Abstract
Life history is a robust correlate of relative brain size: larger-brained mammals and birds have slower life histories and longer lifespans than smaller-brained species. The cognitive buffer hypothesis (CBH) proposes an adaptive explanation for this relationship: large brains may permit greater behavioural flexibility and thereby buffer the animal from unpredictable environmental challenges, allowing for reduced mortality and increased lifespan. By contrast, the developmental costs hypothesis (DCH) suggests that life-history correlates of brain size reflect the extension of maturational processes needed to accommodate the evolution of large brains, predicting correlations with pre-adult life-history phases. Here, we test novel predictions of the hypotheses in primates applied to the neocortex and cerebellum, two major brain structures with distinct developmental trajectories. While neocortical growth is allocated primarily to pre-natal development, the cerebellum exhibits relatively substantial post-natal growth. Consistent with the DCH, neocortical expansion is related primarily to extended gestation while cerebellar expansion to extended post-natal development, particularly the juvenile period. Contrary to the CBH, adult lifespan explains relatively little variance in the whole brain or neocortex volume once pre-adult life-history phases are accounted for. Only the cerebellum shows a relationship with lifespan after accounting for developmental periods. Our results substantiate and elaborate on the role of maternal investment and offspring development in brain evolution, suggest that brain components can evolve partly independently through modifications of distinct developmental phases, and imply that environmental input during post-natal maturation may be particularly crucial for the development of cerebellar function. They also suggest that relatively extended post-natal maturation times provide a developmental mechanism for the marked expansion of the cerebellum in the apes.
Keywords: life history, evolution, mosaic brain evolution, developmental costs, cognitive buffering, neocortex, cerebellum
1. Introduction
Extended lifespan is one of the most consistent correlates of large brain size across mammal species (e.g. [1,2]). Despite being first identified over 100 years ago [1] and confirmed by multiple comparative analyses since (e.g. [2,3]), the biological significance of the brain size–lifespan correlation remains uncertain. The cognitive buffer hypothesis (hereafter ‘CBH') posits that larger brains bestow behavioural flexibility, which in turn reduces mortality by enabling individuals to adjust to environmental contingency and unpredictability, and that this reduction in mortality facilitates longer lifespans [1,2,4,5]. A variant of the CBH proposes an alternative causal scenario in which longer lifespans create conditions favouring the evolution of enlarged brains, because species with longer reproductive periods have greater opportunities to reap the benefits of investment in learning during development [4], termed the ‘delayed benefits' hypothesis [3,5,6]. Under the umbrella of the CBH, various elements of behavioural flexibility are emphasized as beneficial for survival in variable environments, including innovation [4], complex foraging strategies [7] and social learning [8]. Common to all these ideas, however, is the prediction that across species there should be a direct association between relatively enlarged brains and longer lifespans [5].
In addition to adaptive benefits, however, it is increasingly recognized that large brains impose costs, which may provide a sufficient explanation for the positive correlation between brain size and lifespan. The developmental costs hypothesis (hereafter ‘DCH') [9], together with the related maternal energy hypothesis [10,11], proposes that life-history correlates of brain size reflect the need to extend development and maternal investment in order to build a large brain. Previous comparative analyses have provided support for this hypothesis: across mammals, pre-natal brain growth correlates specifically with gestation duration, while post-natal brain growth correlates specifically with lactation duration. Once these effects are accounted for, the brain size–lifespan correlation becomes non-significant, suggesting it is a side effect of developmental costs [9]. Positive correlations between adult brain size and pre- and post-natal developmental periods in birds are also consistent with the DCH [12]. An additional ‘costs-based' hypothesis, the expensive brain framework, proposes a trade-off between the energetic costs of large brains and reproduction, such that large-brained species must spread higher costs of reproduction over longer lifespans [11]. Common to both the expensive brain hypothesis and the DCH is the prediction that the brain size–lifespan correlation is indirect, mediated by relationships of both variables to protracted developmental periods.
To date, tests of these hypotheses have focused on variation in whole brain size. However, the brain is composed of functionally and anatomically heterogeneous structures which show heterochronicity in their developmental scheduling [13–16], influenced by structure-specific genes [17]. The DCH therefore predicts that different brain structures should have specific developmental correlates across species. A comparative analysis in primates provides general support for this idea, finding that some brain components correlate more strongly with lifespan and some with age at first reproduction [1]. However, this study did not examine specific developmental periods relevant to different aspects of brain growth, nor did it explicitly consider contrasting predictions made by costs-based and adaptive hypotheses. Furthermore, it did not control for phylogenetic non-independence in comparative data [1].
Here, we test the predictions of the DCH and CBH by examining life-history correlates of brain size and the size of two major brain structures which together make up a substantial proportion of total brain size, and which have expanded relative to other structures during primate evolution: the neocortex and cerebellum [18]. These two structures have to some extent evolved in a coordinated fashion, congruent with their anatomical and functional connectivity [19], but also partly independently, in a mosaic fashion, with a notable acceleration in the rate of cerebellar expansion in the ape clade [20]. These patterns of coordinated and mosaic brain component evolution are reflected in patterns of change at the molecular level, with similar numbers of changes in genes annotated during neocortical and cerebellar ontogeny in non-ape anthropoids, but more changes in cerebellar than neocortical genes during ape evolution [17]. These phenotypic and genetic patterns imply that developmental mechanisms can be adjusted to facilitate a complex pattern of both coordinated and mosaic evolution of the cerebellum and neocortex.
Neurodevelopmental studies on humans and other primates suggest that one ontogenetic mechanism facilitating divergent evolution of these two structures is a substantial difference in the allocation of growth to pre- versus post-natal phases. While growth and neurogenesis of the neocortex is predominantly pre-natal, the cerebellum exhibits relatively rapid and prolonged post-natal neurogenesis and volumetric growth [21,22]. At birth, the human cerebellum is approximately 25% of its volume at 2 years of age, increasing by 240% in the first year post-natally, while the neonatal neocortex is already 46% of its volume at 2 years, increasing by a relatively modest 88% in the first year after birth [21]. In terms of neurogenesis, the cerebellum is unusual in that the large majority (85%) of human cerebellar granule cells—the most numerous class of neurons in the brain—are generated post-natally [22]. Post-natal growth of the human cerebellum is particularly extended relative to the cortex, attaining its peak volume at around 13.5 years [23]. By contrast, the cortex reaches this milestone almost 5 years earlier at approximately 8.7 years [24].
Based on the literature discussed, we derive and test the following predictions. The CBH predicts that overall brain volume will be positively associated with lifespan, even when accounting for the effects of other life-history phases. While previous tests of the CBH have focused on whole brain volume, some authors have suggested it may apply specifically to the neocortex, on the assumption that this region is particularly implicated in aspects of behavioural flexibility [5] such as innovation [25,26], while the cerebellum has not been predicted to play a major role. The DCH, by contrast, predicts that lifespan will not be positively associated with brain or brain component volumes after accounting for developmental effects. Further, owing to the differential allocation of developmental costs to pre- versus post-natal phases between the neocortex and cerebellum respectively, the DCH predicts that post-natal developmental periods (lactation duration and juvenile period) will be more strongly associated with cerebellum than with neocortex volume, and, vice versa, pre-natal life-history phases (gestation) will be more strongly associated with neocortex than with cerebellum volume. Given the markedly protracted post-natal development of the cerebellum, the DCH implies that the evolution of extended post-natal development in primates may be explained by the developmental costs associated with growing and maturing a large cerebellum in particular. Therefore, the DCH also predicts that apes, a group with substantially expanded cerebella [20], will have significantly longer post-natal developmental periods than other primates, after accounting for allometric effects.
2. Methods
(a). Brain volume data
We obtained whole brain, neocortex and cerebellum volumes (in mm3) for anthropoid species from an existing compilation [20], together with additional data for non-anthropoids compiled by the same authors (originally from [27,28]), for 55 species. We analysed overall brain volume in addition to structure volumes to allow comparisons with previous work which has primarily investigated the whole brain (e.g. [1,2,8,9]), and to give context to structure analyses in terms of how relationships between structure sizes and life history may be related to overall brain size. We did not use measures from a recent comparative dataset based on MRI scans [29] due to incompatible measures of neocortex volume (neocortical grey matter only in [29] versus whole neocortex volume in [20]; see [30]).
(b). Life-history data
We obtained life-history data from the PanTHERIA [31] and AnAge databases [32]. For most life-history traits—body mass (grams), gestation length (days), weaning age (days) and age at first birth (days)—we prioritized data from PanTHERIA, supplementing missing values with data from AnAge where possible. AnAge provides age of female sexual maturity rather than age of first birth estimates, but the two are very closely correlated among species with data for both variables (PGLS: β = 0.85, p < 0.001, λ = 0.00, n = 43). For lifespan data (estimated as maximum longevity), we prioritized records from AnAge due to higher data quality and longer estimates for many species compared with other datasets [33]. Longevity records were converted from years (AnAge) or months (PanTHERIA) to days for comparability between datasets and with other life-history traits. Some of these life-history variables represent phases of life nested within one another (i.e. weaning age within age at first birth, age at first birth within lifespan). To avoid autocorrelation when including multiple life-history predictors in the same model, we calculated two additional life-history variables that do not overlap with any other for use in analyses: juvenile period and adult lifespan. We calculated juvenile period length by subtracting weaning age from age at first birth, and adult lifespan by subtracting age at first birth from maximum longevity.
After matching species across different datasets and to the 10ktrees primate phylogeny [34], the main sample contained 48 species (excluding humans) with complete data on all life history and brain volume variables. This dataset is available in the electronic supplementary material.
(c). Statistical analyses
(i). Phylogenetic comparative methods
We tested predictions using comparative statistical methods that account for the influence of phylogeny, specifically phylogenetic generalized least-squares (PGLS) regression using functions from the caper R package [35]. We used a consensus phylogeny from 10ktrees [34]. Pagel's lambda (λ), a measure of phylogenetic signal, was estimated by maximum likelihood. All continuous variables were log10 transformed to reduce positive skew and improve fit to statistical assumptions. In all analyses, we treated brain or brain structure volumes as the outcome variable and life-history traits as the predictors. Additionally, in all analyses, we included body mass to control for allometric scaling of both brain structure volumes and life-history traits with body size [36–38]. For analyses of structure volumes, we did not attempt to additionally control for remaining brain volume for both theoretical and statistical reasons. The DCH rests on the assumption that additional neural tissue, relative to body mass (reflective of energetic capacity), requires longer developmental periods [9], and therefore does not make direct predictions about the size of brain components relative to one another. We also focused on the size of brain components relative to body mass in order to facilitate direct comparisons with prior tests of the DCH and CBH which have examined whole brain relative to body mass (e.g. [2–4,8,9]). Further, measures of remaining brain volume are too highly correlated with body mass to obtain confident estimates of the independent contributions of both remaining brain and body size to structure volumes. Variance inflation factors (VIFs) were at least 15 when both body mass and remaining brain volume were included as predictors of individual structures, exceeding commonly used thresholds for problematic levels of collinearity (usually 5 or 10 [39]).
We tested predictions by examining coefficients reported in global models and by using model comparison to identify more parsimonious models. For each brain volume measure, we first fitted a global model including all four life-history traits plus body mass as predictor variables. Model performance was deemed acceptable for all global models based on visual examination of diagnostic plots. VIFs for the global model ranged from 2.04 to 5.20, indicating moderate to potentially problematic levels of collinearity (although thresholds vary widely in practice) [39]. Then for each brain measure, we created a candidate set of models using functions from the R package MuMIn [40]. Candidate sets consisted of the full model, a null allometric (body mass only) model and models containing all possible combinations of one to three life-history predictors (total n = 16 models for each structure). Body mass was included in all candidate models to account for allometric relationships with brain volumes and life-history variables. Comparing PGLS models is complicated by the effect of phylogeny, since both changes in the predictor variables and the influence of phylogeny can affect model fit. Therefore, to simplify interpretation of life-history effects, we fixed λ across all models in the candidate set for a given structure to the same value as that estimated by maximum likelihood in the global model (as recommended in [35]). We used Bayesian information criterion (BIC) rather than AIC(c) scores to rank models, as the former applies a higher penalty for additional parameters and is thus better suited to identifying the most parsimonious model [41]. We report selected effect sizes to aid the interpretation of the relative effects of life-history phases, illustrating comparisons that are particularly important in relation to predictions. We compare effects only between growth periods (i.e. gestation, lactation and juvenile period) as these comparisons are most biologically meaningful, and only when at least one of these variables has a significant or marginal effect (p < 0.10) in global models. Effect sizes for selected parameters were estimated by raising 10 to the power of their coefficients from the global models, which gives the amount of change in the dependent variable for a unit change a given predictor variable on the same scale as the data (e.g. number of additional mm3 in neocortex volume for an additional day of gestation), assuming the effects of all other predictors are held constant.
(ii). Differences between apes and other primates
We assessed the potential influence of apes (hominoidea) on the relationship between the cerebellum and life-history variables in two different ways. First, we tested for differences in life-history traits relative to body mass between apes and non-apes by fitting models predicting each life-history trait in turn from body mass and a factor representing membership of the ape clade (following [20] which used this approach to examine such ‘grade shifts' in cerebellar evolution). Here, we compared models in which either both slopes and intercepts, or intercepts only, are allowed to vary between apes and non-apes, using BIC scores. Second, we re-ran the global cerebellum model removing ape species (n = 5) from the sample. To establish whether any differences in results were due specifically to removing apes versus reduced statistical power, we also re-ran the model 1000 times, removing five random non-ape species from the sample at each iteration. If the relationship between cerebellum volume and a particular life-history variable is strongly contingent on the apes, we should expect that it generally remains significant when five random non-ape species are removed, but not when the five apes are removed.
3. Results
Figure 1 summarizes the results of global models for all brain volume measures. Global models are reported in full in tables 1–3 while model selection tables are included in the electronic supplementary material, tables S1–S3.
Figure 1.
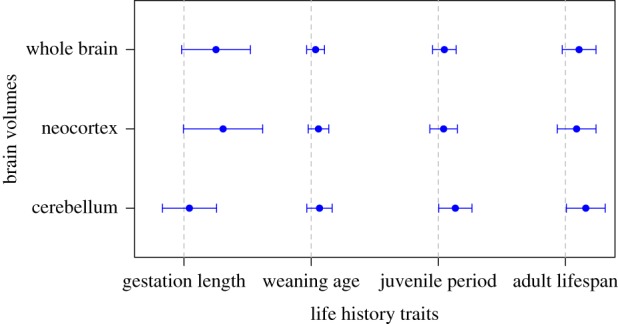
PGLS regression coefficients (points) with 95% confidence intervals (whiskers) for life-history predictors of brain structure volumes, from global models. Dashed vertical lines indicate zero. Each row corresponds to a separate global model in which the brain structure volume on the y-axis is the outcome variable, predicted by the four life-history traits on the x-axis plus body mass (not shown). (Online version in colour.)
Table 1.
Results of the global model for brain volume, predicted by all four life-history traits and body mass (n = 48, R2 = 0.88, λ = 1).
| parameter | estimate | s.e. | T-value | p-value |
|---|---|---|---|---|
| intercept | 0.28 | 0.72 | 0.39 | 0.70 |
| gestation | 0.50 | 0.28 | 1.82 | 0.08 |
| lactation | 0.07 | 0.07 | 0.97 | 0.34 |
| juvenile period | 0.10 | 0.10 | 1.00 | 0.32 |
| adult lifespan | 0.22 | 0.14 | 1.59 | 0.12 |
| body mass | 0.51 | 0.05 | 10.10 | <0.001 |
Table 2.
Results of the global model for neocortex volume, predicted by all four life-history traits and body mass (n = 48, R2 = 0.87, λ = 1).
| parameter | estimate | s.e. | T-value | p-value |
|---|---|---|---|---|
| intercept | −0.19 | 0.83 | −0.23 | 0.82 |
| gestation | 0.61 | 0.32 | 1.93 | 0.06 |
| lactation | 0.12 | 0.08 | 1.39 | 0.17 |
| juvenile period | 0.08 | 0.11 | 0.76 | 0.45 |
| adult lifespan | 0.18 | 0.16 | 1.13 | 0.27 |
| body mass | 0.53 | 0.06 | 9.07 | <0.001 |
Table 3.
Results of the global model for cerebellum volume, predicted by all four life-history traits and body mass (n = 48, R2 = 0.96, λ = 0).
| parameter | estimate | s.e. | T-value | p-value |
|---|---|---|---|---|
| intercept | −0.94 | 0.65 | −1.44 | 0.16 |
| gestation | 0.08 | 0.22 | 0.39 | 0.70 |
| lactation | 0.13 | 0.10 | 1.29 | 0.20 |
| juvenile period | 0.27 | 0.13 | 2.01 | 0.05 |
| adult lifespan | 0.32 | 0.16 | 2.06 | 0.05 |
| body mass | 0.58 | 0.05 | 11.03 | <0.001 |
(a). Cognitive buffer hypothesis
Contrary to the predictions of the CBH, we do not find a strong effect of adult lifespan on whole brain volume in the global model, in which the effects of pre- and post-natal development are taken into account (table 1). In model comparisons, adult lifespan is retained in the best-supported model for overall brain volume, but the second-ranked model, containing gestation length only, is similarly well supported (electronic supplementary material, table S1). We also find little support for the predictions of the CBH as applied to the neocortex. Gestation length is the strongest predictor of neocortex volume in the global model, while adult lifespan has little effect (table 2). In model comparisons, adult lifespan is absent from the three highest-ranking models (electronic supplementary material, table S2). We do, however, find a significant effect of adult lifespan on cerebellum volume in the global model, after accounting for developmental periods (table 3). In model comparisons, adult lifespan is included in the model with the lowest BIC score for cerebellum volume (electronic supplementary material, table S3), although the second-ranked, similarly supported model does not contain adult lifespan.
(b). Developmental costs hypothesis
In the global model, gestation length is the strongest predictor of whole brain volume, while lactation and juvenile period have negligible effects (table 1). In model comparisons, the two highest-ranked models retain gestation length, but not lactation duration or juvenile period (electronic supplementary material, table S1). Effect sizes from the model predict that adult brain volume increases by 3.18 mm3 for each additional day of gestation, and by 1.17 mm3 for each additional day of lactation, for a species with average life-history traits and body mass. Gestation length is the only near-significant predictor of neocortex volume in the global model, while lactation and juvenile period have little to no effects (table 2). Gestation length is the sole predictor included in the top-ranked model for neocortex volume, although the second highest-ranking model contains both gestation length and weaning age (electronic supplementary material, table S2). Effect sizes predict a greater increase in neocortex volume for each additional day of gestation (4.11 mm3) than lactation (1.30 mm3), all else being equal. Conversely, cerebellum volume significantly increases with juvenile period in the global model, while gestation and lactation have negligible effects (table 3). Juvenile period is retained in the five top-ranked models for cerebellum volume, and is the sole predictor in the second highest-ranked model (electronic supplementary material, table S3). Effect sizes from the global model suggest that for an average species, every additional day of life prior to first reproduction is associated with an increase of 1.85 mm3 in cerebellum volume. We obtain estimates of zero phylogenetic signal in global models of cerebellum volume (table 3). While a lack of phylogenetic signal is unexpected for evolutionarily conserved traits such as brain structure volumes, we show in supplementary analyses that this is unlikely to be the result of statistical artefacts (electronic supplementary material, appendix).
(c). Differences between apes and other primates
Relative to their body sizes, apes have significantly longer lactation and juvenile periods, and marginally longer adult lifespans, than non-apes (tables 4–6; figures 2 and 3). By contrast, apes do not significantly differ from non-apes in relative gestation time (table 7). For all life-history variables, BIC scores favoured intercept-only models over those in which both slopes and intercepts were allowed to vary between the clades (table 8). When re-running the global cerebellum model without apes (n = 43), the association between juvenile period and cerebellum volume becomes non-significant (table 9). However, removing five randomly selected non-ape species from the sample also often results in a weakened relationship: p-values for juvenile period are 0.05 or greater in 64.7% of 1000 iterations. This suggests that the relationship between the juvenile period and cerebellum volume is not solely contingent on the ape clade.
Table 4.
Results of the intercept-only model comparing lactation duration relative to body mass in ape versus non-ape species (n = 48, R2 = 0.75, λ = 0.05).
| parameter | estimate | s.e. | T-value | p-value |
|---|---|---|---|---|
| intercept | 0.91 | 0.15 | 6.12 | <0.001 |
| body mass | 0.40 | 0.05 | 8.61 | <0.001 |
| ape v. non-ape | 0.27 | 0.11 | 2.46 | 0.02 |
Table 5.
Results of the intercept-only model comparing juvenile period length relative to body mass in ape versus non-ape species (n = 48, R2 = 0.53, λ = 0.26).
| parameter | estimate | s.e. | T-value | p-value |
|---|---|---|---|---|
| intercept | 2.32 | 0.13 | 18.58 | <0.001 |
| body mass | 0.19 | 0.04 | 4.84 | <0.001 |
| ape v. non-ape | 0.24 | 0.09 | 2.56 | 0.01 |
Table 6.
Results of the intercept-only model comparing adult lifespan relative to body mass in ape versus non-ape species (n = 48, R2 = 0.52, λ = 0).
| parameter | estimate | s.e. | T-value | p-value |
|---|---|---|---|---|
| intercept | 3.56 | 0.09 | 40.45 | <0.001 |
| body mass | 0.12 | 0.03 | 4.51 | <0.001 |
| ape v. non-ape | 0.13 | 0.07 | 1.96 | 0.06 |
Figure 2.
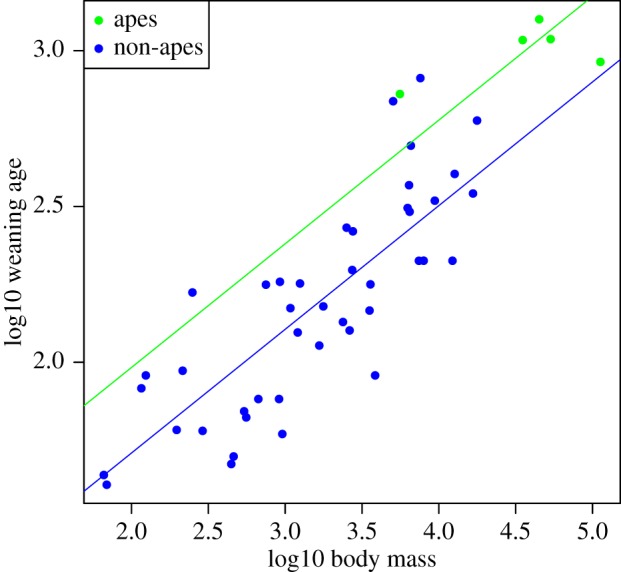
Raw data (points) and regression slopes (lines) from a model predicting lactation duration from body mass, fitting separate intercepts for ape (green) and non-ape species (blue). Apes have significantly longer lactation periods relative to their body size than do non-apes.
Figure 3.
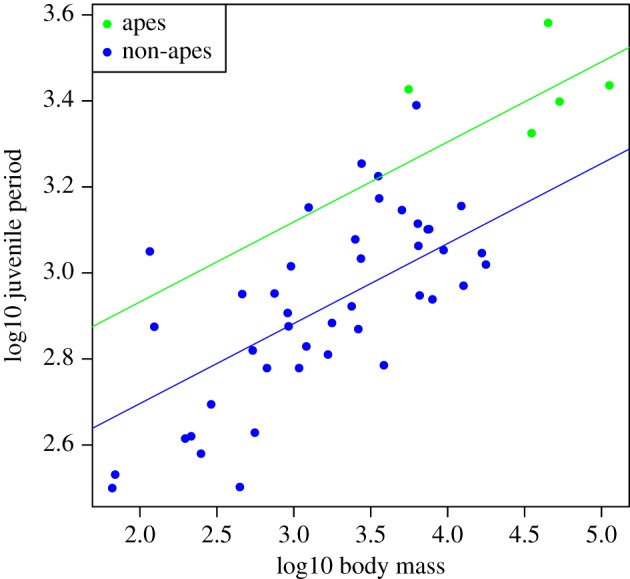
Raw data (points) and regression slopes (lines) from a model predicting juvenile period length from body mass, fitting separate intercepts for ape (green) and non-ape species (blue). Apes have significantly longer juvenile periods relative to their body size than do non-apes.
Table 7.
Results of the intercept-only model comparing gestation length relative to body mass in ape versus non-ape species (n = 48, R2 = 0.34, λ = 1).
| parameter | estimate | s.e. | T-value | p-value |
|---|---|---|---|---|
| intercept | 1.93 | 0.08 | 25.55 | <0.001 |
| body mass | 0.09 | 0.02 | 4.26 | <0.001 |
| ape versus non-ape | 0.08 | 0.07 | 1.19 | 0.24 |
Table 8.
BIC scores for models comparing gestation, lactation, juvenile period and adult lifespan relative to body size between apes and non-apes. In intercept-only models, only intercepts were allowed to vary between apes and non-apes, while in different slopes models, both intercepts and slopes were allowed to vary between apes and non-apes.
| outcome variable | intercept-only | different slopes |
|---|---|---|
| gestation | −121.79 | −118.06 |
| lactation | −11.80 | −10.21 |
| juvenile period | −37.60 | −35.26 |
| adult lifespan | −59.57 | −56.47 |
Table 9.
Results of the global model for cerebellum volume repeated without the inclusion of ape species (n = 43, R2 = 0.93, λ = 0).
| parameter | estimate | s.e. | T-value | p-value |
|---|---|---|---|---|
| intercept | −0.67 | 0.72 | −0.92 | 0.36 |
| gestation | 0.09 | 0.23 | 0.38 | 0.70 |
| lactation | 0.09 | 0.11 | 0.79 | 0.44 |
| juvenile period | 0.25 | 0.15 | 1.70 | 0.10 |
| adult lifespan | 0.28 | 0.17 | 1.67 | 0.10 |
| body mass | 0.59 | 0.06 | 10.19 | <0.001 |
4. Discussion
Rather than a general extension of lifespan in large-brained species, we find that specific aspects of life history are correlated with the volumes of different structures according to their developmental trajectories. Our results are therefore primarily consistent with predictions of the DCH and Expensive brain hypothesis rather than the CBH, in that correlations between lifespan and brain size or neocortex volume appear to be by-products of the relationship of these structures with developmental periods. In support of the DCH more specifically, we find that brain structures with different emphases on pre-versus post-natal growth show predicted associations with those periods of investment. Maternal investment, specifically pre-natal investment, has an independent relationship with the relative volume of the neocortex. By contrast, cerebellum volume has an independent positive correlation with juvenile period length, congruent with the idea that interaction with the environment during maturation provides crucial input to the development of this structure, through play, for example. In summary, the correlation between brain size and life history in primates may require no specific adaptive explanation, instead reflecting the developmental mechanisms by which enlarged brains and brain components evolve.
(a). Cognitive buffer hypothesis
We do not find evidence of a strong, direct correlation between brain size and lifespan, contradicting a central prediction of the CBH. Rather, the association between the two is weak when controlling for other life-history phases. This finding is consistent with the interpretation that the brain size–lifespan correlation is confounded by the duration of maternal investment, as previously found across mammals [9] and more recently confirmed for primates in particular [42]. We also find no evidence to support a direct association between lifespan and neocortex volume, contradicting the idea that the neocortex plays a key role in extending lifespan via behavioural flexibility [5]. We do find, however, an independent positive association of the cerebellum with adult lifespan, unanticipated by prior literature on the CBH, which has focused on whole brain or neocortex volume. Our findings could therefore be construed as consistent with a cognitive buffering effect specifically of the cerebellum. In support of this interpretation, the cerebellum is increasingly recognized to play a role not only in fine motor control and coordination but a wide diversity of cognitive functions including working memory, planning and decision-making [18]. However, this finding is also consistent with the neuronal investment hypothesis, which posits that longer-lived animals require larger brain volumes to compensate for longer periods of decline in neuronal function over their lifetimes [5]. The cerebellum in particular may be implicated due to its potentially greater susceptibility to neuronal loss with age compared with other structures [5]. Further evidence, most crucially a relationship between cerebellum volume and survival, is thus required to distinguish between different explanations for this finding.
While we find little support for the CBH in terms of a direct link between overall brain size or neocortex size and lifespan in primates, our findings do not exclude the possibility that this hypothesis is supported by other lines of evidence and in other taxonomic groups. For example, primate species with larger brains experience less variation in net energy intake than expected based on environmental seasonality compared with smaller-brained species, consistent with a cognitive buffering effect [43]. Prior comparative work suggests that the extent to which brain size correlates with lifespan may vary between clades, finding greater support for the prediction among haplorrhine than strepsirrhine primates [1]. A more recent comparative study finds support for the correlation within primates and rodents, but not other mammalian orders [3]. Neither of these studies, however, accounted for the effects of developmental periods and therefore do not directly test the DCH. In birds, cognitive buffering effects are consistent with the findings that relatively large-brained species have lower adult mortality rates [44], experience more environmental variation [45] and have more stable populations in variable environments [46]. Directly comparable tests of the DCH in primates and birds would therefore be a productive avenue of future research.
(b). Developmental costs hypothesis
Our findings suggest that the relationship between lifespan and both overall brain volume and neocortical volume in primates is a by-product of maternal investment, primarily in pre-natal, rather than post-natal, offspring development. This result probably reflects the predominant role of pre-natal investment in neocortical growth specifically, given that this structure accounts for such a large proportion of overall brain volume. Consistent with this interpretation, and as predicted from the fact that neurogenesis and a relatively large proportion of neocortical growth is completed before birth, the life-history variable most strongly associated with adult neocortex size was gestation length. Our results suggest that evolutionary expansion of the neocortex is supported primarily by increased pre-natal investment, while cerebellar expansion requires greater investment in post-natal development. Despite the shared functional roles and correlated evolution of the cerebellum and neocortex [18–20,47–49], a degree of independent (or mosaic) evolution of the two structures has been documented [20]. Our results thus provide developmental mechanisms for the expansion of the neocortex and cerebellum, complementing evidence of distinct genetic mechanisms supporting such mosaic evolution [17]. The patterns may be yet more complex, however: since the neocortex is composed of many heterogeneous systems, an interesting avenue for future work would be to investigate whether specific neocortical components or tissue types correlate with different aspects of maternal investment. Indeed, developmental scheduling varies across the neocortex, with occipital grey matter maturing earlier than that in the prefrontal cortex [50]. Those specific areas and tissues which continue to grow post-natally may therefore be associated with post-natal developmental phases including lactation and juvenile period.
Adult cerebellum volume correlated positively with post-natal (juvenile) development, after accounting for variation in other life-history phases. This pattern fits with evidence indicating late volumetric growth and maturation of this structure, extending through infancy and beyond in humans [23,51,52]. At a cellular level, the post-natal genesis of the majority of cerebellar granule cells followed by synaptogenesis indicates high functional plasticity during this time, making environmental stimuli potentially critical in cerebellar maturation [22]. Further evidence for the importance of environmental input in cerebellar development includes the low heritability of cerebellum volume compared to that of other brain structures [53], and effects of an impoverished post-natal environment on the volume of superior-posterior cerebellar lobes [54]. Infancy and juvenility are periods of social learning, practice and play in an environment of reduced risk [55]. Behaviourally, play is correlated with cerebellum volume [56] and with the volume of structures comprising the cortico-cerebellar system [57] across primates, and within species, there are concurrent increases in the rate of play and formation of cerebellar synapses during post-natal development [58]. The correlation between play and both post-natal brain growth and behavioural flexibility in primates is thus likely to involve cerebellar maturation [59,60]. Converging lines of evidence therefore suggest that many environmental influences on post-natal learning and development may be mediated by effects on the cerebellum.
(c). Cerebellar expansion and extended maturation time in apes
When apes were removed from the PGLS analyses, the effect of juvenile period duration on cerebellum volume became non-significant. However, the same was true in the majority of cases when five random non-ape species were removed from the analyses, suggesting that this may be due to a loss of statistical power rather than contingency of results on the ape clade. We do, however, find that apes have a distinct life-history profile compared with other primates, with significantly longer lactation and juvenile periods, and marginally longer adult lifespans. Together with prior evidence of accelerated cerebellar expansion in the apes [20], these results suggest that apes may have evolved extended post-natal maturation in part due to the need to invest in development of a large cerebellum and the time required for its experience-dependent maturation. The absence of a difference in relative gestation duration between the apes and other primates further suggests that ape life histories are distinct specifically in terms of their post-natal developmental trajectories. Consistent with this interpretation, cerebellar expansion in the apes is largely driven by enlargement of the cerebellar hemispheres: late-developing structures that are strongly implicated in the organization and control of complex motor patterns [61,62]. Together, these results may help to explain the combination of unusually large cerebella [20], extended periods of immaturity [63], delayed locomotor independence [64], and high levels of social learning [65], play [66], extractive foraging and tool use [20] that characterizes the ape clade. Future comparative analyses could investigate whether similar developmental profiles help explain independent cerebellar expansion in other mammalian lineages, such as elephants and cetaceans [67].
5. Conclusion
Developmental costs appear to provide the best explanation for the pattern of correlations between primate brain structures and life history. The central prediction of the CBH was not supported by strong, direct associations of lifespan and whole brain or neocortex volume; instead, these structures correlated most strongly with gestation length. The cerebellum does correlate with lifespan after accounting for developmental periods, consistent with an unanticipated cognitive buffering effect of this structure in particular, although alternative explanations are possible. Overall, we provide the first evidence that primate brain components exhibit distinct life-history correlates that are congruent with their divergent developmental profiles. These divergent patterns support the view that selection on particular functional capacities can result in mosaic brain evolution mediated by complex developmental mechanisms [68].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
All data and code associated with this paper are available in the electronic supplementary material.
Authors' contributions
L.E.P. conceived and designed the study, performed the original analyses and wrote the paper. R.A.B. co-conceived and co-developed the study, advised on all stages of the research and helped draft the manuscript. S.E.S. reanalysed the data and revised the paper for resubmission. All authors commented on manuscript drafts and contributed to the study throughout the research process. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
Financial support for the study was provided to L.E.P. from the Durham University Postgraduate Publication Bursary Scheme.
References
- 1.Allman J, Mclaughlin T, Hakeem A. 1993. Brain structures and life-span in primate species. Neurobiology 90, 3559–3563. ( 10.1073/pnas.90.8.3559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Lagos C, Sol D, Reader SM. 2010. Large-brained mammals live longer. J. Evol. Biol. 23, 1064–1074. ( 10.1111/j.1420-9101.2010.01976.x) [DOI] [PubMed] [Google Scholar]
- 3.DeCasien AR, Thompson NA, Williams SA, Shattuck MR. 2018. Encephalization and longevity evolved in a correlated fashion in Euarchontoglires but not in other mammals. Evolution 72, 2617–2631. ( 10.1111/evo.13633) [DOI] [PubMed] [Google Scholar]
- 4.Sol D. 2009. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol. Lett. 5, 130–133. ( 10.1098/rsbl.2008.0621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deaner RO, Barton RA, van Schaik CP. 2003. Primate brains and life histories: renewing the connection. In Primate life histories and socioecology (eds Kappeler PM, Pereira ME), pp. 233–265. Chicago, IL: University of Chicago Press. [Google Scholar]
- 6.Eliassen S, Jørgensen C, Mangel M, Giske J. 2007. Exploration or exploitation: life expectancy changes the value of learning in foraging strategies. Oikos 116, 513–523. ( 10.1111/j.2006.0030-1299.15462.x) [DOI] [Google Scholar]
- 7.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. () [DOI] [Google Scholar]
- 8.Street SE, Navarrete AF, Reader SM, Laland KN. 2017. Coevolution of cultural intelligence, extended life history, sociality, and brain size in primates. Proc. Natl Acad. Sci. USA 114, 7908–7914. ( 10.1073/pnas.1620734114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton RA, Capellini I. 2011. Maternal investment, life histories, and the costs of brain growth in mammals. Proc. Natl Acad. Sci. USA 108, 6169–6174. ( 10.1073/pnas.1019140108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RD. 1996. Scaling of the mammalian brain: the maternal energy hypothesis. News Physiol. Sci. 11, 149–156. [Google Scholar]
- 11.Isler K, van Schaik CP. 2009. The expensive brain: a framework for explaining evolutionary changes in brain size. J. Hum. Evol. 57, 392–400. ( 10.1016/j.jhevol.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 12.Iwaniuk AN, Nelson JE. 2003. Developmental differences are correlated with relative brain size in birds: a comparative analysis. Can. J. Zool. 81, 1913–1928. ( 10.1139/z03-190) [DOI] [Google Scholar]
- 13.Sherwood CC, Omez-Robles A. 2017. Brain plasticity and human evolution. Annu. Rev. Anthropol. 46, 399–419. ( 10.1146/annurev-anthro-102215-100009) [DOI] [Google Scholar]
- 14.Charvet CJ, Finlay BL. 2012. Embracing covariation in brain evolution: large brains, extended development, and flexible primate social systems. Prog. Brain Res. 195, 71–87. ( 10.1016/B978-0-444-53860-4.00004-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. 2013. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. 33, 7368–7383. ( 10.1523/JNEUROSCI.5746-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 387, 167–178. () [DOI] [PubMed] [Google Scholar]
- 17.Harrison PW, Montgomery SH. 2017. Genetics of cerebellar and neocortical expansion in anthropoid primates: a comparative approach. Brain Behav. Evol. 89, 274–285. ( 10.1159/000477432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton RA. 2012. Embodied cognitive evolution and the cerebellum. Phil. Trans. R. Soc. B 367, 2097–2107. ( 10.1098/rstb.2012.0112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting BA, Barton RA. 2003. The evolution of the cortico-cerebellar complex in primates: anatomical connections predict patterns of correlated evolution. J. Hum. Evol. 44, 3–10. ( 10.1016/S0047-2484(02)00162-8) [DOI] [PubMed] [Google Scholar]
- 20.Barton RA, Venditti C. 2014. Rapid evolution of the cerebellum in humans and other great apes. Curr. Biol. 24, 2440–2444. ( 10.1016/j.cub.2014.08.056) [DOI] [PubMed] [Google Scholar]
- 21.DeVito J, Graham J, Schultz G, Sundsten J, Prothero J. 1986. Morphometry of the developing brain in Macaca nemestrina. In Ontogeny, cognition and social behaviour of primates (eds Lee PC, Else JG), pp. 131–139. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Kiessling MC, Büttner A, Butti C, Müller-Starck J, Milz S, Hof PR, Frank HG, Schmitz C. 2014. Cerebellar granule cells are generated postnatally in humans. Brain Struct. Funct. 219, 1271–1286. ( 10.1007/s00429-013-0565-z) [DOI] [PubMed] [Google Scholar]
- 23.Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. 2010. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage 49, 63–70. ( 10.1016/j.neuroimage.2009.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. 2011. How does your cortex grow? J. Neurosci. 31, 7174–7177. ( 10.1523/JNEUROSCI.0054-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reader SM, Hager Y, Laland KN. 2011. The evolution of primate general and cultural intelligence. Phil. Trans. R. Soc. B 366, 1017–1027. ( 10.1098/rstb.2010.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reader SM, Laland KN. 2002. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl Acad. Sci. USA 99, 4436–4441. ( 10.1073/pnas.062041299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephan H, Frahm H, Baron G. 1981. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol. 35, 1–29. ( 10.1159/000155963) [DOI] [PubMed] [Google Scholar]
- 28.Bush EC, Allman JM. 2003. The scaling of white matter to gray matter in cerebellum and neocortex. Brain Behav. Evol. 61, 1–5. ( 10.1159/000068880) [DOI] [PubMed] [Google Scholar]
- 29.Navarrete AF, Blezer EL, Pagnotta M, de Viet ES, Todorov OS, Lindenfors P, Laland KN, Reader SM. 2018. Primate brain anatomy: new volumetric MRI measurements for neuroanatomical studies. Brain Behav. Evol. 91, 109–117. ( 10.1159/000488136) [DOI] [PubMed] [Google Scholar]
- 30.Navarrete AF, et al. 2018. Erratum: Primate brain anatomy: new volumetric MRI measurements for neuroanatomical studies. Brain Behav. Evol. 92, 182–184. ( 10.1159/000496658) [DOI] [PubMed] [Google Scholar]
- 31.Jones KE, et al. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648 ( 10.1890/08-1494.1) [DOI] [Google Scholar]
- 32.Tacutu R, et al. 2018. Human ageing genomic resources: new and updated databases. Nucleic Acids Res. 46(D1), D1083–D1090. ( 10.1093/nar/gkx1042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Magalhaes JP, Costa J. 2009. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 22, 1770–1774. ( 10.1111/j.1420-9101.2009.01783.x) [DOI] [PubMed] [Google Scholar]
- 34.Arnold C, Matthews LJ, Nunn CL. 2010. The 10kTrees website: a new online resource for primate phylogeny. Evol. Anthropol. 19, 114–118. ( 10.1002/evan.20251) [DOI] [Google Scholar]
- 35.Orme D, et al. 2013. caper: comparative analyses of phylogenetics and evolution in R. R package version 0.5.2 See http://cran.r-project.org/package=caper.
- 36.Freckleton RP. 2002. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. J. Anim. Ecol. 71, 542–545. ( 10.1046/j.1365-2656.2002.00618.x) [DOI] [Google Scholar]
- 37.García-berthou E. 2001. On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. J. Anim. Ecol. 70, 708–711. ( 10.1046/j.1365-2656.2001.00524.x) [DOI] [Google Scholar]
- 38.Smith RJ. 1999. Statistics of sexual size dimorphism. J. Hum. Evol. 36, 423–458. ( 10.1006/jhev.1998.0281) [DOI] [PubMed] [Google Scholar]
- 39.Mundry R. 2014. Statistical issues and assumptions of phylogenetic generalized least squares. In Modern phylogenetic comparative methods and their application in evolutionary biology (ed. Garamszegi LZ.), pp. 131–156. Berlin, Germany: Springer. [Google Scholar]
- 40.Bartoń K. 2018. MuMIn: multi-model inference. R package version 1.42.1. See https://cran.r-project.org/web/packages/MuMIn/index.html.
- 41.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 42.Street SE, Navarrete AF, Reader SM, Laland KN. 2019. Correction for Street et al., Coevolution of cultural intelligence, extended life history, sociality, and brain size in primates. Proc. Natl Acad. Sci. USA 116, 3929–3932. ( 10.1073/pnas.1900438116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Woerden JT, Willems EP, van Schaik CP, Isler K. 2012. Large brains buffer energetic effects of seasonal habitats in catarrhine primates. Evolution 66, 191–199. ( 10.1111/j.1558-5646.2011.01434.x) [DOI] [PubMed] [Google Scholar]
- 44.Sol D, Székely T, Liker A, Lefebvre L. 2007. Big-brained birds survive better in nature. Proc. R. Soc. B 274, 763–769. ( 10.1098/rspb.2006.3765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayol F, Maspons J, Lapiedra O, Iwaniuk AN, Székely T, Sol D. 2016. Environmental variation and the evolution of large brains in birds. Nat. Commun. 7, 13971 ( 10.1038/ncomms13971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fristoe TS, Iwaniuk AN, Botero CA. 2017. Big brains stabilize populations and facilitate colonization of variable habitats in birds. Nat. Ecol. Evol. 1, 1706–1715. ( 10.1038/s41559-017-0316-2) [DOI] [PubMed] [Google Scholar]
- 47.Herculano-Houzel S. 2010. Coordinated scaling of cortical and cerebellar numbers of neurons. Front. Neuroanat. 4, 1–8. ( 10.3389/fnana.2010.00012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smaers JB, Steele J, Zilles K. 2011. Modeling the evolution of cortico-cerebellar systems in primates. Ann. N Y Acad. Sci. 1225, 176–190. ( 10.1111/j.1749-6632.2011.06003.x) [DOI] [PubMed] [Google Scholar]
- 49.Lent R, Azevedo FAC, Andrade-Moraes CH, Pinto AVO. 2012. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur. J. Neurosci. 35, 1–9. ( 10.1111/j.1460-9568.2011.07923.x) [DOI] [PubMed] [Google Scholar]
- 50.Gilmore JH, et al. 2007. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci. 27, 1255–1260. ( 10.1523/JNEUROSCI.3339-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knickmeyer RC, et al. 2008. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 28, 12 176–12 182. ( 10.1523/JNEUROSCI.3479-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu KH, Chen CY, Shen EY. 2011. The cerebellar development in Chinese children—a study by voxel-based volume measurement of reconstructed 3D MRI scan. Pediatr. Res. 69, 80–83. ( 10.1203/PDR.0b013e3181ff2f6c) [DOI] [PubMed] [Google Scholar]
- 53.Giedd JN, Schmitt JE, Neale MC. 2007. Structural brain magnetic resonance imaging of pediatric twins. Hum. Brain Mapp. 28, 474–481. ( 10.1002/hbm.20403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. 2009. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biol. Psychiatry 66, 1100–1106. ( 10.1016/j.biopsych.2009.06.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burghardt GM. 2010. The comparative reach of play and brain: perspective, evidence, and implications. Am. J. Play 2, 338–356. [Google Scholar]
- 56.Lewis KP, Barton RA. 2001. Playing for keeps: evolutionary relationships between social play and the cerebellum in nonhuman primates. Hum. Nat. 15, 5–21. ( 10.1007/s12110-004-1001-0) [DOI] [PubMed] [Google Scholar]
- 57.Kerney M, Smaers JB, Schoenemann PT, Dunn JC. 2017. The coevolution of play and the cortico-cerebellar system in primates. Primates 58, 485–491. ( 10.1007/s10329-017-0615-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byers JA, Walker C. 1995. Refining the motor training hypothesis for the evolution of play. Am. Nat. 146, 25–40. ( 10.1086/285785) [DOI] [Google Scholar]
- 59.Montgomery SH. 2014. The relationship between play, brain growth and behavioural flexibility in primates. Anim. Behav. 90, 281–286. ( 10.1016/j.anbehav.2014.02.004) [DOI] [Google Scholar]
- 60.Pellis SM, Iwaniuk AN. 2000. Comparative analyses of the role of postnatal development on the expression of play fighting. Dev. Psychobiol 36, 136–147. () [DOI] [PubMed] [Google Scholar]
- 61.MacLeod CE, Zilles K, Schleicher A, Rilling JK, Gibson KR. 2003. Expansion of the neocerebellum in Hominoidea. J. Hum. Evol. 44, 401–429. ( 10.1016/S0047-2484(03)00028-9) [DOI] [PubMed] [Google Scholar]
- 62.Cantalupo C, Hopkins W. 2010. The cerebellum and its contribution to complex tasks in higher primates: a comparative perspective. Cortex 46, 821–830. ( 10.1016/j.cortex.2009.10.004) [DOI] [PubMed] [Google Scholar]
- 63.Kelley J. 2004. Life history and cognitive evolution in the apes. In The evolution of thought: evolutionary origins of great ape intelligence (eds Begun DR, Russon AE), pp. 280–297. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 64.Young JW, Shapiro LJ. 2018. Developments in development: what have we learned from primate locomotor ontogeny? Am. J. Phys. Anthropol. 165, 37–71. ( 10.1002/ajpa.23388) [DOI] [PubMed] [Google Scholar]
- 65.van Schaik CP, Burkart JM. 2011. Social learning and evolution: the cultural intelligence hypothesis. Phil. Trans. R. Soc. B 366, 1008–1016. ( 10.1098/rstb.2010.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramsey JK, McGrew WC. 2005. Object play in great apes: studies in nature and captivity. In The nature of play: great apes and humans (eds Pellegrini AD, Smith PK), pp. 89–138. New York, NY: Guilford Press. [Google Scholar]
- 67.Maseko BC, Spocter MA, Haagensen M, Manger PR. 2012. Elephants have relatively the largest cerebellum size of mammals. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 295, 661–672. ( 10.1002/ar.22425) [DOI] [PubMed] [Google Scholar]
- 68.Barton RA, Harvey PH. 2000. Mosaic evolution of brain structure in mammals. Nature 405, 1055–1058. ( 10.1038/35016580) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code associated with this paper are available in the electronic supplementary material.


