Abstract
Objective:
Our initial understanding of the menopause transition (MT) has been framed by clinical samples of women seeking treatment rather than by population-based studies. The Study of Women's Health Across the Nation (SWAN) initiated in 1996 with an overall goal to define the MT, to characterize its biological and psychosocial antecedents and sequelae in an ethnically and racially diverse sample of midlife women.
Methods:
This review summarizes the central findings of SWAN to date that can inform women and their healthcare providers about the impact of the MT and midlife aging on overall health and well-being.
Results:
SWAN characterized changes in reproductive axis and menstrual cycle patterns that informed the development of the reproductive aging staging system Staging of Reproductive Aging Workshop+10; MT-related symptoms and mental health (vasomotor symptoms, sleep complaints, psychological symptoms, cognitive performance, and urogenital and sexual health); and physiological systems and functions (cardiovascular and cardiometabolic health, bone health, physical function performance) that are influenced by the MT. SWAN demonstrated substantial interrelations among these changes and significant racial/ethnic differences in the rate and magnitude of change in multiple health indictors in midlife women. The findings point to midlife as a critical stage for adopting healthy behavior and preventive strategies.
Conclusions:
Over the past 23 years, SWAN has advanced our understanding of the impact of the MT and midlife aging on health and well-being in women. SWAN will be instrumental to determine whether MT-related changes during midlife are related to unfavorable health and well-being in early old age.
Keywords: Midlife, Menopause transition, Women, Aging, Symptoms
The menopause transition (MT) is a major health milestone for women with influences that extend far beyond reproduction. In addition to the symptoms that accompany menopause, concomitant biological, psychological, behavioral, and social changes shape women's midlife and future health. Much of what we now know about the MT, its characteristics, and its consequences is the product of a series of seminal cohorts that were established in the 1980s and 1990s.1-6 These longitudinal studies broadened our understanding of the MT, which was formerly based on reports of women seeking clinical care and was not reflective of the more general MT experience.
Begun in 1996, the Study of Women's Health Across the Nation's (SWAN's) mission was to define the MT, to characterize its biological and psychosocial antecedents and sequelae, and to do so in an ethnically and racially diverse sample of women (Black, Chinese, Hispanic, Japanese, and White). The principal content areas in SWAN included the reproductive axis; menstrual cycle characterization; MT-related symptoms and mental health; sleep; bone health; body composition; cardiovascular risk factors and outcomes; physical function and performance; cognitive performance; and vaginal and urogenital health.
The goal of this article is to summarize central findings of SWAN to date that can inform women and their healthcare providers about the impact of the MT and midlife aging on overall health and well-being. First, we provide an overview of the design of the SWAN study including a description of relevant ancillary studies. Next, we summarize SWAN contributions to our understanding of changes in (1) reproductive axis and menstrual cycle patterns that informed staging of the MT; (2) symptoms and mental health; and (3) physiological systems and functions that are influenced by the MT. In each of these main areas, we provide, if known, how they are different (or similar) in each of the SWAN's racial/ethnic groups. We provide the clinical and research implications of the reviewed findings and conclude with the next steps SWAN will take to understand women's health in midlife and early old age (defined as the time when adverse changes in health and function start to accumulate7). To aid the use of the reviewed information for clinical care, teaching, and/or research, we include a “take home messages box” for each topic area and provide a central illustration figure that summarizes the impact of the MT and midlife aging on women's health and well-being.
OVERVIEW OF THE SWAN STUDY DESIGN
SWAN is a multisite, multiracial/ethnic, longitudinal cohort study.6 Starting in 1996, SWAN enrolled 3,302 women at seven geographically distinct sites across the United States: Boston, MA; Chicago, IL; Pittsburgh, PA; Detroit, MI; Oakland, CA; Los Angeles, CA; Hudson County, NJ. The Coordinating Center is located at the University of Pittsburgh and the Central Laboratory is located at the University of Michigan. At enrollment, women were between 42 and 52 years of age, had an intact uterus and at least one ovary, were not receiving hormone therapy, were not pregnant or lactating, and had at least one menstrual period in the 3 prior months. Each site enrolled Non-Hispanic White women and women from one other predetermined racial/ethnic group. SWAN enrolled White (n = 1,550), Black (n = 935), Japanese (n = 281), Chinese (n = 250), and Hispanic (n = 286) women. Since enrollment, a baseline visit and 16 follow-up visits have been conducted through 2017.
At each visit, SWAN participants completed a standard protocol that included interview- and self-administered questionnaires focused on menstrual bleeding patterns, clinical health history, health behaviors, and psychosocial factors; sexual health, assessment of anthropometric measures, physical performance, and cognitive function; and provision of blood and urine samples. Self-administered monthly calendars for recording of menstrual flow heaviness as well as end-of-month questions on symptoms, gynecological surgery, and exogenous hormone use were collected through the MT. Each clinical site also elected to collect additional measures related to local scientific resources and interest at selected evaluations. Thus, some sites assessed body composition and bone density; radiographic knee osteoarthritis; subclinical cardiovascular atherosclerosis; and objective measures of sleep. At the most recent visit, a subset of women at all sites participated in protocols of accelerometer-assessed physical activity and measures of vaginal health.
The annual assessments of menstrual bleeding patterns and reproductive hormones—as well as monthly menstrual calendars—were of particular importance to the SWAN design because they allowed for precise identification of menopausal stages and the timing of the final menstrual period (FMP). SWAN defined the following stages: premenopausal (no change in bleeding patterns); early perimenopause (change in length of bleed or interbleed interval); late perimenopause (no bleeding in 3-11 months); natural postmenopause (no bleeding in 12 months not due to hysterectomy), surgical menopause (bilateral oophorectomy with or without hysterectomy), hysterectomy with one or two ovaries retained, and hormone use before FMP. To disentangle the effects of MT and chronological aging, analyses included measures of the MT and aging into the same models to identify independent effects. Because hormone use may mask the FMP, SWAN took into account hormone use in models at the various MT stages as well.
The depth and breadth of SWAN's contribution to understanding the MT and midlife aging extend through several existing sub- and ancillary studies. Of relevance to this report are the Daily Hormone Substudy (DHS) and the SWAN Sleep, the SWAN Mental Health, the SWAN Heart, and the SWAN Cardiovascular Fat ancillary studies. The DHS included a daily collection of first morning voided urine for an entire menstrual cycle or up to 50 days (whichever comes first) for 10 annual visits to characterize patterns of urinary hormone metabolites throughout menstrual cycles over the MT among a subset of SWAN participants (n = 848) across all clinical sites. The SWAN Sleep ancillary study collected in-home polysomnography (PSG) and actigraphy measures of sleep among a subset of SWAN participants (n = 370) across four clinical sites. The SWAN Mental Health ancillary study measured lifetime psychiatric diagnoses at baseline and occurrences of depression annually among SWAN participants from one site (n = 443). The SWAN Heart ancillary study measured subclinical markers of atherosclerosis over two visits among participants from two SWAN sites (n = 608) and the SWAN Cardiovascular Fat ancillary study quantified volumes of cardiovascular heart fat depots among SWAN Heart participants at one visit (n = 564).
WHAT WE HAVE LEARNED FROM SWAN
The reproductive axis, menstrual cycle patterns, and stages of the MT
As one of the largest and longest studies of the MT, SWAN has provided precise characterization of the main hormonal alterations and menstrual cycle changes that accompany the MT and define stages of reproductive aging (Box 1).8
Box 1.
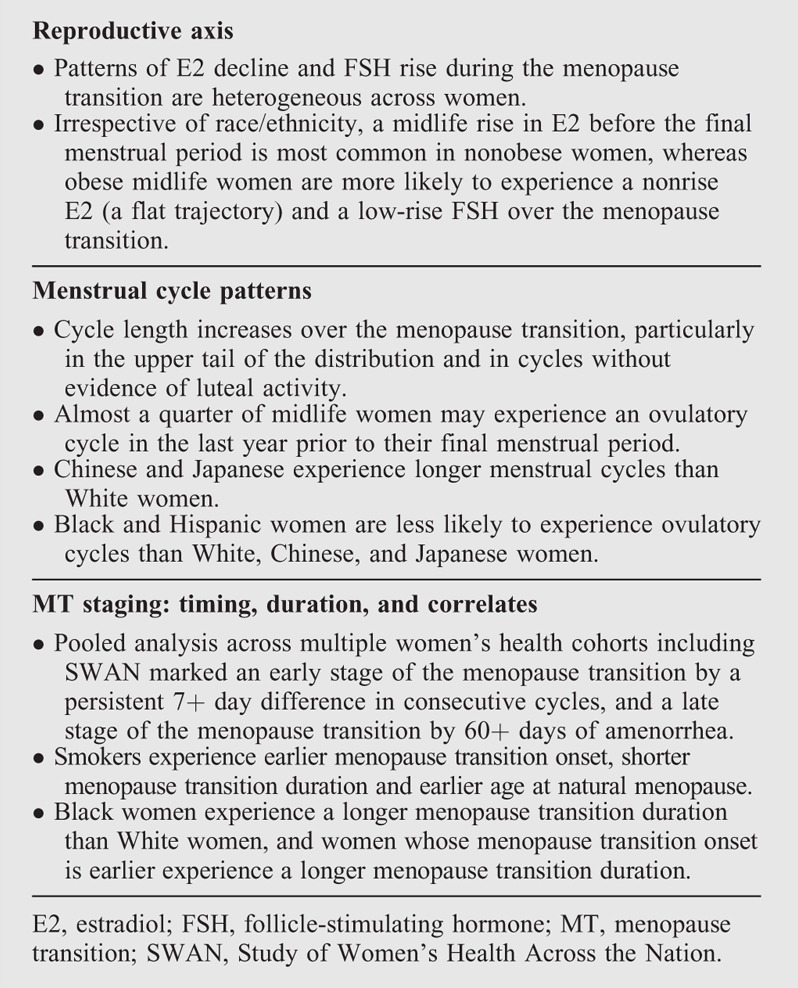
Take-home messages: the reproductive axis, menstrual cycle patterns, and stages of the menopause transition
The reproductive axis over the MT
Estradiol (E2) and follicle-stimulating hormones (FSHs) changed dramatically over the MT. Overall, E2 decreased across the FMP beginning at 2 years before the FMP and continuing for 2 years after the FMP, with a stabilization 2 years after the FMP. On the contrary, FSH increases across the MT with a gradual increase beginning 7 before the FMP and continuing until approximately 2 years before the FMP, followed by a remarkable increase in the time period of 2 years before to 2 years after the FMP, and finally a stabilization 2 years after the FMP.9
Interestingly, the time course and pattern of these hormonal changes across the MT did not vary by age at FMP or obesity, and they were conserved across racial/ethnic groups.9 Instead, the levels of circulating E2 and FSH varied by body mass index (BMI) and race/ethnicity. Obesity markedly attenuated the rise in FSH level after FMP and was negatively associated with E2 level before FMP, but positively associated after FMP. Race/ethnicity was not associated with the span of FSH changes across the FMP, but Chinese and Japanese women had a shorter span of E2 changes and a slightly lower E2 levels than White or Black women.9
SWAN demonstrated for the first time that not all midlife women experienced one pattern of E2 decline or FSH rise over the MT.10 Among 31.5% of SWAN women, E2 rose around 5.5 years before the FMP with a steep decline almost 1 year before the FMP. A similar rise of E2 was observed among 13.1% of SWAN women, but with a slow decline over 2 years after the FMP. A third group of 26.9% experienced a slow-decline pattern with no E2 rise before FMP, and a last group of 28.6% followed a limited flat decline across FMP.10 For FSH rise, three patterns were observed across the MT, with 40.7% of the evaluated participants followed a high FSH rise, 48.7% a medium rise, and 10.6% followed a low rise FSH pattern. Interestingly, the rise pattern of E2 before the FMP was mainly observed among non-obese women of all racial/ethnic groups, whereas the flat E2 and low rise FSH patterns were more pronounced among obese women of all racial/ethnic groups (Box 1).10
Menstrual cycle patterns
The menstrual calendar data showed that the menstrual cycle length increased beginning 7.5 years before the FMP, with the steepest increase starting 4 years pre-FMP, and increased variability in cycle length beginning 2 years pre-FMP.11 Consistent with this finding, analyses of the SWAN DHS found cycle length increased primarily in cycles without evidence of luteal activity (ELA, presumed ovulatory) as determined by the Kassam algorithm,12 whereas the mean cycle length in ELA cycles was highly stable over time at 27 to 29 days.13 In turn, the percentage of ELA cycles declined from almost 100% 10 years pre-FMP to 88% 5 years later, followed by a sharper decline to 23% in the last year pre-FMP.13 Chinese and Japanese participants had consistently longer cycle length than non-Hispanic Whites, as did women with an older age at MT onset, but these factors did not modify the time trends.11 Black and Hispanic women had consistently lower percentages of ELA cycles (overall 73% and 66%, respectively) than non-Hispanic Whites, Chinese, and Japanese (81%-83%), with no racial/ethnic effect modification of MT-related declines (Box 1).13
MT stages: timing, duration, and correlates
SWAN participated in the ReSTAGE Collaboration—with the Melbourne Women's Midlife Health Project, the Seattle Midlife Women's Health Study, and the Treloar data—which investigated aspects of menstrual cycle regularity as potential markers of the early and late MT stages, proposed in the first Staging of Reproductive Aging Workshop (STRAW).14 Pooled analyses of monthly menstrual calendar data suggested a persistent difference in consecutive menstrual cycle length of seven or more days as the optimal marker of entry into early stage of the MT, as this predicted subsequent FMP, was observed in a high proportion of women, and occurred before other candidate markers.15 ReSTAGE recommended at least 60 days of amenorrhea as the marker of entry into late stage of the MT,16 as both 60+ days and 90+ days—used by many past studies17—were similarly predictive of subsequent FMP, but 60+ days was observed in a higher proportion of women and is a more straightforward indicator for women and clinicians.17
Using the above STRAW criteria of early and late stages of the MT, analyses of menstrual calendar data showed that women who smoked had both an earlier onset of the MT and a shorter total MT duration (defined as time from the onset of the early MT to the FMP). Total MT duration was longer in those with earlier age at MT onset and in Black women. Shorter premenopausal cycle length was linked to shorter MT duration, consistent with faster follicle depletion, whereas women with irregular—and perhaps longer—cycles in midreproductive age had a longer early-stage duration.18 Median age at natural menopause was 51.4 years in the cross-sectional screener data from SWAN19 and slightly higher at 52.5 years among longitudinal cohort participants.20 In multivariate modeling, race/ethnicity was not independently associated with age at natural menopause,20 whereas time-varying current smoking—but not baseline smoking—was linked to an earlier age at natural menopause (Box 1).20
Symptoms and mental health
Vasomotor symptoms
Up to 80% of SWAN women reported vasomotor symptoms (VMS), defined as hot flashes and night sweats occurring during the past 2 weeks, at some point during the MT, with the highest reporting occurring during the transition from early to late perimenopause.21 In contrast to long-held beliefs that VMS last only a few years, frequent VMS persisted for a median of 7.4 years, with even longer durations for some women.22 Analyses examining individual trajectories of VMS over the MT found four trajectories, with women falling into approximately equal proportions across four groups.23 An early onset group of women had VMS early in the transition that continued after the FMP, but then declined. A later onset group had VMS peaking at the FMP and continuing, though declining, into their postmenopausal years. A third group had few or no VMS throughout the MT, and a fourth group of women, which we dubbed the “super flashers,” started VMS well before their FMP that continued well into the postmenopause. These findings provide insight into different patterns of VMS experienced by women.
SWAN's diverse cohort has provided additional insight into racial/ethnic differences in VMS. Black women had the highest prevalence and longest duration of VMS, and were most bothered by their VMS.21,22,24 Asian women had the lowest VMS prevalence, whereas Hispanic and White women fell between the Black and Asian groups. Women in lower socioeconomic positions were more likely to have VMS, independent of race/ethnicity.21
Several other demographic and psychological factors were associated with VMS. Less education, smoking, greater depressive symptoms, greater anxiety, and more symptom sensitivity were all significantly related to greater subsequent reporting of VMS.21 Depressed mood and anxiety were also associated with greater perceived bother of VMS, independent of VMS frequency.24 Women with a history of childhood abuse or neglect were more likely to report VMS over the MT.25
SWAN has elucidated and helped resolve a complex relation between VMS and body weight/adiposity. In SWAN, higher adiposity was a risk factor for VMS early in the transition,26 but this relation reversed after women became postmenopausal, with greater adiposity associated with lower VMS.27 A similar reversal by menopause stage was noted between VMS and adipokines or inflammatory substances released by body fat.28
Although the physiology of VMS is not fully understood, reproductive hormones likely play an integral role. In serum samples from the full SWAN cohort and urine samples from the DHS, lower E2 (or estrone conjugates in the case of urine samples) and higher FSH levels were associated with VMS reporting, particularly in the setting of non-ELA cycles.29 Variants in genes that encode for estrogen receptor alpha and enzymes involved in synthesis and conversion of estrogens predicted greater likelihood of VMS (Box 2).30
Box 2.
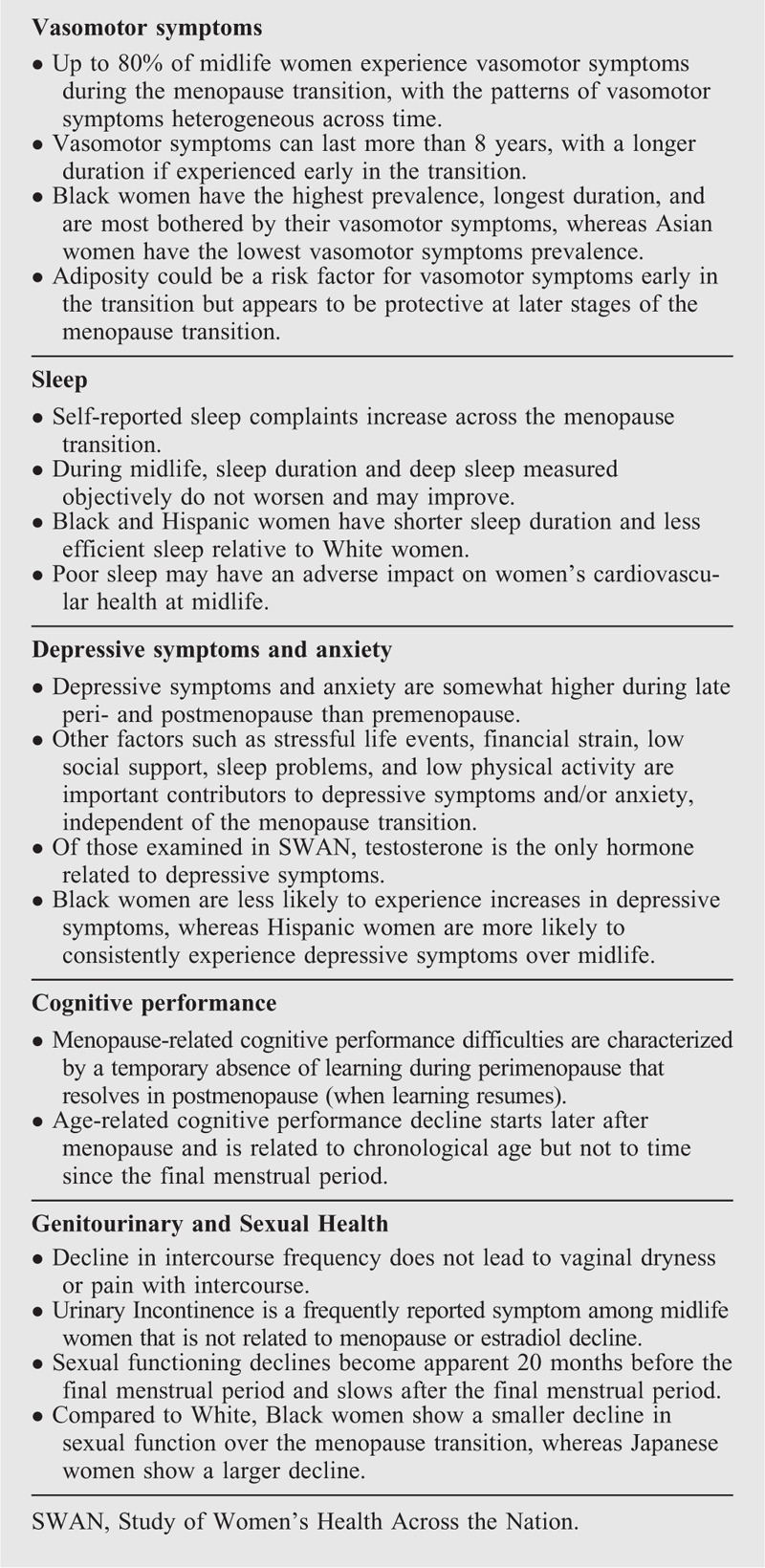
Take-home messages: symptoms and mental health
Sleep
Sleep is a multidimensional concept. Major dimensions of sleep include duration, continuity, timing, regularity, and satisfaction, in addition to sleep disorders. Some theorists include daytime sleepiness and napping as indicators of poor nighttime sleep in their models. Sleep dimensions can be assessed by self-report (questionnaire, interview, diary), behavioral movement (actigraphy), and PSG (multichannel physiological recordings, including electroencephalogram [EEG]). Not surprisingly, different measures of the same dimension do not yield the same results. Thus, it is necessary to take a comprehensive approach to understand the impact of the MT on sleep.
In the SWAN initial cross-sectional survey, late perimenopause and natural postmenopausal women reported more sleep difficulties than did premenopausal women, regardless of the occurrence of VMS. Postmenopausal hormone therapy users had similar rates of sleep difficulties to premenopausal women.31 A longitudinal analysis of the first 7 years of SWAN showed a similar pattern of MT for self-reported sleep disturbance, defined as trouble falling asleep, waking up several times, and waking up earlier than planned at least three nights weekly in the last 2 weeks, with several exceptions. Early perimenopause was also associated with more sleep disturbance and hormone therapy use did not protect postmenopausal women from sleep disturbance. Frequent reporting of VMS, E2 decline, and FSH rise were also associated with sleep difficulties.32 Waking up several times at night showed the most pronounced association with menopause stage.
Most studies of menopause and sleep complaints report averages across women by menopausal stage, which does not address whether a subset of women is especially vulnerable to the development of sleep disturbance near the FMP. Using group-based trajectory modeling, four different patterns of sleep complaint trajectories were apparent over the MT. Only 15% reported increasing sleep complaints with the MT; specifically, waking up frequently around the time of the FMP. The other groups showed stability over the transition or a linear increase consistent with aging-related increases in sleep problems not specific to the MT.33 Black and White women were more likely to experience an increasing prevalence of waking up frequently around the FMP, relative to the other racial/ethnic groups.
Sleep data gathered by PSG within the SWAN Sleep ancillary study paint another picture. More rapid increases in FSH in the 7 years before the sleep protocol was associated with longer sleep time and more deep sleep, but consistent with the self-reported sleep disturbance, less favorable self-reported sleep quality.34 A spectral analysis of non-rapid eye movement sleep showed that beta EEG power, a measure of cognitive arousal, was greater in late perimenopausal and postmenopausal women than in pre- and early perimenopausal women, with hot flashes explaining part of this relationship of cognitive arousal with menopausal status.35
Race/ethnic differences in sleep characteristics and their determinants are substantial. White women reported the most sleep complaints and Hispanic women the fewest in the full SWAN cohort.32 US-born Hispanic, Chinese, and Japanese women had more sleep complaints compared to their first-generation immigrant ethnic counterparts.36 On the contrary, Black women had shorter sleep duration, longer sleep latency, and lower sleep efficiency measured by PSG compared to both White and Chinese women in the SWAN Sleep ancillary study.37 In addition, EEG beta power was higher and delta power lower in Black compared to White women. Actigraphy measures of sleep in the SWAN cohort show similar results: Black and Hispanic women had shorter sleep and lower sleep efficiency than White women.38 The major factors that mediated race/ethnic differences in actigraphy were financial hardship and increasing health problems, and for Black women, VMS.
The associations of sleep at midlife with health outcomes have focused thus far primarily on cardiovascular health. In the SWAN Sleep ancillary study, women who had the metabolic syndrome had less PSG-assessed sleep efficiency, more beta power, and greater apnea/hyponea scores (an indicator of sleep disordered breathing), compared to those who did not.39 Women with multiple indicators of sleep disordered breathing had elevated inflammatory and coagulation biomarkers.40 In addition, Black women who had higher levels of C-reactive protein also had shorter PSG sleep duration and those who had higher levels of fibrinogen had lower sleep efficiency. Women with lower total delta power showed increased diastolic blood pressure over time.41 A delayed bedtime, relative to keeping one's usual bedtime, was related to increased insulin resistance, but not to change in BMI over time (Box 2).42
Depressive symptoms and anxiety
A long-held belief is that women experience increased depression and anxiety during the MT. Researchers continue to evaluate the scientific evidence for this belief and whether VMS, sleep disruption, and other stressful factors contribute to negative moods at midlife.
SWAN has measured depressive symptoms using the Center for Epidemiological Studies of Depression (CES-D) scale at baseline and at all subsequent visits. At baseline, 23% of SWAN women scored 16 or higher on the CES-D, the cut-point for indicating potential clinical depression.43 In longitudinal analyses,44 the odds of having high depressive symptoms were significantly greater in the early peri-, late peri-, or postmenopause, as compared to the premenopause, with highest odds in the late perimenopause.43,44 However, stressful life events, being Hispanic, and having low education conveyed greater risk for high depressive symptoms than did menopausal status.44 Testosterone was the only reproductive hormone related to depressive symptoms and was positively related to higher odds of depressive symptoms.44
Most studies of menopause and depression report mean levels of depressive symptoms over the MT, which does not address the heterogeneity of patterns of depressive symptoms experienced by women. Using group-based trajectory modeling, five different trajectories of depressive symptoms over midlife aging were identified. Half (50%) of the women had very low CES-D scores (scores <5), 29% had scores less than 16 over the entire time, 11% of women initially had high CES-D scores that decreased over time, and small groups of women showed an increasing trend over time (5%) or consistently high scores (5%) over time.45 Black women were less likely to be in the increasing group and more likely to be in the decreasing symptom trajectory. Hispanic women were more likely to be in the consistently high group. The group with increasing scores was characterized by lower physical activity, an increase in sleep problems, a smaller reduction in stressful life events, and a decrease in social support. Baseline menopausal status (ie, pre- or early perimenopausal) was not associated with trajectory group membership.
In the SWAN Mental Health ancillary study, women were two to four times as likely to experience a major depressive episode in the MT or early postmenopause than in premenopause,46 although menopause status was not a significant predictor of first episode of major depression.47
Although anxiety symptoms are highly prevalent among midlife women and may precede the onset of depressive symptoms, they have received much less attention than depression or depressive symptoms. SWAN has examined anxiety longitudinally using a measure of anxiety based on a composite of four anxiety symptoms: irritability, nervousness or tension, feeling fearful for no reason, and heart pounding or racing. Internal consistency of these items is good (Cronbach alpha of 0.77) and the scores are highly correlated with the Generalized Anxiety Disorder-7 (Spearman r = 0.71), a commonly used measure of anxiety. The odds of high anxiety symptoms were significantly greater for early peri-, late peri-, and postmenopause compared to premenopause, and peaked in the late perimenopause.48 However, menopausal stage was no longer significantly related to anxiety when analyses were adjusted for frequent VMS. Among women who did not have anxiety before the MT there was a greater likelihood of reporting anxiety over the MT compared to when premenopausal, independent of multiple risk factors, including upsetting life events, financial strain, fair/poor perceived health, and VMS.48 Odds ratios for anxiety symptoms increased from early peri- to late peri-, but then declined in postmenopause, suggesting that increased levels of anxiety may be a temporary occurrence. Perceived health less than very good and at least one upsetting event in the past year conveyed greater risk of anxiety symptoms than did menopause stage (Box 2).
Cognitive performance
Approximately 60% of midlife women report problems with memory during the MT, yet studies of measured cognitive performance during the transition are rare.49-52 To address unanswered questions about midlife cognitive function, the SWAN protocol included in-person serial tests of cognitive processing speed, verbal episodic memory, and working memory. SWAN's initial, 4-year longitudinal analysis of the relation between the MT and cognitive performance disclosed a temporary decrement in both processing speed and verbal episodic memory during the perimenopause; the decrement resolved in postmenopause.51 The negative perimenopause effect was subtle, manifest as the absence of a learning effect, meaning that cognitive test scores did not get better with repeated administrations. Improvement with repeated testing is normative in this age range.53 But even in perimenopause, test scores did not drop—they simply did not improve during this MT stage. It is important to make the distinction between not getting better and an actual decline, which was observed in a later analysis (see below).
It appears intuitive that symptoms associated with the MT, specifically VMS, depressive symptoms, anxiety, and sleep disturbance symptoms, would contribute to the cognitive difficulties observed during perimenopause,54-56 but a subsequent, 6-year, longitudinal analysis from SWAN evaluating a direct relation between these four MT symptoms and cognitive performance did not uphold this thesis.52 There was no association between cognitive performance and either VMS or poor sleep, but women with depressive symptoms did score lower in the domain of cognitive processing speed and those with more anxiety symptoms had worse verbal episodic memory. When all four symptoms were added to the models of MT stage and cognition, the negative effect of late perimenopause on cognitive performance was unaltered, suggesting that the presence of these symptoms does not account for the perimenopause learning decrement.52
As the SWAN cohort aged and continued to undergo serial waves of cognitive tests, age-related cognitive decline (also known as cognitive aging) studies have been undertaken. Age-related cognitive decline is a decline in cognitive performance that can be a normal part of aging.57 There is debate about whether age-related cognitive decline starts in midlife.58
To circumvent the practice effects related to the initial two testing occasions, age-related cognitive decline evaluation started at the third cognitive assessment; starting after practice effects have abated, one can observe an actual decline in cognitive performance (if present).58
At analysis outset, the median age was 54 and 60% had reached menopause. Cognitive processing speed and verbal episodic memory declined with time, adjusted for MT stage, MT symptoms, diabetes, race/ethnicity, education, and attrition, consistent with age-related cognitive decline. To consider whether the menopause, apart from chronological aging, influenced cognitive decline, an alternate analysis strategy was used, gauging cognitive performance as a function of time before and after the FMP. The decline in cognitive processing speed and verbal memory per year of chronological age was almost identical to the average, between-women difference in cognitive processing speed by age at time of FMP, supporting the thesis that the declines in cognitive performance are not related to menopause, but rather, are a function of chronological aging (Box 2).
Genitourinary and Sexual Health
Vulvovaginal symptoms
In longitudinal analyses, vaginal dryness increased across the MT, from about 19% in pre- and early perimenopause to 34% in postmenopause years. Furthermore, vaginal dryness contributed to pain during sexual intercourse.59 Advancing menopausal stage was more strongly associated with vaginal dryness than E2 decline, confirming a more complex etiology of this symptom than low E2 levels alone. Naturally postmenopausal women were less likely to develop vaginal dryness than surgically menopausal women.59 Contrary to popular belief that reduced intercourse frequency leads to poorer vaginal function, reduced intercourse frequency did not precede the development of vaginal dryness or pain with intercourse.59 Importantly, sexual lubricant use was associated with better sexual functioning scores around the FMP and reduced the likelihood of developing sexual pain (Box 2).59,60
Urinary incontinence
About 68% of SWAN women reported monthly or more frequent urinary incontinence (UI; assessed as number of times a participant had leaked urine, even a small amount during the month before each annual visit) symptoms at least once over the first 9 years of follow-up.61 Although SWAN women who were continent at baseline had higher odds of reporting new onset monthly or less frequent UI in early and late perimenopause, women who were incontinent at baseline actually reported greater improvement in their UI symptoms during this same time frame, that is, mild UI symptoms were just as likely to resolve as develop. Importantly, women in early and late perimenopause were not more likely to develop weekly or more frequent UI or experience worsening UI symptoms than before or after the MT.62,63 Neither UI development nor worsening was associated with E2 level or change in E2 levels over time.64 Instead, aging, being overweight, weight gain, and incident diabetes increased the risk of developing UI and having weekly or more frequent UI symptoms.62-64
Less than 40% of incontinent women sought treatment for UI from a healthcare provider. Treatment seeking was most strongly related to longer duration and greater frequency of UI symptoms and was not associated with race/ethnicity, education level, or socioeconomic status.61 Women with frequent UI who did not seek treatment were more likely to report inaccurate beliefs about UI (eg, UI is normal consequence of aging) or motivational barriers (eg, provider never asked about UI) as reasons for not seeking treatment (Box 2).65
Sexual functioning
Sexual functioning is an important component of women's lives with 75% of SWAN women at baseline reporting sex as moderately to extremely important.66 Pain during sexual intercourse increased and sexual desire decreased over the MT, independent of sociodemographic characteristics, or changes in health and psychological functioning.67 However, menopausal stage was not associated with frequency of sexual intercourse, emotional satisfaction with partner, or physical pleasure, although vaginal dryness was an important factor for all in adjusted analyses. Longitudinal data showed that concurrent E2 level was not related to any measured sexual function domain, but concurrent testosterone level was positively associated with sexual desire, arousal, and masturbation.68
SWAN has also conducted analysis of sexual function relative to the FMP.60 Using a composite measure of sexual functioning among naturally menopausal women who remained sexually active throughout the MT, a decline in sexual functioning became apparent 20 months before the FMP and slowed 1 year after the FMP through 5 years afterwards. This decline was not explained by factors such as poorer health, VMS, vaginal dryness, depression, or partner status.
Sexual functioning in midlife varied by race/ethnicity. Compared to White women, Chinese and Japanese women reported lower importance of sex, desire, masturbation, arousal, and more sexual pain, whereas Black women reported greater importance of sex, higher sexual intercourse frequency, but more sexual pain, less arousal, less emotional satisfaction, and less physical pleasure.67,69 Sexually active Black women also had a smaller decline, whereas Japanese women had a larger decline in sexual functioning across the MT (Box 2).60
Physiological systems and functions
Cardiovascular and cardiometabolic health
Over the past 25 years, our understanding of whether and how the MT could influence women's cardiovascular disease (CVD) risk has significantly evolved. Menopause is now listed as a female-specific CVD risk factor per the American Heart Association,70 and has been recognized as a risk-enhancing factor favoring statin therapy initiation in the 2018 updated guidelines of blood cholesterol management (Box 3).71
Box 3.
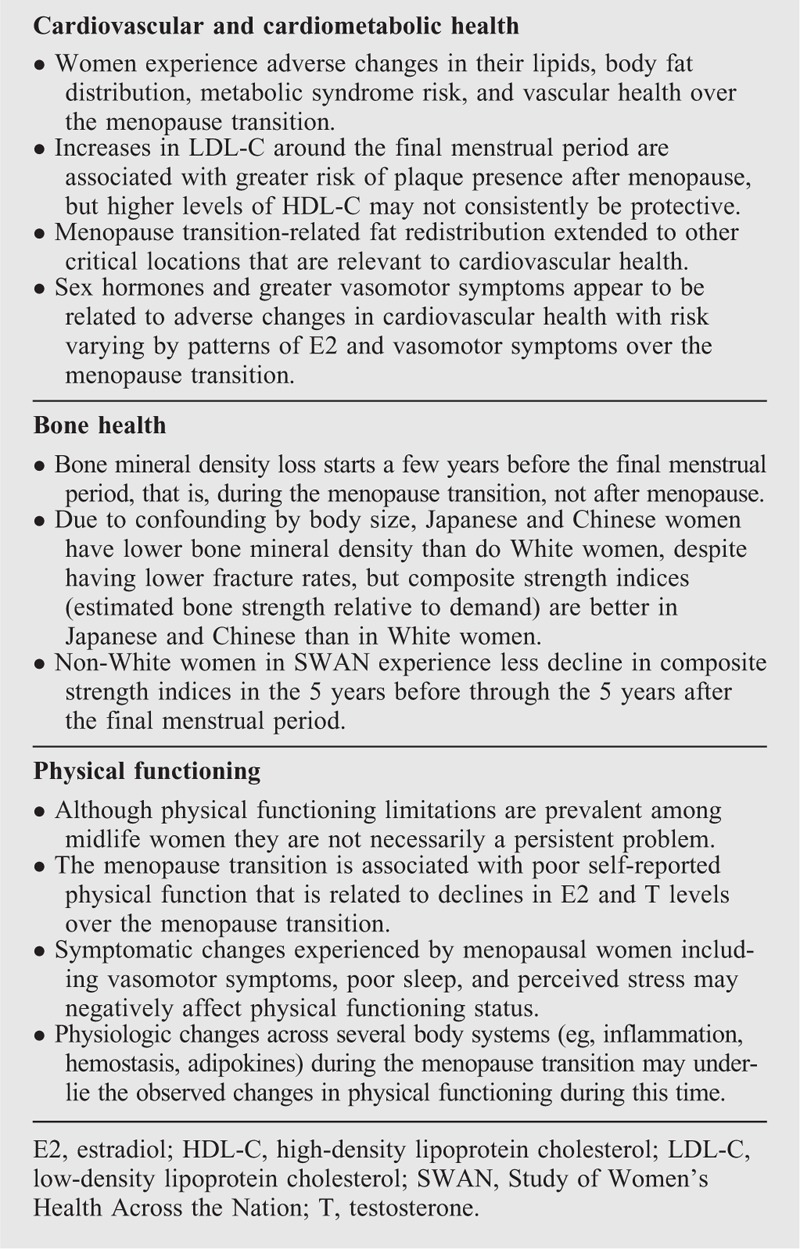
Take-home messages: physiological systems and functions
Traditional and novel CVD risk factors
By following women over the MT, SWAN documented sharp increases in total cholesterol, low-density lipoprotein cholesterol (LDL-C) and apolipoprotein (Apo)B levels within a 1-year interval surrounding the FMP.72 Importantly, the menopause-related acceleration in LDL-C was associated with greater risk of carotid plaque later in life in a follow-up analysis.73 Conversely, high-density lipoprotein cholesterol (HDL-C) and ApoA did not decline over the MT.72 In fact, a SWAN analysis by menopausal stage showed HDL-C to be the highest during the late peri- and early postmenopause stages.74 Analysis from the SWAN Heart ancillary study suggested potential changes in HDL quality over the MT, with increases in HDL-C associated with less carotid intima medial thickness (IMT) before, but with greater carotid IMT after the FMP.75 Recent preliminary work showed that the ability of HDL particles to promote cholesterol efflux capacity, the main function by which HDL removes cholesterol from peripheral cells, was reduced over the MT.76 Unlike lipids, changes in blood pressure, insulin, glucose, and BMI during midlife were not associated with the MT, independent of chronological aging.72,77,78 However, the odds of developing metabolic syndrome were the highest during the perimenopause independent of age.79 Moreover, SWAN found a relation between body composition, fat distribution, and the MT.80-82 Using dual energy x-ray absorptiometry, SWAN examined change in body composition, beginning 9 years before and concluding 9 years after the FMP.80 About 2 years before the FMP, the rate of fat gain doubled and lean mass started to decline. Fat gains and lean losses continued until 2 years after the FMP and then flattened. Weight increased linearly during the MT, but also plateaued 2 years after the FMP.80 Black and White women had similar patterns of change in body composition and weight in relation to the FMP. Japanese women lost some lean mass during the MT but, unlike White and Black women, did not gain fat mass. Uniquely, Chinese women gained lean mass and lost fat mass after menopause.80
Menopause-related fat distribution extended to other critical locations that are relevant to cardiovascular health. In SWAN Cardiovascular Fat ancillary study, postmenopausal women had greater volumes of heart fat within and outside the pericardial sac,81 with the latter fat, known as paracardial fat, associated with greater risk of coronary artery calcification in postmenopausal than premenopausal women.82 The proximity of heart fat to the myocardium and the vasculature may result in a higher pro-atherogenic contribution for this fat compared to other fat depots.83 Heart fat has been associated with worse CVD risk factors and a greater risk of CVD events.84
VMS, the hallmark of menopause, were associated with a more adverse CVD risk factor profile, independent of age or sex hormones. Reporting of VMS over the course of the MT was associated with higher LDL-C, Apo B, triglycerides,85 and insulin-resistance,86 and with a greater risk for incident hypertension (Box 3).87
Subclinical vascular health
SWAN reported detrimental changes to vascular health over the MT. In SWAN Heart ancillary study, yearly increases in carotid IMT and interadventitial diameters, markers of carotid atherosclerosis and remodeling, accelerated during the late perimenopause stage independent of age.88 Additional work from SWAN Heart ancillary study showed that metrics of vascular function also were compromised over the MT. Women who transitioned over a mean of 2.4 years of follow-up experienced greater progression in aortic pulse wave velocity, a marker of arterial stiffness, than women who remained premenopausal or those who were postmenopausal at baseline.89
Further work showed that changes in E2 and FSH might contribute to vascular remodeling over the MT. Lower levels of E2 and higher levels of FSH were associated with a wider adventitial diameter or a thicker IMT over time independent of age.90 When considering patterns of E2 and FSH changes over the MT91; women who experienced a rise in E2 5 years pre-FMP with an early decline at 1 year pre-FMP had lower risk of carotid plaque after menopause than women with a flat E2 decline trajectory. Furthermore, women with a lower FSH rise over the MT had thinner IMT postmenopause.91
Similar to being adversely associated with traditional CVD risk factors (described above), VMS might be a marker of adverse underlying vascular changes. Women with hot flashes had a lower flow-mediated dilation and a greater aortic calcification independent of CVD risk factors and E2.92 Moreover, reporting more frequent VMS was associated with thicker IMT.93 The timing of VMS across the MT might be relevant for midlife vascular health according to a recent work from SWAN.94 Women with early-onset VMS had thicker IMT after menopause, independent of demographics and CVD risk factors (Box 3).94
Bone mineral density, bone strength indices, and fractures
Although bone loss has long been considered a hallmark of menopause, before SWAN, longitudinal examinations of how bone mineral density (BMD) changed in relation to the MT were scarce. In 1992, a landmark study of 75 US White women illustrated that the term “post-menopausal bone loss” was misleading: BMD loss started a few years before the FMP, that is, during the MT, not after menopause.95 SWAN solidified and generalized this finding by quantifying changes in spine and femoral neck BMD in relation to time before and after the FMP in more than 2,000 Black, Chinese, Japanese, and White women; in each group, BMD loss began 1 year before the FMP and decelerated, but did not cease, ∼2 years after it.96
Other results offered insight into the biology underlying this temporal pattern of MT-related bone loss: 2 years before the FMP, a sharp decline in serum E2 was accompanied by a marked increase in urinary N-telopeptide (a bone turnover marker), suggesting that a drop in E2 is one of the triggers of the greater-bone-turnover-greater-bone-loss cascade.97,98 Approximately 1.5 years after the FMP, urinary N-telopeptide levels stabilized and serum E2 reached its nadir level, presaging the lesser rate of BMD loss that commences at about 2 years post-FMP.
In predominantly cortical bone, bone loss is characterized by endosteal (inner surface) resorption and compensatory periosteal (outer surface) formation, which increases the bone diameter.99 SWAN showed that this cortical bone remodeling also began 1 year before the FMP.100 However, gain in bone diameter did not counterbalance the BMD loss, resulting in a 0.7% average annual decline in femoral neck strength (estimated using composite strength indices, which quantify bone strength relative to loading demand).100,101
Racial/ethnic variation in osteoporotic fracture risk is recognized—for example, fracture rates among US Black and Asian women are approximately half those of White women —but our comprehension of the biology underlying the fracture differential remains limited.102 Moreover, in some instances, between-group differences in BMD do not parallel between-group fracture risks; for example, average BMDs of Japanese and Chinese women are lower than White women's. SWAN baseline, cross-sectional analyses documented the paradoxical differences in BMD between its Asian and White participants.103 But BMD alone is insufficient to capture fracture risk.104 Composite indices of femoral neck strength, which forecast fracture risk in older adults, combine BMD, femoral neck width, body height, and weight to estimate bone strength relative to the demand experienced during a fall from standing height. These indices help remove the confounding of BMD by body size and bone size, which is a particular problem in crossracial/ethnic BMD comparisons.101 On average, baseline composite strength indices were greater in Chinese and Japanese compared to White women, concordant with ethnic/racial differences in fracture risk.105 Baseline values of composite strength indices predicted incident fracture during 9 years of follow-up. Race/ethnicity predicted incident fracture independent of BMD, but did not predict fracture independent of composite strength estimates.106
Longitudinal analysis of change in composite indices in relation to FMP-time disclosed a possible etiology of lesser fracture risk in Asian and Black women compared to White women.100 On average, at the end of the 10-year interval bracketing the FMP (5 years prior through 5 years after it), values of the composite strength indices were less in White women than those of each of the three non-White subgroups (Box 3).100
Physical functioning
Limitations in physical functioning have been widely studied among older adults, but data from SWAN has been instrumental in characterizing the midlife as a critical window for the onset of poor physical functioning. In a cross-sectional analysis of SWAN screening data, almost one-fifth of the participants reported either moderate or substantial limitations using the SF-36 physical functioning questionnaire.107 When considering the federal gait speed standard requirement for safely crossing a pedestrian intersection (<1.22 m/s) as a benchmark for physical functioning mobility concerns, an analysis among SWAN women from the Michigan site showed that a surprisingly high percent of women, 31% (mean age 47 years), had mobility issues that may affect their pedestrian safety.108
Although physical functioning limitations are prevalent among midlife women, findings from SWAN demonstrated that they are not necessarily a persistent issue across time. In a prospective analysis of five biennial measures of physical functioning, only one-third of women never reported physical functioning limitations, whereas 42% and 26% of women reported ever experiencing some and substantial limitations, respectively.109 On average, the probability of improving in functioning was greater than the probability of worsening and older age and high levels of economic strain were found to be predictive of greater worsening.109
Changes in endogenous sex hormones during the MT, including greater reductions in E2 and testosterone were predictive of poorer physical functioning in SWAN women.110 The burden of physical functioning limitations differs by menopausal stage. Late perimenopause, postmenopause, or surgical menopause women reported poorer levels of physical functioning111,112 as compared to premenopausal women, adjusted for age. With respect to the impact of symptoms on physical functioning, women experiencing greater burden of VMS, urine leakage, poor sleep, and perceived stress had poorer physical functioning.112 Thus, the physiologic and symptomatic changes experienced by menopausal women may negatively affect physical functioning status.
The rapid physiologic changes occurring across several body systems during the MT may underlie the observed changes in physical functioning during this time. In site-specific analyses, the systemic inflammatory biomarker, C-reactive protein, was associated with poor self-reported physical functioning113 and high levels of leptin, a proinflammatory adipokine, predicted poor performance on mobility-related tasks.114 Inflammation was associated with poor physical functioning in SWAN study-wide analyses as well; adverse levels of C-reactive protein and hemostatic markers including plasminogen-activator inhibitor 1, tissue plasminogen activator antigen, and factor VIIc predicted poor performance-based outcomes of physical functioning.115
Physical functioning is hypothesized to be not only an outcome of adverse health factors during the midlife, but as a marker of poor health. Women with poor self-reported physical functioning were at greater risk for metabolic syndrome116 and those with low grip strength had greater risk for diabetes.117 Poor physical functioning may also be a marker of early cardiovascular risk; walking speed was inversely associated with carotid adventitial diameter among SWAN women, a finding that was independent of traditional cardiovascular risk factors.118 It is likely that the relationships between health behaviors, health outcomes, and physical functioning are bidirectional, and so longitudinal studies such as SWAN are critical to our understanding of the complexities of these processes (Box 3).
CLINICAL IMPLICATIONS AND NEXT STEPS
Since its beginning in 1996, SWAN has made wide-ranging contributions to women's health and well-being at midlife. SWAN has elaborated on the biological, symptomatic, and psychosocial changes that accompany the MT, and characterized the health sequelae of the MT. SWAN findings emphasize the diversity of the MT experiences among women, especially those of different racial/ethnic backgrounds, and underscore the multifactorial nature of the MT as a landmark event in women's lives. In addition, the findings point to midlife as a critical stage for adopting healthy behavior and preventive strategies.
SWAN's characterization of the MT was instrumental to the development of the criterion standard staging system, STRAW+10, which characterizes the stages of reproductive aging. Use of such a standardized reproductive aging staging system is critical to improving comparability across studies of midlife and menopause and providing uniform clinical terminology. SWAN findings underscore the heterogeneity of the endocrinologic and symptomatic experience of the MT as defined by patterns and timing of changes in sex hormones, VMS, and sleep complaints. The heterogeneous patterns of changes in E2 and VMS over the MT in SWAN were associated with differences in cardiovascular health outcomes after menopause, supporting the role of these factors in cardiovascular risk stratification. The between-women heterogeneity documented by SWAN underscores the appropriateness of implementing a personalized approach in both research and clinical practice when evaluating MT health sequelae or designing MT-related therapeutic or behavioral interventions.
SWAN's strong design and the breadth of information collected over approximately two decades of follow-up time enabled SWAN to unravel the contribution of the MT versus midlife aging in several physiological systems and health domains (Fig. 1; Central Illustration Figure: blue bars depicting changes that were more driven by midlife aging while light orange bars depicting changes that were more driven by the MT). The adverse changes observed in markers of cardiovascular (eg, lipids, vascular remodeling, body fat deposition) and musculoskeletal (eg, bone density and physical function) health during midlife were more influenced by the MT, whereas increases in blood pressure and BMI were more influenced by midlife aging. Transient rise in cognitive difficulties occurring during perimenopause, decline in sexual functioning due to increased pain and decreased sexual desire, and increases in sleep complaints and vaginal dryness were more driven by the MT. Although women reported greater depressive and anxiety symptoms during the MT, those symptoms were transient and more impacted by stressful life events, sleep problems, and low physical activity than the MT.
FIG. 1.
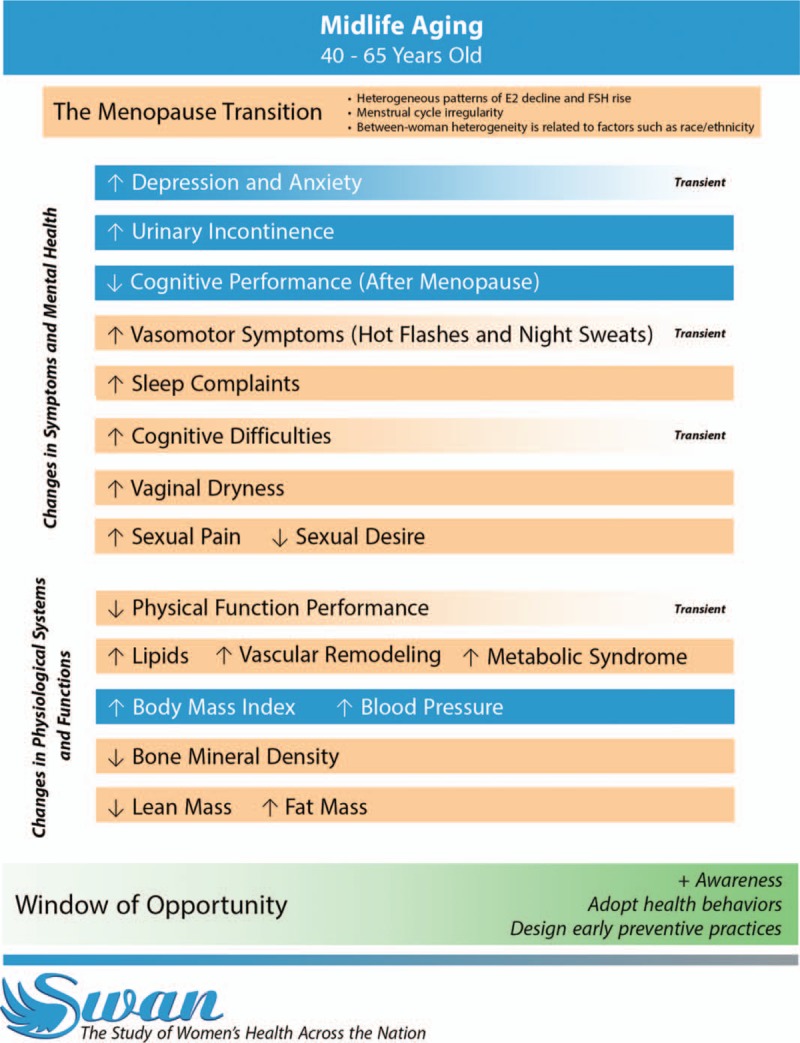
Central Illustration Figure. The Contribution of the Menopause Transition and Midlife Aging to Women's Health and Well-being: What Have We Learned from the Study of Women's Health Across the Nation (SWAN)?; SWAN contributed to our understanding of the impacts of the menopause transition versus midlife aging on women's health and well-being in different domains. The figure lists changes occurring during the menopause transition that were reviewed in this paper, and differentiates whether these changes were more driven by the menopause transition or midlife aging using different color schemes. Light orange bars depict changes that were mostly driven by the menopause transition, whereas blue bars depict changes that were mostly driven by midlife aging. Transient changes are identified when applicable by fading color bars and listing the word “Transient” at the end of relevant bars. SWAN has identified midlife as a critical stage for adopting healthy behavior and preventive strategies.
The reported findings from SWAN along with those of other studies focusing on midlife1-5 point strongly to midlife as a window of opportunity for maintaining or establishing positive health behaviors to delay or prevent poor health outcomes or disability later in life (Fig. 1). For example, smoking cessation, healthy diet, and regular physical activity are three important modifiable factors that can significantly reduce cardiovascular risk.119 SWAN documented extremely low prevalence of each of these modifiable factors with only 1.7% of the women evidencing positive healthy behaviors over time.120 These findings call for more effort to educate women about MT-related acceleration in their cardiovascular risk and the importance of implementing a heart-healthy lifestyle to reduce risk of CVD in later life.
Similar to cardiovascular health, the midlife is a critical stage for musculoskeletal health. The decline in dual energy x-ray absorptiometry-assessed BMD starts several years before the FMP. This finding again points to the potential for early intervention, including lifestyle modification along with possibly prophylactic therapeutics that may delay and/or reduce the burden of osteoporosis and fracture risk later in life. Being physically active was associated with greater peak femoral neck strength relative to load in pre- and early perimenopausal women.121 SWAN also showed serum 25-hydroxyvitamin D level below 20 ng/mL, which is partially determined by diet, was associated with 85% hazard for nontraumatic fracture development.122
The burden of physical functioning limitations among SWAN women also call for identifying ways in which women can improve midlife physical functioning. Health behaviors and the prevention of certain health conditions may be key targets for potential interventions given their association with midlife physical functioning. SWAN women who had a better diet quality123 as well as an overall healthier lifestyle,124 and particularly more physical activity125 had better midlife physical functioning. Furthermore, women free from health conditions that commonly onset during the midlife, including depressive symptoms, vision impairment, knee osteoarthritis, and peripheral nerve impairment, have better functioning than women afflicted with these conditions.126-129
Taken together, adopting healthy lifestyle behaviors is critical to midlife women who face several changes in their physical and psychological well-being, many of which are highly related to the MT. Although this article has reviewed findings by distinct scientific areas, it is essential to point out that these domains are interrelated. For example, changes in sex hormones have been linked to changes in cardiovascular and physical functioning health.81,90,91,110 Both cardiovascular health and physical functioning are tightly linked and SWAN demonstrated their interconnection.116,118 Sleep and VMS symptomatology are interconnected and both have been linked to cardiometabolic health.39,41,85,94 It is critical to understand the impact of symptomatology changes on physiologic changes as well as how changes within a body system might impact changes in another system. Such knowledge will elucidate mechanistic pathways of how midlife and the MT might impact disease development and the aging process.
The MT experience of midlife women is strongly influenced by their racial/ethnic background. SWAN has documented significant racial/ethnic differences in the rate and magnitude of change in multiple health indictors, including patterns and timing of sex hormones changes and VMS reporting, declines in BMD, body composition, sleep characteristics, depressive symptoms, and sexual health. The reported racial/ethnic differences at midlife may drive the widening of health disparities later in life; a hypothesis that should be tested in future studies. It is critical that clinicians and researchers realize the diversity in endocrinologic, symptomatic, and physiologic changes of the MT among women of different racial/ethnic backgrounds and how might this diversity impact women future risk of disease and disability as well as their therapeutic and prevention options.
Important limitations of the SWAN study included the limited sample size of Hispanic participants which may reduce ability to detect significant differences in MT characteristics and its correlates among this ethnic group; recruiting women aged 42 to 52 years old (mean age 47 years old) at baseline making SWAN unable to study early menopause and its sequels; and limited generalizability to geographical locations not included in the study, for example, southeast.
Next steps
SWAN has advanced our understanding of the impact of the MT on health and well-being in midlife women. Whether the MT-related changes during midlife set the stage for poor health and well-being in early old age or represent a temporary set of physical and mental challenges is not known. Owing to its long duration, SWAN participants have now reached early old age. Therefore, SWAN now has the opportunity to examine the relation between prospectively characterized MT and midlife health measures and later life health and functional outcomes. This previously unavailable link between the MT, midlife, and older age will allow identification of interventions that may optimally preserve health and function as women age.
CONCLUSION
Over the past 23 years, SWAN has advanced our understanding of the impact of the MT and midlife aging on health and well-being in women. SWAN will be instrumental to determine whether MT-related changes during midlife are related to unfavorable health and well-being in early old age.
Acknowledgments
The authors thank Mohammed R. El Khoudary for designing the central illustration figure of this article. The authors thank the study staff at each site and all the women who participated in SWAN.
Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011-present, Mary Fran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999-present; Robert Neer, PI 1994-1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009-present; Lynda Powell, PI 1994-2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011-present, Rachel Wildman, PI 2010-2011; Nanette Santoro, PI 2004-2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994-2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD—Chhanda Dutta 2016-present; Winifred Rossi 2012-2016; Sherry Sherman 1994-2012; Marcia Ory 1994-2001; National Institute of Nursing Research, Bethesda, MD—Program Officers. Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012-present; Kim Sutton-Tyrrell, PI 2001-2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995-2001. Steering Committee: Susan Johnson, Current Chair. Chris Gallagher, Former Chair.
Footnotes
Funding/support: The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH); DHHS, through the National Institute on Aging (NIA); the National Institute of Nursing Research (NINR); and the NIH Office of Research on Women's Health (ORWH) (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Financial disclosure/conflicts of interest: S.R.K., G.G., S.L.C., N.E.A., M.M.B., C.K., L.E.W., and K.M. have nothing to disclose. R.C.T. discloses MAS Innovations, Pfizer, Procter & Gamble (consulting).
REFERENCES
- 1.Kaufert PA. Women and their health in the middle years: a Manitoba project. Soc Sci Med 1984; 18:279–281. [DOI] [PubMed] [Google Scholar]
- 2.McKinlay JB, McKinlay SM, Brambilla DJ. Health status and utilization behavior associated with menopause. Am J Epidemiol 1987; 125:110–121. [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med 1989; 321:641–646. [DOI] [PubMed] [Google Scholar]
- 4.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: a 9-year prospective population-based study. The Melbourne Women's Midlife Health Project. Climacteric 2004; 7:375–389. [DOI] [PubMed] [Google Scholar]
- 5.Woods NF, Mitchell ES. The Seattle Midlife Women's Health Study: a longitudinal prospective study of women during the menopausal transition and early postmenopause. Womens Midlife Health 2016; 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego, CA: Academic press, 2000:175–188. [Google Scholar]
- 7.Zaidi A. Life cycle transitions and vulnerabilities in old age: a review (Occasional Paper). UNDP Human Development Report Office 2014. [Google Scholar]
- 8.Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10 addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012; 97:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randolph JF, Jr, Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab 2011; 96:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tepper PG, Randolph JF, Jr, McConnell DS, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women's Health across the Nation (SWAN). J Clin Endocrinol Metab 2012; 97:2872–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paramsothy P, Harlow SD, Elliott MR, et al. Influence of race/ethnicity, body mass index, and proximity of menopause on menstrual cycle patterns in the menopausal transition: the Study of Women's Health Across the Nation. Menopause 2015; 22:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassam A, Overstreet JW, Snow-Harter C, DeSouza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect 1996; 104:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santoro N, Crawford SL, El Khoudary SR, et al. Menstrual cycle hormone changes in women traversing menopause: study of Women's Health Across the Nation. J Clin Endocrinol Metab 2017; 102:2218–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril 2001; 76:874–878. [DOI] [PubMed] [Google Scholar]
- 15.Harlow SD, Mitchell ES, Crawford S, et al. The ReSTAGE Collaboration: defining optimal bleeding criteria for onset of early menopausal transition. Fertil Steril 2008; 89:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlow SD, Crawford S, Dennerstein L, Burger HG, Mitchell ES, Sowers MF. ReSTAGE Collaboration. Recommendations from a multi-study evaluation of proposed criteria for staging reproductive aging. Climacteric 2007; 10:112–119. [DOI] [PubMed] [Google Scholar]
- 17.Harlow SD, Paramsothy P. Menstruation and the menopausal transition. Obstet Gynecol Clin N Am 2011; 38:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paramsothy P, Harlow SD, Nan B, et al. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women's Health Across the Nation. Menopause 2017; 24:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001; 153:865–874. [DOI] [PubMed] [Google Scholar]
- 20.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013; 178:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold E, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health 2006; 96:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avis NE, Crawford SL, Greendale G, et al. Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tepper PG, Brooks MM, Randolph JF, Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause 2016; 23:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: who is most bothered by vasomotor symptoms? Menopause 2008; 15:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurston RC, Bromberger J, Chang Y, et al. Childhood abuse or neglect is associated with increased vasomotor symptom reporting among midlife women. Menopause 2008; 15:16–22. [PMC free article] [PubMed] [Google Scholar]
- 26.Thurston RC, Sowers MR, Chang Y, et al. Adiposity and reporting of vasomotor symptoms among midlife women: the study of women's health across the nation. Am J Epidemiol 2008; 167:78–85. [DOI] [PubMed] [Google Scholar]
- 27.Gold EB, Crawford SL, Shelton JF, et al. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms: the Study of Women’ Health Across the Nation (SWAN). Menopause 2017; 24:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurston RC, Chang Y, Mancuso P, Matthews KA, Adipokines adiposity, and vasomotor symptoms during the menopause transition: findings from the Study of Women's Health Across the Nation. Fertil Steril 2013; 100:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randolph JF, Jr, Sowers M, Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab 2005; 90:6106–6112. [DOI] [PubMed] [Google Scholar]
- 30.Crandall CJ, Crawford SL, Gold EB. Vasomotor symptom prevalence is associated with polymorphisms in sex steroid-metabolizing enzymes and receptors. Am J Med 2006; 119:S52–S60. [DOI] [PubMed] [Google Scholar]
- 31.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause 2003; 10:19–28. [DOI] [PubMed] [Google Scholar]
- 32.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 2008; 31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 33.Kravitz HM, Janssen I, Bromberger JT, et al. Sleep trajectories before and after the final menstrual period in the Study of Women's Health Across the Nation (SWAN). Curr Sleep Med Rep 2017; 3:235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep 2008; 31:1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell IG, Bromberger JT, Buysse DJ, et al. Evaluation of the association of menopausal status with delta and beta EEG activity during sleep. Sleep 2011; 34:1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hale L, Troxel WM, Kravitz HM, Hall MH, Matthews KA. Acculturation and sleep among a multiethnic sample of women: the Study of Women's Health Across the Nation (SWAN). Sleep 2014; 37:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN Sleep Study. Sleep 2009; 32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews KA, Hall M, Lee L, et al. Racial/ethnic disparities in women's sleep duration, continuity, and quality and their statistical mediators: Study of Women's Health Across the Nation. Sleep 2019; 42:pii: zsz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall MH, Okun ML, Sowers M, Matthews KA, Kravitz HM. Sleep is associated with the metabolic syndrome in a multi-ethnic cohort of midlife women: the SWAN Sleep Study. Sleep 2012; 35:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews KA, Zheng H, Kravitz HM, et al. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: Study of Women's Health Across the Nation sleep study. Sleep 2010; 33:1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews KA, Chang Y, Kravitz HM, et al. Sleep and risk for high blood pressure and hypertension in midlife women: the SWAN (Study of Women's Health Across the Nation) sleep study. Sleep Med 2014; 15:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor BJ, Matthews KA, Hasler BP, et al. Bedtime variability and metabolic health in midlife women: the SWAN sleep study. Sleep 2016; 39:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN). J Affect Disord 2007; 103:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women's Health Across the Nation (SWAN). Arch Gen Psychiatry 2010; 67:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bromberger JT, Schott LL, Avis NE, et al. Psychosocial and health-related risk factors for depressive symptom trajectories among midlife women over 15 years: Study of Women's Health Across the Nation (SWAN). Psychol Med 2019; 49:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bromberger JT, Kravitz HM, Chang Y, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women's Health Across the Nation. Psychol Med 2011; 41:1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bromberger JT, Kravitz HM, Matthews K, Youk A, Brown C, Feng W. Predictors of first lifetime episodes of major depression in midlife women. Psychol Med 2009; 39:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bromberger JT, Kravitz HM, Chang Y, et al. Does risk for anxiety increase during the menopausal transition? Study of Women's Health Across the Nation. Menopause 2013; 20:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan Mitchell E, Fugate Woods N. Midlife women's attributions about perceived memory changes: observations from the Seattle Midlife Women's Health Study. J Womens Health Gend Based Med 2001; 10:351–362. [DOI] [PubMed] [Google Scholar]
- 50.Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas 2006; 53:447–453. [DOI] [PubMed] [Google Scholar]
- 51.Greendale GA, Huang MH, Wight RG, et al. Effects of the menopause transition and hormone use on cognitive performance in mid-life women. Neurology 2009; 72:1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greendale GA, Wight RG, Huang MH, et al. Menopause-associated symptoms and cognitive performance: results from the Study of Women's Health Across the Nation. Am J Epidemiol 2010; 171:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamar M, Resnick SM, Zonderman AB. Longitudinal changes in verbal memory in older adults: distinguishing the effects of age from repeat testing. Neurology 2003; 60:82–86. [DOI] [PubMed] [Google Scholar]
- 54.Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol 1998; 11:111–119. [PubMed] [Google Scholar]
- 55.Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev 2009; 13:309–321. [DOI] [PubMed] [Google Scholar]
- 56.Beaudreau SA, O’Hara R. Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry 2008; 16:790–803. [DOI] [PubMed] [Google Scholar]
- 57.Blazer DG, Yaffe K, Liverman CT, et al. Cognitive Aging: Progress in Understanding and Opportunities for Action. Washington, DC: The National Academies Press, 2015. [PubMed] [Google Scholar]
- 58.Karlamangla AS, Lachman ME, Han W, Huang M, Greendale GA. Evidence for cognitive aging in midlife women: Study of Women's Health Across the Nation. PLoS One 2017; 12:e0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waetjen LE, Crawford SL, Chang P, et al. Study of Women's Health Across the Nation (SWAN). Factors associated with developing vaginal dryness symptoms in women transitioning through menopause: a longitudinal study. Menopause 2018; 25:1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avis NE, Colvin A, Karlamangla AS, et al. Change in sexual function over the menopause transition: results from the Study of Women's Health Across the Nation (SWAN). Menopause 2017; 24:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waetjen LE, Xing G, Johnson WO, Melnikow J, Gold EB. Study of Women's Health Across the Nation (SWAN). Factors associated with seeking treatment for urinary incontinence across the menopausal transition. Obstet Gynecol 2015; 125:1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waetjen LE, Feng WY, Ye J, et al. Study of Women's Health Across the Nation (SWAN). Factors associated with worsening and improving urinary incontinence across the menopausal transition. Obstet Gynecol 2008; 111:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waetjen LE, Ye J, Feng WY, et al. Study of Women's Health Across the Nation (SWAN). Association between menopausal transition and the development of urinary incontinence. Obstet Gynecol 2009; 114:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waetjen LE, Johnson WO, Xing G, Feng WY, Greendale GA, Gold EB. Study of Women's Health Across the Nation. Serum estradiol levels are not associated with urinary incontinence in midlife women transitioning through menopause. Menopause 2011; 18:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waetjen LE, Xing G, Johnson WO, Melnikow J, Gold EB. Study of Women's Health Across the Nation (SWAN). Factors associated with reasons incontinent women report for not seeking urinary incontinence treatment over 9 years across the menopausal transition. Menopause 2018; 25:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cain V, Johannes C, Avis NE, et al. Sexual functioning and practices in a multi-ethnic sample of midlife women: Baseline results from SWAN. J Sex Res 2003; 40:266–276. [DOI] [PubMed] [Google Scholar]
- 67.Avis NE, Brockwell S, Randolph JF, Jr, et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women's Health Across the Nation. Menopause 2009; 16:442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Randolph JF, Zheng H, Avis NE, Greendale GA, Harlow SD. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab 2015; 100:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Avis NE, Zhao X, Johannes C, Ory M, Brockwell S, Greendale G. Correlates of sexual functioning among mid-aged women: results from the Study of Women's Health Across the Nation (SWAN). Menopause 2005; 12:385–398. [DOI] [PubMed] [Google Scholar]
- 70.Benjamin EJ, Muntner P, Alonso A, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 update: a report from the American Heart Association. Circulation 2019; 139:e56–e66. [DOI] [PubMed] [Google Scholar]
- 71.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018 2019; 73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 72.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 2009; 54:2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matthews KA, El Khoudary SR, Brooks MM, et al. Lipid changes around the final menstrual period predict carotid subclinical disease in postmenopausal women. Stroke 2017; 48:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am J Epidemiol 2009; 169:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El Khoudary SR, Wang L, Brooks MM, Thurston RC, Derby CA, Matthews KA. Increase HDL-C level over the menopausal transition is associated with greater atherosclerotic progression. J Clin Lipidol 2016; 10:962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Khoudary SR, Hutchins PM, Matthews KA, et al. Cholesterol efflux capacity and subclasses of HDL particles in healthy women transitioning through menopause. J Clin Endocrinol Metab 2016; 101:3419–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matthews KA, Abrams B, Crawford S, et al. Body mass index in mid-life women: relative influence of menopause, hormone use, and ethnicity. Int J Obes Relat Metab Disord 2001; 25:863–873. [DOI] [PubMed] [Google Scholar]
- 78.Sternfeld B, Wang H, Quesenberry CP, Jr, et al. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women's Health Across the Nation. Am J Epidemiol 2004; 160:912–922. [DOI] [PubMed] [Google Scholar]
- 79.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med 2008; 168:1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greendale GA, Sternfeld B, Huang MH, et al. Changes in body composition and weight during the menopause transition. JCI Insight 2019; 4:e124865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El Khoudary SR, Shields KJ, Janssen I, et al. Cardiovascular fat, menopause, and sex hormones in women: the SWAN Cardiovascular Fat Ancillary Study. J Clin Endocrinol Metab 2015; 100:3304–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El Khoudary SR, Shields KJ, Janssen I, et al. Postmenopausal women with greater paracardial fat have more coronary artery calcification than premenopausal women: the Study of Women's Health Across the Nation (SWAN) Cardiovascular Fat Ancillary Study. J Am Heart Assoc 2017; 6:pii: e004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iacobellis G, Gao YJ, Sharma AM. Do cardiac and perivascular adipose tissue play a role in atherosclerosis? Curr Diab Rep 2008; 8:20–24. [DOI] [PubMed] [Google Scholar]
- 84.Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol 2013; 61:1388–1395. [DOI] [PubMed] [Google Scholar]
- 85.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol 2012; 119:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and insulin resistance in the Study of Women's Health Across the Nation. J Clin Endocrinol Metab 2012; 97:3487–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jackson EA, El Khoudary SR, Crawford SL, et al. Hot flash frequency and blood pressure: data from the Study of Women's Health Across the Nation. J Womens Health (Larchmt) 2016; 25:1204–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause 2013; 20:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan ZA, Janssen I, Mazzarelli JK, et al. Serial studies in subclinical atherosclerosis during menopausal transition (from the Study of Women's Health Across the Nation). Am J Cardiol 2018; 122:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis 2012; 225:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El Khoudary SR, Santoro N, Chen HY, et al. Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. Eur J Prev Cardiol 2016; 23:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women's Health Across the Nation Heart Study. Circulation 2008; 118:1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Powell LH, Matthews KA. Hot flashes and carotid intima media thickness among midlife women. Menopause 2011; 18:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thurston RC, El Khoudary SR, Tepper PG, et al. Trajectories of vasomotor symptoms and carotid intima media thickness in the Study of Women's Health Across the Nation. Stroke 2016; 47:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Recker RR, Lappe JM, Davies KM, Kimmel DB. Change in bone mass immediately before menopause. J Bone Miner Res 1992; 7:857–862. [DOI] [PubMed] [Google Scholar]
- 96.Greendale GA, Sowers MF, Han WJ, et al. Bone mineral density loss in relation to the final menstrual period in a multi-ethnic cohort: results from the Study of Women's Health Across the Nation (SWAN). J Bone Miner Res 2012; 27:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sowers MR, Zheng H, Greendale GA, et al. Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab 2013; 98:2854–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 2004; 89:1555–1561. [DOI] [PubMed] [Google Scholar]
- 99.Heaney RP, Barger-Lux MJ, Davies KM, Ryan RA, Johnson ML, Gong G. Bone dimensional change with age: interactions of genetic, hormonal, and body size variables. Osteoporos Int 1997; 7:426–431. [DOI] [PubMed] [Google Scholar]
- 100.Ishii S, Cauley JA, Greendale GA, et al. Trajectories of femoral neck strength in relation to the final menstrual period in a multi-ethnic cohort. Osteop Int 2013; 24:2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karlamangla AS, Barrett-Connor E, Young J, Greendale GA. Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporos Int 2004; 15:62–70. [DOI] [PubMed] [Google Scholar]
- 102.Cauley JA, Wu L, Wampler NS, et al. Clinical risk factors for fractures in multi-ethnic women: the Women's Health Initiative. J Bone Miner Res 2007; 22:1816–1826. [DOI] [PubMed] [Google Scholar]
- 103.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008; 93:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.World Health Organization Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser 1994; 843:1–129. [PubMed] [Google Scholar]
- 105.Ishii S, Cauley JA, Greendale GA, Danielson ME, Safaei Nili N, Karlamangla A. Ethnic differences in composite indices of femoral neck strength. Osteoporos Int 2012; 23:1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishii S, Greendale G, Cauley J, et al. Fracture risk assessment without race/ethnicity information. J Clin Endocrin Metab 2012; 97:3593–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sowers M, Pope S, Welch G, Sternfeld, Albrecht G. The association of menopause and physical functioning in women at midlife. J Am Geriatr Soc 2001; 49:1485–1492. [DOI] [PubMed] [Google Scholar]
- 108.Sowers M, Jannausch ML, Gross M, et al. Performance-based physical functioning in African-American and Caucasian women at midlife: considering body composition, quadriceps strength, and knee osteoarthritis. Am J Epidemiol 2006; 163:950–958. [DOI] [PubMed] [Google Scholar]
- 109.Ylitalo KR, Karvonen-Gutierrez CA, Fitzgerald N, et al. Relationship of race-ethnicity, body mass index, and economic strain with longitudinal self-report of physical functioning: the Study of Women's Health Across the Nation. Ann Epidemiol 2013; 23:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.El Khoudary SR, McClure CK, VoPham T, et al. Longitudinal assessment of the menopausal transition, endogenous estradiol, and perception of physical functioning: the Study of Women's Health Across the Nation. J Gerontol A Biol Sci Med Sci 2014; 69:1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tseng LA, El Khoudary SR, Young EA, et al. The association of menopause status with physical function: the Study of Women's Health Across the Nation. Menopause 2012; 19:1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women's Health Across the Nation. Menopause 2009; 16:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tomey K, Sowers M, Zheng H, Jackson EA. Physical functioning related to C-reactive protein and fibrinogen levels in mid-life women. Exp Gerontol 2009; 44:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karvonen-Gutierrez CA, Zheng H, Mancuso P, Harlow SD. Higher leptin and adiponectin concentrations predict poorer performance-based physical functioning in midlife women: the Michigan Study of Women's Health Across the Nation. J Gerontol A Biol Sci Med Sci 2016; 71:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McClure CK, El Khoudary SR, Karvonen-Gutierrez C, et al. Prospective associations between inflammatory and hemostatic markers and physical functioning limitations in mid-life women: longitudinal results of the Study of Women's Health Across the Nation (SWAN). Exp Gerontol 2014; 49:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ylitalo KR, Karvonen-Gutierrez C, McClure C, et al. Is self-reported physical functioning associated with incident cardiometabolic abnormalities or the metabolic syndrome? Diabetes Metab Res Rev 2016; 32:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karvonen-Gutierrez CA, Peng Q, Peterson M, Duchowny K, Nan B, Harlow S. Low grip strength predicts incident diabetes among mid-life women: the Michigan Study of Women's Health Across the Nation. Age Ageing 2018; 47:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.El Khoudary SR, Chen HY, Barinas-Mitchell E, et al. Simple physical performance measures and vascular health in late midlife women: the Study of Women's Health Across the Nation. Int J Cardiol 2015; 182:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343:16–22. [DOI] [PubMed] [Google Scholar]
- 120.Wang D, Jackson EA, Karvonen-Gutierrez CA, et al. Healthy lifestyle during the midlife is prospectively associated with less subclinical carotid atherosclerosis: the Study of Women's Health Across the Nation. J Am Heart Assoc 2018; 7:e010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mori T, Ishii S, Greendale GA, et al. Physical activity as determinant of femoral neck strength relative to load in adult women: findings from the hip strength across the menopause transition study. Osteoporos Int 2014; 25:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cauley JA, Greendale GA, Ruppert K, et al. Serum 25 hydroxyvitamin D, bone mineral density and fracture risk across the menopause. J Clin Endocrinol Metab 2015; 100:2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tomey S, Sowers MR, Crandall C, Johnston J, Jannausch M, Yosef M. Dietary intake related to prevalent functional limitations in midlife women. Am J Epidemiol 2008; 167935–167943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sternfeld B, Colvin A, Stewart A, et al. The impact of a healthy lifestyle on future physical functioning in midlife women. Med Sci Sports Exerc 2017; 49:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pettee Gabriel K, Sternfeld B, Colvin A, et al. Physical activity trajectories during midlife and subsequent risk of physical functioning decline in late mid-life: the Study of Women's Health Across the Nation (SWAN). Prev Med 2017; 105:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tomey S, Sowers MR, Harlow S, Jannausch M, Zheng H, Bromberger J. Physical functioning among mid-life women: associations with trajectory of depressive symptoms. Soc Sci Med 2010; 71:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chandrasekaran N, Harlow S, Moroi S, Musch D, Peng Q, Karvonen-Gutierrez C. Visual impairment at baseline is associated with future poor physical functioning among middle-aged women: the Study of Women's Health Across the Nation, Michigan Site. Maturitas 2017; 96:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am 2011; 93:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ylitalo KR, Herman WH, Harlow SD. Performance-based physical functioning and peripheral neuropathy in a population-based cohort of women at midlife. Am J Epidemiol 2013; 177:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]


