Abstract
We review the involvement of a small molecule, oxytocin, in various effects of physical stimulation of somatosensory organs, mindfulness meditation, emotion and fragrance on humans, and then propose a hypothesis that complex human states and behaviors, such as well-being, social bonding, and emotional behavior, are explained by oxytocin. We previously reported that oxytocin can induce pain relief and described the possibility how oxytocin in the dorsal horn and/or the dorsal root ganglion relieves joint and muscle pain. In the present article, we expand our research target from the physical analgesic effects of oxytocin to its psychologic effects to upregulate well-being and downregulate stress and anxiety. For this purpose, we propose a “hypothalamic-pituitary-adrenal (HPA) axis-oxytocin model” to explain why mindfulness meditation, placebo, and fragrance can reduce stress and anxiety, resulting in contentment. This new proposed model of HPA axis-oxytocin in the brain also provides a target to address other questions regarding emotional behaviors, learning and memory, and excess food intake leading to obesity, aimed at promoting a healthy life.
Keywords: hypothalamic-pituitary-adrenal axis, oxytocin, pain, placebo, stress
Significance.
Oxytocin has been thought to relieve pain of joint and muscle. In the present article, the analgesic effects of oxytocin by physical stimulation are expanded to its psychologic effects. We propose that the hypothalamic-pituitary-adrenal (HPA) axis and oxytocin comprise a candidate model to promote well-being in humans. For example, it can explain why mindfulness meditation, placebo, and fragrance can reduce stress and anxiety, resulting in contentment. The HPA axis-oxytocin model also addresses questions regarding emotional behaviors.
Oxytocin, a peptide hormone comprising 9 amino acids, is synthesized in neurons of the supraoptic nucleus and paraventricular nucleus of the hypothalamus after specific stimulation of the brain. These neurons project to the posterior pituitary, where oxytocin is released into the blood for delivery to the peripheral tissues as well as into the brain [1,2]. In the peripheral case known as a neurohormone, the function of oxytocin is very much expanded, such as stimulation of epididymal and uterine muscle contraction and stimulation of the nipples from breastfeeding [3]. Oxytocin is, therefore, an important factor regulating the human life cycle and species propagation [4]. Oxytocin facilitates birth, lactation, maternal behavior, neocortical growth, and maintenance of the cortical blood supply [5].
Recently, the function of oxytocin in the brain is strongly noticed, because oxytocin causes the following two special actions: (a) physiologic integrity (parturition, sexual contact, aggressive attack, unpredictable threatening events, and interaction) and (b) enhanced sociality (affiliation, trust, mind reading, and social memory) [6]. In the case of enhanced sociality, changes in oxytocin levels in the central nervous system (CNS) and the peripheral nervous system (PNS) are considered a marker of social functioning [7]. Even in humans, oxytocin is thought to be released in the CNS as a social effector that is brought about by positive emotion or mood [8], and oxytocin stimulates various types of social interactions and promotes healing [9].
In the present review, we first discuss how oxytocin levels are increased in the human body by physical stimulation (e.g., vibration and massage of hair and hairless [glabrous] skin) via somatosensory organs. It has already been known that oxytocin release is induced by several types of nonn-oxious sensory stimuli [10]. Then, we propose a hypothesis that oxytocin levels are also increased by psychologic stimulation (e.g., mindfulness meditation, placebo, emotion, mood, and fragrance) via visual, olfactory, and auditory sensory organs.
Effects of physical stimulation of somatosensory organs on oxytocin levels
To produce the effects of oxytocin in the body, oxytocin must modify the corresponding neural circuits in the CNS as well as in the dorsal horn (DH), and probably also the dorsal root ganglion (DRG) [11]. Oxytocin also moderates the autonomic nervous system and the vagal pathway, and has anti-inflammatory effects [12], and is well known to induce anti-stress effects, such as blood pressure and cortisol level reductions [13]. We recently reported the contribution of oxytocin to physical analgesia [14], which means that physical stimulation of the somatosensory organs induces an increase in oxytocin levels. In 2010, Morrison et al. proposed that skin can be considered as a social organ, because touches mediate social perceptions [15]. Our previous finding that physical stimulation of cutaneous receptors leads to the release of oxytocin may support this notion [14].
In a study of pain relief, we found that physical stimulation of hairy and glabrous skin relieves joint and muscle pain [16]. In patients with tennis elbow, pain was eliminated within four treatments with pyramidal thorn patches. The adhesion of a pyramidal thorn patch is thought to represent a gentle touch. Thus, we hypothesized that gentle stimulation by adhesion of pyramidal thorn patches activates Merkel cells directly under the skin as well as Merkel cell-neurite complexes around the hair follicles by deflecting hair, and its impulse signaling by a gentle touch is conveyed via Aβ fibers to alleviate pain sensations originally delivered via C and Aδ fibers [16]. This interaction between Aβ fibers and C/Aδ fibers occurs in the DH [17] and/or the DRG, and the pain reduction system is thought to include oxytocin [18]. The reduced pain signal is sent to the CNS, resulting in the perception of less pain. In analogy with our oxytocin hypothesis, massage, which is the most well-known method of systematic touching to soften skin tissues, including the back, neck, arms, and legs, can be considered to promote the release of oxytocin [19,20].
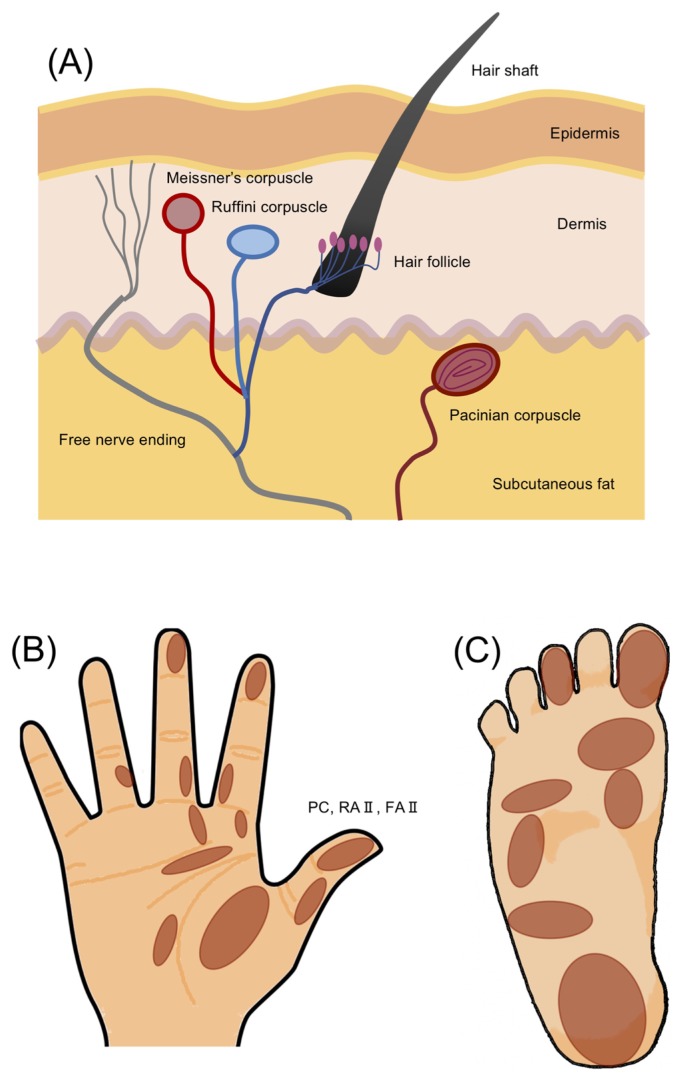
In glabrous skin, Pacinian corpuscles play a leading role in the response to mechanical pressure, especially vibrational stimulation [21]. Vibration receptors are known to respond in a frequency-dependent manner, and the most sensitive vibration frequency is around 200 Hz [22]. In humans, the most typical glabrous skin areas are the palm of the hand and the sole of the foot, both of which are common targets of alternative medicine therapies [23]. The skin structure, including tissues and tactile receptors and the specific tactile receptors in the palm of the hand and the sole of the foot, are illustrated in Figure 1. The classification of fast- and slow-adapting response receptor types is determined by the difference in mechanical impedance [24]. According to the numerous findings, it is reasonably suggested that transient receptor potential (TRP) channels act as tactile receptors [25]. The TRP channels activated by various extracellular and intracellular stimuli play variously physiological and pathological roles. There are seven families of TRPs including TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPP (polycystin), TRPML (mucolipin), and TRPN (Drosophila NOMPC) in mammals [26]. These channels are distributed on every cell in human bodies and activated by various harmful signals such as high temperature, UV radiation and toxic chemicals [e.g., reactive oxygen species (ROS)]. When these channels are activated, Na+ and Ca2+ enter into the cells with the different ratios, resulting in a change in the physiological states of cells. It has been recently suggested that TRPM2 are involved in the release of oxytocin from the nerve [27].
Figure 1.
Schematics of skin structure and tactile receptors in hairy and hairless (glabrous) skin. (A) Skin structure including tissues and tactile receptors. This drawing includes both hairy and glabrous skins. (B) Distribution of receptive fields for an afferent fiber type (called Pacinian corpuscle [PC], rapidly adapting type II nerve fiber [RA II], and fast-adapting type II nerve fiber [FA II]) in the palm of the hand and sole of the foot (i.e., glabrous skin) [24,78,79]. Here, PC, RA II and FA II are the same but are used in a different way by different researchers.
Effects of mindfulness meditation, emotion, and fragrance on oxytocin release
Everybody has had an experience in which slow tempo music and a pleasant fragrance in a warm room made us relax. It is expected that oxytocin is released in the brain under such conditions [28,29]. This is a type of emotional and/or mood stimulation of the brain that contributes to relaxation, trust, psychologic stability, and reduction of stress responses, including anxiety [8]. Oxytocin also induces an emotional sense of safety and high levels of social sensitivity [30]. Further, oxytocin affects prosocial behaviors. Prosocial behavior is a social behavior that benefits other people or society as a whole, such as helping, sharing, donating, co-operating, and volunteering [31].
On the other hand, exogenously administered oxytocin, such as by central administration or by nasal application, improves several social behaviors such as anxiety reduction and perceptual selectivity, thereby inducing various social effects [32,33]. Pleasure is thought to be a social effect induced by oxytocin, and thus soft vibrational stimuli of glabrous skin (i.e., massage) probably induces oxytocin for relaxation [34]. Based on a similar logic, various types of brain stimulation by psychologic mechanisms, such as mindfulness meditation, are hypothesized to be accompanied by the release of oxytocin in the body [35]. In the meditation, the visual, olfactory and auditory senses are used to induce the psychologic effects, and thus the cortices in the CNS corresponding to these senses should be carefully examined.
There are many types of meditation, such as mindfulness meditation, mantra meditation, yoga, tai chi, and chi gong, in human society [36]. Among them, mindfulness meditation is commonly practiced for attention control, regulation of emotion, self-awareness and stress reduction [37]. Although many researchers have tried to uncover the brain function related to these types of meditation using functional magnetic resonance imaging (fMRI), electroencephalography (EEG), and magnetoencephalography (MEG) [38], the underlying neural mechanisms remain unclear. The fMRI, however, gives us only the static information of images. The EEG, on the other hand, gives us the dynamic information, but the signals measured by channels indicate a complex sum of huge number of excited neurons that have no information of activated regions of the brain. The MEG was initially thought as an ultimate apparatus to obtain brain imaging, but it has eventually betrayed our expectation because of the extremely small signals.
Thus, we have begun to use near-infrared spectroscopy (NIRS) to clarify the underlying brain mechanisms [14,39]. NIRS is a noninvasive neuroimaging apparatus with several potential advantages, especially in the fields of psychiatry and rehabilitation because of its dynamic images [40]. For example, fMRI apparatus is expensive and non-portable and the operation is limited in the magnetically-shielded room, whereas NIRS can be easily used without a special room and used as a portable apparatus. Further, the important difference between fMRI and NIRS is that subjects must assume the supine position for fMRI but they can keep the sitting or standing position for NIRS. This difference of a posture is thought to cause a large alternation of the autonomic nervous system [41]. Further progress with NIRS is expected in the near future, even though NIRS can only detect the information a few cm beneath the brain surface.
Neurobiological mechanism of placebo
The neurobiological mechanism for placebo effects is of deep interest. Placebo was initially presented as a result of treatment by pseudo medicine [42]. Approximately 25% to 30% of variance is observed in placebo analgesic responses [43]. Conditioning and expectancy are two of the most accepted theories in placebo response research. For example, an authoritative doctor’s visit in which both the process of being treated (conditioning) and the physician’s verbal suggestions that a treatment may be beneficial (expectancy) may promote a placebo response. Benedetti suggests that by examining placebo studies from the perspective of these different learning and verbal mechanisms, studies can be designed to investigate the effect of the placebo response on medical care [44]. That is, the placebo effect is thought to represent the manifestation of a proactive mind-body link that evokes an innate protective response.
The placebo effect seems to be a real neurobiological phenomenon [45], and the brain’s ‘inner pharmacy’ is a critical determinant for the occurrence of psychobiologic and behavioral changes relevant to healing processes and well-being [43]. The placebo effect can induce relaxation responses by the activation of noradrenaline, nitric oxide (NO), and opioid signaling, both in the CNS and PNS [45]. Stefano et al. found that NO controls noradrenaline processes on many levels, including synthesis, release and actions, and finally proposed a model of peripheral relaxation with NO having the lead role [45]. Oxytocin is also thought to be involved in placebo. Harnessing the advantages of the placebo effect in healthcare, however, remains a challenge [46].
The neurobiological effects of mindfulness meditation, placebo and fragrance were thought to occur mainly by glia-neuron interactions in the DH and DRG [14], and the functions of the prefrontal cortex, the anterior cingulate cortex, the primary and secondary somatosensory cortices, and the periaqueductal gray in the CNS [47]. In addition, another neurobiological explanation for the effects of mindfulness meditation, placebo and fragrance may be provided by the concept proposed by Leknes and Tracey [48]. They proposed that pain and pleasure, which are considered opposites, can be sensed as “subjective utility” based on the studies of molecular imaging and animal use. They postulated that pain and pleasure can be described by activation of the opioid and/or dopamine systems. This proposal by Leknes and Tracey [48] and the proposal by Stefano et al. [45], as described above, led us to present our new model based on oxytocin.
Hypothetical model of well-being in humans by oxytocin-activated paraventricular nucleus and hypothalamic-pituitary-adrenal axis
Some previous studies have suggested that when the paraventricular nucleus is activated, the oxytocin level increases, even during parturition [49–51], namely pain makes oxytocin, which may be a kind of innate protection system. This system may be a positive feedback system, although the detailed mechanisms are not yet known. If the oxytocin level in the CNS is controlled by such a system, it should be called “a well-being circuit” for the promotion of a healthy life, including the functions of increasing the saliency of social information and modulating pleasure [52].
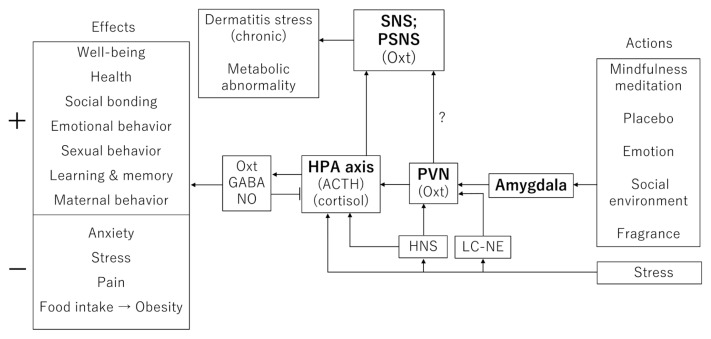
As described previously, pain analgesia modulated by oxytocin in the DH and/or the DRG may be a key component of the peripheral part of the model. The role of oxytocin in the CNS is more complex, however, because it modulates neural circuits according to different types of behaviors such as sexual behavior, partner and maternal behavior, pair and social bonding, affiliative behavior, preference formation, grooming, nociception, sensory processing, anxiety, and feeding [53]. To understand the diversity of oxytocin functions in the CNS alone, we introduce the model that oxytocin activates the hypothalamic-pituitary-adrenal (HPA) axis and the paraventricular nucleus of the hypothalamus and stimulates NO release [54]. Figure 2 shows an improved model based on Esch and Stefano [55] for the formation of social bonding by oxytocin. The HPA axis is involved in the control of stress using adrenocorticotropic hormone (ACTH) and cortisol. However, as far as we know, the involvement of oxytocin in the pathways of well-being or stress reduction before and after the HPA axis has not yet been examined well. This is the important point in the present article. Repeatedly, we claim that the effects of oxytocin on the HPA axis are complex, for example oxytocin inhibits the basal activity of the HPA axis [56] and rather enhances the activity of the HPA axis during exposure to stress [57].
Figure 2.
Diagram of HPA axis-oxytocin-GABA-NO from actions (e.g., mindfulness meditation and placebo) to effects (e.g., well-being upregulation and anxiety downregulation) in humans. Abbreviations: OXT, oxytocin; PVN, paraventricular nucleus; HPA axis, hypothalamic-pituitary-adrenal axis; LC-NE, locus coeruleus-norepinephrine (noradrenaline); HNS, hypothalamic-neurohypophyseal system; SNS, sympathetic nervous system; PSNS, parasympathetic nervous system. + indicates upregulation, and − indicates downregulation.
Psychologic stimulation, such as by mindfulness meditation, emotion, and fragrance, activates the HPA-axis via the amygdala and the paraventricular nucleus, leading to the release of oxytocin in the brain (Fig. 2). We do not exclude the involvement of higher brain regions but now pay attention to the basic pathways expected in stress-responsive cascades in our present model. If mood, emotion, and relaxation adequately activate the HPA axis, the release of oxytocin is thought to be increased, resulting in a feeling of well-being [10]. Stress activates the paraventricular nucleus and HPA axis via either the locus coeruleus-norepinephrine (i.e., noradrenaline) or the hypothalamic-neurohypophyseal system [58,59]. When oxytocin is produced in the CNS and partially released from the pituitary into the blood, stress is relieved [10,60]. On the other hand, if an oxytocin release level is not sufficiently produced by mindfulness meditation, placebo, or fragrance, the sympathetic nervous system is thought to be stimulated by GABA and/or probably NO, which is released in the PNS [61]. As shown in Figure 2, oxytocin increases appetitive motivation, social attachment, and well-being. These effects are characterized by the upregulation of the PNS.
Mechanism underlying the oxytocin-mediated regulation of neuronal functions
The mechanisms underlying the oxytocin-mediated regulation of neuronal functions have been studied [62]. First, we should notice that a classical, autoradiographical study showed that the putative oxytocin receptors were abundantly present in several brain regions [63]. For example, the receptors were located in the olfactory nucleus, the hypothalamus, the amygdala, the septum, the paraventricular nucleus, and so forth. In particular, oxytocin receptors have been recently confirmed in the autonomic nervous system [64]. More recently, a study using oxytocin receptor-Venus mice provided a detailed distribution of oxytocin receptors at the cellular level [65]. Second, we would like to insist that oxytocin receptor is a G-protein coupled receptor (GPCR) [66]. GPCR is coupled with Gq proteins. That is, stimulation of GPCR produces inositol trisphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol 4,5-bisphosphate (PIP2) via activation of Gq protein and phospholipase C (PLC) [14], resulting in activation of several types of TRP channels and eventually an increase in intracellular Ca2+ concentration from endoplasmic reticulum (ER) and from the outside of cells [67]. In the case of a GPCR-related Ca2+ entry from the outside of cells, transient receptor potential canonical (TRPC) channels are known to play an important role. TRPC carries not only Ca2+ but also Na+ from the outside to the inside of cells. Na+ entry via TRPC channels evokes a change in membrane potential (i.e., depolarization) and further induces Ca2+ entry via Na+/Ca2+ exchangers and voltage-dependent Ca2+ channels. Finally, oxytocin-induced Ca2+ elevation hyperpolarizes the cells by open of Ca2+-activated K+ channels (BK channels) [68].
Because endoplasmic reticulum (ER)-original and TRPC-related Ca2+ elevations are not a long-lasting phenomenon, the involvement of GABA receptors and the function of NO should be also taken into account [14] to prolong hyperpolarization as a supporting system, because GABA hyperpolarizes neurons, and NO is permeable to membranes and relaxes blood vessels [69]. That is, the functions of GABA and NO are thought to be associated with oxytocin. Recently, a role of the DH in inflammation-induced hyperalgesia was investigated, and phosphatidylinositol 3 (PI3)-kinase and its subsequent signaling were found to be involved [17]. Our model proposed in the present review is consistent with this view.
Relationship between oxytocin and mental disorders
Recently, strong attention has been paid to the effects of oxytocin as a treatment on mental disorders. For example, studies in patients with schizophrenia that have investigated the effects of oxytocin at various levels, such as the levels of clinical symptoms, social cognitive function as assessed with experimental and neuropsychological tasks, and brain function as assessed using fMRI, showed that oxytocin was an ideal treatment [70]. Further, about developmental disability, changes in brain activity during judgments of socially and nonsocially meaningful pictures in children with autism spectrum disorder were examined using fMRI after intranasal administration of oxytocin [71]. In this study, oxytocin increased activity in the striatum and the other regions that have been previously implicated in reward; social attention, perception, and cognition; and detecting, decoding, and reasoning about mental states [72]. In particular, oxytocin increased activity during social judgments and decreased activity during nonsocial judgments. In the future, oxytocin will be used as a treatment more for mental disorders before evidence-based behavioral treatments.
Conclusions
The HPA axis and oxytocin together comprise a strong candidate model to explain how, for example, mindfulness meditation, placebo and fragrance promote well-being. The causality between oxytocin level and well-being/stress reduction seems to be proved by fMRI examinations after intranasal administration of oxytocin [71]. As a biomarker for well-being, social bonding and so forth, quantitative measurement of oxytocin has been attempted in the blood, saliva, or urine [28]. The sensitivity of commercially available detection kits, however, is not sufficient. Therefore, we are currently developing a new ultrasensitive ELISA for detecting trace amounts of oxytocin [73–75]. There may be other types of small molecules sensitive to psychophysiologic activity of the CNS.
Finally, the role of the HPA axis in regulating the functions of the liver, stomach, and other organs via the sympathetic and parasympathetic nervous systems must be clarified. Generally, the activity of the autonomic nervous system can be measured by heart rate variability, respiratory wave pattern, and electrodermal activity [76]. These methods, however, cannot be used to directly obtain information about CNS activity. For this purpose, EEG and MEG are used to obtain the information of neural activity in the CNS, but they are not very good for quantitative analysis [77]. Further, as described earlier, fMRI is applied only to subjects in a recumbent posture, and thus this measurement is affected by the autonomic nervous system [41]. We believe that NIRS is also useful if a dynamic pattern analysis method is applied [39]. The combination between an ultrasensitive ELISA measurement of oxytocin and a NIRS detection of brain function will confirm our hypothesis.
Acknowledgments
This work was partially supported by JSPS KAKENHI [grant number: 19H00633] to E. I.
Abbreviations
- CNS
central nervous system
- DH
dorsal horn
- DRG
dorsal root ganglion
- EEG
electroencephalography
- fMRI
functional magnetic resonance imaging
- HPA axis
hypothalamic-pituitaryadrenal axis
- MEG
magnetoencephalography
- NIRS
near-infrared spectroscopy
- NO
nitric oxide
- PNS
peripheral nervous system
- TRP channel
transient receptor potential channel.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author Contributions
E. I. and T. Y. designed the manuscript components, and R. S. prepared the figures. All the authors wrote the manuscript and approved the submitted version.
References
- 1.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Sivukhina EV, Jirikowski GF. Magnocellular hypothalamic system and its interaction with the hypothalamo-pituitary-adrenal axis. Steroids. 2016;111:21–28. doi: 10.1016/j.steroids.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Hawker RW, Robertson PA. Oxytocin and lactation. J Clin Endocrinol Metab. 1957;17:448–451. doi: 10.1210/jcem-17-3-448. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: The great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter CS. Oxytocin and human evolution. Curr Top Behav Neurosci. 2018;35:291–319. doi: 10.1007/7854_2017_18. [DOI] [PubMed] [Google Scholar]
- 6.Campbell A. Oxytocin and human social behavior. Pers Soc Psychol Rev. 2010;14:281–295. doi: 10.1177/1088868310363594. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman ER, Brownley KA, Hamer RM, Bulik CM. Plasma, salivary, and urinary oxytocin in anorexia nervosa: a pilot study. Eat Behav. 2012;13:256–259. doi: 10.1016/j.eatbeh.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lonstein JS, Maguire J, Meinlschmidt G, Neumann ID. Emotion and mood adaptations in the peripartum female: Complementary contributions of GABA and oxytocin. J Neuroendocrinol. 2014;26:649–664. doi: 10.1111/jne.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersson M, Uvnäs-Moberg K. Effects of an acute stressor on blood pressure and heart rate in rats pretreated with intracerebroventricular oxytocin injections. Psychoneuroendocrinology. 2007;32:959–965. doi: 10.1016/j.psyneuen.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Donadon MF, Martin-Santos R, Osório FL. The associations between oxytocin and trauma in humans: A systematic review. Front Pharmacol. 2018;9:154. doi: 10.3389/fphar.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González-Hernández A, Rojas-Piloni G, Condés-Lara M. Oxytocin and analgesia: Future trends. Trends Pharmacol Sci. 2014;35:549–551. doi: 10.1016/j.tips.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Fujita M, Boraschi D. Endotoxin contamination in nanomaterials leads to the misinterpretation of immunosafety results. Front Immunol. 2017;8:472. doi: 10.3389/fimmu.2017.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, Devries AC. Oxytocin increases autonomic cardiac control: Moderation by loneliness. Biol Psychol. 2010;86:174–180. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Saito N, Shima R, Yamada Y, Nagaoka M, Ito E, Yoshioka T. A proposed molecular mechanism for physical analgesia in chronic pain. Neural Plast. 2018;2018:1260285. doi: 10.1155/2018/1260285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison I, Löken LS, Olausson H. The skin as a social organ. Exp Brain Res. 2010;204:305–314. doi: 10.1007/s00221-009-2007-y. [DOI] [PubMed] [Google Scholar]
- 16.Saito N, Shima R, Yen CT, Yang RC, Ito E, Yoshioka T. Adhesive pyramidal thorn patches provide pain relief to athletes. Kaohsiung J Med Sci. 2019;35:230–237. doi: 10.1002/kjm2.12044. [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Fitzsimons B, Steinauer J, O’Neill A, Newton AC, Hua X-Y, et al. Spinal phosphinositide 3-kinase-AktmTOR signaling cascades in inflammation-induced hyperalgesia. J Neurosci. 2011;31:2113–2124. doi: 10.1523/JNEUROSCI.2139-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, et al. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89:1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morhenn V, Beavin LE, Zak PJ. Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Altern Ther Health Med. 2012;18:11–18. [PubMed] [Google Scholar]
- 20.Lloyd DM, McGlone FP, Yosipovitch G. Somatosensory pleasure circuit: from skin to brain and back. Exp Dermatol. 2015;24:321–324. doi: 10.1111/exd.12639. [DOI] [PubMed] [Google Scholar]
- 21.Quindlen-Hotek JC, Barocas VH. A finite-element model of mechanosensation by a Pacinian corpuscle cluster in human skin. Biomech Model Mechanobiol. 2018;17:1053–1067. doi: 10.1007/s10237-018-1011-1. [DOI] [PubMed] [Google Scholar]
- 22.Manfredi LR, Baker AT, Elias DO, Dammann JF, III, Zielinski MC, Polashock VS, et al. The effect of surface wave propagation on neural responses to vibration in primate glabrous skin. PLoS One. 2012;7:e31203. doi: 10.1371/journal.pone.0031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu CC, Jan YM, Li TC, Hsieh CL. Electroacupuncture induces differential effects between Yin and Yang: a study using cutaneous blood flow and temperature recordings of the hand’s dorsum and palm. Am J Chin Med. 2009;37:639–645. doi: 10.1142/S0192415X09007120. [DOI] [PubMed] [Google Scholar]
- 24.Strzalkowski NDJ, Peters RM, Inglis JT, Bent LR. Cutaneous afferent innervation of the human foot sole: What can we learn from single-unit recordings? J Neurophysiol. 2018;120:1233–1246. doi: 10.1152/jn.00848.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho JC, Lee CH. TRP channels in skin: From physiological implications to clinical significances. Biophysics. 2015;11:17–24. doi: 10.2142/biophysics.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H. TRP channel classification. Adv Exp Med Biol. 2017;976:1–8. doi: 10.1007/978-94-024-1088-4_1. [DOI] [PubMed] [Google Scholar]
- 27.Liu HX, Ma S, Nan Y, Yang WH. Transient receptor potential melastatin-2 and temperature participate in the process of CD38-regulated oxytocin secretion. Neuroreport. 2016;27:935–939. doi: 10.1097/WNR.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 28.Ooishi Y, Mukai H, Watanabe K, Kawato S, Kashino M. Increase in salivary oxytocin and decrease in salivary cortisol after listening to relaxing slow-tempo and exciting fast-tempo music. PLoS One. 2017;12(e0189075) doi: 10.1371/journal.pone.0189075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oettl LL, Kelsch W. Oxytocin and olfaction. Curr Top Behav Neurosci. 2018;35:55–75. doi: 10.1007/7854_2017_8. [DOI] [PubMed] [Google Scholar]
- 30.Grinevich V, Stoop R. Interplay between oxytocin and sensory systems in the orchestration of socio-emotional behaviors. Neuron. 2018;99:887–904. doi: 10.1016/j.neuron.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Brief AP, Motowidlo SJ. Prosocial organizational behavior. Acad Manage Rev. 1986;11:710–725. [Google Scholar]
- 32.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 33.Veening JG, Olivier B. Intranasal administration of oxytocin: behavioral and clinical effects, a review. Neurosci Biobehav Rev. 2013;37:1445–1465. doi: 10.1016/j.neubiorev.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellingsen DM, Wessberg J, Chelnokova O, Olausson H, Laeng B, Leknes S. In touch with your emotions: oxytocin and touch change social impressions while others’ facial expressions can alter touch. Psychoneuroendocrinology. 2014;39:11–20. doi: 10.1016/j.psyneuen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Gu J, Strauss C, Bond R, Cavanagh K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clin Psychol Rev. 2015;37:1–12. doi: 10.1016/j.cpr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16:213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa H, Mieda T, Oshio A, Koshikawa F. The relationship between decentering and adaptiveness of response styles in reducing depression. Mindfulness. 2018;9:556–563. [Google Scholar]
- 38.Tomasino B, Chiesa A, Fabbro F. Disentangling the neural mechanisms involved in Hinduism- and Buddhism-related meditations. Brain Cogn. 2014;90:32–40. doi: 10.1016/j.bandc.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Lee C-H, Sugiyama T, Kataoka A, Kudo A, Fujino F, Chen Y-W, et al. Analysis for distinctive activation patterns of pain and itchy in the human brain cortex measured using near infrared spectroscopy (NIRS) PLoS One. 2013;8:e75360. doi: 10.1371/journal.pone.0075360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohi K, Shimada T, Kihara H, Yasuyama T, Sawai K, Matsuda Y, et al. Impact of familial loading on prefrontal activation in major psychiatric disorders: A near-infrared spectroscopy (NIRS) study. Sci Rep. 2017;7:44268. doi: 10.1038/srep44268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaughlin LJ, Goldman H, Kleinman KM, Korbol B. The influence of body position on autonomic nervous system function. Pavlov J Biol Sci. 1978;13:29–41. doi: 10.1007/BF03005155. [DOI] [PubMed] [Google Scholar]
- 42.Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159:1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- 43.Meissner K, Kohls N, Colloca L. Introduction to placebo effects in medicine: Mechanisms and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2011;366:1783–1789. doi: 10.1098/rstb.2010.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiol Rev. 2013;93:1207–1246. doi: 10.1152/physrev.00043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefano GB, Fricchione GL, Slingsby BT, Benson H. The placebo effect and relaxation response: Neural processes and their coupling to constitutive nitric oxide. Brain Res Brain Res Rev. 2001;35:1–19. doi: 10.1016/s0165-0173(00)00047-3. [DOI] [PubMed] [Google Scholar]
- 46.Colloca L, Miller FG. Harnessing the placebo effect: the need for translational research. Philos Trans R Soc Lond B Biol Sci. 2011;366:1922–1930. doi: 10.1098/rstb.2010.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colloca L, Klinger R, Flor H, Bingel U. Placebo analgesia: Psychological and neurobiological mechanisms. Pain. 2013;154:511–514. doi: 10.1016/j.pain.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 49.Neumann I, Schwarzberg H, Landgraf R. Measurement of septal release of vasopressin and oxytocin by the push-pull technique following electrical stimulation of the paraventricular nucleus of rats. Brain Res. 1988;462:181–184. doi: 10.1016/0006-8993(88)90603-8. [DOI] [PubMed] [Google Scholar]
- 50.Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 51.Neumann I, Douglas AJ, Pittman QJ, Russell JA, Landgraf R. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J Neuroendocrinol. 1996;8:227–233. doi: 10.1046/j.1365-2826.1996.04557.x. [DOI] [PubMed] [Google Scholar]
- 52.Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, et al. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Baribeau DA, Anagnostou E. Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front Neurosci. 2015;9:335. doi: 10.3389/fnins.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 55.Esch T, Stefano GB. Love promotes health. Neuro Endocrinol Lett. 2005;26:264–267. [PubMed] [Google Scholar]
- 56.Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 57.Torner L, Plotsky PM, Neumann ID, de Jong TR. Forced swimming-induced oxytocin release into blood and brain: Effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology. 2017;77:165–174. doi: 10.1016/j.psyneuen.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Giustino TF, Maren S. Noradrenergic modulation of fear conditioning and extinction. Front Behav Neurosci. 2018;12:43. doi: 10.3389/fnbeh.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jurek B, Slattery DA, Hiraoka Y, Liu Y, Nishimori K, Aguilera G, et al. Oxytocin regulates stress-induced Crf gene transcription through CREB-regulated transcription coactivator 3. J Neurosci. 2015;35:12248–12260. doi: 10.1523/JNEUROSCI.1345-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Streeter CC, Jensen JE, Perlmutter RM, Cabral HJ, Tian H, Terhune DB, et al. Yoga Asana sessions increase brain GABA levels: A pilot study. J Altern Complement Med. 2007;13:419–426. doi: 10.1089/acm.2007.6338. [DOI] [PubMed] [Google Scholar]
- 62.Kim SC, Lee JE, Kang SS, Yang HS, Kim SS, An BS. The regulation of oxytocin and oxytocin receptor in human placenta according to gestational age. J Mol Endocrinol. 2017;59:235–243. doi: 10.1530/JME-16-0223. [DOI] [PubMed] [Google Scholar]
- 63.Elands J, Beetsma A, Barberis C, de Kloet ER. Topography of the oxytocin receptor system in rat brain: an autoradiographical study with a selective radioiodinated oxytocin antagonist. J Chem Neuroanat. 1988;1:293–302. [PubMed] [Google Scholar]
- 64.Lancaster K, Goldbeck L, Puglia MH, Morris JP, Connelly JJ. DNA methylation of OXTR is associated with parasympathetic nervous system activity and amygdala morphology. Soc Cogn Affect Neurosci. 2018;13:1155–1162. doi: 10.1093/scan/nsy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma K, LeBlanc R, Haque M, Nishimori K, Reid MM, Teruyama R. Sexually dimorphic oxytocin receptor-expressing neurons in the preoptic area of the mouse brain. PLoS One. 2019;14(e0219784) doi: 10.1371/journal.pone.0219784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim SH, Bennett PR, Terzidou V. Advances in the role of oxytocin receptors in human parturition. Mol Cell Endocrinol. 2017;449:56–63. doi: 10.1016/j.mce.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 67.Kirchner MK, Foehring RC, Wang L, Chandaka GK, Callaway JC, Armstrong WE. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulates afterhyperpolarizations in oxytocin neurons of the supraoptic nucleus. J Physiol. 2017;595:4927–4946. doi: 10.1113/JP274219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Che T, Sun H, Li J, Yu X, Zhu D, Xue B, et al. Oxytocin hyperpolarizes cultured duodenum myenteric intrinsic primary afferent neurons by opening BKCa channels through IP3 pathway. J Neurochem. 2012;121:516–525. doi: 10.1111/j.1471-4159.2012.07702.x. [DOI] [PubMed] [Google Scholar]
- 69.Ikeda M, Yoshioka T, Allen CN. Developmental and circadian changes in Ca2+ mobilization mediated by GABAA and NMDA receptors in the suprachiasmatic nucleus. Eur J Neurosci. 2003;17:58–70. doi: 10.1046/j.1460-9568.2003.02427.x. [DOI] [PubMed] [Google Scholar]
- 70.Ettinger U, Hurlemann R, Chan RCK. Oxytocin and schizophrenia spectrum disorders. Curr Top Behav Neurosci. 2018;35:515–527. doi: 10.1007/7854_2017_27. [DOI] [PubMed] [Google Scholar]
- 71.Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci USA. 2013;110:20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jurek B, Neumann ID. The oxytocin receptor: From intracellular signaling to behavior. Physiol Rev. 2018;98:1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- 73.Watabe S, Kodama H, Kaneda M, Morikawa M, Nakaishi K, Yoshimura T, et al. Ultrasensitive enzyme-linked immunosorbent assay (ELISA) of proteins by combination with the thio-NAD cycling method. Biophysics. 2014;10:49–54. doi: 10.2142/biophysics.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morikawa M, Naito R, Mita K, Watabe S, Nakaishi K, Yoshimura T, et al. Subattomole detection of adiponectin in urine by ultrasensitive ELISA coupled with thio-NAD cycling. Biophys Physicobiol. 2015;12:79–86. doi: 10.2142/biophysico.12.0_79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamakado S, Cho H, Inada M, Morikawa M, Jiang Y-H, Saito K, et al. Urinary adiponectin as a new diagnostic index for chronic kidney disease due to diabetic nephropathy. BMJ Open Diabetes Res Care. 2019;7(e000661) doi: 10.1136/bmjdrc-2019-000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Penzel T, Kantelhardt JW, Bartsch RP, Riedl M, Kraemer JF, Wessel N, et al. Modulations of heart rate, ECG, and cardio-respiratory coupling observed in polysomnography. Front Physiol. 2016;7:460. doi: 10.3389/fphys.2016.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He B, Sohrabpour A, Brown E, Liu Z. Electrophysiological source imaging: A noninvasive window to brain dynamics. Annu Rev Biomed Eng. 2018;20:171–196. doi: 10.1146/annurev-bioeng-062117-120853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lowrey CR, Strzalkowski NDJ, Bent LR. Cooling reduces the cutaneous afferent firing response to vibratory stimuli in glabrous skin of the human foot sole. J Neurophysiol. 2013;109:839–850. doi: 10.1152/jn.00381.2012. [DOI] [PubMed] [Google Scholar]