Abstract
Background:
Myopia has become a global public health problem. High myopia is linked to pathologic myopia (PM). As the severity of myopia increases, excessive axial elongation of the globe exerts a biomechanical stretch on the posterior pole, followed by a series of retinopathy which can lead to marked visual impairment. Posterior scleral reinforcement (PSR) is the only way that may have the potential to prevent the progression of axial elongation. Some scholars expressed satisfaction with the efficacy and safety of PSR. In contrast, other surgeons had negative conclusions on the outcomes for the surgery.
Objectives:
The aims of this review are to provide an update on the current knowledge of posterior scleral reinforcement to prevent progression of high myopia and to discuss clinical trials examining the potential utility of PSR in treating this disease.
Methods:
We searched Ovid MEDLINE, Embase, PubMed and China National Knowledge Infrastructure (CNKI) (all years to 1 July 2019). We also conducted a gray literature search and handsearched reference lists of included studies and pertinent review articles.
Results:
26 clinical trials were included. 20 trials were designed as only one eye of each patient had posterior scleral reinforcement surgery. After 3 to 5 years of follow-up, the results are very satisfactory. 6 randomized controlled trials, which have conservatively treated groups, showed statistically significant differences between the eyeball axial length progression in the study group and the control group, where surgery was not performed. Most clinical trials reached a positive influence. But the efficacy of different clinical trials varies greatly.
Conclusions:
PSR, is safe and effective to slowdown myopia progression, especially for high myopia.
Keywords: efficacy, high myopia, posterior scleral reinforcement
INTRODUCTION
Myopia is a common cause of vision loss. The increasing prevalence of high myopia has already been noted. In 2010, it was estimated that uncorrected myopia was the most common cause of distance vision impairment, affecting 108 million persons, and the second most common cause of blindness globally.1 Up to 2015, 216.6 million persons were affected by uncorrected myopia, which became the leading cause of moderate or severe vision impairment.2Vitale et al3 found that the prevalence of myopia in the United States appeared to be substantially higher from 1999 to 2004 than 30 years ago, reaching an 8-fold increase (≤−7.90 D) from 0.2% to 1.6%. Asian countries have a higher incidence of high myopia. In the study of the Israeli population by Bar Dayan et al4, the overall prevalence of myopia increased from 20.3% in 1990 to 28.3% in 2002. Lin et al5 found that 21% of 18-year-old Taiwanese students in 2000 had high myopia (<−6.00 D) compared with 10.9% of that in 1983. The respective numbers of people affected by blindness, and moderate and severe vision impairment owing to uncorrected myopia are increasing. Myopic retinopathy has become the first reason causing irreversible blindness. Therefore, preventing the progression of myopia may help reduce the blindness rate of these patients. The definition of high myopia varies among studies and is mostly defined as spherical equivalent of <−6.00 D. High myopia is linked to pathologic myopia. The prevalence of myopia retinopathy increased significantly with increasing myopic refractive error or axial length,6 from 3.8% in eyes with a myopic refractive error of <−4.0 D to 89.6% in eyes with a myopic refractive error of at least −10.0 D.7
As the severity of myopia increases, excessive axial elongation of the globe exerts a biomechanical stretch on the posterior pole. This stretch will form an outpouching of a circumscribed region of the posterior fundus, and it has a curvature radius which is smaller than that of the adjacent eye wall, and this is posterior staphyloma.8In a series of interrelated retinopathy of high myopia, posterior scleral staphyloma is one of the most basic lesions. Gentle et al9 showed a decrease in messenger ribonucleic acid (mRNA) expression of type I collagen and a relatively small change in mRNA expression of types III and V collagen in the biochemical examination of the scleral tissue of the myopic myopia model. The ratio of collagen III/I to V/I increases in a short-term cause of more small diameter collagen fibers in the sclera of myopia. Posterior staphylomas are a hallmark of high myopia and are one of the major causes of developing myopic maculopathy.10–12 The association of staphylomas with other macular complications, like myopic choroidal neovascularization (CNV)8 and myopic macular retinoschisis,13 has also been reported. Posterior staphylomas are directly or indirectly associated with the high myopia-associated glaucoma-like or glaucomatous optic neuropathy.14,15 Subsequent complications may seriously affect the quality of life of patients, and even lead to blindness.
TREATMENTS FOR HIGH MYOPIA
High myopia treatment usually means ceasing or decreasing the myopia progression. Treatments for high myopia include conservative treatments and surgical treatments.
Proposed conservative treatments include atropine, pirenzepine, orthokeratology, peripheral defocus modifying contact lenses, rigid gas-permeable contact lenses, soft contact lenses, and undercorrected single-vision lenses. A network meta-analysis involving 30 randomized controlled trials determines the effectiveness of different interventions in slowing down the progression of myopia.16 It is found that the most effective intervention showing a marked reduction in myopia progression was atropine, followed by pirenzepine, orthokeratology, peripheral defocus modifying contact lenses showing moderate effects, and progressive addition spectacle lenses showing minimal effects on slowing myopia progression. In addition, rigid gas-permeable contact lenses, soft contact lenses, and undercorrected single-vision lenses were all ineffective in reducing myopia progression. Notwithstanding this result, it is likely that more large data trials are necessary to support these conclusions.
Surgical treatment includes laser corneal refractive surgery, intraocular lens implantation, implantable collamer lens, and posterior scleral reinforcement. In terms of axial length, only posterior scleral reinforcement is effective among these surgical methods. In recent years, scientists have also proposed the concept of subscleral injection of mesenchymal stem cells and dopamine injection for the treatment of high myopia,17 representing a promising new strategy to halt the progression of myopia.
This review focuses on the current knowledge of posterior scleral reinforcement, the only existing surgical method to prevent progression of high myopia.
POSTERIOR SCLERAL REINFORCEMENT
As the pathogenesis of high myopia progression is not clear yet, the targeted deletion of etiology is deficient. Excluding correction of refractive errors, the therapeutic efficacy of treatment strategies directed on inhibiting the extension of the eye axis. Posterior scleral reinforcement surgery, using biological or nonbiological materials to strengthen the scleral weak area in the posterior pole and block the continuous elongation of the axial length, was first proposed by Shevelev in 1930.18 Since then, Snyder and Thompson have modified the technique.19,20
Mechanism of Posterior Scleral Reinforcement
According to animal experiments,21 the histopathological changes after posterior scleral reinforcement were divided into 4 phases: inflammatory reaction period (1–2 weeks after surgery); granuloma formation stage, angiogenesis stage (2–4 weeks after surgery); collagen fiber-formation stage (1–3 months after surgery) and connective tissue proliferative stage (>3 months after surgery). The histopathological changes in the early period after surgery were manifested by the inflammatory response and the dissolution of collagen fibers. Almost at the same time, the implanted sclera also began the repairing process. Neovascularization began to appear on surfaces of the donor and acceptor sclera, and between them 1 week after surgery, and it grew deeper into the sclera over time. The neovascularization reaches a peak in the posterior segment of the eye 1 to 3 months postoperatively. Thereafter, the inflammatory response completely subsides, some new blood vessels will occlude, and the remaining new blood vessels will continue to be functional. The neovascularization improves the nutritional status of the posterior pole of high myopia, thereby improving the visual function of the patient. After a long time of repair and reconstruction, the implanted scleral graft finally fuses with the recipient sclera. The scleral thickness increases significantly, and so does the hardness, achieving the purpose of mechanically reinforcing the sclera. During the reconstruction of the implant material, the axial length of the eye can be slightly shortened because of the pulling of the collagen fibers.
Types of Posterior Scleral Reinforcement
There have been several so-called posterior scleral reinforcement or sclera fortification surgical methods in previous clinical practice, such as posterior scleral reinforcement with quadratus interscalene, single band posterior scleral reinforcement, widen band posterior scleral reinforcement or macular thickening, nasal augmentation, and so on. However, confirmed by various clinical trials, single band posterior scleral reinforcement has become a safe and effective treatment for progressive myopia.
The Efficacy of PSR on Preventing High Myopia
There is a summary of trial results evaluating the efficacy of PSR in high myopia, which is provided in Table 1.
TABLE 1.
Summary of the Results of Trials Evaluating the Efficacy of PSR in High Myopia
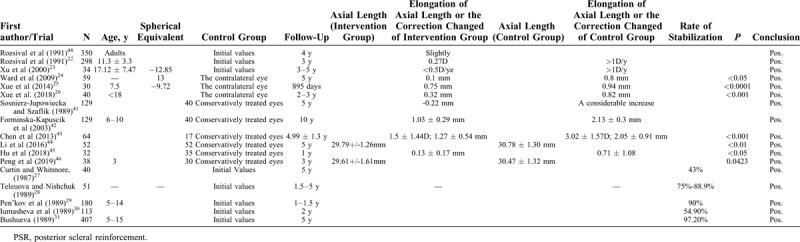
Some trials were designed as only 1 eye of each patient had posterior scleral reinforcement surgery. After 3 to 5 years of follow-up, the results are very satisfactory. Rozsival et al22 revealed 3 years after correction only changed by 0.27 D on average, as indication for operation was progression of myopia by at least 1 D per year before operation. This is consistent with the results found by Xu et al,23 that the increment of refractive diopter was <0.50 D/year. In terms of axial length, the mean elongation was significantly less in the surgery eye group than that in the contralateral eye group.24–26 The rate of stabilization varied, including correction and axial length, from 43% to 97.2%,27–40 and such a large fluctuation range may be because of different surgical materials and surgical techniques. Those clinical randomized controlled trials, which have conservatively treated group, always showed less myopic progression and less eye elongation.41–46 There was statistically significant difference between the eyeball axial length progression in the study group and the control group, where surgery was not performed.
As shown in Table 2, most clinical trials reached a positive influence. But the efficacy of different clinical trials varies greatly, the reasons might be different surgical procedure and experience of the surgeons. It has been documented that PSR may lead to some serious complications27; postoperative complications mainly are ocular hypertension, conjunctival tissue edema, vitreous hemorrhage, retinal hemorrhage or choroidal hemorrhage, diplopia or eye movement disorder, retinal detachment, and optic atrophy. It was possible that the strengthening bands compressed the optic nerves and injured vortex veins, or the bands were not anchored surrounding the posterior pole toward the macula. Reinforcement material expulsion, symblepharon, and choroidal effusions may result. Intraoperative complications may include injury of vortex vein and penetration of sclera. However, common complications were temporary. Therefore, whether the operation is performed by an experienced doctor is crucial.
TABLE 2.
Summary of the Results of Trials Evaluating the Efficacy of PSR in High Myopia

Therefore, PSR is controversial, and more studies are needed to confirm its therapeutic benefits.
CONCLUSIONS
PSR, a surgical approach modifying the sclera remodeling causing direct mechanical reinforcement of the wall of eyeball, is of great importance to slowdown myopia progression, especially for high myopia. PSR is safe and effective to stabilize the vision, prevent the axial elongation, halt the further myopia development, and delay the chorioretinal degeneration to a certain extent. The long-term effects remain to be further verified through a large-sample clinical research.25,26,47
Footnotes
Supported by Beijing Municipal Administration of Hospitals Incubating Program. Code: PX2016006
The authors report no conflicts of interest to disclose.
REFERENCES
- 1.Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health 2013; 1:e339–e349. [DOI] [PubMed] [Google Scholar]
- 2.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017; 5:e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 3.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol 2009; 127:1632–1639. [DOI] [PubMed] [Google Scholar]
- 4.Bar Dayan Y, Levin A, Morad Y, et al. The changing prevalence of myopia in young adults: a 13-year series of population-based prevalence surveys. Invest Ophthalmol Vis Sci 2005; 46 (8):2760–2765. [DOI] [PubMed] [Google Scholar]
- 5.Lin LL, Shih YF, Hsiao CK, et al. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acadof Med 2004; 33:27–33. [PubMed] [Google Scholar]
- 6.Gozum N, Cakir M, Gucukoglu A, et al. Relationship between retinal lesions and axial length, age and sex in high myopia. Eur J Ophthalmol 1997; 7:277–282. [DOI] [PubMed] [Google Scholar]
- 7.Liu HH, Xu L, Wang YX, et al. Prevalence and progression of myopic retinopathy in Chinese adults: the Beijing Eye Study. Ophthalmology 2010; 117:1763–1768. [DOI] [PubMed] [Google Scholar]
- 8.Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology 2014; 121:1798–1809. [DOI] [PubMed] [Google Scholar]
- 9.Gentle A, Liu Y, Martin JE, et al. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem 2003; 278:16587–16594. [DOI] [PubMed] [Google Scholar]
- 10.Fang Y, Yokoi T, Nagaoka N, et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology 2018; 125:863–877. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology 2010; 117:1595–1611. 611.e1-4. [DOI] [PubMed] [Google Scholar]
- 12.Yan YN, Wang YX, Yang Y, et al. Ten-year progression of myopic maculopathy: The Beijing Eye Study 2001-2011. Ophthalmology 2018; 125:1253–1263. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara K, Tanaka N, Jonas JB, et al. Ultrawide-field OCT to investigate relationships between myopic macular retinoschisis and posterior staphyloma. Ophthalmology 2018; 125:1575–1586. [DOI] [PubMed] [Google Scholar]
- 14.Nagaoka N, Jonas JB, Morohoshi K, et al. Glaucomatous-type optic discs in high myopia. PLoS One 2015; 10:e0138825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L, Wang Y, Wang S, et al. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology 2007; 114:216–220. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology 2016; 123:697–708. [DOI] [PubMed] [Google Scholar]
- 17.Janowski M, Bulte JW, Handa JT, et al. Concise review: using stem cells to prevent the progression of myopia-a concept. Stem Cells (Dayton, Ohio) 2015; 33:2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shevelev MM. Operation against high myopia and sclera with aid of the transplantation of fascia lata on thinned sclera. Russian Oftalmol 1930; 1930:107–110. [Google Scholar]
- 19.Snyder AA, Thompson FB. A simplified technique for surgical treatment of degenerative myopia. Am J Ophthalmol 1972; 74:273–277. [DOI] [PubMed] [Google Scholar]
- 20.Thompson FB. A simplified scleral reinforcement technique. Am J Ophthalmol 1978; 86:782–790. [DOI] [PubMed] [Google Scholar]
- 21.Ma D, Liu S. Study on mechanism of posterior scleral reinforcement. China Journal of Modern Medicine 2004; 10: 96-7+101. [Google Scholar]
- 22.Rozsival P, Mericka P, Zaydlar K. [Scleroplasty surgery. I. Results in children]. Cesk Oftalmol 1991; 47:246–257. [PubMed] [Google Scholar]
- 23.Xu Y, Liu H, Niu T, et al. [Long-term observation of curative effects of posterior scleral reinforcement surgery in patients with juvenile progressive myopia]. Zhonghua Yan Ke Za Zhi 2000; 36:455–458. [PubMed] [Google Scholar]
- 24.Ward B, Tarutta EP, Mayer MJ. The efficacy and safety of posterior pole buckles in the control of progressive high myopia. Eye (Lond) 2009; 23:2169–2174. [DOI] [PubMed] [Google Scholar]
- 25.Xue A, Bao F, Zheng L, et al. Posterior scleral reinforcement on progressive high myopic young patients. Optom Vis Sci 2014; 91:412–418. [DOI] [PubMed] [Google Scholar]
- 26.Xue A, Zheng L, Tan G, et al. Genipin-crosslinked donor sclera for posterior scleral contraction/reinforcement to fight progressive myopia. Invest Ophthalmol Vis Sci 2018; 59:3564–3573. [DOI] [PubMed] [Google Scholar]
- 27.Curtin BJ, Whitmore WG. Long-term results of scleral reinforcement surgery. Am J Ophthalmol 1987; 103:544–548. [DOI] [PubMed] [Google Scholar]
- 28.Teleuova TS, Nishchuk ZV. [Scleroplasty as a prophylaxis against blindness and asthenopia in children with progressive myopia]. Oftalmol Zh 1989; 207–209. [PubMed] [Google Scholar]
- 29.Pen’kov MA, Morozova TA, Miroshnik DM. [The surgical results in progressive myopia in children]. Oftalmol Zh 1989; 202–203. [PubMed] [Google Scholar]
- 30.Iumasheva AA, Belous VI, Lishchenko BM. [Scleroplasty results in progressive myopathy with the use of allogeneic amnion]. Oftalmol Zh 1989; 352–354. [PubMed] [Google Scholar]
- 31.Bushueva NN. [The late results of different methods of sclera-reinforcing operations on children and adolescents suffering from progressive myopia]. Oftalmol Zh 1989; 194–198. [PubMed] [Google Scholar]
- 32.Tarutta EP, Andreeva LD, Markosian GA, et al. [Reinforcement of the sclera with new types of synthetic materials in progressive myopia]. Vestn Oftalmol 1999; 115:8–10. [PubMed] [Google Scholar]
- 33.Stepanov VK. [A new method of scleroplasty in progressive myopia]. Vestn Oftalmol 1990; 106:19–21. [PubMed] [Google Scholar]
- 34.Medvetskaia GA, Golychev VN. [The late results of Murmamedov-Atameredova's modified scleroplasty in children]. Vestn Oftalmol 1993; 109:15–16. [PubMed] [Google Scholar]
- 35.Gerinec A, Belanova L. [Effectiveness of posterior scleroplasty in progressive myopia in children]. Cesk Slov Oftalmol 1996; 52:220–225. [PubMed] [Google Scholar]
- 36.Avetisov ES, Tarutta EP, Iomdina EN, et al. Nonsurgical and surgical methods of sclera reinforcement in progressive myopia. Acta Ophthalmol Scand 1997; 75:618–623. [DOI] [PubMed] [Google Scholar]
- 37.Balazs K, Bekesi L, Berta A, et al. Scleral reinforcement in progressive myopia and intraoperative ultrasound control of the cadaver fascia lata strip. Acta Chir Hung 1997; 36:14–15. [PubMed] [Google Scholar]
- 38.Gonchar PA, Dushin NV, Beliaev VS, et al. [The optimization of a surgical intervention to stabilize progressive myopia]. Vestn Oftalmol 1999; 115:6–8. [PubMed] [Google Scholar]
- 39.Gerinec A, Slezakova G. Posterior scleroplasty in children with severe myopia. Bratisl Lek Listy 2001; 102:73–78. [PubMed] [Google Scholar]
- 40.Miao Z, Li L, Meng X, et al. Modified posterior scleral reinforcement as a treatment for high myopia in children and its therapeutic effect. Biomed Res Int 2019; 2019:5185780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sosnierz-Jupowiecka A, Szaflik J. [Evaluation of the effect of Snyder-Thompson scleroplasty based on the behavior of the axial dimension of the eye ball]. Klin Oczna 1989; 91:19–20. [PubMed] [Google Scholar]
- 42.Forminska-Kapuscik M, Kaminska-Olechnowicz B, Sosnierz-Jupowiecka A, et al. [Retrospective evaluation of eyes with high progressive myopia in children and youth ten years after Snyder and Thompson's scleroplasty]. Klin Oczna 2003; 105:151–154. [PubMed] [Google Scholar]
- 43.Chen M, Dai J, Chu R, et al. The efficacy and safety of modified Snyder-Thompson posterior scleral reinforcement in extensive high myopia of Chinese children. Graefes Arch Clin Exp Ophthalmol 2013; 251:2633–2638. [DOI] [PubMed] [Google Scholar]
- 44.Li XJ, Yang XP, Li QM, et al. Posterior scleral reinforcement for the treatment of pathological myopia. Int J Ophthalmol 2016; 9:580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu H, Zhao G, Wu R, et al. Axial s. Ophthalmologica 2018; 239:128–132. [DOI] [PubMed] [Google Scholar]
- 46.Peng C, Xu J, Ding X, et al. Effects of posterior scleral reinforcement in pathological myopia: a 3-year follow-up study. Graefes Arch Clin Exp Ophthalmol 2019; 257:607–617. [DOI] [PubMed] [Google Scholar]
- 47.Shen ZM, Zhang ZY, Zhang LY, et al. Posterior scleral reinforcement combined with patching therapy for pre-school children with unilateral high myopia. Graefes Arch Clin Exp Ophthalmol 2015; 253:1391–1395. [DOI] [PubMed] [Google Scholar]
- 48.Rozsival P, Mericka P, Zaydlar K. [Scleroplasty surgery. II. Results in adults]. Cesk Oftalmol 1991; 47:258–269. [PubMed] [Google Scholar]
- 49.Tarutta EP, Shamkhalova E, Val'skii VV. [An analysis of the late results of scleroplasty in progressive myopia]. Oftalmol Zh 1989; 204–207. [PubMed] [Google Scholar]


