Watch a video presentation of this article
Answer questions and earn CME
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- CDR
cirrhosis/dysbiosis ratio
- HE
hepatic encephalopathy
- ICU
intensive care unit
- LDA
linear discriminant analysis
- outpts
outpatients
- PPI
proton pump inhibitor
- SBP
spontaneous bacterial peritonitis
Patients with cirrhosis are predisposed to infections, which often have a very poor prognosis.1 Most of this poor prognosis stems from the development of end‐stage organ diseases and nosocomial infections that result in acute‐on‐chronic liver failure (ACLF).2 Infections are one of the most common precipitants of ACLF in Western countries.2 Once this organ failure cycle sets in, it is difficult to reverse, which is why we need to determine prognostic markers that predict the early development of infections and subsequent ACLF. A large proportion of infections in cirrhosis are related to gut barrier changes such as spontaneous bacterial peritonitis (SBP), and gut microbiota could be a relevant focus of study.3, 4
Gut Microbiota Changes in Cirrhosis
Patients with cirrhosis have an altered gut‐liver axis related to changes in liver disease severity, gut barrier impairment, and intestinal and systemic inflammation that are associated with changes in gut microbiota composition and function. Cirrhosis is associated with an altered immune response that potentially allows for dysbiosis or an altered microbiota in the stool, intestinal mucosa, ascites, liver, serum, and saliva.5 In the gut specifically, there is an increased relative abundance of bacterial taxa belonging to Enterobacteriaceae (includes gram‐negative rods such as Escherichia coli and Klebsiella), Enterococcaceae (includes Enterococcus faecalis and E. faecium), Streptococcaceae, and lower potentially beneficial taxa such as Lachnospiraceae and Ruminococcaceae in advancing cirrhosis.3, 6 In addition to these changes in microbial composition, there are also changes in bacterial function, including higher endotoxin, lower conversion from primary to secondary bile acids, and reduced short‐chain fatty acids. These changes in cirrhosis can have real‐world consequences.7
Gut Microbiota Changes and Clinical Consequences in Outpatients With Cirrhosis
In outpatients with cirrhosis, there are several outcomes of interest such as hospitalizations, cognitive dysfunction, and hepatic encephalopathy (HE). Studies on stool and colonic mucosal and salivary microbiota are associated with 90‐day hospitalizations in cirrhosis independent of clinical covariates.7 This is similar regardless of whether the DNA or RNA content of the stool was used. Stool and colonic mucosal microbiota are also linked with brain function on cognitive tests and magnetic resonance imaging, with oral‐origin microbiota such as Porphyromonadaceae associated with neuronal and Enterobacteriaceae, Lachnospiraceae, and Ruminococcaceae with astrocytic changes.8
Gut Microbiota Changes in Inpatients With Infection With Cirrhosis
The cirrhosis/dysbiosis ratio (CDR) was created to simplify the enormous complexity of gut microbiota. CDR is the ratio of potentially beneficial bacteria (Lachnospiraceae + Ruminococcaceae + Clostridium Cluster XIV + Veillonellaceae) in the numerator and potentially pathogenic taxa (Enterobacteriaceae + Bacteroidaceae) as the denominator.3 Therefore, a high ratio indicates healthy microbiota. As shown in Fig. 1, the best CDR was in controls and the worst/lowest one was in inpatients with infection, the majority of whom had gut‐associated infections such as SBP. This ratio also had prognostic value and was stable in clinically stable patients over time.
Figure 1.
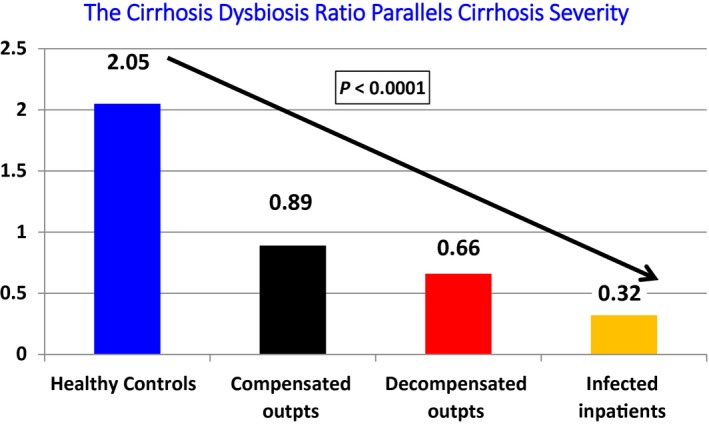
Changes in CDR in different groups. CDR was also stable over time, worsened with the development of the first episode of HE, and was worse in those who were subsequently hospitalized. Adapted from Journal of Hepatology.3 Copyright 2014, European Association for the Study of the Liver.
Because patients with infection are often more clinically advanced and are usually receiving antibiotics, the analysis of gut microbiota in these patients needs careful interpretation. Antibiotic therapy can affect culture results relatively quickly, but the 16srRNA/bacterial DNA over 48 to 72 hours in these patients remains similar to preantibiotic levels.3 In addition, when inpatients with infection were Model for End‐Stage Liver Disease matched to inpatients without infection, the patients with infection still had more dysbiosis than the uninfected ones.3 In multicenter studies, patients with infection whose stool was collected within 24 hours of admission had greater relative abundance of potentially pathogenic Enterococcaceae and lower Bifidobacteriaceae compared with patients without infection. A specific group of patients can acquire fungal infections, which carry an even worse prognosis than bacterial infections.9 The rate of fungal infections can be 10% and are almost always preceded by bacterial infections or antibiotic use. Antibiotics, whether in the outpatient or inpatient setting, can result in the collapse of bacterial diversity, which in turn reduces the fungal diversity and encourages the growth of Candida.10 These can also have an independent prognostic impact related to 90‐day hospitalizations. Therefore, indiscriminate use of antibiotics can not only predispose to multidrug‐resistant bacteria but also fungi.
These findings suggest that dysbiosis likely predisposes to the development of infection rather than being a consequence of this. Therefore, measures to curb this dysbiosis (see the following section) in the outpatient setting may be relevant.
Gut Microbiota Changes and Association with Inpatient OUTCOMES
In a single‐center study, patients with cirrhosis who experienced development of ACLF, individual organ failures, or those who died within 30 days had a significantly different gut microbiota on admission compared with patients who were free of these outcomes.3 Specifically, lower Lachnospiraceae, higher serum endotoxin, and lower CDR were associated with these outcomes. In another study from China where more frequent monitoring of gut microbiota was performed, there was a clear separation between patients who died or survived, with higher Pasteurellaceae in those who died.11 Given that practices vary across centers, a multicenter approach is necessary. A recent study from multiple US and Canadian centers found that admission microbiota was different in those who had ACLF, required intensive care unit (ICU) transfer, experienced individual organ failures, or died within 30 days12 (Fig. 2). Specific microbiota found in relatively higher abundance in those who experienced negative outcomes belonged to Enterococcaceae and the phylum Proteobacteria with relatively lower Lachnospiraceae. However, despite maximum standard of care, the patients with dysbiosis on admission continued to have these negative outcomes. Therefore, it is likely that once the patient is hospitalized, it may be too late to effect changes in his or her natural history, and beneficial alterations in gut microbiota in outpatients might be needed.
Figure 2.
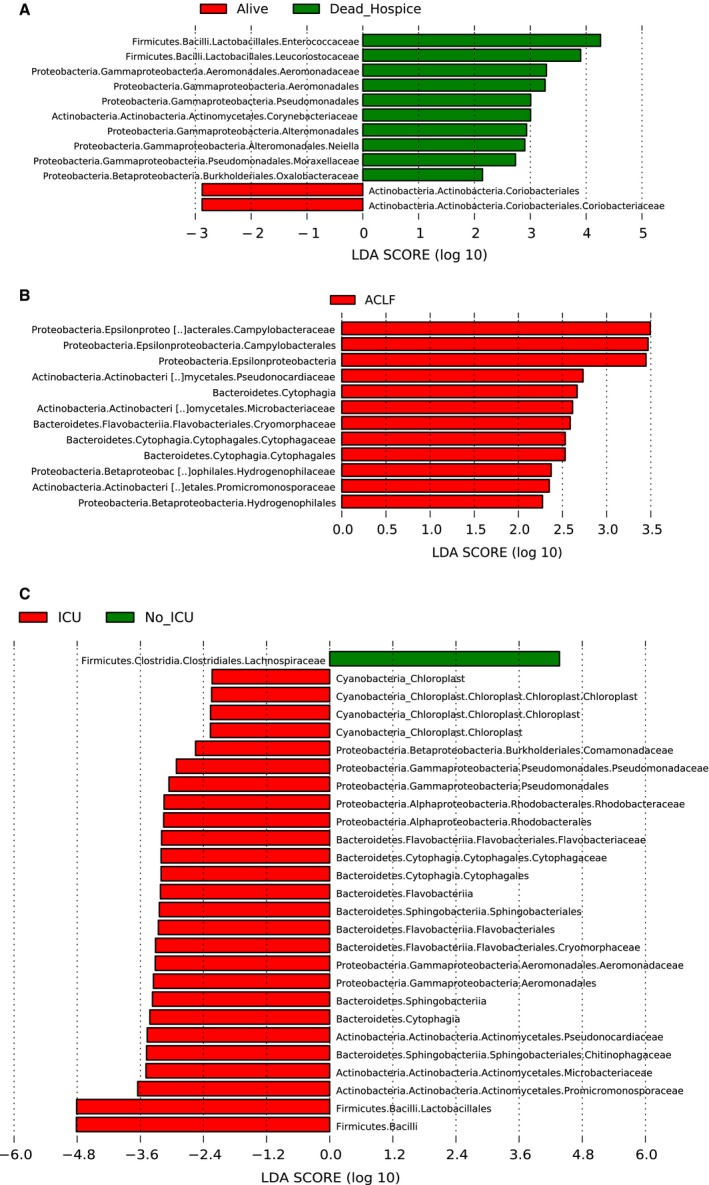
Admission microbiota was associated with inpatient outcomes in a multicenter study. Linear discriminant analysis (LDA) effect size comparisons were performed between patients who did or did not develop outcomes. (A) Alive (red) and dead or hospice (green). (B) Red represents the taxa higher in those who had ACLF. (C) ICU transfer (red) compared with those without ICU stay (green). Reproduced with permission from Clinical Gastroenterology and Hepatology.12 Copyright 2019, Elsevier.
Measures to Potentially Improve Gut Microbiota to Affect Outcomes
Gut microbiota can be affected by several inputs including diet, ethnicity, stages of disease, and medications.7 In outpatients with cirrhosis, gut microbiota diversity is associated with a Middle Eastern diet that has daily fermented milk product intake compared with a Western diet.13 In addition, coffee/tea and vegetables were associated with lower rate of hospitalizations, along with the microbial diversity when Turkish and American cohorts were compared.13 A major reason for dysbiosis with bacteria usually found in the oral cavity (i.e., Streptococcaceae, Veillonellaceae, and Porphyromonadaceae being found in the stool) is due to the use of proton pump inhibitors (PPIs). In compensated and decompensated cirrhosis, the initiation of 14 days of omeprazole therapy was associated with an increase in these oral‐origin bacteria, and importantly, withdrawal of these bacteria was associated with resolution of this dysbiosis.14, 15 In advanced cirrhosis, PPI withdrawal could beneficially impact gut microbial dysbiosis.14 In addition to oral origin microbiota, the oral cavity itself is often a source of inflammation and endotoxemia because of poor dental hygiene and periodontitis. In a small study, dental cleaning was associated with improvement in salivary and gut microbial dysbiosis with cognitive and quality‐of‐life improvement in those with prior HE.13 In addition, alcohol misuse, which has also been associated with dysbiosis, should be discouraged.
Conclusions
Alterations in gut microbiota in cirrhosis can play an important role in the progression of disease from the outpatient to inpatient settings (Table 1). Favorable modification of the microbiota could potentially reduce these negative outcomes. These steps could include: (1) withdraw unnecessary PPI use; (2) encourage probiotics in foods with a Mediterranean dietary pattern; (3) avoid therapies, including over‐the‐counter probiotics that are not approved by the US Food and Drug Administration; and (4) encourage dental cleaning and taking care of the oral health of patients.
Table 1.
Potential Measures to Beneficially Alter Gut Microbiota in Cirrhosis
| 1. Reduce unnecessary antibiotic use. |
| 2. Withdraw unneeded PPI therapy and replace with H2 receptor blockers. |
| 3. Encourage fermented food intake with potential probiotic properties. |
| 4. Encourage regular oral cleaning and dental appointments. |
| 5. Avoid alcohol misuse. |
Potential conflict of interest: Nothing to report.
References
- 1. Tandon P, Garcia‐Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 2008;28:26‐42. [DOI] [PubMed] [Google Scholar]
- 2. Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute‐on‐chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2018;67:1870‐1880. [DOI] [PubMed] [Google Scholar]
- 3. Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bajaj JS, O'Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: The North American consortium for the study of end‐stage liver disease (NACSELD) experience. Hepatology 2012;56:2328‐2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albillos A, Lario M, Alvarez‐Mon M. Cirrhosis‐associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol 2014;61:1385‐1396. [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011;54:562‐572. [DOI] [PubMed] [Google Scholar]
- 7. Acharya C, Bajaj JS. The microbiome in cirrhosis and its complications. Clin Gastroenterol Hepatol 2019;17(2):307‐321. doi: 10.1016/j.cgh.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahluwalia V, Betrapally NS, Hylemon PB, et al. Impaired gut‐liver‐brain axis in patients with cirrhosis. Sci Rep 2016;6:26800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bajaj JS, Reddy KR, Tandon P, et al. Prediction of fungal infection development and their impact on survival using the NACSELD Cohort. Am J Gastroenterol 2018;113:556‐563. [DOI] [PubMed] [Google Scholar]
- 10. Bajaj JS, Liu EJ, Kheradman R, et al. Fungal dysbiosis in cirrhosis. Gut 2018;67:1146‐1154. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Guo J, Qian G, et al. Gut dysbiosis in acute‐on‐chronic liver failure and its predictive value for mortality. J Gastroenterol Hepatol 2015;30:1429‐1437. [DOI] [PubMed] [Google Scholar]
- 12. Bajaj JS, Vargas HE, Reddy KR, et al. Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis. Clin Gastroenterol Hepatol 2019;17:756‐765.e3. [DOI] [PubMed] [Google Scholar]
- 13. Bajaj JS, Idilman R, Mabudian L, et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology 2018;68:234‐247. [DOI] [PubMed] [Google Scholar]
- 14. Bajaj JS, Acharya C, Fagan A, et al. Proton pump inhibitor initiation and withdrawal affects gut microbiota and readmission risk in cirrhosis. Am J Gastroenterol 2018;113:1177‐1186. [DOI] [PubMed] [Google Scholar]
- 15. Bajaj JS, Cox IJ, Betrapally NS, et al. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol 2014;307:G951‐G957. [DOI] [PMC free article] [PubMed] [Google Scholar]


