Abstract
We conducted a randomized controlled trial of an individually-tailored, virtual perspective-taking intervention to reduce race and socioeconomic (SES) disparities in providers’ pain treatment decisions. Physician residents and fellows (n=436) were recruited from across the United States for this two-part online study. Providers first completed a bias assessment task in which they made treatment decisions for virtual patients with chronic pain who varied by race (Black/White) and SES (low/high). Providers who demonstrated a treatment bias were randomized to the intervention or control group. The intervention consisted of personalized feedback about their bias, real-time dynamic interactions with virtual patients, and videos depicting how pain impacts the patients’ lives. Treatment bias was re-assessed one week later. Compared to the control group, providers who received the tailored intervention had 85% lower odds of demonstrating a treatment bias against Black patients and 76% lower odds of demonstrating a treatment bias against low SES patients at follow-up. Providers who received the intervention for racial bias also showed increased compassion for patients compared to providers in the control condition. Group differences did not emerge for provider comfort in treating patients. Results suggest an online intervention that is tailored to providers according to their individual treatment biases, delivers feedback about these biases, and provides opportunities for increased contact with Black and low SES patients, can produce substantial changes in providers’ treatment decisions, resulting in more equitable pain care. Future studies should examine how these effects translate to real-world patient care, and the optimal timing/dose of the intervention.
Introduction
Racial disparities are prevalent in pain care [50,68]. Black patients are especially likely to be under-treated for pain [68], which has numerous deleterious biopsychosocial consequences [51]. These consequences are particularly devastating for Black patients who are disproportionately affected by pain, have worse pain-related outcomes, and often face other social hardships (e.g., financial, occupational) [51]. Socioeconomic status (SES) also affects pain perception and treatment. Low SES is associated with greater pain and disability across conditions and settings [3,15,34,39,52,64,84,94], and this relationship is independent of race [31]. Low SES patients are also at increased risk for poor pain care. They are less likely to receive thorough and accurate clinical assessments, as well as guideline-concordant treatment [9,20,53,55,62,75]. Low SES patients are also especially vulnerable to the deleterious consequences of under-treated pain, given that these patients are already at increased risk for all-cause morbidity and mortality [33,65,89,101] and face other psychosocial hardships.
Healthcare, including for pain, is influenced by providers’ ability to take the perspectives of their patients [91]. Perspective-taking is particularly important when treating racial minority and socioeconomically disadvantaged patients [17]. Consistent with the tenets of Intergroup Contact Theory (ICT), perspective-taking facilitates provider empathy and comfort in treating Black and low SES patients, thereby reducing biases and discrimination [80]. Research supports this notion [7,8,32], and experts have noted that enhancing provider perspective-taking is a promising strategy for improving the care of disadvantaged patients [17]. Moreover, increasing people’s awareness of their biases can motivate them to “correct” these biases [6,17]. Thus, if providers are made aware of situations in which disparity group members receive inferior care, they may be motivated to reexamine their initial treatment decisions for the possibility of bias and correct their decisions accordingly [17]. The impact of increased awareness is likely to be particularly powerful in this context because most providers self-identify as egalitarian, most are trained and aspire to provide equitable care, and the implications of such biases are meaningful. Thus, discovering discrepancies between their clinical decisions and their egalitarian ideals may instigate self-regulatory processes to bring their behavior in line with their attitudes [71,72].
Despite these theoretical tenets and empirical findings, provider-focused interventions to decrease health disparities are rare [22]. When interventions are designed, they are often simplistic (e.g., didactic lectures) and/or prescriptive (e.g., instructions to avoid stereotyping), yielding modest and sometimes counterproductive results [18]. Unfortunately, more potent interventions (e.g., role-playing, small group exercises) are often impractical for widespread provider engagement. It is in this context that we developed an innovative alternative intervention to reduce racial and SES disparities in pain treatment. The current intervention emerged from our prior work using virtual human (VH) technology and computer-simulated environments to examine providers’ biases and pain treatment disparities. Our previous studies established that these computer-simulations have high ecological validity, yield clinically-relevant data about biases and treatment disparities, and facilitate the efficient delivery of assessments and interventions that target providers [1,41-49,90,93,95-97,99]. The current study presents the results of a randomized controlled trial (RCT) testing an individually-tailored virtual perspective-taking intervention to reduce racial and SES disparities in pain treatment.
Methods
Overall design
All study procedures were approved by the institutional review board at Indiana University. The study was a 2-arm RCT and was conducted entirely online. The study flow diagram is presented in Figure 1. The study included two sessions, spaced one week apart. For each session, participants (“providers”) watched videos and read vignettes for 12 VH patients presenting with chronic back pain. The patients systematically varied by race (Black, White) and SES (low, high), and providers made pain assessment and treatment decisions for each patient. Based on real-time statistical analyses of their pain management decisions at Session 1, providers were classified into one of two groups: (1) no treatment bias, or (2) treatment bias. Providers in the ‘no treatment bias’ group were informed that they had concluded the study. Providers in the ‘treatment bias’ group were immediately randomized to a control or intervention group. For providers in the control group, the procedures for Session 1 were concluded. Providers in the intervention group were immediately directed to the virtual perspective-taking intervention where they were given feedback about their treatment biases and engaged in individually-tailored, dynamic interactions with two VH patients. Following the one-week interval, providers in both groups (control and intervention) completed Session 2. This session was similar to the first one in that providers made pain management decisions for 12 VH patients with chronic pain who varied by race and SES. No feedback or intervention was provided in Session 2.
Fig. 1.
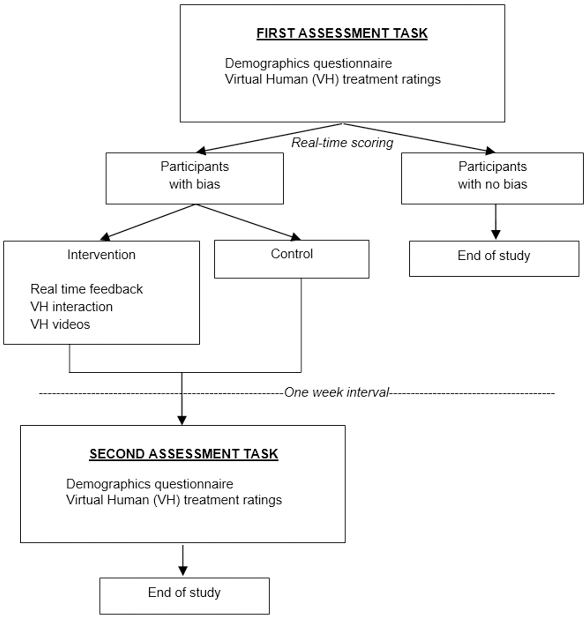
Study flow diagram
Participant recruitment
Physician residents and fellows were recruited from university-affiliated training programs across the United States. Recruitment occurred primarily through email. Program administrators were contacted, provided standardized study announcement information (stating that the study purpose is to better understand how healthcare providers make decisions for patients with pain), and encouraged to distribute the announcement to residents/fellows through program listservs. Snowball sampling procedures were also used for recruitment. To be eligible, providers had to be 18 years of age or older, currently enrolled in a physician residency or fellowship program, and able to read and write in English. Inclusion criteria also required that providers had access to a personal computer with high-speed internet. Individuals were excluded if they had previously participated in a study using VH technology to investigate pain decision-making. Individuals who were interested in participating were instructed (in the study announcement) to contact the research team via email or phone. During this correspondence, potential participants were provided additional information about the study procedures and screened for eligibility. Those who were interested and eligible were provided unique login credentials to access the study website.
Session 1
After logging into the study website, providers completed informed consent procedures, followed by a demographics questionnaire assessing personal and professional characteristics. Providers then completed the VH decision-making assessment task.
Measures
Demographics questionnaire
Providers reported their sex, age, race/ethnicity, state of residence, current income, and parental income. They also provided information about their medical training program, including specialty, clinical experience, and experience with pain.
Pain treatment decision task
Virtual patient stimuli
Providers were presented full-motion videos and text vignettes of patients with chronic pain (Figure 2). The videos consisted of animated virtual patients from our catalogue of standardized and validated patients that have been used in prior studies. The virtual patients were created with AutoDesk Character Generator, which allows developers to create characters by adjusting the anthropometric parameters of human faces; parameters that are reliably different between racial groups were incorporated into the algorithm. Race was represented visually by White and Black patients. SES was represented visually by work attire – low SES patients wore clothing consistent with a low income/prestige job (e.g., fast food uniform), and high SES patients wore clothing consistent with a high income/prestige job (e.g., business suit). Similar visual representations have been used in prior studies [29,102]. We pilot tested these stimuli to ensure the validity of our representations. With the exception of patient race and SES, other demographic characteristics were balanced across patients. All patients dynamically expressed a high level of pain in the videos; we achieved this by manipulating the facial and bodily features to be characteristic of non-verbal expressions of pain [25,59,82]. Character Generator was used to create polygonal models, and a professional animator created the patient movements and pain behavior.
Fig. 2.
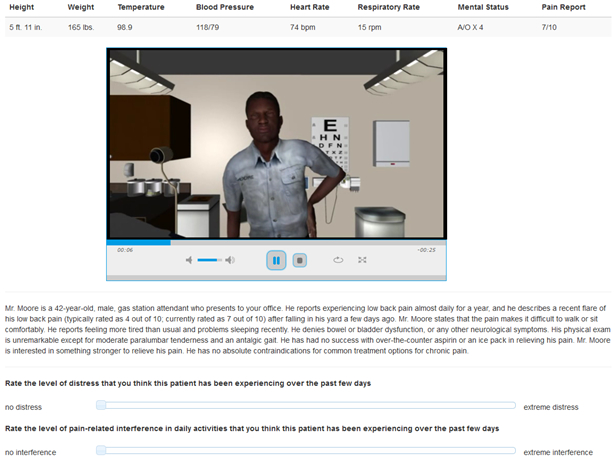
Interface for the pain treatment decision task
Text vignettes accompanied each patient video. Patients were described as having chronic low back pain for over 1 year that was recently aggravated while performing a common lifting/moving/carrying behavior. Specific features of the pain were described, including objective findings, exacerbating/palliating factors, and medical history. The patients’ usual (i.e., chronic) and recent (i.e., aggravated) pain intensities were noted to be in the moderate and severe range, respectively [13]. Vital signs varied minimally across patients and were always within normal limits. The specific text (e.g., patient name, details of the recent aggravation) also varied to increase study realism and provider engagement; however, with the exception of SES (i.e., occupational type) this information was equivalent across patients. Because occupational status is commonly used as an indicator of SES [69], patient SES was represented by text describing patients’ occupation; these descriptions were consistent with the work attire worn by patients in the accompanying videos. Low SES patients were stated to be employed in low income/prestige jobs, such as food service or retail cashier. High SES patients were stated to be employed in high income/prestige jobs, such as business executive or software engineer. The specific occupations were selected according to the Nam-Powers-Boyd Occupational Status Scale [74].
Four unique patients were needed to represent each combination of race and SES. We tripled this number so that providers viewed and made decisions for 12 unique patients. This permitted us to present each cue combination 3 times (2 male patients and 1 female patient), which increased task sensitivity and statistical power. Our extensive prior work [1,24,27,41-49,54,83,86,90,93,95-99] supports the ecological validity of the virtual patient stimuli. This work has established that diverse participants (laypersons, trainees, and providers) (1) consider the expressions to approximate real depictions of pain, the vignettes to reflect real clinical scenarios, and the contextual information provided in the visual and written stimuli to be similar to real clinical settings; and (2) respond to and treat virtual patients similar to how they make decisions for real patients, demonstrating high correlations between their communication patterns with virtual and real humans.
Provider decisions
For each patient, providers made four treatment decisions: (1) oral opioid analgesic, (2) referral to a pain specialist, (3) physical therapy, and (4) oral non-opioid analgesic. For these treatments, providers were prompted to: “Rate the likelihood that you would use the following treatments to relieve the patient’s pain.” Treatment decisions were registered on separate 0–100 VASs anchored by “not at all likely” on the left and “very likely” on the right. We have used similar VASs to assess pain treatment decisions in prior work. Providers also used 0–100 VASs to rate the extent to which they felt compassion towards the patients (“not at all” to “extremely”), and their level of comfort in providing care for the patients (“not at all comfortable” to “extremely comfortable”).
Statistical analyses – Session 1
The programming code included real-time statistical analysis of providers’ treatment decision data during Session 1. This allowed us to model treatment decisions at the individual provider level, identify which providers demonstrated a treatment bias, characterize the specific nature of these biases, and randomize providers to the control group or to an individually-tailored intervention – all of this occurred in real-time during Session 1. To determine treatment bias, statistical analyses occurred at the individual provider level, similar to our prior work. We examined providers’ cue use (i.e., the extent that patient race and/or SES predicted treatment decisions) in making pain treatment decisions across the 12 patients. Repeated measures ANOVAs (rmANOVAs) were computed for each provider on each treatment outcome variable (opioid analgesic, referral to a pain specialist, physical therapy, and non-opioid analgesic). Patient race and SES were the independent variables in these analyses. Consistent with prior work, including the Wandner et al. [98] intervention study, a p threshold of .10 was used to determine statistically reliable cue use (i.e., treatment bias). Providers who had at least one individual-level rmANOVA that met this threshold were included in the ‘treatment bias’ group and randomized to the intervention or control; all other providers were included in the ‘no treatment bias’ group and were finished with the study.
Bias determination for the ‘treatment bias’ group
Because providers made decisions across 4 treatment modalities for each patient, treatments were rank ordered to determine the nature of providers’ bias. Based on prior work indicating that pain treatment disparities are particularly prominent for opioid analgesics [68], this treatment was assigned the highest priority when determining provider bias. Referral to a pain specialist was assigned the next highest priority because this option suggests that the referring provider considers the patient’s pain to be legitimate, severe, and/or in need of specialty care [77,87]. Previous work suggests that providers’ perceptions of legitimacy vary across patient demographic groups [50,51], thus, making this treatment domain (i.e., specialty referral) susceptible to biased decision-making. The next highest priority was assigned to physical therapy, followed by non-opioid analgesics. Although consistent with evidence-based guidelines for chronic pain care, these treatments are considered less potent (particularly compared to opioids) and, thus, may not elicit biased decision-making as readily as the other treatment modalities.
Providers in the ‘treatment bias’ group were randomized to the intervention or control. For providers randomized to the intervention, the nature of their treatment biases were determined so that the intervention could be tailored accordingly. The simplest case involves a provider who had one individual-level rmANOVA indicating statistically-reliable cue use for one treatment decision. In this case, the programming code characterized the nature of this cue use (i.e., which race or SES group received a lower treatment likelihood rating), and the provider received the corresponding intervention. For example, a provider who gave lower opioid ratings to Black than White patients would receive the intervention involving Black patients – that is, they would receive feedback that they gave lower treatment ratings to Black patients, and they would interact with two virtual Black patients to better understand how pain has impacted their lives (see below). A more complex case involves a provider who had more than one rmANOVA indicating cue use. If a provider’s results involved the same patient cue and were all in the same direction (e.g., the provider gave consistently lower treatment ratings to Black than White patients), the provider would receive the tailored intervention as described in the previous example. However, if a provider’s results involved different patient cues (e.g., the provider gave lower ratings to low SES patients for some treatments and lower ratings to Black patients for other treatments), the treatment priority ranking system described above would be implemented to determine which intervention was delivered. Specifically, the rmANOVA results for the highest priority treatment that elicited statistically-reliable cue use would be used to determine the intervention. Thus, each participant randomized to the intervention arm would receive a tailored intervention aimed at reducing race bias or SES bias based upon which bias was ranked greater using this priority ranking system.
Intervention
Providers who did not demonstrate a statistically reliable treatment bias at Session 1 were informed that they had concluded the study; they were debriefed about the study purpose and compensated with a gift card for an online retailor. Providers who demonstrated a statistically reliable treatment bias at Session 1 were immediately randomized to a control or intervention group. The control group received on-screen instructions stating that their participation in the first part of the study was complete. They were also told to access the study website again in 1 week, and that they would receive follow-up email reminders for this purpose.
The intervention group proceeded to the next series of tasks, all of which occurred during Session 1. First, providers received individualized feedback about the nature of their treatment bias demonstrated in the just-completed pain decision task. This feedback was delivered via on-screen text, similar to a previous study involving tailored feedback about participants’ treatment biases [98].
Next, providers in the intervention group engaged in real-time, dynamic interactions with 2 virtual patients – 1 man and 1 woman. Figure 3 presents a static image of the virtual patient interaction interface. Each interaction was one-on-one between provider and patient and was individually-tailored according to providers’ specific treatment bias as described above. For example, a provider who demonstrated a treatment bias against Black patients in Session 1 would interact with 2 different Black patients (1 man and 1 woman) during the intervention. These interactions occurred in succession, and the order was counterbalanced across providers. Providers were instructed that the purpose of these interactions was to gain a better understanding of the patient’s pain condition and how it affects their life, particularly regarding social, occupational, and family domains.
Fig. 3.
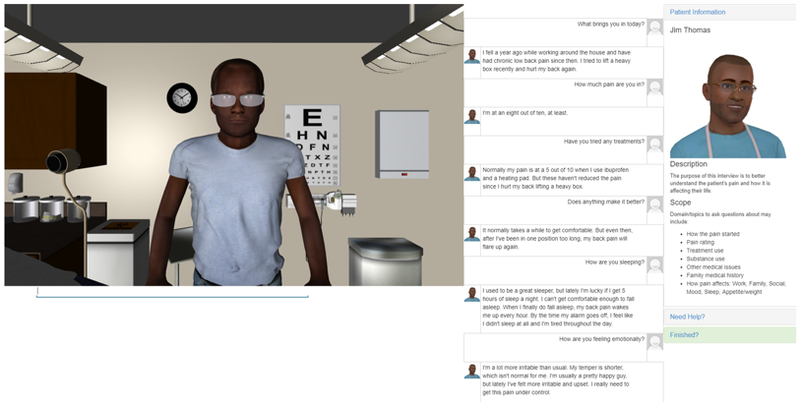
Interface for the perspective-taking intervention
The interaction used a typed interface to a 3D virtual patient presented in a web browser using the Virtual People Factory (VPF) system developed by the research team. Providers interacted with 3D rendered and animated patients who display gestural and facial expressions that are triggered by providers’ questions/statements (“inputs”). The provider-to-patient interaction is through typed questions/statements similar in format to instant messaging. For example, if a provider types “How is your pain today?” the patient replies “I’m at a seven out of ten, at least.” with both on-screen text and fully animated lip-synced audio.
The patient-to-provider interaction uses an un-annotated corpus retrieval approach consisting of keyword matching to find a list of corpus stimuli that are most similar to input stimuli [27,61,86]. We developed the initial stimuli based on our experience working in clinical settings with chronic pain patients, as well as our research and applied experience with VH interactions. These stimuli were then refined through a systematic, iterative process involving crowdsourcing through the Amazon Mechanical Turk platform [14]. This crowdsourcing process uses non-experts to transform a clinical case description into a virtual patient script. Crowdsourcing involves dividing one large task – e.g., creating a virtual patient script – into a series of smaller tasks that can be distributed across a network of people working in parallel. This distribution process has been demonstrated to be a valid and cost-effective method of script development for virtual patients [14].
The ultimate goal of the script development process is for the virtual patient to give the correct response (“output”) to the diverse array of domain-specific questions/statements (“inputs”) from the provider. For example, in the above interaction, the provider asked “How is your pain today?” This is only one of the many ways a provider might inquire about a patient’s current pain. A different provider might elicit this information by asking “What is your pain like right now?” whereas another provider might request the patient to “Rate your current pain.” Thus, our aim was to develop an interaction script such that when a provider inquires about the patient’s pain – using any number of differently-worded inputs – the patient would respond with the single correct output; in this case, “I’m at a seven out of ten, at least.”
By accumulating inputs from a large distribution network of non-experts, our crowdsourcing approach produces a script that is robust to a diverse array of inputs. The final interaction script for the current trial represented the common and important domains of provider-patient interactions for chronic pain, including general medical history, pain complaint, treatment experience, and physical, psychological, and social functioning, as well as information about family, work, and recreational activities. The final interaction script yielded high conversational accuracy in the current study – both the male and female patient scripts produced a correct output for over 80% of user inputs. In the event that a user input failed to elicit a patient output, a drop-down menu appeared on the screen, listing an array of topical domains from which the user selects the one that matches the domain of their intended input. This drop-down menu consisted of exact questions/statements from the virtual patient script so as to guarantee an accurate patient response. After a question/statement was chosen from the drop-down menu, the patient then responded with the appropriate output, and the interaction proceeded.
In addition to these one-on-one interactions, providers in the intervention group viewed videos depicting how the patients’ daily lives are affected by pain. Five videos, approximately 20 seconds each, were presented during the provider-patient interactions. These videos showed the patient struggling to engage in valued life activities on account of his/her pain. The following domains were depicted: performing household activities, attending recreational events involving the patient’s children, performing work-related activities, engaging in social activities with friends, and assisting an elderly parent with household activities. The specific behaviors depicted in the videos were matched to the specific patients in the interaction. When the provider inquired about one of the 5 domains of life depicted in the videos, the patient responded with on-screen text and audio as described above. While the patient responded, a pop-up video appeared showing how pain has impacted that particular domain of life. At the conclusion of the video, the provider-patient interaction resumed.
The videos were created using the Sims 4 gaming platform and iMovie. The Sims 4 [92] is a life simulation game in which players create and maintain Sims – virtual characters that have needs similar to those of humans in the real world, such as eating, going to work, and socializing with others. In addition to normal gameplay, Sims 4 users can also create videos of their Sims, which can be saved and edited in film-editing software. Edited Sims 4 videos have previously been used in virtual patient interactions as a way to build virtual patients with “backstory” and have been linked to greater empathy in subsequent interviews with standardized patients [24].
Each provider-patient interaction was 10-minutes long. At the end of 10 minutes, providers were informed with a pop-up message that the interaction had concluded. Providers who had prompted all 5 of the patient videos were then advanced to the next patient interaction (if they had just completed the first patient interaction) or to the next phase of the study (if they had just completed the second patient interaction). Providers who did not cue all of the patient videos were instructed to direct their attention to a column on the right side of the screen. This column presented separate buttons labeled with the life domains for the videos that were not cued in the previous interaction. Providers were instructed to click these buttons to view the videos and listen to the accompanying patient descriptions. Once all the videos had been viewed, providers were advanced to the next task as described above.
At the conclusion of the intervention phase of the study, providers in this group were informed that their participation in the first part of the study was complete. Similar to the control group, they received instructions on how to access the study website again in 1 week and were informed that they would receive follow-up email reminders.
Session 2
One week after participating in Session 1, providers (intervention and control) logged back into the study website to compete Session 2. Website access was restricted until one week had elapsed. The study procedures for Session 2 were similar to those for Session 1; however, no intervention or feedback was provided. The same pain treatment decision task used in Session 1 was used in Session 2, such that providers made pain treatment decisions for 12 unique virtual patients who varied by race and SES. The patient videos and text vignettes paralleled those used in Session 1; however, different patients were used in Session 2. The same treatment decisions were made in both sessions. At the conclusion of Session 2, providers were debriefed about the purpose of the study and compensated with a gift card for an online retailor.
Statistical analyses – Session 2
The same analyses involving rmANOVAs, as well as the same bias determination procedure, as used in Session 1 were used in Session 2.
Statistical analyses – Intervention efficacy
Logistic regression analyses were conducted to test the effects of the intervention on change in pain treatment bias from Session 1 to Session 2. Based on previous literature indicating that Black and low SES patients are at particular risk for suboptimal pain care, we focused on intervention effectiveness in reducing treatment bias against Black and low SES patients. A logistic regression was fitted with the binary variable of treatment bias against Black patients in Session 2 (yes/no) as the outcome variable, and treatment bias against Black patients at Session 1 (yes/no) and group assignment (tailored intervention/control) as predictor variables. Whether providers received a different intervention for treatment bias (yes/no) was also included as a predictor variable, which allowed us to determine whether another type of intervention (e.g., intervention for SES bias) affected providers’ treatment decisions for Black patients. For SES, a parallel logistic regression model was fitted with the binary variable of treatment bias against low SES patients in Session 2 (yes/no) as the outcome variable, and treatment bias against low SES patients at Session 1 (yes/no), group assignment (tailored intervention/control), and other intervention (yes/no) as predictor variables.
Results
Sample characteristics
Five hundred two providers were recruited for Session 1 of the study. Excluding those who did not complete the decision-making task or did not meet eligibility requirements yielded a final sample of 436 providers who completed Session 1 (Figure 4). Of the 436 participants, 255 (58.5%) were male, and the mean (SD) age was 29.7 years (3.1). In terms of race, 297 (68.1%) were White, 110 (25.3%) Asian, 9 (2.1%) Black, and 19 (4.4%) other; 1 did not report race. Twenty (4.6%) participants were Hispanic. The highest represented clinical specialties included primary/internal/family medicine (17%), pediatrics (17%), anesthesiology (16%), and physiatry (9%). Providers reported a moderate amount of clinical experience with pain, with a mean (SD) of 41.4 (23.7) on a 0 (“not at all experienced”) to 100 (“very experienced”) visual analogue scale.
Fig. 4.
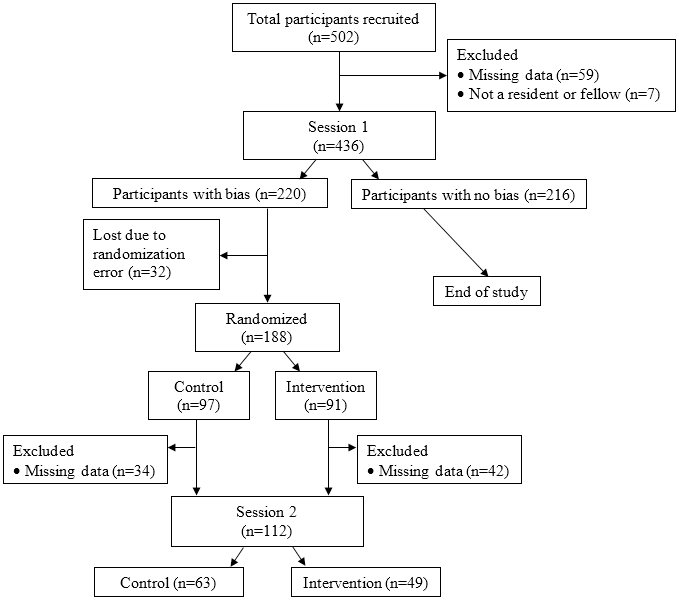
Consort diagram for participant enrollment
Session 1 treatment bias
Results of the individual-level rmANOVAs indicated that 220 providers (approximately 50% of total sample) demonstrated a statistically-reliable treatment bias during the pain treatment decision task at Session 1. The distribution of providers in the ‘no treatment bias’ and ‘treatment bias’ groups did not differ by provider sex (χ2(1)=0.11, p=0.75) or race (White vs. Asian vs. all others; χ2(2)=0.61, p=0.74), nor did the groups differ in age (F(1,434)=1.05, p=0.31) or clinical experience with pain (F(1,434)=1.02, p=0.31). Due to an error in the randomization code, 32 providers with a treatment bias did not get randomized to the intervention or control group, resulting in 188 providers who were randomized (Figure 4).
Session 2 treatment bias
One-hundred twelve providers completed the pain treatment decision task at Session 2 (Figure 4). Results of the individual-level rmANOVAs indicated that 59 of these providers demonstrated a treatment bias at Session 2. This represents 53% of the 112 providers who showed a treatment bias at Session 1, were randomized, and completed Session 2. In other words, 47% of providers who were biased at baseline and were randomized did not show a statistically-reliable treatment bias one week later.
Intervention efficacy
Providers who were randomized to the intervention or control group, and who completed both Sessions 1 and 2, did not differ on key demographic or clinical characteristics (Table 1). Results of the logistic regression models testing the effects of the intervention on pain treatment bias are presented in Table 2. Compared to the control group, providers who received the tailored intervention for bias against Black patients had an 85% (1 – exp [–1.875]) lower odds of demonstrating a treatment bias against Black patients in Session 2. A similar, though smaller effect, was observed for SES bias. Compared to the control group, providers who received the tailored intervention for bias against low SES patients had a 76% (1 – exp [–1.420]) lower odds of demonstrating a treatment bias against low SES patients in Session 2.
Table 1.
Sample characteristics stratified by intervention arm
| Control | Intervention | p-value | |
|---|---|---|---|
| N (%) | 63 (56%) | 49 (44%) | |
| Sex | 0.28 | ||
| Female, n (%) | 22 (35%) | 22 (45%) | |
| Male, n (%) | 41 (65%) | 27 (55%) | |
| Race | 0.30 | ||
| White, n (%) | 48 (76%) | 33 (67%) | |
| Other, n (%) | 15 (24%) | 16 (33%) | |
| Age | 0.31 | ||
| Mean (SD) | 29.7 (2.8) | 29.2 (2.2) | |
| Clinical experience with pain | 0.60 | ||
| Mean (SD) | 46.7 (24.5) | 44.4 (21.3) |
Table 2.
Logistic regression analyses for intervention effects on overall bias
| Bias Against Black Patients | Bias Against Low SES Patients | |||||
|---|---|---|---|---|---|---|
| Estimate | OR | p-value | Estimate | OR | p-value | |
| (Intercept) | −1.53 | -- | <.00 | |||
| Race bias S1 | 0.38 | 1.46 | .28 | |||
| Int. Race | −1.88 | 0.15 | .06 | |||
| Int. Other | 0.54 | 1.72 | .17 | |||
| (Intercept) | −2.80 | -- | <.00 | |||
| SES bias S1 | 1.85 | 6.34 | .01 | |||
| Int. SES | −1.42 | 0.24 | .08 | |||
| Int. Other | 1.13 | 3.09 | .07 | |||
Race bias S1 – categorical race bias score at session one; SES bias S1 – categorical SES bias score at session one; OR – odds ratio; Int. Black – tailored intervention for race bias; Int. SES – tailored intervention for SES bias; Int. Other – non-race/SES intervention.
To better understand the nature of these effects, we conducted a series of linear models that were similar to the logistic models described above. However, whereas the logistic models tested the intervention effects on change in overall treatment bias, these linear models tested the intervention effects on change in the individual treatments. Specifically, these models examined the extent to which providers in the intervention vs. control groups showed differential change in their individual treatment ratings (opioid, pain specialist referral, physical therapy, non-opioid analgesic) from Session 1 to Session 2 (Table 3). Compared to the control group, providers who received the tailored intervention for bias against Black patients demonstrated a greater increase in their use of opioids (coefficient=9.59, SE=3.90) and pain specialist referral (coefficient=7.63, SE=5.05) for Black patients. No group differences emerged for change in providers’ use of physical therapy or non-opioid analgesics. Providers who received the tailored intervention for bias against low SES patients demonstrated a greater increase in their use of non-opioid analgesics (coefficient=6.79, SE=4.16) for low SES patients than did the control group, but no group differences were found for change in the other treatments.
Table 3.
Linear regression analyses for intervention effects on individual treatments
| Bias Against Black Patients | Bias Against Low SES Patients | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Estimate | SE | p-value | Estimate | SE | p-value | |
| Opioid | (Intercept) | 6.13 | 2.35 | .01 | |||
| Black rating S1 | 0.82 | 0.06 | <.00 | ||||
| Int. Race | 9.59 | 3.89 | .01 | ||||
| Int. Other | −6.22 | 2.71 | .02 | ||||
| (Intercept) | 6.81 | 2.45 | .00 | ||||
| SES rating S1 | 0.81 | 0.06 | <.00 | ||||
| Int. SES | −3.19 | 3.68 | .19 | ||||
| Int. Other | −1.42 | 2.94 | .32 | ||||
| Pain Specialist | (Intercept) | 9.84 | 2.76 | <.00 | |||
| Black rating S1 | 0.81 | 0.17 | <.00 | ||||
| Int. Race | 7.63 | 5.05 | .07 | ||||
| Int. Other | −5.53 | 3.52 | .06 | ||||
| (Intercept) | 8.87 | 2.75 | .00 | ||||
| SES rating S1 | 0.81 | 0.07 | <.00 | ||||
| Int. SES | 1.77 | 4.54 | .35 | ||||
| Int. Other | −0.67 | 3.63 | .43 | ||||
| Physical Therapy | (Intercept) | 8.75 | 5.43 | .06 | |||
| Black rating S1 | 0.81 | 0.07 | <.00 | ||||
| Int. Race | 3.96 | 5.24 | .23 | ||||
| Int. Other | 2.67 | 3.70 | .24 | ||||
| (Intercept) | 6.90 | 5.36 | .10 | ||||
| SES rating S1 | 0.84 | 0.07 | <.00 | ||||
| Int. SES | 0.19 | 4.84 | .48 | ||||
| Int. Other | 1.63 | 3.87 | .34 | ||||
| Non-opioid | (Intercept) | 16.90 | 8.90 | .03 | |||
| Black rating S1 | 0.77 | 0.10 | <.00 | ||||
| Int. Race | 0.99 | 4.79 | .42 | ||||
| Int. Other | 1.42 | 3.34 | .34 | ||||
| (Intercept) | 14.70 | 8.93 | .05 | ||||
| SES rating S1 | 0.80 | 0.10 | <.00 | ||||
| Int. SES | 6.79 | 4.16 | .05 | ||||
| Int. Other | −1.25 | 3.33 | .71 | ||||
Race bias S1 – treatment rating for Black patients at session one; SES bias S1 – treatment rating for low SES patients at session one; SE – standard error of the estimate; Int. Black – tailored intervention for race bias; Int. SES – tailored intervention for SES bias; Int. Other – non-race/SES intervention.
Finally, we tested the theoretical underpinnings of our intervention – based on Intergroup Contact Theory (ICT) – that perspective-taking would facilitate provider empathy and comfort in treating Black and low SES patients. Two linear models were conducted employing a similar approach as used for the individual treatments above. One model examined the extent to which providers in the intervention vs. control groups showed differential change in their compassion ratings for Black and low SES patients, and the other model examined differential change in providers’ comfort level treating Black and low SES patients (Table 4). For bias against Black patients, providers who received the tailored intervention reported a greater increase in their compassion compared to the control group (coefficient=8.12, SE=3.78) but no differential change in comfort level. No group differences emerged for the low SES analyses.
Table 4.
Linear regression analyses for intervention effects on pain judgments
| Bias Against Black Patients | Bias Against Low SES Patients | ||||||
|---|---|---|---|---|---|---|---|
| Judgment | Estimate | SE | p-value | Estimate | SE | p-value | |
| Compassion | (Intercept) | 17.96 | 4.87 | <.00 | |||
| Black rating S1 | 0.71 | 0.08 | <.00 | ||||
| Int. Race | 8.12 | 3.78 | .02 | ||||
| Int. Other | −0.03 | 2.64 | .10 | ||||
| (Intercept) | 15.20 | 4.80 | .00 | ||||
| SES rating S1 | 0.75 | 0.07 | <.00 | ||||
| Int. SES | −3.03 | 3.34 | .18 | ||||
| Int. Other | 4.65 | 2.67 | .04 | ||||
| Comfort | (Intercept) | 13.47 | 4.24 | .00 | |||
| Black rating S1 | 0.75 | 0.06 | <.00 | ||||
| Int. Race | −1.27 | 3.98 | .38 | ||||
| Int. Other | 6.81 | 2.78 | .01 | ||||
| (Intercept) | 15.77 | 4.46 | .00 | ||||
| SES rating S1 | 0.73 | 0.06 | <.00 | ||||
| Int. SES | 4.41 | 3.90 | .13 | ||||
| Int. Other | 3.11 | 3.12 | .16 | ||||
Race bias S1 – judgment rating for Black patients at session one; SES bias S1 – judgment rating for low SES patients at session one; SE – standard error of the estimate; Int. Black – tailored intervention for race bias; Int. SES – tailored intervention for SES bias; Int. Other – non-race/SES intervention.
Discussion
Black and low SES individuals are at risk for suboptimal pain care [50,55,62,68,75], yet few interventions have been tested to reduce these disparities. We conducted an RCT of an individually-tailored virtual perspective-taking intervention to reduce pain treatment bias in providers. Compared to the control group, providers who received the tailored intervention had 85% lower odds of demonstrating a treatment bias against Black patients and 76% lower odds of demonstrating a treatment bias against low SES patients. Providers who received the intervention for racial bias showed increased compassion compared to providers in the control condition. Group differences did not emerge for provider comfort. Results suggest that an online intervention tailored to providers according to individual treatment biases, delivers feedback about these biases, and provides opportunities for increased contact with Black and low SES patients, can produce substantial changes in providers’ treatment decisions resulting in more equitable pain care.
The results are encouraging given the longstanding nature of pain disparities, particularly for Black individuals. At the patient level, Black and low SES individuals may be more reluctant to take opioids due to concerns about addiction, side effects, and safe storage [12,70]. At the systems level, Black and low SES patients face considerable barriers to accessing pain providers [50,51], which is compounded by the fact that pharmacies in predominately nonwhite and poorer neighborhoods are less likely to stock analgesics [35,67,73]. Providers also contribute to disparities when their pain-related decisions systematically vary by patient race and SES. We intervened at this level because provider bias is a major contributor to pain disparities [17,18], and providers have rarely been targeted in this context. The potential impact of a positive effect at the provider level is substantial – a single provider may treat thousands of patients in a career.
The dearth of empirically-tested interventions addressing provider bias in pain care is surprising. Drwecki et al. [28] is a notable exception. In that laboratory-based study, graduate-level nursing students (n=40) watched videos and made treatment decisions for Black and White patients undergoing a painful shoulder exam [63]. The intervention group was instructed to “imagine how each of your patients feels while you are examining them” and “imagine how your patient feels about his or her pain and how this pain is affecting his or her life.” The control group was instructed to make the “best, most accurate treatment decisions for each patient.” Results indicated that the control group showed a treatment bias favoring Whites, whereas the intervention group did not [28]. The Drwecki intervention was verbally-based and delivered to all participants regardless of bias. These features facilitate a brief and easily-delivered format; however, they lack the richness and individual-tailoring of our intervention. These differences may explain why our intervention increased provider compassion whereas Drwecki’s did not, as well as the larger overall effect observed in the current study (85% and 76% reductions in race and SES bias, respectively) than in the Drwecki study (55% reduction in race bias).
Although the tailored intervention reduced the odds of bias against low SES patients, the effects were less potent than for race – the only rating that changed was for non-opioid treatment. Potential explanations are that low SES bias is more entrenched or the intervention failed to impact compassion and comfort treating low SES patients. Two implications warrant further research. First, our intervention may lack the key ingredients to enhance compassion and comfort with low SES patients. Future studies may focus on identifying optimal ways to elicit such changes. Second, ICT may apply less to SES bias and, hence, compassion and comfort are not key intervention targets to improve pain care for low SES patients. The foundation and application of ICT has largely focused on race relations [79,81]. Future studies may determine whether ICT principles apply to SES disparities in pain care, as well as other theories (e.g., ambivalence-amplification, mutual differentiation) that may contribute important insights to understanding and reducing these disparities [40,57].
The current intervention was based on research and theory suggesting that feedback is necessary but insufficient to decrease treatment bias [47,48,98,100] and that interpersonal contact is needed to elicit these changes [16,79]. Facilitating “real-life” contact is difficult. By using an online platform and high-fidelity, computer-simulated patients, the current intervention sidestepped many practical constraints. Our intervention is also noteworthy for its methodological rigor. The provider-patient interaction was controlled and topically-focused, thus, avoiding another limitation of live contact – divergence from the intended focus – that dampens the effect [4,38,76,78]. Despite this increased control, our intervention allowed for individualized interactions where providers could elicit information in ways that are natural for them. We acknowledge the virtual interaction does not capture the full complexity of human-human contact. Nevertheless, we are encouraged by the effects of this first trial, especially for racial bias.
Another advantage of our intervention is it provided video supplements to patients’ verbal report. We consider these to be potent perspective-taking facilitators, allowing providers to better understand how pain affects patients’ lives. These videos highlight a distinction between two perspective-taking orientations. Self-oriented perspective-taking involves “me” (i.e., the provider) imagining what it is like to be me in “your” (i.e., the patient) situation. It is a form of identification [11], akin to sympathy [23,26]. Providers who adopt this perspective assume the thoughts and feelings of patients are synonymous with their own (“I know how you feel. I know what you’re going through.”), which can interfere with true appreciation of what it is like to live with pain from the patient’s perspective. Self-orientation also focuses provider attention on their own distress, leading to social distancing in an attempt at emotion regulation [23]. It may also increase burnout [58], which is common in pain providers [60,85]. In contrast, other-oriented perspective-taking involves imagining what it is like to be the patient living in the patient’s world. It requires mental flexibility, emotion regulation, and willingness to prioritize the patient’s viewpoint. It also requires historical-contextual knowledge of the patient’s experience – the “tell” portion of the provider-patient interaction imparts this information. Other-oriented perspective-taking is a decentering process that privileges the patient’s perspective while preserving the self(provider)-other(patient) boundary [37]. Enhancing providers’ other-oriented perspective is important in the context of pain disparities. Providers are typically affluent White individuals [19,56] and, thus, may have had little contact with racial minority and low-income individuals outside of healthcare settings. Indeed, segregation along race and socioeconomic lines remains a prominent feature of contemporary life in America [5,10,21,30,36,66]. Thus, providers may have a particularly difficult time achieving and maintaining an other-orientation with minority and low-income patients. A scalable perspective-taking intervention such as ours is ideal in this context.
Further examination of the results yielded partial support for central tenets of Intergroup Contact Theory – i.e., that interacting with members of the group against whom one is biased would facilitate increased perspective-taking, thereby leading to greater empathy for and comfort level with these individuals. The intervention moved the needle on provider empathy (i.e., compassion) for Black patients but did not enhance their comfort treating Black or low SES patients. Compassion is a foundational component of quality healthcare [88], enshrined in Principle 1 of the American Medical Association Code of Ethics: “A physician shall be dedicated to providing competent medical care, with compassion and respect for human dignity and rights” [2]. Research and theory indicate that humans experience less compassion for different vs. similar others [26], as in the case of affluent White providers treating racial minority and socioeconomically-disadvantaged patients. We interpret our findings that by hearing (from patients themselves) and seeing (with one’s own eyes) how pain impacts patients’ lives, our intervention increased providers’ compassion. What then is to be made about the lack of change in provider comfort? In hindsight, these results are not altogether surprising. The intervention was not skills-based per se. It did not include tips or activities specifically intended to enhance patient care. Interventions that combine perspective-taking and skills-based components may be needed to enhance both compassion and comfort level among providers, thereby yielding even greater effects than observed herein.
The current study has a number of advantages in addition to those noted above. The sample was geographically diverse, including providers from across the US. The trial was efficient from a resource-allocation standpoint in that we tested the intervention among the subset of providers who demonstrated treatment bias. Several limitations should also be noted. Although representative of physicians in the US, our sample lacked diversity across race and ethnic categories. The sample also consisted of physician residents and fellows who, despite being licensed to provide medical care, may differ from independent physicians who are not in training.
Several empirical questions are in need of future study. Do these effects translate to real-world patient care? What is the optimal timing and dose of the intervention? What is the long-term effectiveness and/or are follow-up “booster sessions” needed to sustain the effects? Should the intervention target only providers who evince a treatment disparity or be delivered to all providers, as a prevention strategy? These outstanding issues speak to the nascent nature of intervention research on pain disparities. Continued work in this area is vital to improving the lives of patients with pain.
Supplementary Material
Acknowledgments
The authors thank Shankar Manamalkav for his excellent technical assistance in the development and implementation of this project. Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD008931. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. There are no conflicts of interest that might be seen as influencing or prejudicing the research.
References
- [1].Alqudah AF, Hirsh AT, Stutts LA, Scipio CD, Robinson ME. Sex and race differences in rating other’s pain, pain-related negative mood, pain coping, and recommending medical help. J Cyber Ther Rehabil 2010;3:63. [PMC free article] [PubMed] [Google Scholar]
- [2].American Medical Association. Code of Medial Ethics overview. 2019. Available at: https://www.ama-assn.org/delivering-care/ethics/code-medical-ethics-overview. Accessed March 12, 2019.
- [3].Andersson HI, Ejlertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain 1993;9:174–182. [DOI] [PubMed] [Google Scholar]
- [4].Ashton LJ, Gordon SE, Reeves RA. Key ingredients—target groups, methods and messages, and evaluation—of local-level, public interventions to counter stigma and discrimination: a lived experience informed selective narrative literature review. Community Ment Health J 2018;54:312–333. [DOI] [PubMed] [Google Scholar]
- [5].Bader MD, Warkentien S. The fragmented evolution of racial integration since the civil rights movement. Sociol Sci 2016;3:135–166. [Google Scholar]
- [6].Batson C The altruism question: toward a social-psychological answer. Hillsdale, NJ: Erlbaum, 1991. [Google Scholar]
- [7].Batson CD, Chang J, Orr R, Rowland J. Empathy, attitudes, and action: can feeling for a member of a stigmatized group motivate one to help the group? Pers Soc Psychol Bull 2002;28:1656–1666. [Google Scholar]
- [8].Batson CD, Polycarpou MP, Harmon-Jones E, Imhoff HJ, Mitchener EC, Bednar LL, Klein TR, Highberger L. Empathy and attitudes: can feelings for a member of a stigmatized group improve feelings toward the group? J Pers Soc Psychol 1997;72:105. [DOI] [PubMed] [Google Scholar]
- [9].Bigal ME, Buse DC, Chen YT, Golden W, Serrano D, Chu MK, Lipton RB. Rates and predictors of starting a triptan: results from the American Migraine Prevalence and Prevention Study. Headache 2010;50:1440–1448. [DOI] [PubMed] [Google Scholar]
- [10].Bischoff K, Reardon SF. Residential segregation by income, 1970–2009 In: Logan J, editor. Diversity and disparities: America enters a new century. New York: Russell Sage Foundation, 2014. pp. 208–233. [Google Scholar]
- [11].Bondi L Understanding feelings: Engaging with unconscious communication and embodied knowledge. Emot Space Soc 2014;10:44–54. [Google Scholar]
- [12].Booker SQ. African Americans’ perceptions of pain and pain management: a systematic review. J Transcult Nurs 2016;27:73–80. [DOI] [PubMed] [Google Scholar]
- [13].Boonstra AM, Preuper HRS, Balk GA, Stewart RE. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain 2014;155:2545–2550. [DOI] [PubMed] [Google Scholar]
- [14].Borish M, Lok B. Rapid low-cost virtual human bootstrapping via the crowd. ACM Trans Intell Syst Technol 2016;7:47. [Google Scholar]
- [15].Brekke M, Hjortdahl P, Kvien TK. Severity of musculoskeletal pain: relations to socioeconomic inequality. Soc Sci Med 2002;54:221–228. [DOI] [PubMed] [Google Scholar]
- [16].Burgess D, Van Ryn M, Dovidio J, Saha S. Reducing racial bias among health care providers: lessons from social-cognitive psychology. J Gen Intern Med 2007;22:882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Burgess DJ, Fu SS, van Ryn M. Why do providers contribute to disparities and what can be done about it? J Gen Intern Med 2004;19:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Burgess DJ, van Ryn M, Crowley-Matoka M, Malat J. Understanding the provider contribution to race/ethnicity disparities in pain treatment: insights from dual process models of stereotyping. Pain Med 2006;7:119–134. [DOI] [PubMed] [Google Scholar]
- [19].Castillo-Page L. Diversity in the physician workforce: facts and figures 2010. Washington, D.C.: Association of American Medical Colleges, 2010. [Google Scholar]
- [20].Chu MK, Buse DC, Bigal ME, Serrano D, Lipton RB. Factors associated with triptan use in episodic migraine: results from the American Migraine Prevalence and Prevention Study. Headache 2012;52:213–223. [DOI] [PubMed] [Google Scholar]
- [21].Clark WA. Changing residential preferences across income, education, and age: findings from the multi-city study of urban inequality. Urban Aff Rev 2009;44:334–355. [Google Scholar]
- [22].Clarke AR, Goddu AP, Nocon RS, Stock NW, Chyr LC, Akuoko JA, Chin MH. Thirty years of disparities intervention research: what are we doing to close racial and ethnic gaps in health care? Med Care 2013;51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coplan A, Goldie P. Empathy: philosophical and psychological perspectives. Oxfrod, UK: Oxford University Press, 2011. [Google Scholar]
- [24].Cordar A, Borish M, Foster A, Lok B. Building virtual humans with back stories: training interpersonal communication skills in medical students. In: Bickmore T, Marsella S, Sidner S, editors. Lecture notes in computer science. Proc. 14th International Conference on Intelligent Virtual Agents, Vol. 8637. Cham: Springer International Publishing, 2014. pp. 144–153. [Google Scholar]
- [25].Craig KD, Prkachin KM, Grunau RV. The facial expression of pain In: Turk D, Melzack R, editors. Handbook of pain assessment. New York: Guilford Press, 1992. pp. 257–276. [Google Scholar]
- [26].Davis M Empathy: A social psychological approach. New York: Routledge, 2018. [Google Scholar]
- [27].Dickerson R, Johnsen K, Raij A, Lok B, Hernandez J, Stevens A, Lind D. Evaluating a script-based approach for simulating patient-doctor interaction. In: Proceedings of the International Conference of Human-Computer Interface Advances for Modeling and Simulation, Vol. 1 New York, NY: Association for Computing Machinery, 2005. pp. 79–84. [Google Scholar]
- [28].Drwecki BB, Moore CF, Ward SE, Prkachin KM. Reducing racial disparities in pain treatment: the role of empathy and perspective-taking. Pain 2011;152:1001–1006. [DOI] [PubMed] [Google Scholar]
- [29].Freeman JB, Penner AM, Saperstein A, Scheutz M, Ambady N. Looking the part: social status cues shape race perception. PLoS One 2011;6:e25107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fry R, Taylor P. The rise of residential segregation by income. Washington, DC: Pew Research Center; 2012. [Google Scholar]
- [31].Fuentes M, Hart-Johnson T, Green CR. The association among neighborhood socioeconomic status, race and chronic pain in black and white older adults. J Natl Med Assoc 2007;99:1160. [PMC free article] [PubMed] [Google Scholar]
- [32].Galinsky AD, Moskowitz GB. Perspective-taking: decreasing stereotype expression, stereotype accessibility, and in-group favoritism. J Pers Soc Psychol 2000;78:708. [DOI] [PubMed] [Google Scholar]
- [33].Gold R, Michael YL, Whitlock EP, Hubbell FA, Mason ED, Rodriguez BL, Safford MM, Sarto GE. Race/ethnicity, socioeconomic status, and lifetime morbidity burden in the women’s health initiative: a cross-sectional analysis. J Womens Health 2006;15:1161–1173. [DOI] [PubMed] [Google Scholar]
- [34].Green CR, Hart-Johnson T. The association between race and neighborhood socioeconomic status in younger Black and White adults with chronic pain. J Pain 2012;13:176–186. [DOI] [PubMed] [Google Scholar]
- [35].Green CR, Ndao-Brumblay SK, West B, Washington T. Differences in prescription opioid analgesic availability: comparing minority and white pharmacies across Michigan. J Pain 2005;6:689–699. [DOI] [PubMed] [Google Scholar]
- [36].Greene S, Turner MA, Gourevitch R. Racial residential segregation and neighborhood disparities. Washington, DC: US Partnership on Mobility from Poverty, 2017. [Google Scholar]
- [37].Halpern J From detached concern to empathy: humanizing medical practice. New York: Oxford University Press, 2001. [Google Scholar]
- [38].Harwood J The contact space: a novel framework for intergroup contact research. J Lang Soc Psychol 2010;29:147–177. [Google Scholar]
- [39].Heistaro S, Vartiainen E, Heliövaara M, Puska P. Trends of back pain in eastern Finland, 1972–1992, in relation to socioeconomic status and behavioral risk factors. Am J Epidemiol 1998;148:671–682. [DOI] [PubMed] [Google Scholar]
- [40].Hewstone M, Brown R. Contact is not enough: an intergroup perspective on the ‘contact hypothesis’ In: Hewstone M, Brown R, editors. Contact and conflict in intergroup encounters Oxford, UK: Blackwell, 1986. pp. 1–44. [Google Scholar]
- [41].Hirsh A, Hollingshead N, Bair M, Matthias M, Wu J, Kroenke K. The influence of patient’s sex, race and depression on clinician pain treatment decisions. Eur J Pain 2013;17:1569–1579. [DOI] [PubMed] [Google Scholar]
- [42].Hirsh AT, Alqudah AF, Stutts LA, Robinson ME. Virtual human technology: capturing sex, race, and age influences in individual pain decision policies. Pain 2008;140:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hirsh AT, Callander SB, Robinson ME. Patient demographic characteristics and facial expressions influence nurses’ assessment of mood in the context of pain: a virtual human and lens model investigation. Int J Nurs Stud 2011;48:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hirsh AT, George SZ, Robinson ME. Pain assessment and treatment disparities: a virtual human technology investigation. Pain 2009;143:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hirsh AT, Hollingshead NA, Ashburn-Nardo L, Kroenke K. The interaction of patient race, provider bias, and clinical ambiguity on pain management decisions. J Pain 2015;16:558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hirsh AT, Hollingshead NA, Bair MJ, Matthias MS, Kroenke K. Preferences, experience, and attitudes in the management of chronic pain and depression: a comparison of physicians and medical students. Clin J Pain 2014;30:766–774. [DOI] [PubMed] [Google Scholar]
- [47].Hirsh AT, Jensen MP, Robinson ME. Evaluation of nurses’ self-insight into their pain assessment and treatment decisions. J Pain 2010;11:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hollingshead NA, Matthias MS, Bair MJ, Hirsh AT. Impact of race and sex on pain management by medical trainees: a mixed methods pilot study of decision making and awareness of influence. Pain Med 2015;16:280–290. [DOI] [PubMed] [Google Scholar]
- [49].Hollingshead NA, Meints S, Middleton SK, Free CA, Hirsh AT. Examining influential factors in providers’ chronic pain treatment decisions: a comparison of physicians and medical students. BMC Med Educ 2015;15:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: The National Academies Press, 2003. [PubMed] [Google Scholar]
- [51].Institute of Medicine and the Committee on Advancing Pain Research. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington DC: Academies Press, 2011. [PubMed] [Google Scholar]
- [52].Jablonska B, Soares JJ, Sundin Ö. Pain among women: associations with socio‐economic and work conditions. Eur J Pain 2006;10:435–435. [DOI] [PubMed] [Google Scholar]
- [53].Ji GY, Oh CH, Jung N-Y, An SD, Choi W-S, Kim JH. Interference of detection rate of lumbar disc herniation by socioeconomic status. Asian Spine J 2013;7:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Johnsen K, Raij A, Stevens A, Lind DS, Lok B. The validity of a virtual human experience for interpersonal skills education. In: Proceedings of the SIGCHI Conference on Human Factors in Computing Systems New York, NY: Association for Computing Machinery, 2006. pp.1049–1058. [Google Scholar]
- [55].Joynt M, Train MK, Robbins BW, Halterman JS, Caiola E, Fortuna RJ. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J Gen Intern Med 2013;28:1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kane L Medscape Physician Compensatin Report 2018. 2018. Available at: https://www.medscape.com/slideshow/2018-compensation-overview-6009667. Accessed February 2, 2019.
- [57].Katz I, Wackenhut J, Glass DC. An ambivalence-amplification theory of behavior toward the stigmatized In: Worchel S, Austin W, editors. Psychology of intergroup relations. Chicago: Nelson-Hall, 1986. pp. 103–117. [Google Scholar]
- [58].Kearney MK, Weininger RB, Vachon ML, Harrison RL, Mount BM. Self-care of physicians caring for patients at the end of life:“Being connected... a key to my survival”. JAMA 2009;301:1155–1164. [DOI] [PubMed] [Google Scholar]
- [59].Keefe FJ, Block AR. Development of an observation method for assessing pain behavior in chronic low back pain patients. Behav Ther 1982. [Google Scholar]
- [60].Knoll H, Macaulay T, Jesse M. A preliminary survey examining predictors of burnout in pain medicine physicians in the United States. Pain Physician 2016;19:E689–E696. [PubMed] [Google Scholar]
- [61].Leuski A, Patel R, Traum D, Kennedy B. Building effective question answering characters. In: Proceedings of the 7th SIGdial Workshop on Discourse and Dialogue Stroudsburg: Association for Computational Linguistics, 2009. pp. 18–27. [Google Scholar]
- [62].Lipton RB, Serrano D, Holland S, Fanning KM, Reed ML, Buse DC. Barriers to the diagnosis and treatment of migraine: effects of sex, income, and headache features. Headache 2013;53:81–92. [DOI] [PubMed] [Google Scholar]
- [63].Lucey P, Cohn JF, Prkachin KM, Solomon PE, Matthews I. Painful data: The UNBC-McMaster shoulder pain expression archive database. In: Ninth IEEE International Conference on Automatic Face & Gesture Recognition and Workshops (FG 2011) Santa Barbara: IEEE, 2011. pp. 57–64. [Google Scholar]
- [64].Macfarlane GJ, Norrie G, Atherton K, Power C, Jones GT. The influence of socioeconomic status on the reporting of regional and widespread musculoskeletal pain: results from the 1958 British Birth Cohort Study. Ann Rheum Dis 2009;68:1591–1595. [DOI] [PubMed] [Google Scholar]
- [65].Mackenbach JP, Bos V, Andersen O, Cardano M, Costa G, Harding S, Reid A, Hemström Ö, Valkonen T, Kunst AE. Widening socioeconomic inequalities in mortality in six Western European countries. Int J Epidemiol 2003;32:830–837. [DOI] [PubMed] [Google Scholar]
- [66].Massey DS, Rothwell J, Domina T. The changing bases of segregation in the United States. Ann Am Acad Pol Soc Sci 2009;626:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mayer JD, Kirlin B, Rehm CD, Loeser JD. Opioid availability in outpatient pharmacies in Washington State. Clin J Pain 2008;24:120–123. [DOI] [PubMed] [Google Scholar]
- [68].Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med 2012;13:150–174. [DOI] [PubMed] [Google Scholar]
- [69].Meghani SH, Chittams J. Controlling for socioeconomic status in pain disparities research: all-else-equal analysis when “all else” is not equal. Pain Med 2015;16:2222–2225. [DOI] [PubMed] [Google Scholar]
- [70].Meghani SH, Keane A. Preference for analgesic treatment for cancer pain among African Americans. J Pain Symptom Manage 2007;34:136–147. [DOI] [PubMed] [Google Scholar]
- [71].Monteith MJ. Self-regulation of prejudiced responses: implications for progress in prejudice-reduction efforts. J Pers Soc Psychol 1993;65:469. [Google Scholar]
- [72].Monteith MJ, Ashburn-Nardo L, Voils CI, Czopp AM. Putting the brakes on prejudice: on the development and operation of cues for control. J Pers Soc Psychol 2002;83:1029. [DOI] [PubMed] [Google Scholar]
- [73].Morrison RS, Wallenstein S, Natale DK, Senzel RS, Huang L-L. “We don’t carry that”—failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. N Engl J Med 2000;342:1023–1026. [DOI] [PubMed] [Google Scholar]
- [74].Nam CB, Boyd M. Occupational status in 2000; over a century of census-based measurement. Popul Res Policy Rev 2004;23:327–358. [Google Scholar]
- [75].Nampiaparampil DE, Nampiaparampil JX, Harden RN. Pain and prejudice. Pain Med 2009;10:716–721. [DOI] [PubMed] [Google Scholar]
- [76].Nancy K The unfocused focus group: benefit or bane? Qual Rep 2011;16:1380. [Google Scholar]
- [77].Nicholas MK. When to refer to a pain clinic. Best Pract Res Clin Rheumatol 2004;18:613–629. [DOI] [PubMed] [Google Scholar]
- [78].Pettigrew TF. Intergroup contact theory. Annu Rev Psychol 1998;49:65–85. [DOI] [PubMed] [Google Scholar]
- [79].Pettigrew TF, Tropp LR. A meta-analytic test of intergroup contact theory. J Pers Soc Psychol 2006;90:751. [DOI] [PubMed] [Google Scholar]
- [80].Pettigrew TF, Tropp LR. How does intergroup contact reduce prejudice? Meta‐analytic tests of three mediators. Eur J Soc Psychol 2008;38:922–934. [Google Scholar]
- [81].Pettigrew TF, Tropp LR, Wagner U, Christ O. Recent advances in intergroup contact theory. Int J Intercult Relat 2011;35:271–280. [Google Scholar]
- [82].Prkachin KM. The consistency of facial expressions of pain: a comparison across modalities. Pain 1992;51:297–306. [DOI] [PubMed] [Google Scholar]
- [83].Raij A, Johnsen K, Dickerson R, Lok B, Cohen M, Bernard T, Oxendine C, Wagner P, Lind S. Interpersonal scenarios: Virtual\approx real? In: Proceedings of the IEEE Conference on Virtual Reality Piscataway, NJ: Institute of Electrical and Electronic Engineers, Inc, 2006. pp.59–66. [Google Scholar]
- [84].Riley JL, Gilbert GH, Heft MW. Socioeconomic and demographic disparities in symptoms of orofacial pain. J Public Health Dent 2003;63:166–173. [DOI] [PubMed] [Google Scholar]
- [85].Riquelme I, Chacón J-I, Gándara A-V, Muro I, Traseira S, Monsalve V, Soriano J-F. Prevalence of burnout among pain medicine physicians and its potential effect upon vlinical outcomes in patients with oncologic pain or chronic pain of nononcologic origin. Pain Med 2018:2398–2407. [DOI] [PubMed] [Google Scholar]
- [86].Rossen B, Lind S, Lok B. Human-centered distributed conversational modeling: Efficient modeling of robust virtual human conversations. In: Ruttkay Z, Kipp M, Nijholt A, Vilhjalmasson H, editors. Lecture notes in computer science. Proc. International Workshop on Intelligent Virtual Agents, Vol. 5773 Berlin, Heidelberg: Springer, September 2009. pp. 474–481. [Google Scholar]
- [87].Schulte E, Hermann K, Berghöfer A, Hagmeister H, Schuh‐Hofer S, Schenk M, Kopf A, Vilain M, Martus P, Willich SN. Referral practices in patients suffering from non‐malignant chronic pain. Eur J Pain 2010;14:308. e301–308. e310. [DOI] [PubMed] [Google Scholar]
- [88].Sinclair S, Norris JM, McConnell SJ, Chochinov HM, Hack TF, Hagen NA, McClement S, Bouchal SR. Compassion: a scoping review of the healthcare literature. BMC Palliat Care 2016;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Stringhini S, Sabia S, Shipley M, Brunner E, Nabi H, Kivimaki M, Singh-Manoux A. Association of socioeconomic position with health behaviors and mortality. JAMA 2010;303:1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Stutts LA, Hirsh AT, George SZ, Robinson ME. Investigating patient characteristics on pain assessment using virtual human technology. Eur J Pain 2010;14:1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sullivan M The new subjective medicine: taking the patient’s point of view on health care and health. Soc Sci Med 2003;56:1595–1604. [DOI] [PubMed] [Google Scholar]
- [92].The Sims 4 [software]. Redwood City, CA: Electronic Arts, 2014. [Google Scholar]
- [93].Torres CA, Bartley EJ, Wandner LD, Alqudah AF, Hirsh AT, Robinson ME. The influence of sex, race, and age on pain assessment and treatment decisions using virtual human technology: a cross-national comparison. J Pain Res 2013;6:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Vargas CM, Macek MD, Marcus SE. Sociodemographic correlates of tooth pain among adults: United States, 1989. Pain 2000;85:87–92. [DOI] [PubMed] [Google Scholar]
- [95].Wandner L, Hirsh A, Torres C, Lok B, Scipio C, Heft M, Robinson M. Using virtual human technology to capture dentists’ decision policies about pain. J Dent Res 2013;92:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wandner LD, George SZ, Lok BC, Torres CA, Chuah JH, Robinson ME. Pain assessment and treatment decisions for virtual human patients. Cyberpsychol Behav Soc Netw 2013;16:904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wandner LD, Heft MW, Lok BC, Hirsh AT, George SZ, Horgas AL, Atchison JW, Torres CA, Robinson ME. The impact of patients’ gender, race, and age on health care professionals’ pain management decisions: an online survey using virtual human technology. Int J Nurs Stud 2014;51:726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wandner LD, Letzen JE, Torres CA, Lok B, Robinson ME. Using virtual human technology to provide immediate feedback about participants′ use of demographic cues and knowledge of their cue use. J Pain 2014;15:1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wandner LD, Stutts LA, Alqudah AF, Craggs JG, Scipio CD, Hirsh AT, Robinson ME. Virtual human technology: patient demographics and healthcare training factors in pain observation and treatment recommendations. J Pain Res 2010;3:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wigton RS. Applications of judgment analysis and cognitive feedback to medicine In: Amsterdam N, editor. Human judgment: The SJT view, Vol. 54 Amsterdam, Netherlands: Elsevier, 1988. pp. 227–245. [Google Scholar]
- [101].Williams DR, Collins C. US socioeconomic and racial differences in health: patterns and explanations. Annu Rev Sociol 1995;21:349–386. [Google Scholar]
- [102].Woo JK, Ghorayeb SH, Lee CK, Sangha H, Richter S. Effect of patient socioeconomic status on perceptions of first-and second-year medical students. Can Med Assoc J 2004;170:1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


