Watch a video presentation of this article
Answer questions and earn CME
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- BT
bacterial translocation
- CSPH
clinically significant portal hypertension
- GI
gastrointestinal
- HE
hepatic encephalopathy
- HRS
hepatorenal syndrome
- MDRO
multidrug‐resistant organism
- PH
portal hypertension
- RCT
randomized controlled trial
- SBP
spontaneous bacterial peritonitis
- SID
selective intestinal decontamination
- TMP/SMZ
trimethoprim/sulfamethoxazole
- VH
variceal hemorrhage
Why Bacterial Infections are Important in Cirrhosis?
Cirrhosis is classified into two main prognostic stages: compensated and decompensated, with decompensation defined by the presence of overt complications, specifically ascites, variceal hemorrhage (VH), and encephalopathy (Fig. 1). The compensated stage is the asymptomatic stage with a median survival longer than 12 years. Compensated patients usually transition to the decompensated stage before dying. However, a percentage of them (albeit low) dies before experiencing a decompensating event either from a nonhepatic cause or because of the development of a superimposed acute injury that is often a bacterial infection.1 Patients in the decompensated stage have a much higher mortality outcome (median survival is 2 years), and the main driver of death is an inflammatory state leading to worsening vasodilatation/heart function that results in a stage of “further” decompensation characterized by the development of complications (refractory ascites, hyponatremia, recurrent VH, recurrent hepatic encephalopathy [HE]) and/or to worsening liver function (jaundice, encephalopathy, and/or coagulopathy)2 (Fig. 1). This inflammatory state is most probably the result of the passage of bacteria or bacterial products from the gut lumen to extraintestinal sites, a phenomenon called bacterial translocation (BT).
Figure 1.
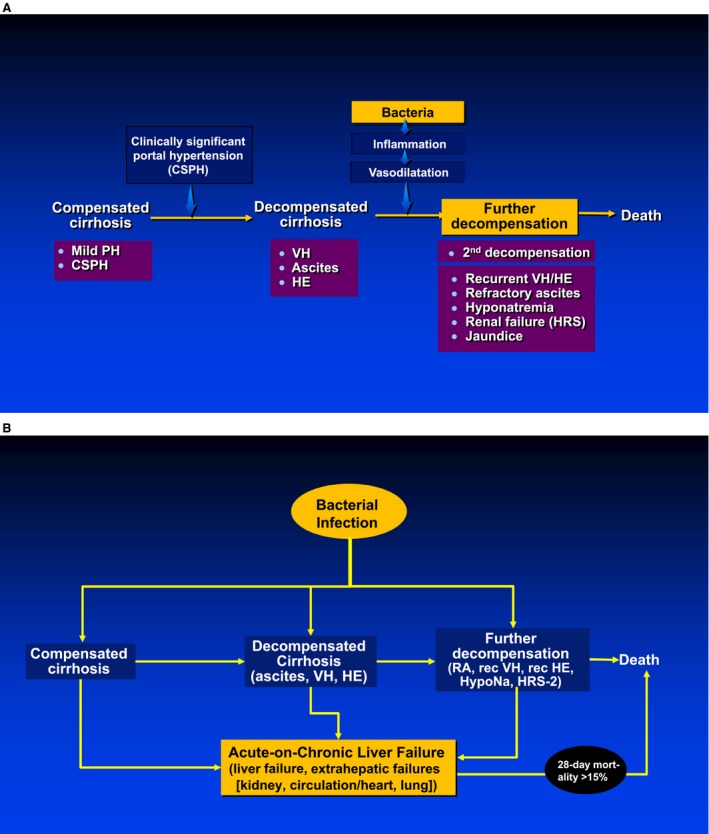
Bacteria (A) and bacterial infections (B) are the main driver of “further” decompensation of cirrhosis and multiorgan failure at every stage of cirrhosis. Both figures 1 and 2 were adapted and reprinted with permission from figures 1 and 2 from Yan and Garcia‐Tsao.17
The development of an overt infection in cirrhosis (at the compensated, decompensated, or further decompensated stages) is associated with an acute inflammatory state that leads to the so‐called acute‐on‐chronic liver failure (ACLF) defined by the presence of liver failure and/or extrahepatic organ failures (kidney, lung, circulation) that, depending on the number of “organs” affected, will lead to a progressively higher short‐term mortality.1, 3 Bacterial infections not only lead to worsening of circulatory dysfunction in cirrhosis, but may also lead to liver injury either through hypovolemia (with consequent ischemic hepatitis) and/or sepsis (with consequent ischemia and hepatocyte apoptosis) (Fig. 1).
The persistent translocation of bacterial products and/or bacterial infections that initially lead to a proinflammatory state eventually leads to an exhaustion of the immune response (immune “paralysis”), leading to an immunodeficient state that favors the development of overt bacterial infections.4 In fact, a recent study showed a high predisposition of patients with ACLF to acquire bacterial infections during hospitalization. Patients with ACLF and bacterial infections (either at diagnosis or during follow‐up) showed a lower 90‐day probability of survival (49%) than patients with ACL F without infection (72%).5
Therefore, if infections (or BT) could be prevented, the downstream complications (further decompensation, repeat infections, ACLF, death) of cirrhosis could also be prevented.
Do Prophylactic Antibiotics Prevent Infections and the Downstream Deleterious Effects Ascribed to Inflammation?
BT is not only the main pathogenic mechanism underlying the development of systemic inflammation in cirrhosis, but it is also the main pathogenic mechanism in the development of overt bacterial infections in cirrhosis. The rate of BT (defined by positive bacteriological cultures from mesenteric lymph nodes) has been shown to be significantly increased in rats with cirrhosis and ascites and in patients with decompensated cirrhosis. Mechanisms of increased BT consist of a combination of impaired gut microbiota (dysbiosis), increased intestinal permeability, and decreased intestinal phagocytic activity (Fig. 2). Intestinal dysbiosis in cirrhosis is characterized by a larger proportion of Bacteroidetes (that includes Enterobacteriaceae) with a lower proportion of Firmicutes (autochthonous potentially beneficial bacteria), a reversal of the distribution in healthy individuals.
Figure 2.
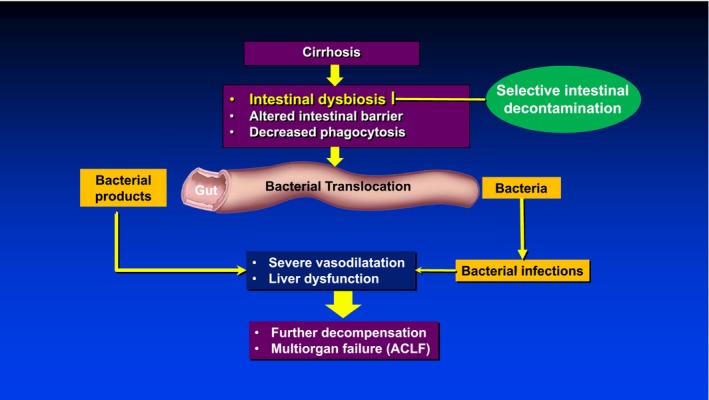
BT is the main pathogenic mechanism leading to systemic inflammation and the cardiocirculatory and liver alterations that lead to multiorgan failure in cirrhosis. The hyperactivation of the immune system leads to immune “paralysis” that, in turn, decreases bacterial clearance and promotes infections. The main mechanisms responsible for BT in cirrhosis are intestinal dysbiosis, altered intestinal barrier, and decreased phagocytosis of intestinal macrophages. Reversing the microbiota alterations through the use of oral antibiotics (SID), mainly norfloxacin, should decrease BT and its downstream deleterious effects in cirrhosis.
Selective intestinal decontamination (SID) consists of the administration of oral antibiotics to inhibit intestinal gram‐negative flora while preserving the remaining flora, especially anaerobic bacteria that are key in preventing intestinal overgrowth and extraintestinal spread of pathogenic bacteria.
In cirrhosis, SID with oral nonabsorbable antibiotics (such as polymyxin, neomycin, gentamycin, colistin) was first used effectively in preventing bacterial infections in patients with cirrhosis and gastrointestinal (GI) hemorrhage. These are no longer recommended because in patients with neutropenia they have been associated with poor tolerance and poor patient compliance. Although trimethoprim‐sulfamethoxazole (TMP/SMZ), another form of SID, has shown to prevent infections in cirrhosis, trials are limited and have included a heterogeneous population of patients with cirrhosis, and in patients with neutropenia, it has been associated with further myelosuppression.
Orally administered quinolones are the form of SID for which there is the most evidence in cirrhosis.6 Quinolones have a broad antimicrobial spectrum (increased activity against gram‐negative bacteria, including Pseudomonas aeruginosa), preservation of the gut anaerobic flora, high concentration in stool, systemic bactericidal activity, good tolerability, and lack of myelosuppression. In patients with neutropenia with cancer, this strategy has been associated with an improvement in mortality.7
In cirrhosis, randomized controlled trials (RCTs) of SID have been shown to reduce infections in patients with GI hemorrhage and to prevent spontaneous bacterial peritonitis (SBP) in high‐risk patients (prior history of SBP or in those with advanced cirrhosis with low ascites protein).6 Importantly, a survival benefit has been observed in those with GI hemorrhage, and a decrease in hepatorenal syndrome (HRS) has been observed in those with advanced cirrhosis and low ascites protein.6 In these studies, prevention of infection was the primary outcome, and mortality (or HRS) was a secondary endpoint. Only one recent RCT investigates the effect of oral norfloxacin (versus placebo) on 6‐month survival in patients with decompensated (Child C) cirrhosis.8 Although there were no differences in overall mortality, survival was greater with norfloxacin in those with an ascites protein level less than 1 g/dL.
Norfloxacin is the quinolone that has been mostly used because of its low solubility, low permeability, and therefore low bioavailability,9 characteristics that theoretically allow it to selectively decontaminate the bowel. However, it was withdrawn from the US market as of April 2014 (not because of safety concerns). Ciprofloxacin, which is widely bioavailable, has been used instead, but its effect on preventing SBP was investigated in only one RCT that failed to show a significant difference in the development of SBP or other bacterial infections when compared with placebo, but was associated with an improvement in survival.10
Therefore, SID prevents infections in patients with cirrhosis and, in selected patients, may prevent the downstream effects of BT/infection, including death.
Can Prophylactic Antibiotics Promote Infections?
A widespread and growing problem in patients with cirrhosis is the development of infections due to multidrug‐resistant organisms (MDRO), manifested clinically as a lack of response to first‐line recommended empiric antibiotics. It has been uniformly shown that infections with MDRO are associated with a high mortality.11
Two epidemiological single‐center studies showed that the prevalence of infections caused by MDRO doubled from less than 10% between 1998 and 2000 to 23% between 2010 and 2011. In the first study, long‐term (>1 month) norfloxacin prophylaxis was associated with a higher rate of SBP because of quinolone‐resistant and/or TMP/SMZ‐resistant organisms.12 In the second study, long‐term norfloxacin prophylaxis together with nosocomial acquisition of infection, recent infection by MDRO, and recent use of β‐lactams were independent predictors of MDRO infections.13 The association between SID and a higher rate of MDRO infections in cirrhosis has also been demonstrated in a single‐center US study14 and in a multicenter prospective study.15 In the more recent prospective “global” study, the prevalence rate of infections due to MDRO in hospitalized patients with cirrhosis was 34%, with independent risk factors being an infection in India, use of therapeutic antibiotics in the previous 3 months, and nosocomial or health care–associated infections. Contrary to previous publications, norfloxacin prophylaxis was not associated with a higher rate of MDRO infections.16
SID in cirrhosis can be associated with asymptomatic intestinal colonization with MDRO, which could not only constitute a source of infection in the individual patient but could also contribute to nosocomial spread of MDRO infections. Therefore, until prospective multicenter studies examine the effect of SID on MDR rates at the individual and population levels, SID should be used only in patients with cirrhosis at the highest risk for development of an infection, and perhaps only in those in whom SID has been associated with a survival benefit. The search for nonantibiotic strategies that target BT should continue to be actively sought.
This study was supported by the Yale Liver Center via National Institutes of Health grant P30 DK34989.
Potential conflict of interest: Nothing to report.
References
- 1. Moreau R, Jalan R, Gines P, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426‐1437. [DOI] [PubMed] [Google Scholar]
- 2. Turco L, Garcia‐Tsao G, Magnani I, et al. Cardiopulmonary hemodynamics and C‐reactive protein as prognostic indicators in compensated and decompensated cirrhosis. J Hepatol 2018;68:949‐958. [DOI] [PubMed] [Google Scholar]
- 3. Bajaj JS, O'Leary JG, Reddy KR, et al. Survival in infection‐related acute‐on‐chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albillos A, Lario M, Alvarez‐Mon M. Cirrhosis‐associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol 2014;61:1385‐1396. [DOI] [PubMed] [Google Scholar]
- 5. Fernandez J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute‐on‐chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2018;67:1870‐1880. [DOI] [PubMed] [Google Scholar]
- 6. Fernández J, Tandon P, Mensa J, et al. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology 2016;63:2019‐2031. [DOI] [PubMed] [Google Scholar]
- 7. Gafter‐Gvili A, Fraser A, Paul M, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev 2012;1:CD004386. [DOI] [PubMed] [Google Scholar]
- 8. Moreau R, Elkrief L, Bureau C, et al.; NORFLOCIR Trial Investigators . Effects of long‐term norfloxacin therapy in patients with advanced cirrhosis. Gastroenterology 2018;155:1816‐1827.e9. [DOI] [PubMed] [Google Scholar]
- 9. Mendes C, Meirelles GC, Silva MAS, et al. Intestinal permeability determinants of norfloxacin in Ussing chamber model. Eur J Pharm Sci 2018;121:236‐242. [DOI] [PubMed] [Google Scholar]
- 10. Terg R, Fassio E, Guevara M, et al. Ciprofloxacin in primary prophylaxis of spontaneous bacterial peritonitis: A randomized, placebo‐controlled study. J Hepatol 2008;48:774‐779. [DOI] [PubMed] [Google Scholar]
- 11. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: A position statement based on the EASL Special Conference 2013. J Hepatol 2014;60:1310‐1324. [DOI] [PubMed] [Google Scholar]
- 12. Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002;35:140‐148. [DOI] [PubMed] [Google Scholar]
- 13. Fernandez J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology 2012;55:1551‐1561. [DOI] [PubMed] [Google Scholar]
- 14. Tandon P, Delisle A, Topal JE, et al. High prevalence of antibiotic‐resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol 2012;10:1291‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salerno F, Borzio M, Pedicino C, et al. The impact of infection by multidrug‐resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int 2017;37:71‐79. [DOI] [PubMed] [Google Scholar]
- 16. Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol 2013;59:482‐489. [DOI] [PubMed] [Google Scholar]
- 17. Yan K, Garcia‐Tsao G. Novel prevention strategies for bacterial infections in cirrhosis. Expert Opin Pharmaco 2016;17:689‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]


