p + c + b | m | 488 | 6 | 2.4 | 0.12 |
| l + p + m + b | c | 491 | 6 | 5.2 | 0.02 |
| l + c + m + b | p | 489 | 6 | 3.5 | 0.06 |
| p + c + m + b | l | 486 | 6 | 0.3 | 0.56 |
| l + p + c + m + c|b | c × b | 492 | 9 | 0.2 | 0.91 |
| l + p + c + m + p|b | p × b | 491 | 9 | 1.2 | 0.54 |
| l + p + c + m + l|b | l × b | 490 | 9 | 1.6 | 0.46 |
| l + p + c + c|m + b | c × m | 492 | 9 | 0.4 | 0.83 |
| l + p + c + p|m + b | p × m | 492 | 9 | 0.1 | 0.95 |
| l + p + c + l|m + b | l × m | 483 | 9 | 8.5 | 0.01 |
| l + p + c + p|c + m + b | p × c | 490 | 8 | <0.1 | 0.84 |
| l + p + c + l|c + m + b | l × c | 488 | 8 | 1.5 | 0.23 |
| l + p + l|p + c + m + b | l × p | 490 | 8 | <0.1 | 0.99 |
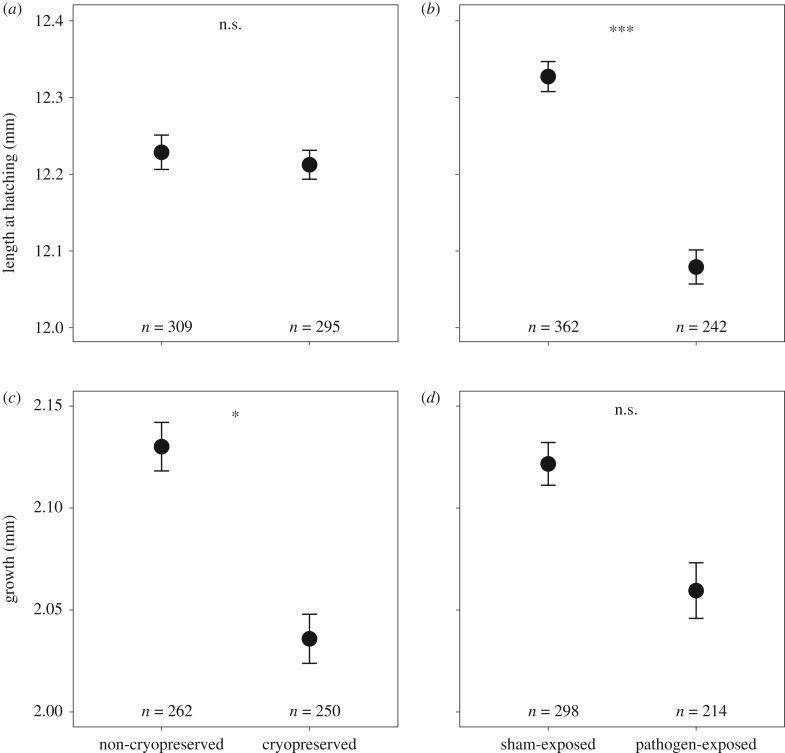
Offspring produced with cryopreserved sperm did not suffer significant extra mortality compared with their siblings at any stage (table 1b; electronic supplementary material, table S1A,B; and figure S4A–C), but they hatched slightly earlier than those produced with non-cryopreserved sperm (table 1c; electronic supplementary material, figure S4D). Sperm cryopreservation treatment did not significantly affect larval length at hatching (table 2a and figure 2a) nor its initial yolk sac volume (table 2b; electronic supplementary material, figure S5A), and yolk sac consumption over the 14 days was not significantly affected by the treatment of the sperm (electronic supplementary material, table S1 and figure S5B). However, larvae produced with cryopreserved sperm showed reduced growth (−4.2%) compared with their full sibs fertilized with non-cryopreserved sperm (figure 2c and table 2c). The effect of sperm treatment did not significantly interact with any other term for any of the traits we investigated (tables 1 and 2; electronic supplementary material, table S1).
Figure 2.
Effects of (a,c) sperm cryopreservation and (b,d) exposure to pathogen on (a,b) larval length at hatching and (c,d) larval growth during 14 days after hatching. The figure gives the means and 95% CI (based on family means) and the total number of larvae that could be measured per treatment group. Asterisks indicate the levels of significance (***p < 0.001; *p < 0.05; ‘n.s.’, not significant). See table 2a,c for statistics.
Exposure to the pathogen caused strong effects on embryos. Embryo mortality was about four times higher for embryos exposed to A. salmonicida than for sham-exposed embryos (table 1b; electronic supplementary material, figure S4A). After transfer of the freshly hatched larvae to sterile new plates, pathogen-linked larval mortality dropped below the one observed in the controls (electronic supplementary material, table S1A and figure S4B), but the cumulative pathogen-induced mortality was still 1.8 times higher at the end of the experiment (electronic supplementary material, table S1 and figure S4C). Embryos exposed to the pathogen also hatched later (table 1c; electronic supplementary material, figure S4D) and at smaller size (table 2a and figure 2b) than their sham-treated siblings despite having similar yolk sac sizes at hatching (table 2b; electronic supplementary material, figure S5A). The pathogen stress reduced larval growth by about 3.6% compared with the controls (figure 2d and table 2c), and both, growth and pathogen stress, affected yolk consumption (g × p interaction in electronic supplementary material, table S1C; and figure S7). Breeding block affected tolerance to A. salmonicida with regard to mortality at the embryonic stage, hatching time and cumulative mortality after two weeks of growth (see p × b interactions in table 1b,c; electronic supplementary material, table S1B), but not for length at hatching, yolk volume at hatching or consumption and larval growth (table 2a–c; electronic supplementary material, table S1C). Male identities significantly affected pathogen-induced changes in hatching time (table 2c) and cumulative mortality as determined two weeks after hatching (electronic supplementary material, table S1B).
Importantly, no measure of pathogen virulence was enhanced as a consequence of being fertilized by cryopreserved sperm. There was no significant interaction between the pathogen treatment and the sperm treatment in any of the studied traits (see p × c interactions in tables 1 and 2; electronic supplementary material, table S1).
4. Discussion
We tested experimentally whether sperm cryopreservation affects offspring performance in the presence or absence of environmental stress, and if so, whether such effects are male-specific. We did not find significant effects of cryopreservation on embryo mortality, on embryo tolerance to infection nor on embryo growth until hatching. However, we found significant effects on larval development after hatching. Larvae produced with cryopreserved sperm grew on average about 4.2% slower than their siblings produced with non-cryopreserved sperm despite using up their yolk sac content at a similar rate. This will lead to a smaller size at, and/or a delayed time of, emergence from gravel when larvae have used up their yolk and start exogenous feeding. These two variables can be fitness-relevant in salmonids. Size at emergence affects competition for feeding territories [50,51], and mortality due to predation or hydroclimatic events is often size selective [52].
The deleterious effect of cryopreservation that we found at the larval stage was independent of the environmental stress the embryos had been exposed to (i.e. we found no significant interaction between the sperm treatment and the effect of the pathogen). However, we found significant additive genetic variance for tolerance to the pathogen. Some males produced offspring that seemed less affected by the pathogen than others. Despite this genetic diversity, and despite significant genetic divergence between the populations from which the males were sampled [42], the offspring of all males reacted similarly to cryopreservation.
We used an experimental design and a model organism that will reveal even small genetic effects if they exist. Such effects are difficult to demonstrate in internal fertilizers, where genetic effects need to be disentangled from maternal effects that include, for instance, differential maternal investment [18]. Our within-male comparisons also allowed us to disentangle genetic from environmental effects. Salmonid males only fertilize eggs and show no parental care; hence, the paternal effect on a given offspring phenotype is a useful proxy of additive genetic variance [13,53,54]. In order to detect genetic effects, we raised large numbers of embryos singly, keeping track of their pedigree, exposure to treatments and individual performance. The statistical power given by such experimental set-ups could be used in previous studies to demonstrate genetic variance for tolerance to various types of biotic and abiotic stressors [12,37,44,55] or to demonstrate that there is no significant additive genetic variance for tolerance to an environmental stressor [39]. Moreover, we used males that vary genetically (the significant sire effects in our models). We therefore argue that we had the genetic diversity, the experimental design and the statistical power to detect relevant additive genetic variance for tolerance to cryopreservation if it existed, and hence the likelihood of a type II error (false negative) is small in our case.
As expected [7,8], cryopreservation reduced sperm motility and viability. Nonetheless, we obtained fertilization rates that were very close to that of fresh sperm by using a protocol developed by Ciereszko et al. [40], tested before in our group on brown trout [41], and rated as the most promising protocol for salmonids by Judycka et al. [56] in their recent review. As also expected (see Introduction), we did not find any deleterious effect of sperm cryopreservation on early embryo viability nor on early development. However, cryopreservation affected larval development. Because sperm cryopreservation is a procedure that includes dilution of sperm in an extender, equilibration, freezing, storage usually over longer periods and thawing for final use, it remains to be shown which step(s) in the protocol is/are responsible for the observed effects on offspring growth. If storage time creates such negative effects, the short time frame used in our study would lead to an underestimation of the effects that would be relevant in medicine and population management.
Previous breeding experiments with gametes from the study populations or from populations within the same drainage system [42] concluded that males differ in their genetic quality [37,45,57–59]. Here, we found again evidence for variation in genetic quality among males within wild populations: the offspring of some males grew faster and hatched earlier than the offspring of other males. However, the negative effects of cryopreservation on larval development seemed not mitigated by an overall good genetic quality (‘good genes effects’ [13]) of the sperm donor. We found no male effect on the tolerance to damage induced by cryopreservation.
We suggest two possible explanations for the observed effect of cryopreservation. First, cryopreservation could select against certain types of spermatozoa in the milt sample (the ‘sperm selection hypothesis'). There is typically much phenotypic variation among the sperm of an ejaculate, and this variation often clusters into several identifiable subpopulations of sperm [60,61]. The proportion of these subpopulations in the milt seems typically affected by cryopreservation, and the factors responsible for this differential tolerance to cryopreservation are not clear yet [60]. However, for such an effect of selection to translate into variation in offspring phenotype, there needs to be a relationship between sperm phenotype and factors that affect offspring growth. Such relationships have been observed in various taxa [10,11,62,63]. Considering the evidence for haploid selection [64], it is therefore possible that the effect we detected is due to cryopreservation-mediated selection against certain sperm phenotypes and the haplotypes carried by these sperm.
Second, cellular damage induced by cryopreservation could affect offspring phenotype (the ‘cryodamage hypothesis’). Cryodamage is well documented and can be due to physico-chemical stress [65]. It can affect the cellular integrity [65], the sperm DNA integrity [66,67], the sperm epigenome [68] and the sperm transcriptome [69,70]. DNA integrity can vary due to genotoxic products released during freezing-induced membrane peroxidation [65,71]. Sperm with damaged DNA can be viable and fertilize eggs [72], but there can also be selection against them, such as in the mouse by the female reproductive tract and/or the zona pellucida [73]. The zygote may be able to repair some degree of DNA fragmentation [72,74,75], but cryodamage could still have consequences for the offspring [66,76,77]. Such consequences could be species-specific [67]. So far, there seems to be little consensus in the literature about cryopreservation-mediated DNA fragmentation in humans and its effect on the progeny [14]. Moreover, cryodamage may not only affect genomic DNA. There is growing evidence that non-genomic information is transmitted by sperm to the embryo in various mammals [78] and that paternal epigenome can play a role during development through at least six ways [79]. This epigenetic information can play a role in embryo development [80] and can affect embryo phenotype [81]. Importantly, such non-genomic information can also be altered by cryopreservation [70].
These two hypotheses lead to predictions that can be tested with our observations. In the cryodamage hypothesis, one possibility is that cryopreservation affects the genome randomly. We would then expect genes involved in larval growth and in pathogen resistance to be about equally affected. However, we found no significant interaction between the sperm treatment and the pathogen treatment, suggesting that genes may be differentially affected by cryopreservation based on their location in the nucleus, on their size or on other aspects that make them susceptible to damage [82,83]. Indeed, Fernández-Díez & Herráez [69] found that DNA damage induced by cryopreservation mostly affected the transcription of genes related to metabolic and cellular processes. Future studies could explore the possible links between an induced loss of DNA integrity, offspring growth and tolerance to infection in brown trout.
The sperm selection hypothesis predicts a relationship between sperm phenotype and the genetic or non-genetic information it carries, but it remains unclear what type of sperm-mediated information would be selected. One possibility is that post-thaw sperm viability (i.e. tolerance of sperm to cryopreservation) reveals mainly haploid information linked to development [10,11]. Our observations seem to support this possibility, as sperm cryopreservation affected offspring growth but not their tolerance to the pathogen. However, further studies will be necessary to test the sperm selection hypothesis.
To conclude, we demonstrate that milt cryopreservation affects offspring performance at a late stage of development (i.e. after the first developmental stages that have typically been considered for the validation of cryopreservation). In these earlier developmental stages, cryopreservation shows no significant damaging effects and does not even affect the tolerance to an infection. The late effects of cryopreservation seem independent of variation in overall genetic quality among males (i.e. they seem unlikely to be mitigated by ‘good genes’ effects). Sperm cryopreservation reduces the average genetic quality or the haplotype diversity when compared with fresh sperm. Further studies are necessary to evaluate the consequences and the applicability of our findings for the use of sperm cryopreservation in reproductive medicine, livestock production and conservation biology.
Supplementary Material
Acknowledgements
We thank C. Küng from the Fishery Inspectorate Bern for permission, U. Gutmann and B. Bracher for catching the fish and stripping them, A. Uppal, D. Maitre, I. Castro and D. Zeugin for assistance and discussion, and J. Merilä and three reviewers for constructive comments.
Ethics
The sampling of adults, the stripping, the experimental breeding and the raising of embryos were approved by the Fishery Inspectorate of the Bern canton and, where necessary, also by the Veterinary Office of the Bern canton (approval number BE188/14).
Data accessibility
The data used in this study are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.q6t8k07 [84].
Authors' contributions
D.N., L.M.d.C. and C.W. designed the experiment and organized the fieldwork. D.N. measured milt characteristics. D.N. and L.M.d.C. treated the sperm, did the in vitro fertilizations, distributed the fertilized eggs to the 24-well plates, monitored the embryos and determined larval growth. All authors analysed the data and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project was supported by Swiss National Science Foundation (grant nos 31003A_159579 and 31003A_182265).
References
- 1.Bailey JL, Blodeau J-F, Cormier N. 2000. Semen cryopreservation in domestic animals: a damaging and capacitating phenomenon minireview. J. Androl. 21, 1–7. ( 10.1002/j.1939-4640.2000.tb03268.x) [DOI] [PubMed] [Google Scholar]
- 2.Gwo J-C. 2000. Cryopreservation of aquatic invertebrate semen: a review. Aquacult. Res. 31, 259–271. ( 10.1046/j.1365-2109.2000.00462.x) [DOI] [Google Scholar]
- 3.Watson P, Holt WV. 2001. Cryobanking the genetic resource: wildlife conservation for the future? Boca Raton, FL: CRC Press. [Google Scholar]
- 4.Horváth Á, et al. 2012. Application of sperm cryopreservation to hatchery practice and species conservation: a case of the Adriatic grayling (Thymallus thymallus). Aquaculture 358–359, 213–215. ( 10.1016/j.aquaculture.2012.07.012) [DOI] [Google Scholar]
- 5.Mocé E, Fajardo AJ, Graham JK. 2016. Human sperm cryopreservation. Eur. Med. J. 1, 86–91. [Google Scholar]
- 6.Martínez-Páramo S, et al. 2017. Cryobanking of aquatic species. Aquaculture 472, 156–177. ( 10.1016/j.aquaculture.2016.05.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabrita E, Martínez-Páramo S, Gavaia PJ, Riesco MF, Valcarce DG, Sarasquete C, Herráez MP, Robles V. 2014. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 432, 389–401. ( 10.1016/j.aquaculture.2014.04.034) [DOI] [PubMed] [Google Scholar]
- 8.Kopeika J, Thornhill A, Khalaf Y. 2015. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum. Reprod. Update 21, 209–227. ( 10.1093/humupd/dmu063) [DOI] [PubMed] [Google Scholar]
- 9.Asturiano JF, Cabrita E, Horváth Á. 2016. Progress, challenges and perspectives on fish gamete cryopreservation: a mini-review. Gen. Comp. Endocrinol. 245, 69–76. ( 10.1016/j.ygcen.2016.06.019) [DOI] [PubMed] [Google Scholar]
- 10.Immler S, Hotzy C, Alavioon G, Petersson E, Arnqvist G. 2014. Sperm variation within a single ejaculate affects offspring development in Atlantic salmon. Biol. Lett. 10, 20131040 ( 10.1098/rsbl.2013.1040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alavioon G, Hotzy C, Nakhro K, Rudolf S, Scofield DG, Zajitschek S, Maklakov AA, Immler S. 2017. Haploid selection within a single ejaculate increases offspring fitness. Proc. Natl Acad. Sci. USA 114, 8053–8058. ( 10.1073/pnas.1705601114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark ES, Pompini M, Marques da Cunha L, Wedekind C.. 2014. Maternal and paternal contributions to pathogen resistance dependent on development stage in a whitefish (Salmonidae). Funct. Ecol. 28, 714–723. ( 10.1111/1365-2435.12214) [DOI] [Google Scholar]
- 13.Neff BD, Pitcher TE. 2005. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38. ( 10.1111/j.1365-294X.2004.02395.x) [DOI] [PubMed] [Google Scholar]
- 14.Di Santo M, Tarozzi N, Nadalini M, Borini A.. 2012. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv. Urol. 2012, 12 ( 10.1155/2012/854837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Z, Wang Y, Lin J, Xu J, Ding G, Huang H. 2017. Genetic and epigenetic risks of assisted reproduction. Best Pract. Res. Clin. Obstet. Gynaecol. 44, 90–104. ( 10.1016/j.bpobgyn.2017.07.004) [DOI] [PubMed] [Google Scholar]
- 16.Haig D. 1996. Placental hormones, genomic imprinting, and maternal–fetal communication. J. Evol. Biol. 9, 357–380. ( 10.1046/j.1420-9101.1996.9030357.x) [DOI] [Google Scholar]
- 17.Forbes S. 2017. Embryo quality: the missing link between pregnancy sickness and pregnancy outcome. Evol. Hum. Behav. 38, 265–278. ( 10.1016/j.evolhumbehav.2016.10.003) [DOI] [Google Scholar]
- 18.Ratikainen II, Haaland TR, Wright J. 2018. Differential allocation of parental investment and the trade-off between size and number of offspring. Proc. R. Soc. B 285, 20181074 ( 10.1098/rspb.2018.1074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alencoão I, et al. 2019. Intracytoplasmatic sperm injection cycles with cryopreserved versus fresh testicular sperm: is there any difference? Eur. J. Obstet. Gynecol. Reprod. Biol. 234, e179–e180. ( 10.1016/j.ejogrb.2018.08.553) [DOI] [Google Scholar]
- 20.Adams DH, Clark RA, Davies MJ, de Lacey S.. 2017. A meta-analysis of sperm donation offspring health outcomes. J. Dev. Orig. Health Dis. 8, 44–55. ( 10.1017/S2040174416000489) [DOI] [PubMed] [Google Scholar]
- 21.Ogonuki N, et al. 2006. Spermatozoa and spermatids retrieved from frozen reproductive organs or frozen whole bodies of male mice can produce normal offspring. Proc. Natl Acad. Sci. USA 103, 13 098–13 103. ( 10.1073/pnas.0605755103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakayama S, et al. 2017. Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc. Natl Acad. Sci. USA 114, 5988–5993. ( 10.1073/pnas.1701425114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Gonzalez R, et al. 2008. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol. Reprod. 78, 761–772. ( 10.1095/biolreprod.107.065623) [DOI] [PubMed] [Google Scholar]
- 24.Akter S, Hassan MM, Nahiduzzaman M, Hossain MAR. 2016. Growth and survival of olive barb, Puntius sarana (Hamilton 1822) larvae produced with cryopreserved versus fresh sperm. Aquacult. Res. 47, 228–233. ( 10.1111/are.12484) [DOI] [Google Scholar]
- 25.Linhart O, Billard R, Proteau JP. 1993. Cryopreservation of European catfish (Silurus glanis L.) spermatozoa. Aquaculture 115, 347–359. ( 10.1016/0044-8486(93)90148-R) [DOI] [Google Scholar]
- 26.Rahman F, Sarder MRI, Rouf MA. 2009. Comparison of growth performance between cryopreserved and fresh sperm-originated fry of Barbonymus gonionotus. J. Bangladesh Agric. Univ. 7, 145–149. ( 10.3329/jbau.v7i1.4977) [DOI] [Google Scholar]
- 27.Rana KJ, McAndrew BJ. 1989. The viability of cryopreserved tilapia spermatozoa. Aquaculture 76, 335–345. ( 10.1016/0044-8486(89)90085-9) [DOI] [Google Scholar]
- 28.Viveiros ATM, Isaú ZA, Caneppele D, Leal MC. 2012. Sperm cryopreservation affects postthaw motility, but not embryogenesis or larval growth in the Brazilian fish Brycon insignis (Characiformes). Theriogenology 78, 803–810. ( 10.1016/j.theriogenology.2012.03.028) [DOI] [PubMed] [Google Scholar]
- 29.Young WP, Frenyea K, Wheeler PA, Thorgaard GH. 2009. No increase in developmental deformities or fluctuating asymmetry in rainbow trout (Oncorhynchus mykiss) produced with cryopreserved sperm. Aquaculture 289, 13–18. ( 10.1016/j.aquaculture.2008.12.031) [DOI] [Google Scholar]
- 30.Galo JM, Streit-Junior DP, Oliveira CA, Povh JP, Fornari DC, Digmayer M, Ribeiro RP. 2019. Quality of fresh and cryopreserved semen and their influence on the rates of fertilization, hatching and quality of the larvae of Piaractus mesopotamicus. Braz. J. Biol. 79, 438–445. ( 10.1590/1519-6984.182391) [DOI] [PubMed] [Google Scholar]
- 31.Miller ME, Kemski M, Grayson JD, Towne K, Dabrowski K. 2018. Yellow perch sperm motility, cryopreservation, and viability of resulting larvae and juveniles. North Am. J. Aquacult. 80, 3–12. ( 10.1002/naaq.10001) [DOI] [Google Scholar]
- 32.Bokor Z, Ittzés I, Mosonyi G, Kotrik L, Müller T, Urbányi B, Horváth Á. 2015. Survival and growth rates of wels catfish (Silurus glanis Linnaeus, 1758) larvae originating from fertilization with cryopreserved or fresh sperm. J. Appl. Ichthyol. 31, 164–168. ( 10.1111/jai.12730) [DOI] [Google Scholar]
- 33.Hayes MC, Rubin SP, Hensleigh JE, Reisenbichler RR, Wetzel LA. 2005. Performance of juvenile steelhead trout (Oncorhynchus mykiss) produced from untreated and cryopreserved milt. Aquaculture 249, 291–302. ( 10.1016/j.aquaculture.2005.04.035) [DOI] [Google Scholar]
- 34.Martínez-Páramo S, Pérez-Cerezales S, Gómez-Romano F, Blanco G, Sánchez JA, Herráez MP. 2009. Cryobanking as tool for conservation of biodiversity: effect of brown trout sperm cryopreservation on the male genetic potential. Theriogenology 71, 594–604. ( 10.1016/j.theriogenology.2008.09.034) [DOI] [PubMed] [Google Scholar]
- 35.Queitsch C, Sangster TA, Lindquist S. 2002. Hsp90 as a capacitor of phenotypic variation. Nature 417, 618–624. ( 10.1038/nature749) [DOI] [PubMed] [Google Scholar]
- 36.Jacob A, Evanno G, Von Siebenthal BA, Grossen C, Wedekind C.. 2010. Effects of different mating scenarios on embryo viability in brown trout. Mol. Ecol. 19, 5296–5307. ( 10.1111/j.1365-294X.2010.04884.x) [DOI] [PubMed] [Google Scholar]
- 37.Pompini M, Clark ES, Wedekind C. 2013. Pathogen-induced hatching and population-specific life-history response to waterborne cues in brown trout (Salmo trutta). Behav. Ecol. Sociobiol. 67, 649–656. ( 10.1007/s00265-013-1484-y) [DOI] [Google Scholar]
- 38.Kortet R, Vainikka A, Janhunen M, Piironen J, Hyvärinen P. 2014. Behavioral variation shows heritability in juvenile brown trout Salmo trutta. Behav. Ecol. Sociobiol. 68, 927–934. ( 10.1007/s00265-014-1705-z) [DOI] [Google Scholar]
- 39.Marques da Cunha L, Uppal A, Seddon E, Nusbaumer D, Vermeirssen ELM, Wedekind C.. 2019. No additive genetic variance for tolerance to ethynylestradiol exposure in natural populations of brown trout (Salmo trutta). Evol. Appl. 12, 940–950. ( 10.1111/eva.12767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciereszko A, Dietrich GJ, Nynca J, Dobosz S, Zalewski T. 2014. Cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture 420–421, 275–281. ( 10.1016/j.aquaculture.2013.11.014) [DOI] [Google Scholar]
- 41.Nusbaumer D, Marques da Cunha L, Wedekind C.2018. Comparing methanol-glucose and dimethyl-sulfoxide based extender for milt cryopreservation of brown trout (Salmo trutta). bioRxiv 289736. (doi:10.1101/289736)
- 42.Stelkens RB, Jaffuel G, Escher M, Wedekind C. 2012. Genetic and phenotypic population divergence on a microgeographic scale in brown trout. Mol. Ecol. 21, 2896–2915. ( 10.1111/j.1365-294X.2012.05581.x) [DOI] [PubMed] [Google Scholar]
- 43.OECD. 1992. OECD guideline for the testing of chemicals 203 (fish, acute toxicity test), p. 9 Paris, France: OECD. [Google Scholar]
- 44.von Siebenthal BA, Jacob A, Wedekind C.. 2009. Tolerance of whitefish embryos to Pseudomonas fluorescens linked to genetic and maternal effects, and reduced by previous exposure. Fish Shellfish Immunol. 26, 531–535. ( 10.1016/j.fsi.2009.02.008) [DOI] [PubMed] [Google Scholar]
- 45.Clark ES, Stelkens RB, Wedekind C. 2013. Parental influences on pathogen resistance in brown trout embryos and effects of outcrossing within a river network. PLoS ONE 8, e57832 ( 10.1371/journal.pone.0057832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen LF, Hansen MM, Pertoldi C, Holdensgaard G, Mensberg K-LD, Loeschcke V. 2008. Local adaptation in brown trout early life-history traits: implications for climate change adaptability. Proc. R. Soc. B 275, 2859–2868. ( 10.1098/rspb.2008.0870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. ArXiv14065823 Stat.
- 49.RStudio Team. 2015. RStudio: Integrated development environment for R. Boston, MA: RStudio; See http://www.rstudio.com/. [Google Scholar]
- 50.Cutts CJ, Metcalfe NB, Taylor AC. 1999. Competitive asymmetries in territorial juvenile Atlantic salmon, Salmo salar. Oikos 86, 479–486. ( 10.2307/3546652) [DOI] [Google Scholar]
- 51.Fausch KD. 1984. Profitable stream positions for salmonids: relating specific growth rate to net energy gain. Can. J. Zool. 62, 441–451. ( 10.1139/z84-067) [DOI] [Google Scholar]
- 52.Good SP, Dodson JJ, Meekan MG, Ryan DA. 2001. Annual variation in size-selective mortality of Atlantic salmon (Salmo salar) fry. Can. J. Fish. Aquat. Sci. 58, 1187–1195. ( 10.1139/f01-069) [DOI] [Google Scholar]
- 53.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer; See http://www.invemar.org.co/redcostera1/invemar/docs/RinconLiterario/2011/febrero/AG_8.pdf. [Google Scholar]
- 54.Hendry AP, et al. 2011. Evolutionary principles and their practical application. Evol. Appl. 4, 159–183. ( 10.1111/j.1752-4571.2010.00165.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brazzola G, Chèvre N, Wedekind C. 2014. Additive genetic variation for tolerance to estrogen pollution in natural populations of Alpine whitefish (Coregonus sp., Salmonidae). Evol. Appl. 7, 1084–1093. ( 10.1111/eva.12216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Judycka S, Nynca J, Ciereszko A. 2019. Opportunities and challenges related to the implementation of sperm cryopreservation into breeding of salmonid fishes. Theriogenology 132, 12–21. ( 10.1016/j.theriogenology.2019.03.022) [DOI] [PubMed] [Google Scholar]
- 57.Jacob A, Nusslé S, Britschgi A, Evanno G, Müller R, Wedekind C. 2007. Male dominance linked to size and age, but not to ‘good genes’ in brown trout (Salmo trutta). BMC Evol. Biol. 7, 207 ( 10.1186/1471-2148-7-207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkins LGE, Fumagalli L, Wedekind C. 2016. Effects of host genetics and environment on egg-associated microbiotas in brown trout (Salmo trutta). Mol. Ecol. 25, 4930–4945. ( 10.1111/mec.13798) [DOI] [PubMed] [Google Scholar]
- 59.Wilkins LGE, da Cunha L Marques, Glauser G, Vallat A, Wedekind C.. 2017. Environmental stress linked to consumption of maternally derived carotenoids in brown trout embryos (Salmo trutta). Ecol. Evol. 7, 5082–5093. ( 10.1002/ece3.3076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Labbé C, Robles V, Herráez MP. 2013. Cryopreservation of gametes for aquaculture and alternative cell sources for genome preservation. In Advances in aquaculture hatchery technology (ed. Burnell G), pp. 76–116. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 61.Gallego V, Vílchez MC, Peñaranda DS, Pérez L, Herráez MP, Asturiano JF, Martínez-Pastor F. 2015. Subpopulation pattern of eel spermatozoa is affected by post-activation time, hormonal treatment and the thermal regimen. Reprod. Fertil. Dev. 27, 529–543. ( 10.1071/RD13198) [DOI] [PubMed] [Google Scholar]
- 62.Crean AJ, Dwyer JM, Marshall DJ. 2012. Fertilization is not a new beginning: the relationship between sperm longevity and offspring performance. PLoS ONE 7, e49167 ( 10.1371/journal.pone.0049167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borowsky R, Luk A, He X, Kim RS. 2018. Unique sperm haplotypes are associated with phenotypically different sperm subpopulations in Astyanax fish. BMC Biol. 16, 72 ( 10.1186/s12915-018-0538-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Immler S, Otto SP. 2018. The evolutionary consequences of selection at the haploid gametic stage. Am. Nat. 192, 241–249. ( 10.1086/698483) [DOI] [PubMed] [Google Scholar]
- 65.Hezavehei M, Sharafi M, Kouchesfahani HM, Henkel R, Agarwal A, Esmaeili V, Shahverdi A. 2018. Sperm cryopreservation: a review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 37, 327–339. ( 10.1016/j.rbmo.2018.05.012) [DOI] [PubMed] [Google Scholar]
- 66.Labbé C, Martoriati A, Devaux A, Maisse G. 2001. Effect of sperm cryopreservation on sperm DNA stability and progeny development in rainbow trout. Mol. Reprod. Dev. 60, 397–404. ( 10.1002/mrd.1102) [DOI] [PubMed] [Google Scholar]
- 67.Migaud H, Bell G, Cabrita E, McAndrew B, Davie A, Bobe J, Herráez MP, Carrillo M. 2013. Gamete quality and broodstock management in temperate fish. Rev. Aquacult. 5, S194–S223. ( 10.1111/raq.12025) [DOI] [Google Scholar]
- 68.Labbé C, Robles V, Herráez MP. 2017. Epigenetics in fish gametes and early embryo. Aquaculture 472, 93–106. ( 10.1016/j.aquaculture.2016.07.026) [DOI] [Google Scholar]
- 69.Fernández-Díez C, Herráez MP. 2018. Changes in transcriptomic profile of trout larvae obtained with frozen sperm. Aquaculture 492, 306–320. ( 10.1016/j.aquaculture.2018.04.022) [DOI] [Google Scholar]
- 70.Guerra SM, Valcarce DG, Cabrita E, Robles V. 2013. Analysis of transcripts in gilthead seabream sperm and zebrafish testicular cells: mRNA profile as a predictor of gamete quality. Aquaculture 406–407, 28–33. ( 10.1016/j.aquaculture.2013.04.032) [DOI] [Google Scholar]
- 71.Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN.. 2014. Oxidative stress and male reproductive health. Asian J. Androl. 16, 31–38. ( 10.4103/1008-682X.122203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pérez-Cerezales S, Martínez-Páramo S, Beirão J, Herráez MP. 2010. Fertilization capacity with rainbow trout DNA-damaged sperm and embryo developmental success. Reproduction 139, 989–997. ( 10.1530/REP-10-0037) [DOI] [PubMed] [Google Scholar]
- 73.Hourcade JD, Pérez-Crespo M, Fernández-González R, Pintado B, Gutiérrez-Adán A. 2010. Selection against spermatozoa with fragmented DNA after postovulatory mating depends on the type of damage. Reprod. Biol. Endocrinol. 8, 9 ( 10.1186/1477-7827-8-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernández-Díez C, González-Rojo S, Lombó M, Herráez MP. 2018. Tolerance to paternal genotoxic damage promotes survival during embryo development in zebrafish (Danio rerio). Biol. Open 7, bio030130 ( 10.1242/bio.030130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ménézo Y, Dale B, Cohen M. 2010. DNA damage and repair in human oocytes and embryos: a review. Zygote 18, 357–365. ( 10.1017/S0967199410000286) [DOI] [PubMed] [Google Scholar]
- 76.Aitken RJ, Krausz C. 2001. Oxidative stress, DNA damage and the Y chromosome. Reproduction 122, 497–506. ( 10.1530/rep.0.1220497) [DOI] [PubMed] [Google Scholar]
- 77.Gavriliouk D, Aitken RJ. 2015. Damage to sperm DNA mediated by reactive oxygen species: its impact on human reproduction and the health trajectory of offspring. In The male role in pregnancy loss and embryo implantation failure (ed. Bronson R), pp. 23–47. Cham, Switzerland: Springer. [DOI] [PubMed] [Google Scholar]
- 78.Herráez MP, Ausió J, Devaux A, González-Rojo S, Fernández-Díez C, Bony S, Saperas N, Robles V. 2017. Paternal contribution to development: sperm genetic damage and repair in fish. Aquaculture 472, 45–59. ( 10.1016/j.aquaculture.2016.03.007) [DOI] [Google Scholar]
- 79.Yamauchi Y, Shaman JA, Ward WS. 2011. Non-genetic contributions of the sperm nucleus to embryonic development. Asian J. Androl. 13, 31 ( 10.1038/aja.2010.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teperek M, et al. 2016. Sperm is epigenetically programmed to regulate gene transcription in embryos. Genome Res. 26, 1034–1046. ( 10.1101/gr.201541.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, Gillot I, Cuzin F. 2006. RNA-mediated non-Mendelian inheritance of an epigenetic change in the mouse. Nature 441, 469–474. ( 10.1038/nature04674) [DOI] [PubMed] [Google Scholar]
- 82.Bermejo R, Kumar A, Foiani M. 2012. Preserving the genome by regulating chromatin association with the nuclear envelope. Trends Cell Biol. 22, 465–473. ( 10.1016/j.tcb.2012.05.007) [DOI] [PubMed] [Google Scholar]
- 83.Riesco MF, Robles V. 2013. Cryopreservation causes genetic and epigenetic changes in zebrafish genital ridges. PLoS ONE 8, e67614 ( 10.1371/journal.pone.0067614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nusbaumer D, Marques da Cunha L, Wedekind C.. 2019. Data from: Sperm cryopreservation reduces offspring growth Dryad Digital Repository. ( 10.5061/dryad.q6t8k07) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nusbaumer D, Marques da Cunha L, Wedekind C.. 2019. Data from: Sperm cryopreservation reduces offspring growth Dryad Digital Repository. ( 10.5061/dryad.q6t8k07) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data used in this study are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.q6t8k07 [84].