Abstract
Purpose of the Review:
The two main forms of large-vessel vasculitis (LVV) are giant cell arteritis (GCA) and Takayasu’s arteritis (TAK). Vascular imaging can characterize disease activity and disease extent in LVV. This review critically analyzes the clinical utility of vascular imaging in LVV and highlights how imaging may be incorporated into the management and study of these conditions.
Recent Findings:
There are multiple imaging modalities available to assess LVV including ultrasonography, CT angiography (CTA), magnetic resonance angiography (MRA), and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET). As these techniques are refined, imaging may be increasingly useful to evaluate the cranial arteries and the aorta and its primary branches. In addition, vascular imaging may be useful to monitor disease activity and may have prognostic value to predict future clinical events.
Summary:
There are strengths and weaknesses associated with vascular imaging that should be considered when evaluating patients with LVV. Vascular imaging will likely play an increasingly important role in the clinical management of patients and the conduct of research in LVV and may ultimately be incorporated as outcome measures in clinical trials in these conditions.
Keywords: large-vessel vasculitis, giant cell arteritis, Takayasu’s arteritis, positron emission tomography, angiography, ultrasound
Introduction:
Giant cell arteritis (GCA) and Takayasu’s arteritis (TAK) are the two main forms of large-vessel vasculitis (LVV), defined by inflammation involving the aorta and its major branches[1]. Traditionally, GCA is considered a disease of the cranial arteries, with temporal artery biopsy constituting the diagnostic the gold standard. The 1990 American College of Rheumatology (ACR) criteria for the classification of GCA reflect the importance of cranial features of the disease, including headache, jaw claudication, and abnormal temporal artery findings [2] [3]. However, many patients with GCA may have disease involving the aorta and branch arteries of the thorax. In cases of large-vessel GCA, unless the cranial arteries are also affected, these patients would not be classified as GCA by the current 1990 ACR criteria. The ACR criteria for TAK were also developed in 1990 and rely on vascular abnormalities for disease classification which were typically characterized at that time by catheter-based arteriogram, an invasive procedure that can detect luminal changes (i.e. stenosis, aneurysm, occlusion) without detailing vessel wall morphology[4].
Widespread adoption of imaging-based angiography and development of specific vascular imaging sequences and protocols have enabled non-invasive characterization of disease extent and arterial wall morphology in LVV. For both GCA and TAK, several imaging modalities have become increasingly essential to evaluate arterial disease. Current modalities available for the assessment of LVV include ultrasonography, CT angiography (CTA), magnetic resonance angiography (MRA), and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET). The extent to which different imaging modalities provide unique versus redundant information about vascular disease is unclear, and there are strengths, weaknesses, and potential pitfalls associated with each modality.
Uncertainty remains about which imaging technique to choose to evaluate a patient with suspected LVV, how to incorporate imaging in the assessment of disease activity in a patient with established LVV to monitor disease and response to treatment, and the prognostic capacity of imaging to predict future clinical and adverse angiographic events. Data regarding the clinical utility of imaging-based assessment of disease in LVV is starting to emerge, accompanied by initial efforts to develop evidence-based guidelines for the use of vascular imaging in the management of patients with GCA and TAK. Despite this progress, tremendous gaps in knowledge remain that limit the universal application of imaging-based disease assessment in LVV. The objective of the current review is to detail existing evidence regarding the clinical utility of vascular imaging in LVV and to highlight ways in which imaging may be better incorporated into management approaches in these conditions.
Imaging as a Diagnostic Modality in LVV
Suspected Predominantly Cranial GCA
Vascular imaging has traditionally been used to evaluate TAK and the large-vessel variant of GCA, but as imaging techniques have become more sophisticated, it is increasingly possible to directly evaluate the cranial arteries (i.e. temporal arteries). For patients with suspected GCA with predominantly cranial symptoms, vascular ultrasound or high resolution MRA can be used to profile the cranial arteries. As spatial resolution improves on next-generation platforms for advanced molecular imaging, use of FDG-PET to detect vascular inflammation in the cranial arteries may also soon be within reach.
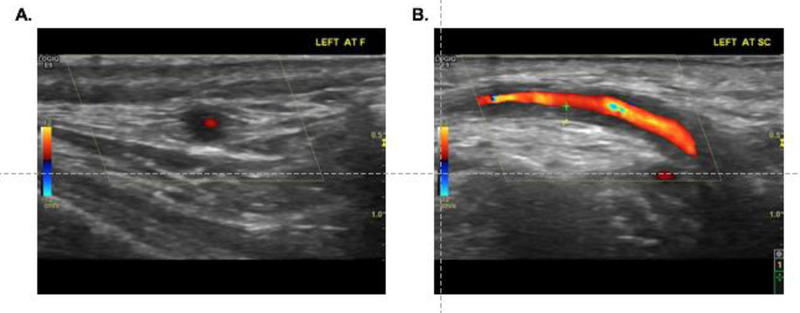
The characteristics on ultrasound consistent with vascular inflammation include the “halo” sign which refers to hypoechoic thickening of the vessel wall (FIGURE 1), and the “compression” sign, which refers to incompressibility upon application of pressure with the ultrasound probe [5–7]. In 1997, Schmidt, et al were the first to evaluate the use of ultrasonography in the diagnosis of GCA and found that 73% patients with clinically diagnosed GCA had evidence of the “halo” sign[8]. Since that study, there have been variable results reported for the performance characteristics of temporal artery ultrasound in the diagnosis of GCA[9–14]. A large meta-analyses of eight studies involving 575 patients found that a unilateral halo sign achieved an overall sensitivity of 68% and specificity of 91% for GCA, rising to 100% in presence of bilateral halos [9], whereas a smaller study of 77 patients found a sensitivity of only 10–17%, but a specificity of 100%[10]. In the TABUL study by Luqmani, et al, the largest study of its kind, the clinical effectiveness of ultrasound was compared with temporal artery biopsy in 381 patients with suspected GCA who underwent ultrasound followed by biopsy[11]. Using clinical diagnosis as a reference standard, ultrasound had a sensitivity of 54% and specificity of 81%, whereas temporal artery biopsy had a sensitivity of 39% and specificity of 100%. While the sensitivity of biopsy was noted to be inferior to ultrasound, the sensitivity of temporal artery biopsy was also significantly lower in this study (39%) than what has been previously typically reported (>70%) [11, 15]. The relatively low sensitivity observed for both ultrasound and temporal artery biopsy illustrate that many cases of clinically diagnosed GCA are still missed by each of these methods. Advantages to ultrasound include cost-effectiveness [11], ability to be obtained quickly and non-invasively often at the bedside, and absence of radiation or contrast exposure. Several studies have proposed a fast-track outpatient GCA clinic which incorporates ultrasonography in evaluating suspected LVV [16, 17, 7]. Use of ultrasonography in these settings may significantly reduce risk of permanent visual impairment and inpatient hospital days in patients with GCA [16].
Figure 1.
Ultrasound images from a patient with newly diagnosed giant cell arteritis showing a “halo sign” in a cross-sectional view of the left frontal branch of the temporal artery (A) and increased wall thickness seen on transverse view of the left common superficial branch of the temporal artery (B). Image courtesy of Cristina Ponte, MD, Hospital de Santa Maria, Lisbon, Portugal.
An alternative imaging method to evaluate patients with suspected GCA with predominantly cranial symptoms is high-resolution MRI of scalp arteries using high-field 3T MRI. Four prior studies have demonstrated high sensitivity of high-resolution MRI to detect cranial arteritis in suspected GCA [18] [19] [20] [21]. The largest of these studies included 171 patients who underwent MRI [18]. Using temporal artery biopsy as the diagnostic gold standard, the sensitivity of MRI was 94% and specificity was 78%. In addition, the negative predictive value of MRI was 98%, which indicates that temporal artery biopsy may be avoided in patients with a normal scalp MRI. However, when clinical diagnosis of GCA was used as a reference standard, MRI had poor sensitivity at 39% and a specificity of 82%. This highlights that, similar to ultrasound, many cases of clinically diagnosed GCA may be missed by high-resolution MRI of the scalp. Advantages of MRI scalp imaging include the ability to standardize the imaging protocol and evaluate multiple cranial and extracranial arteries at the same time; however, technical challenges of implementing and interpreting these protocols may limit widespread adoption of these approaches.
Whether or not imaging studies are an acceptable surrogate for temporal artery biopsy in GCA remains controversial. Comparison of the two largest studies regarding the diagnostic performance characteristics of ultrasound and high resolution MRA highlights that interpretation of study results is dependent on the diagnostic reference standard (Table 1) [11, 18]. When compared against temporal artery biopsy as the diagnostic reference standard, data from these two large studies shows that ultrasound has moderate sensitivity (73%) and specificity (69%), while high-resolution MR has excellent sensitivity (94%) and moderate specificity (78%). Therefore, a normal temporal artery by high-resolution MR closely correlates to a negative temporal artery biopsy, and a number of cases with an abnormal ultrasound or MRA of the temporal arteries may not have corresponding histologic evidence of vasculitis. When using clinical diagnosis as the reference standard, there is low sensitivity for both ultrasound (54%) and MR (39%) but good specificity for ultrasound (81%) and MR (82%). The low sensitivity indicates that most patients in these studies who were clinically diagnosed with GCA had normal ultrasound or MR studies of the temporal arteries. It is likely that clinical heterogeneity of GCA underlies the low diagnostic sensitivity observed in these studies and suggests that better standardization of the diagnostic reference standard in GCA is needed[22]. The good specificity observed in these studies was due to a low rate of false positive findings, meaning that most, although not all, patients with abnormalities on these modalities likely are clinically diagnosed with GCA. Based on the good to excellent specificity of ultrasound findings in relationship to the clinical diagnosis of GCA observed across a range of studies, recent recommendations have suggested that a positive ultrasound of the temporal arteries could be a diagnostic surrogate for GCA, particularly in patients with a high index of clinical suspicion for the disease[23].
Table 1:
Diagnostic Performance Characteristics of Ultrasound and High Resolution MRI in Two Large Studies of Giant Cell Arteritis
CT and PET are not currently recommended to assess cranial artery involvement in GCA, but these methods are being evaluated in a few studies. A small, retrospective case-control study including 14 patients with GCA evaluated the utility of cranial CTA to diagnose GCA based upon evaluation of the superficial temporal artery abnormalities, with a sensitivity of 71.4% and specificity of 85.7% when compared to a clinical diagnosis of GCA as the reference standard[24]. FDG-PET is more commonly used to evaluate the aorta and primary branch arteries. Traditionally, spatial resolution limitations have precluded the ability to detect arterial FDG uptake in the temporal arteries; however, recent studies have shown that newer generation PET/CT scanners and delayed imaging acquisition protocols may be useful detect increased FDG uptake in cranial arteries in cases of suspected GCA [25, 26]. As scanner capabilities and imaging protocols are further refined, use of CTA and PET may be incorporated into the diagnostic evaluation of cranial GCA.
Suspected TAK or GCA with predominantly large-artery involvement
Imaging-based assessment of the aorta and its primary branches is needed in patients with TAK and patients with GCA who have disease predominantly affecting the large arteries. Studies on the extent of large artery involvement by either MRA or FDG-PET have shown strong similarities in the patterns of arterial disease between patients with TAK and GCA[27] [28]. The incidence of large-artery involvement in GCA varies based on timing of imaging acquisition relative to disease onset and initiation of treatment. Incidence also varies depending upon the imaging modality used to screen for disease (angiography 20–30%, positron emission tomography 30–80%, ultrasound 30%) [29]. Despite these estimates, there are no current guidelines regarding the need for screening for large-artery disease in GCA, and reliance on the vascular physical examination to screen for large artery involvement will miss a significant burden of arteriographic disease [30].
Ultrasound can be used to evaluate the carotid, axillary, and renal arteries but has less value to evaluate the descending aorta. Compared to TAK, patients with GCA have more involvement of the bilateral axillary arteries and longer stenotic lesions of the upper extremity arteries, which can be detected by ultrasonographic evaluation of the axillary arteries[27, 31].
CTA can evaluate mural thickening, stenosis, and aneurysm of the aorta and branch vessels [32]. In one study, CTA was shown to have a sensitivity of 73% and specificity of 78% to diagnose GCA [33]. CTA has also been used in the diagnosis of TAK with high accuracy [34]. Advantages of CT include its non-invasiveness in comparison to conventional catheter-based angiography and the ability to detect structural lesions with a higher resolution and shorter scanning time than MRA, allowing for more arterial regions to be evaluated in one session. Additionally, CTA is a preferred non-invasive imaging modality to monitor changes in aortic aneurysm morphology over time [35]. Multiple studies have shown MRI imaging to be useful to diagnose LVV[36, 37]. MRA detects structural lesions (i.e. stenosis, occlusion, aneurysm) and arterial wall abnormalities reflective of ongoing vascular inflammation, including edema and contrast enhancement (FIGURE 2). MRA is advantageous given the absence of radiation, which is particularly important in younger patients with TAK. Recently, gadolinium-based contrast agents have been shown to be retained in the brain and other tissues for one year or longer after contrast-based MR studies[38, 39]. While the clinical significance of this observation is unknown, the Federal Drug Administration has issued recommendations to minimize the use of these agents unless clinically necessary. Limitations of both MRA and CTA include cost of imaging studies, lack of specificity to differentiate arterial lesions due to vasculitis from atherosclerotic changes, dependence on expertise of the center and radiologist, adverse events related to the administration of contrast agents, and lack of standardized definitions of arteriographic abnormalities, notably wall thickness.
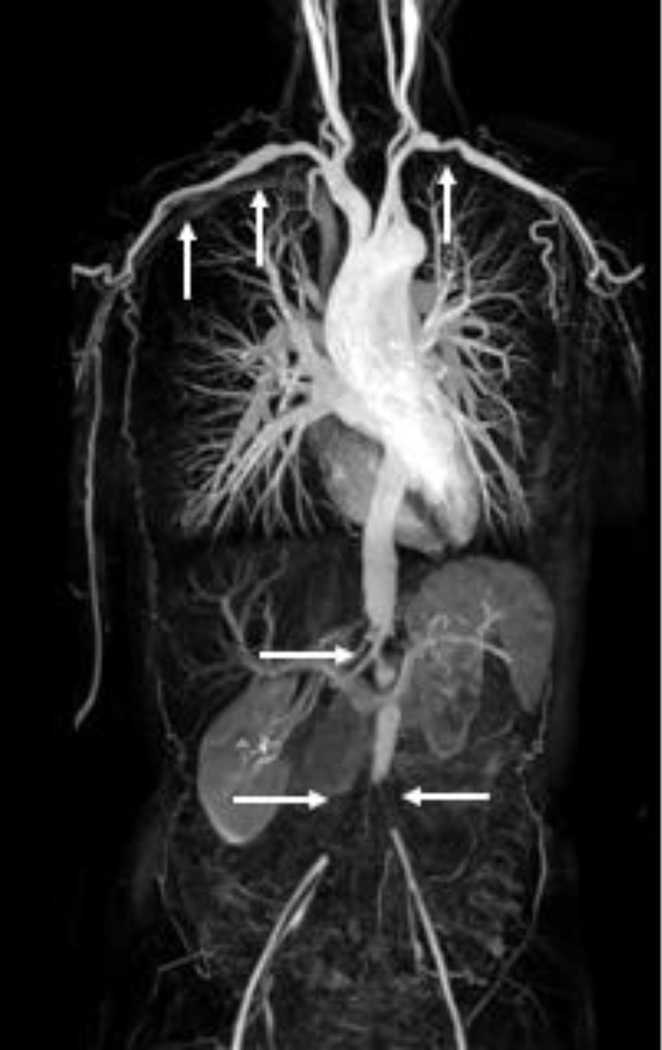
Figure 2.
Maximum intensity projection (MIP) reconstruction image from three-station magnetic resonance angiography (MRA) of a patient with Takayasu’s arteritis. The white arrows indicate areas of vascular pathology in the subclavian, axillary, abdominal, and iliofemoral arteries. Image from the National Institutes of Health Vasculitis Translational Research Program.
FDG-PET is a type of nuclear medicine imaging currently established for use in the diagnosis and monitoring of cancer. Metabolically active cells, such as malignant cells, utilize more glucose than other tissues, resulting in greater FDG uptake on PET scans. Use of FDG-PET has been proposed in patients with LVV, as abnormal metabolic activity in the walls of large arteries, presumably from activated immune cells with enhanced glycolytic capacity, can be a surrogate for vascular inflammation in LVV (FIGURE 3) [32]. A meta-analysis of 8 studies [40] [41–47], including 170 patients with LVV, showed a pooled sensitivity of 76% and specificity of 93% for FDG-PET to diagnose LVV[48]. Most of these studies are limited by retrospective study designs which introduce selection bias and comparison against healthy controls or patients with cancer rather than a more targeted population of patients with conditions that mimic LVV. A more recent prospective study of 88 patients found that PET distinguished patients with active LVV from disease-relevant comparators with a sensitivity of 85% and specificity of 83%[49]. A recent study showed that many patients thought to have isolated polymyalgia rheumatica, without associated cranial symptoms, often have evidence of LVV on FDG-PET.[50] These studies indicate that PET has good sensitivity for detecting vascular inflammation, but its imperfect specificity highlights that vascular PET activity may be present in other conditions, particularly atherosclerosis, and can be similar to LVV on imaging. Other limitations to PET include cost, radiation exposure, limitations to access, and lack of a standardized approach for analysis of vascular activity. One main advantage of FDG-PET is that it can be used in combination with CT or MR to supplement assessment of LVV.
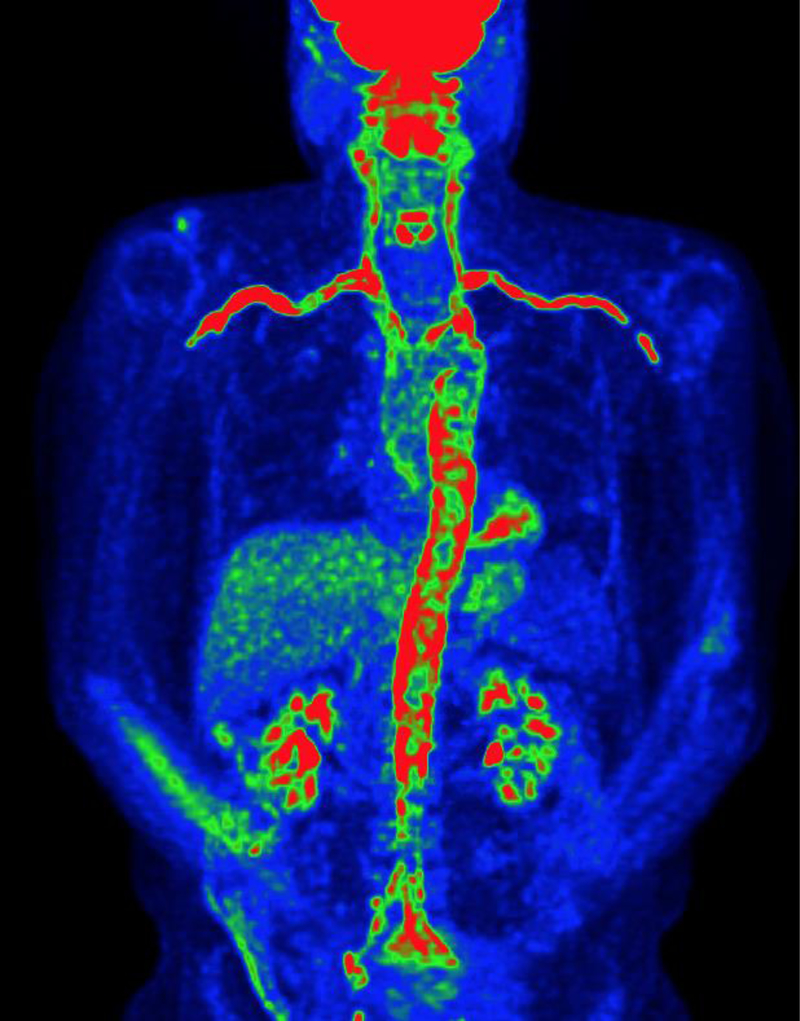
Figure 3.
Maximum intensity projection (MIP) reconstruction image from 18-F-fluorodeoxyglucose positron emission tomography of a patients with biopsy-confirmed giant cell arteritis. There is severe FDG uptake (red) throughout the aorta and branch arteries reflective of vascular inflammation. Image from the National Institutes of Health Vasculitis Translational Research Program.
Vascular Imaging to Assess Disease Activity:
Patients with LVV often experience disease relapses following initial remission. Some studies have recommended periodic imaging to monitor for disease activity, but there are no accepted guidelines regarding frequency of imaging or the preferred imaging modality. A small prospective study evaluated carotid ultrasound in patients with LVV and found it useful as a marker of disease activity[51], and several other studies have suggested ultrasound of the large vessels can detect disease activity[52, 53] [51, 54], whereas other studies have shown abnormalities such as wall thickness may persist for years on imaging following diagnosis[55]. Arterial wall thickening and contrast enhancement on both CTA and MRA have been proposed as features of active disease, although it also remains unknown how long these changes persist in patients with previously active disease[56] [57]. Other studies have suggested wall thickening alone is not associated with active disease and have reported conflicting data on the presence of vascular wall enhancement for identifying disease activity [58]. There have been varying reports about the utility of FDG-PET to monitor disease activity, with some studies suggesting interpretation of FDG-PET is less reliable once immunosuppressive therapy is initiated and noting persistence of FDG uptake in absence of clinically active disease [59, 60] [61, 62] [63]. Lack of standardized definitions of abnormal uptake on FDG-PET makes interpretation of multiple studies difficult[58].
Patients with LVV may have evidence to suggest active disease on imaging in the absence of clinical symptoms, with prior studies demonstrating ongoing vascular disease activity during remission on MRA [64] [65] [66] [67] and PET [60, 49, 68]. In one study, while PET was shown to be active in 34 out of 40 patients with clinically active disease, PET was also active in 41 out of 71 (58%) patients with LVV in clinical remission [49]. A follow-up study found that approximately 50% patients with LVV have what appears to be ongoing disease activity on both MRA and PET studies obtained concurrently during clinical remission[57]. In absence of corresponding histology, a typical challenge for these types of studies, it remains unclear to what extent vascular abnormalities observed during clinical remission represent active vascular inflammation, non-specific changes related to vascular damage, including atherosclerosis, or both. Clinical and laboratory assessment of disease activity does not always correlate well with ongoing vascular inflammation, and patients can develop new arterial lesions on angiography during periods of clinical remission[69]. In addition, persistent activity on imaging substantiate autopsy data and findings from temporal artery biopsies performed during clinical remission that have shown active, ongoing vasculitis[49, 70].
Vascular imaging abnormalities may be responsive to treatment [71]. One study evaluated changes on CTA in patients with LVV following glucocorticoid treatment at 1-year follow up and found that contrast enhancement resolved in 94% patients[56]. While wall thickening was still present in 68% patients, the number of affected segments and aortic wall thickness significantly decreased in response to treatment. Ultrasound abnormalities of the temporal arteries can normalize with treatment [11]. Reichenbach and colleagues evaluated MRA signals following treatment with tocilizumab, and found that vessel wall enhancement normalized in 1/3 of patients at week 52 [67]. Another study showed qualitative PET scores significantly improved in response to increased treatment, remained unchanged when there was no change in treatment, and significantly worsened following decreased treatment over 6-month imaging assessment intervals [71]. The improvements seen on imaging in response to treatment, suggests that vascular activity by PET and MRA may constitute an imaging-based biomarker of disease activity in LVV.
Prognostic Value of Vascular Imaging:
There is limited prospective data about whether vascular imaging obtained at the time of diagnosis or during a period of clinical remission may predict long term clinical outcomes in LVV. Data regarding PET activity has been particularly interesting in this regard. In one pilot study of 17 patients with LVV, increased intensity and extent of arterial FDG uptake at diagnosis predicted a less favorable response to treatment [72]. A prospective study in 35 patients with GCA failed to demonstrate value of FDG-PET to predict clinical relapse; however, patients were studied early in the disease course while taking moderate doses of glucocorticoids[60]. In a recent study of 39 patients with LVV who underwent FDG-PET during clinical remission with subsequent prospective clinical evaluation, patients with increased vascular PET activity were 4x more likely to experience clinical relapse over 15 months average follow-up [49]. Patients with LVV are at substantially increased risk to develop aortic dilation and aneurysms, often occurring years after initial diagnosis[73][74]. Use of imaging, in particular FDG-PET, to identify and monitor disease activity may be able to identify subclinical inflammation prior to development of permanent structural damage to the aorta and its major branches, including thoracic and abdominal aortic aneurysms and dissections. However, long term prospective studies that monitor angiographic progression of disease in relationship to vascular PET activity are lacking in LVV. In the absence of a histologic gold standard, these studies are needed to evaluate whether disease activity on imaging correlates with future disease progression and vascular complications.
Imaging in Clinical Practice:
Given the various imaging options, there have been recommendations for the use of imaging in LVV. EULAR proposed guidelines, published in 2018, recommending an early imaging test in patients with suspected GCA or TAK[23]. The guidelines propose that for a patient with suspected GCA and a positive imaging test, the diagnosis of GCA can be made without an additional test, including temporal artery biopsy or further imaging. Furthermore, they advocate ultrasound of temporal and/or axillary arteries as first-line imaging for suspected GCA, with high resolution MRI cranial arteries as an alternative option, and ultrasound, PET, MRI, and/or CT as secondary diagnostic options. For patients with suspected TAK, MRI was recommended to evaluate mural inflammation and luminal pathology as first-line imaging, and PET, CT, and/or ultrasound as alternative imaging modalities. Joint procedural recommendations out of Europe regarding use of FDG-PET/CTA for LVV stress the importance of patient preparation and image acquisition and propose standardized imaging interpretation criteria for clinical use [32].
Timing of image acquisition relative to initiation of treatment likely influences performance characteristics of vascular imaging in LVV. For example, in a serial imaging study, diagnostic accuracy of FDG-PET to detect vascular inflammation in patients with LVV was preserved 3 days after initiation of glucocorticoid treatment but declined by 10 days into therapy[75]. Given that prompt initiation of glucocorticoids is standard of care in patients with suspected LVV with symptoms of disease activity, accompanying vascular imaging studies should be performed expeditiously, and results should be interpreted in the context of ongoing treatment.
Clinicians who employ imaging-based strategies to assess LVV should be aware of various modality-specific pitfalls that may complicate the interpretation of these studies. Incorrect probe angle can create a false “halo” by ultrasonography, and an angle between sound waves and artery of ≤60 degrees has been recommended[23]. On MRA, concentrated gadolinium in the adjacent subclavian vein can create a susceptibility artifact termed a “pseudostenosis” in the adjacent subclavian artery that could be misinterpreted as a true arterial stenosis[76]. FDG uptake by prosthetic grafts is not specific for active disease and may limit the utility of FDG-PET to monitor vascular inflammation at sites of previous surgical intervention[77]. Differences in the interpretation of temporal artery ultrasound and biopsy findings by expert review emphasize a need to standardize assessment of vascular imaging studies and highlight that even the diagnostic gold standard of temporal artery biopsy is subject to interpretation[11].
As imaging interpretation and expertise are variable among different regions of the world and various centers, it is difficult to follow specific guidelines when incorporating imaging into clinical practice. For example in the EULAR guidelines[23], imaging preference is given to ultrasound for GCA, perhaps reflecting the regional expertise in vascular ultrasonography common to many expert European centers [32]. The preferred vascular imaging study in a patient with LVV may differ depending on the resources and expertise available at each center. Center-specific algorithms that incorporate ultrasound and other imaging modalities in the diagnosis of GCA have been proposed [78].
Recommendations for the use of imaging to monitor disease activity over time are less defined than recommendations concerning diagnostic use of vascular imaging in LVV. Patients with TAK often undergo serial angiography at regular intervals to monitor vascular damage, particularly in the early phases of disease. In the recent EULAR guidelines, repeat imaging for patients in established clinical remission was not routinely recommended. Guidelines for use of FDG-PET emphasize potential value of FDG-PET/CT to evaluate response to treatment by monitoring functional metabolic information and detecting structural vascular changes [32].
Imaging as an Outcome Measure in Clinical Trials
Imaging may be incorporated as novel outcome measures in clinical trials and other research studies to standardize more objectively the assessment of disease activity. To date, defining disease activity and disease remission in clinical trials of TAK and GCA has been difficult, as using clinical impression as a reference standard can lead to variability in physician-dependent subjective interpretation of symptoms. Previous measures of disease activity in TAK and GCA have attempted to incorporate imaging into disease indices as outlined in TABLE 2 [79, 69, 49, 80, 66]. The NIH criteria for TAK in 1994[69] was the first disease activity measure to incorporate imaging, where new or worsening angiographic features were considered to be a marker of active disease in TAK. Since that time, other indices have incorporated imaging [66, 80, 79], but most continue to focus on angiographic progression of disease, with emphasis given to new areas of arterial stenosis, occlusion, or aneurysm. Many of these outcome measures are difficult to implement in observational cohorts of patients who do not have prior imaging for comparison, as assessing angiographic lesions to differentiate active inflammation from vascular damage is challenging. More recently, PETVAS [49] has been proposed as a disease activity index which incorporates assessment of FDG uptake in arterial territories, by summing PET activity in 9 vascular territories. This measure has the potential ability to evaluate and monitor vascular disease activity prior to development of angiographic lesions and should be tested in clinical trials.
Table 2:
Incorporation of Vascular Imaging into Disease Assessment Indices in Large-Vessel Vasculitis
| Name | Population | Imaging Measure | Advantages | Disadvantages |
|---|---|---|---|---|
| Kerr, et al.[69] (NIH criteria) | TAK | New onset or worsening of typical angiographic features | Angiogram allows for direct visualization of vascular pathology | Angiographic changes not noted until there is significant stenosis or occlusion |
| Aydin, et al. [82] (DEI-Takayasu) | TAK | No imaging component | N/A | N/A |
| Misra, et al.[66] (ITAS2010) | TAK | Free text component | Any new imaging is noted | Not standardized or incorporated into scoring system |
| Nakagomi et al. [80] (Combined Arteritis Damage Score, CARDS) | GCA + TAK | Uses MRA + CTA to evaluate arterial stenosis, occlusion, and aneurysm | Quantifies extent of damage using novel score | Does not incorporate wall thickness; only evaluates damage |
| Grayson, et al. [49] (PETVAS) | GCA + TAK | Summary score of qualitative assessment of FDG uptake in arterial territories (4 segments of aorta +11 branch arteries) | Useful in assessing degree of PET activity; higher scores were predictive of clinical relapse | Subject to visual interpretation without standardized semiquantitative values |
| Tombetti, et al. [79] | GCA + TAK | Uses MRA + CTA to evaluate arterial stenosis and dilation | Quantifies arterial involvement to provide an overview of pattern of arterial disease and severity | Does not incorporate wall thickness or edema of vascular walls, exclusively uses luminal data |
Concluding Remarks:
Vascular imaging will likely play an increasingly important role in the clinical management of patients and the conduct of research in LVV. Implementation of imaging-based diagnostic algorithms may potentially spare patients from more invasive diagnostic procedures. Improvements in advanced molecular imaging may enable the visualization and quantification of cell-specific immune subsets, an emerging field of study known as “immuno-PET” [81]. Novel PET ligands specific for monocytes hold the promise of improved specificity to detect vascular inflammation; however, these efforts may be limited by an inability to differentiate tracer uptake from circulating myeloid cells within the blood pool from mononuclear cellular infiltrates within the arterial wall. Incorporating imaging-based outcomes in clinical trials of LVV in comparison to clinical and serologic assessments of disease activity may provide a more nuanced understanding of drug efficacy in LVV. Exploring circulating and tissue-specific biomarkers in relationship to both clinical assessment and imaging-based assessment of vascular disease activity may enable discovery of novel therapeutics capable of inducing more durable remission in LVV.
The large-vessel vasculitides have historically posed significant management challenges to clinicians. Understanding the benefits of imaging-based assessment in relationship to cost and potential risks are critical unmet needs in LVV. Carefully designed longitudinal studies will be needed to evaluate whether imaging-based assessments are predictive of long term clinical outcomes in LVV, including clinical response, angiographic progression of disease, and mortality. Ultimately, an improved understanding of the relationships between clinical, serologic, and imaging-based assessments of disease will likely be critical to promote advancements in the lives of patients with these complex, potentially life-threatening conditions.
Acknowledgments
Financial supports of conflicts disclosure:
This research was supported through the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Compliance with Ethics Guidelines
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Hunder GG, Arend WP, Bloch DA, Calabrese LH, Fauci AS, Fries JF et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthritis Rheum. 1990;33(8):1065–7. [DOI] [PubMed] [Google Scholar]
- 3.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–8. [DOI] [PubMed] [Google Scholar]
- 4.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–34. [DOI] [PubMed] [Google Scholar]
- 5.Chrysidis S, Duftner C, Dejaco C, Schafer VS, Ramiro S, Carrara G et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open. 2018;4(1):e000598. doi: 10.1136/rmdopen-2017-000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aschwanden M, Imfeld S, Staub D, Baldi T, Walker UA, Berger CT et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol. 2015;33(2 Suppl 89):S-113–5. [PubMed] [Google Scholar]
- 7.Monti S, Floris A, Ponte C, Schmidt WA, Diamantopoulos AP, Pereira C et al. The use of ultrasound to assess giant cell arteritis: review of the current evidence and practical guide for the rheumatologist. Rheumatology (Oxford). 2018;57(2):227–35. doi: 10.1093/rheumatology/kex173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt WA, Kraft HE, Vorpahl K, Volker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337(19):1336–42. doi: 10.1056/NEJM199711063371902. [DOI] [PubMed] [Google Scholar]
- 9.Arida A, Kyprianou M, Kanakis M, Sfikakis PP. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord. 2010;11:44. doi: 10.1186/1471-2474-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldini C, Depinay-Dhellemmes C, Tra TT, Chauveau M, Allanore Y, Gossec L et al. Limited value of temporal artery ultrasonography examinations for diagnosis of giant cell arteritis: analysis of 77 subjects. J Rheumatol. 2010;37(11):2326–30. doi: 10.3899/jrheum.100353. [DOI] [PubMed] [Google Scholar]
- 11.Luqmani R, Lee E, Singh S, Gillett M, Schmidt WA, Bradburn M et al. The Role of Ultrasound Compared to Biopsy of Temporal Arteries in the Diagnosis and Treatment of Giant Cell Arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess. 2016;20(90):1–238. doi: 10.3310/hta20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvarani C, Silingardi M, Ghirarduzzi A, Lo Scocco G, Macchioni P, Bajocchi G et al. Is duplex ultrasonography useful for the diagnosis of giant-cell arteritis? Ann Intern Med. 2002;137(4):232–8. [DOI] [PubMed] [Google Scholar]
- 13.Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med. 2005;142(5):359–69. [DOI] [PubMed] [Google Scholar]
- 14.Ball EL, Walsh SR, Tang TY, Gohil R, Clarke JM. Role of ultrasonography in the diagnosis of temporal arteritis. Br J Surg. 2010;97(12):1765–71. doi: 10.1002/bjs.7252. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt WA, Gromnica-Ihle E. Incidence of temporal arteritis in patients with polymyalgia rheumatica: a prospective study using colour Doppler ultrasonography of the temporal arteries. Rheumatology (Oxford). 2002;41(1):46–52. [DOI] [PubMed] [Google Scholar]
- 16.Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology (Oxford). 2016;55(1):66–70. doi: 10.1093/rheumatology/kev289. [DOI] [PubMed] [Google Scholar]
- 17.Patil P, Williams M, Maw WW, Achilleos K, Elsideeg S, Dejaco C et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol. 2015;33(2 Suppl 89):S-103–6. [PubMed] [Google Scholar]
- 18.Rheaume M, Rebello R, Pagnoux C, Carette S, Clements-Baker M, Cohen-Hallaleh V et al. High-Resolution Magnetic Resonance Imaging of Scalp Arteries for the Diagnosis of Giant Cell Arteritis: Results of a Prospective Cohort Study. Arthritis Rheumatol. 2017;69(1):161–8. doi: 10.1002/art.39824. [DOI] [PubMed] [Google Scholar]
- 19.Bley TA, Uhl M, Carew J, Markl M, Schmidt D, Peter HH et al. Diagnostic value of high-resolution MR imaging in giant cell arteritis. AJNR Am J Neuroradiol. 2007;28(9):1722–7. doi: 10.3174/ajnr.A0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiger J, Bley T, Uhl M, Frydrychowicz A, Langer M, Markl M. Diagnostic value of T2-weighted imaging for the detection of superficial cranial artery inflammation in giant cell arteritis. J Magn Reson Imaging. 2010;31(2):470–4. doi: 10.1002/jmri.22047. [DOI] [PubMed] [Google Scholar]
- 21.Klink T, Geiger J, Both M, Ness T, Heinzelmann S, Reinhard M et al. Giant cell arteritis: diagnostic accuracy of MR imaging of superficial cranial arteries in initial diagnosis-results from a multicenter trial. Radiology. 2014;273(3):844–52. doi: 10.1148/radiol.14140056. [DOI] [PubMed] [Google Scholar]
- 22.Craven A, Robson J, Ponte C, Grayson PC, Suppiah R, Judge A et al. ACR/EULAR-endorsed study to develop Diagnostic and Classification Criteria for Vasculitis (DCVAS). Clin Exp Nephrol. 2013;17(5):619–21. doi: 10.1007/s10157-013-0854-0. [DOI] [PubMed] [Google Scholar]
- 23.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636–43. doi: 10.1136/annrheumdis-2017-212649. [DOI] [PubMed] [Google Scholar]
- 24.Conway R, Smyth AE, Kavanagh RG, O’Donohoe RL, Purcell Y, Heffernan EJ et al. Diagnostic Utility of Computed Tomographic Angiography in Giant-Cell Arteritis. Stroke. 2018;49(9):2233–6. doi: 10.1161/STROKEAHA.118.021995. [DOI] [PubMed] [Google Scholar]
- 25.Sammel AHE, Schembri G, Nguyen K, Brewer J, Schrieber L, Janssen B, Youssef P, Fraser C, Bailey E, Bailey D, Roach P, Laurent R. The Diagnostic Accuracy of PET/CT Scan of the Head, Neck and Thorax Compared with Temporal Artery Biopsy in Patients Newly Suspected of Having GCA [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 10). [Google Scholar]
- 26.Nielsen BDTHI, Keller KK, Therkildsen P, Hauge EM, Gormsen LC. FDG PET/CT Visualization of Inflammation in Temporal and Maxillary Arteries in Treatment-Naive GCA Patients [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). [Google Scholar]
- 27.Grayson PC, Maksimowicz-McKinnon K, Clark TM, Tomasson G, Cuthbertson D, Carette S et al. Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Ann Rheum Dis. 2012;71(8):1329–34. doi: 10.1136/annrheumdis-2011-200795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soriano A, Pazzola G, Boiardi L, Casali M, Muratore F, Pipitone N et al. Distribution patterns of 18F-fluorodeoxyglucose in large vessels of Takayasu’s and giant cell arteritis using positron emission tomography. Clin Exp Rheumatol. 2018;36 Suppl 111(2):99–106. [PubMed] [Google Scholar]
- 29.Grayson PC. Lumpers and splitters: ongoing issues in the classification of large vessel vasculitis. J Rheumatol. 2015;42(2):149–51. doi: 10.3899/jrheum.141376. [DOI] [PubMed] [Google Scholar]
- 30.Grayson PC, Tomasson G, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA et al. Association of vascular physical examination findings and arteriographic lesions in large vessel vasculitis. J Rheumatol. 2012;39(2):303–9. doi: 10.3899/jrheum.110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuta S, Cousins C, Chaudhry A, Jayne D. Clinical features and radiological findings in large vessel vasculitis: are Takayasu arteritis and giant cell arteritis 2 different diseases or a single entity? J Rheumatol. 2015;42(2):300–8. doi: 10.3899/jrheum.140562. [DOI] [PubMed] [Google Scholar]
- 32.Slart R, Writing g, Reviewer g, Members of EC, Members of EI, Inflammation et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45(7):1250–69. doi: 10.1007/s00259-018-3973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lariviere D, Benali K, Coustet B, Pasi N, Hyafil F, Klein I et al. Positron emission tomography and computed tomography angiography for the diagnosis of giant cell arteritis: A real-life prospective study. Medicine (Baltimore). 2016;95(30):e4146. doi: 10.1097/MD.0000000000004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada I, Nakagawa T, Himeno Y, Numano F, Shibuya H. Takayasu arteritis: evaluation of the thoracic aorta with CT angiography. Radiology. 1998;209(1):103–9. doi: 10.1148/radiology.209.1.9769819. [DOI] [PubMed] [Google Scholar]
- 35.Sparks AR, Johnson PL, Meyer MC. Imaging of abdominal aortic aneurysms. Am Fam Physician. 2002;65(8):1565–70. [PubMed] [Google Scholar]
- 36.Yamada I, Nakagawa T, Himeno Y, Kobayashi Y, Numano F, Shibuya H. Takayasu arteritis: diagnosis with breath-hold contrast-enhanced three-dimensional MR angiography. J Magn Reson Imaging. 2000;11(5):481–7. [DOI] [PubMed] [Google Scholar]
- 37.Meller J, Grabbe E, Becker W, Vosshenrich R. Value of F-18 FDG hybrid camera PET and MRI in early takayasu aortitis. Eur Radiol. 2003;13(2):400–5. doi: 10.1007/s00330-002-1518-8. [DOI] [PubMed] [Google Scholar]
- 38.Tedeschi E, Caranci F, Giordano F, Angelini V, Cocozza S, Brunetti A. Gadolinium retention in the body: what we know and what we can do. Radiol Med. 2017;122(8):589–600. doi: 10.1007/s11547-017-0757-3. [DOI] [PubMed] [Google Scholar]
- 39.Ramalho M, Ramalho J, Burke LM, Semelka RC. Gadolinium Retention and Toxicity-An Update. Adv Chronic Kidney Dis. 2017;24(3):138–46. doi: 10.1053/j.ackd.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Besson FL, de Boysson H, Parienti JJ, Bouvard G, Bienvenu B, Agostini D. Towards an optimal semiquantitative approach in giant cell arteritis: an (18)F-FDG PET/CT case-control study. Eur J Nucl Med Mol Imaging. 2014;41(1):155–66. doi: 10.1007/s00259-013-2545-1. [DOI] [PubMed] [Google Scholar]
- 41.Prieto-Gonzalez S, Depetris M, Garcia-Martinez A, Espigol-Frigole G, Tavera-Bahillo I, Corbera-Bellata M et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis. 2014;73(7):1388–92. doi: 10.1136/annrheumdis-2013-204572. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann P, Buchtala S, Achajew N, Haerle P, Ehrenstein B, Lighvani H et al. 18F-FDG PET as a diagnostic procedure in large vessel vasculitis-a controlled, blinded re-examination of routine PET scans. Clin Rheumatol. 2011;30(1):37–42. doi: 10.1007/s10067-010-1598-9. [DOI] [PubMed] [Google Scholar]
- 43.Fuchs M, Briel M, Daikeler T, Walker UA, Rasch H, Berg S et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging. 2012;39(2):344–53. doi: 10.1007/s00259-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 44.Hautzel H, Sander O, Heinzel A, Schneider M, Muller HW. Assessment of large-vessel involvement in giant cell arteritis with 18F-FDG PET: introducing an ROC-analysis-based cutoff ratio. J Nucl Med. 2008;49(7):1107–13. doi: 10.2967/jnumed.108.051920. [DOI] [PubMed] [Google Scholar]
- 45.Walter MA, Melzer RA, Schindler C, Muller-Brand J, Tyndall A, Nitzsche EU. The value of [18F]FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32(6):674–81. doi: 10.1007/s00259-004-1757-9. [DOI] [PubMed] [Google Scholar]
- 46.Meller J, Strutz F, Siefker U, Scheel A, Sahlmann CO, Lehmann K et al. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging. 2003;30(5):730–6. doi: 10.1007/s00259-003-1144-y. [DOI] [PubMed] [Google Scholar]
- 47.Henes JC, Muller M, Krieger J, Balletshofer B, Pfannenberg AC, Kanz L et al. [18F] FDG-PET/CT as a new and sensitive imaging method for the diagnosis of large vessel vasculitis. Clin Exp Rheumatol. 2008;26(3 Suppl 49):S47–52. [PubMed] [Google Scholar]
- 48.Lee YH, Choi SJ, Ji JD, Song GG. Diagnostic accuracy of 18F-FDG PET or PET/CT for large vessel vasculitis : A meta-analysis. Z Rheumatol. 2016;75(9):924–31. doi: 10.1007/s00393-015-1674-2. [DOI] [PubMed] [Google Scholar]
- 49.Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ et al. (18) F-Fluorodeoxyglucose-Positron Emission Tomography As an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients With Large Vessel Vasculitis. Arthritis Rheumatol. 2018;70(3):439–49. doi: 10.1002/art.40379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prieto-Pena D, Martinez-Rodriguez I, Loricera J, Banzo I, Calderon-Goercke M, Calvo-Rio V et al. Predictors of positive (18)F-FDG PET/CT-scan for large vessel vasculitis in patients with persistent polymyalgia rheumatica. Semin Arthritis Rheum. 2018. doi: 10.1016/j.semarthrit.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Schinkel AF, van den Oord SC, van der Steen AF, van Laar JA, Sijbrands EJ. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging. 2014;15(5):541–6. doi: 10.1093/ehjci/jet243. [DOI] [PubMed] [Google Scholar]
- 52.Dikkes A, Aschwanden M, Imfeld S, Glatz K, Messerli J, Staub D et al. Takayasu arteritis: active or not, that’s the question. Rheumatology (Oxford). 2017;56(10):1818–9. doi: 10.1093/rheumatology/kex213. [DOI] [PubMed] [Google Scholar]
- 53.Germano G, Macchioni P, Possemato N, Boiardi L, Nicolini A, Casali M et al. Contrast-Enhanced Ultrasound of the Carotid Artery in Patients With Large Vessel Vasculitis: Correlation With Positron Emission Tomography Findings. Arthritis Care Res (Hoboken). 2017;69(1):143–9. doi: 10.1002/acr.22906. [DOI] [PubMed] [Google Scholar]
- 54.Herlin B, Baud JM, Chadenat ML, Pico F. Contrast-enhanced ultrasonography in Takayasu arteritis: watching and monitoring the arterial inflammation. BMJ Case Rep. 2015;2015. doi: 10.1136/bcr-2015-211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt WA. Ultrasound in the diagnosis and management of giant cell arteritis. Rheumatology (Oxford). 2018;57(suppl_2):ii22–ii31. doi: 10.1093/rheumatology/kex461. [DOI] [PubMed] [Google Scholar]
- 56.Prieto-Gonzalez S, Garcia-Martinez A, Tavera-Bahillo I, Hernandez-Rodriguez J, Gutierrez-Chacoff J, Alba MA et al. Effect of glucocorticoid treatment on computed tomography angiography detected large-vessel inflammation in giant-cell arteritis. A prospective, longitudinal study. Medicine (Baltimore). 2015;94(5):e486. doi: 10.1097/MD.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quinn KA, Ahlman MA, Malayeri AA, Marko J, Civelek AC, Rosenblum JS et al. Comparison of magnetic resonance angiography and (18)F-fluorodeoxyglucose positron emission tomography in large-vessel vasculitis. Ann Rheum Dis. 2018;77(8):1165–71. doi: 10.1136/annrheumdis-2018-213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barra L, Kanji T, Malette J, Pagnoux C, CanVasc. Imaging modalities for the diagnosis and disease activity assessment of Takayasu’s arteritis: A systematic review and meta-analysis. Autoimmun Rev. 2018;17(2):175–87. doi: 10.1016/j.autrev.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Blockmans D, Bley T, Schmidt W. Imaging for large-vessel vasculitis. Curr Opin Rheumatol. 2009;21(1):19–28. doi: 10.1097/BOR.0b013e32831cec7b. [DOI] [PubMed] [Google Scholar]
- 60.Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55(1):131–7. doi: 10.1002/art.21699. [DOI] [PubMed] [Google Scholar]
- 61.Both M, Ahmadi-Simab K, Reuter M, Dourvos O, Fritzer E, Ullrich S et al. MRI and FDG-PET in the assessment of inflammatory aortic arch syndrome in complicated courses of giant cell arteritis. Ann Rheum Dis. 2008;67(7):1030–3. doi: 10.1136/ard.2007.082123. [DOI] [PubMed] [Google Scholar]
- 62.Lee KH, Cho A, Choi YJ, Lee SW, Ha YJ, Jung SJ et al. The role of (18) F-fluorodeoxyglucose-positron emission tomography in the assessment of disease activity in patients with takayasu arteritis. Arthritis Rheum. 2012;64(3):866–75. doi: 10.1002/art.33413. [DOI] [PubMed] [Google Scholar]
- 63.Soussan M, Nicolas P, Schramm C, Katsahian S, Pop G, Fain O et al. Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine (Baltimore). 2015;94(14):e622. doi: 10.1097/MD.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheel AK, Meller J, Vosshenrich R, Kohlhoff E, Siefker U, Muller GA et al. Diagnosis and follow up of aortitis in the elderly. Ann Rheum Dis. 2004;63(11):1507–10. doi: 10.1136/ard.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newman KA, Ahlman MA, Hughes M, Malayeri AA, Pratt D, Grayson PC. Diagnosis of Giant Cell Arteritis in an Asymptomatic Patient. Arthritis Rheumatol. 2016;68(5):1135. doi: 10.1002/art.39517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Misra R, Danda D, Rajappa SM, Ghosh A, Gupta R, Mahendranath KM et al. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010). Rheumatology (Oxford). 2013;52(10):1795–801. doi: 10.1093/rheumatology/ket128. [DOI] [PubMed] [Google Scholar]
- 67.Reichenbach S, Adler S, Bonel H, Cullmann JL, Kuchen S, Butikofer L et al. Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology (Oxford). 2018;57(6):982–6. doi: 10.1093/rheumatology/key015. [DOI] [PubMed] [Google Scholar]
- 68.Arnaud L, Haroche J, Malek Z, Archambaud F, Gambotti L, Grimon G et al. Is (18)F-fluorodeoxyglucose positron emission tomography scanning a reliable way to assess disease activity in Takayasu arteritis? Arthritis Rheum. 2009;60(4):1193–200. doi: 10.1002/art.24416. [DOI] [PubMed] [Google Scholar]
- 69.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919–29. [DOI] [PubMed] [Google Scholar]
- 70.Ostberg G Morphological changes in the large arteries in polymyalgia arteritica. Acta Med Scand Suppl. 1972;533:135–59. [PubMed] [Google Scholar]
- 71.Banerjee SQK, Gribbons KB, Rosenblum JS, Civelek A, Novakovich E, Bagheri A, Merkel PA, Ahlman MA, Grayson PC. Effect of Specific Treatments on Clinical, Serologic, and Imaging Assessments of Disease Activity in Large-Vessel Vasculitis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 10). . [Google Scholar]
- 72.Dellavedova L, Carletto M, Faggioli P, Sciascera A, Del Sole A, Mazzone A et al. The prognostic value of baseline (18)F-FDG PET/CT in steroid-naive large-vessel vasculitis: introduction of volume-based parameters. Eur J Nucl Med Mol Imaging. 2016;43(2):340–8. doi: 10.1007/s00259-015-3148-9. [DOI] [PubMed] [Google Scholar]
- 73.Nuenninghoff DM, Hunder GG, Christianson TJ, McClelland RL, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48(12):3522–31. doi: 10.1002/art.11353. [DOI] [PubMed] [Google Scholar]
- 74.de Boysson H, Daumas A, Vautier M, Parienti JJ, Liozon E, Lambert M et al. Large-vessel involvement and aortic dilation in giant-cell arteritis. A multicenter study of 549 patients. Autoimmun Rev. 2018;17(4):391–8. doi: 10.1016/j.autrev.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 75.Nielsen BD, Gormsen LC, Hansen IT, Keller KK, Therkildsen P, Hauge EM. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur J Nucl Med Mol Imaging. 2018;45(7):1119–28. doi: 10.1007/s00259-018-4021-4. [DOI] [PubMed] [Google Scholar]
- 76.Lee VS, Martin DJ, Krinsky GA, Rofsky NM. Gadolinium-enhanced MR angiography: artifacts and pitfalls. AJR Am J Roentgenol. 2000;175(1):197–205. doi: 10.2214/ajr.175.1.1750197. [DOI] [PubMed] [Google Scholar]
- 77.Youngstein T, Tombetti E, Mukherjee J, Barwick TD, Al-Nahhas A, Humphreys E et al. FDG Uptake by Prosthetic Arterial Grafts in Large Vessel Vasculitis Is Not Specific for Active Disease. JACC Cardiovasc Imaging. 2017;10(9):1042–52. doi: 10.1016/j.jcmg.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 78.Berger CT, Sommer G, Aschwanden M, Staub D, Rottenburger C, Daikeler T. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:smw.2018.14661 10.4414/smw.2018.14661. [DOI] [PubMed] [Google Scholar]
- 79.Tombetti E, Godi C, Ambrosi A, Doyle F, Jacobs A, Kiprianos AP et al. Novel Angiographic Scores for evaluation of Large Vessel Vasculitis. Sci Rep. 2018;8(1):15979. doi: 10.1038/s41598-018-34395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakagomi D, Cousins C, Sznajd J, Furuta S, Mohammad AJ, Luqmani R et al. Development of a score for assessment of radiologic damage in large-vessel vasculitis (Combined Arteritis Damage Score, CARDS). Clin Exp Rheumatol. 2017;35 Suppl 103(1):139–45. [PubMed] [Google Scholar]
- 81.Jiemy WF, Heeringa P, Kamps J, van der Laken CJ, Slart R, Brouwer E. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) imaging of macrophages in large vessel vasculitis: Current status and future prospects. Autoimmun Rev. 2018;17(7):715–26. doi: 10.1016/j.autrev.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 82.Aydin SZ, Yilmaz N, Akar S, Aksu K, Kamali S, Yucel E et al. Assessment of disease activity and progression in Takayasu’s arteritis with Disease Extent Index-Takayasu. Rheumatology (Oxford). 2010;49(10):1889–93. doi: 10.1093/rheumatology/keq171. [DOI] [PubMed] [Google Scholar]