Watch a video presentation of this article
Watch the interview with the author
Answer questions and earn CME
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- AKI
acute kidney injury
- DAMP
damage‐associated molecular pattern
- DILI
drug‐induced liver injury
- EABV
effective arterial blood volume
- HE
hepatic encephalopathy
- HRS
hepatorenal syndrome
- NO
nitric oxide
- N.S.
not significant
- PAMP
pathogen‐associated molecular pattern
- SBP
spontaneous bacterial peritonitis
- SCr
serum creatinine
Bacterial infections are common in cirrhosis, estimated to occur in 25% to 46% of patients hospitalized with acute decompensation of cirrhosis.1 The presence of infection triggers a surge of inflammatory response,2 superimposed on the chronic systemic inflammation related to intestinal dysbiosis, loss of intestinal mucosal integrity, and increased bacterial translocation that is usually present in these patients.3 Many of the pathogen‐associated molecular patterns (PAMPs) released by the bacteria have vasodilatory properties, further compromising the systemic vasodilatation that is already present in these patients, leading to organ hypoperfusion, potentially causing cell injury and cell death. As a result of the deleterious effects of the inflammatory mediators on organ homeostasis, organ failure is a common sequala of bacterial infections in cirrhosis (Fig. 1). The occurrence of organ failures in the setting of acute decompensation of a previously stable patient with cirrhosis describes the syndrome of acute‐on‐chronic liver failure (ACLF), which is associated with high short‐term mortality.4 Once ACLF is present, the patient with cirrhosis is predisposed to the development of second infections, related to immune exhaustion associated with exaggerated systemic inflammatory response observed in ACLF,5 thereby further increasing the mortality risks of these patients.
Figure 1.
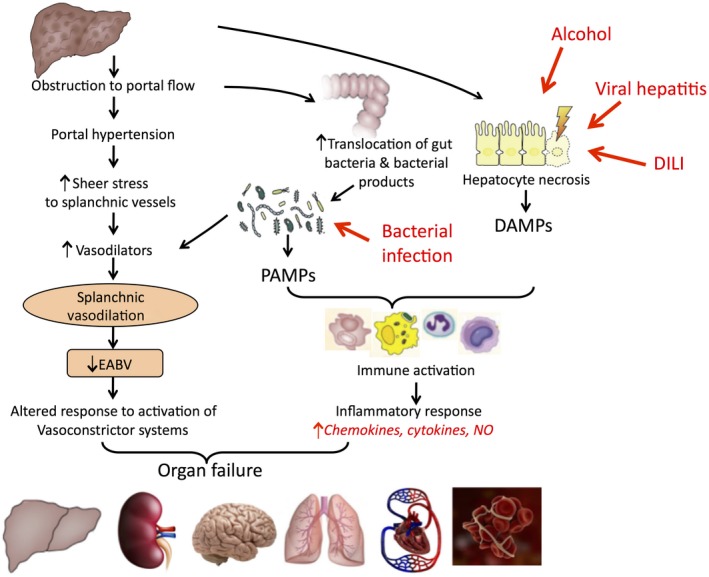
Pathophysiological mechanisms that lead to organ failure in advanced cirrhosis: roles of hemodynamic changes and inflammation.
Incidence of Organ Failures With Bacterial Infections in Cirrhosis
In a multicenter North American study that enrolled only infected patients with cirrhosis admitted to hospital, the incidence rates of various organ failures were as follows: kidney, 15.1%; brain, 55.7%; circulation, 17.6%; and respiratory, 15.8%.6 In contrast, in a European multicenter CLIF Acute‐oN‐ChrONic LIver Failure in Cirrhosis (CANONIC) study that included both infected and noninfected patients with cirrhosis who were hospitalized, the incidence rates of kidney (55.8%) and brain (24.1%) failures were significantly different,7 but those for circulation (16.8%) and respiratory (9.2%) failures were similar. The CANONIC study also described liver failure (43.6%) and coagulation failure (27.7%). The disparity in the incidence of various organ failures is related to the populations of patients enrolled (infected only versus infected + noninfected patients), and the differences in the definition of organ failures.6, 7
Individual Organ Failures
Renal Failure
Bacterial infections are the most common cause of renal failure, accounting for up to 46% of all cases in hospitalized patients with cirrhosis.8 Spontaneous bacterial peritonitis (SBP) and urinary tract infections were the most common precipitants for the development of renal failure in the North American study,6 whereas SBP and respiratory infections were the most common precipitants in the CANONIC study.7 Irrespective of the source of infection, the intense inflammatory reaction evoked by the presence of bacteria with the production of inflammatory cytokines can lead to oxidative stress in the peritubular microcirculation of the kidneys, causing sluggish flow and microthrombi formation. PAMPs and inflammatory cytokines filtered through the glomeruli can trigger further oxidative stress, causing tubular cell death,9 leading to the release of damage‐associated molecular patterns (DAMPs), and further exaggerating the cycle of compromised microcirculation and exposure to cell‐damaging mediators. When superimposed on the relative renal vasoconstriction that is usually observed in patients with decompensated cirrhosis, renal failure commonly ensues.
Therefore, in patients with decompensated cirrhosis who are admitted to hospital with bacterial infection, it is imperative that these patients have their renal function monitored closely for the development of renal failure. This is particularly true in patients who have background renal dysfunction, whose higher baseline serum creatinine (SCr) concentration predisposes them to a much worse prognosis when renal failure occurs.10 Such patients tend to have a higher peak SCr concentration with their acute kidney injury (AKI) episode and are more likely to have a progressive rather than a transient course of their AKI. Patients should also be monitored for other organ failures and preventive measures be applied if possible, because the presence of other organ failures will also make the patient less likely to respond to treatment despite the same severity of baseline renal dysfunction.11
The management of renal failure in cirrhosis begins with prompt identification of the infection and administration of appropriate antibiotics. Other precipitating factors also have to be identified, such as dehydration from excess diuretic use, and treated.12 Any evidence of structural renal disease needs to be diagnosed and managed according to the etiology. It is recommended that albumin should be administered to patients with cirrhosis and AKI12 at a dosage of 1 g/kg of body weight, up to 100 g/day, not only for its oncotic properties to improve the circulatory volume, but also for its antioxidant and immune‐modulatory properties to dampen the inflammatory response.13 For patients who are not responding to these measures, and also meeting the diagnostic criteria of AKI‐hepatorenal syndrome (AKI‐HRS)14 (Table 1), treatment with a vasoconstrictor should be given.12 Terlipressin is the most studied vasoconstrictor to date, followed by norepinephrine, and then midodrine/octreotide combination.15 Terlipressin has been shown to be equally efficacious as norepinephrine in the management of AKI‐HRS,16 and it is much superior than the midodrine/octreotide combination in improving renal function.17 However, in the context of ACLF and other organ failures, terlipressin is better than norepinephrine in terms of achieving an earlier and higher response rate in patients with AKI‐HRS.18
Table 1.
Diagnostic Criteria for and Staging of AKI‐HRS
| Cirrhosis and ascites | ||
| Stage 2 or 3 AKI | ||
| No improvement of SCr concentration (decrease of creatinine ≤0.3 mg/dL of baseline) after at least 48 hours of diuretic withdrawal and volume expansion with albumin (1 g/kg body weight/day for 2 days) | ||
| Absence of hypovolemic shock or severe infection requiring vasoactive drugs to maintain arterial pressure | ||
| No current or recent treatment with nephrotoxic drugs | ||
| Proteinuria <500 mg/day and no microhematuria (<50 red blood cells/mL) | ||
| Staging | Stage 1: | ↑ SCr ≥ 26.4 μmol/L (0.3 mg/dL) or |
| ↑ SCr ≥ 1.5–2.0× from baseline | ||
| Stage 2: | ↑ SCr ≥ 2.0–3.0× from baseline | |
| Stage 3: | ↑ SCr >3.0× from baseline, or | |
| SCr ≥ 352 μmol/L (4.0 mg/dL) with an acute ↑ of ≥26.4 μmol/L), or | ||
| Initiation of renal replacement therapy | ||
Adapted with permission from Gut.14 Copyright 2015, BMJ Publishing Group Ltd & British Society of Gastroenterology.
Brain Failure
Brain failure manifests clinically as overt hepatic encephalopathy (HE), grade ≥3 according to the West Haven criteria. The inflammatory milieu of a patient with bacterial infection increases the permeability of the blood‐brain barrier. The diffusion of ammonia into the brain increases the amidation of glutamate to form glutamine.19 Glutamine is an osmolyte. This ammonia elimination pathway leads to accumulation of glutamine in astrocytes, resulting in astrocyte swelling and impaired neuronal signal transmission.19 Glutamine, in turn, can be transported from the cytoplasm of the astrocyte into its mitochondria and deaminated again to form ammonia, which can lead to collapse of the inner mitochondrial potential, free radical generation, and oxidative damage of mitochondrial components.19 The presence of infection can also significantly increase cerebral blood flow and exaggerate the earlier cycle of events.
Merli et al.20 have shown that cognitive impairment is consistently associated with the presence of bacterial infection, being more pronounced with the development of sepsis, but improved on resolution of infection. Repeated episodes of more than three infections per year can increase the risk for the development of HE by 10‐fold. The infections that are most likely to cause HE are SBP, biliary tract infection, and sepsis.21 Of course, the presence of other organ failures with the infective episode can also exacerbate HE. For example, renal failure will lead to decreased excretion of nitrogenous compounds, liver failure will lead to reduced removal of ammonia to form urea, respiratory failure with respiratory alkalosis will reduce the amount of ammonia being converted to ammonium, and circulatory failure with hypotension will interfere with brain autoregulation.
It is important to recognize that not every patient with cirrhosis with decompensation who presents with a confusional state has HE. The differential diagnosis is wide ranging, including complications related to poor diabetic control such as diabetic ketoacidosis, hypoglycemic coma, or hyperosmolar coma; alcohol intoxication or withdrawal; Wernicke encephalopathy; drug overdose; intracranial bleed; seizure disorder; electrolyte abnormalities such as hyponatremia or hypercalcemia; meningitis; or psychiatric illness. Therefore, it is imperative that patients who present confused or have an impaired conscious state have these conditions excluded. Once HE is diagnosed, treatment of the acute episode usually consists of lactulose, given either orally or rectally. Lactulose is converted to volatile fatty acids by gut bacteria, thereby decreasing the pH of the intestinal contents, favoring the formation of ammonium from ammonia. There are also data suggesting that the use of lactulose can decrease the translocation of bacterial products across the gut wall, associated with improving neurocognitive function.22 Once the patient recovers from the acute episode, regular lactulose use should be maintained to prevent HE recurrence. Rifaximin is a nonabsorbable antibiotic that targets bacterial DNA‐dependent RNA polymerase, thereby blocking one of the steps in transcription. This results in inhibition of bacterial protein synthesis and consequently inhibits bacterial growth, with consequent reduction in ammonia production by colonic bacteria. Rifaximin is not approved for treatment of acute HE, but rather is used in combination with lactulose to prevent HE recurrence.23 Other treatments that have been reported to have some success in the management of acute HE include ammonia scavengers such as polyethylene glycol and glycerol phenylacetate.
Circulatory Failure
Several inflammatory cytokines have direct cardiomyocyte toxic effects, and it is not uncommon to see a mild increase in troponin levels in patients who experience development of sepsis. This, when added to the presence of cirrhotic cardiomyopathy, may lead to reduced ventricular function with lower left ventricular ejection fraction. When coupled with the volume challenge that is frequently given to patients with concomitant renal failure, cardiac failure may ensue.2 The systemic vasodilatation associated with the inflammation from the bacterial infection often makes the cardiac failure more difficult to manage. Prompt antibiotic use and vasopressor support are essential in the management of patients with infection and cardiac failure.
Respiratory Failure
Respiratory failure can occur as a result of bacterial infection in the lung, causing pneumonia, or from aspiration of gastric contents in a patient with high‐grade HE. Infection source other than the lung can cause inflammation‐mediated capillary damage in the lungs, leading to the development of noncardiogenic pulmonary edema. This decreases pulmonary compliance and increases the labor of breathing. The hallmark is tachypnea, and in a sick patient with cirrhosis, respiratory muscle fatigue soon follows, associated with hypoxemia and hypercarbia. A chest radiograph will show bilateral pulmonary infiltrates, indistinguishable from features of cardiac failure. Therefore, these patients will need intubation and mechanical ventilation until recovery from their respiratory failure.
Impact of Organ Failures on Patient Survival
In a North American study enrolling patients hospitalized with bacterial infections, the development of infection‐associated organ failure was associated with a reduced 30‐day survival.6 ACLF, defined as at least two extrahepatic organ failures in this particular study, was the strongest predictor of 30‐day survival associated with infection, which progressively decreased as the number of organ failures increases.6 The presence of circulatory failure, whether alone or in combination with other organ failures, was associated with the worse prognosis.6 Similar findings were reported by a European study, which showed significant reduction in 90‐day transplant‐free survival in patients with bacterial infection and organ failure when compared with those without24 (Fig. 2). Once again, the more organ failures the patient had, the worse the prognosis. It is interesting that in this particular study, bacterial infections not only can precipitate ACLF, but they can also follow the onset of ACLF, triggered by other noninfectious precipitants, with the same unfavorable prognosis. Therefore, it is imperative that physicians caring for patients with decompensated cirrhosis be vigilant for the development of bacterial infections. Once bacterial infection is suspected, patients need to be treated promptly with adequate antibiotics and be supported should ACLF develop. Infection control and prophylactic measures should be part of the treatment strategy.
Figure 2.
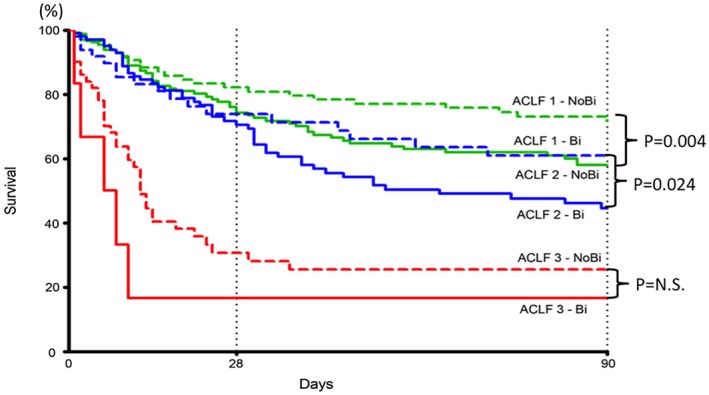
Probability of 90‐day transplant‐free survival in patients with ACLF‐1 (green), ACLF‐2 (blue), and ACLF‐3 (red) with (continuous lines) and without (discontinued lines) bacterial infections either at diagnosis of ACLF or during follow‐up after the diagnosis of ACLF. Adapted with permission from Gut.24 Copyright 2018, BMJ Publishing Group Ltd & British Society of Gastroenterology.
Potential conflict of interest: Nothing to report.
References
- 1. Piano S, Brocca A, Mareso S, Angeli P. Infections complicating cirrhosis. Liver Int 2018;38(suppl 1):126‐133. [DOI] [PubMed] [Google Scholar]
- 2. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nature Rev Dis Primers 2016;2:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63:1272‐1284. [DOI] [PubMed] [Google Scholar]
- 4. Arroyo V, Moreau R, Jalan R, Ginès P; EASL‐CLIF Consortium CANONIC Study . Acute‐on‐chronic liver failure: a new syndrome that will re‐classify cirrhosis. J Hepatol 2015;62:S131‐S143. [DOI] [PubMed] [Google Scholar]
- 5. Clària J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute‐on‐chronic liver failure. Hepatology 2016;64:1249‐1264. [DOI] [PubMed] [Google Scholar]
- 6. Bajaj JS, O'Leary JG, Reddy KR, et al.; North American Consortium for the Study of End‐Stage Liver Disease (NACSELD) . Survival in infection‐related acute‐on‐chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moreau R, Jalan R, Gines P, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426‐1437. [DOI] [PubMed] [Google Scholar]
- 8. Martín‐Llahí M, Guevara M, Torre A, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 2011;140:488‐496. [DOI] [PubMed] [Google Scholar]
- 9. Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis‐induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014;41:3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong F, O'Leary JG, Reddy KR, et al. Acute kidney injury in cirrhosis: baseline serum creatinine predicts patient outcomes. Am J Gastroenterol 2017;112:1103‐1110. [DOI] [PubMed] [Google Scholar]
- 11. Piano S, Schmidt H, Ariza X, et al. Association between grade of acute‐on‐chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol 2018;16:1792‐1800. [DOI] [PubMed] [Google Scholar]
- 12. Wong F, Angeli P. New diagnostic criteria and management of acute kidney injury in cirrhosis. J Hepatol 2017;66:860‐861. [DOI] [PubMed] [Google Scholar]
- 13. Garcia‐Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58:1836‐1846. [DOI] [PubMed] [Google Scholar]
- 14. Angeli P, Ginès P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015;64:531‐537. [DOI] [PubMed] [Google Scholar]
- 15. Facciorusso A, Chandar AK, Murad MH, et al. Comparative efficacy of pharmacological strategies for management of type 1 hepatorenal syndrome: a systematic review and network meta‐analysis. Lancet Gastroenterol Hepatol 2017;2:94‐102. [DOI] [PubMed] [Google Scholar]
- 16. Mattos AZ, Mattos AA, Ribeiro RA. Terlipressin versus noradrenaline in the treatment of hepatorenal syndrome: systematic review with metaanalysis and full economic evaluation. Eur J Gastroenterol Hepatol 2016;28:345‐351. [DOI] [PubMed] [Google Scholar]
- 17. Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology 2015;62:567‐574. [DOI] [PubMed] [Google Scholar]
- 18. Arora V, Maiwall R, Rajan V, et al. Terlipressin is superior to noradrenaline in the management of acute kidney injury in acute on chronic liver failure. Hepatology; 10.1002/hep.30208. [DOI] [PubMed] [Google Scholar]
- 19. Coltart I, Tranah TH, Shawcross DL. Inflammation and hepatic encephalopathy. Arch Biochem Biophy 2013;536:189‐196. [DOI] [PubMed] [Google Scholar]
- 20. Merli M, Lucidi C, Pentassuglio I, et al. Increased risk of cognitive impairment in cirrhotic patients with bacterial infections. J Hepatol 2013;59:243‐250. [DOI] [PubMed] [Google Scholar]
- 21. Yuan LT, Chuah SK, Yang SC, et al. Multiple bacterial infections increase the risk of hepatic encephalopathy in patients with cirrhosis. PLoS ONE 2018;13:e0197127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moratalla A, Ampuero J, Bellot P, et al. Lactulose reduces bacterial DNA translocation, which worsens neurocognitive shape in cirrhotic patients with minimal hepatic encephalopathy. Liver Int 2017;37:212‐223. [DOI] [PubMed] [Google Scholar]
- 23. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362:1071‐1081. [DOI] [PubMed] [Google Scholar]
- 24. Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute‐on‐chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 2018;67:1870‐1880. [DOI] [PubMed] [Google Scholar]


