This systematic review and meta-analysis assesses the association of rotavirus vaccination among neonates and infants with risk of intussusception for up to 2 years after vaccination overall and by type of vaccine.
Key Points
Question
What is the association between rotavirus vaccination and risk of intussusception?
Findings
In this systematic review and meta-analysis of 25 randomized clinical trials including 200 594 participants (104 647 receiving vaccine and 95 947 receiving placebo) in 33 countries from 4 continents, monovalent, pentavalent, monovalent human-bovine, oral bovine pentavalent, and human neonatal rotavirus vaccinations were not associated with an increased risk of intussusception compared with placebo for up to 2 years after vaccination.
Meaning
The findings suggest that rotavirus vaccination is not associated with an increased risk of intussusception for up to 2 years after vaccination among neonates or infants.
Abstract
Importance
The conclusions from the multiple randomized clinical trials exploring the relationship between development of intussusception and rotavirus vaccination among neonates and infants have been controversial.
Objective
To evaluate the association between rotavirus vaccination and risk of intussusception.
Data Sources
For this systematic review and meta-analysis, PubMed, Web of Science, Cochrane library, and Embase databases were searched from January 1, 1999, through December 31, 2018, using no language restrictions. The search terms were rotavirus or RV (rotavirus vaccine) or HRV (human rotavirus vaccine), vaccin*, and intussusception.
Study Selection
Randomized clinical trials of neonates and infants that compared the risk of intussusception after the vaccination with a placebo group were included.
Data Extraction and Synthesis
A fixed-effects model was used to pool the data. Statistical heterogeneity was assessed with Q test and I2 statistic; relative risk (RR), risk difference (RD), and 95% CIs were calculated using the Mantel-Haenszel method.
Main Outcomes and Measures
The main outcome was the diagnosis of intussusception in the analysis. The pooled and subtotal results of RR, RD, and 95% CI for the risk of intussusception were estimated at 31 days, 1 year, and 2 years after vaccination.
Results
A total of 25 randomized clinical trials including 200 594 participants (104 647 receiving vaccine and 95 947 receiving placebo) in 33 countries from 4 continents were included in this meta-analysis. Twenty cases of definite intussusception were diagnosed within 31 days after rotavirus vaccination, with 11 cases (55%) in the vaccine group and 9 cases (45%) in the placebo group (RD, 0.17 per 10 000 infants [95% CI, −1.16 to 1.50 per 10 000 infants], P = .80; RR, 1.14 [95% CI, 0.49 to 2.64], P = .77). Seventy-four cases were reported within 1 year, with 37 cases (50%) in the vaccine group and 37 cases (50%) in the placebo group (RD, −0.65 per 10 000 infants [95% CI, −2.68 to 1.39 per 10 000 infants], P = .53; RR, 0.84 [95% CI, 0.53 to 1.32], P = .45). Fifty-nine cases were reported within 2 years, with 29 cases (49%) in the vaccine group and 30 cases (51%) in the placebo group (RD, −0.48 per 10 000 infants [95% CI, −3.64 to 2.69 per 10 000 infants], P = .77; RR, 0.91 [95% CI, 0.55 to 1.52], P = .73).
Conclusions and Relevance
Results of this systematic review and meta-analysis suggest that monovalent, pentavalent, monovalent human-bovine, oral bovine pentavalent, and human neonatal rotavirus vaccination was not associated with an elevated risk of intussusception among neonates or infants.
Introduction
Vaccines play an important role in the prevention of infectious diseases. Rotavirus (RV) vaccination has significantly reduced the occurrence and severity of RV-related gastroenteritis and mortality among infants and young children.1 Although data from some clinical trials show that the efficacy of RV vaccines for prevention of RV gastroenteritis reaches 36% to 96% within a year of follow-up,2,3 the Global Advisory Committee on Vaccine Safety has noted that the use of RV vaccines may be associated with an increased risk of intussusception.4 Even though the efficacy of the vaccine might outweigh the small potential risk of intussusception, the Global Advisory Committee on Vaccine Safety has also suggested performance of active surveillance to ensure that the long-term benefit and safety of RV vaccines are entirely assessed.4
Data from randomized clinical trials (RCTs) regarding the efficacy and safety of RV vaccines show conflicting evidence on the incidence of intussusception. Whether or not there was an association between vaccination and an increased risk of intussusception, the answer varied across studies.5,6,7,8 The varying data from the RCTs about the difference in the incidence of intussusception could be attributable to multiple variables such as age, sex, geographic and population distribution of the participants, and the different types of RV vaccines used in these studies. However, the reasons behind the differing risk of intussusception are still not clear. Therefore, we conducted this systematic review and meta-analysis of published RCTs to further assess the risk of development of intussusception after RV vaccination.
Methods
This systematic review and meta-analysis followed the Cochrane Collaboration Group9 and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)10 reporting guidelines. The construction of databases, article screening, article quality evaluation, and data extraction were independently completed by 2 of us (H.-L.L. and Y.D.). Discrepancies were resolved by consensus or, if necessary, with the assistance of one of us (H.-G.X.).
Literature Search and Study Selection
The PubMed, Web of Science, Cochrane library, and Embase databases were searched from January 1, 1999, through December 31, 2018, with no language restrictions. The search teams were ([rotavirus or RV (rotavirus) or HRV (human rotavirus vaccine)] and vaccin*) and (intussusception or intestinal invagination or indignation or invagination intextinorum) and (Clinical Trial[PTyp] and (1999/01/01[PDat]:2018/12/31[PDat]). In addition, references from the relevant articles were searched for appropriate studies.
This meta-analysis focused on the association between RV vaccination and the risk of intussusception. Only RCTs that compared the risk of intussusception between the vaccine and placebo groups for neonates or infants were included in the analysis. Other inclusion criteria included the following: (1) use of any type of vaccination, such as monovalent (RV1) (Rotarix; GlaxoSmithKline), pentavalent (RV5) (RotaTeq; Merck & Co, Inc), monovalent human-bovine (116E) (Rotavac; Bharat Biotech), oral bovine pentavalent (BRV-PV) (Rotasiil; Serum Institute of India), and human neonatal (RV3-BB); (2) a sample size of at least 100 participants; and (3) data on the incidence of intussusception. Exclusion criteria were (1) no data on intussusception; (2) no placebo group; (3) use of human reassortant rotavirus tetravalent vaccine (RRV-TV) (Rotashield; Wyeth Laboratories Inc) because the US Advisory Committee on Immunization no longer recommends the use of this vaccine owing to high risk of intussusception; and (4) duplicate publications. Some studies included the participants from the same general population, in which case, the most comprehensive and up-to-date study was selected for inclusion in this meta-analysis. Data from unpublished trials or conference abstracts were also excluded from the final analysis (Figure 1).
Figure 1. Flow Diagram.
BRV-PV indicates oral bovine rotavirus pentavalent vaccine (Rotasiil); RRV-TV, human reassortant rotavirus tetravalent vaccine; RV1, monovalent rotavirus vaccine (Rotarix); RV3-BB, human neonatal rotavirus vaccine; RV5, pentavalent rotavirus vaccine (Rotateq); 116E, monovalent human-bovine rotavirus vaccine (Rotavac).
Data Collection and Extraction
Data were extracted using a standardized data extraction form. Information collected included the study title, authors, publication year, study period, phase and the registration number of the RCT, city, country, continent, sex, weight, vaccine type, time of vaccination, sample size in the vaccine or placebo group, number of intussusception cases, number of days between the administration of the vaccine and diagnosis of intussusception, the risk estimates or data used to calculate the risk estimates, and 95% CIs or data used to calculate 95% CIs. If a trial had more than 2 groups or differed in the vaccine component or concentration, the extracted information and data on the vaccine used were those of the similar composition and with the closest concentration to the approved vaccine.
Statistical Analysis
The association between RV vaccination and intussusception, the pooled results of relative risk (RR), the risk difference (RD), and 95% CIs in the 3 different follow-up periods were calculated using the Mantel-Haenszel method. Because of the very low incidence of intussusception, the number of intussusceptions in the vaccine and placebo groups during the observation period was 0 at the same time in some trials. To accurately and objectively reflect the facts, we evaluated RR and RD. Undefined RRs (ie, RR of 0 or infinity) were not included in the statistical calculations but were included in the calculation of RD.
Separate analyses were performed for RV1 and RV5. Because the 116E, BRV-PV, and RV3-BB vaccines were used only in local areas and there were fewer related trials, these 3 vaccines were combined into 1 group for analysis. If only 1 trial evaluated the vaccine, a systematic review was conducted, and if more than 1 trial evaluated the vaccine, a meta-analysis was conducted. The results were separately calculated as subtotal results. The pooled and subtotal results were presented in forest plots as RRs, RDs, and 95% CIs of intussusception after RV vaccination. Both the pooled and subtotal results of RR and RD of intussusception were estimated at 31 days, 1 year, and 2 years after the vaccination period. If the number of trials was small during a follow-up period (31 days, 1 year, and 2 years), analysis was not conducted on publication bias.
The Q test and I2 statistic index were used to assess the degree of heterogeneity between different studies: low (<25%), moderate (26%-50%), and high (51%-75%). Results were calculated using a fixed- or random-effect model depending on the statistical results of the heterogeneity test. Sensitivity analysis was performed for identifying the heterogeneity among the studies. All statistical analyses were performed using Stata, version 14.2 (StataCorp). A 2-sided P < .05 was considered to indicate statistical significance.
Results
Study Screening
A total of 25 RCTs met the inclusion criteria. The initial search produced 196 studies from all 4 databases. There were 33 studies from PubMed, 49 studies from Web of Science, 59 studies from Cochrane library, and 55 studies from Embase. After the screening of titles and abstracts, 115 studies were removed because of duplicates. The full texts of the remaining 81 studies were reviewed in detail. The reference lists or relevant publications of these articles were also screened based on the eligibility criteria; 58 studies were excluded, and 2 studies were newly identified through the references. The selection process is summarized in Figure 1.
Study Characteristics
As shown in Table 1, a total of 25 RCTs including 200 594 participants (104 647 receiving vaccine and 95 947 receiving placebo) in 33 countries from 4 continents were finally included in this systematic review and meta-analysis. There were 11 trials on the RV1 vaccine, 10 trials on the RV5 vaccine, 2 trials on the BRV-PV vaccine, and 1 trial on the 116E and RV3-BB vaccines. Most RCTs reported intussusception cases from up to 31 days after vaccination. As shown in Table 2, 20 cases of definite intussusception were diagnosed within 31 days after RV vaccination, with 11 cases (55%) in the vaccine group and 9 cases (45%) in the placebo group. A total of 74 cases of intussusception (37 cases [50%] in the vaccine group and 37 cases [50%] in the placebo group) were reported within 1 year and 59 cases (29 cases [49%] in the vaccine and 30 cases [51%] in the placebo group) within 2 years after vaccination.
Table 1. Characteristics of the Included Randomized Clinical Trials.
| Source | Countries or Regions | Vaccine | Study Period | Clinical Trial Phase | Registration No. | Queue, No. | Age at First Dose | Participants, No. | |
|---|---|---|---|---|---|---|---|---|---|
| Vaccine Group | Placebo Group | ||||||||
| Dennehy et al,11 2005 | United States and Canada | RV1 | December 2000-September 2001 | 2 | NA | 2 | 5-15 wk | 209 | 108 |
| Kawamura et al,12 2011 | Japan | RV1 | June 2007-December 2009 | 3 | NCT00480324 | 1 | 7.7 (2.01) wka | 507 | 257 |
| Li et al,13 2014 | China | RV1 | August 2010-December 2010 | 3 | NCT01171963 | 1 | NA | 1666 | 1667 |
| Madhi et al,14 2010 | South Africa and Malawi | RV1 | 2005-2007 | NA | NCT00241644 | 1 | NA | 3298 | 1641 |
| Phua et al,15 2009 | China and Singapore | RV1 | December 2003-August 2005 | 3 | NCT00329745 | 1 | NA | 5359 | 5349 |
| Ruiz-Palacios et al,16 2006 | Latin America (11 countries) and Finland | RV1 | August 2003-March 2004 | 3 | NCT00139347 and NCT00263666 | 1 | 2-4 mo | 31 673 | 31 552 |
| Salinas et al,17 2005 | Latin America (3 countries) | RV1 | May 2001-April 2003 | NA | NA | 3 | 8.3 wka | 540 | 537 |
| Steele et al,18 2010 | South Africa | RV1 | September 2003-October 2004 | 2 | NCT00383903, eTrack 444563/013 | 2 | 5-10 wk | 190 | 96 |
| Tregnaghi et al,19 2011 | Latin America (6 countries) | RV1 | December 2003-March 2007 | 3 | NCT00139347 | 1 | NA | 4376 | 2192 |
| Vesikari et al,20 2004 | Finland | RV1 | August 2000-November 2000 | NA | NA | 1 | 6-12 wk | 270 | 135 |
| Vesikari et al,3 2007 | Europe (6 countries) | RV1 | September 2004-February 2005 | 3b | NCT00140686, eTrack102247 | 1 | 6-14 wk | 2646 | 1348 |
| Armah et al,21 2010 | Ghana, Kenya, and Mali | RV5 | April 2007-May 2009 | NA | NCT00362648 | 1 | NA | 2733 | 2735 |
| Chang et al,22 2009 | China | RV5 | April 2003-June 2004 | 3 | NA | 1 | 6-12 wk | 95 | 93 |
| Grant et al,23 2012 | United States | RV5 | March 2002-October 2003 | NA | NA | 1 | NA | 512 | 494 |
| Iwata et al,24 2013 | Japan | RV5 | August 2008-August 2009 | NA | NCT00718237 | 1 | 6-12 wk | 380 | 381 |
| Kim et al,25 2008 | South Korea | RV5 | August 2005-July 2006 | 3 | NA | 1 | 9 wkb | 115 | 63 |
| Mo et al,26 2017 | China | RV5 | May 2014-October 2014 | NA | NCT02062385 | 1 | 6-12 wk | 2015 | 2019 |
| Rodriguez et al,27 2007 | 11 Countries | RV5 | 2001-2005 | NA | NA | 1 | 6-12 wk | 662 | 696 |
| Vesikari et al,28 2006 | Finland | RV5 | 1998-2001 | 2 | NA | 3 | 2-8 mo | 323 | 322 |
| Vesikari et al,29 2006 | 11 Countries, including United States and Finland | RV5 | 2001-2004 | 3 | NCT00090233 | 1 | 9.8 (1.4) wka | 34 644 | 34 630 |
| Zaman et al,30 2010 | Bangladesh and Vietnam | RV5 | March 2007-March 2009, September 2007-March 2009 | NA | NCT00362648 | 1 | 8.9 (1.5) wka | 1018 | 1018 |
| Bhandari et al,31 2014 | India | 116E | March 2011-November 2012 | NA | NCT01305109 | 1 | 6.8 wka | 4532 | 2267 |
| Isanaka et al,32 2017 | Niger | BRV-PV | August 2014-November 2015 | 3 | NCT02145000 | 1 | 6-8 wk | 2044 | 2047 |
| Kulkarni et al,2 2017 | India | BRV-PV | 2014-2016 | 3 | NCT02133690 | 1 | 48.2 (4.1) da | 3749 | 3751 |
| Bines et al,33 2018 | Indonesia | RV3-BB | January 2013-July 2016 | NA | ACTRN12612001282875 | 1 | 0-5 d and 8-10 wk | 1091 | 549 |
Abbreviations: BRV-PV, oral bovine rotavirus pentavalent vaccine (Rotasiil); NA, not applicable; RV1, monovalent rotavirus vaccine (Rotarix); RV3-BB, human neonatal rotavirus vaccine; RV5, pentavalent rotavirus vaccine (Rotateq); 116E, monovalent human-bovine rotavirus vaccine (Rotavac).
Data are presented as mean or mean (SD).
Data are presented as median.
Table 2. Meta-analysis Results of the Risk of Intussusception After Rotavirus Vaccination.
| Vaccine Type, Source | Intussusception 31 d After Each Dosea | Intussusception at 1 y | Intussusception at 2 y | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | RR (95% CI) | RD (95% CI), per 10 000 Infants | No. of Cases | RR (95% CI) | RD (95% CI), per 10 000 Infants | No. of Cases | RR (95% CI) | RD (95% CI), per 10 000 Infants | ||||
| Vaccine Group | Placebo Group | Vaccine Group | Placebo Group | Vaccine Group | Placebo Group | |||||||
| RV1 | ||||||||||||
| Dennehy et al,11 2005b | 0 | 0 | NA | 0 (−142.96 to 142.96) | 0 | 0 | NA | 0 (−142.96 to 142.96) | NA | NA | NA | NA |
| Kawamura et al,12 2011 | 0 | 0 | NA | 0 (−60.20 to 60.20) | 0 | 0 | NA | 0 (−60.20 to 60.20) | NA | NA | NA | NA |
| Li et al,13 2014 | 0 | 0 | NA | 0 (−11.75 to 11.75) | 1 | 1 | 1.00 (0.06 to 15.98) | 0 (−16.61 to 16.62) | NA | NA | NA | NA |
| Madhi et al,14 2010 | 0 | 0 | NA | 0 (−9.43 to 9.43) | 1 | 0 | 1.49 (0.06 to 36.63) | 3.03 (−8.11 to 14.17) | NA | NA | NA | NA |
| Phua et al,15 2009 | 0 | 0 | NA | 0 (−3.66 to 3.66) | NA | NA | NA | NA | 8 | 4 | 2.00 (0.60 to 6.63) | 7.45 (−5.22 to 20.12) |
| Ruiz-Palacios et al,16 2006 | 6 | 7 | 0.85 (0.29 to 2.54) | −0.32 (−2.56 to 1.91) | 9 | 16 | 0.56 (0.25 to 1.27) | −2.23 (−5.33 to 0.87) | NA | NA | NA | NA |
| Salinas et al,17 2005b | 0 | 0 | NA | 0 (−36.31 to 36.31) | NA | NA | NA | NA | NA | NA | NA | NA |
| Steele et al,18 2010 | 0 | 0 | NA | 0 (−159.87 to 159.87) | NA | NA | NA | NA | NA | NA | NA | NA |
| Tregnaghi et al,19 2011 | NA | NA | NA | NA | 4 | 2 | 1.00 (0.18 to 5.47) | 0.02 (−15.47 to 15.51) | NA | NA | NA | NA |
| Vesikari et al,20 2004 | 0 | 0 | NA | 0 (−113.83 to 113.83) | 0 | 0 | NA | 0 (−113.83 to 113.83) | 0 | 0 | NA | 0 (−113.83 to 113.83) |
| Vesikari et al,3 2007 | 1 | 0 | 1.53 (0.06 to 37.51) | 3.78 (−9.92 to 17.48) | 1 | 0 | 1.53 (0.06 to 37.51) | 3.78 (−9.92 to 17.48) | 2 | 1 | 1.02 (0.09 to 11.23) | 0.14 (−17.77 to 18.05) |
| RV5 | ||||||||||||
| Armah et al,21 2010 | 0 | 0 | NA | 0 (−7.17 to 7.17) | 0 | 0 | NA | 0 (−7.17 to 7.17) | NA | NA | NA | NA |
| Chang et al,22 2009 | 0 | 0 | NA | 0 (−205.80 to 205.80) | 0 | 0 | NA | 0 (−205.80 to 205.80) | NA | NA | NA | NA |
| Grant et al,23 2012 | 0 | 0 | NA | 0 (−38.89 to 38.89) | 0 | 0 | NA | 0 (−38.89 to 38.89) | NA | NA | NA | NA |
| Iwata et al,24 2013 | 0 | 0 | NA | 0 (−51.34 to 51.34) | NA | NA | NA | NA | NA | NA | NA | NA |
| Kim et al,25 2008 | 0 | 0 | NA | 0 (−246.45 to 246.45) | NA | NA | NA | NA | NA | NA | NA | NA |
| Mo et al,26 2017 | 0 | 0 | NA | 0 (−9.71 to 9.71) | 2 | 0 | 5.01 (0.24 to 104.29) | 9.93 (−6.90 to 26.75) | NA | NA | NA | NA |
| Rodriguez et al,27 2007 | 1 | 0 | 3.15 (0.13 to 77.28) | 15.11 (−26.16 to 56.37) | NA | NA | NA | NA | NA | NA | NA | NA |
| Vesikari et al,28 2006b | 0 | 0 | NA | 0 (−60.54 to 60.54) | 0 | 0 | NA | 0 (−60.54 to 60.54) | 0 | 0 | NA | 0 (−60.54 to 60.54) |
| Vesikari et al,29 2006 | 3 | 2 | 1.50 (0.25 to 8.97) | 0.29 (−0.98 to 1.55) | 12 | 15 | 0.80 (0.37 to 1.71) | −0.87 (−3.81 to 2.07) | 12 | 18 | 0.67 (0.32 to 1.38) | −1.73 (−4.83 to 1.36) |
| Zaman et al,30 2010 | 0 | 0 | NA | 0 (−19.23 to 19.23) | 0 | 1 | 0.33 (0.01 to 8.17) | −9.82 (−37.01 to 17.36) | NA | NA | NA | NA |
| 116E, Rotavac | ||||||||||||
| Bhandari et al,31 2014 | 0 | 0 | NA | 0 (−6.83 to 6.83) | 6 | 2 | 1.50 (0.30 to 7.43) | 4.42 (−11.75 to 20.59) | NA | NA | NA | NA |
| BRV-PV, Rotasiil | ||||||||||||
| Isanaka et al,32 2017 | 0 | 0 | NA | 0 (−9.58 to 9.58) | 0 | 0 | NA | 0 (−9.58 to 9.58) | 0 | 0 | NA | 0 (−9.58 to 9.58) |
| Kulkarni et al,2 2017 | 0 | 0 | NA | 0 (−5.22 to 5.22) | NA | NA | NA | NA | 6 | 7 | 0.86 (0.29 to 2.55) | −2.66 (−21.49 to 16.17) |
| RV3-BB | ||||||||||||
| Bines et al,33 2018 | 0 | 0 | 0 (−28.20 to 28.20) | 1 | 0 | 1.51 (0.06 to 37.03) | 9.17 (−24.25 to 42.59) | 1 | 0 | 1.51 (0.06 to 37.03) | 9.17 (−24.25 to 42.59) | |
| Fixed-effects model using Mantel-Haenszel | 11 | 9 | 1.14 (0.49 to 2.64) | 0.17 (−1.16 to 1.50) | 37 | 37 | 0.84 (0.53 to 1.32) | −0.65 (−2.68 to 1.39) | 29 | 30 | 0.91 (0.55 to 1.52) | −0.48 (−3.64 to 2.69) |
| P value | NA | NA | .77 | .80 | NA | NA | .45 | .53 | NA | NA | .73 | .77 |
Abbreviations: BRV-PV, oral bovine rotavirus pentavalent vaccine (Rotasiil); NA, not applicable; RD, risk difference; RR, relative risk; RV1, monovalent rotavirus vaccine (Rotarix); RV3-BB, human neonatal rotavirus vaccine; RV5, pentavalent rotavirus vaccine (Rotateq); 116E, monovalent human-bovine rotavirus vaccine (Rotavac).
From the data extracted for the study, most of the intussusception data description was divided by 31 days; thus, 31 days was chosen as the statistical indicator. We believe that the 31-day follow-up reflected the short-term effect of the vaccine; the long-term effect was shown at 1 and 2 years.
The 3 studies compared different concentrations of vaccine vs placebo. Among 4630 patients, 2 cases of intussusception occurred in the low-concentration group; 1 case was a 7-month-old boy, with occurrence 9 days after the first dose of RV5, and the second case was a 10-month-old boy, with occurrence 6 months after the second dose of RV1.
Study Quality
Quality assessment of the trials was performed according to the Cochrane collaboration′s tool for assessing the risk of bias.9 Of the 25 RCTs, 19 were high quality and 6 were moderate quality.
Meta-analysis
Risk of Intussusception Within 31 Days After Rotavirus Vaccination
As shown in Table 2 and Figure 2, 20 cases of intussusception were diagnosed within 31 days after any RV vaccination, with 11 cases (55%) in the vaccine group and 9 cases (45%) in the placebo group. The RR of intussusception ranged from 0.85 to 3.15 among the 4 studies that reported intussusception risk within 31 days after vaccination. Heterogeneity among these studies was low (Q = 0.78; P = .85; I2 = 0%). The RD of intussusception ranged from −0.32 per 10 000 infants to 15.11 per 10 000 infants (Table 2). Heterogeneity among those studies was also very low (Q = 1.01; P > .99; I2 = 0%). The pooled effects were calculated using the fixed-effect model. The overall estimate of RR for intussusception within 31 days for the fixed-effect model was 1.14 (95% CI, 0.49-2.64; P = .77). The overall estimate of RD of intussusception within 31 days after each dose for the fixed-effect model was 0.17 per 10 000 infants (95% CI, −1.16 to 1.50 per 10 000 infants; P = .80).
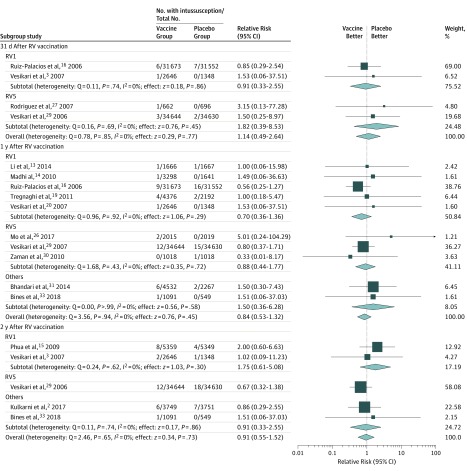
Figure 2. Subgroup Analysis for Intussusception Between Rotavirus (RV) Vaccine and Placebo Groups at Different Follow-up Times.
Relative risk and 95% CIs were calculated using the Mantel-Haenszel method, with a fixed-effects model used to pool data. Randomized clinical trials with 0 cases of intussusception among the vaccine and placebo groups were not included in the relative risk statistics but were included in the statistics of the risk difference. Other vaccines included monovalent human-bovine (116E) (Rotavac), human neonatal (RV3-BB), and oral bovine pentavalent (BRV-PV). Boxes represent means, with the size of the box corresponding with the weight; horizontal lines represent 95% CIs; and diamonds indicate pooled means with the horizontal points of the diamonds representing 95% CIs. RV1 indicates monovalent rotavirus vaccine (Rotarix); RV5, pentavalent rotavirus vaccine (Rotateq).
Fourteen cases of intussusception were diagnosed within 31 days after RV1 vaccination (7 cases [50%] in the vaccine group and 7 cases [50%] in the placebo group). The subtotal estimate of RR of intussusception within 31 days after each dose of RV1 for the fixed-effect model was 0.91 (95% CI, 0.33-2.55; P = .86). The subtotal estimate of RD of intussusception within 31 days after each dose for the fixed-effect model was −0.08 per 10 000 infants (95% CI, −2.22 to 2.06 per 10 000 infants; P = .94).
Six cases of intussusception were diagnosed within 31 days after RV5 vaccination (4 cases [66%] in the vaccine group and 2 cases [33%] in the placebo group). The subtotal estimate of RR of intussusception within 31 days after each RV5 dose for the fixed-effect model was 1.82 (95% CI, 0.39-8.53; P = .45). The subtotal estimate of RD of intussusception in 31 days after each RV5 dose for the fixed-effect model was 0.48 per 10 000 infants (95% CI, −1.32 to 2.27 per 10 000 infants; P = .60).
Risk of Intussusception Within 1 Year of Vaccination
As shown in Table 2 and Figure 2, a total of 74 cases of definite intussusception were diagnosed within 1 year after any RV vaccination (37 cases [50%] in each group). The RR of intussusception ranged from 0.33 to 5.01 among 10 studies that reported intussusception outcome at 1 year. Heterogeneity among these studies was low (Q = 3.56; P = .94; I2 = 0%). The RD of intussusception ranged from −9.82 to 9.93 per 10 000 infants, with low heterogeneity (Q = 4.57; P > .99; I2 = 0%). The pooled effects were calculated using the fixed-effect model. The overall estimate of RR of intussusception within 1 year of RV vaccination for the fixed-effect model was 0.84 (95% CI, 0.53-1.32; P = .45). The overall estimate of RD of intussusception within 1 year after each RV dose for the fixed-effect model was –0.65 per 10 000 infants (95% CI, −2.68 to 1.39 per 10 000 infants; P = .53).
Thirty-five cases of definite intussusception were diagnosed within 1 year after RV1 vaccination (16 cases [46%] in the vaccine group and 19 cases [54%] in the placebo group). The subtotal estimate of RR of intussusception within 1 year after each dose of RV1 for the fixed-effect model was 0.70 (95% CI, 0.36-1.36; P = .29). The subtotal estimate of RD of intussusception within 1 year after receipt of each dose for the fixed-effect model was −1.40 per 10 000 infants (95% CI, −4.38 to 1.59 per 10 000 infants; P = .36).
Thirty cases of intussusception were identified within 1 year after RV5 vaccination (14 cases [47%] in the vaccine group and 16 cases [53%] in the placebo group). The subtotal estimate of RR of intussusception within 1 year after each dose for the fixed-effect model was 0.88 (95% CI, 0.44-1.77; P = .72). The subtotal estimate of RD of intussusception within 1 year after each dose for the fixed-effect model was −0.48 per 10 000 infants (95% CI, −3.33 to 2.36 per 10 000 infants; P = .74).
Nine cases of intussusception were diagnosed within 1 year after 116E and RV3-BB vaccinations (7 cases [78%] in the vaccine group and 2 cases [22%] in the placebo group). The subtotal estimate of RR of intussusception within 1 year after each dose of these vaccines for the fixed-effect model was 1.50 (95% CI, 0.36-6.28; P = .58). The subtotal estimate of RD of intussusception within 1 year after each dose for the fixed-effect model was 3.46 per 10 000 infants (95% CI, −6.55 to 13.47 per 10 000 infants; P = .50).
Risk of Intussusception Within 2 Years of Vaccination
As shown in Table 2 and Figure 2, a total of 59 cases of intussusception were diagnosed in the 5 studies that reported outcome within 2 years after any RV vaccination (29 cases [49%] in the vaccine group and 30 cases [51%] in the placebo group). The RR of intussusception ranged from a minimum of 0.67 to a maximum of 2.00 with low heterogeneity among these studies (Q = 2.46; P = .65; I2 = 0%). The RD of intussusception ranged from −2.66 to 9.17 per 10 000 infants. Heterogeneity among those studies was also low (Q = 2.52; P = .93; I2 = 0%). The pooled effects were calculated using the fixed-effect model. The overall estimate of RR of intussusception within 2 years after vaccination for the fixed-effect model was 0.91 (95% CI, 0.55-1.52; P = .73). The overall estimate of RD of intussusception within 2 years after each dose for the fixed-effect model was −0.48 per 10 000 infants (95% CI, −3.64 to 2.69 per 10 000 infants; P = .77).
Fifteen cases of definite intussusception were diagnosed within 2 years of RV1 vaccination (10 cases [67%] in the vaccine group and 5 cases [33%] in the placebo group). The subtotal estimate of RR of intussusception within 2 years after each dose for the fixed-effect model was 1.75 (95% CI, 0.61-5.08; P = .30). The subtotal estimate of RD of intussusception within 2 years after each dose for the fixed-effect model was 5.48 per 10 000 infants (95% CI, –5.14 to 16.11 per 10 000 infants; P = .31).
Thirty cases of definite intussusception were diagnosed within 2 years of RV5 vaccination (12 cases [40%] in the vaccine group and 18 cases [60%] in the placebo group). The subtotal estimate of RD of intussusception within 2 years after each dose of RV5 for the fixed-effect model was −1.72 per 10 000 infants (95% CI, −4.84 to 1.40 per 10 000 infants; P = .28).
Fourteen cases of definite intussusception were diagnosed within 2 years after BRV-PV and RV3-BB vaccinations (7 cases [50%] in the vaccine group and 7 cases [50%] in the placebo group). The subtotal estimate of RR of intussusception within 2 years after each dose of BRV-PV and RV3-BB vaccines for the fixed-effect model was 0.91 (95% CI, 0.33-2.55; P = .86). The subtotal estimate of RD of intussusception within 2 years after each dose for the fixed-effect model was −0.50 per 10 000 infants (95% CI, −12.34 to 11.34 per 10 000 infants; P = .93).
Discussion
Intussusception is a potentially life-threatening condition in children, and recent evidence has indicated an association between the RV vaccination and intussusception.5,34 Because of this adverse event, careful monitoring for development of intussusception after the administration of RV vaccine is suggested. In this systematic review and meta-analysis of RCTs evaluating the risk of intussusception after RV vaccination found no such significant association. This meta-analysis included the RCTs that used RV1, RV5, 116E, BRV-PV, or RV3-BB vaccine. Analysis of the subtotal group of different vaccine types and the pooled estimated risks of intussusception within 31 days after each dose, and 1 and 2 years after vaccination revealed no association of risk of developing intussusception after receipt of the rotavirus vaccine, a finding that corresponds with the results of some previous studies.6,7,35,36,37
The absence of any significant association between the RV vaccine and intussusception could possibly be attributed to a wide range of RCTs covering a total of 200 594 infants worldwide. The key strength of this meta-analysis was the large number of infants included in the RCTs, which focused on surveillance of vaccine safety. Of the total 108 cases of intussusception, 3 occurred within 7 days16,29 and after the second dose of the vaccine. However, there was no statistical difference in the incidence of intussusception between the vaccine group and the placebo group. Studies5,34,38,39,40 with different methods, such as cohort studies, case-control studies, self-controlled case series (SCCS), or self-controlled risk interval evaluation studies, reported a positive association between RV vaccination and intussusception, whereas RCTs often found no correlation between intussusception and vaccination. A recent meta-analysis41 of 6 cohort studies (4 506 265 first doses) and 5 case-control studies (n = 9643 infants) suggests that the RV vaccination is associated with an increased risk of development of intussusception, which was predominantly seen after the administration of the first dose. Another meta-analysis conducted by Dong et al42 that included children receiving RV1 and RV5 vaccines showed an increased risk of intussusception within 7 days, especially after the first dose. However, only SCCS and self-controlled risk interval studies were included in the analysis. Another meta-analysis of 10 SCCSs showed that RR for intussusception was 5.71 (95% CI, 4.50-7.25) from 1 to 7 days after the first dose, 1.69 (95% CI, 1.33-2.14) after the second dose, and 1.14 (95% CI, 0.75-1.74) after the third dose.43 The SCCS evaluation is increasingly being used during the active vaccine safety surveillance, whereas an SCCS has its own limitations of measuring only the incidence of reported cases with a descriptive design rather than an analytic study. Thus, SCCSs could include potential referral bias because variation of the treatment application has no control.44 When comparing different study designs to determine the best design for surveillance of vaccine safety, the limitations of those studies are evident, especially based on their heterogeneity. Thus, these positive results need to be carefully considered and further investigated.
Another possible reason for no association could be the exclusion of RRV-TV vaccination in this meta-analysis. Many previous studies45,46,47 investigating RRV-TV demonstrated that RRV-TV was associated with a strong increased risk of intussusception; it was suspended in 1999 because of the safety issues.
Limitations
This study has limitations. The power was low for analysis of RCTs assessing the risk of intussusception with 116E, BRV-PV, and RV3-BB vaccines because of the limited number of trials. Another limitation was the inability to assess whether there was a difference in the risk of intussusception among infants from various geographic regions because of unavailability of sufficiently large trials in the same region.
Conclusions
In this systematic review and meta-analysis of RCTs of the RV1, RV5, 116E, BRV-PV, and RV3-BB vaccines, we found no association of vaccination with increased risk of intussusception compared with placebo among infants for up to 2 years after vaccination. Our results contradict the postmarketing monitoring suggestion about the risk of intussusception after the RV vaccination. We suggest that the benefit of the vaccination exceeds the potential risk of intussusception.
References
- 1.Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis. 2017;215(11):-. doi: 10.1093/infdis/jix186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni PS, Desai S, Tewari T, et al. ; SII BRV-PV author group . A randomized phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35(45):6228-6237. doi: 10.1016/j.vaccine.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370(9601):1757-1763. doi: 10.1016/S0140-6736(07)61744-9 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Advisory Committee on Vaccine Safety, 6-7 December 2017. 2017. https://www.who.int/vaccine_safety/committee/reports/Dec_2017/en/. Accessed February 23, 2019.
- 5.Patel MM, López-Collada VR, Bulhões MM, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364(24):2283-2292. doi: 10.1056/NEJMoa1012952 [DOI] [PubMed] [Google Scholar]
- 6.Shui IM, Baggs J, Patel M, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA. 2012;307(6):598-604. doi: 10.1001/jama.2012.97 [DOI] [PubMed] [Google Scholar]
- 7.Tate JE, Mwenda JM, Armah G, et al. ; African Intussusception Surveillance Network . Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med. 2018;378(16):1521-1528. doi: 10.1056/NEJMoa1713909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yih WK, Lieu TA, Kulldorff M, et al. Intussusception risk after rotavirus vaccination in US infants. N Engl J Med. 2014;370(6):503-512. doi: 10.1056/NEJMoa1303164 [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT. Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6 (updated July 2019). 2019. The Cochrane Collaboration. https://training.cochrane.org. Accessed August 24, 2019.
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennehy PH, Brady RC, Halperin SA, et al. ; North American Human Rotavirus Vaccine Study Group . Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatr Infect Dis J. 2005;24(6):481-488. doi: 10.1097/01.inf.0000164763.55558.71 [DOI] [PubMed] [Google Scholar]
- 12.Kawamura N, Tokoeda Y, Oshima M, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine. 2011;29(37):6335-6341. doi: 10.1016/j.vaccine.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 13.Li RC, Huang T, Li Y, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother. 2014;10(1):11-18. doi: 10.4161/hv.26319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289-298. doi: 10.1056/NEJMoa0904797 [DOI] [PubMed] [Google Scholar]
- 15.Phua KB, Lim FS, Lau YL, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine. 2009;27(43):5936-5941. doi: 10.1016/j.vaccine.2009.07.098 [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. ; Human Rotavirus Vaccine Study Group . Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11-22. doi: 10.1056/NEJMoa052434 [DOI] [PubMed] [Google Scholar]
- 17.Salinas B, Pérez Schael I, Linhares AC, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: a randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J. 2005;24(9):807-816. doi: 10.1097/01.inf.0000178294.13954.a1 [DOI] [PubMed] [Google Scholar]
- 18.Steele AD, Reynders J, Scholtz F, et al. Comparison of 2 different regimens for reactogenicity, safety, and immunogenicity of the live attenuated oral rotavirus vaccine RIX4414 coadministered with oral polio vaccine in South African infants. J Infect Dis. 2010;202(suppl):S93-S100. doi: 10.1086/653550 [DOI] [PubMed] [Google Scholar]
- 19.Tregnaghi MW, Abate HJ, Valencia A, et al. ; Rota-024 Study Group . Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J. 2011;30(6):e103-e108. doi: 10.1097/INF.0b013e3182138278 [DOI] [PubMed] [Google Scholar]
- 20.Vesikari T, Karvonen A, Puustinen L, et al. Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J. 2004;23(10):937-943. doi: 10.1097/01.inf.0000141722.10130.50 [DOI] [PubMed] [Google Scholar]
- 21.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606-614. doi: 10.1016/S0140-6736(10)60889-6 [DOI] [PubMed] [Google Scholar]
- 22.Chang CC, Chang MH, Lin TY, Lee HC, Hsieh WS, Lee PI. Experience of pentavalent human-bovine reassortant rotavirus vaccine among healthy infants in Taiwan. J Formos Med Assoc. 2009;108(4):280-285. doi: 10.1016/S0929-6646(09)60067-X [DOI] [PubMed] [Google Scholar]
- 23.Grant LR, Watt JP, Weatherholtz RC, et al. Efficacy of a pentavalent human-bovine reassortant rotavirus vaccine against rotavirus gastroenteritis among American Indian children. Pediatr Infect Dis J. 2012;31(2):184-188. doi: 10.1097/INF.0b013e3182435afe [DOI] [PubMed] [Google Scholar]
- 24.Iwata S, Nakata S, Ukae S, et al. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum Vaccin Immunother. 2013;9(8):1626-1633. doi: 10.4161/hv.24846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim DS, Lee TJ, Kang JH, et al. Immunogenicity and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine in healthy infants in Korea. Pediatr Infect Dis J. 2008;27(2):177-178. doi: 10.1097/INF.0b013e31815aba79 [DOI] [PubMed] [Google Scholar]
- 26.Mo Z, Mo Y, Li M, et al. Efficacy and safety of a pentavalent live human-bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: a randomized, double-blind, placebo-controlled trial. Vaccine. 2017;35(43):5897-5904. doi: 10.1016/j.vaccine.2017.08.081 [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez ZM, Goveia MG, Stek JE, et al. Concomitant use of an oral live pentavalent human-bovine reassortant rotavirus vaccine with licensed parenteral pediatric vaccines in the United States. Pediatr Infect Dis J. 2007;26(3):221-227. doi: 10.1097/01.inf.0000254391.71103.e8 [DOI] [PubMed] [Google Scholar]
- 28.Vesikari T, Clark HF, Offit PA, et al. Effects of the potency and composition of the multivalent human-bovine (WC3) reassortant rotavirus vaccine on efficacy, safety and immunogenicity in healthy infants. Vaccine. 2006;24(22):4821-4829. doi: 10.1016/j.vaccine.2006.03.025 [DOI] [PubMed] [Google Scholar]
- 29.Vesikari T, Matson DO, Dennehy P, et al. ; Rotavirus Efficacy and Safety Trial (REST) Study Team . Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23-33. doi: 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- 30.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615-623. doi: 10.1016/S0140-6736(10)60755-6 [DOI] [PubMed] [Google Scholar]
- 31.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. ; India Rotavirus Vaccine Group . Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9935):2136-2143. doi: 10.1016/S0140-6736(13)62630-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isanaka S, Guindo O, Langendorf C, et al. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N Engl J Med. 2017;376(12):1121-1130. doi: 10.1056/NEJMoa1609462 [DOI] [PubMed] [Google Scholar]
- 33.Bines JE, At Thobari J, Satria CD, et al. Human neonatal rotavirus vaccine (RV3-BB) to target rotavirus from birth. N Engl J Med. 2018;378(8):719-730. doi: 10.1056/NEJMoa1706804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weintraub ES, Baggs J, Duffy J, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014;370(6):513-519. doi: 10.1056/NEJMoa1311738 [DOI] [PubMed] [Google Scholar]
- 35.Kim KY, Kim DS. Relationship between pentavalent rotavirus vaccine and intussusception: a retrospective study at a single center in Korea. Yonsei Med J. 2017;58(3):631-636. doi: 10.3349/ymj.2017.58.3.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Layton JB, Butler AM, Panozzo CA, Brookhart MA. Rotavirus vaccination and short-term risk of adverse events in US infants. Paediatr Perinat Epidemiol. 2018;32(5):448-457. doi: 10.1111/ppe.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loughlin J, Mast TC, Doherty MC, Wang FT, Wong J, Seeger JD. Postmarketing evaluation of the short-term safety of the pentavalent rotavirus vaccine. Pediatr Infect Dis J. 2012;31(3):292-296. doi: 10.1097/INF.0b013e3182421390 [DOI] [PubMed] [Google Scholar]
- 38.Carlin JB, Macartney KK, Lee KJ, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clin Infect Dis. 2013;57(10):1427-1434. doi: 10.1093/cid/cit520 [DOI] [PubMed] [Google Scholar]
- 39.Yung CF, Chan SP, Soh S, Tan A, Thoon KC. Intussusception and monovalent rotavirus vaccination in Singapore: self-controlled case series and risk-benefit study. J Pediatr. 2015;167(1):163-168.e1. doi: 10.1016/j.jpeds.2015.03.038 [DOI] [PubMed] [Google Scholar]
- 40.Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England: a self-controlled case-series evaluation. Vaccine. 2016;34(32):3684-3689. doi: 10.1016/j.vaccine.2016.04.050 [DOI] [PubMed] [Google Scholar]
- 41.Kassim P, Eslick GD. Risk of intussusception following rotavirus vaccination: an evidence based meta-analysis of cohort and case-control studies. Vaccine. 2017;35(33):4276-4286. doi: 10.1016/j.vaccine.2017.05.064 [DOI] [PubMed] [Google Scholar]
- 42.Dong R, Yang YF, Chen G, et al. Risk of intussusception after rotavirus vaccination: a meta-analysis. Int J Clin Exp Med. 2016;9(2):1306-1313. [Google Scholar]
- 43.Koch J, Harder T, von Kries R, Wichmann O. Risk of intussusception after rotavirus vaccination. Dtsch Arztebl Int. 2017;114(15):255-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClure DL, Glanz JM, Xu S, Hambidge SJ, Mullooly JP, Baggs J. Comparison of epidemiologic methods for active surveillance of vaccine safety. Vaccine. 2008;26(26):3341-3345. doi: 10.1016/j.vaccine.2008.03.074 [DOI] [PubMed] [Google Scholar]
- 45.Verstraeten T, Baughman AL, Cadwell B, Zanardi L, Haber P, Chen RT; Vaccine Adverse Event Reporting System Team . Enhancing vaccine safety surveillance: a capture-recapture analysis of intussusception after rotavirus vaccination. Am J Epidemiol. 2001;154(11):1006-1012. doi: 10.1093/aje/154.11.1006 [DOI] [PubMed] [Google Scholar]
- 46.Kramarz P, France EK, Destefano F, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J. 2001;20(4):410-416. doi: 10.1097/00006454-200104000-00008 [DOI] [PubMed] [Google Scholar]
- 47.Murphy TV, Gargiullo PM, Massoudi MS, et al. ; Rotavirus Intussusception Investigation Team . Intussusception among infants given an oral rotavirus vaccine [published correciton appears in N Engl J Med. 2001;344(20):1564]. N Engl J Med. 2001;344(8):564-572. doi: 10.1056/NEJM200102223440804 [DOI] [PubMed] [Google Scholar]