This cohort study examines the association of mobility impairment, measured during hospitalization, as a risk marker for functional decline among older patients with acute myocardial infarction.
Key Points
Question
Is mobility impairment, measured during hospitalization, a “geriatric biomarker” for functional decline among older adults with acute myocardial infarction?
Findings
In this cohort study of 2587 patients 75 years or older hospitalized for acute myocardial infarction, mobility impairment measured by the Timed “Up and Go” was significantly associated in a dose-response manner with risk of functional decline.
Meaning
This study’s findings suggest that a brief and easy-to-administer mobility assessment may be useful in the inpatient setting to identify older patients with acute myocardial infarction who are at risk for functional decline.
Abstract
Importance
Many older survivors of acute myocardial infarction (AMI) experience functional decline, an outcome of primary importance to older adults. Mobility impairment has been proposed as a risk factor for functional decline but has not been evaluated to date in older patients hospitalized for AMI.
Objective
To examine the association of mobility impairment, measured during hospitalization, as a risk marker for functional decline among older patients with AMI.
Design, Setting, and Participants
Prospective cohort study among 94 academic and community hospitals in the United States. Participants were 2587 hospitalized patients with AMI who were 75 years or older. The study dates were January 2013 to June 2017.
Main Outcomes and Measures
Mobility was evaluated during AMI hospitalization using the Timed “Up and Go,” with scores categorized as preserved mobility (≤15 seconds to complete), mild impairment (>15 to ≤25 seconds to complete), moderate impairment (>25 seconds to complete), and severe impairment (unable to complete). Self-reported function in activities of daily living (ADLs) (bathing, dressing, transferring, and walking around the home) and walking 0.4 km (one-quarter mile) was assessed at baseline and 6 months after discharge. The primary outcomes were worsening of 1 or more ADLs and loss of ability to walk 0.4 km from baseline to 6 months after discharge. The association between mobility impairment and risk of functional decline was evaluated with multivariable-adjusted logistic regression.
Results
Among 2587 hospitalized patients with AMI, the mean (SD) age was 81.4 (4.8) years, and 1462 (56.5%) were male. More than half of the cohort exhibited mobility impairment during AMI hospitalization (21.8% [564 of 2587] had mild impairment, 16.0% [414 of 2587] had moderate impairment, and 15.2% [391 of 2587] had severe impairment); 12.8% (332 of 2587) reported ADL decline, and 16.7% (431 of 2587) reported decline in 0.4-km mobility. Only 3.8% (30 of 800) of participants with preserved mobility experienced any ADL decline compared with 6.9% (39 of 564) of participants with mild impairment (adjusted odds ratio [aOR], 1.24; 95% CI, 0.74-2.09), 18.6% (77 of 414) of participants with moderate impairment (aOR, 2.67; 95% CI, 1.67-4.27), and 34.7% (136 of 391) of participants with severe impairment (aOR, 5.45; 95% CI, 3.29-9.01). Eleven percent (90 of 800) of participants with preserved mobility declined in ability to walk 0.4 km compared with 15.2% (85 of 558) of participants with mild impairment (aOR, 1.51; 95% CI, 1.04-2.20), 19.0% (78 of 411) of participants with moderate impairment (aOR, 2.03; 95% CI, 1.37-3.02), and 24.6% (95 of 386) of participants with severe impairment (aOR, 3.25; 95% CI, 2.02-5.23).
Conclusions and Relevance
This study’s findings suggest that mobility impairment assessed during hospitalization may be a potent risk marker for functional decline in older survivors of AMI. These findings also suggest that brief, validated assessments of mobility should be part of the care of older hospitalized patients with AMI to identify those at risk for this important patient-centered outcome.
Introduction
Acute myocardial infarction (AMI) is increasingly a condition of older adults, with more than one-third of AMIs occurring in patients 75 years or older.1 Owing to increased use of therapies such as percutaneous coronary intervention among older adults,2 survival after AMI in this age group has improved.3,4 However, many older survivors of AMI face another major challenge to their health, namely, functional decline (ie, loss of ability to independently perform everyday activities). Thirty percent of survivors of AMI report functional decline after AMI,5,6,7 and AMI is recognized as a common contributor to the development of disability in older adults.6,7,8,9,10 Functional decline, in addition to directly diminishing independence and quality of life,11 is a harbinger of poor outcomes, such as readmissions,12 institutionalization,13 and mortality.14
Maintenance of function is an outcome valued most by many older adults.15 Despite this, little is known about factors that put older adults at risk for functional decline after hospitalization for AMI. One common vulnerability experienced by many older hospitalized adults is impairment in mobility (ie, difficulty with transfers and ambulation).16 Research has linked poor mobility to increased risk of mortality,17 frailty,18 and functional decline,19 and emerging evidence suggests that mobility impairment is prevalent and predictive of poor outcomes among older patients with AMI.5,20 However, prior studies have been constrained by limited generalizability (eg, single site) or untimely collection of mobility data (ie, after hospitalization). Therefore, we explored the association between mobility, assessed during AMI hospitalization, and risk of functional decline at 6 months after discharge in a prospective nationwide cohort of adults 75 years or older.
Methods
Design, Setting, and Participants
Data were obtained from the Comprehensive Evaluation of Risk Factors in Older Patients With Acute Myocardial Infarction (SILVER-AMI) study, a prospective cohort study of 3041 adults 75 years or older hospitalized with AMI (ClinicalTrials.gov identifier R01HL115295). Details of the study have been published previously.21 The study dates were January 2013 to June 2017. Briefly, participants were recruited from 94 academic and community hospitals across the United States. Site coordinators reviewed hospital admission records daily to identify potentially eligible participants and performed medical record review to confirm AMI diagnosis in accord with the Third Universal Definition of Myocardial Infarction.22 Site coordinators approached eligible patients, explained the scope of the study, and obtained written informed consent. The University of California, San Diego Brief Assessment of Capacity to Consent23 was administered to patients with decisional capacity concerns, and proxy consent was obtained for patients with diminished capacity. Patients were ineligible if they had initial troponin elevation more than 24 hours after admission, were transferred from another hospital after more than 24 hours, were incarcerated, or were unable to provide informed consent, with no proxy available. All study protocols were approved by the institutional review boards at the coordinating site (Yale School of Medicine, New Haven, Connecticut) and all participating sites.
Baseline Assessment
Participants underwent a structured interview and physical assessment during AMI hospitalization. Information was collected on geriatric impairments (mobility, hearing, vision, cognition, etc), functional status, demographics, and psychosocial characteristics.
Mobility status was assessed during the baseline interview (median day of admission, day 2; interquartile range, days 2-4) using the Timed “Up and Go” (TUG),24 a performance-based assessment with demonstrated validity in populations with cardiac concerns.25 To complete the TUG, participants were instructed to rise from a seated position, walk 3 m, turn, walk 3 m back to the chair, and sit down. Participants could use assistive devices but were not allowed assistance from another person. We used the time it took participants to complete the maneuver (in seconds) as the score on the TUG. We applied cut points (based on historical TUG scores in hospitalized older adults26,27 and adapted from prior studies28,29) to classify participants into the following groups: preserved mobility (≤15 seconds to complete), mild impairment (>15 to ≤25 seconds to complete), moderate impairment (>25 seconds to complete), and severe impairment (unable to complete assessment, verified via medical record review and communication with site coordinators). Of note, these thresholds differ from those commonly used for the TUG (eg, <10 to 15 seconds),24,30,31 which were established in samples of community-dwelling older adults and may be too conservative for characterizing mobility impairment in acutely ill hospitalized older patients. Missing TUG scores were multiply imputed (by chained equations) for the 16.2% (418 of 2587) of participants who were able to complete the assessment (based on documentation in the medical record review and communication with site coordinators) but did not (eg, refused owing to fear of falling or fatigue).
Demographic characteristics (race/ethnicity, educational level, and marital status) were assessed via self-report during the baseline interview or via medical record abstraction. Geriatric impairments (hearing,32 vision,33 grip strength,34 and cognition35) and psychosocial characteristics (depressive symptoms36 and social support37) were assessed via self-report or performance-based testing during the baseline interview.
Coordinators at each enrollment site and a research nurse at the coordinating center abstracted data from participants’ medical records on presenting clinical characteristics, medical history, procedures (cardiac catheterization only, percutaneous coronary intervention, and coronary artery bypass graft), and complications (arrhythmia, heart failure, bleeding event, and acute kidney injury). Follow-up interviews were conducted with all participants at 6 months after discharge. A physician panel (J.A.D. and S.I.C. and other nonauthors) confirmed deaths between hospital discharge and the 6-month interview via review of medical records and death certificates.
Outcomes
Primary outcomes were defined as a decrease in ability to independently perform 1 or more essential activities of daily living (ADLs) and loss of ability to walk 0.4 km (one-quarter mile) at 6 months after hospital discharge relative to premorbid functional status. During the baseline interview (participants reported on function 1 month before admission) and the 6-month follow-up interview, participants were asked how much help they needed from another person to bathe, dress, transfer (get in and out of a chair), and walk around their home.38 Response options were “no help,” “help,” and “unable to do.” Decline in ADLs was characterized as any decrease in ability to perform these tasks from baseline to 6 months after discharge (ie, transition from “no help” to “help,” “help” to “unable to do,” or “no help” to “unable to do”). Participants were also asked about their ability to walk 0.4 km (or 2-3 blocks), an established marker of neighborhood-level mobility,39 at baseline (reporting on 1 month before admission) and 6 months after discharge. Decline in ability to walk 0.4 km was defined as reporting yes at baseline and no at 6 months after discharge. Declines in individual ADLs were examined as secondary outcomes, as was a count outcome of the number of functional activities in which participants declined (range, 0-5) from baseline to 6 months after discharge.
Statistical Analysis
Baseline characteristics were compared according to level of mobility impairment (ie, preserved mobility [≤15 seconds to complete], mild impairment [>15 to ≤25 seconds to complete], moderate impairment [>25 seconds to complete], or severe impairment [unable to complete]) using analysis of variance for continuous variables, Kruskal-Wallis test for nonnormal/ordinal variables, and χ2 test for categorical variables. Multivariable-adjusted logistic regression was used to model odds of functional decline associated with degree of mobility impairment, with preserved mobility used as the reference category. Negative binomial regression was used to examine the association of mobility impairment with the count of functional activities in which participants declined. Selection of covariates for inclusion in multivariable-adjusted models was guided by the literature,5,40,41 review of bivariate associations between covariates and the outcomes, and clinical judgment and included the following: demographics (age, sex, race/ethnicity, educational level, marital status, and cohabitation status), AMI characteristics (AMI type, peak troponin ratio, and in-hospital revascularization), comorbidities (arrhythmia, heart failure, hypertension, peripheral vascular disease, stroke, prior AMI, chronic obstructive pulmonary disease, cancer, smoking history, Charlson Comorbidity Index, prior revascularization, and chronic lung disease), in-hospital complications (arrhythmia, heart failure, bleeding event, and acute kidney injury), discharge location, geriatric impairments (preadmission impairment in ADLs or 0.4-km mobility, hearing impairment, visual impairment, grip strength impairment, and cognitive impairment), and psychosocial characteristics (depression36 and social support).
Missing data on covariates, ranging from less than 1% to 16% (mobility status), were addressed using multiple imputation by chained equations.42 Hosmer-Lemeshow tests were applied to multivariable-adjusted logistic regression models to confirm goodness of fit; Pearson goodness-of-fit statistic was used for the negative binomial model.
To account for the competing risk of death during follow-up in the SILVER-AMI study (10% at 6 months after discharge), we conducted secondary analyses of our primary outcomes via the approach recommended by Murphy et al,43 in which we simulated extreme outcomes for decedents (eg, all decedents experienced the outcome or no decedents experienced the outcome), in addition to standard multiple imputation, to evaluate the robustness of our associations of mobility impairment and functional outcomes to bias from death. Analyses were performed using statistical software (Stata 14; StataCorp LLC). Statistical significance was set at 2-tailed P < .05.
Results
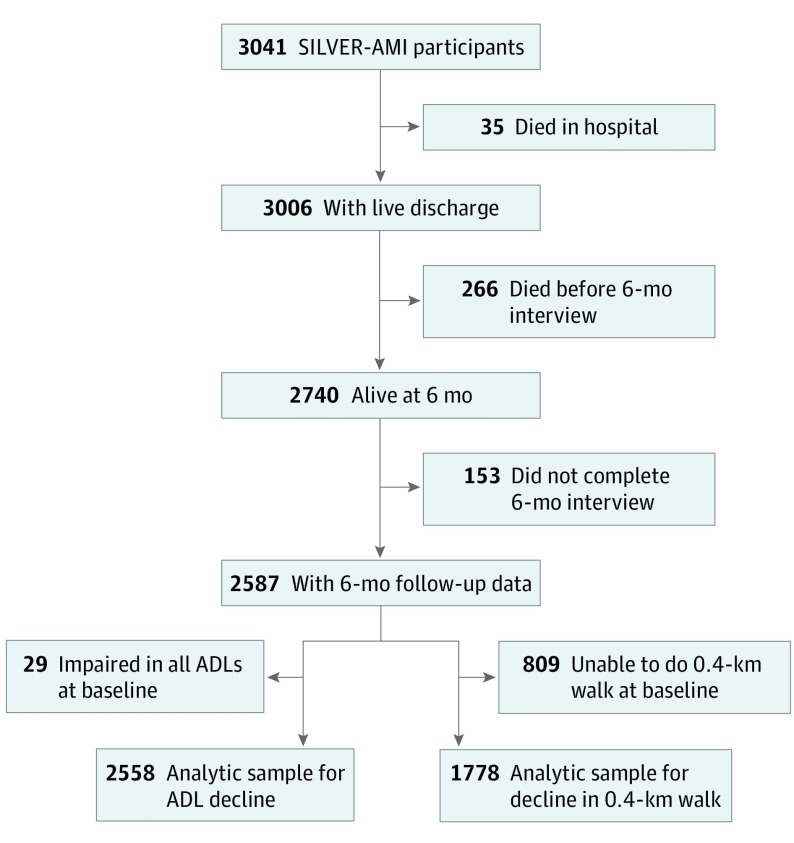
Of the 3041 participants in the study sample, 35 died during the index hospitalization, 266 died before the 6-month interview, and 153 were lost to follow-up for reasons other than death (5.6% loss to follow-up), leaving 2587 hospitalized patients with AMI who were 75 years or older with 6-month assessments (Figure 1). Twenty-nine participants were impaired in all 4 ADLs at baseline, and 809 participants could not walk 0.4 km at baseline; these participants were excluded from analyses of ADL decline and decline in 0.4-km mobility, respectively. Excluded participants did not differ significantly in terms of sex or AMI type but were slightly older, less likely to be of white race/ethnicity or to have undergone percutaneous coronary intervention, and more likely to be functionally impaired at baseline.
Figure 1. Derivation of the Analytic Sample.
Data on the 0.4-km walk were missing in 8 participants. ADL indicates activity of daily living; SILVER-AMI, Comprehensive Evaluation of Risk Factors in Older Patients With Acute Myocardial Infarction.
The mean (SD) age of the sample was 81.4 (4.8) years, and more than half of the sample (56.5% [1462 of 2587]) were male. Eighty-nine percent (2296 of 2587) were of white race/ethnicity, and 43.4% (1114 of 2587) were educated beyond high school. Almost three-quarters of the sample (72.6% [1881 of 2587]) were initially seen with non–ST-segment elevation myocardial infarction, and slightly more than one-third (35.4% [916 of 2587]) were impaired in ADLs or 0.4-km mobility before admission.
Thirty-one percent of the sample (n = 800) were free of mobility impairment (ie, completed the TUG in ≤15 seconds) as measured during the in-hospital assessment (Table). The remainder of participants had mild impairment (564 of 2587 [21.8%]), moderate impairment (414 of 2587 [16.0%]), or severe impairment (391 of 2587 [15.1%]). Differences in characteristics among participants according to mobility status are listed in the Table. Participants with impaired mobility were older and more likely to be female, be of nonwhite race/ethnicity, and live alone (Table). Participants with impaired mobility were also more likely to initially be seen with non–ST-segment elevation myocardial infarction and had higher rates of comorbidities, complications, and impairments in physical, cognitive, and psychosocial characteristics (Table).
Table. Patient Characteristics, by Level of Mobility Impairment During Hospitalizationa.
| Patient Characteristic | Mobility Level, Measured via Timed “Up and Go,” No. (%) | ||||
|---|---|---|---|---|---|
| Preserved Mobility, ≤15 s to Complete (n = 800) | Mild Impairment, >15 to ≤25 s to Complete (n = 564) | Moderate Impairment, >25 s to Complete (n = 414) | Severe Impairment, Unable to Complete (n = 391) | P Value | |
| Demographics | |||||
| Age, mean (SD), y | 80.4 (4.5) | 80.9 (4.5) | 82.1 (5.0) | 83.0 (5.3) | <.001 |
| Male sex | 530 (66.3) | 333 (59.0) | 183 (44.2) | 183 (46.8) | <.001 |
| Nonwhite race/ethnicity | 44 (5.5) | 59 (10.5) | 53 (12.8) | 40 (10.2) | <.001 |
| Educational level ≤12 y | 391 (48.9) | 330 (58.5) | 246 (59.4) | 244 (62.4) | <.001 |
| Married/living as married | 495 (61.9) | 320 (56.7) | 169 (40.8) | 157 (40.2) | <.001 |
| Live alone | 273 (34.1) | 195 (34.6) | 173 (41.8) | 165 (42.2) | .005 |
| Clinical | |||||
| Acute myocardial infarction type | |||||
| NSTEMI | 532 (66.5) | 427 (75.7) | 322 (77.8) | 290 (74.2) | <.001 |
| STEMI | 268 (33.5) | 137 (24.3) | 92 (22.2) | 101 (25.8) | |
| Peak troponin ratio, median (IQR)b | 59 (14-261) | 47 (12-201) | 41 (11-153) | 78 (16-299) | <.001 |
| Comorbidities | |||||
| Arrhythmia | 150 (18.8) | 138 (24.5) | 114 (27.5) | 113 (28.9) | <.001 |
| Heart failure | 71 (8.9) | 84 (14.9) | 91 (22.0) | 113 (28.9) | <.001 |
| Hypertension | 627 (78.4) | 484 (85.8) | 366 (88.4) | 339 (86.7) | <.001 |
| Peripheral vascular disease | 51 (6.4) | 68 (12.1) | 56 (13.5) | 46 (11.8) | <.001 |
| Stroke | 79 (9.9) | 81 (14.4) | 86 (20.8) | 69 (17.6) | <.001 |
| Prior acute myocardial infarction | 200 (25.0) | 160 (28.4) | 114 (27.5) | 114 (29.2) | .15 |
| Chronic obstructive pulmonary disease | 85 (10.6) | 60 (10.6) | 61 (14.7) | 73 (18.7) | <.001 |
| Chronic kidney disease | 434 (54.3) | 315 (55.9) | 251 (60.6) | 266 (68.0) | <.001 |
| Diabetes | 236 (29.5) | 212 (37.6) | 183 (44.2) | 163 (41.7) | <.001 |
| Cancer | 168 (21.0) | 129 (22.9) | 93 (22.5) | 77 (19.7) | .63 |
| Ever smoker | 451 (56.4) | 302 (53.5) | 206 (49.8) | 210 (53.7) | .19 |
| Charlson Comorbidity Index, median (IQR) | 3 (1-4) | 3 (2-5) | 3 (2-5) | 3 (2-5) | <.001 |
| In-hospital procedures | |||||
| Noninvasive management only | 53 (6.6) | 62 (11.0) | 61 (14.7) | 93 (23.8) | <.001 |
| Cardiac catheterization only | 118 (14.8) | 94 (16.7) | 77 (18.6) | 56 (14.3) | |
| Percutaneous coronary intervention | 564 (70.5) | 340 (60.3) | 213 (51.4) | 176 (45.0) | |
| Coronary artery bypass graft | 65 (8.1) | 68 (12.1) | 63 (15.2) | 66 (16.9) | |
| In-hospital complications | |||||
| Arrhythmia | 116 (14.5) | 91 (16.1) | 81 (19.6) | 95 (24.3) | <.001 |
| Heart failure | 55 (6.9) | 75 (13.3) | 64 (15.5) | 82 (21.0) | <.001 |
| Bleeding event | 146 (18.3) | 127 (22.5) | 113 (27.3) | 155 (39.6) | <.001 |
| Acute kidney injury | 100 (12.5) | 111 (19.7) | 91 (22.0) | 132 (33.8) | <.001 |
| Discharge location to home | 763 (95.4) | 519 (92.0) | 320 (77.3) | 154 (39.4) | <.001 |
| Geriatric | |||||
| Impairment in ≥1 activity of daily living before admissionc | 28 (3.5) | 44 (7.8) | 67 (16.2) | 126 (32.2) | <.001 |
| Inability to walk 0.4 km | 122 (15.3) | 173 (30.7) | 189 (45.7) | 195 (49.9) | <.001 |
| Hearing impairmentd | 92 (11.5) | 70 (12.4) | 66 (15.9) | 61 (15.6) | .02 |
| Visual impairmente | 37 (4.6) | 36 (6.4) | 41 (9.9) | 59 (15.1) | <.001 |
| Grip strength impairmentf | 370 (46.3) | 330 (58.5) | 303 (73.2) | 277 (70.8) | <.001 |
| Cognitive impairmentg | 34 (4.3) | 68 (12.1) | 86 (20.8) | 115 (29.4) | <.001 |
| Psychosocial | |||||
| Depressionh | 60 (7.5) | 64 (11.3) | 80 (19.3) | 92 (23.5) | <.001 |
| Social support, median (IQR)i | 25 (21-25) | 23 (19-25) | 23 (19-25) | 23 (19-25) | <.001 |
Abbreviations: IQR, interquartile range; NSTEMI, non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
Analyses performed using analysis of variance for continuous variables, Kruskal-Wallis test for nonnormal/ordinal variables, and χ2 test for categorical variables. The Timed Up and Go results were missing in 16% of participants.
Ratio to upper limit of normal at recruitment site.
Essential activities of daily living (bathing, dressing, transferring, and walking).
Responses of “a lot” or “a moderate amount” to the question “How much does your hearing interfere with normal day-to-day activities?” from the Cognitive Function and Aging Studies.32
Responses of “poor,” “very poor,” or “completely blind” to select questions about vision from the National Eye Institute Visual Function Questionnaire.33
Less than 18.5 kg for women and less than 28.5 kg for men on best of 3 grip tests via dynamometer.
Score less than 27 on the Telephone Interview for Cognitive Status.35
Score on the Patient Health Questionnaire of 8 of 10 or higher.36
Five-item Medical Outcomes Study Social Support Scale.37
One-quarter (648 of 2587) of participants reported functional decline at 6 months after discharge (compared with 4 weeks before admission), 12.8% (332 of 2587) reported ADL decline, and 16.7% (431 of 2587) reported decline in 0.4-km mobility. The most common ADL in which participants declined was bathing (8.5% [219 of 2587]), followed by dressing (6.5% [169 of 2587]), transferring (4.4% [113 of 2587]), and walking around the home (4.1% [105 of 2587]). Among participants who experienced decline, 67.4% (433 of 642) declined in 1 activity, 17.6% (113 of 642) declined in 2 activities, 6.5% (42 of 642) declined in 3 activities, and 8.4% (54 of 642) declined in 4 or 5 activities.
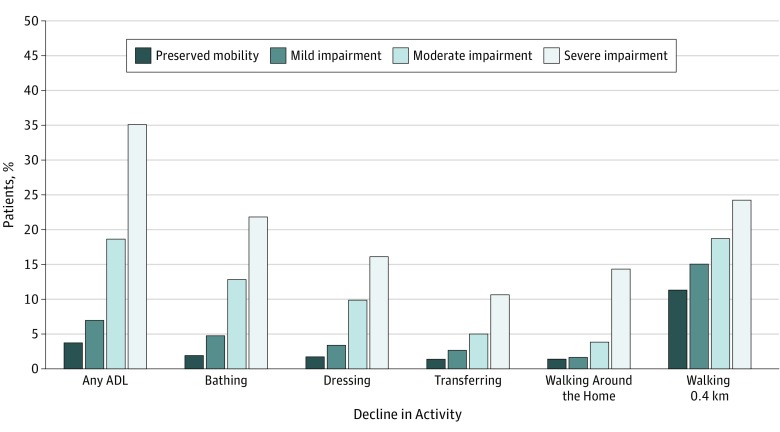
Mobility impairment during hospitalization was associated with decline in ADLs and 0.4-km mobility at 6 months after discharge. Only 3.8% (30 of 800) of participants with preserved mobility reported ADL decline at 6 months after discharge compared with rates of 6.9% (39 of 564) among participants with mild impairment, 18.6% (77 of 414) among participants with moderate impairment, and 34.7% (136 of 391) among participants with severe impairment (P < .001) (Figure 2). Compared with participants with preserved mobility and after adjustment for demographic, clinical, other physical and cognitive impairments, and psychosocial characteristics, adjusted odds ratios (aORs) for any ADL decline were 1.24 (95% CI, 0.74-2.09) for mild impairment, 2.67 (95% CI, 1.67-4.27) for moderate impairment, and 5.45 (95% CI, 3.29-9.01) for severe impairment (Figure 3 and eTable 1A in the Supplement). For decline in ability to walk 0.4 km, aORs were 1.51 (95% CI, 1.04-2.20) among participants with mild impairment, 2.03 (95% CI, 1.37-3.02) among participants with moderate impairment, and 3.25 (95% CI, 2.02-5.23) among participants with severe impairment relative to preserved mobility (Figure 3 and eTable 1B in the Supplement). Sensitivity analyses accounting for the competing risk of death were consistent with the main analyses (eFigure 1 in the Supplement).
Figure 2. Percentage of Older Patients With Acute Myocardial Infarction Experiencing Functional Decline at 6 Months After Discharge, According to Mobility Status.
ADL indicates activity of daily living.
Figure 3. Multivariable-Adjusted Odds Ratios (aORs) for Functional Decline After Acute Myocardial Infarction (AMI), According to In-Hospital Mobility Status.
Adjusted for age, sex, race/ethnicity, educational level, marital status, AMI type, peak troponin ratio, comorbidities, smoking history, in-hospital procedures, in-hospital complications, discharge location, preadmission functional impairment, hearing impairment, visual impairment, grip strength impairment, cognitive impairment, depression, and social support. ADL indicates activity of daily living.
We also examined the association of mobility impairment with the count of activities in which each participant declined (range, 0-5). Greater severity of mobility impairment was associated in a dose-response manner with increasing counts of functional decline after AMI, ranging from an incident rate ratio of 1.30 (95% CI, 1.02-1.65) for mild impairment to 2.83 (95% CI, 2.16-3.72) for severe impairment in multivariable-adjusted analyses (eTable 2 in the Supplement).
The incidence of decline in each functional activity according to mobility status is shown in Figure 2. Incidences of decline in each activity were monotonically higher across worsening levels of mobility impairment. For example, only 1.9% (15 of 800) of participants with preserved mobility declined in ability to bathe compared with incidence rates of decline in bathing ability of 4.8% (27 of 564) among participants with mild impairment, 12.8% (53 of 414) among participants with moderate impairment, and 21.9% (86 of 391) among participants with severe impairment.
Multivariable-adjusted ORs for the association of mobility impairment and risk of decline in each essential ADL are shown in eFigure 2 in the Supplement. In adjusted analyses, severe impairment was consistently associated with risk of decline in each activity, with the greatest risk observed for declines in bathing (aOR, 5.28; 95% CI, 2.75-10.14) and dressing (aOR, 4.53; 95% CI, 2.17-9.45). Moderate impairment was associated with significantly increased risks of decline in bathing (aOR, 3.07; 95% CI, 1.61-5.84) and dressing (aOR, 3.24; 95% CI, 1.59-6.60). Mild impairment was not significantly associated with decline in individual ADLs.
Discussion
In this multicenter prospective cohort study, we found that mobility impairment, measured during AMI hospitalization with the TUG, was associated with increased risk of functional decline at 6 months after discharge. More than half (1369 of 2587) of our older adult cohort exhibited impaired mobility during hospitalization, and impaired mobility was consistently associated with decline in several functions encompassing “essential” ADLs and neighborhood-level mobility. Our findings suggest that the TUG score may be a useful “geriatric biomarker” for identifying older patients with AMI at risk for functional decline.
Mobility status, including gait and balance, is emerging as an important predictor of outcomes among older adults with cardiovascular disease. A recent study20 found slow gait speed to be associated with a nearly 2-fold risk of mortality and hospital readmissions among older patients with AMI. Another study44 reported the TUG score to be a better predictor of complications and mortality after cardiac surgery than established surgery risk scores. The TUG score is considered a surrogate marker for frailty,45 a strong risk factor for clinical outcomes in older patients with AMI.46 The present work adds evidence to the utility of inpatient mobility, and the TUG score specifically, as a marker for poor patient-centered outcomes in older patients with cardiovascular disease.
We found that mobility impairment was associated with decline in all activities we evaluated, including the essential ADLs and neighborhood-level mobility. Mobility impairment showed the greatest risk in its association with declines in bathing and dressing; this finding is important because the loss of ability to independently perform these ADLs may signal a critical transition for older adults from independence to dependence.47,48,49 Losses of independence in bathing and dressing are associated, more so than loss of independence in other ADLs, with increased risk of hospital admissions50 and institutionalization51; therefore, a brief tool (eg, the TUG) that identifies patients at risk of decline in these domains could be useful in tailoring treatment plans to prevent poor clinical outcomes.
Strengths, Limitations, and Future Directions
This study is strengthened by the use of data from the largest prospective cohort study to date of patients 75 years or older hospitalized for AMI, recruited from a nationwide network of academic and community hospitals. The SILVER-AMI study rigorously collected a rich array of demographic, cardiac, and geriatric data, allowing us to examine new risk factors in this population while accounting for important traditional risk factors. Follow-up was complete in 94.4% (2587 of 2740) of participants who survived to 6 months, limiting the risk of attrition bias. We also robustly accounted for the competing risk of death in secondary analyses.
These strengths are balanced by some limitations. We operationalized “baseline” functional status as participants’ report of function 1 month before hospitalization. As such, we are unable to precisely identify whether the functional decline reported by participants at 6-month follow-up first occurred immediately before AMI hospitalization, during hospitalization, or afterward. Prior evidence has shown that retrospective report of premorbid functional status (ie, from a time before onset of illness) is a better indicator of baseline function than functional status during hospitalization and is associated with posthospitalization outcomes.52 We acknowledge that some participants may have experienced functional decline around the time of their hospitalization for AMI but subsequently recovered before the 6-month assessment. Other researchers have reported rates of functional decline closer to 30% among older adults shortly after hospitalization, with one-third recovering by 6 months after discharge.53 The 25% rate of functional decline reported in the present study is likely an underestimation of the incidence of functional decline, which potentially biases our findings toward the null and focuses our inquiry on patients with persistent, unrecovered functional decline.
Because most older adults value function and independence as health outcomes of greater priority than longevity,54 clinicians caring for older adults with AMI must recognize and address threats to these important patient-centered outcomes. Our findings suggest that mobility assessment during hospitalization may be useful for evaluating risk of functional decline among older patients with AMI. The TUG is a brief, freely available, and easy assessment that can be administered by clinicians or support staff without extensive training. The assessment requires minimal equipment (a chair with arms, a 3-m [10-ft] floor span, and a clock), all of which are already available in most hospital rooms. We advocate for all clinicians caring for older patients with AMI to administer assessments of balance and gait, such as the TUG.
Despite mobility status demonstrating utility as a potent marker of risk for functional decline, the course of action for mitigating this risk remains elusive. There has been a surge in interest regarding progressive mobilization interventions in the hospital, which show evidence of being safe and feasible for older hospitalized patients, including those with cardiovascular disease.55 However, a recent trial56 of inpatient mobilization found no difference in postdischarge ADLs between experimental and control groups. Other interventions, such as cardiac rehabilitation57,58 and postdischarge case management,59 have shown modest results for improving physical function after AMI, but the low participation rates60,61,62 of older adults in these programs, particularly those with mobility impairment,63 suggest that further work is needed to develop effective interventions that are suited to the needs and preferences of older adults.
Conclusions
This study’s findings showed that mobility impairment was common among older adults hospitalized for AMI and was associated with risk of functional decline at 6 months after discharge. The TUG is a brief and easy-to-administer point-of-care mobility assessment that may be a useful tool to identify older hospitalized adults at risk of functional decline after AMI.
eTable 1A. Multivariable-Adjusted Odds Ratios for Risk of ADL Decline After AMI
eTable 1B. Multivariable-Adjusted Odds Ratios for Risk of Decline in ¼-Mile Mobility After AMI
eTable 2. Multivariable-Adjusted Associations of Mobility Status With Count of Activities in Which Participants Decline at 6 Months After AMI
eFigure 1. Sensitivity Analyses of Associations Between Mobility Impairment and Risk of Functional Decline, Accounting for Competing Risk of Death
eFigure 2. Multivariable-Adjusted Odds Ratios for Risk of Decline in Independence in Each Activity of Daily Living After AMI, According to Mobility Status
References
- 1.Kozak LJ, DeFrances CJ, Hall MJ. National hospital discharge survey: 2004 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2006;(162):1-209. [PubMed] [Google Scholar]
- 2.Tisminetzky M, Erskine N, Chen HY, et al. Changing trends in, and characteristics associated with, not undergoing cardiac catheterization in elderly adults hospitalized with ST-segment elevation acute myocardial infarction. J Am Geriatr Soc. 2015;63(5):925-931. doi: 10.1111/jgs.13399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen HL, Saczynski JS, Gore JM, et al. Long-term trends in short-term outcomes in acute myocardial infarction. Am J Med. 2011;124(10):939-946. doi: 10.1016/j.amjmed.2011.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krumholz HM, Wang Y, Chen J, et al. Reduction in acute myocardial infarction mortality in the United States: risk-standardized mortality rates from 1995-2006. JAMA. 2009;302(7):767-773. doi: 10.1001/jama.2009.1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodson JA, Arnold SA, Reid KJ, et al. Physical function and independence 1 year after myocardial infarction: observations from the Translational Research Investigating Underlying Disparities in Recovery From Acute Myocardial Infarction: Patients’ Health Status Registry. Am Heart J. 2012;163(5):790-796. doi: 10.1016/j.ahj.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamper AM, Stott DJ, Hyland M, Murray HM, Ford I; PROSPER Study Group . Predictors of functional decline in elderly people with vascular risk factors or disease. Age Ageing. 2005;34(5):450-455. doi: 10.1093/ageing/afi137 [DOI] [PubMed] [Google Scholar]
- 7.Levine DA, Davydow DS, Hough CL, Langa KM, Rogers MA, Iwashyna TJ. Functional disability and cognitive impairment after hospitalization for myocardial infarction and stroke. Circ Cardiovasc Qual Outcomes. 2014;7(6):863-871. doi: 10.1161/HCQ.0000000000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota RTC, Van der Heyden J, Demarest S, et al. Contribution of chronic diseases to the mild and severe disability burden in Belgium. Arch Public Health. 2015;73(1):37. doi: 10.1186/s13690-015-0083-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171-2179. doi: 10.1111/j.1532-5415.2008.02023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med. 2009;25(4):563-577, vii. doi: 10.1016/j.cger.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barile JP, Thompson WW, Zack MM, Krahn GL, Horner-Johnson W, Haffer SC. Activities of daily living, chronic medical conditions, and health-related quality of life in older adults. J Ambul Care Manage. 2012;35(4):292-303. doi: 10.1097/JAC.0b013e31826746f5 [DOI] [PubMed] [Google Scholar]
- 12.Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. doi: 10.1001/jamainternmed.2014.7756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portegijs E, Buurman BM, Essink-Bot ML, Zwinderman AH, de Rooij SE. Failure to regain function at 3 months after acute hospital admission predicts institutionalization within 12 months in older patients. J Am Med Dir Assoc. 2012;13(6):569.e1-569.e7. doi: 10.1016/j.jamda.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 14.Huerre C, Guiot A, Maréchaux S, et al. Functional decline in elderly patients presenting with acute coronary syndromes: impact on midterm outcome. Arch Cardiovasc Dis. 2010;103(1):19-25. doi: 10.1016/j.acvd.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 15.Fried TR, Tinetti M, Agostini J, Iannone L, Towle V. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ Couns. 2011;83(2):278-282. doi: 10.1016/j.pec.2010.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi: 10.1111/j.1532-5415.1990.tb03451.x [DOI] [PubMed] [Google Scholar]
- 17.Lum HD, Studenski SA, Degenholtz HB, Hardy SE. Early hospital readmission is a predictor of one-year mortality in community-dwelling older Medicare beneficiaries. J Gen Intern Med. 2012;27(11):1467-1474. doi: 10.1007/s11606-012-2116-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60(10):1901-1905. doi: 10.1111/j.1532-5415.2012.04145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266-273. doi: 10.1111/j.1532-5415.2010.03276.x [DOI] [PubMed] [Google Scholar]
- 20.Dodson JA, Arnold SV, Gosch KL, et al. Slow gait speed and risk of mortality or hospital readmission after myocardial infarction in the Translational Research Investigating Underlying Disparities in Recovery From Acute Myocardial Infarction: Patients’ Health Status Registry. J Am Geriatr Soc. 2016;64(3):596-601. doi: 10.1111/jgs.14016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodson JA, Geda M, Krumholz HM, et al. Design and rationale of the Comprehensive Evaluation of Risk Factors in Older Patients With AMI (SILVER-AMI) study. BMC Health Serv Res. 2014;14:506. doi: 10.1186/s12913-014-0506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, et al. ; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; ESC Committee for Practice Guidelines (CPG) . Third Universal Definition of Myocardial Infarction. Eur Heart J. 2012;33(20):2551-2567. doi: 10.1093/eurheartj/ehs184 [DOI] [PubMed] [Google Scholar]
- 23.Jeste DV, Palmer BW, Appelbaum PS, et al. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry. 2007;64(8):966-974. doi: 10.1001/archpsyc.64.8.966 [DOI] [PubMed] [Google Scholar]
- 24.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148. doi: 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 25.Hwang R, Morris NR, Mandrusiak A, et al. Timed up and go test: a reliable and valid test in patients with chronic heart failure. J Card Fail. 2016;22(8):646-650. doi: 10.1016/j.cardfail.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 26.Trombetti A, Hars M, Herrmann F, Rizzoli R, Ferrari S. Effect of a multifactorial fall-and-fracture risk assessment and management program on gait and balance performances and disability in hospitalized older adults: a controlled study. Osteoporos Int. 2013;24(3):867-876. doi: 10.1007/s00198-012-2045-3 [DOI] [PubMed] [Google Scholar]
- 27.Laver K, George S, Ratcliffe J, et al. Use of an interactive video gaming program compared with conventional physiotherapy for hospitalised older adults: a feasibility trial. Disabil Rehabil. 2012;34(21):1802-1808. doi: 10.3109/09638288.2012.662570 [DOI] [PubMed] [Google Scholar]
- 28.Aubert CE, Folly A, Mancinetti M, Hayoz D, Donzé JD. Performance-based functional impairment and readmission and death: a prospective study. BMJ Open. 2017;7(6):e016207. doi: 10.1136/bmjopen-2017-016207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roopchand S, Fung J, Barbeau H. Locomotor training and the effects of unloading on overground locomotion following stroke In: Ruper GD, ed. New Developments in Stroke Research. Hauppauge, NY: Nova Science Publishers Inc; 2007:127-148. [Google Scholar]
- 30.Donoghue OA, Savva GM, Cronin H, Kenny RA, Horgan NF. Using Timed Up and Go and usual gait speed to predict incident disability in daily activities among community-dwelling adults aged 65 and older. Arch Phys Med Rehabil. 2014;95(10):1954-1961. doi: 10.1016/j.apmr.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 31.Lusardi MM, Pellecchia GL, Schulman M. Functional performance in community living older adults. J Geriatr Phys Ther. 2003;26(3):14-22. doi: 10.1519/00139143-200312000-00003 [DOI] [Google Scholar]
- 32.Hayman KJ, Kerse N, Dyall L, et al. Life and living in advanced age: a cohort study in New Zealand: e Puāwaitanga o Nga Tapuwae Kia Ora Tonu, LiLACS NZ: study protocol [published correction appears in BMC Geriatr. 2017;17(1):127]. BMC Geriatr. 2012;12:33. doi: 10.1186/1471-2318-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD; National Eye Institute Visual Function Questionnaire Field Test Investigators . Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050-1058. doi: 10.1001/archopht.119.7.1050 [DOI] [PubMed] [Google Scholar]
- 34.Wang CY, Chen LY. Grip strength in older adults: test-retest reliability and cutoff for subjective weakness of using the hands in heavy tasks. Arch Phys Med Rehabil. 2010;91(11):1747-1751. doi: 10.1016/j.apmr.2010.07.225 [DOI] [PubMed] [Google Scholar]
- 35.Brandt J, Spencer M, Folstein MF. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(2):111-117. https://jhu.pure.elsevier.com/en/publications/the-telephone-interview-for-cognitive-status-3. Accessed August 2014. [Google Scholar]
- 36.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163-173. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 37.McCarrier KP, Bushnell DM, Martin ML, Paczkowski R, Nelson DR, Buesching D. PRM16: validation and psychometric evaluation of a 5-item measure of perceived social support. Value Health. 2011;14(3):A148. doi: 10.1016/j.jval.2011.02.824 [DOI] [Google Scholar]
- 38.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727. doi: 10.1111/j.1532-5415.1983.tb03391.x [DOI] [PubMed] [Google Scholar]
- 39.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26(2):130-135. doi: 10.1007/s11606-010-1543-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendes de Leon CF, Rajan KB. Psychosocial influences in onset and progression of late life disability. J Gerontol B Psychol Sci Soc Sci. 2014;69(2):287-302. doi: 10.1093/geronb/gbt130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. doi: 10.1007/s11606-012-2226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 43.Murphy TE, Gill TM, Leo-Summers LS, Gahbauer EA, Pisani MA, Ferrante LE. The competing risk of death in longitudinal geriatric outcomes. J Am Geriatr Soc. 2019;67(2):357-362. http://www.ncbi.nlm.nih.gov/pubmed/30537050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson TN, Wu DS, Sauaia A, et al. Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann Surg. 2013;258(4):582-588. doi: 10.1097/SLA.0b013e3182a4e96c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savva GM, Donoghue OA, Horgan F, O’Regan C, Cronin H, Kenny RA. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci. 2013;68(4):441-446. doi: 10.1093/gerona/gls190 [DOI] [PubMed] [Google Scholar]
- 46.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non–ST-segment elevation myocardial infarction. Circulation. 2011;124(22):2397-2404. doi: 10.1161/CIRCULATIONAHA.111.025452 [DOI] [PubMed] [Google Scholar]
- 47.Edjolo A, Proust-Lima C, Delva F, Dartigues JF, Pérès K. Natural history of dependency in the elderly: a 24-year population-based study using a longitudinal item response theory model. Am J Epidemiol. 2016;183(4):277-285. doi: 10.1093/aje/kwv223 [DOI] [PubMed] [Google Scholar]
- 48.Morris JN, Berg K, Fries BE, Steel K, Howard EP. Scaling functional status within the interRAI suite of assessment instruments. BMC Geriatr. 2013;13:128. doi: 10.1186/1471-2318-13-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 50.Caplan GA, Brown A, Croker WD, Doolan J. Risk of admission within 4 weeks of discharge of elderly patients from the emergency department: the DEED study. Age Ageing. 1998;27(6):697-702. doi: 10.1093/ageing/27.6.697 [DOI] [PubMed] [Google Scholar]
- 51.Fong JH, Mitchell OS, Koh BSK. Disaggregating activities of daily living limitations for predicting nursing home admission. Health Serv Res. 2015;50(2):560-578. doi: 10.1111/1475-6773.12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451-458. doi: 10.1046/j.1532-5415.2003.51152.x [DOI] [PubMed] [Google Scholar]
- 53.Boyd CM, Ricks M, Fried LP, et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the Women’s Health and Aging Study I. J Am Geriatr Soc. 2009;57(10):1757-1766. doi: 10.1111/j.1532-5415.2009.02455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171(20):1854-1856. doi: 10.1001/archinternmed.2011.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peixoto TC, Begot I, Bolzan DW, et al. Early exercise-based rehabilitation improves health-related quality of life and functional capacity after acute myocardial infarction: a randomized controlled trial. Can J Cardiol. 2015;31(3):308-313. doi: 10.1016/j.cjca.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 56.Brown CJ, Foley KT, Lowman JD Jr, et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial. JAMA Intern Med. 2016;176(7):921-927. doi: 10.1001/jamainternmed.2016.1870 [DOI] [PubMed] [Google Scholar]
- 57.Audelin MC, Savage PD, Ades PA. Exercise-based cardiac rehabilitation for very old patients (≥75 years): focus on physical function. J Cardiopulm Rehabil Prev. 2008;28(3):163-173. doi: 10.1097/01.HCR.0000320066.58599.e5 [DOI] [PubMed] [Google Scholar]
- 58.Johnston M, MacDonald K, Manns P, Senaratne M, Rodgers W, Haennel RG. Impact of cardiac rehabilitation on the ability of elderly cardiac patients to perform common household tasks. J Cardiopulm Rehabil Prev. 2011;31(2):100-104. doi: 10.1097/HCR.0b013e3181f1fd8c [DOI] [PubMed] [Google Scholar]
- 59.Hunger M, Kirchberger I, Holle R, et al. Does nurse-based case management for aged myocardial infarction patients improve risk factors, physical functioning and mental health? the KORINNA trial. Eur J Prev Cardiol. 2015;22(4):442-450. doi: 10.1177/2047487314524682 [DOI] [PubMed] [Google Scholar]
- 60.Dolansky MA, Moore SM, Visovsky C. Older adults’ views of cardiac rehabilitation program: is it time to reinvent? J Gerontol Nurs. 2006;32(2):37-44. doi: 10.3928/0098-9134-20060201-10 [DOI] [PubMed] [Google Scholar]
- 61.Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med. 2015;175(10):1700-1702. doi: 10.1001/jamainternmed.2015.3819 [DOI] [PubMed] [Google Scholar]
- 62.Tolmie EP, Lindsay GM, Kelly T, Tolson D, Baxter S, Belcher PR. Are older patients’ cardiac rehabilitation needs being met? J Clin Nurs. 2009;18(13):1878-1888. doi: 10.1111/j.1365-2702.2009.02798.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flint K, Kennedy K, Arnold SV, Dodson JA, Cresci S, Alexander KP. Slow gait speed and cardiac rehabilitation participation in older adults after acute myocardial infarction. J Am Heart Assoc. 2018;7(5). doi: 10.1161/JAHA.117.008296 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1A. Multivariable-Adjusted Odds Ratios for Risk of ADL Decline After AMI
eTable 1B. Multivariable-Adjusted Odds Ratios for Risk of Decline in ¼-Mile Mobility After AMI
eTable 2. Multivariable-Adjusted Associations of Mobility Status With Count of Activities in Which Participants Decline at 6 Months After AMI
eFigure 1. Sensitivity Analyses of Associations Between Mobility Impairment and Risk of Functional Decline, Accounting for Competing Risk of Death
eFigure 2. Multivariable-Adjusted Odds Ratios for Risk of Decline in Independence in Each Activity of Daily Living After AMI, According to Mobility Status