Abstract
The US remains at the forefront of a global obesity epidemic with a significant negative impact on public health. While it is well known that a balance between energy intake and expenditure is homeostatically regulated to control weight, growing evidence points to multifactorial social, neurobehavioral and metabolic determinants of food intake that influence obesity risk. This review presents factors such as the ubiquitous presence of rewarding foods in the environment and increased salience of such foods that stimulate brain reward motivation and stress circuits to influence eating behaviors. These rewarding foods via conditioned and reinforcing effects stimulate not only metabolic, but also stress hormones, that, in turn, hijack the brain emotional (limbic) and motivational (striatal) pathways, to promote food craving and excessive food intake. Furthermore, the impact of high levels of stress and trauma and altered metabolic environment (e.g. higher weight, altered insulin sensitivity) on prefrontal cortical self-control processes that regulate emotional, motivational and visceral homeostatic mechanisms of food intake and obesity risk are also discussed. A heuristic framework is presented in which the interactive dynamic effects of neurobehavioral adaptations in metabolic, motivation and stress neurobiology may further support food craving, excessive food intake and weight gain in a complex feed-forward manner. Implications of such adaptations in brain addictive-motivational and stress pathways and their effects on excessive food intake and weight gain are discussed to highlight key questions that requires future research attention in order to better understand and address the growing obesity epidemic.
Keywords: Addiction, Stress, Neurobiology, Food intake, Obesity
1. Multifactorial determinants of body weight and food intake
Obesity is a global epidemic with more than 500 million people worldwide classified as obese (body mass index, BMI ≥ 30 kg/m2) (2011). The United States is at the forefront of the pandemic with two-thirds of its population classified as overweight or obese (BMI ≥ 25 kg/m2) (Flegal, Carroll, Ogden, & Curtin, 2010). Thus, most Americans are above the recommended normal weight (lean BMI 18.5–24.9 kg/m2) and are predisposed to obesity-related conditions including cardiovascular disease, type 2 diabetes (T2DM), and various cancers (Ogden, Carroll, McDowell, & Flegal, 2007). This serious and worrying pandemic has thrown into question previously believed notions that body weight is regulated by the hypothalamus with homeostatic and physiologic metabolic processes that maintain a balance between energy intake and energy expenditure (Berthoud, 2012; Yeo&Heisler, 2012). There is growing acknowledgment that a number of social factors such as the easy availability and relatively low cost of high calorie foods that are highly palatable (HP), widespread marketing of such HP foods, increased use of sugars, sugar substitutes, preservatives and sugar sweetened beverages, altered eating patterns as well as the promotion of sedentary lifestyles all contribute to this pandemic (Hill & Peters, 1998; Ogden et al., 2007; Wang, Volkow, Thanos, & Fowler, 2004). In addition, genetic and other social and biological variables may also contribute towards vulnerability to obesity and weight gain (Stice, Spoor, Bohon, Veldhuizen, & Small, 2008; von Deneen, Gold, & Liu, 2009), but these alone do not explain the pandemic levels of obesity. Thus, this review focuses on the dynamic interplay between, (a) environmental variables of overabundance of rewarding foods and food cues (e.g. advertising), (b) the neurobiological effects of consuming such foods on the hypothalamic and extrahypothalamic reward/motivation and stress pathways, and, (c) effects on metabolic hormones, to impact food craving and motivation, excessive food intake and weight gain.
2. Highly palatable (HP) foods and related cues, food seeking and intake
Highly palatable (HP) foods are more liked, preferred and found to be rewarding in taste. These include foods high in sugar and sweet taste, highly processed foods high in saturated fats or high carbohydrates making up savory tastes and combination of food groups prepared in ways that enhance taste and value or ‘salience’ of such foods. These foods are ubiquitous in our current obesogenic environment and their related sensory (including both discrete and context related) and autobiographical associations such as sights, smells, tastes, place where eaten, with whom, when and time and other context factors serve as conditioned cues that may increase liking and preference for such foods, tendency towards seeking them, thereby resulting in facilitating food craving and intake of these foods. For example, in a cross sectional study with a large community sample, we found higher food craving for these HP foods. Furthermore, higher food craving was significantly associated with greater intake of these foods, and those with higher body mass index (BMI) reported greater levels of food craving (Chao, Grilo, White, & Sinha, 2014). In other population-based research, fast foods such as potato chips, processed meats, sugar sweetened beverages all predicted long term weight gain in large prospective cohorts of US men and women (Mozaffarian, Hao, Rimm, Willett, & Hu, 2011). Such highly palatable and processed foods and their related associations or ‘cues’ stimulate the brain reward and motivation pathways just as reinforcing drugs of abuse and via learning/conditioning mechanisms increase the likelihood of HP food seeking and consumption (Alsio, Olszewski, Levine, & Schioth, 2012; Avena, Rada, & Hoebel, 2009; Berthoud, 2012; Coelho, Jansen, Roefs, & Nederkoorn, 2009; Lutter & Nestler, 2009; Weingarten, 1983). The conditioned properties of these HP foods and related increases in their intake promotes their heightened salience, and, in turn, results in greater ‘wanting’ and seeking of HP foods, similar to the incentive salience processes that occur with increasing alcohol and drug intake (Robinson & Berridge, 2008; Sinha & Jastreboff, 2013). Research with animals and humans has documented activation of brain reward regions and increased dopaminergic transmission with HP food cue exposure, along with concomitant increases in food craving and motivation (Kelley, Schiltz, & Landry, 2005; Small, Zatorre, Dagher, Evans, & Jones-Gotman, 2001; Wang et al., 2001), with greater responsivity of brain reward regions and food craving among individuals with higher BMI (Pelchat, Johnson, Chan, Valdez, & Ragland, 2004; Saelens & Epstein, 1996; Simansky, 2005; Stice et al., 2008; Stice, Spoor, Ng, & Zald, 2009; Tetley, Brunstrom, & Griffiths, 2009).
3. Overeating of HP foods and metabolic and stress hormone responses
Intake of balanced meals with healthy foods or even small amounts of HP foods in healthy individuals will lead to food-related rise in plasma glucose that stimulates insulin secretion and enables glucose uptake into peripheral tissues. Previous evidence indicates that in lean healthy individuals, peripheral and even central infusion of insulin suppresses appetite and feeding (Kahn, Hull, & Utzschneider, 2006; Konner et al., 2011; Schwartz, Woods, Porte, Seeley, & Baskin, 2000; Sherwin, 2008; Woods, Lotter, McKay, & Porte, 1979). However, interesting new evidence suggests that with greater consumption of HP foods, the concomitant changes in carbohydrate and fat metabolism, insulin sensitivity and appetite hormones may influence neural reward regions involved in increasing salience and motivation for food intake, that, in turn, may alter energy homeostasis (Alsio et al., 2012; Chuang et al., 2011; DiLeone, 2009; Dossat, Lilly, Kay, & Williams, 2011; Farooqui, 2009; Figlewicz & Sipols, 2010; Malik, McGlone, Bedrossian, & Dagher, 2008). For example, chronic high levels of peripheral insulin and insulin resistance, as is observed in many individuals with obesity, may promote rather than suppress food craving and intake as well as increase brain activation in dopamine-rich reward regions such as the ventral tegmental area (VTA), nucleus accumbens and dorsal striatum (Anthony et al., 2006; Chechlacz et al., 2009; Jastreboff et al., 2013; Konner et al., 2011; Kullmann et al., 2012). Similar adaptations are noted with leptin in obesity, with evidence indicating that leptin and ghrelin influence dopaminergic transmission in brain reward regions and food seeking behavior in animals, and activate brain reward regions in humans (Chuang et al., 2011; DiLeone, 2009; Farooqui, 2009; Malik et al., 2008). Interestingly, we have also shown that higher leptin levels (associated with obesity) predicted blunted ventromedial prefrontal cortex and rostral anterior cingulate (VmPFC/rACC) activation to high fat food images and to glucose and fructose intake in adolescents, suggesting a role for leptin in central control of food intake (Jastreboff et al., 2014; Jastreboff et al., 2016). Furthermore, higher activity in the insula and dorsal striatum correlated with higher insulin levels, insulin resistance and with food craving when participants were placed in their favorite food contexts via imagined exposure (Jastreboff et al., 2013). Together, these findings support the notion that there may be parallel and related adaptations in metabolic and neural reward and motivation circuits that closely interact to dynamically influence hunger, food motivation and choice and subsequent overeating of HP foods.
In other evidence, we found in a recent longitudinal study of community volunteers that higher ghrelin was associated with greater food cravings, while higher chronic stress, insulin and cortisol responses predicted greater weight gain at a future 6-month follow-up assessment (Chao, Grilo, White, & Sinha, in press). There is also evidence that laboratory chow and specific macronutrients such as carbohydrates, fats and proteins stimulate autonomic and cortisol responses in animals and humans (Dallman, 2010; Stimson et al., 2014). The source of cortisol increases appears to be via both adrenal and extra-adrenal production (Stimson et al., 2014). These findings suggest that cortisol rise with macronutrient intake may serve to promote gluconeogenesis underscoring the critical role of glucocorticoids in energy homeostasis. On the other hand, there is also basic science evidence in laboratory animals that sucrose and palatable snack foods dampen HPA axis stress responses (Christiansen, Dekloet, Ulrich-Lai, & Herman, 2011; Ulrich-Lai et al., 2010). These findings suggest that specific types of foods may significantly affect both hypothalamic and extrahypothalamic peripheral sources of cortisol and may modulate stress responses to promote food motivation and intake.
Overconsumption of HP food intake is also known to reduce reward thresholds along with an upregulation of the extrahypothalamic Corticotrophin Releasing Factor (CRF) in the amygdala and related limbic striatal pathways, which, in turn, may promote food craving and associated greater neural reactivity to food cues in these brain regions thereby increasing risk of overeating of HP foods (Cifani, Polidori, Melotto, Ciccocioppo, & Massi, 2009; Cottone et al., 2009; Ghitza, Gray, Epstein, Rice, & Shaham, 2006). Thus, exposure to high fats diets, yo–yo dieting and withdrawal from such diets, may alter extrahypothalamic CRF pathways involved in the regulation of stress responses and also disrupt brain reward motivation responses to increase compulsive food seeking and stress-induced HP food seeking (Cifani et al., 2009; Cottone et al., 2009). Such findings are consistent with the Koob allostasis model of addiction (Koob & Le Moal, 1997) which posits that binge or heavy substance use results in allostatic load and adaptations in brain reward pathways, which, in turn, increases compulsive reward seeking and intake, suggesting similar effects with overconsumption of rewarding drugs and HP foods.
On the basis of evidence cited above, it is possible that higher intake of HP comfort foods may alter cortisol responses to such food intake, with glucocorticoid-related alterations that not only influence homeostatic functions of glucocorticoids in energy balance, but also affect hypothalamic and extrahypothalamic regulation of food intake. For example, it is well known that binge and heavy alcohol use, but also high nicotine and other psychostimulant use, reduces the well-known increased cortisol responses observed with acute alcohol/drug intake (Koob & Kreek, 2007; Lee & Rivier, 1997; Richardson, Lee, O'Dell, Koob, & Rivier, 2008; Sinha, 2001). These HPA axis adaptations with binge levels of alcohol and drug abuse have been identified as “neuroendocrine tolerance” (Lu & Richardson, 2014; Richardson et al., 2008), and raise the possibility that such blunted HPA axis responses may contribute to the well-known blunted reward and low dopamine state commonly associated with addiction (Koob & Griebel, 2015; Volkow, Wang, Fowler, Tomasi, & Baler, 2012). Interestingly, HPA axis adaptations with low cortisol awakening responses have been reported in higher BMI groups (Hillman, Dorn, Loucks, & Berga, 2012; Packard, Egan, & Ulrich-Lai, 2016; Sinha & Jastreboff, 2013; Tyrka, Walters, Price, Anderson, & Carpenter, 2012), and reduced dopamine activity has been associated with obesity (Wang et al., 2003). Finally, we have shown that in response to a standard glucose drink, healthy lean individuals showed increased connectivity between the hypothalamus and dopamine-rich regions of the ventral and dorsal striatum as well as the insula (Page, Sinha, & Sherwin, 2013), suggesting significant associations between the hypothalamic circuitry and extrahypothalamic striatal limbic circuits in response to food intake. In previous work, we also showed that experimentally reducing blood glucose to mild hypoglycemic levels relative to normal euglycemic levels, not only increases plasma cortisol but also increases wanting of HP foods and brain activation of reward-motivational (striatal) and emotion-stress (limbic) regions in response to high fat, high sugar HP versus low fat and non-food pictures. Furthermore, higher cortisol responses in the hypoglycemic state was associated with higher striatal-limbic brain activation during concurrent assessment of these responses (Page et al., 2011). Together, these findings implicate a broader role for cortisol and glucocorticoids centrally in food craving and intake beyond their role in stress, suggesting the need to evaluate alterations in HPA axis response and metabolic hormones on eating behaviors and food intake.
4. Metabolic and stress adaptations with increased body weight
Increasing body weight above healthy lean levels results in changes in glucose metabolism, insulin sensitivity and hormones, such as leptin, ghrelin, NPY, orexin and hypocretin, [GLP-1] which, in turn, regulate appetite and energy homeostasis (Alsio et al., 2012; Gao & Horvath, 2007; Lutter & Nestler, 2009). As indicated in the previous section, such alterations in metabolic state may influence both responses of brain reward regions to impact motivation, but also affect hypothalamic circuits that interact with overlapping stress and energy regulation circuitry (Sinha & Jastreboff, 2013; Ulrich-Lai & Ryan, 2014). Thus, it is not surprising that growing evidence from animal and human research indicates that increased weight, insulin resistance and high fat diets are associated with blunted glucocorticoid responses, and also altered autonomic and peripheral catecholamine responses to stress challenge (Appelhans, Pagoto, Peters, & Spring, 2010; Benson et al., 2009; Hillman et al., 2012; Keltikangas-Jarvinen, Ravaja, Raikkonen, & Lyytinen, 1996; Packard et al., 2016; Sinha & Jastreboff, 2013; Tyrka et al., 2012). As noted previously, high levels of stress and glucocorticoids increase glucose and insulin levels and also promote insulin resistance. Similarly, chronic high levels of insulin have been shown to downregulate HPA axis responses and increase basal sympathetic tone (Greenfield & Campbell, 2008; Keltikangas-Jarvinen et al., 1996; Tamashiro et al., 2007; Warne, 2009). Additionally, evidence indicates that stress affects glucose levels and increased glucose variability in both patients with type 1 and 2 diabetes (Faulenbach et al., 2012; Hermanns et al., 2007; Wiesli et al., 2005), while ghrelin, which, via signaling of reward pathways promotes appetite and feeding (Malik et al., 2008), is also involved in stress-induced food reward and food seeking (Chuang & Zigman, 2010). We also recently showed that acute stress increases amygdala and blunts medial orbito-frontal cortex response to milkshake vs tasteless receipt, but this effect was moderated by high cortisol levels and by high BMI respectively (Rudenga, Sinha, & Small, 2013). Thus, weight-related metabolic shifts may increase allostatic load with increased autonomic basal tone and altered HPA axis activity to affect food reward and food seeking (Dallman, 2010; Kuo et al., 2007; McEwen, 2007; Sinha & Jastreboff, 2013; van Dijk & Buwalda, 2008).
5. Stress, trauma, adversity and obesity risk
Considerable evidence from population-based and clinical studies indicates a significant and positive association of high uncontrollable stressful events and chronic stress states with both substance addiction (Sinha, 2008) and with adiposity, BMI and weight gain(Block, He, Zaslavsky, Ding, & Ayanian, 2009; Epel, Lapidus, McEwen, & Brownell, 2001; Greeno & Wing, 1994; Sinha, 2008; Smith, Baum, & Wing, 2005). Stressful events such as job strain, unemployment, family caregiving, marital conflicts and chronic adversity including poverty are associated with weight gain and obesity (Adam & Epel, 2007; Block et al., 2009; Dallman, Pecoraro, &la Fleur, 2005; Greeno & Wing, 1994; Smith et al., 2005; Torres & Nowson, 2007). Evidence also indicates that this relationship appears to be strongest among individuals who are overweight and those who binge eat (Block et al., 2009; Dallman et al., 2005; Gluck, 2006; Gluck, Geliebter, Hung, & Yahav, 2004; Sinha & Jastreboff, 2013). There is also significant evidence suggesting potentially detrimental effects of stress and adversity on eating patterns (e.g., skipping meals, restraining intake, binging) and food preference (Torres & Nowson, 2007). For example, research indicates that stress and adversity can increase consumption of fast food (Steptoe, Lipsey, & Wardle, 1998), snacks (Oliver & Wardle, 1999), calorie-dense and highly-palatable foods (Epel et al., 2001), and stress has been associated with increased binge eating (Freeman & Gil, 2004). The effects of stress may be different in lean as compared to obese individuals (Block et al., 2009; Greeno & Wing, 1994; Jastreboff et al., 2011; Laitinen, Ek, & Sovio, 2002; Lemmens, Rutters, Born, & Westerterp-Plantenga, 2011). Stress-driven eating has been found to be exacerbated in obese women (Laitinen et al., 2002) whereas stress-driven eating appears to have an inconsistent effect on food consumption in lean individuals (Greeno & Wing, 1994). Following exposure to psychological stress, satiated overweight people have been shown to have greater craving for desserts and snacks and have higher caloric intake as compared to lean individuals (Lemmens et al., 2011). Compared with individuals with lower BMIs, those with higher BMIs demonstrate stronger associations between psychological stress and future weight gain (Block et al., 2009). Furthermore, changes in eating patterns may relate to carbohydrate metabolism and insulin sensitivity (Farshchi, Taylor, & Macdonald, 2004). In healthy lean women, binge eating increases fasting glucose, insulin response, and alters the diurnal pattern of leptin secretion (Taylor, Hubbard, & Anderson, 1999). Irregular meal frequency has been found to increase peak insulin and AUC insulin in response to a test meal after a period of irregular eating patterns (Farshchi et al., 2004). This research suggests that stress, trauma and adversity may promote obesity risk, irregular eating patterns and alter food preference, but that overweight and obese individuals may be more vulnerable to such effects, possibly via weight-related adaptations in energy regulation and homeostasis.
6. The overlapping neurobiology of stress, reward and energy homeostasis
6.1. Peripheral and central stress biology, energy homeostasis and food intake
The physiological responses to acute stress are manifest via two interacting stress pathways. The first is the HPA axis, in which CRF is released from the paraventricular nucleus (PVN) of the hypothalamus, stimulating secretion of adrenocorticotrophin hormone (ACTH) from the anterior pituitary, which subsequently stimulates the secretion of glucocorticoids (GC) (cortisol or corticosterone) from the adrenal glands. The second is the autonomic nervous system, which is coordinated by the sympathoadrenal medullary (SAM) and the parasympathetic pathways. Both components of these stress pathways also influence inflammatory cytokines and immunity (Kyrou & Tsigos, 2007).
The release of CRF and ACTH from the hypothalamus and the anterior pituitary during stress results in GC release from the adrenal cortex, which in turn, supports energy mobilization and gluconeogenesis. Stress-related sympathetic arousal results in blood pressure increases and a diversion of blood flow from the gastrointestinal tract to skeletal muscles and the brain. The acute effects of stress on CRF and ACTH is terminated by GC negative feedback, supporting a return to homeostasis, and under such acute stress conditions, there is significant evidence that there is a decrease, rather than an increase, in food intake (Dallman et al., 2005; Harris et al., 1998; Marti, Marti, & Armario, 1994; Pecoraro, Reyes, Gomez, Bhargava, & Dallman, 2004).
In addition to the hypothalamus being responsive to GCs via negative feedback, it is also responsive to insulin, secreted from the pancreas and integral to glucose metabolism and energy storage (Dallman et al., 2005; Schwartz, Figlewicz, Baskin, Woods, & Porte, 1992), and to hormones like leptin, which inhibits appetite, and ghrelin, which promotes appetite (Currie, Mirza, Fuld, Park, & Vasselli, 2005; Schwartz et al., 1996). Glucocorticoids increase plasma leptin and ghrelin levels, while ghrelin which also increases with stress, appears to contribute to regulating anxiety and mood (Chuang & Zigman, 2010; Currie et al., 2005; Schwartz et al., 1996; Schwartz et al., 1992). Furthermore, a number of hypothalamic neuropeptides, such as CRF, propriomelanocortin (POMC), the orexigenic neuropeptide Y (NPY), and agouti-related peptide (AgRP), as well as the melanocortin receptors (Lu, 2001) involved in regulating the stress response, also play a role in feeding. Glucocorticoids alter the expression of these neuropeptides that regulate energy intake (Hanson & Dallman, 1995; Maniam & Morris, 2012). For example, bilateral adrenalecomy reduces food intake, and GC administration increases food intake by stimulating the release of NPY and inhibiting CRF release (Cavagnini, Croci, Putignano, Petroni, & Invitti, 2000). Thus, the hypothalamus is a critical region for coordinating both the stress response and in the regulation of feeding and energy balance.
Chronic and high levels of repeated and uncontrollable stress results in dysregulation of the HPA axis, with changes in GC gene expression (Lupien, McEwen, Gunnar, & Heim, 2009; McEwen, 2007). Food restriction and high fat diets not only alter HPA axis responses to stress (Appelhans et al., 2010; Dallman, 2010; Hillman et al., 2012; Tyrka et al., 2012), but also alter GC gene expression in a number of brain regions involved in energy homeostasis and stress (Guarnieri et al., 2012). Chronic activation of the HPA axis is known to alter glucose metabolism, promote insulin resistance, with changes in a number of appetite-related hormones (e.g. leptin, ghrelin) and feeding neuropeptides (e.g. NPY) (Bjorntorp, 1992; Kaplan, Adams, Clarkson, & Koritnik, 1984; Kuo et al., 2007; Marin et al., 1992; Qi & Rodrigues, 2007; Rebuffe-Scrive, Walsh, McEwen, & Rodin, 1992; Rosmond, Dallman, & Bjorntorp, 1998; Shively & Clarkson, 1988). Chronic stress persistently increases GCs and promotes abdominal fat, which in the presence of insulin, decreases HPA axis activity (Benson et al., 2009; Dallman et al., 2005; Tyrka et al., 2012). Basic science studies have shown that adrenal steroids increase glucose and insulin levels as well as selection and intake of high caloric foods (Chrousos, 2000; Dallman et al., 2003; Tataranni et al., 1996; Tempel, McEwen, & Leibowitz, 1992). Chronic high GCs and increases in insulin have synergistic effects on increasing HP food intake and abdominal fat deposition (Epel et al., 2001; Pecoraro et al., 2004; Warne, 2009). High levels of repeated stress also result in sympathetic overactivity, and stress-related increases in autonomic responses are related to insulin levels and insulin resistance in adolescents and adults (Keltikangas-Jarvinen et al., 1996).
6.2. Extrahypothalamic neural regulation of food reward, motivation and intake
The hypothalamic stress circuits are under the regulation of extrahypothalamic cortico-limbic pathways modulated by CRF, NPY and noradrenergic pathways. The stress response is initiated via the amygdala and stress regulation occurs partially via GC negative feedback to the hippocampus and medial prefrontal cortical (mPFC) regions (Heinrichs, 2005; McEwen, 2007). The extrahypothalamic projections of CRF are involved in subjective and behavioral responses to stress (Heinrichs, 2005), while release of orexigenic NPY during stress and increased NPY mRNA in the arcuate nucleus of the hypothalamus, amygdala and hippocampus, increase feeding, but also decrease anxiety and stress (Maniam & Morris, 2012). Stress and GCs potentiate dopaminergic transmission and impact reward seeking and intake in laboratory animals (Dallman, 2010; Dallman et al., 2003; Piazza, 1991; Piazza et al., 1996). Acute stress increases acquisition of food reward, intake of high fat diets (Adam & Epel, 2007; Wilson et al., 2008), and compulsive food seeking of HP foods (Lemmens et al., 2011), and promotes reward dependent habits (Schwabe & Wolf, 2009, 2011). Stress also potentiates craving for desserts, snacks and higher HP food intake in satiated overweight individuals relative to lean individuals (Lemmens et al., 2011).
Increased drug taking and high fat diets alter CRF, GC and noradrenergic activity to increase sensitization of reward pathways (including the ventral tegmental area [VTA], nucleus accumbens [NAc], dorsal striatum and the mPFC regions) which influences preference for addictive substances and HP foods and increases drug/food craving and intake (Aston-Jones & Kalivas, 2008; Cottone et al., 2009; Shaham & Hope, 2005; Sinha, 2008). More importantly, this motivational circuit overlaps with limbic/emotional regions (eg. the amygdala, hippocampus, and insula) that play a role in experiencing emotions and stress, and in learning and memory processes that contribute to negotiating behavioral and cognitive responses critical for adaptation and homeostasis (Arnsten, 2009; Paulus, 2007; Phan et al., 2005). For example, amygdala, hippocampus and insula play an important role in encoding of reward, reward cue-based learning and memory for high emotional and reward cues and potentiating emotion and reward cue-based feeding (Berthoud, 2012; Holland, Petrovich, & Gallagher, 2002; Small, 2002). On the other hand, the medial and lateral components of the prefrontal cortex (PFC) are involved in higher cognitive and executive control functions and also in regulating emotions, visceral and physiological responses, impulses, desires and craving (Arnsten, Mazure, & Sinha, 2012). High and repeated stress alters structural and functional responses in these prefrontal and limbic brain regions, providing some basis for the effects of chronic stress on cortico-limbic regions that modulate food reward and craving (Arnsten, 2009; Arnsten et al., 2012; Dias-Ferreira et al., 2009; Liston, McEwen, & Casey, 2009; Liston et al., 2006). These findings are consistent with behavioral and clinical research which shows that high levels of stress or negative affect decrease emotional, visceral and behavioral control and increase impulsivity (Mischel, 1996; Sinha, 2001; Tice, Bratslavsky, & Baumeister, 2001), which, in turn, is associated with greater engagement in alcohol, smoking, and other drug abuse (Baler & Volkow, 2006; Fishbein et al., 2006; Laucht et al., 2009; Wills, Ainette, Mendoza, Gibbons, & Brody, 2007; Wills & Stoolmiller, 2002; Wills, Walker, Mendoza, & Ainette, 2006), as well as increased intake of HP foods (Epel et al., 2001; Klein, 1996; Roberts, 2008; Willner et al., 1998). Therefore, prefrontal circuits involved in self-control, emotion regulation and decision making as well as the emotional and motivational brain pathways are key targets for the brain and body’s stress chemicals, that, in turn, influence addiction vulnerability and also obesity risk.
On the basis of the converging lines of evidence suggesting that ubiquitous HP food cues and their intake may alter eating behaviors (e.g., restraint and skipping meals, bingeing and overeating of HP foods), which, in turn, may result in metabolic changes and also affect the brain reward and motivation pathways to increase craving and motivation for HP foods. Chronic stress and trauma may further affect stress and metabolic hormones to further modify food motivation and intake. A dynamic interactive framework of these complex pathways is presented in Fig. 1 as a modification of the previously proposed sensitized feed-forward heuristic model (Sinha & Jastreboff, 2013). This framework centralizes the role of both HP foods and modified food products and their ubiquity on metabolic, stress and reward-motivation pathways in the brain and body via food-responsive (e.g., NPY, AgRP), energy and stress-responsive (CRF, norepinephrine, GCs) and metabolic (insulin, ghrelin, leptin) hormones and peptides, that altered energy homeostasis and food intake behaviors to promote greater food intake and weight gain. Stress and increased weight impinge on these same pathways to alter energy homeostasis and alter food motivation and related behaviors to impact intake, weight gain and obesity risk. In addition to higher weight and BMI, individual differences in genetic and individual susceptibility to obesity, eating patterns, insulin resistance, chronic stress, and other psychological variables may further moderate this process.
Fig. 1.
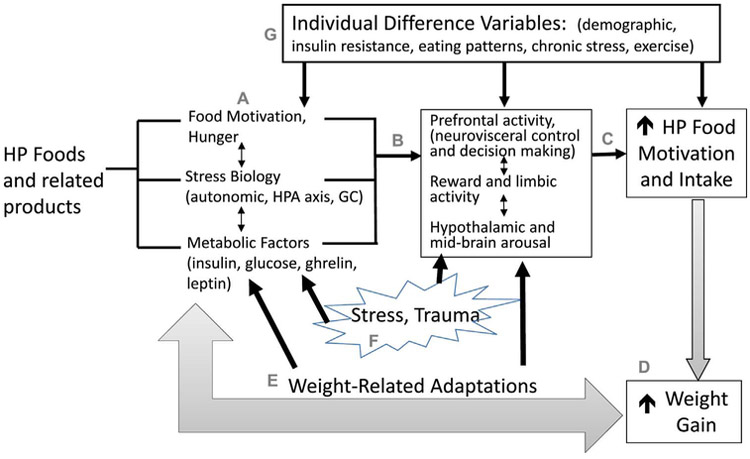
A heuristic framework is presented to show the putative effects of highly palatable (HP) food Intake and exposure to their related products (e.g., additives, preservatives, advertisement) on (i) motivation and hunger for specific foods, (ii) metabolic hormones, and (iii) stress and energy regulating peptides to promote HP food motivation and intake (A). Stress-responsive hormones and peptides (e.g., CRF, ACTH, cortisol, NPY) and metabolic factors (e.g., insulin, ghrelin, leptin) influence brain limbic and striatal reward/motivation regions to influence dopaminergic signaling, activate hypothalamic and midbrain arousal regions and prefrontal cortical circuits involved in reward prediction, self control and decision making (B). With activation of metabolic, neuroendocrine and emotion and motivational pathways, there is risk of increased ‘wanting’ and intake of such HP foods (C). Thus HP food intake promotes a sensitized process with increased HP food motivation and intake that would, in turn, also promote weight gain (D), thereby potentiating the cycle of overeating of HP food intake leading to alterations in stress and metabolic pathways that drive weight gain. Increased weight gain would, in turn result in weight related adaptations in brain stress and motivation pathways as well as in metabolic responses to further promote HP food motivation and intake (E). Furthermore, stress and trauma further activate stress and motivation neuroendocrine, metabolic and subjective/behavioral responses and in those with sensitized food motivation circuits may promote increase stress-induced craving and wanting of HP foods, thereby promoting food intake and weight gain (F). Individual difference variables may further moderate these relationships as shown in G.
7. Summary and future directions
This review focuses on the obesity pandemic and provides an overview of research on the role of addiction and stress neurobiology, namely learning/conditioning, reward/motivation and central and peripheral glucocorticoids, in food craving and excessive intake of highly palatable foods. The influence of metabolic hormones such as insulin, leptin and ghrelin and the role of regulation of glucose metabolism and energy homeostasis on brain hypothalamic and extra-hypothalamic circuits is presented, particularly in the context of weight-related adaptations in energy balance and alterations in metabolic and stress hormones in overweight and obese individuals. As glucocorticoids are involved in gluconeogenesis, energy balance and in stimulation of motivated behaviors, adaptations in GC/cortisol responses with palatable foods is discussed to highlight their involvement in food motivation and intake separate from their role in stress responses. Stress and trauma is discussed in the context of vulnerability factors that significantly impact glucocorticoids to alter food craving and intake in those at risk for obesity and weight gain. Thus, we also describe the neurobiological effects of high stress load on metabolic function and on brain reward pathways in the context of an obesogenic environment, thereby suggesting the neurobiological basis for the association between addiction, stress, excessive food intake and obesity risk.
While there have been significant advances in knowledge on the neurobiology of obesity, there remain gaps in our understanding of the multifactorial factors driving obesity risk. In particular, the link between glucocorticoids, ghrelin, insulin and leptin on food motivation and intake in humans is not clear. For example, it is known that chronic stress downregulates the HPA axis responses, but the influence of specific food types on related peripheral and central glucocorticoids and their effects on food craving and intake is not known. Similarly, with increases in weight there is increasing insulin insensitivity and alterations in leptin and ghrelin signaling, but their influence on reward and food motivation and intake is not well understood. Whether these addictive motivation and stress-relevant adaptations are different for sweets, fats and carbohydrates versus proteins and their impact on excessive intake of certain foods and nutrients to promote obesity risk is also not clear. Similarly the role of food related products such as additives and preservatives on stress and metabolic changes are not well studied and need further attention. Longitudinal studies that measure metabolic and stress hormone adaptations and their impact on food intake and weight gain in community samples are needed. In addition, experimental studies assessing neural and biobehavioral changes relating to food cues, stress and food intake have the potential to identify mechanisms that drive excessive food intake and future weight gain. For example, neuromolecular changes that occur in stress and metabolic pathways as they pertain to high-fat or high sugar diets, and chronic stress, and how they relate to food intake and weight gain, would be critical in understanding the role that reward, stress and metabolic adaptations plays in food motivation, overeating and weight gain.
Future research that addresses these types of questions has the potential to not only increase our scientific knowledge of the biobehavioral mechanisms that may contribute to excessive food intake and obesity risk, but also reveal potential novel therapies to attenuate excessive HP food motivation, intake and weight gain. There is also the possibility that we will be able to identify specific vulnerable subgroups of overweight and obese individuals who show changes in these processes to affect weight gain. Vulnerable subgroups such as individuals with high trauma and chronic stress or those with high levels of obesity may have somewhat distinct mechanisms driving excessive weight gain with a need for different types of interventions for such specific subgroups. Thus, a more comprehensive understanding of the multifactorial pathways driving food intake and weight gain will likely better equip the field with prevention and treatment interventions to help curb the obesity epidemic.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health grant R01-DK099039 and the National Institutes of Health Roadmap for Medical Research Common FundGrants UL1-DE019586, UL1-RR024139 (Yale Clinical and Translational Science Award), and the PL1-DA024859. Dr. Sinha is on the Scientific Advisory Board for Embera Neurotherapeutics.
References
- Adam T, & Epel E (2007). Stress, eating and the reward system. Physiology & Behavior, 91(4), 449–458. 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Alsio J, Olszewski PK, Levine AS, & Schioth HB (2012). Feed-forward mechanisms: Addiction-like behavioral and molecular adaptations in overeating. Frontiers in Neuroendocrinology, 33(2), 127–139. 10.1016/j.yfrne.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Anthony K, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, & Amiel S (2006). Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: The cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes, 55(11), 2986–2992. 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Pagoto SL, Peters EN, & Spring BJ (2010). HPA axis response to stress predicts short-term snack intake in obese women. Appetite, 54(1), 217–220. 10.1016/j.appet.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A, Mazure CM, & Sinha R (2012). This is your brain in meltdown. Scientific American, 306(4), 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–422. 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, & Kalivas PW (2008). Brain norepinephrine rediscovered in addiction research. Biological Psychiatry, 63(11), 1005–1006. 10.1016/j.biopsych.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, & Hoebel BG (2009). Sugar and fat bingeing have notable differences in addictive-like behavior. The Journal of Nutrition, 139(3), 623–628. 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, & Volkow ND (2006). Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine, 12(12), 559–566. 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Benson S, Arck PC, Tan S, Mann K, Hahn S, Janssen OE, … Elsenbruch S (2009). Effects of obesity on neuroendocrine, cardiovascular, and immune cell responses to acute psychosocial stress in premenopausal women. Psychoneuroendocrinology, 34(2), 181–189. 10.1016/j.psyneuen.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Berthoud HR (2012). The neurobiology of food intake in an obesogenic environment. The Proceedings of the Nutrition Society, 1–10. 10.1017/s0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P (1992). Metabolic abnormalities in visceral obesity. Annals of Medicine, 24(1), 3–5. [DOI] [PubMed] [Google Scholar]
- Block JP, He Y, Zaslavsky AM, Ding L, & Ayanian JZ (2009). Psychosocial stress and change in weight among US adults. American Journal of Epidemiology, 170(2), 181–192. 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagnini F, Croci M, Putignano P, Petroni ML, & Invitti C (2000). Glucocorticoids and neuroendocrine function. International Journal of Obesity and Related Metabolic Disorders, 24(Suppl. 2), S77–S79. [DOI] [PubMed] [Google Scholar]
- Chao A, Grilo CM, White MA, & Sinha R (2014). Food cravings, food intake, and weight status in a community-based sample. Eating Behaviors, 15(3), 478–482. 10.1016/j.eatbeh.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AM, Jastreboff AM, White MA, Grilo CM, & Sinha R (2017). Stress, cortisol and appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity, 25(4), 703–720 April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Klamer S, Porubska K, Higgs S, Booth D, … Nouwen A (2009). Diabetes dietary management alters responses to food pictures in brain regions associated with motivation and emotion: A functional magnetic resonance imaging study. Diabetologia, 52(3), 524–533. 10.1007/s00125-008-1253-z. [DOI] [PubMed] [Google Scholar]
- Christiansen AM, Dekloet AD, Ulrich-Lai YM, & Herman JP (2011). Snacking causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiology & Behavior, 103(1), 111–116. 10.1016/j.physbeh.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP (2000). The stress response and immune function: Clinical implications. The 1999 Novera H. spector lecture. Annals of the New York Academy of Sciences, 917, 38–67. [DOI] [PubMed] [Google Scholar]
- Chuang JC, & Zigman JM (2010). Ghrelin’s roles in stress, mood, and anxiety regulation. International Journal of Peptides, 2010. 10.1155/2010/460549 pii: 460549. Epub 2010 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, & Zigman JM (2011). Ghrelin mediates stress-induced food-reward behavior in mice. The Journal of Clinical Investigation, 121(7), 2684–2692. 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifani C, Polidori C, Melotto S, Ciccocioppo R, & Massi M (2009). A preclinical model of binge eating elicited by yo–yo dieting and stressful exposure to food: Effect of sibutramine, fluoxetine, topiramate, and midazolam. Psychopharmacology, 204(1), 113–125. 10.1007/s00213-008-1442-y. [DOI] [PubMed] [Google Scholar]
- Coelho JS, Jansen A, Roefs A, & Nederkoorn C (2009). Eating behavior in response to food-cue exposure: Examining the cue-reactivity and counteractive-control models. Psychology of Addictive Behaviors, 23(1), 131–139. 10.1037/a0013610. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, … Zorrilla EP (2009). CRF system recruitment mediates dark side of compulsive eating. Proceedings of the National Academy of Sciences of the United States of America, 106(47), 20016–20020. 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie PJ, Mirza A, Fuld R, Park D, & Vasselli JR (2005). Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 289(2), R353–R358. 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana S, la Fleur S, Gomez F, Houshyar H, … Manalo S (2003). Chronic stress and obesity: A new view of comfort food. Proceedings of the National Academy of Sciences of the United States of America, 100(20), 11696–11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, & la Fleur SE (2005). Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain, Behavior, and Immunity, 19(4), 275–280. 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Dallman MF (2010). Stress-induced obesity and the emotional nervous system. Trends in Endocrinology and Metabolism: TEM, 21(3), 159–165. 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ (2009). The influence of leptin on the dopamine system and implications for ingestive behavior. International Journal of Obesity, 33(Suppl. 2), S25–S29. 10.1038/ijo.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, … Sousa N (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science, 325(5940), 621–625. 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dossat AM, Lilly N, Kay K, & Williams DL (2011). Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. The Journal of Neuroscience, 31(41), 14453–14457. 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, & Brownell K (2001). Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology, 26(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Farooqui AA (2009). Lipid mediators in the neural cell nucleus: their metabolism, signaling, and association with neurological disorders. The Neuroscientist: a Review Journal Bringing Neurobiology, Neurology and Psychiatry, 15(4), 392–407. 10.1177/1073858409337035. [DOI] [PubMed] [Google Scholar]
- Farshchi HR, Taylor MA, & Macdonald IA (2004). Regular meal frequency creates more appropriate insulin sensitivity and lipid profiles compared with irregular meal frequency in healthy lean women. European Journal of Clinical Nutrition, 58(7), 1071–1077. 10.1038/sj.ejcn.1601935. [DOI] [PubMed] [Google Scholar]
- Faulenbach M, Uthoff H, Schwegler K, Spinas GA, Schmid C, & Wiesli P (2012). Effect of psychological stress on glucose control in patients with Type 2 diabetes. Diabetic Medicine: Ajournal of the British Diabetic Association, 29(1), 128–131. 10.1111/j.1464-5491.2011.03431.x. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, & Sipols AJ (2010). Energy regulatory signals and food reward. Pharmacology, Biochemistry, and Behavior, 97(1), 15–24. 10.1016/j.pbb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Herman-Stahl M, Eldreth D, Paschall MJ, Hyde C, Hubal R, … Ialongo N (2006). Mediators of the stress-substance-use relationship in urban male adolescents. Prevention Science, 7(2), 113–126. 10.1007/s11121-006-0027-4. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, & Curtin LR (2010). Prevalence and trends in obesity among US adults, 1999–2008. JAMA: the Journal of the American Medical Association, 303(3), 235–241. 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Freeman LM, & Gil KM (2004). Daily stress, coping, and dietary restraint in binge eating. The International Journal of Eating Disorders, 36(2), 204–212. 10.1002/eat.20012. [DOI] [PubMed] [Google Scholar]
- Gao Q, & Horvath TL (2007). Neurobiology of feeding and energy expenditure. Annual Review of Neuroscience, 30, 367–398. 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, & Shaham Y (2006). The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: A role of CRF1 receptors. Neuropsychopharmacology, 31(10), 2188–2196. 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck ME, Geliebter A, Hung J, & Yahav E (2004). Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosomatic Medicine, 66(6), 876–881. 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- Gluck ME (2006). Stress response and binge eating disorder. Appetite, 46(1), 26–30. http://dx.doi.Org/10.1016/j.appet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, & Campbell LV (2008). Role of the autonomic nervous system and neuropeptides in the development of obesity in humans: Targets for therapy? Current Pharmaceutical Design, 14(18), 1815–1820. [DOI] [PubMed] [Google Scholar]
- Greeno CG, & Wing RR (1994). Stress-induced eating. Psychological Bulletin, 115(3), 444–464. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, Brayton CE, Richards SM, Maldonado-Aviles J, Trinko JR, Nelson J, … DiLeone RJ (2012). Gene profiling reveals a role for stress hormones in the molecular and behavioral response to food restriction. Biological Psychiatry, 71 (4), 358–365. 10.1016/j.biopsych.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ES, & Dallman MF (1995). Neuropeptide Y (NPY) may integrate responses of hypothalamic feeding systems and the hypothalamo-pituitary-adrenal axis. Journal of Neuroendocrinology, 7(4), 273–279. [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, & Ryan DH (1998). Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. The American Journal of Physiology, 275(6 Pt. 2), R1928–1938. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC (2005). Behavioral consequences of altered corticotropin-relaeasing factor activation in brian; a functionalist view of affective neuroscience In Steckler T, Calin NH, & Reul JMHM (Vol. Eds.), Handbook of stress and the brain. Vol 15, (pp. 155–178). New York: Elsevier. [Google Scholar]
- Hermanns N, Scheff C, Kulzer B, Weyers P, Pauli P, Kubiak T, & Haak T (2007). Association of glucose levels and glucose variability with mood in type 1 diabetic patients. Diabetologia, 50(5), 930–933. 10.1007/s00125-007-0643-y. [DOI] [PubMed] [Google Scholar]
- Hill JO, & Peters JC (1998). Environmental contributions to the obesity epidemic. Science, 280(5368), 1371–1374. [DOI] [PubMed] [Google Scholar]
- Hillman JB, Dorn LD, Loucks TL, & Berga SL (2012). Obesity and the hypothalamic-pituitary-adrenal axis in adolescent girls. Metabolism: Clinical and Experimental, 61(3), 341–348. 10.1016/j.metabol.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, & Gallagher M (2002). The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & Behavior, 76(1), 117–129. [DOI] [PubMed] [Google Scholar]
- Jastreboff AM, Potenza MN, Lacadie C, Hong KA, Sherwin RS, & Sinha R (2011). Body mass index, metabolic factors, and striatal activation during stressful and neutral-relaxing states: An FMRI study. Neuropsychopharmacology, 36(3), 627–637. 10.1038/npp.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, & Potenza MN (2013). Neural correlates of stress- and food cue-induced food craving in obesity: Association with insulin levels. Diabetes Care, 36(2), 394–402. 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff AM, Lacadie C, Seo D, Kubat J, Van Name MA, Giannini C, … Sinha R (2014). Leptin is associated with exaggerated brain reward and emotion responses to food images in adolescent obesity. Diabetes Care, 37(11), 3061–3068. 10.2337/dc14-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Arora J, Giannini C, Kubat J, Malik S, … Caprio S (2016). Altered brain response to drinking glucose and fructose in obese adolescents. Diabetes, 65(7), 1929–1939. 10.2337/db15-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, & Utzschneider KM (2006). Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature, 444(7121), 840–846. 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, & Koritnik DR (1984). Psychosocial influences on female ‘protection’ among cynomolgus macaques. Atherosclerosis, 53(3), 283–295. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, & Landry CF (2005). Neural systems recruited by drug- and food-related cues: Studies of gene activation in corticolimbic regions. Physiology & Behavior, 86(1–2), 11–14. 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Keltikangas-Jarvinen L, Ravaja N, Raikkonen K, & Lyytinen H (1996). Insulin resistance syndrome and autonomically mediated physiological responses to experimentally induced mental stress in adolescent boys. Metabolism: Clinical and Experimental, 45(5), 614–621. [DOI] [PubMed] [Google Scholar]
- Klein W (1996). Gender differences in eating after exposure to noise stressor. Annals of Behavioural Medicine, 18, S103. [Google Scholar]
- Konner AC, Hess S, Tovar S, Mesaros A, Sanchez-Lasheras C, Evers N, … Bruning JC (2011). Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metabolism, 13(6), 720–728. 10.1016/j.cmet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Koob G, & Griebel G (2015). Pharmacology, biochemistry and behavior: The 2015 transition. Pharmacology, Biochemistry, and Behavior, 131, iii 10.1016/j.pbb.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Koob G, & Kreek MJ (2007). Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American Journal of Psychiatry, 164(8), 1149–1159. 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, & Le Moal M (1997). Drug abuse: Hedonic homeostatic dysregulation. Science, 278(5335), 52–58. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring HU, … Preissl H (2012). The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Human Brain Mapping, 33(5), 1052–1061. 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, … Zukowska Z (2007). Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nature Medicine, 13(7), 803–811. 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- Kyrou I, & Tsigos C (2007). Stress mechanisms and metabolic complications. Hormone and Metabolic Research, 39(6), 430–438. 10.1055/s-2007-981462. [DOI] [PubMed] [Google Scholar]
- Laitinen J, Ek E, & Sovio U (2002). Stress-related eating and drinking behavior and body mass index and predictors of this behavior. Preventive Medicine, 34(1), 29–39. 10.1006/pmed.2001.0948. [DOI] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmid B, Becker K, … Banaschewski T (2009). Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: Evidence from a high-risk community sample of young adults. The International Journal of Neuropsychopharmacology, 12(6), 737–747. 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- Lee S, & Rivier C (1997). Alcohol increases the expression of type 1, but not type 2 alpha corticotropin-releasing factor (CRF) receptor messenger ribonucleic acid in the rat hypothalamus. Brain Research. Molecular Brain Research, 52(1), 78–89. [DOI] [PubMed] [Google Scholar]
- Lemmens SG, Rutters F, Born JM, & Westerterp-Plantenga MS (2011). Stress augments food ‘wanting’ and energy intake in visceral overweight subjects in the absence of hunger. Physiology & Behavior, 103(2), 157–163. 10.1016/j.physbeh.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, … McEwen BS (2006). Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. The Journal of Neuroscience, 26(30), 7870–7874. 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, & Casey BJ (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America, 106(3), 912–917. 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, & Richardson HN (2014). Alcohol, stress hormones, and the prefrontal cortex: A proposed pathway to the dark side of addiction. Neuroscience, 277, 139–151. 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY (2001). Role of central melanocortin signaling in eating disorders. Psychopharmacology Bulletin, 35(4), 45–65. [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience, 10(6), 434–445. 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lutter M, & Nestler EJ (2009). Homeostatic and hedonic signals interact in the regulation of food intake. The Journal of Nutrition, 139(3), 629–632. 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, & Dagher A (2008). Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metabolism, 7(5), 400–409. 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Maniam J, & Morris MJ (2012). The link between stress and feeding behaviour. Neuropharmacology, 63(1), 97–110. 10.1016/j.neuropharm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Marin P, Hogh-Kristiansen I, Jansson S, Krotkiewski M, Holm G, & Bjorntorp P (1992). Uptake of glucose carbon in muscle glycogen and adipose tissue triglycerides in vivo in humans. The American Journal of Physiology, 263(3 Pt. 1), E473–E480. [DOI] [PubMed] [Google Scholar]
- Marti O, Marti J, & Armario A (1994). Effects of chronic stress on food intake in rats: Influence of stressor intensity and duration of daily exposure. Physiology & Behavior, 55(4), 747–753. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87(3), 873–904. [DOI] [PubMed] [Google Scholar]
- Mischel W (1996). From goood intenetions to willpower. New York: Guildford Press. [Google Scholar]
- Mozaffarian D, Hao T, Rimm EB, Willett WC, & Hu FB (2011). Changes in diet and lifestyle and long-term weight gain in women and men. The New England Journal of Medicine, 364(25), 2392–2404. 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, McDowell MA, & Flegal KM (2007). Obesity among adults in the United States-no statistically significant chance since 2003–2004. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- Oliver G, & Wardle J (1999). Perceived effects of stress on food choice. Physiology & Behavior, 66(3), 511–515. [DOI] [PubMed] [Google Scholar]
- Packard AE, Egan AE, & Ulrich-Lai YM (2016). HPA axis interactions with behavioral systems. Comprehensive Physiology, 6(4), 1897–1934. 10.1002/cphy.c150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, … Sinha R (2011). Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. The Journal of Clinical Investigation, 121(10), 4161–4169. 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KA, Sinha R, & Sherwin RS (2013). Differential effects of fructose and glucose on cerebral blood flow-reply. JAMA: the Journal of the American Medical Association, 309(17), 10.1001/jama.2013.3367. [DOI] [PubMed] [Google Scholar]
- Paulus MP (2007). Decision-making dysfunctions in psychiatry-altered homeostatic processing? Science, 318(5850), 602–606. 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, & Dallman MF (2004). Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology, 145(8), 3754–3762. 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, & Ragland JD (2004). Images of desire: Food-craving activation during fMRI. Neuroimage, 23(4), 1486–1493. 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, & Tancer ME (2005). Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry, 57(3), 210–219. 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, & Le Moal M (1996). Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proceedings of the National Academy of Sciences of the United States of America, 93(16), 8716–8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV (1991). Individual vulnerability to drug self-administration: action of corticosterone on dopaminergic systems as possible pathophysiological mechanism In Scheel-Kruger PWAJ (Ed.), The mesolimbic dopaminergic system: From motivation to action (pp. 473–495). New York: Wiley. [Google Scholar]
- Qi D, & Rodrigues B (2007). Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. American Journal of Physiology. Endocrinology and Metabolism, 292(3), E654–E667. 10.1152/ajpendo.00453.2006. [DOI] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Walsh UA, McEwen B, & Rodin J (1992). Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Physiology & Behavior, 52(3), 583–590. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob G, & Rivier CL (2008). Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. European Journal of Neuroscience, 28(8), 1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CJ (2008). The effects of stress on food choice, mood and body weight in healthy women. Nutrition Bulletin: British Nutrition Foundation, 33, 33–39. [Google Scholar]
- Robinson TE, & Berridge KC (2008). Review. The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3137–3146. 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R, Dallman MF, & Bjorntorp P (1998). Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. The Journal of Clinical Endocrinology and Metabolism, 83(6), 1853–1859. [DOI] [PubMed] [Google Scholar]
- Rudenga KJ, Sinha R, & Small DM (2013). Acute stress potentiates brain response to milkshake as a function of body weight and chronic stress. International Journal of Obesity, 37(2), 309–316. 10.1038/ijo.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelens BE, & Epstein LH (1996). Reinforcing value of food in obese and non-obese women. Appetite, 27(1), 41–50. 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Schwabe L, & Wolf OT (2009). Stress prompts habit behavior in humans. The Journal of Neuroscience, 29(22), 7191–7198. 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, & Wolf OT (2011). Stress-induced modulation of instrumental behavior: From goal-directed to habitual control of action. Behavioural Brain Research, 219(2), 321–328. 10.1016/j.bbr.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, & Porte D Jr. (1992). Insulin in the brain: A hormonal regulator of energy balance. Endocrine Reviews, 13(3), 387–414. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, … Weigle DS (1996). Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes, 45(4), 531–535. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D Jr., Seeley RJ, & Baskin DG (2000). Central nervous system control of food intake. Nature, 404(6778), 661–671. 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shaham Y, & Hope BT (2005). The role of neuroadaptations in relapse to drug seeking. Nature Neuroscience, 8(11), 1437–1439. 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Sherwin RS (2008). Bringing light to the dark side of insulin: A journey across the blood-brain barrier. Diabetes, 57(9), 2259–2268. 10.2337/db08-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, & Clarkson TB (1988). Regional obesity and coronary artery atherosclerosis in females: A non-human primate model. Acta Medica Scandinavica. Supplementum, 723, 71–78. [DOI] [PubMed] [Google Scholar]
- Simansky KJ (2005). NIH symposium series: Ingestive mechanisms in obesity, substance abuse and mental disorders. Physiology & Behavior, 86(1–2), 1–4. 10.1016/j.physbeh.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Sinha R, & Jastreboff AM (2013). Stress as a common risk factor for obesity and addiction. Biological Psychiatry, 73(9), 827–835. 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology, 158(4), 343–359. 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R (2008). Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences, 1141, 105–130. 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, & Jones-Gotman M (2001). Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain: a Journal of Neurology, 124(Pt. 9), 1720–1733. [DOI] [PubMed] [Google Scholar]
- Small DM (2002). Toward an understanding of the brain substrates of reward in humans. Neuron, 33(5), 668–671. [DOI] [PubMed] [Google Scholar]
- Smith AW, Baum A, & Wing RR (2005). Stress and weight gain in parents of cancer patients. International Journal of Obesity, 29(2), 244–250. 10.1038/sj.ijo.0802835. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Lipsey Z, & Wardle J (1998). Stress, hassles and variations in alcohol consumption, food choice and physical exercise: A diary study. British Journal of Health Psychology, 3, 51–63. [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, & Small DM (2008). Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. Journal of Abnormal Psychology, 117(4), 924–935. 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, & Zald DH (2009). Relation of obesity to consummatory and anticipatory food reward. Physiology & Behavior, 97(5), 551–560. 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson RH, Mohd-Shukri NA, Bolton JL, Andrew R, Reynolds RM, & Walker BR (2014). The postprandial rise in plasma cortisol in men is mediated by macronutrient-specific stimulation of adrenal and extra-adrenal cortisol production. The Journal of Clinical Endocrinology and Metabolism, 99(1), 160–168. 10.1210/jc.2013-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, & Sakai RR (2007). Dynamic body weight and body composition changes in response to subordination stress. Physiology & Behavior, 91(4), 440–448. 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, & Ravussin E (1996). Effects of glucocorticoids on energy metabolism and food intake in humans. The American Journal of Physiology, 271(2 Pt. 1), E317–325. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Hubbard J, & Anderson EJ (1999). Impact of binge eating on metabolic and leptin dynamics in normal young women. The Journal of Clinical Endocrinology and Metabolism, 84(2), 428–434. [DOI] [PubMed] [Google Scholar]
- Tempel DL, McEwen BS, & Leibowitz SF (1992). Effects of adrenal steroid agonists on food intake and macronutrient selection. Physiology & Behavior, 52(6), 1161–1166. [DOI] [PubMed] [Google Scholar]
- Tetley A, Brunstrom J, & Griffiths P (2009). Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite, 52(3), 614–620. 10.1016/j.appet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, & Baumeister RF (2001). Emotional distress regulation takes precedence over impulse control: If you feel bad, do it!. Journal of Personality and Social Psychology, 80(1), 53–67. [PubMed] [Google Scholar]
- Torres SJ, & Nowson CA (2007). Relationship between stress, eating behavior, and obesity. Nutrition, 23(11–12), 887–894. 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Walters OC, Price LH, Anderson GM, & Carpenter LL (2012). Altered response to neuroendocrine challenge linked to indices of the metabolic syndrome in healthy adults. Hormone and Metabolic Research, 44(7), 543–549. 10.1055/s-0032-1306342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, & Ryan KK (2014). Neuroendocrine circuits governing energy balance and stress regulation: Functional overlap and therapeutic implications. Cell Metabolism, 19(6), 910–925. 10.1016/j.cmet.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, … Herman JP (2010). Pleasurable behaviors reduce stress via brain reward pathways. Proceedings of the National Academy of Sciences of the United States of America, 107(47), 20529–20534. 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk G, & Buwalda B (2008). Neurobiology of the metabolic syndrome: An allostatic perspective. European Journal of Pharmacology, 585(1), 137–146. 10.1016/j.ejphar.2007.11.079. [DOI] [PubMed] [Google Scholar]
- von Deneen KM, Gold MS, & Liu Y (2009). Food addiction and cues in prader-willi syndrome. Journal of Addiction Medicine, 3(1), 19–25. 10.1097/ADM.0b013e31819a6e5f. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, & Baler R (2012). Food and drug reward: Overlapping circuits in human obesity and addiction. Current Topics in Behavioral Neurosciences, 11, 1–24. 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, … Fowler JS (2001). Brain dopamine and obesity. Lancet, 357(9253), 354–357. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Franceschi D, Wong CT, Pappas NR, … Ma Y (2003). Alcohol intoxication induces greater reductions in brain metabolism in male than in female subjects. Alcoholism: Clinical and Experimental Research, 27(6), 909–917. 10.1097/01.ALC.0000071740.56375.BA. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, & Fowler JS (2004). Similarity between obesity and drug addiction as assessed by neurofunctional imaging: A concept review. Journal of Addictive Diseases, 28(3), 39–53. 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Warne JP (2009). Shaping the stress response: Interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Molecular and Cellular Endocrinology, 300(1–2), 137–146. 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Weingarten HP (1983). Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science, 220(4595), 431–433. [DOI] [PubMed] [Google Scholar]
- Wiesli P, Schmid C, Kerwer O, Nigg-Koch C, Klaghofer R, Seifert B, … Schwegler K (2005). Acute psychological stress affects glucose concentrations in patients with type 1 diabetes following food intake but not in the fasting state. Diabetes Care, 23(8), 1910–1915. [DOI] [PubMed] [Google Scholar]
- Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, & Morgan M (1998). Depression increases craving for sweet rewards in animal and human models of depression and craving. Psychopharmacology, 136(3), 272–283. [DOI] [PubMed] [Google Scholar]
- Wills TA, & Stoolmiller M (2002). The role of self-control in early escalation of substance use: A time-varying analysis. Journal of Consulting and Clinical Psychology, 70(4), 986–997. [DOI] [PubMed] [Google Scholar]
- Wills TA, Walker C, Mendoza D, & Ainette MG (2006). Behavioral and emotional self-control: Relations to substance use in samples of middle and high school students. Psychology of Addictive Behaviors, 20(3), 265–278. 10.1037/0893-164X.20.3.265. [DOI] [PubMed] [Google Scholar]
- Wills TA, Ainette MG, Mendoza D, Gibbons FX, & Brody GH (2007). Self-control, symptomatology, and substance use precursors: Test of a theoretical model in a community sample of 9-year-old children. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors, 21(2), 205–215. 10.1037/0893-164X.21.2.205. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, & Bartness TJ (2008). Quantifying food intake in socially housed monkeys: Social status effects on caloric consumption. Physiology & Behavior, 94(4), 586–594. 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Lotter EC, McKay LD, & Porte D Jr. (1979). Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature, 282(5738), 503–505. [DOI] [PubMed] [Google Scholar]
- Yeo GS, & Heisler LK (2012). Unraveling the brain regulation of appetite: Lessons from genetics. Nature Neuroscience, 15(10), 1343–1349. 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]


