Abstract
PURPOSE
Patients with human epidermal growth factor receptor 2 (HER2)–positive metastatic breast cancer eventually develop resistance to dual-antibody therapy with trastuzumab plus pertuzumab. Mechanisms of resistance have not been well elucidated. We evaluated the safety, tolerability, and efficacy of ado-trastuzumab emtansine (T-DM1) plus neratinib in patients who progressed on trastuzumab plus pertuzumab.
PATIENTS AND METHODS
In this 3 + 3 dose-escalation study, patients with metastatic breast cancer who progressed on trastuzumab, pertuzumab, and a taxane were treated with T-DM1 at 3.6 mg/kg intravenously every 3 weeks and dose-escalating neratinib at 120, 160, 200, or 240 mg/d orally.
RESULTS
Twenty-seven patients were treated across four dose-levels of neratinib. Dose-limiting toxicity in cycle 1 was grade 3 diarrhea in six patients and grade 3 nausea in one; no patient experienced grade 4 diarrhea, and there were no grade 5 toxicities. Other grade 3 to 4 toxicities included nausea (11%), dehydration (11%), electrolyte abnormality (19%), thrombocytopenia (15%), elevated transaminase levels (7%), and fatigue (7%). Twelve (63%) of 19 evaluable patients had an objective response. Responses occurred at all neratinib doses. Plasma cell–free DNA at baseline showed ERBB2 (HER2) amplification in 10 of 27 patients. Deep and more durable responses occurred in patients with cell-free DNA ERBB2 amplification. Two complete responders had high expression of total HER2 and p95HER2 in baseline tissue.
CONCLUSION
We report the recommended phase II dose of T-DM1 3.6 mg/kg and neratinib 160 mg/d for this combination. Possible resistance mechanisms to HER2 antibodies may be loss of the HER2 receptor and high expression of p95HER2. These data provide the basis for an ongoing phase II study to better define the activity of this regimen.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2) is overexpressed in 20% of breast cancers. The blocking of HER2 activity with trastuzumab, which binds to the extracellular domain IV of the HER2 receptor, prevents dimerization and inhibits downstream signaling, which results in cell cycle arrest and apoptosis and leads to improved outcomes for patients with HER2-positive disease.1 However, in women with metastatic breast cancers (MBCs), resistance usually occurs. To overcome resistance, additional anti-HER2 therapies, monoclonal antibodies, antibody-drug conjugates, and oral tyrosine kinase inhibitors (TKIs) have been developed. Pertuzumab, another monoclonal antibody, binds to extracellular domain II of HER2, prevents heterodimerization with HER3 and other HER receptors, and acts in a complementary fashion with trastuzumab to provide a more complete signaling blockade. This activity has been confirmed in clinical trials,2-4 which led to US Food and Drug Administration (FDA) approval of pertuzumab in metastatic and neoadjuvant settings.
Ado-trastuzumab emtansine (T-DM1), developed to treat trastuzumab-resistant patients, is a conjugated antibody composed of the cytotoxic agent DM1, a maytansinoid derivative attached to trastuzumab through a stable thioether linker.5 This first-in-class antibody-drug conjugate received US FDA approval for second-line anti-HER2 therapy on the basis of findings from the Trastuzumab Emtansine Versus Capecitabine Plus Lapatinib in Patients With Previously Treated HER2-Positive Advanced Breast Cancer (EMILIA) trial.6 Patients treated with multiple lines of anti-HER2 therapies had lower objective responses (ORs) and shorter progression-free survival with T-DM1 monotherapy than those observed in EMILIA.7 Although no prospective data of T-DM1 activity exist in patients whose cancer progresses on trastuzumab plus pertuzumab, a retrospective analysis showed tumor response to T-DM1 of less than 20%.8
Several TKIs show activity after progression on trastuzumab. Neratinib, an oral, small-molecule TKI of HER family members, was approved for extended adjuvant treatment in early-stage HER2-overexpressing/amplified breast cancer after completion of adjuvant trastuzumab-based therapy.9 In an earlier phase II study, neratinib as monotherapy in HER2-positive MBC demonstrated an overall response rate of 24% in trastuzumab-refractory patients and 56% in trastuzumab-naïve patients.10
To be effective, trastuzumab, pertuzumab, and T-DM1 must bind to the extracellular domain of HER2. The conjugated antibody T-DM1 binds to the HER2 receptor to allow for intracellular drug delivery of the potent cytotoxic agent DM1. In contrast, neratinib binds irreversibly to the intracellular ATP pocket of the HER2 tyrosine kinase domain. A potential advantage of the TKI is that truncated HER2 (p95HER2) lacks trastuzumab and pertuzumab binding sites while retaining the kinase domain, which potently drives downstream signaling. Expression of p95HER2 occurs in up to 30% of HER2-positive MBCs.11 Thus, high-p95HER2 expression is expected to result in resistance to trastuzumab, pertuzumab, and T-DM1 but retain sensitivity to several TKIs.
Taken together, T-DM1 plus neratinib combines agents with different mechanisms of action and toxicity profiles. As monotherapy, both agents have been shown to overcome trastuzumab resistance. The purpose of this study was to determine the safety and preliminary efficacy of the combination in patients previously treated with trastuzumab plus pertuzumab and to explore potential predictors of sensitivity and mechanisms of resistance.
PATIENTS AND METHODS
Patients
Eligible patients included women 18 years of age or older with an Eastern Cooperative Oncology Group performance status of 0 to 1; HER2-positive breast cancer (determined by local testing using the ASCO/College of American Pathologists HER2 test guideline)12; hormone receptor positivity or negativity; measurable metastatic disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; and progression on anti-HER–based therapy with trastuzumab plus pertuzumab given in the neoadjuvant/adjuvant or first-line setting, irrespective of time to progression. Adequate hematologic parameters were absolute neutrophil count of 1,000/mm3 or greater, platelet count of 100,000/mm3 or greater, hemoglobin of 9 g/dL or greater, adequate renal function (defined as serum creatinine of less than or equal to 1.5 × the upper limit of normal [ULN]), and adequate hepatic function (defined as total bilirubin of less than or equal to 1.5 × ULN, AST and ALT of less than or equal to 2.5 × ULN, or 5 × ULN if liver metastases). Left ventricular ejection fraction (LVEF) of 50% or greater assessed by either two-dimensional echocardiogram or multiple-gated acquisition scan was required. Patients were excluded if they had received prior T-DM1 or TKI therapy; could not swallow medications; had symptomatic brain metastases (patients with known, stable brain metastases were allowed; CNS imaging in asymptomatic patients was not an entry requirement); active hepatitis B or C with abnormal liver function tests; persistent grade 2 or higher diarrhea; or active cardiac disease, including angina pectoris.
FB-10 was approved by the institutional review boards at participating institutions; written informed consent was required. The study was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki.
Study Design
This phase Ib, multicenter, open-label, dose-escalation study evaluated T-DM1 plus neratinib in women with HER2-positive MBC. On day 1, patients received 3.6 mg/kg T-DM1 intravenously on a 3-week cycle and began daily oral neratinib in one of four dose-escalation cohorts (120, 160, 200, or 240 mg). Up to two dose reductions after cycle 1 were allowed for both T-DM1 and neratinib.
All patients who during cycle 1 experienced a dose-limiting toxicity (DLT) or received 75% of neratinib were considered evaluable for a DLT. Patients who did not complete cycle 1 for reasons other than toxicity were replaced. Patients who completed one cycle of therapy but not evaluated by imaging were considered nonevaluable for efficacy. DLT was defined as one or more of the following: diarrhea (any grade) with fever or dehydration requiring intravenous fluids, grade 3 diarrhea lasting more than 2 days on optimal medical therapy, grade 4 diarrhea of any duration, grade 3/4 neutropenia with fever, grade 4 neutropenia lasting more than 7 days, grade 4 thrombocytopenia, grade 3/4 nonhematologic toxicity (excluding grade 3 rash or allergic reaction/hypersensitivity), or toxicity-related delay of more than 2 weeks to initiate cycle 2. Progressive disease (PD) or grade 2 or higher toxicity, as defined for specific adverse events (AEs), would lead to permanent discontinuation of both T-DM1 and neratinib. The primary aim was to evaluate the safety of T-DM1 plus neratinib and to determine the recommended phase II dose (RP2D), which is the highest dose of neratinib with no more than one in six patients experiencing a DLT. Secondary end points were determination of rates of complete response (CR), partial response (PR), stable disease (SD), duration of response, overall toxicity, and exploratory studies to better understand therapy sensitivity or resistance. Because diarrhea is expected with neratinib, primary prophylaxis with loperamide was mandated to begin with the first dose and to continue throughout cycle 1 with subsequent titration of antidiarrheal therapy (guidance provided in the Data Supplement).
Safety Assessment
Safety was assessed by physical examination, interim history, and laboratory assessment. Patients continued therapy until PD or discontinuation as a result of withdrawal, physician discretion, or toxicity. AE assessment occurred on days 1, 8, and 15 of cycle 1; on day 1 of each cycle; and for 30 days after therapy discontinuation or when alternate therapy began. AE reporting was according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Patient safety and AEs were continuously monitored and reviewed by the NSABP medical review team and during weekly teleconferences with designated participating site staff and investigators.
Response Assessment
Clinical activity assessed by tumor measurements using RECIST version 1.1 was performed at baseline and after every two cycles (6 weeks). Patients whose first scan (at 6 weeks) indicated CR, PR, or SD underwent a confirmatory scan at 12 weeks. After six cycles, responding patients were permitted to have the scanning interval increased to every third cycle.
Correlative Studies
Blood samples for neratinib pharmacokinetic (PK) determination were required on day 1, cycle 1 (pretreatment), and day 1, cycle 2. Optional blood samples for more comprehensive neratinib PK determination were collected during cycle 1 at 1, 2, 4, 6, and approximately 22 to 24 hours after neratinib administration (n = 8). PK parameters were determined noncompartmentally with PK Solutions 2.0 (Summit Research Services, Montrose, CO). Plasma concentrations of neratinib were quantitated with an assay fully validated per US FDA guidance.13 Neratinib trough concentrations were extrapolated to the 24-hour value assuming a 14.9-hour half-life as reported previously.14,15 Statistical analyses were performed using SPSS version 22 software (IBM Corporation, Armonk, NY). Additional blood samples were collected in Streck tubes at baseline, during therapy, and at progression for cell-free DNA (cfDNA) and future analyses. cfDNA next-generation sequencing analyses were performed on all baseline samples (N = 27); selected day 1, cycle 2, samples (n = 9); and at progression (n = 2) at Guardant Health (Guardant360 assay), a Clinical Laboratory Improvement Amendments–certified, College of American Pathologists–accredited, New York State Department of Health–approved laboratory. The Guardant360 assay16 detects single-nucleotide variants, indels, fusions, and copy number alterations in 73 genes. For ERBB2 amplification, a cutoff of more than 2.14 was used.
Two patients consented for optional tissue biopsies upon enrollment. These samples were assayed for total HER2 (H2T) using the HERmark assay17 and for p95HER2 using the VeraTag assay18 (Monogram Biosciences, South San Francisco, CA).
Statistical Analyses
FB-10 was intended to determine the safety, tolerability, and RP2D of neratinib in combination with T-DM1. Categorical data were summarized by presenting frequencies and percentages. End point analyses were descriptive. No interim analyses were planned or conducted.
RESULTS
Patient Characteristics
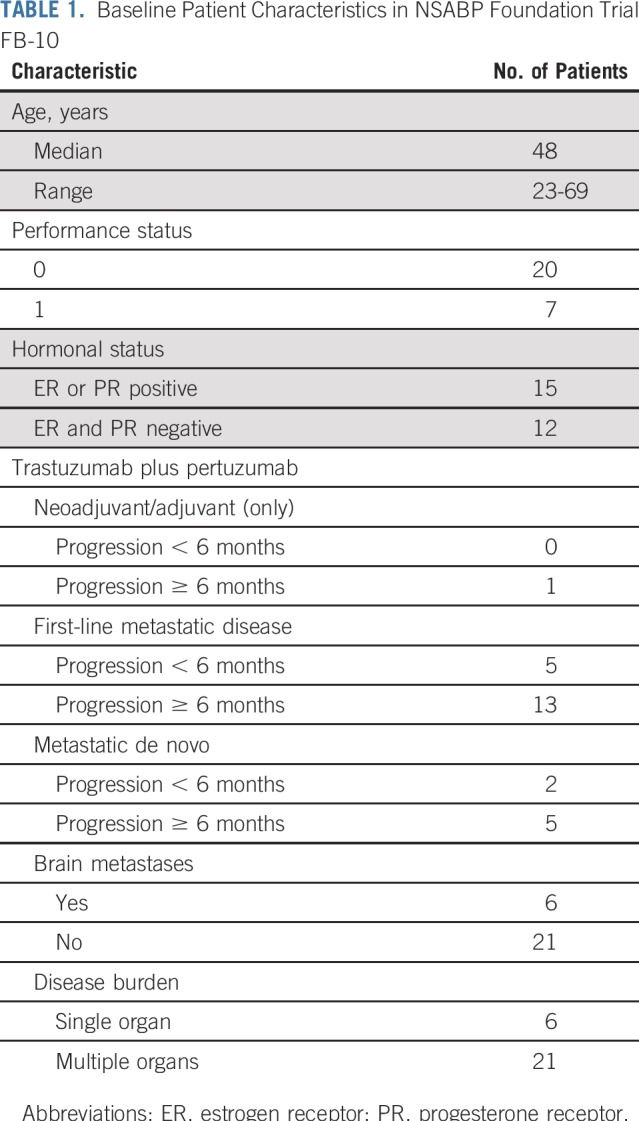
From February 2015 to July 2017, five institutions enrolled 27 patients. Table 1 lists baseline patient characteristics. Median age was 48 years (range, 23 to 69 years); Eastern Cooperative Oncology Group performance status was 0 in 20 patients and 1 in seven. Hormone receptor status was positive in 15 patients and negative in 12. All patients had a history of HER2-positive disease and had received trastuzumab, pertuzumab, and taxane. Six patients with stable brain metastases were enrolled.
TABLE 1.
Baseline Patient Characteristics in NSABP Foundation Trial FB-10
Safety Assessment
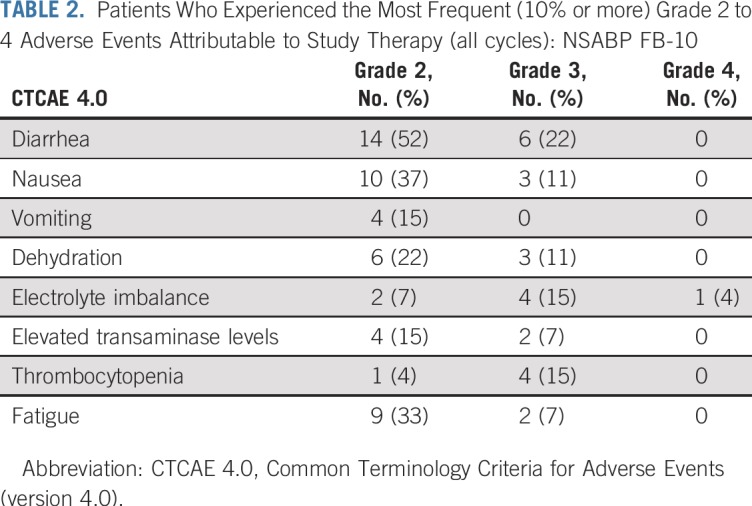
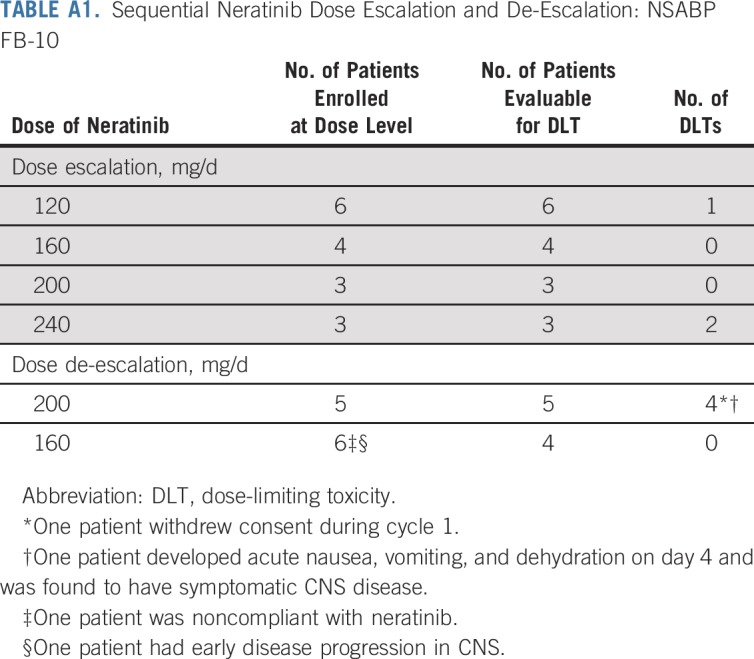
Twenty-five patients were evaluable for a DLT and 27 for AEs. All patients experienced at least one AE (grades 1 to 4). One patient had grade 4 hypokalemia secondary to GI toxicity. The most common grade 3 toxicities were diarrhea (22%), electrolyte imbalance (15%), thrombocytopenia (15%), dehydration (11%), and nausea (11%). Diarrhea was the most common AE, with all grades occurring in 23 patients (93%): grade 1 in 19%, grade 2 in 52%, and grade 3 in 22% (Table 2). At the RP2D of neratinib (160 mg/d) with full-dose T-DM1, there were no DLTs (zero of eight patients). For other doses of neratinib, there were seven DLTs: one of six patients at 120 mg/d; four of eight at 200 mg/d; and two of three at 240 mg/d (Appendix Table A1, online only). All patients had a baseline LVEF 50% or greater (median, 60%) and were monitored every 3 months. No patients had an LVEF decline below 50%.
TABLE 2.
Patients Who Experienced the Most Frequent (10% or more) Grade 2 to 4 Adverse Events Attributable to Study Therapy (all cycles): NSABP FB-10
Efficacy Assessment
Nineteen patients were evaluable for response. Six were considered nonevaluable because of a DLT in cycle 1 followed by withdrawal (one patient with a DLT continued with a dose reduction and was considered evaluable). One patient was noncompliant, and one had early disease complication before first tumor assessment. Best response was CR in three patients with durations of 364, 510, and more than 969 days; confirmed PR in nine; SD in two; and PD in five.
Of the six patients with known brain metastases, five had PD outside the CNS, and the sixth had PR that lasted 330 days before progressing in the CNS. An additional patient developed new brain metastases at 510 days while in systemic CR. ORs were observed at all doses of neratinib.
PK
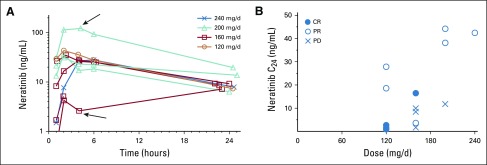
During the 24 hours after the initial dose of neratinib, eight patients had intensive neratinib PK sampling. No dose response was apparent. Two outliers were observed: one, with the highest peak concentration at 200 mg/d, experienced DLT during cycle 1, and the second, with the lowest peak concentration at 160 mg/d, was taking delayed-release pantoprazole (Fig 1A).
FIG 1.
Neratinib pharmacokinetics. (A) Neratinib concentration versus time over 24 hours. The outlier at 200 mg/d (upper arrow) developed a dose-limiting toxicity; the outlier at 160 mg/d (lower arrow) was taking pantoprazole. (B) Neratinib trough concentration at 24 hours (C24) on day 1, cycle 2, after dosing, with indication of response. CR, complete response; PD, progressive disease; PR, partial response.
Fifteen patients had evaluable steady-state neratinib plasma trough samples collected between 19 and 37 hours after their last neratinib dose. Samples in two patients had values of 0.9 and 1.2 ng/mL, which were below the assay lower limit of quantitation of 2 ng/mL.13 Calculation of trough concentrations at 24 hours (C24) resulted in median absolute concentration adjustments of 8.3% (range, −20% to 87%). Mean C24 was 15.3 ng/mL with 102% coefficient of variation, with large variability of exposure at any given dose (Fig 1B). Dose normalization of C24 resulted in a mean of 0.089 ng/mL/mg with 93% coefficient of variation. Patients who experienced PD seemed to have lower trough levels relative to those with responses (P = .661 by Mann-Whitney U test).19 Of note, two of the three CRs also had very low trough exposures.
Blood Samples for cfDNA
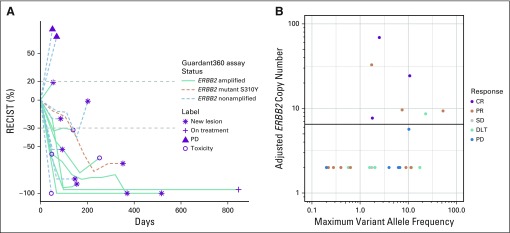
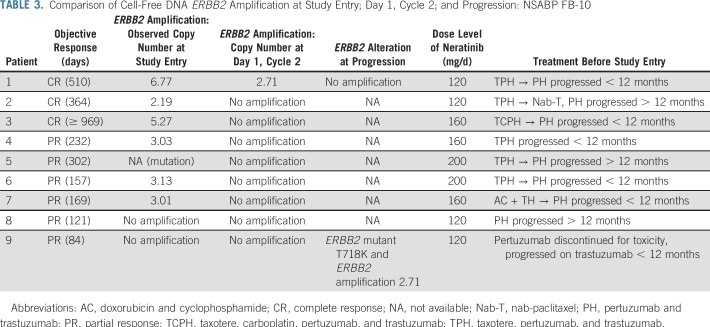
Twenty-seven baseline blood samples were analyzed by Guardant360 assay for ERBB2 amplification in cfDNA. Nine were ERBB2 amplified with a copy number cutoff of 2.14 in plasma. One had the ERBB2-activating mutation S310Y. Amplification was not detected in the remaining 17 samples. Seven (70%) of 10 patients with amplification or mutation showed CR (n = 3) or PR (n = 4) documented on the second confirmatory imaging evaluation versus four (24%) of 17 without amplification (P = .04, Fisher’s exact test).20 Furthermore, only PRs and no CRs were observed among those without amplification. Duration of OR in ERBB2-amplified patients ranged from 18 to more than 132 weeks; responses in nonamplified patients ranged from 12 to 24 weeks (Fig 2A). Nine blood samples drawn at cycle 2 and two collected at progression were analyzed for ERBB2 amplification and/or mutations (Table 3). Five of six patients whose plasma samples were initially ERBB2 amplified had undetectable ERBB2 amplification by day 1, cycle 2. The sixth sample had an amplification decrease from 6.77 to 2.71 and was no longer amplified at progression. In the patient with an activating ERBB2 mutation detected on day 1, cycle 1, the mutation was no longer detected on day 1, cycle 2.
FIG 2.
Response Evaluation Criteria in Solid Tumors (RECIST) response and duration. (A) Best response and duration in cell-free DNA in patients with and without ERBB2 amplification. Although response by dose level is not shown here, objective responses and stable disease (SD) were seen at all dose levels, including two complete responses (CRs) and three partial responses (PRs) in six patients treated with neratinib 120 mg/d, one CR and three PRs in nine patients treated with 160 mg/d, two PRs and one SD in eight patients treated with 200 mg/d, and one PR and one SD in three patients treated with 240 mg/d. (B) Best response above and below the adjusted ERBB2 copy number threshold of 6.5. DLT, dose-limiting toxicity; PD, progressive disease.
TABLE 3.
Comparison of Cell-Free DNA ERBB2 Amplification at Study Entry; Day 1, Cycle 2; and Progression: NSABP FB-10
Analysis of Tissue Samples for H2T and p95HER2
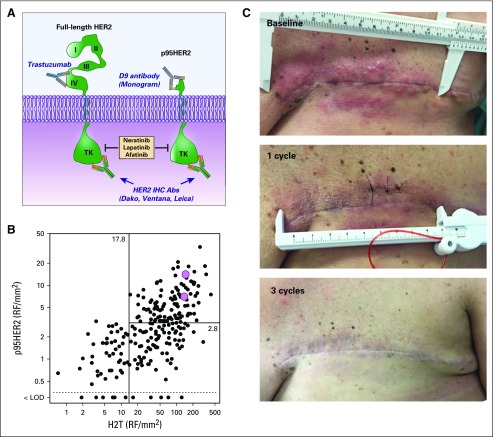
Two patients with extensive chest wall recurrence had adequate tissue biopsy samples for quantitative analysis of H2T and p95HER2 using the HERmark and VeraTag assays, respectively (Fig 3A), and were well above previously established cutoffs21 (Fig 3B). Both patients achieved CR (Fig 3C).
FIG 3.
Response in a patient who expressed p95HER2. (A) Anti–human epidermal growth factor receptor 2 (HER2) antibodies from Dako (Agilent, Santa Clara, CA), Ventana Medical Systems (Oro Valley, AZ), and Leica Biosystems (Wetzlar, Germany), currently used for diagnostic immunohistochemical (IHC) evaluation of breast cancer, do not distinguish between expression of full-length HER2 and the truncated p95HER2. In contrast, the antibody developed by Monogram Biosciences specifically detects the truncated p95HER2 form of the receptor and is used in the VeraTag assay. (B) Pink circles indicate the pretreatment total HER2 (H2T) and p95HER2 levels for two patients who achieved complete response (one of whom is represented in the photos in panel C) superimposed for comparison on a previously published scattergram of values obtained from a similar cohort of patients with trastuzumab-treated, metastatic HER2-positive breast cancer (LOD, p95HER2 below the limit of detection). (C) The patient initially presented with locally advanced disease with progressive chest wall disease on trastuzumab plus pertuzumab therapy. By cycle 3, the patient had complete disappearance of chest wall disease and had a clinical complete response. Abs, antibodies; LOD, logarithm of odds; RF, relative fluorescence; TK, tyrosine kinase. Panel B scattergram reproduced with permission.21
DISCUSSION
In this study, the DLT of T-DM1 plus neratinib is diarrhea, which is similar to reports of neratinib when used as a single agent or in combination with other agents.10,22 Diarrhea is most prominent in cycle 1 and is manageable with primary antidiarrheal prophylaxis. Although no DLTs were experienced at the RP2D of neratinib (160 mg/d), seven of 10 patients experienced early-onset diarrhea, including grade 2 (n = 5) and grade 3 (n = 2) that lasted less than 24 hours. Although the mechanism of neratinib-associated diarrhea is not well understood, preliminary investigations suggested an inflammatory component.23-27 One report suggested that budesonide, an oral, poorly absorbed corticosteroid, decreased grade 3 neratinib-associated diarrhea from 50% to 16% and evaluated the addition of the bile salt–binding agent colestipol in mitigating diarrhea.28
The median neratinib trough concentration observed was 15 ng/mL (0.09 ng/mL/mg) with a range of 0.9 to 44 ng/mL, which is somewhat lower than that reported previously with 240 mg daily dosing (50 to 60 ng/mL; 0.2 ng/mL/mg).10 However, exposure reached preclinical target concentrations of 28 ng/mL, which represents the neratinib half maximal inhibitory concentration for the inhibition of ligand-independent receptor phosphorylation in BT474 cells.29 As noted previously, two of three CRs occurred in patients who received the lowest dose of neratinib; these patients also had the lowest trough levels.
The outlying low exposure in the patient who took pantoprazole is consistent with a report of the impact of lansoprazole on maximum serum concentration (reduced by 71%) and area under the curve (reduced by 65%).30 Although more frequent DLTs at higher neratinib doses (200 mg and 240 mg) were observed, this was not reflected in peak concentrations of neratinib during the first 24 hours after treatment. Furthermore, responses were seen at all dose levels and steady-state concentrations. In this combination, lower neratinib doses were equally as efficacious as higher, more toxic doses, although any effect of exposure on response might have been obscured by the activity of T-DM1.
An OR to T-DM1 plus neratinib was observed in 12 (63%) of 19 patients, and SD was observed in two patients (one PR was not confirmed on a second imaging study). PD was demonstrated on the first scan in five patients. There are multiple mechanisms of resistance to anti-HER2 therapies in patients treated with previous chemotherapy and dual anti-HER2 interventions. One resistance mechanism seems to be selective elimination of HER2-overexpressing clones, which leaves residual HER2-negative metastatic disease.31,32 In patients with HER2-positive metastatic disease treated with trastuzumab-based chemotherapy, 35% showed loss of HER2 amplification in post-therapy tissue biopsy specimens.31
Because we have no baseline pretreatment cfDNA determination, we can only speculate that the absence of cfDNA ERBB2 amplification in 17 (63%) of 27 patients may represent loss of ERBB2 amplification in patients who received trastuzumab and pertuzumab–based therapy. Although absence of ERBB2 cfDNA amplification may be partly due to false-negatives, the limited information demonstrated a high degree of concordance between ERBB2 amplification in cfDNA and in contemporaneous tissue samples.33
Our clinical findings suggest that deeper and more durable responses occur in patients in whom ERBB2 amplification is detected in blood. Six responding patients with cfDNA ERBB2 amplification had either a reduction in amplification (n = 1) or undetectable amplification (n = 5) by day 1, cycle 2, which suggests that molecular response in cfDNA may provide an early indication of clinical response. These results are consistent with and support the utility of cfDNA molecular response in several studies, including response to neratinib monotherapy in patients with ERBB2-mutated but nonamplified MBC.34
Three patients achieved a CR. Of note, two patients, who agreed to tissue biopsy, had assays performed for p95HER2 and H2T, which showed that both were highly expressed. This finding suggests that resistance to previous HER2 antibody therapy may have been the result of a high level of expression of the truncated p95HER2, which lacks the extracellular domain essential for the binding of anti-HER2 antibodies. In ERBB2-amplified cancers, additional analysis of both p95HER2 and full-length HER2 components may have important therapeutic implications.
T-DM1 has been paired with a number of other agents. For example, in a phase I study of T-DM1 and tucatinib, a reversible HER2-specific TKI, the overall response rate (CR and PR) was 47% (16 of 34 evaluable patients).35 Twenty of 57 patients received both pertuzumab and trastuzumab; however, the response in this group of patients was not specified. In another phase I study,36 T-DM1 was combined with alpelisib, a phosphatidylinositol 3-kinase α-isoform–specific inhibitor. The overall response rate was 43% (six of 14). Biomarker-driven studies will be needed to determine whether these regimens target specific subpopulations.
In summary, the RP2D of neratinib with full-dose T-DM1 was determined to be 160 mg/d. With appropriate prophylaxis, manageable diarrhea occurred during the first cycle of therapy and abated thereafter. Responses were seen across all dose levels. On the basis of historical data, the combination of T-DM1 plus neratinib seems more active than either agent alone. In addition, our results highlight the potential benefit of cfDNA in personalizing the selection of cancer treatment and in monitoring response as well as genomic evolution under pressure of therapy (eg, eight of 27 patients had a phosphatidylinositol 3-kinase pathway aberration detected in cfDNA). Alterations in the expression of specific HER2 species in ERBB2-amplified cancers, including p95HER2, may have therapeutic implications and require further investigation.
APPENDIX
TABLE A1.
Sequential Neratinib Dose Escalation and De-Escalation: NSABP FB-10
Footnotes
Presented at the American Association for Cancer Research 2017 Annual Meeting, Washington, DC, April 1-5, 2017; American Society of Clinical Oncology 2018 Annual Meeting, Chicago, IL, June 1-5, 2018; and 2018 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4-8, 2018.
Supported in part by PUMA Biotechnology, Los Angeles, CA. This project used the University of Pittsburgh Medical Center Hillman Cancer Pharmacokinetics and Pharmacodynamics Facility and was supported in part by award P30-CA47904.
Clinical trial information: NCT02236000.
AUTHOR CONTRIBUTIONS
Conception and design: Jame Abraham, Rachel C. Jankowitz, Jan H. Beumer, Carmen J. Allegra, Ashok Srinivasan, Samuel A. Jacobs
Provision of study material or patients: Jame Abraham, Mohamad Adham Salkeni
Collection and assembly of data: Jame Abraham, Rachel C. Jankowitz, Mohamad Adham Salkeni, Jan H. Beumer, Brian F. Kiesel, Laura M. Adamson, Rebecca J. Nagy, Richard B. Lanman, Ashok Srinivasan, Samuel A. Jacobs
Data analysis and interpretation: Jame Abraham, Alberto J. Montero, Rachel C. Jankowitz, Jan H. Beumer, Brian F. Kiesel, Fanny Piette, Rebecca J. Nagy, Richard B. Lanman, Jeff Sperinde, Weidong Huang, Carmen J. Allegra, Ashok Srinivasan, Ying Wang, Katherine L. Pogue-Geile, Peter C. Lucas, Samuel A. Jacobs
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety and Efficacy of T-DM1 Plus Neratinib in Patients With Metastatic HER2-Positive Breast Cancer: NSABP Foundation Trial FB-10
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jame Abraham
Honoraria: Pfizer, Genentech, Roche
Speakers’ Bureau: Pfizer
Research Funding: Seattle Genetics (Inst), Pfizer (Inst)
Alberto J. Montero
Honoraria: Celgene
Consulting or Advisory Role: New Century Health, Welwaze
Rachel C. Jankowitz
Honoraria: Eisai
Mohamad Adham Salkeni
Research Funding: Pfizer (Inst), Genentech (Inst), Seattle Genetics (Inst), AstraZeneca (Inst), MedImmune (Inst), Eli Lilly (Inst), Novartis (Inst), Quintiles (Inst)
Travel, Accommodations, Expenses: Pfizer
Jan H. Beumer
Employment: Cardinal Health (I), Voisin Consulting (I)
Stock and Other Ownership Interests: GlaxoSmithKline (I)
Research Funding: AbbVie (Inst), Spectrum Pharmaceuticals (Inst), Taiho Pharmaceutical (Inst)
Patents, Royalties, Other Intellectual Property: Sulforaphane for melanoma chemoprevention (Inst)
Rebecca J. Nagy
Employment: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Richard B. Lanman
Employment: Guardant Health, Veracyte
Leadership: Guardant Health, Biolase
Stock and Other Ownership Interests: Guardant Health, Biolase, Forward Medical
Consulting or Advisory Role: Forward Medical
Research Funding: Guardant Health
Jeff Sperinde
Employment: LabCorp, Roche (I), Genentech (I)
Stock and Other Ownership Interests: LabCorp, Roche (I), Genentech (I)
Weidong Huang
Employment: Monogram Biosciences-LabCorp Specialty Testing Group
Stock and Other Ownership Interests: Monogram Biosciences-LabCorp Specialty Testing Group
Patents, Royalties, Other Intellectual Property: Patents of Monogram Biosciences-LabCorp Specialty Testing Group pending
Travel, Accommodations, Expenses: Monogram Biosciences-LabCorp Specialty Testing Group
Katherine L. Pogue-Geile
Research Funding: NSABP (Inst)
Patents, Royalties, Other Intellectual Property: Submitted US Patent Application Serial No. 14/738,757 entitled “Methods of Subtyping CRC and Their Association With Treatment of Colon Cancer Patients With Oxaliplatin” published December 24, 2015
Peter C. Lucas
Stock and Other Ownership Interests: Amgen
Consulting or Advisory Role: Bayer AG (I), Loxo Oncology (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 6.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–699. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 8.Dzimitrowicz H, Berger M, Vargo C, et al. T-DM1 activity in metastatic human epidermal growth factor receptor 2-poisitive breast cancers that received prior therapy with trastuzumab and pertuzumab. J Clin Oncol. 2016;34:3511–3517. doi: 10.1200/JCO.2016.67.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367–377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 10.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 11.Scaltriti M, Chandarlapaty S, Prudkin L, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010;16:2688–2695. doi: 10.1158/1078-0432.CCR-09-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 13.Kiesel BF, Parise RA, Wong A, et al. LC-MS/MS assay for the quantitation of the tyrosine kinase inhibitor neratinib in human plasma. J Pharm Biomed Anal. 2017;134:130–136. doi: 10.1016/j.jpba.2016.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong KK, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Chia YL, Nedelman J, et al. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther Drug Monit. 2009;31:579–584. doi: 10.1097/FTD.0b013e3181b2c8cf. [DOI] [PubMed] [Google Scholar]
- 16.Odegaard JI, Vincent JJ, Mortimer S, et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res. 2018;24:3539–3549. doi: 10.1158/1078-0432.CCR-17-3831. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Reinholz M, Weidler J, et al. Comparison of central HER2 testing with quantitative total HER2 expression and HER2 homodimer measurements using a novel proximity-based assay. Am J Clin Pathol. 2010;134:303–311. doi: 10.1309/AJCP3BZY4YAFNTRG. [DOI] [PubMed] [Google Scholar]
- 18.Sperinde J, Jin X, Banerjee J, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16:4226–4235. doi: 10.1158/1078-0432.CCR-10-0410. [DOI] [PubMed] [Google Scholar]
- 19. Mann H, Whitney D: On a test of whether one of two random variables is stochastically larger than the other. Ann Math Statistics 18:50-60, 1947. [Google Scholar]
- 20. Fisher RA: On the interpretation of χ2 from contingency tables, and the calculation of P. J Royal Stat Soc Series A (Stats in Soc) 85:87-94, 1922.
- 21.Duchnowska R, Sperinde J, Chenna A, et al. Quantitative measurements of tumoral p95HER2 protein expression in metastatic breast cancer patients treated with trastuzumab: Independent validation of the p95HER2 clinical cutoff. Clin Cancer Res. 2014;20:2805–2813. doi: 10.1158/1078-0432.CCR-13-2782. [DOI] [PubMed] [Google Scholar]
- 22.Jankowitz RC, Abraham J, Tan AR, et al. Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2‑positive breast cancer: An NSABP Foundation Research Program phase I study. Cancer Chemother Pharmacol. 2013;72:1205–1212. doi: 10.1007/s00280-013-2262-2. [DOI] [PubMed] [Google Scholar]
- 23.Bowen JM, Mayo BJ, Plews E, et al. Development of a rat model of oral small molecule receptor tyrosine kinase inhibitor-induced diarrhea. Cancer Biol Ther. 2012;13:1269–1275. doi: 10.4161/cbt.21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wardill HR, Gibson RJ, Van Sebille YZ, et al. Irinotecan-induced gastrointestinal dysfunction and pain are mediated by common TLR4-dependent mechanisms. Mol Cancer Ther. 2016;15:1376–1386. doi: 10.1158/1535-7163.MCT-15-0990. [DOI] [PubMed] [Google Scholar]
- 25.Van Sebille YZA, Gibson RJ, Wardill HR, et al. Dacomitinib-induced diarrhoea is associated with altered gastrointestinal permeability and disruption in ileal histology in rats. Int J Cancer. 2017;140:2820–2829. doi: 10.1002/ijc.30699. [DOI] [PubMed] [Google Scholar]
- 26.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 27.Barcenas C, Olek E, Hunt D, et al. Incidence and severity of diarrhea with neratinib + intensive loperamide prophylaxis in patients (pts) with HER2+ early stage breast cancer (EBC): Interim analysis from the multicenter, open-label, phase II CONTROL trial; 2016 San Antonio Breast Cancer Symposium; San Antonio, TX. December 6-10, 2016. [Google Scholar]
- 28. Ibrahim E, Tripathy D, Wilkinson M, et al: Effects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients (pts) with HER2+ early-stage breast cancer (eBC): The CONTROL trial. Proceedings of the American Association for Cancer Research Annual Meeting 2017. Cancer Res 77, 2017 (suppl; abstr CT128) [Google Scholar]
- 29.Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 30.Keyvanjah K, DiPrimeo D, Li A, et al. Pharmacokinetics of neratinib during coadministration with lansoprazole in healthy subjects. Br J Clin Pharmacol. 2017;83:554–561. doi: 10.1111/bcp.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30:593–599. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–7388. doi: 10.1158/1078-0432.CCR-09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang DH, Ensor JE, Liu ZB, et al. Cell-free DNA as a molecular tool for monitoring disease progression and response to therapy in breast cancer patients. Breast Cancer Res Treat. 2016;155:139–149. doi: 10.1007/s10549-015-3635-5. [DOI] [PubMed] [Google Scholar]
- 34.Ma CX, Bose R, Gao F, et al. Neratinib efficacy and circulating tumor DNA detection of HER2 mutations in HER2 nonamplified metastatic breast cancer. Clin Cancer Res. 2017;23:5687–5695. doi: 10.1158/1078-0432.CCR-17-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borges VF, Ferrario C, Aucoin N, et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: A phase Ib clinical trial. JAMA Oncol. 2018;4:1214–1220. doi: 10.1001/jamaoncol.2018.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain S, Shah AN, Santa-Maria CA, et al. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat. 2018;171:371–381. doi: 10.1007/s10549-018-4792-0. [DOI] [PubMed] [Google Scholar]