Purpose:
To provide an overview of the currently available retreatment methods after myopic small-incision lenticule extraction (SMILE).
Design:
Systematic literature review.
Methods:
The PubMed library was searched for articles containing the terms “small-incision lenticule extraction” and “enhancement” or “retreatment”. The last search was performed on May 1, 2019.
Results:
In contrast to laser in-situ keratomileusis (LASIK), which can be retreated by a flap relift, repeat SMILE retreatment is currently not approved and only seldomly performed. As substitutes, surface ablation, cap-to-flap conversion using the CIRCLE program in the VisuMax platform, and thin-flap LASIK have been recently established. While all options offer safety and efficacy comparable to LASIK retreatments, each has its patient-specific advantages and disadvantages. While surface ablation preserves the flap-free approach of the primary procedure, the aspect of pain and a slow visual recovery might render it less attractive as compared with CIRCLE and thin-flap LASIK which offer quick recovery, however at the price of flap creation. Besides, each retreatment method generates specific tissue responses and has a different impact on corneal biomechanics, which is strongly dependent on the previous SMILE parameters, especially the cap thickness.
Conclusions:
Refractive enhancement after SMILE is currently mostly performed by surface ablation, CIRCLE cap-to-flap conversion or thin-flap LASIK, which all offer safety and efficacy comparable to LASIK retreatments. In this review, a detailed overview over each method, its technical aspects, and specific advantages and disadvantages is given.
Keywords: CIRCLE, enhancement, laser in situ keratomileus, small incision lenticule extraction, surface ablation
In the treatment of myopia and myopic astigmatism, SMILE and fs-LASIK have been established as equivalently safe and effective treatment options.1,2 In contrast to LASIK, which can be retreated by a flap relift, reSMILE retreatment is, however, currently neither approved nor commercially available in the VisuMax platform (Carl Zeiss Meditec AG, Jena, Germany), with sparse data on its safety and efficacy.3 As a substitute, a multitude of alternative enhancement options have been proposed and established, including surface ablation,4 cap-to-flap conversion using the CIRCLE program,5 and thin-flap LASIK.6 This review will focus on the epidemiology of enhancement after SMILE and elucidate the advantages and disadvantages of each available retreatment method.
EPIDEMIOLOGY AND RISK FACTORS OF SMILE ENHANCEMENT
Enhancement rates after SMILE are very similar to the ones known from fs-LASIK. In the largest study on the epidemiology of SMILE enhancement, Liu et al7 reported an incidence of 2.1% and 2.9% after 1 and 2 years, respectively, resulting in an overall prevalence of 2.7%. These numbers have been recently confirmed by Siedlecki et al.8 reporting a prevalence of 2.3% in 2803 eyes, and Reinstein et al6,15, reporting a prevalence 4.4% in 2643 eyes. 71% of retreatments are performed within the first year with a mean latency of 10 to 11 months.5,7,8 Retreatment is much more likely to be performed for under- (79%) than overcorrection (21%), and 86% represent spherocylindrical retreatments.7
The most important risk factors for enhancement after SMILE are age >35 years (odds ratio [OR]: 5.6), preSMILE refractive spherical equivalent (SEQ) >−6.0 diopters (D) (OR 4.8), astigmatism >−3.0 D (OR 3.1), and suction loss during surgery (OR 2.1).7 In the age group above 40 years, Liu et al7 found an enhancement prevalence of 13%, whereas patients with >8.0 D SEQ before surgery had a prevalence of 7.1%.
OPTIONS OF ENHANCEMENT AFTER SMILE
Repeat Small-incision Lenticule Extraction
Surgical Technique
Theoretically, secondary SMILE can be performed anteriorly or posteriorly to the original interface. As cap thickness is usually set to 120 to 140 μm, there is usually not enough tissue left for an anterior reSMILE, and presently no peer-reviewed data on its feasibility exist. In contrast, reSMILE enhancement posterior to the original interface was first described in a case report by Donate and Thaëron in 2015.3 This so-called “subcap lenticule extraction” reuses the primary SMILE interface as the cap cut of the secondary SMILE, and thus only introduces a new posterior plane and a new lenticule sidecut to create a new lenticule (by aborting the laser procedure after the lenticule sidecut; Fig. 1).3 Moreover, the primary SMILE opening incision can also be reused for secondary lenticule extraction.3
FIGURE 1.
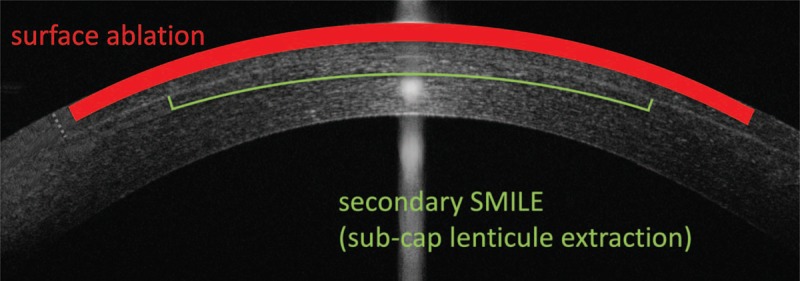
Schematic illustration of SMILE enhancement using surface ablation, and a secondary reSMILE procedure posteriorly to the primary interface. This so-called “sub-cap lenticule extraction” reuses the primary SMILE interface as the cap cut of the secondary SMILE, and thus only introduces a new posterior plane and a new lenticule sidecut to create a new lenticule. The primary SMILE opening incision can also be reused for secondary lenticule extraction (grey dotted line).
Clinical outcomes
In the original case report, target accuracy with +0.25 D SEQ and uncorrected and corrected distance visual acuity (UDVA/CDVA) with both 20/16 were excellent.3 Nevertheless, further data reproducing these results are still scarce. In the only follow-up study by Sedky et al,9 9 eyes were retreated with so-called “cap-preserving” reSMILE. In this cohort, 78% of eyes were within 1.0 D of target after retreatment and 89% had UDVA matching or exceeding CDVA. To achieve correct centration alignment of both treatments, the use of a custom SMILE retreatment centering marker has been described.9 Moreover, the diameter of the treatment zone was found to be optimal if being programmed 0.2 mm less than the original diameter to facilitate lenticule dissection.9 Nevertheless, enhancement using reSMILE is currently off-label and considered as cumbersome by most surgeons, and has not yet been proven to be equivalent to alternative established options. Moreover, reSMILE retreatment is difficult or even impossible for enhancement of low residual refractive errors, as the lenticule will eventually become too thin for a successful dissection. In these cases, it can be helpful to deliberately increase minimum border thickness.10
Surface Ablation
Surgical Technique
Although limited by some disadvantages, surface ablation represents the easiest and most straightforward enhancement option after SMILE. In a study by Siedlecki et al., 40 postSMILE eyes were retreated with epi-off laser epithelial keratomileusis (LASEK) with mitomycin C (MMC) using tissue saving, Triple A, topography-guided, and aspherically optimized (ASA) profiles on MEL 80 and 90 excimer lasers (Carl Zeiss Meditec AG). The surgical procedure differs very little from conventional state-of-the-art surface ablation in virgin eyes (Fig. 1).
Clinical Outcomes
In this study, the mean preenhancement SEQ of −0.86 ± 0.43 D had improved to 0.03 ± 0.57 D at 3 months, and the number of eyes within ±0.50 and ±1.00 D of target refraction increased from 22.5% to 80% and from 72.5% to 92.5%, respectively. An UDVA of 20/20 was achieved in 63% of eyes. Interestingly, the ASA profile led to significant overcorrection, which can be explained by the fixed amount of induced asphericity regardless of the myopic correction, thus increasing ablation depth nonproportionally in low myopia.11 Transient haze was observed in 1 eye (2.5%); no eye lost ≥2 lines of CDVA. The resulting safety and efficacy indices at 3 months were 1.06 and 0.90, respectively, which are comparable to surface ablation retreatments after LASIK.12 Another recent matched-pair study by Siedlecki et al. seeking to compare state-of-the art epi-off LASEK after SMILE versus the CIRCLE cap-to-flap procedure found even better results.8 Excluding ASA from the retreatment algorithm, all eyes were within 0.50 D of target refraction at 3 months.
From a clinical perspective, surface ablation might be less attractive to patients because of its slow visual recovery and painful nature. Moreover, the stronger induction of inflammatory reactions after surface ablation might be of increased significance in eyes treated with previous refractive surgery (see below, Enhancement Options From a Tissue Perspective). On the contrary, surface ablation preserves the flap-free nature of SMILE, which will usually be one of the main reasons for most patients to prefer this technique over LASIK.
CIRCLE cap-to-flap
Surgical Technique
The proprietary CIRCLE software of the VisuMax platform (presently not available in the United States) has been specifically developed for enhancements and conversion of the SMILE cap into a full flap for LASIK-like excimer laser enhancement.5 Presently, 4 CIRCLE patterns with different sequential laser cuts are available.13 Pattern A represents the simplest one, creating a side cut in the clearance zone of the cap plane between the end of the optical zone and the outer circumference of the cap (Fig. 2).13 In a study in 12 eyes of 6 New Zealand white rabbits, Riau et al,13 found that this reduces the area available for retreatment, which might be especially problematic in hyperopic retreatments.13 To overcome this problem, patterns B, C, and D all create a lamellar ring outside the optical zone to increase the retreatment area; both old (cap) and new treatment plane (lamellar ring) are then linked by a vertical junction cut, and made accessible as a flap by circular incision with the exception of a hinge area. In patterns B and C, the lamellar rings are introduced posteriorly (B) or, anteriorly (C) to the cap, which however makes the entry into the right dissection plane more difficult, as the new and old treatment plane are at different depths.13 Pattern D, which creates the lamellar ring at the same depth as the cap, has therefore been shown to produce flaps easiest to dissect and lift.13
FIGURE 2.
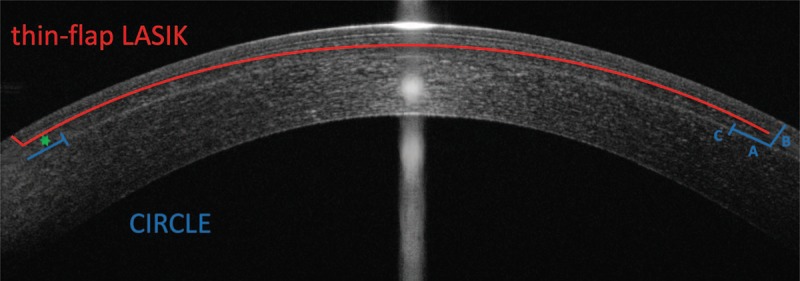
Schematic illustration of SMILE enhancement using CIRCLE and thin-flap LASIK. In CIRCLE, 3 laser cuts are performed to generate a lamellar ring around the cap cut, A, a side cut with exception of a hinge area, B, and a vertical junction cut, C, to link both treatment planes. In thin-flap LASIK, a new flap with usually 100 μm is generated. Any cross-talk with the epithelium (above the Bowman's layer, seen as a highly reflective line above the LASIK interface) and the SMILE interface (highly reflective line below the LASIK interface) has to be avoided. The green star symbolizes the delta of anterior stroma affected depending on the method chosen.
For these reasons, pattern D is currently recommended by the manufacturer and has been adopted by most surgeons. Chansue et al14 and Siedlecki et al.5,8 have reported on the clinical efficacy of CIRCLE pattern D and have found excellent results with flap handling very similar to conventional fs-LASIK. To facilitate surgical manipulation, the outer diameter of the CIRCLE procedure is recommended to be programmed to extend beyond the SMILE interface (eg, 8.2 mm over ≥7.9 mm, depending on the white-to-white diameter).8 The inner diameter should be smaller than the lenticule (eg, 6.2 mm within 6.5 mm). To avoid cross-talk with the SMILE incision, the flap should be orientated in a fashion that the new hinge area does not overlap with the former side cut incision (eg, SMILE incision at 130 degree and CIRCLE flap at 50 degree).5 After femtosecond laser application, the new flap can be lifted using a blunt spatula like a regular LASIK flap, followed by excimer laser treatment. The use of MMC has been reported in the first few published cases on CIRCLE,5 however, is now deemed obsolete, as the inflammatory reaction after CIRCLE is much less severe than that after surface ablation, making haze a rare complication.8
Clinical Outcomes
In a study on the surgical outcomes of CIRCLE, Siedlecki et al.5 retreated 22 eyes with CIRCLE (pattern D) for a mean SEQ of −0.51 ± 1.08 D. Safe flap lifting was possible in all eyes with no complications. At 3 months, mean SEQ had improved to 0.18 ± 0.31 D. The number of eyes within 0.50 and 1.00 D from target refraction increased from 31.8% to 90.9% and from 77.3% to 100%, respectively. An UDVA of 20/20 was achieved in 77.3% of eyes. No eye lost >2 lines of CDVA. The resulting safety and efficacy indices at 3 months were 1.03 and 0.97, respectively.
Presently, both surface ablation and CIRCLE represent the easiest and most widely adopted enhancement methods after myopic SMILE.5 In a recent matched comparative study by Siedlecki et al.,8 CIRCLE and surface ablation yielded equivalent results at 3 months concerning target accuracy (100% within 0.50 D of target in both groups) and visual acuity (83% of eyes with 20/20 UDVA or better in both groups), resulting in equivalent safety (1.06 vs 1.00) and efficacy indices (1.03 vs 0.95). Although neither procedure has been reported to cause significant loss of CDVA, CIRCLE seems to convey lower risk of losing ≥1 lines of CDVA.4,5,8
The largest clinical difference between both options as perceivable by patients lies within the aspect of pain and speed of visual recovery. In this respect, CIRCLE represents a major improvement to surface ablation. In the matched comparative study,8 CIRCLE was superior to surface ablation concerning UDVA (approximately 2 lines) and CDVA (1 line) at week 1 after enhancement.8 Unfortunately, pain was not assessed in the study. As a disadvantage, CIRCLE sacrifices the idea of a flap-free approach, and, especially in deeper caps, might cause inappropriate biomechanical weakening (see below, Enhancement Options From a Tissue Perspective). Therefore, the choice of preserving a flap-free approach versus a painless, quick recovery should be thoroughly discussed in preoperative counseling.
Thin-flap LASIK
Surgical Technique
The first studies on the surgical technique and outcomes of thin-flap LASIK enhancement after myopic SMILE were recently published by Reinstein et al.6,15. The choice between CIRCLE cap-to-flap conversion and thin-flap LASIK is mainly made by cap thickness, as thin caps of 100 to 120 μm make it nearly impossible to introduce another LASIK treatment plane above, and on the contrary, thick caps of ≥160 μm make it unreasonable to create a thick flap at the corresponding cap depth, as the biomechanical impact on the anterior stroma will be disproportionate in most cases (Fig. 2).14
Thin-flap LASIK after SMILE poses two main challenges. First, any interference of the thin flap with the epithelium or the previous SMILE interface must be avoided to prevent the generation of cryptic buttonholes or tissue slivers.16 As suggested by Reinstein et al,14 flaps can usually be safely generated if there is at least a delta of 40 μm between maximum epithelial thickness and minimum cap thickness on optical coherence tomography or very high-frequency ultrasound (Fig. 3). Secondly, flap dissection and lifting might be more difficult as the previous SMILE incision or interface might be accidentally accessed, causing tears and potentially traumatic scarring.14 Reinstein et al have therefore developed a so-called “bimanual inferior pseudo-hinge fulcrum technique.” As a first step, the side cut of the flap is opened in its inferior part with a flap lifter, which is then pushed to the opposite side, creating an entry location for a McPherson forceps. Counteracting with the flap lifter at the inferior aspect, the flap is separated superiorly from the flap lifter with the McPherson forceps. Finally, the inferior part of the flap is separated from the flap lifter, with counteraction in the superior aspect using the McPherson forceps.14
FIGURE 3.
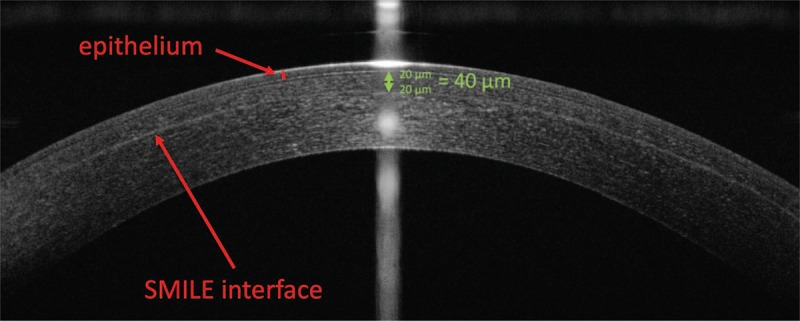
Caveats in planning thin-flap LASIK after SMILE. Using optical coherence tomography or very high-frequency ultrasound, a minimum distance of 20 μm above the new LASIK interface toward the epithelium, and 20 μm below the new LASIK interface toward the SMILE cap must be respected to avoid cryptic buttonholing and tissue slivers. As a rule of thumb, a delta of >40 μm between the maximum epithelial thickness and the cap are sufficient.
Clinical Outcomes
Using mainly 100-μm-thick flaps, Reinstein et al6 retreated 100 eyes for a mean SEQ of −0.05 ± 0.99 D. Of these, 42% were treated for a mean myopia of −1.06 ± 0.34 D, whereas 58% were treated for a mean hyperopia of +0.69 ± 0.57 D. SMILE interface-related complications were observed in 9% of eyes, with SMILE interface access seen in 5%, and SMILE interface sliver separation and incision tear each seen in 2%. These complications, however, mostly occurred in the early learning phase, and were greatly reduced by the introduction of the above mentioned bimanual inferior pseudo-hinge fulcrum technique.6,15 At 1 year, the mean SEQ had improved to 0.19 ± 0.49 D. The number of eyes within 0.50 and 1.00 D from target refraction increased to 74% and 95%, respectively. An UDVA of 20/20 was achieved in 81% of eyes. No eye lost >2 lines of CDVA.
In contrast to CIRCLE, thin-flap LASIK probably preserves corneal biomechanical stability better because of less involvement of anterior stroma (see below, chapter Enhancement options from a tissue perspective). In contrast to regular LASIK, thin-flap LASIK might, however, show a higher incidence of intraoperative and postoperative complications, such as flap tear, free cap, bubble escape and flap folds, and later on, diffuse lamellar keratitis or epithelial ingrowth.17 Moreover, thin-flap LASIK can be seldomly complicated by persistent haze that is usually not observed in regular flap thicknesses. This is probably because of possible damages in Bowman layer resulting from the superficial flap position, allowing proinflammatory epithelium-derived cytokines to infiltrate the corneal stroma.18
ENHANCEMENT OPTIONS FROM A TISSUE PERSPECTIVE
Corneal Biomechanics
Within the cornea, the anterior stroma contributes most to its biomechanical stability because of dense fiber packing,19 interlacing,20 and insertion into Bowman membrane.21 Owing to the absence of a flap, SMILE thus results in less biomechanical impact on the cornea than LASIK.22
With surface ablation and reSMILE retreatments after SMILE, this advantage can be preserved. In contrast, CIRCLE and thin-flap LASIK separate the anterior stroma above the flap from the posterior corneal structures, and thus induce more biomechanical weakening. In an ex vivo study retreating porcine eyes with reSMILE, surface ablation, and CIRCLE after SMILE,23 Kling et al recently showed that the introduction of a flap by CIRCLE retreatment resulted in a significantly higher impact on corneal biomechanical integrity than both other methods. Resulting from the introduction of a sidecut, the impact on corneal biomechanics will further increase with flap thickness.24 For this reason, CIRCLE retreatment will inevitably be more detrimental to corneal biomechanical stability than thin LASIK flaps, especially in deeper caps (eg, 150–160 μm).25 Presently, no data on the resulting clinical relevance exist, and it is unclear whether thick flaps used for SMILE retreatments will eventually increase the risk of iatrogenic ectasia.
Tissue Responses
SMILE and LASIK are generally thought to induce comparable inflammatory and wound healing reactions in the human cornea.26 In the case of additional secondary treatments, these responses might however escalate in an disproportional manner. In an in vivo model in 15 New Zealand white rabbits, Riau et al27 studied the different tissue responses in the early postoperative period after SMILE retreatment using anterior reSMILE, CIRCLE pattern D, and surface ablation. In comparison to reSMILE and CIRCLE, surface ablation caused the strongest tissue reaction with the highest amount of edema and haze. Moreover, surface ablation showed the highest amount of inflammatory CD11b- and apoptotic TUNEL-positive cells in the central superficial stroma. For these reasons, the use of MMC in surface ablation retreatments is regarded as obligatory. Comparing reSMILE with CIRCLE retreatments, Riau et al27 also found that CIRCLE showed more inflammatory and apoptotic cellular reactions than reSMILE in the early postoperative period. However, the response was mild, and MMC is not recommended as a routine treatment after CIRCLE.
DISCUSSION AND CONCLUSIONS
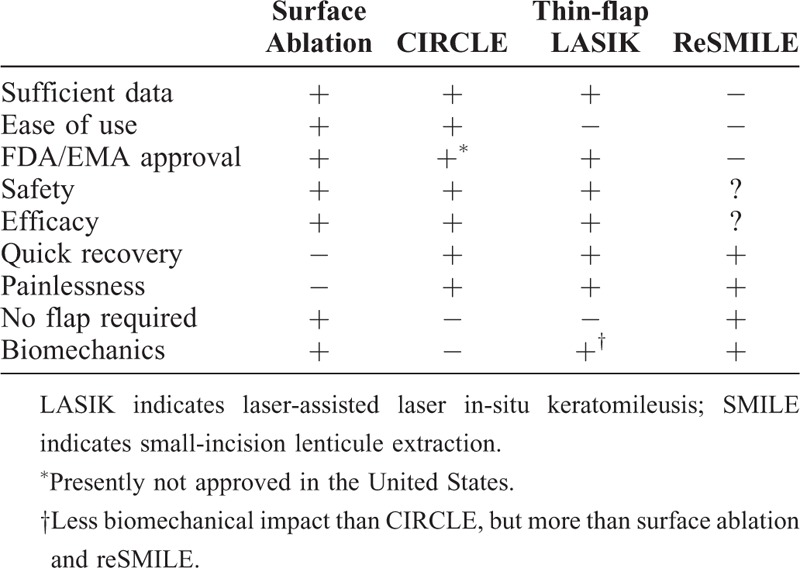
As suggested by a growing body of evidence, enhancement after SMILE is just as safe and effective as retreatment after fs-LASIK. In conclusion, surface ablation, CIRCLE and thin-flap LASIK can all be applied equal as effective substitutes.12 Thorough patient counseling is of utmost importance, as every enhancement method has its advantages and downsides (Table 1). Mostly, the aspect of a painless procedure with quick visual recovery (CIRCLE and thin-flap LASIK) will have to be weighed against the preservation of a flap-free procedure with surface ablation. From a surgical perspective, cap thickness can be used as a guidance in weighing CIRCLE versus thin-flap LASIK, with thinner caps suggesting CIRCLE, and thicker caps suggesting thin-flap LASIK above the SMILE interface. We believe that because of the low enhancement rates of 2.3% to 4.4% after SMILE,4,6,7 patients should be counseled that by choosing SMILE they chose a likelihood of >95% to stay flap-free, and that in the case of retreatment, a flap-based approach might be necessary, which however, as a primary flap procedure, will have a better risk profile than retreatments utilizing a flap-relift, especially concerning epithelial ingrowth among others.28,29
TABLE 1.
Advantages and Disadvantages of Each SMILE Enhancement Method
Footnotes
Financial Interests: J.S.: Speaker honoraria and travel reimbursement from Carl Zeiss Meditec AG, Novartis Pharma GmbH, Bayer AG, Pharm-Allergan GmbH, Oculentis OSD Medical GmbH Travel reimbursement: Roche AG.
S.P.: Personal fees and travel reimbursement from Carl Zeiss Meditec AG
All other authors: None.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Han T, Xu Y, Han X, et al. Three-year outcomes of small incision lenticule extraction (SMILE) and femtosecond laser-assisted laser in situ keratomileusis (FS-LASIK) for myopia and myopic astigmatism. Br J Ophthalmol 2019; 103:565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Shen Q, Jia Y, et al. Clinical outcomes of SMILE and FS-LASIK used to treat myopia: a meta-analysis. J Refract Surg 2016; 32:256–265. [DOI] [PubMed] [Google Scholar]
- 3.Donate D, Thaëron R. Preliminary evidence of successful enhancement after a primary SMILE procedure with the sub-cap-lenticule-extraction technique. J Refract Surg 2015; 31:708–710. [DOI] [PubMed] [Google Scholar]
- 4.Siedlecki J, Luft N, Kook D, et al. Enhancement after myopic small incision lenticule extraction (smile) using surface ablation. J Refract Surg 2017; 33:513–518. [DOI] [PubMed] [Google Scholar]
- 5.Siedlecki J, Luft N, Mayer WJ, et al. CIRCLE enhancement after myopic SMILE. J Refract Surg 2018; 34:304–309. [DOI] [PubMed] [Google Scholar]
- 6.Reinstein DZ, Carp GI, Archer TJ, et al. Outcomes of re-treatment by LASIK after SMILE. J Refract Surg 2018; 34:578–588. [DOI] [PubMed] [Google Scholar]
- 7.Liu YC, Rosman M, Mehta JS. Enhancement after small-incision lenticule extraction: incidence, risk factors, and outcomes. Ophthalmology 2017; 124:813–821. [DOI] [PubMed] [Google Scholar]
- 8.Siedlecki JSM, Luft N, Kook D, et al. Surface ablation vs. CIRCLE for myopic enhancement after SMILE: a matched comparative study. J Refract Surg 2019; 35:294–300. [DOI] [PubMed] [Google Scholar]
- 9.Sedky AN, Wahba SS, Roshdy MM, et al. Cap-preserving SMILE Enhancement Surgery. BMC Ophthalmol 2018; 18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siedlecki J, Luft N, Keidel L, et al. Variation of lenticule thickness for SMILE in low myopia. J Refract Surg 2018; 34:453–459. [DOI] [PubMed] [Google Scholar]
- 11.Dausch D, Dausch B, Wottke M, et al. Comparison of clinical outcomes in PRK with a standard and aspherical optimized profile: a full case analysis of 100 eyes with 1-year follow-up. Clini Ophthalmol 2014; 8:2251–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beerthuizen JJ, Siebelt E. Surface ablation after laser in situ keratomileusis: retreatment on the flap. J Cataract Refract Surg 2007; 33:1376–1380. [DOI] [PubMed] [Google Scholar]
- 13.Riau AK, Ang HP, Lwin NC, et al. Comparison of four different VisuMax circle patterns for flap creation after small incision lenticule extraction. J Refract Surg 2013; 29:236–244. [DOI] [PubMed] [Google Scholar]
- 14.Chansue E, Tanehsakdi M, Swasdibutra S, et al. Safety and efficacy of VisuMax(R) circle patterns for flap creation and enhancement following small incision lenticule extraction. Eye Vis (Lond) 2015; 2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinstein DZ, Carp GI, Archer TJ, et al. Inferior pseudo-hinge fulcrum technique and intraoperative complications of laser in situ keratomileusis retreatment after small-incision lenticule extraction. J Cataract Refract Surg 2018; 44:1355–1362. [DOI] [PubMed] [Google Scholar]
- 16.Reinstein DZ, Srivannaboon S, Gobbe M, et al. Epithelial thickness profile changes induced by myopic LASIK as measured by Artemis very high-frequency digital ultrasound. J Refract Surg 2009; 25:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JS. Complications of sub-Bowman's keratomileusis with a femtosecond laser in 3009 eyes. J Refract Surg 2008; 24:S97–S101. [DOI] [PubMed] [Google Scholar]
- 18.Hafezi F, Seiler T. Persistent subepithelial haze in thin-flap LASIK. J Refract Surg 2010; 26:222–225. [DOI] [PubMed] [Google Scholar]
- 19.Bergmanson JP, Horne J, Doughty MJ, et al. Assessment of the number of lamellae in the central region of the normal human corneal stroma at the resolution of the transmission electron microscope. Eye Contact Lens 2005; 31:281–287. [DOI] [PubMed] [Google Scholar]
- 20.Radner W, Zehetmayer M, Aufreiter R, et al. Interlacing and cross-angle distribution of collagen lamellae in the human cornea. Cornea 1998; 17:537–543. [DOI] [PubMed] [Google Scholar]
- 21.Morishige N, Petroll WM, Nishida T, et al. Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals. J Cataract Refract Surg 2006; 32:1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damgaard IB, Reffat M, Hjortdal J. Review of corneal biomechanical properties following LASIK and SMILE for myopia and myopic astigmatism. Open Ophthalmol J 2018; 12:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kling S, Spiru B, Hafezi F, et al. Biomechanical weakening of different re-treatment options after Small Incision Lenticule Extraction (SMILE). J Refract Surg 2017; 33:193–198. [DOI] [PubMed] [Google Scholar]
- 24.Knox Cartwright NE, Tyrer JR, Jaycock PD, et al. Effects of variation in depth and side cut angulations in LASIK and thin-flap LASIK using a femtosecond laser: a biomechanical study. J Refract Surg 2012; 28:419–425. [DOI] [PubMed] [Google Scholar]
- 25.Moshirfar M, Shah TJ, Masud M, et al. Surgical options for retreatment after small-incision lenticule extraction: advantages and disadvantages. J Cataract Refract Surg 2018; 44:1384–1389. [DOI] [PubMed] [Google Scholar]
- 26.Luft N, Schumann RG, Dirisamer M, et al. Wound healing, inflammation, and corneal ultrastructure after SMILE and femtosecond laser-assisted LASIK: a human ex vivo study. J Refract Surg 2018; 34:393–399. [DOI] [PubMed] [Google Scholar]
- 27.Riau AK, Liu YC, Lim CHL, et al. Retreatment strategies following Small Incision Lenticule Extraction (SMILE): in vivo tissue responses. PLoS One 2017; 12:e0180941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bragheeth MA, Fares U, Dua HS. Re-treatment after laser in situ keratomileusis for correction of myopia and myopic astigmatism. Br J Ophthalmol 2008; 92:1506–1510. [DOI] [PubMed] [Google Scholar]
- 29.Ortega-Usobiaga J, Llovet-Osuna F, Katz T, et al. Comparison of 5468 retreatments after laser in situ keratomileusis by lifting the flap or performing photorefractive keratectomy on the flap. Arch Soc Esp Oftalmol 2018; 93:60–68. [DOI] [PubMed] [Google Scholar]


