Abstract
Objective:
To assess overall adherence to Centers for Disease Control and Prevention and American College of Obstetrics and Gynecology recommended guidelines for syphilis testing among women who delivered a stillbirth and compare it with other tests recommended for stillbirth evaluation.
Methods:
We used MarketScan claims data with 40 million commercially insured and 8 million Medicaid enrollees annually to estimate prenatal care and follow-up testing among women who had stillbirths between January 1, 2013, and December 24, 2013. Stillbirth was identified if women had any International Classification of Disease, Ninth Revision codes related to a stillbirth outcome. Among women with stillbirths, we estimated the proportions of women who received prenatal care and prenatal syphilis testing within 280 days before stillbirth, and testing at the time of stillbirth (syphilis testing, complete blood count, placental examination and autopsy) using Physician’s Current Procedural Terminology codes.
Results:
We identified 3672 Medicaid-insured women and 6023 commercially insured women with stillbirths in 2013. Approximately, 61.7% of Medicaid-insured women and 66.0% of commercially insured women had claims data indicating prenatal syphilis testing. At the time of stillbirth, Medicaid-insured and commercially insured women had similar rates of syphilis testing (6.5% vs 9.3%), placental examination (61.6% vs 57.8%), and complete blood count (31.9% vs 37.6%). Autopsies were too infrequent to be reported. Approximately, 34.6% of Medicaid-insured women and 29.7% of commercially insured women had no syphilis testing either prenatally or at the time of stillbirth.
Conclusions:
Syphilis testing among women after stillbirth was less than 10%, illustrating limited adherence to Centers for Disease Control and Prevention and American College of Obstetrics and Gynecology recommendations. Such low prenatal and delivery syphilis testing rates may impact the number of stillbirth cases identified as congenital syphilis cases and reported to the national surveillance system. Our results emphasize the need to improve syphilis testing to improve diagnosis of syphilitic stillbirths, identify women with syphilis infection, and provide treatment to these women to avoid syphilis-related adverse outcomes.
Stillbirth remains one of the most challenging pregnancy outcomes in the United States. In some cases, stillbirth is preventable. Stillbirth, defined as spontaneous intrauterine death of a fetus at 20 weeks of gestation or more in the United States, is often reported as “fetal death after 20 weeks gestation” in national statistics. About 24,000 stillbirths were reported in the United States in 2013.1 For years 2012 and 2013, the stillbirth rate was 6.0 and 6.1 per 1000 live births, respectively.1 These estimates indicate more progress needed to achieve the US Healthy People 2020 targets of reducing fetal deaths or stillbirths to 5.6 per 1000 live births.2 The highest stillbirth rates were reported among teenagers, unmarried women, women 35 years or older, and women with multiple gestation pregnancy.1
Potentially preventable causes of stillbirth include congenital infections, such as syphilis, toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, hepatitis viruses, human immunodeficiency virus, varicella, malaria, and parvovirus B19.3–7 Untreated syphilis infection during pregnancy may also result in spontaneous abortion, prematurity, low birth weight, or congenital syphilis (manifest either as stillbirth or a live but affected infant). Among infants congenitally infected with syphilis, approximately 10% result in neonatal death.8,9 Syphilis can be identified through serological testing of pregnant women. Congenital syphilis, including syphilitic stillbirths, generally can be prevented by treatment with a penicillin regimen that is appropriate for the mother’s stage of syphilis.10–12 A systematic review and meta-analysis found that fetal loss (before 20 weeks) and stillbirth were 21% more frequent among untreated pregnant women with syphilis than pregnant women without syphilis.9 A recent surveillance report showed that during 2012 to 2014, the number of reported congenital syphilis cases, including syphilitic stillbirths, rose from 334 to 458, and the rate increased from 8.4 to 11.6 syphilitic stillbirths per 100,000 live births.13
The prevention of congenital syphilis, including syphilitic stillbirths, requires syphilis screening in early pregnancy and timely identification of syphilis followed by appropriate treatment. An example of inadequate prenatal care and potentially adverse pregnancy outcome is demonstrated in a study where of the 92 Chinese infants born with congenital syphilis, 83% had mothers with no prenatal care.14 The Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force, and the American College of Obstetrics and Gynecology (ACOG) recommend syphilis screening for all pregnant women at their first prenatal visit.6,11 All 3 organizations recommend additional syphilis testing early in the third trimester and at the time of delivery for women at increased risk (eg, uninsured, living in poverty, sex workers, STD diagnosis, living in a community of high syphilis morbidity, or who use illicit drugs).14–16 To identify syphilitic stillbirth and to prevent syphilis transmission, both ACOG and CDC further recommend that any woman who delivers a stillbirth at 20 weeks of gestation or more should be tested for syphilis.4,11
There has been little evidence available in the literature regarding provider adherence to recommendations for syphilis testing among women with stillbirth. The main objective of this study was to evaluate, among women who delivered a stillbirth, the proportion who received syphilis testing within 1 week before or after stillbirth diagnosis, and to compare this with other evaluations commonly performed as part of a post-stillbirth workup, such as complete blood count (CBC), placental examination, and fetal autopsy. Because adequate testing at stillbirth may be associated with prenatal care services received, the second objective was to estimate, among women who delivered a stillbirth, the proportion who received prenatal care and prenatal syphilis testing. Finally, we aimed to explore differences in key outcomes by variables that may influence provider practice, such as demographics and insurance enrollment status.
METHODS
For this study, Truven Health MarketScan Medicaid and commercial claims data were used for the years 2012 and 2013. Annually, an average of 40 million Americans with commercial health care plans, such as health maintenance organizations, preferred provider organizations, point-of-services and fee-for-services are enrolled in the MarketScan database. Also, an average of 8 million enrollees from 10 unknown states are available in the MarketScan Medicaid database.17 Both databases capture person-specific enrollment and medical service utilization information, such as outpatient or inpatient visits, demographics, places and dates of service, diagnosis codes (International Classification of Disease, Ninth Revision [ICD-9]), procedural codes (Physician’s Current Procedural Terminology), prescription drug use, provider details, and other billing-related information.17 The race variable was only available in the Medicaid claims data, whereas the geographic variables, such as region, state, and 3-digit zip code, were only available in the commercial claims data. MarketScan data obtained for analysis consisted of de-identified patient service utilization records.17 This study was approved by CDC internal review board as research that did not involve identifiable human subjects.
Using the MarketScan database, ICD-9 codes were used to identify women aged 15 to 44 years with stillbirth diagnoses in 2013. Because there is no standardized case definition of stillbirth that is universally adopted for claims data, we used a previously validated method to classify women with stillbirths.1,18–20 We hierarchically classified women into 3 stillbirth subgroups by ICD-9 codes: (1) V27.x codes, (2) 779.9 code, and (3) 656.4 code (Table 1).
TABLE 1.
Diagnostic and Procedure Codes Used to Evaluate Stillbirth, Syphilis Testing, and Other Testing Among Women Aged 15–44 Years Who Had Stillbirth During January 1, 2013, to December 24, 2013, MarketScan Medicaid and Commercial Insurance Database, 2012–2013
| ICD-9 Codes | Description for Stillbirth |
|---|---|
| V27.1* | Single stillborn |
| V27.3 | Twins, 1 live born and 1 stillborn |
| V27.4 | Twins, both stillborn |
| V27.6 | Other multiple birth, some live-born |
| V27.7 | Other multiple births, all stillborn |
| 779.9 | Unspecified condition originating in the perinatal period |
| 656.4 | Intrauterine death |
| CPT codes | Description for testing or evaluation |
| 80055, 86780, 86781, 86592, 86593 | Syphilis test |
| 80055, 86900, 86901, 86762 | Prenatal care (Obstetric panel, or rubella and ABO blood type/Rh (D) factor tests) |
| 88305, 88307, 59414 | Placental examination (Gross microscopic exam and placenta delivery) |
| 88000–88099 | Fetal autopsy |
| 85004–85049, 80050, 80055, 86762, 86900, 86901 | CBC (performed solely or as a part of bundle testing) |
The V codes are used to indicate circumstances leading to a medical encounter deemed as “Supplementary Classification of Factors Influencing Health Status and Contact with Health Services.”
We determined the earliest date of stillbirth diagnosis as an index date. We defined “at the time of stillbirth” to be a 14-day period that was 7 days before and 7 days after the index date. To capture syphilis testing at the time of stillbirth, our study population is limited to women whose index dates were between January 1, 2013, and December 24, 2013. To validate if a 14-day interval is sufficient, we also assessed testing practices at delivery to be a 28-day period that was 14 days before and 14 days after the index date. Because gestational age was not available in the database, an interval of 8 to 280 days before the index date was considered the maximum period for prenatal care. Although women in our sample were selected from 2013 database, their prenatal care claims were retrieved back to 2012 if necessary. We used previously described methods to classify women with any prenatal care if they had any claims indicating prenatal care services during the maximum period21 (Table 1). Prenatal services might not be captured if these services occurred before women enrolled in health plans. We used 210 days as the threshold for defining continuous insurance enrollment that has been used in the previous study to assess the association between prenatal services and enrollment coverage.21
In this claims data, all testing refers to maternal tests and is billed to the mother (even fetal autopsy). Women who had prenatal syphilis testing were identified if they had any claims that included syphilis tests during the maximum period for prenatal care (Table 1). Our assessment also included 3 of the tests recommended by ACOG at the time of stillbirth: CBC, placental examination, and fetal autopsy. Although the CBC is nonspecific, it might indicate an infection or abruption of placenta as an etiology for a stillbirth. We assessed prenatal care among these women by their enrollment coverage and by type of insurance (Medicaid vs commercial). We also assessed key outcomes before stillbirth (prenatal syphilis testing) and at the time of stillbirth (syphilis testing, CBC, placental examination, and fetal autopsy) for the whole group and by type of insurance. We used Pearson χ2 tests to detect significant differences in bivariate analyses of categorical data; patients’ demographic characteristics and clinical testing performed during prenatal care and at the time of stillbirth. For all statistical tests, we considered an alpha level P < 0.05 to be significant. We used SAS software version 9.3 (SAS Institute, Cary, NC) for all statistical analyses.
RESULTS
A total of 3672 Medicaid-insured women and 6023 commercially insured women with stillbirth were identified between January 1 2013, and December 24, 2013. Of these women, 1507 (41.0%) Medicaid-insured and 1892 (31.4%) commercially insured women were hierarchically classified as the V-code group, 193 (5.3%) Medicaid-insured and 468 (7.8%) commercially insured women as the 779.9 code group, and 1972 (53.7%) Medicaid and 3663 (60.8%) commercially insured women as the 656.4 code group.
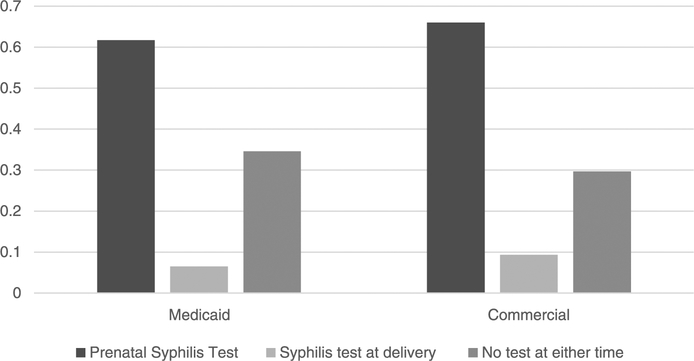
At the time of stillbirth, syphilis testing was performed in 6.5% of Medicaid-insured women and 9.3% of commercially insured women (Table 2). Among women with Medicaid, those age 15 to 24 years had a higher proportion of prenatal syphilis testing than women aged 25 to 34 years and 35 to 44 years. Among commercially insured women, those aged 25 to 34 years had a higher proportion of prenatal syphilis testing than women aged 15 to 24 years and 35 to 44 years (Table 2). Overall, prenatal syphilis testing was significantly higher than syphilis testing at the time of stillbirth: 61.7% versus 6.5% among women with Medicaid (P < 0.001) and 66.0% versus 9.3% (P < 0.001) among commercially insured women (Table 2). Among those women who had a prenatal syphilis test, 4.2% women in Medicaid and 7.5% women with commercial insurance received a syphilis test at the time of stillbirth. Among women without a prenatal syphilis test, 10.2% women in Medicaid and 12.8% women with commercial insurance received a syphilis test at the time of stillbirth. Approximately, 34.6% of Medicaid-insured women and 29.7% of commercially insured women had neither prenatal syphilis testing nor syphilis testing at the time of stillbirth (Fig. 1).
TABLE 2.
Diagnostic Testing Rate Among Women Aged 15–44 Years Who Had Stillbirth During January 1, 2013, to December 24, 2013, by Age Groups, Race/Ethnicity and Region, MarketScan Medicaid and Commercial Database, 2012–2013
| Medicaid | Commercial | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | (%) | Prenatal Syphilis Test | Syphilis Test at Stillbirth | Placental Exam at Stillbirth | CBC at Stillbirth | Total | (%) | Prenatal Syphilis Test | Syphilis Test at Stillbirth | Placental Examination at Stillbirth | CBC at Stillbirth | |
| Total | 3672 | 100 | 61.7 | 6.5 | 61.6 | 31.9 | 6023 | 100 | 66.0 | 9.3 | 57.8 | 37.6 |
| Age groups, y | ||||||||||||
| 15–24 | 1,495 | 40.7 | 67.0* | 6.4 | 62.7* | 28.7* | 830 | 13.8 | 58.3* | 10.4 | 57.4 | 37.5 |
| 25–34 | 1,677 | 45.7 | 60.1 | 6.6 | 62.6 | 34.3 | 3,222 | 53.5 | 67.9 | 8.9 | 56.8 | 36.7 |
| 35–44 | 500 | 13.6 | 50.8 | 6.2 | 54.8 | 33.4 | 1,971 | 32.7 | 66.0 | 9.5 | 59.5 | 39.1 |
| Race ethnicity | ||||||||||||
| White, non-Hispanic | 1,453 | 39.6 | 61.8† | 5.4 | 59.9† | 33.7 | n/a | n/a | n/a | n/a | n/a | n/a |
| Black, non-Hispanic | 1,720 | 46.8 | 63.8 | 7.2 | 64.0 | 30.6 | n/a | n/a | n/a | n/a | n/a | n/a |
| Hispanic | 155 | 4.2 | 47.7 | 7.7 | 53.6 | 27.1 | n/a | n/a | n/a | n/a | n/a | n/a |
| Other | 344 | 9.4 | 56.7 | 7.0 | 60.5 | 32.9 | n/a | n/a | n/a | n/a | n/a | n/a |
| Region | ||||||||||||
| Northeast | n/a | n/a | n/a | n/a | n/a | n/a | 1,147 | 19.0 | 64.1 | 6.6‡ | 55.9‡ | 33.6* |
| North-Central | n/a | n/a | n/a | n/a | n/a | n/a | 1,183 | 19.6 | 66.1 | 7.1 | 60.1 | 38.2 |
| South | n/a | n/a | n/a | n/a | n/a | n/a | 2,176 | 36.1 | 67.6 | 14.8 | 61.9 | 42.1 |
| West | n/a | n/a | n/a | n/a | n/a | n/a | 1,354 | 22.5 | 65.4 | 4.9 | 50.6 | 33.2 |
| Unknown | n/a | n/a | n/a | n/a | n/a | n/a | 163 | 2.7 | 62.0 | 8.0 | 58.9 | 38.7 |
P < 0.05 by age group.
P < 0.05 by race/ethnicity.
P < 0.05 by region.
Figure 1.
Differences in prenatal syphilis testing, syphilis testing at the time of stillbirth and no syphilis testing at either time for women aged 15–44 years who had stillbirth during January 1, 2013, to December 24, 2013, MarketScan Medicaid and commercial database, 2012–2013.
Placental examination was performed at the time of stillbirth in 61.6% of Medicaid and 57.8% of commercially insured women with stillbirth. The proportions of women receiving placental examination decreased as age category increased among Medicaid-insured women; in contrast, proportions increased as age category increased among commercially insured women. During the course of pregnancy, CBC was 85.2% for Medicaid insured and 86.5% for commercially insured women (between 280 days before and 7 days after stillbirth), whereas CBC at the time of stillbirth (between 7 days before and 7 days after stillbirth) was 31.9% for Medicaid and 37.6% for commercially insured women (Table 2).
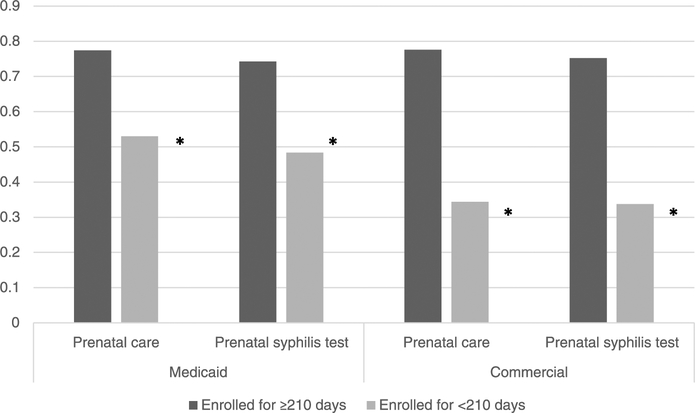
A significantly higher proportion of commercially insured women had insurance coverage of equal or greater than 210 days before stillbirth than Medicaid-insured women (77.9% vs 51.7%, P < 0.05). The proportion of women receiving prenatal care was significantly (P < 0.001) higher for women with equal or greater than 210 days of enrollment coverage than those without 210 days of enrollment coverage among Medicaid-insured women (77.4% vs 53.0%) and among commercially insured women (77.6% vs 34.4%) (Fig. 2). Similarly, the prenatal syphilis testing rate was significantly (P < 0.001) higher for women with equal or greater than 210 days of enrollment coverage than those without 210 days of enrollment coverage among Medicaid-insured women (74.3% vs 48.4%) and among commercially insured women (75.2% vs 33.8%) (Fig. 2).
Figure 2.
Differences in prenatal care and prenatal syphilis testing for women aged 15–44 years who had stillbirth during January 1, 2013, to December 24, 2013, based on days of enrollment, MarketScan Medicaid, and commercial database, 2012–2013.
We found that the maternal syphilis testing rates were not significantly different from the 14-day interval to the 28-day interval: 6.5% to 9.8% for the Medicaid and 9.3% to 12.1% for the commercially insured.
DISCUSSION
Prenatal syphilis testing is crucial for the prevention of congenital syphilis, including stillbirths.3 Our results indicate suboptimal prenatal syphilis screening among insured women who deliver a stillbirth regardless of their insurance source. Over one third of women with a stillbirth did not have prenatal syphilis testing and the proportions were higher in those with inadequate prenatal care or shorter enrollment coverage. Without prenatal syphilis screening, pregnant women are unlikely to be appropriately diagnosed and treated for syphilis at early stages of pregnancy to prevent adverse health outcomes.3,9,22 Therefore, to reduce fetal mortality rates in the United States, missed opportunities to diagnose and treat prenatal syphilis must be addressed.
Our results also indicate that despite CDC and ACOG recommendations, few women (<10%) are tested for syphilis at the time of stillbirth.4,11 Additionally, our results showed that 34.6% of Medicaid-insured women and 29.7% of commercially insured women had neither prenatal syphilis testing nor syphilis testing at the time of stillbirth. This suggests that syphilitic stillbirths could be underdiagnosed. This is concerning because it indicates a quality gap in current practice which reduces the number of congenital syphilis cases reported in the national STD surveillance system.
Because stillbirth may be caused by multiple factors, it is sometimes a challenge to determine the most probable cause.20,23 Experts specifically recommend a thorough investigation including fetal autopsy and karyotype, with placental, cord and membrane examination, and maternal CBC, syphilis, and rubella testing.6,23 One study reported that the cause of fetal or perinatal death could be determined in 94% of cases when an autopsy was performed.24 Our findings reveal low rates of placental examination, CBC, syphilis testing, and virtually no fetal autopsies performed in either Medicaid or commercial plans, illustrating a lack of adherence to ACOG guidelines. Several factors may contribute to poor adherence. Institutions might lack a standardized protocol for workup after stillbirth, and providers might be unaware of current recommendations. The underutilization of fetal autopsy and placental examination may be attributable to provider reluctance to order the test, difficulties obtaining consent for the procedure, inadequate availability of trained pathologists to perform the study, and low reimbursement rates or no reimbursement. The low CBC tests at the time of stillbirth suggest that many providers may rely on available CBC results for patient evaluation rather than order new CBC test at the time of stillbirth.
There are variations on the definition of stillbirth between the World Health Organization, USA (adopted by CDC, ACOG) as well as among states in United States. The World Health Organization defines stillbirth as fetal demise occurring after 28 weeks of gestation or birthweight of 500 g.25 The US national stillbirth reporting requirements vary significantly from state to state: 42 states require reporting fetal death of 20 weeks of gestation or more or 350 g birthweight or more, 7 states require reporting all fetal deaths, 2 states require reporting fetal deaths over 500 g, and 1 requires reporting fetal deaths after 16 weeks gestation.1,20 This variation impacts the ability to accurately capture stillbirths across the United States.
There are 46 states and District of Columbia that have laws that require prenatal syphilis testing for all pregnant women and 12 laws specifically include third trimester testing for all women or high-risk women.26 The low compliance with CDC and ACOG prenatal testing guidance that we found suggests that more vigorous provider education and greater oversight in enforcing laws may be needed.
There were several limitations in our study. First, the MarketScan databases do not completely represent the US population due to its data collection methods. Commercial claims data only include large employers so small businesses that provide insurance are underestimated and uninsured persons are not captured. However, the MarketScan research database includes a large convenient sample of medical claims of about 40 million covered lives in commercial plans and 8 million enrollees in Medicaid. When weighted, information such as gender, age, and geographic distribution allow us to estimate a nationally representative sample of Americans enrolled in different commercial plans.17 Second, our data might not have captured the entire population (stillbirths) or the conditions of interest (testing). There are inherent limitations with claims data such as administrative errors, incomplete, inaccurate, or missing data, and lack of specific billing codes for some conditions.27,28 Third, prenatal care services may be underestimated due to women switching insurance plans or becoming enrolled after confirmed pregnancy. Fourth, our rate of prenatal syphilis testing among women with stillbirths (61%−66%) is lower than that reported for women with live-born infants (85%–96%), although both studies used the MarketScan database.21,29 The difference in prenatal syphilis testing rate might be partly explained by (1) the fact that many stillbirths had ended the pregnancy before the full term of 40 weeks, and (2) our study included all women with stillbirth regardless of length of enrollment coverage or prenatal care, whereas the other studies included only women who had had at least 210 days of enrollment coverage before delivery. Finally, MarketScan data do not allow maternal records to be linked to the fetal death records to identify what ultimately was reported as the cause of death.6
Our study found relatively low number of women being tested for syphilis at the time of stillbirth and during prenatal care suggesting low adherence to the ACOG and CDC guidelines. Prenatal syphilis testing is important to identify potentially preventable mortality in infants attributable to congenital syphilis. To meet public health surveillance and prevention objectives, it is critical that pregnant women be routinely screened to identify maternal syphilis. Adequate and timely treatment of pregnant women for syphilis can reduce the incidence of congenital syphilis and subsequent stillbirths. Future studies to identify strategies to improve adherence to testing recommendations for pregnant women are clearly needed.
Acknowledgements:
The authors thank Dr. Sarah Kidd for her insight and suggestions regarding limitations of administrative claims data associated with syphilis testing among women.
Footnotes
Conflict of Interest and Sources of Funding: None declared.
Publisher's Disclaimer: DISCLAIMER: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.MacDorman MF, Gregory EC. Fetal and perinatal mortality: United States, 2013. Natl Vital Stat Rep 2015; 64:1–24. [PubMed] [Google Scholar]
- 2.Office of Disease Prevention and Health Promotion USDoHaHS. Healthy People 2020—Maternal, infant, and child health objectives. Washington, DC: U.S. Department of Health and Human Services, 2014. [Google Scholar]
- 3.Neu N, Duchon J, Zachariah P. TORCH infections. Clin Perinatol 2015; 42:77–103, viii. [DOI] [PubMed] [Google Scholar]
- 4.Owusu-Edusei K, Bohm MK, Kent CK. Diagnostic methodologies for chlamydia screening in females aged 15 to 25 years from private insurance claims data in the United States, 2001 to 2005. Sex Transm Dis 2009; 36:419–421. [DOI] [PubMed] [Google Scholar]
- 5.Causes of death among stillbirths. JAMA 2011; 306(22):2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley L, AAPCo, Practice ACOG, et al. Guidelines for Perinatal Care; 2012.
- 7.Lawn JE, Blencowe H, Pattinson R, et al. Stillbirths: Where? When? Why? How to make the data count? Lancet 2011; 377:1448–1463. [DOI] [PubMed] [Google Scholar]
- 8.Qin JB, Feng TJ, Yang TB, et al. Risk factors for congenital syphilis and adverse pregnancy outcomes in offspring of women with syphilis in Shenzhen, China: A prospective nested case-control study. Sex Transm Dis 2014; 41:13–23. [DOI] [PubMed] [Google Scholar]
- 9.Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: A systematic review and meta-analysis. Bull World Health Organ 2013; 91:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: An update for physicians. Infect Dis Clin North Am 2013; 27: 705–722. [DOI] [PubMed] [Google Scholar]
- 11.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 12.Genç M, Ledger WJ. Syphilis in pregnancy. Sex Transm Infect 2000; 76:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen V, Su J, Torrone E, et al. Increase in incidence of congenital syphilis—United States, 2012–2014. MMWR Morb Mortal Wkly Rep 2015; 64:1241–1245. [DOI] [PubMed] [Google Scholar]
- 14.Wolff T, Shelton E, Sessions C, et al. Screening for syphilis infection in pregnant women: evidence for the U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 2009; 150: 710–716. [DOI] [PubMed] [Google Scholar]
- 15.Screening for syphilis infection in pregnancy: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 2009; 150:705–709. [DOI] [PubMed] [Google Scholar]
- 16.Preventive US Services Task Force. Screening for syphilis infection in pregnancy: U.S. preventive services task force reaffirmation recommendation statement. Ann Intern Med 2009; 150:705–709. [DOI] [PubMed] [Google Scholar]
- 17.Hansen L CS 2012;Pages. Accessed at http://truvenhealth.com/portals/0/assets/PH_11238_0612_TEMP_MarketScan_WP_FINAL.pdf.
- 18.Likis FE, Sathe NA, Carnahan R, et al. A systematic review of validated methods to capture stillbirth and spontaneous abortion using administrative or claims data. Vaccine 2013; 31 Suppl 10:K74–K82. [DOI] [PubMed] [Google Scholar]
- 19.King-Hele S, Webb RT, Mortensen PB, et al. Risk of stillbirth and neonatal death linked with maternal mental illness: A national cohort study. Arch Dis Child Fetal Neonatal Ed 2009; 94:F105–F110. [DOI] [PubMed] [Google Scholar]
- 20.Silver RM, Varner MW, Reddy U, et al. Work-up of stillbirth: A review of the evidence. Am J Obstet Gynecol 2007; 196:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross CE, Tao G, Patton M, et al. Screening for human immunodeficiency virus and other sexually transmitted diseases among U.S. women with prenatal care. Obstet Gynecol 2015; 125:1211–1216. [DOI] [PubMed] [Google Scholar]
- 22.Newman L, Kamb M, Hawkes S, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: Analysis of multinational antenatal surveillance data. PLoS Med 2013; 10:e1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silver RM. Optimal “work-up” of stillbirth: evidence! Am J Obstet Gynecol 2012; 206:1–2. [DOI] [PubMed] [Google Scholar]
- 24.Faye-Petersen OM, Guinn DA, Wenstrom KD. Value of perinatal autopsy. Obstet Gynecol 1999; 94:915–920. [DOI] [PubMed] [Google Scholar]
- 25.Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: Rates, risk factors, and acceleration towards 2030. Lancet 2016; 387:587–603. [DOI] [PubMed] [Google Scholar]
- 26.Hollier LM, Hill J, Sheffield JS, et al. State laws regarding prenatal syphilis screening in the United States. Am J Obstet Gynecol 2003; 189:1178–1183. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Hilsden R, Hossain S, et al. Validation of administrative data sources for endoscopy utilization in colorectal cancer diagnosis. BMC Health Serv Res 2012; 12:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason SA, Nathens AB, Byrne JP, et al. The accuracy of burn diagnosis codes in health administrative data: Avalidation study. Burns 2017; 43:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neblett Fanfair R, Tao G, Owusu-Edusei K, et al. Suboptimal Prenatal Syphilis Testing Among Commercially Insured Women in the United States, 2013. Sex Transm Dis 2017; 44:219–221. [DOI] [PubMed] [Google Scholar]