Abstract
It has been estimated that autism spectrum disorder (ASD) now affects 1 in 59 children in the United States. Although the cause(s) of ASD remain largely unknown, it is becoming increasingly apparent that ASD can no longer be defined simply as a behavioral disorder, but is in effect a rather complex and highly heterogeneous biological disorder. Up until recently the brain was thought to be “immune privileged.” However, it is now known that the immune system plays critical roles in the development and functioning of the brain throughout life. Recent evidence from multiple investigators has illustrated the deleterious role that dysregulation of the maternal immune system during gestation can play in the manifestation of changes in neurodevelopment, resulting in the development of neurobehavioral disorders such as ASD. One potential etiologic pathway through which the maternal immune system can interfere with neurodevelopment is through maternal autoantibodies that recognize proteins in the developing fetal brain. This mechanism of pathogenesis is now thought to lead to a subphenotype of ASD that has been termed maternal autoantibody related (MAR) ASD. This review provides an overview of the current research implicating the presence of brain-reactive maternal autoantibodies as a risk factor for MAR ASD.
Introduction
Autism spectrum disorder (ASD) are a highly heterogeneous set of neurodevelopmental disorders classified by core impairments in social interaction and communication behaviors that are accompanied by restrictive and repetitive interests and behaviors [1]. While genetic factors are thought to have an important role in the etiology of ASD, recent evidence suggests that environmental influences, especially during gestation or early postnatal periods, also contribute to the development of ASD [2–4]. One potential non-genetic contributing factor is immune system dysregulation, which has been frequently described in individuals with ASD as well as their family members. Such immune-associated findings reported in ASD include a family history of autoimmune disease [5–7], exposure to prenatal immune challenges such as bacterial or viral infections [8, 9], and skewed cytokine and chemokines profiles observed in both individuals with ASD and their mothers [10–14]. Most notably, some mothers of children with ASD have been reported to have circulating autoantibodies reactive to fetal brain proteins [15]. In the following review, readers will be provided with an overview of the current literature concerning brain-reactive maternal autoantibodies and the associated risk for having a child with ASD.
Gestational maternal immune environment and neurodevelopment
Under normal conditions, the maternal immune system is uniquely regulated during pregnancy to maintain a pathogen-free, yet non-inflammatory, environment for the developing fetus [16, 17]. Among the components of the maternal immune system that enter the fetal compartment, maternal immunoglobulin G (IgG) antibodies transfer at high concentrations across the placenta beginning around gestational week 17 in humans, thereby providing the immunologically naive fetus with passive protection against pathogens [18]. These maternal IgG antibodies are also transferred to the newborn during lactation through breast milk, although at much lower levels than IgA, enabling maternal IgG to persist in the newborn through early infancy [19]. In addition to immunoprotective IgG antibodies specific to external pathogens, maternal IgG auto-antibodies that react to proteins (antigens) within the fetus (termed “self”-proteins) can also cross the placenta. While antibodies are normally unable to cross the blood–brain barrier (BBB) to access the brain, the BBB is permissive during early brain development and thus permits maternal antibodies to access the developing fetal brain [20]. Antibodies that react to fetal brain proteins therefore have the potential to exert substantial effects on the fetal brain through their interaction with target antigens. For example, the gestational transfer of maternal autoantibodies in maternal myasthenia gravis can lead to transient neonatal myasthenia gravis and, in rare cases, arthrogryposis multiplex congenita, a disorder that is often fatal and is characterized by severe joint contractures in the offspring [21, 22]. Therefore prenatal exposure to maternal antibodies or autoantibodies that react against fetal brain proteins has been suggested as a mechanism for altering the normal brain development [23].
Identification of fetal brain-reactive maternal autoantibodies specific to risk of ASD
Initial discoveries
Historically, the interest in looking for an association of maternal autoantibodies and risk of ASD was initially spurred by a study conducted by Warren and colleagues [24], which found that exposure to fetal lymphocyte antigens elicited an antibody response in some mothers of children with ASD. These maternal antibodies were originally thought to arise through exposure to paternal antigens during a previous pregnancy and/or parturition, similar to the manner in which the production of anti-Rh antibodies occurs. This interest was furthered by an intriguing pilot study in which sera from a mother of a child with ASD was injected into pregnant mice, producing alterations to exploratory behavior and motor coordination in the prenatally exposed offspring relative to controls [25].
Several studies have since expanded upon this earlier work, aiming to characterize the maternal antibodies in more detail. Utilizing samples from a large, well-characterized case-control study, Braunschweig et al. [26] identified autoantibody reactivity against fetal brain proteins at ~37 and 73 kDa in plasma from mothers of children with ASD (11.5%), but not in mothers of typically developing (TD) children (0%) or in mothers of children with non-ASD developmental delay (DD) (0%).
In a similar preliminary study by Zimmerman and colleagues [27], maternal sera from 11 mothers of children with ASD and 10 mothers of TD children were examined for antibody reactivity to prenatal, early postnatal, and adult brain proteins derived from rats. A distinct pattern of antibody reactivity to prenatal brain proteins was observed in the mothers of children with ASD relative to mothers of TD children, whereas reactivity to postnatal and adult rat brain proteins were variable and did not differentiate between groups of subjects [27]. To expand upon these findings in a larger sample set, serum antibody reactivity against fetal and adult brain proteins derived from both humans and rats was examined in 100 mothers of children with ASD and 100 age-matched mothers of TD children [28]. While numerous bands were identified in all subjects in response to fetal and adult brain proteins from both human and rodent sources, significantly more mothers of children with ASD demonstrated reactivity to a 36 kDa protein antigen in both human and rodent fetal brain tissue than control mothers [28]. Maternal reactivity to proteins at 39 and 61 kDa in human fetal brain tissues and to proteins at 73 kDa in rodent fetal brain tissue was additionally greater in mothers of children with ASD than control mothers [28]. The results observed in both the Braunschweig and Singer studies suggest autoantibody reactivity to fetal brain antigens at ~36–39 kDa and 73 kDa are significantly more prevalent amongst mothers of children with ASD, with minor variances in band definition across the studies most likely due to different tissue and western blot protocols used for analyses.
In an effort to determine whether these ASD-specific maternal autoantibodies are present in mothers from different geographic locations, Rossi et al. [29] measured plasma reactivity in mothers from a regionally specific cohort from the Basque Country of Spain. Maternal reactivity to fetal brain proteins at both 37 and 73 kDa was observed exclusively in mothers of children with ASD (37/73 kDa reactivity: 5.4% mothers of children with ASD; 0% mothers of TD children). Similarly, maternal autoantibody reactivity at both 39 and 73 kDa was only observed in mothers of children with ASD and not in mothers of TD children (39/73 kDa reactivity: 2.7% mothers of children with ASD; 0% mothers of TD children) [29].
In the largest cohort reported to date, maternal antibody reactivity to fetal and adult mouse brain proteins was examined in plasma from 2431 mothers of children with ASD and 653 control women of child-bearing age [30]. Mothers of children with ASD were significantly more likely to have brain-reactive antibodies in their circulation than the unselected control women of child-bearing age (10.7 vs. 2.6%) [30]. Furthermore, antibody-positive mothers of children with ASD were significantly more likely to also be positive for anti-nuclear autoantibodies, as well as have an autoimmune disease, suggesting that these brain-reactive antibodies may be associated with autoimmunity [30]. However, Brimberg and colleagues were unable to comment on specific band reactivity, as the protein bands identified on western blots in this study were variable and maternal brain-reactive antibodies were primarily detected using immunofluorescence staining of brain tissue. Furthermore, their results unfortunately lacked true specificity for ASD as control subjects were not comprised of mothers of children verified as typically developing, but instead were control plasma from women of child-bearing age collected during a previously characterized cancer-focused research cohort [30, 31].
While these initial studies were informative in examining the association of maternal autoantibodies reactive to fetal brain proteins and risk of ASD, specimen collection typically occurred after the child’s diagnosis. As the overall circulating IgG profile of any individual changes over time as a result of subsequent exposures and immune responses, it was not clear whether the autoantibodies measured in the mother’s blood several years after her child’s delivery accurately represent the autoantibody profile present during gestation. To address this concern, Croen et al. screened archived mid-gestational maternal serum specimens from a large case-control study for reactivity toward fetal brain proteins [32]. Maternal mid-gestational autoantibody reactivity to a band at 39 kDa was found to be more common in mothers of children with ASD than in mothers of DD and general population (GP) control children, and paired reactivity to bands at 39 and 73 kDa was exclusively found in mothers of children with ASD, thus confirming the gestational presence of these ASD-specific maternal autoantibodies [32]. Reactivity to bands at 39 and 73 kDa was additionally found to be specific to mothers of children with ASD, but not mothers of TD children, in several subsequent studies [29, 33]. Studies aimed at a longitudinal examination throughout pregnancy are currently underway to determine if the presence of maternal autoantibodies is consistent throughout pregnancy.
Associated risk factors
The identification of any risk factors that are associated with maternal autoantibody related risk of ASD would provide valuable insight on the underlying biological mechanisms responsible for the generation of these brain-reactive autoantibodies in women. As genetics are thought to play a large role in the etiology of ASD, certain genetic polymorphisms related to immune function may contribute to the potential mechanism through which these maternal autoantibodies arise. One such genetic risk candidate is a functional variant in the 5′ promoter of the gene encoding the tyrosine kinase MET receptor. In addition to its roles in neurodevelopment and gastrointestinal repair, the MET receptor serves as a key regulator in immune responsiveness [34, 35]. Further, a functional variant in the 5′ promoter region of the MET gene has been found to be significantly enriched in individuals with ASD [35–39]. This variant, rs1858830, is a common G-to-C single-nucleotide polymorphism (SNP); in ASD, the MET ‘C’ allele is inherited by individuals with ASD more often than predicted by chance and is more common in individuals with ASD than in the general population. In a study comprised of 365 mothers of children with ASD and TD children, Heuer et al. [40] discovered the MET C allele was strongly associated with the presence of maternal autoantibodies with paired reactivity to bands at 37 and 73 kDa in mothers of children with ASD relative to TD mothers. The MET C allele was additionally associated with decreased MET protein expression and decreased levels of interleukin-10 (IL-10) in activated peripheral blood mononuclear cells from these mothers [40]. A decrease in the immunosuppressive cytokine IL-10 may promote an environment conductive to the loss of immune tolerance, which could predispose the production of these ASD-specific maternal autoantibodies [40].
Behavioral associations in humans
Efforts towards identifying subphenotypes of ASD would not only greatly advance our understanding of the development of ASD, but would also provide essential information for the development of precision-based medical treatment strategies. Therefore, it is important for researchers to note the behavioral variability associated with a diagnosis of ASD in order to potentially identify such subphenotypes.
In a population-based case-control study comprised of 560 mothers, ASD-specific paired maternal reactivity to bands at 37 and 73 kDa was found to be significantly correlated with lower expressive language scores in the child [33]. Maternal autoantibody reactivity to both 39 and 73 kDa bands, however, was found to be significantly associated with a broader diagnosis of ASD and was correlated with increased irritability scores in the child [33]. In a separate study conducted in an Italian cohort, Piras et al. [41] reported that children with autism whose mothers are autoantibody positive for the 37 kDa band display an abnormal sleep/wake cycle more frequently, as well as have deficits in both non-verbal and verbal language acquisition. Furthermore, affected children whose mothers have reactivity to the 73 kDa band were found to display overall verbal language deficits relative to neurotypical children, with significantly lower rates of typical language development, enhanced rates of language regression, and a lack of verbal acquisition [41]. Finally, maternal reactivity to paired bands at 39 and 73 kDa was significantly associated with broad motor coordination deficits in their children with ASD [41].
Several studies have investigated the association between brain-reactive maternal autoantibodies and the clinical onset of autistic behaviors in children, finding mixed results. The regressive phenotype of ASD, defined as clear loss of previously required language and/or social skills, has been identified as significantly associated with paired maternal autoantibody reactivity to 37 and 73 kDa [26], as well as maternal reactivity at 36 and 39 kDa [28]. Croen and colleagues [32] reported that within their mid-gestational maternal samples, paired reactivity to 39 and 73 kDa bands was found to be significantly more common amongst mothers of children with early onset autism. Early onset autism is defined by no statement of loss of social and/or language skills, or early and sustained delays or plateauing of skills without actual loss. Finally, approximately equal proportions of both early onset and regressive onset ASD classifications were observed in children of mothers with paired reactivity to 37 and 73 kDa bands in a study by Braunschweig et al. [33].
While ASD is more commonly diagnosed in males than in females, the identification of sex and gender differences in ASD has emerged as area of research priority [42]. Of the studies conducted on brain-reactive maternal autoantibodies and risk for ASD, none have identified any evidence of sex differences in children of ASD whose mothers have these autoantibodies in their circulation. These studies support ongoing research efforts to emphasize the need to include more females with ASD in study cohorts to better identify and understand potential sex differences in ASD, especially given the recent concern that females with ASD may be under-diagnosed.
Biological effects
In an effort to identify etiological subtypes of ASD, it is important to describe and characterize medical comorbidities and other biological associations within children with maternal autoantibody related ASD. For example, while abnormal brain enlargement has been observed in some preschool-aged children with ASD, there is considerable individual variation regarding brain volume within the ASD study population [43–45]. To better characterize the neural phenotype of children with ASD whose mothers have circulating brain-reactive autoantibodies, Nordahl and colleagues evaluated total cerebral volume of preschool-aged male children using structural magnetic resonance imaging (MRI). Boys with ASD whose mothers did not have circulating brain-reactive autoantibodies were found to exhibit a modest, yet significant, 4.4% brain enlargement relative to age-matched TD control children [46]. However, boys with ASD whose mothers were positive for autoantibodies reactive to bands at 37 and 73 kDa exhibited a substantially greater brain enlargement relative to both TD controls (12.1%) and boys with ASD whose mothers who were negative for brain-reactive autoantibodies (7.4%) [46]. In addition to the differences observed in comparing total cerebral volume, boys with maternal autoantibody related ASD exhibited abnormal enlargement in all four cerebral lobes relative to TD controls, as well as in the frontal lobe relative to boys with non-maternal autoantibody related ASD [46]. These results suggest that reactivity to fetal brain proteins by maternal autoantibodies may contribute to a physiologic change in the brains of a subset of children with ASD.
A number of epidemiological studies have found an association between a maternal history of autoimmune disease and subsequent risk of having a child with ASD, including the autoimmune related diseases rheumatoid arthritis, psoriasis, and celiac disease [5, 6, 47]. Several researchers have therefore tested the hypothesis that the production of ASD-specific brain-reactive maternal autoantibodies may be associated with features of autoimmunity. In analyzing a subset of their sample population, Brimberg and colleagues reported that mothers of a child with ASD that were positive for anti-brain antibodies were significantly more likely to be positive for anti-nuclear autoantibodies, which are commonly present in individuals with many autoimmune diseases. Furthermore, they found a significantly increased incidence of autoimmune diseases, particularly rheumatoid arthritis and systemic lupus erythematosus, in mothers with circulating ASD-specific anti-brain antibodies relative to mothers of ASD and TD children who are negative for anti-brain antibodies [30]. Similarly, Piras et al. [41] reported that children of ASD whose mothers are positive for autoantibodies with reactivity to both the 37 and 73 kDa antigens (now known to be LDH, STIP1 and CRMP1) had significantly higher rates of first-degree relatives diagnosed with an autoimmune disorder relative to children with non-maternal autoantibody related ASD.
To replicate and expand upon the original findings by Warren and colleagues, Bressler et al. compared cell-surface binding of maternal serum IgG to peripheral blood monocytes of their children with autism compared to cells from an unaffected sibling using flow cytometry. Maternal IgG binding was additionally examined in specific lymphocyte subpopulations, including CD4, CD8, CD19, and macrophages. In comparing the mean levels of maternal IgG binding to their children’s peripheral blood monocytes, no significant differences were found between their affected and unaffected children, thus contradicting earlier findings by Warren and colleagues [48]. Furthermore, no differences in the percentages of different cell populations were observed in the peripheral blood from children with ASD relative to their control siblings [48]. It is important to note that the sample populations utilized in both studies were small, and therefore the disparity in their results may be a consequence of the heterogeneity of ASD. In addition, while lymphocytes share several proteins and signaling pathways in common with brain tissue, they differ in many ways from fetal brain tissue and manifest a more limited repertoire of protein antigens. Therefore, the use of maternal IgG reactivity against lymphocytes does not appear to be a reliable method for the detection of maternal autoantibody related risk of ASD.
Early animal models
While the early observational studies described above were instrumental in revealing an association between maternal autoantibodies reactive to fetal brain proteins and risk of ASD, their results are more supportive of a correlative relationship and the potential for the maternal autoantibodies as a biomarker of autism risk. To provide support for the pathologic significance of these autoantibodies and the mechanisms by which they may exert their effects, various animal model studies were conducted. One technique often used to generate animal models of autoimmunity is passive transfer, in which circulating autoantibodies from an affected individual are collected and subsequently transferred to a healthy animal, allowing researchers to determine whether the autoantibodies of interest directly exert pathogenic effects in the exposed animals [23]. In the first non-human primate study of maternal autoantibodies in ASD, Martin and colleagues [49] utilized passive transfer techniques by injecting pregnant rhesus macaques with purified pooled IgG from mothers of children with ASD or from control mothers at three separate times during early gestation. Relative to offspring prenatally exposed to control maternal IgG, rhesus monkeys prenatally exposed to the IgG from mothers of children with ASD were hyperactive and consistently demonstrated dramatic whole-body stereotypies across multiple testing paradigms [49]. While gestational exposure to IgG from mothers of children with ASD did not produce profound differences in social behaviors, a small number of subtle differences in social behaviors were noted in these offspring, including a significant decrease in the amount of contact with familiar peers in the months immediately following weaning [49].
In a separate experiment conducted in mice, pregnant dams were administered with purified pooled IgG from mothers of children with ASD or from control mothers during gestational days 13 through 18 (E13-E18) [50]. Offspring prenatally exposed to maternal ASD IgG displayed a variety of abnormal behaviors relative to those exhibited by control offspring. In particular, the maternal ASD IgG exposed mice exhibited impaired social interactions, a greater magnitude of startle response following acoustic stimulation, hyperactivity, and increased anxiety-like behaviors [50]. Furthermore, maternal ASD IgG exposure significantly increased cell proliferation in the subventricular (SVZ) and subgranular zones on postnatal day 7 (PD 7) [51]. Cell densities in layers 2–4 of the frontal and parietal cortices, however, were significantly decreased on PD 7 in mice prenatally exposed to maternal ASD IgG relative to controls [51].
To demonstrate the effects of a single exposure to the targeted maternal IgG autoantibodies during gestation, pregnant female mice were intravenously injected on E12 with purified IgG from mothers of children with ASD positive for autoantibodies reactive to both 37 and 73 kDa bands, or with purified IgG from autoantibody negative mothers of TD children. Injected maternal IgG was detected at highest relative concentrations in fetal brain and liver, supporting the notion that there is direct interaction between the developing fetal brain and maternal IgG [52]. Subsequent male and female offspring resulting from this single IV exposure to ASD-specific maternal IgG showed slower motor and sensory development during pre-weaning periods, with modest altered social and anxiety-like behaviors as juveniles [52].
Protein antigens of maternal autoantibody related (MAR) ASD
Identification and behavioral correlations of MAR ASD antigenic targets
As findings from previous observational and animal studies strongly suggest a role of maternal autoantibodies in the etiology of ASD, the identification of the target protein antigens for maternal autoantibody related (MAR) autism was a critical step in advancing this area of autism research. In the first study of its type, Braunschweig et al. [53] successfully determined the identity of several proteins targeted by the maternal autoantibodies in fetal brain tissue. Through tandem mass spectrometry peptide sequencing, the target proteins corresponding to the 37-, 39-, and 73-kDa bands were identified as: lactate dehydrogenase A and B (LDH-A, LDH-B), guanine deaminase (GDA), stress-induced phosphoprotein 1 (STIP1), collapsin response mediator proteins 1 and 2 (CRMP1, CRMP2), and Y-box binding protein 1 (YBX1) [53]. Each of these identified protein antigens is expressed at significant levels in the human fetal brain and has an established role in neurodevelopment (Table 1), such as the required role of CRMP1 for proper cell migration and growth cone collapse [54, 55]. Maternal reactivity by western blot to any of the antigens, individually or in combination, was found to be significantly associated with an outcome of ASD in the child. Furthermore, there were several combinations of reactivity that were highly specific to mothers of children with ASD and not found in the plasma of mothers of TD children. For example, the most common pattern of maternal reactivity exclusive to mothers of children with ASD was to LDH-A, LDH-B, STIP1, and CRMP1 in combination (5% ASD mothers vs. 0% TD mothers) [53]. Interestingly, these primary antigens (LDH-A, LDH-B, STIP1, and CRMP1) were demonstrated to correspond to the previously described 37/73 kDa proteins recognized by these ASD-specific maternal autoantibodies. When all antigen reactivity patterns were combined, a total of 23% of mothers of children with ASD had one of the autoantibody patterns containing two or more of the target proteins relative to only 1% of control mothers [53].
Table 1.
Neurodevelopmental roles for each of the MAR ASD antigenic protein targets
| Antigenic protein target | Neurodevelopmental role |
|---|---|
| LDH | Metabolism, astrocytic glycogen and glucose metabolisms, and their coupling to neuronal functions via lactate for long-term memory formation [77, 78] |
| STIP1 | Neuritogenesis and neuronal survival [79–82] |
| CRMP1 | Cell migration, growth cone collapse [54, 55, 83, 84] |
| CRMP2 | Axon outgrowth, growth cone collapse, basal dendrite patterning [54, 83] |
| YBX1 | Transcription regulation, neural tube formation, cell division [85] |
| GDA | Dendritic branching [86, 87] |
LDH lactate dehydrogenase, STIP1 stress-induced phosphoprotein 1, CRMP collapsin response mediator protein, YBX1 Y-box-binding protein 1, GDA guanine deaminase
In order to determine whether specific patterns of antigen reactivity were associated with distinct behavioral phenotypes, behavioral outcome measures of children with ASD and TD children were compared between children of maternal autoantibody positive and negative mothers. Significantly increased stereotypical behaviors were observed in children of mothers possessing autoantibodies reactive to either type of LDH, CRMP1, as well as combined reactivity to LDH-A/LDH-B/STIP1 or to LDH-A/LDH-B/STIP1/CRMP1 in comparison to children with ASD of autoantibody negative mothers [53]. An increased overall impairment was additionally reflected in the composite Aberrant Behavior Checklist (ABC) score for children of mothers reactive against LDH and CRMP1, as well as LDH-A/LDH-B/STIP1/CRMP1 [53]. While the identification of the antigenic proteins further supports the potential role of maternal autoantibodies in the etiology of a subtype of ASD, the precise mechanism(s) underlying alterations to neurodevelopment and behavior remains under investigation.
Associated risk factors
As previously mentioned, the identification of associated risk factors provides further insight onto the underlying biological mechanisms of MAR ASD. One potential non-genetic susceptibility factor is metabolic conditions (MCs) experienced during pregnancy, which have independently been associated with increased risk of having child diagnosed with ASD [56–59]. In particular, maternal obesity during gestation, gestational diabetes, and hypertensive disorders during gestation have been identified as risk factors for ASD [56–59]. Further, MCs are often characterized by chronic low-grade inflammation and insulin resistance [60–63]. As both the metabolic and immune systems share common signaling pathways, alterations in one system might reflect similar changes in the other [64, 65]. To test this hypothesis, Krakowiak and colleagues sought to determine whether ASD-specific maternal autoantibodies identified postnatally are more prevalent amongst women that experienced MCs during pregnancy. In assessing 227 mothers of children with ASD, maternal autoantibody prevalence was observed to be higher among mothers with diabetes, hypertensive disorders, or overweight relative to healthy mothers, although these differences did not reach statistical significance. However, when examining a subset of 145 mothers whose children were characterized as having severe ASD, ASD-specific autoantibodies were found to be significantly more prevalent among mothers diagnosed with diabetes (type 2 or gestational), mothers diagnosed with hypertensive disorders, as well as among those mothers who were moderately overweight (body mass index (BMI) of 27.0–29.9) relative to healthy mothers [56]. These findings therefore suggest that some MCs may contribute to a breakdown of maternal immune tolerance and the subsequent production of ASD-specific maternal autoantibodies.
Animal models
As previously discussed, animal models offer the chance to demonstrate the pathological significance of maternal autoantibodies while testing hypotheses related to the biological mechanisms through which they exert their effects. To further progress towards understanding the potential neuropathology associated with MAR ASD, several animal studies have been conducted that incorporate the newly identified protein antigen targets of MAR ASD. These include both passive IgG antibody transfer from human samples and more recently, an active immunization of female mice with the salient peptides to induce autoantibodies in the dam prior to breeding. One advantage of passive transfer animal models is the ability to focus on a particular aspect of the model outcome, such as the neuropathological changes that may result from MAR ASD IgG exposure. To investigate the potential pathogenic effects on neurodevelopment, ASD-specific maternal autoantibodies were directly injected into the cerebral ventricles of embryonic mice during a critical period of cortical neurogenesis (E14.5) in a recent animal model of MAR ASD [66–68]. When assessing the fetal mouse brains two hours post-injection, the ASD-specific maternal autoantibodies reactive to LDH-A, LDH-B, STIP1, and CRMP1 in combination were observed to penetrate into the cortical parenchyma and strongly bind to radial glial cells, but not neuronal or other glial cells, compared to no observed signal detected in the cells of control mice [66]. This finding was of great interest, as it confirms for the first time that these maternal autoantibodies are specifically directed towards intracellular antigenic targets as characterized by the intracellular staining of developing radial glial stem cells (Fig. 1). Moreover, this single and direct prenatal exposure to ASD-specific maternal autoantibodies resulted in increased stem cell proliferation in the SVZ of the embryonic neocortex two days following injection [66]. When the embryonically injected MAR ASD mice were allowed to mature and reach six months of age, their adult brains were significantly increased in size and weight with a corresponding increase the somal volume of adult cortical cells relative to control animals [66]. A recent examination of potential alterations to dendritic morphology via Neurolucida reconstruction of Golgi stained neurons found that exposure to MAR ASD IgG in utero produced a consistent decrease in the number of dendritic spines in the infra-granular layers (IV–V) of both prefrontal and occipital cortices from adult mice [67]. Specifically, basal dendrites of layer V neurons were decreased by 48% in length and 67% in volume in the frontal cortex, and both the total number and density of spines were reduced compared to neurons in the same region of controls. Neurons in the occipital cortex layer VI similarly presented with a 37% decrease in the total number of spines, with a 32% decrease in the spine density in the apical dendrite, as well as decrease in the number of mature spines in the apical and basal dendrites [67]. Finally, the influence of MAR ASD maternal IgG on adult offspring behavior was examined using the intraventricular mouse model of MAR ASD. Relative to controls, mice intraventricularly injected with ASD-specific maternal IgG in utero displayed significantly more stereotypical behaviors, as well as modest alterations in their social approach behaviors [68]. These findings suggest that prenatal exposure to ASD-specific maternal autoantibodies directly affects radial glial cell development and produces long-lasting alterations to neurodevelopment and behaviors highly relevant to ASD, thus providing a viable pathologic mechanism for MAR risk of ASD (Fig. 2). Others have shown that a reduction in one of the key autoantigens, STIP1, results in significant intentional deficits as well as hyperactivity relative to controls [69].
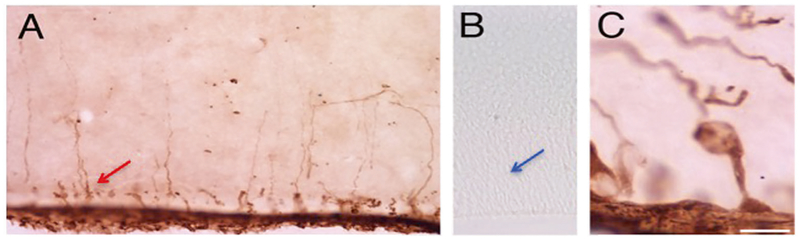
Fig. 1.
Human biotinylated IgG from mothers of children with MAR ASD (a, c) or from mothers of TD children (b) was injected intra-ventricularly into embryonic mice on gestational day 14 (E14), sectioned, and labeled with avidin-peroxidase 2 h later. Radial glial stem cells within the ventricular zone were heavily stained with MAR ASD maternal IgG (red arrow), whereas the corresponding area in fetal brains of mice injected with TD maternal IgG were completely lacking in any intracellular staining (blue arrow)
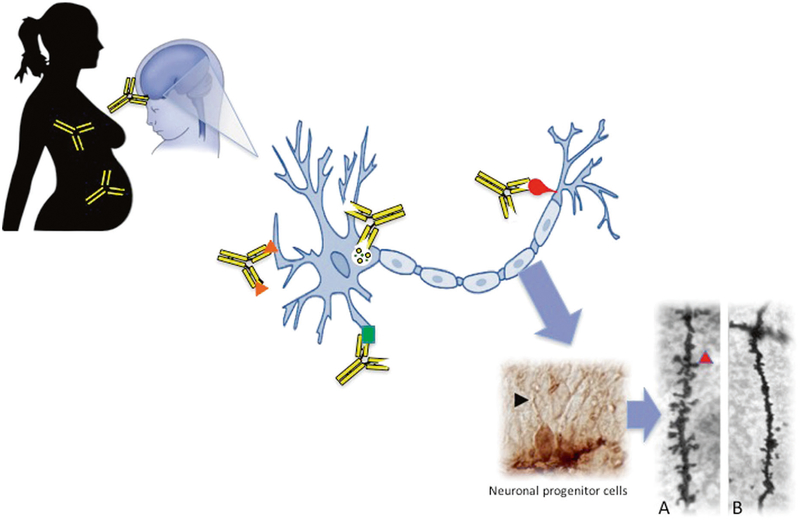
Fig. 2.
Proposed mechanism of maternal autoantibody related (MAR) ASD. Brain-reactive maternal autoantibodies cross the blood–brain barrier during gestation and bind to the various autoantigens on developing neurons (represented by orange triangles, green square, red bulb, and the intracellular antigen, LDH, by the yellow circles). We have shown that antibodies can bind intracellularly to neuronal progenitor cells in the developing fetal brain (black arrowhead) on embryonic day 14. Upon binding to their intracellular targets, these maternal autoantibodies elicit adverse effects to neurodevelopment such as a significant decrease in the number and density of dendritic spines on neurons in the developing cortex. This is illustrated in Panels a and b, as basal dendrites in frontal cortex layer V from an adult control mouse shows numerous dendritic spines (Panel a; red arrowhead), whereas offspring exposed on embryonic day 14 to human IgG with reactivity to LDH, CRMP1, and STIP1 lack mature spines (Panel b)
While there are many benefits to studying rodent models of ASD, non-human primate models offer unique translational advantages that mouse models are unable to fully recapitulate, such as their ability to demonstrate many features of human physiology, anatomy, and behavior. Utilizing these advantages, Bauman and colleagues recently developed a non-human primate model of MAR ASD to further characterize alterations to behavior and neurodevelopment. Pregnant rhesus macaques were administered targeted IgG from mothers of children with ASD that was specific for the dominant 37- and 73-kDa band pattern (now known to correspond to LDH-A, LDH-B, STIP1, and CRMP1) or mothers of TD children with no autoantibody reactivity. Subsequent MAR ASD offspring exhibited a variety of aberrant social behaviors relative to control offspring, including heightened maternal protectiveness of MAR ASD offspring during early development and increased approach behaviors that were not reciprocated by either their familiar peers or by unfamiliar peer monkeys [70]. Longitudinal magnetic resonance imaging analyses additionally revealed that male offspring prenatally exposed to MAR ASD-specific IgG had significantly larger total brain, frontal lobe, and occipital lobe volumes compared to controls. Furthermore, MAR ASD male monkeys had significantly increased total white matter volume than control male offspring [70]. Each of these passive transfer studies provides crucial insight into the underlying mechanisms and pathogenic significance of these maternal autoantibodies on behavioral and neuronal development specific to MAR risk of ASD. To take these studies to the next level, the development of an animal model that replicates the immune response seen in women with MAR ASD autoantibodies was critical.
A novel mouse model for understanding MAR ASD
In addition to the benefit of antigen-specific autoantibodies as a potential biomarker for risk of having a child on the autism spectrum, efforts towards characterizing the targeted interaction between the various autoantigens and the maternal autoantibodies are important in understanding the underlying mechanism(s) of MAR ASD. Further, the development of a highly translational animal model that recapitulates the maternal immune environment as well as a behavioral phenotype relevant to MAR ASD would provide both the ability to map the ontogeny of the neurodevelopmental changes related to the autoantibodies as well as provide support for the development of potential precision-based therapeutic strategies.
Autoantibodies recognize and bind to a specific portion of their antigenic protein target, termed the epitope, and it is this binding specificity that often plays a role the pathologic effect of the autoantibody [71]. For example, if the antigenic epitope is at a critical site of the protein, such as a co-factor binding site, autoantibody binding could then interfere with the function of the protein. Furthermore, the way in which a self-protein is presented to the immune system can affect what epitopes are exposed and therefore targeted by autoantibodies [71]. In addition, most antigens possess several different epitopes and an autoantibody can be specific for one or more of these peptides per protein antigen [72]. Thus, for individuals that produce autoantibodies to a given autoantigen, the epitopes recognized by pathogenic autoantibodies may differ from those recognized by someone producing benign autoantibodies. For example, two people that are immunopositive for the same protein could have completely different reactivity to different parts of the protein. In fact, this is what was noted when mapping the epitopes for each of the autoantigens: there were individual peptides that were recognized by the ASD maternal samples only and not by the autoantibodies in the TD maternal plasma samples, even though they were positive for the same full-length autoantigen [73]. This could potentially manifest as differential peptide epitope reactivity profiles between mothers of TD children and mothers of children with MAR ASD, thereby providing an additional benefit through the potential to identify MAR ASD-specific peptide epitopes that are not found in the TD maternal samples. This would both help with a better understanding of the mechanism of action of the autoantibodies based on their functional aspects, as well as potentially provide a precise biomarker platform for both the pre-conceptual assessment of risk of having a child with ASD and diagnostic tool for the early identification of children at increased risk for developing MAR ASD. In order to define the most prevalent (immunodominant) epitopes for each of the autoantigens recognized by MAR ASD autoantibodies, overlapping peptide microarrays were used to determine the specific portion of the antigenic proteins to which MAR ASD autoantibodies recognize [73]. In addition to providing information regarding distinct peptide reactivity profiles between mothers of children with ASD and mothers of typically developing children, this study thus formed the basis for the next translational mouse model.
As noted previously, while the passive transfer models provided support for the role of these maternal autoantibodies as a risk factor for ASD, they do not reflect a constant exposure throughout gestation, as would be the case in the clinical setting. In contrast, the development of an antigen-driven animal model would provide a targeted and constant exposure to the relevant autoantibodies throughout gestation. However, one must keep in mind that immunization with the full-length sequence of the targeted proteins would generate autoantibodies reactive against multiple areas throughout the protein sequence that do not confer specificity for MAR ASD. Therefore, the use of the recently identified target peptides for the human maternal autoantibodies is essential in preserving the specificity of MAR ASD in an animal model.
Considering that some patterns of antigen reactivity were previously associated with distinct behavioral phenotypes within MAR ASD [53], establishing the antigen-driven model specific to one of these maternal reactivity patterns would greatly increase its translational potential as a preclinical testing platform. As combined reactivity to LDH-A, LDH-B, STIP1, and CRMP1, which corresponds to the original 37/73 kDa pattern, was found to be the most selective and abundant pattern associated with MAR risk for ASD, the initial effort therefore focused on the peptides specific to this set of antigens [74]. Sexually naive female mice were immunized with the peptides, then bred once it was ascertained that their autoantibody titers were sufficient; control dams were treated with a saline preparation. Subsequent male and female offspring were tested in a comprehensive sequence of ASD-relevant behaviors and physical developmental milestones from an early postnatal period through adulthood. Relative to control offspring, offspring prenatally exposed to the epitope-specific maternal autoantibodies (“MAR-ASD” offspring) displayed several alterations in behavior that are highly relevant to ASD (Table 2) [74]. Most notably, MAR-ASD offspring displayed robust deficits in social interactions and increased repetitive self-grooming behaviors as juveniles and adults during dyadic social interactions relative to controls [74]. Furthermore, MAR-ASD males were found to emit significantly fewer ultrasonic vocalizations as adults during the introduction of a novel female in estrus, suggesting alterations in social communication relative to controls [74]. In assessing physical developmental milestones, during early postnatal life, offspring prenatally exposed to the epitope-specific maternal autoantibodies (“MAR-ASD” offspring) were found to have a significantly larger head width relative to control offspring on PD 10 and PD 12 [74]. By generating the MAR ASD-specific epitope antibodies in female mice prior to breeding, this antigen-driven model demonstrates for the first time that these ASD-specific maternal autoantibodies are directly responsible for alterations in behaviors. In addition, this antigen-driven model not only appears to represent the first highly specific and biologically relevant mouse model of MAR ASD, but is one of a few animal models shown to successfully recapitulate several behaviors relevant to ASD.
Table 2.
ASD-relevant behaviors in the antigen-driven mouse model of MAR ASD
| Behavioral assay | Time point | Observed behavioral phenotype of MAR-ASD offspring |
|---|---|---|
| Developmental milestones | Pre-weaning (PD 4–14) | Increased biparietal width (head size) |
| Separation-induced ultrasonic vocalizations | Pre-weaning (PD 4–12) | Increased number of emitted USVs in MAR-ASD females |
| Juvenile reciprocal social interactions | Adolescence (PD 25) | Decreased social behaviors |
| Increased repetitive self-grooming behaviors | ||
| Male-female social interactions | Adulthood (PD 45+) | Decreased social behaviors |
| Decreased number of emitted USVs by males | ||
| Increased repetitive self-grooming behaviors | ||
| Three-chambered social approach task | Adulthood (PD 45+) | No differences |
| Measures of general health and neurological reflexes | Adulthood (PD 45+) | No differences |
| Empty cage self-grooming behaviors | Adulthood (PD 45+) | Increased repetitive self-grooming behaviors |
| Marble bury task | Adulthood (PD 45+) | No differences |
| Morris water maze | Adulthood (PD 45+) | No differences |
Summary of a selection of behavioral results generated in the antigen-driven mouse model of MAR ASD [74].
Remaining questions
There are several remaining questions regarding the relationship between maternal autoantibodies and the risk of having a child with ASD. For example, studies in nulliparous women to determine the presence of the autoantibodies prior to conception would be of great interest as we do not yet understand the etiology of these autoantibodies. Moreover, is pregnancy a necessary factor in the generation of MAR autoantibodies? Such a study is clearly important but would require large numbers of participants and expansive resources. Another epidemiological question of interest is the contribution of ancillary autoimmune disorders to MAR autism or risk of MAR autoantibody production. In the first study by Braunschweig et al. [26], no association was noted between the presence of antibodies to fetal brain antigens and a history of autoimmune disease in the mothers at the time of sample collection. However, that does not preclude the presence of an undiagnosed autoimmune condition in the mother. Further, others have found that over 50% of mothers with one specific anti-brain antibody, found to be elevated but not exclusive for ASD, also had the presence of anti-nuclear autoantibodies compared with 13% of mothers of an ASD child without anti-brain antibodies, and 15% of age appropriate control women. Analysis of the medical history of ASD mothers with this autoantibody also revealed an increased prevalence of an autoimmune disease diagnosis such as rheumatoid arthritis and systemic lupus erythematosus [30].
Another compelling area of future research is the contribution of other factors to the generation of MAR antibodies. This would include both the fetus itself, as well as paternal elements, much like what is noted with Rh disease [75]. While Rh disease has not yet been implicated in ASD [76], there could be similar mechanisms involved in the induction of MAR autoantibodies. Finally, studies are underway to address the birth order of affected children whose mothers are positive for MAR autoantibodies, which will address several unanswered questions regarding the predictive value of these antibodies.
Conclusions
As ASD is a highly heterogeneous neurodevelopmental disorder, efforts towards identifying and understanding potential subtypes of ASD would greatly facilitate the application of precision medicine for therapeutic interventions for both the affected children and their mothers. As discussed herein, there is a growing body of compelling evidence supporting the association between maternal autoantibodies reactive to fetal brain proteins and risk of ASD (summarized in Fig. 3). The protein autoantigens and associated immunodominant epitope sequences targeted by these ASD-specific maternal autoantibodies were recently identified, and it is the recognition of various combinations of these proteins by maternal autoantibodies that confers the specificity of MAR ASD providing the possibility of a biomarker for the risk have having a child with autism. While it appears that specific patterns of autoantigen reactivity are associated with unique behavioral phenotypes, human observational and animal studies alike have consistently noted increased repetitive stereotypies, inappropriate social approach behaviors, and alterations in the neurodevelopmental trajectory of exposed offspring. For example, the most recent antigen-driven mouse model of MAR ASD successfully recapitulated the clinically observed phenotype of children with MAR ASD-specific to the LDH-A, LDH-B, STIP1, and CRMP1 maternal reactivity pattern. Moreover, this mouse model is a necessary first step for the clinical application of MAR ASD antibody studies in human and as such represents the first biomarker-driven animal model of ASD to successfully capture the core behavioral domains of ASD (sociocommunicative deficits and repetitive behaviors). The production of an endogenous animal model will allow for the future investigation of downstream effects of having these autoantibodies present throughout gestation. For example, such a model will allow researcher to identify the direct effects of maternal autoantibodies on neuronal development, and which regions of the brain are most affected. Furthermore, the identification of which autoantibodies are pathologically significant and which are merely biomarkers can be better ascertained through a direct, endogenous model.
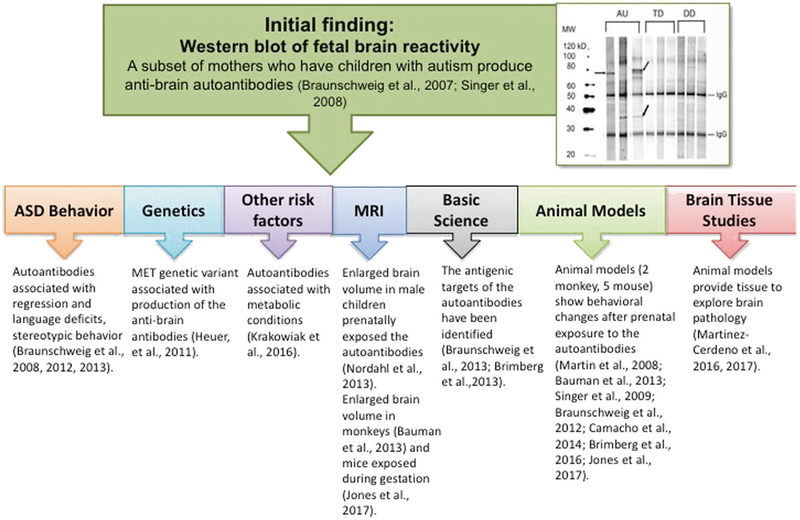
Fig. 3.
Overview of MAR ASD findings. Numerous studies provide evidence of the role for maternal autoantibodies to proteins in the developing brain in ASD. Beginning with the first western blots of fetal brain proteins, studies have shown a relationship between the presence of the MAR ASD autoantibodies and behavioral deficits, the cMET allele polymorphism, metabolic conditions during pregnancy, enlarged brain volume in both humans and animal models, and changes in the way neurons develop following exposure
Finally, animal studies that utilize an antigen-driven approach to examine the effects of maternal autoantibodies reactive to the immunodominant epitope sequences of the remaining autoantigens CRMP2, GDA, and YBX1 are also necessary. Additional research is also necessary to further characterize the behavioral and biological phenotype of children with MAR ASD, which would subsequently aid the development and/or selection of optimal individualized treatment(s) for each affected child. Similarly, research is underway to identify potential contributing risk factors that are associated with MAR risk of ASD in an effort to identify factors that drive the genesis of these brain-reactive maternal autoantibodies.
Acknowledgements
We thank the staff of the CHARGE study, and all of the families involved in this research.
Funding This study was funded by the NIEHS Center for Children’s Environmental Health and Environmental Protection Agency (EPA) grants (2P01ES011269–11, 83543201 respectively), the NIEHS-funded CHARGE study (R01ES015359), the NICHD funded IDDRC 054 (U54HD079125), and the Hartwell and Hearst Foundations.
Footnotes
Conflict of interest VdW is the founder of a startup company that will focus on the development of the MAR ASD autoantibody profile as an assessment of risk for a child developing ASD. VdW received consulting fees from Pediatric Bioscience (no longer in operation) from Jan 1, 2015 until October 2015. The remaining authors declare no conflict of interest.
References
- 1.APA. Diagnostic and statistical manual of mental disorders: DSM-V, vol. 5th Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68: 1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronborg TK, Schendel DE, Parner ET. Recurrence of autism spectrum disorders in full-and half-siblings and trends over time: a population-based cohort study. JAMA Pediatr. 2013;167: 947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YS, Leventhal BL. Genetic epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biol Psychiatry. 2015;77:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009; 124:687–94. [DOI] [PubMed] [Google Scholar]
- 6.Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005;159:151–7. [DOI] [PubMed] [Google Scholar]
- 7.Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ. Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003;112: e420. [DOI] [PubMed] [Google Scholar]
- 8.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–30. [DOI] [PubMed] [Google Scholar]
- 9.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdallah MW, Larsen N, Grove J, Bonefeld-Jorgensen EC, Norgaard-Pedersen B, Hougaard DM, et al. Neonatal chemokine levels and risk of autism spectrum disorders: findings from a Danish historic birth cohort follow-up study. Cytokine. 2013;61:370–6. [DOI] [PubMed] [Google Scholar]
- 11.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011;232:196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zerbo O, Yoshida C, Grether JK, Van de Water J, Ashwood P, Delorenze GN, et al. Neonatal cytokines and chemokines and risk of Autism Spectrum Disorder: the Early Markers for Autism (EMA) study: a case-control study. J Neuroinflamm. 2014;11:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbo O, et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry. 2017;22:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Arch Neurol. 2012;69:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. [DOI] [PubMed] [Google Scholar]
- 17.Chaouat G The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol. 2007;29:95–113. [DOI] [PubMed] [Google Scholar]
- 18.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van de Perre P Transfer of antibody via mother’s milk. Vaccine. 2003;21:3374–6. [DOI] [PubMed] [Google Scholar]
- 20.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Carroll P, Bertorini TE, Jacob G, Mitchell CW, Graff J. Transient neonatal myasthenia gravis in a baby born to a mother with new-onset anti-MuSK-mediated myasthenia gravis. J Clin Neuromuscul Dis. 2009;11:69–71. [DOI] [PubMed] [Google Scholar]
- 22.Hoff JM, Daltveit AK, Gilhus NE. Artrogryposis multiplex congenita–a rare fetal condition caused by maternal myasthenia gravis. Acta Neurol Scand Suppl. 2006;183:26–7. [DOI] [PubMed] [Google Scholar]
- 23.Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Brain-reactive antibodies and disease. Annu Rev Immunol. 2013;31: 345–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren RP, Cole P, Odell JD, Pingree CB, Warren WL, White E, et al. Detection of maternal antibodies in infantile autism. J Am Acad Child Adolesc Psychiatry. 1990;29:873–7. [DOI] [PubMed] [Google Scholar]
- 25.Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, et al. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–7. [DOI] [PubMed] [Google Scholar]
- 26.Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2007;29: 226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, et al. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–7. [DOI] [PubMed] [Google Scholar]
- 28.Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194:165–72. [DOI] [PubMed] [Google Scholar]
- 29.Rossi CC, Fuentes J, Van de Water J, Amaral DG. Brief report: antibodies reacting to brain tissue in basque spanish children with autism spectrum disorder and their mothers. J Autism Dev Disord. 2013;25:1123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brimberg L, Sadiq A, Gregersen PK, Diamond B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry. 2013;18:1171–7. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell MK, Gregersen PK, Johnson S, Parsons R, Vlahov D. New York Cancer P. The New York Cancer Project: rationale, organization, design, and baseline characteristics. J Urban Health. 2004;81:301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64:583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, et al. Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord. 2012; 42: 1435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Futamatsu H, Suzuki J, Mizuno S, Koga N, Adachi S, Kosuge H, et al. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: a potential role for induction of T helper 2 cytokines. Circ Res. 2005;96:823–30. [DOI] [PubMed] [Google Scholar]
- 35.Eagleson KL, Xie Z, Levitt P. The Pleiotropic MET receptor network: circuit development and the neural-medical interface of autism. Biol Psychiatry. 2017;81:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci USA. 2006;103: 16834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1:158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson PB, Boccuto L, Skinner C, Collins JS, Neri G, Gurrieri F, et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Res. 2009;2:232–6. [DOI] [PubMed] [Google Scholar]
- 39.Thanseem I, Nakamura K, Miyachi T, Toyota T, Yamada S, Tsujii M, et al. Further evidence for the role of MET in autism susceptibility. Neurosci Res. 2010;68:137–41. [DOI] [PubMed] [Google Scholar]
- 40.Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB. Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Transl Psychiatry. 2011;1:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piras IS, Haapanen L, Napolioni V, Sacco R, Van de Water J, Persico AM. Anti-brain antibodies are associated with more severe cognitive and behavioral profiles in Italian children with Autism Spectrum Disorder. Brain Behav Immun. 2014;38: 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, et al. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism. 2015;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–54. [DOI] [PubMed] [Google Scholar]
- 44.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–76. [DOI] [PubMed] [Google Scholar]
- 45.Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordahl CW, Braunschweig D, Iosif AM, Lee A, Rogers S, Ashwood P, et al. Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain Behav Immun. 2013;30:61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edmiston E, Ashwood P, Van de Water J. Autoimmunity, autoantibodies, and autism spectrum disorder. Biol Psychiatry. 2017;81:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bressler JP, Gillin PK, O’Driscoll C, Kiihl S, Solomon M, Zimmerman AW. Maternal antibody reactivity to lymphocytes of offspring with autism. Pediatr Neurol. 2012;47:337–40. [DOI] [PubMed] [Google Scholar]
- 49.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J Neuroimmunol. 2009;211:39–48. [DOI] [PubMed] [Google Scholar]
- 51.Kadam SD, French BM, Kim ST, Morris-Berry CM, Zimmerman AW, Blue ME, et al. Altered postnatal cell proliferation in brains of mouse pups prenatally exposed to IgG from mothers of children with autistic disorder. J Exp Neurosci. 2013;7:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braunschweig D, Golub MS, Koenig CM, Qi L, Pessah IN, Van de Water J, et al. Maternal autism-associated IgG antibodies delay development and produce anxiety in a mouse gestational transfer model. J Neuroimmunol. 2012;252:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, et al. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry. 2013;3:e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quach TT, Duchemin AM, Rogemond V, Aguera M, Honnorat J, Belin MF, et al. Involvement of collapsin response mediator proteins in the neurite extension induced by neurotrophins in dorsal root ganglion neurons. Mol Cell Neurosci. 2004; 25:433–43. [DOI] [PubMed] [Google Scholar]
- 55.Charrier E, Reibel S, Rogemond V, Aguera M, Thomasset N, Honnorat J. Collapsin response mediator proteins (CRMPs): involvement in nervous system development and adult neurodegenerative disorders. Mol Neurobiol. 2003;28:51–64. [DOI] [PubMed] [Google Scholar]
- 56.Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu G, Jing J, Bowers K, Liu B, Bao W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 2015;169:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Connolly N, Anixt J, Manning P, Ping ILD, Marsolo KA, Bowers K. Maternal metabolic risk factors for autism spectrum disorder-an analysis of electronic medical records and linked birth data. Autism Res. 2016;9:829–37. [DOI] [PubMed] [Google Scholar]
- 60.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. [DOI] [PubMed] [Google Scholar]
- 61.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Preeclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korkmazer E, Solak N. Correlation between inflammatory markers and insulin resistance in pregnancy. J Obstet Gynaecol. 2015;35:142–5. [DOI] [PubMed] [Google Scholar]
- 63.Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matarese G, Procaccini C, De Rosa V. At the crossroad of T cells, adipose tissue, and diabetes. Immunol Rev. 2012;249:116–34. [DOI] [PubMed] [Google Scholar]
- 65.Procaccini C, De Rosa V, Galgani M, Carbone F, La Rocca C, Formisano L, et al. Role of adipokines signaling in the modulation of T cells function. Front Immunol. 2013;4:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez-Cerdeno V, Camacho J, Fox E, Miller E, Ariza J, Kienzle D, et al. Prenatal exposure to autism-specific maternal autoantibodies alters proliferation of cortical neural precursor cells, enlarges brain, and increases neuronal size in adult animals. Cereb Cortex. 2016;26:374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ariza J, Hurtado J, Rogers H, Ikeda R, Dill M, Steward C, et al. Maternal autoimmune antibodies alter the dendritic arbor and spine numbers in the infragranular layers of the cortex. PLoS ONE. 2017;12:e0183443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Camacho J, Jones KL, Miller E, Ariza J, Noctor S, Van de Water J, et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav Brain Res. 2014;266:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beraldo FH, Thomas A, Kolisnyk B, Hirata PH, De Jaeger X, Martyn AC, et al. Hyperactivity and attention deficits in mice with decreased levels of stress-inducible phosphoprotein 1 (STIP1). Dis Models. 2015;8:1457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bauman MD, Iosif AM, Ashwood P, Braunschweig D, Lee A, Schumann CM, et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry. 2013;3:e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy K Janeway’s Immunobiology. 8 edn, vol. 1 Garland Science: New York, 2011, 868 pp. [Google Scholar]
- 72.Van Regenmortel MH. From absolute to exquisite specificity. Reflections on the fuzzy nature of species, specificity and antigenic sites. J Immunol Methods. 1998;216:37–48. [DOI] [PubMed] [Google Scholar]
- 73.Edmiston E, Jones KL, Vu T, Ashwood P, Van de Water J. Identification of the antigenic epitopes of maternal autoantibodies in autism spectrum disorders. Brain Behav Immun. 2018;69: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones K, Pride M, Edmiston E, Yang M, Silverman J, Crawley J, et al. Autism-specific maternal autoantibodies produce behavioral abnormalities in an endogenous antigen-driven mouse model of autism. Molecular Psychiatry. 2018. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costumbrado J, Ghassemzadeh S. Rh Incompatibility. StatPearls: Treasure Island (FL), 2017. [PubMed]
- 76.Croen LA, Matevia M, Yoshida CK, Grether JK. Maternal Rh D status, anti-D immune globulin exposure during pregnancy, and risk of autism spectrum disorders. Am J Obstet Gynecol. 2008; 199:234.e231–6. [DOI] [PubMed] [Google Scholar]
- 77.Steinman MQ, Gao V, Alberini CM. The Role of Lactate-Mediated Metabolic Coupling between Astrocytes and Neurons in Long-Term Memory Formation. Frontiers in integrative neuroscience 2016;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS ONE 2008;3:e2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hajj GN, Santos TG, Cook ZS, Martins VR. Developmental expression of prion protein and its ligands stress-inducible protein 1 and vitronectin. J Comp Neurol 2009;517:371–84. [DOI] [PubMed] [Google Scholar]
- 80.Lopes MH, Hajj GN, Muras AG, Mancini GL, Castro RM, Ribeiro KC et al. Interaction of cellular prion and stress-inducible protein 1 promotes neuritogenesis and neuroprotection by distinct signaling pathways. J Neurosci 2005;25:11330–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roffé M, Beraldo FH, Bester R, Nunziante M, Bach C, Mancini G et al. Prion protein interaction with stress-inducible protein enhances neuronal protein synthesis via mTOR. Proc Natl Acad Sci U S A 2010;107:13147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coitinho AS, Lopes MH, Hajj GN, Rossato JI, Freitas AR, Castro CC et al. Short-term memory formation and long-term memory consolidation are enhanced by cellular prion association to stress-inducible protein 1. Neurobiol Dis 2007;26: 282–90. [DOI] [PubMed] [Google Scholar]
- 83.Yamashita N, Ohshima T, Nakamura F, Kolattukudy P, Honnorat J, Mikoshiba K et al. Phosphorylation of CRMP2 (collapsin response mediator protein 2) is involved in proper dendritic field organization. J Neurosci 2012;32:1360–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurnellas MP, Li H, Jain MR, Giraud SN, Nicot AB, Ratnayake A et al. Reduced expression of plasma membrane calcium ATPase 2 and collapsin response mediator protein 1 promotes death of spinal cord neurons. Cell Death Differ 2010;17:1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eliseeva IA, Kim ER, Guryanov SG, Ovchinnikov LP, Lyabin DN. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry (Mosc) 2011;76:1402–33. [DOI] [PubMed] [Google Scholar]
- 86.Akum BF, Chen M, Gunderson SI, Riefler GM, Scerri-Hansen MM, Firestein BL. Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat Neurosci 2004;7:145–52. [DOI] [PubMed] [Google Scholar]
- 87.Tseng CY, Firestein BL. The role of PSD-95 and cypin in morphological changes in dendrites following sublethal NMDA exposure. J Neurosci 2011;31:15468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]