Abstract
PURPOSE
Locally advanced or metastatic urothelial carcinoma is an incurable disease with limited treatment options, especially for patients who were previously treated with platinum and anti–programmed death 1 or anti–programmed death ligand 1 (PD-1/L1) therapy. Enfortumab vedotin is an antibody–drug conjugate that targets Nectin-4, which is highly expressed in urothelial carcinoma.
METHODS
EV-201 is a global, phase II, single-arm study of enfortumab vedotin 1.25 mg/kg (intravenously on days 1, 8, and 15 of every 28-day cycle) in patients with locally advanced or metastatic urothelial carcinoma who were previously treated with platinum chemotherapy and anti–PD-1/L1 therapy. The primary end point was objective response rate per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 by blinded independent central review. Key secondary end points were duration of response, progression-free survival, overall survival, safety, and tolerability.
RESULTS
Enfortumab vedotin was administered to 125 patients with metastatic urothelial carcinoma. Median follow-up was 10.2 months (range, 0.5 to 16.5 months). Confirmed objective response rate was 44% (95% CI, 35.1% to 53.2%), including 12% complete responses. Similar responses were observed in prespecified subgroups, such as those patients with liver metastases and those with no response to prior anti–PD-1/L1 therapy. Median duration of response was 7.6 months (range, 0.95 to 11.30+ months). The most common treatment-related adverse events were fatigue (50%), any peripheral neuropathy (50%), alopecia (49%), any rash (48%), decreased appetite (44%), and dysgeusia (40%). No single treatment-related adverse events grade 3 or greater occurred in 10% or more of patients.
CONCLUSION
Enfortumab vedotin demonstrated a clinically meaningful response rate with a manageable and tolerable safety profile in patients with locally advanced or metastatic urothelial carcinoma who were previously treated with platinum and anti–PD-1/L1 therapies.
INTRODUCTION
Locally advanced or metastatic urothelial carcinoma of the renal pelvis, ureters, bladder, or urethra is an incurable disease with poor long-term survival.1 Platinum-based therapies are the first-line treatment for most patients, with objective response rates of 41% to 50% and median progression-free survival of 7.6 months.2-4 In the postplatinum setting, phase III studies of anti–programmed death 1 or anti–programmed death ligand 1 (PD-1/L1) therapy demonstrated objective response rates of 21% and 13%, respectively, with an overall survival advantage compared with second-line chemotherapy demonstrated in one of two studies conducted to date.5,6
For patients who have experienced progression after platinum-based therapy and anti–PD-1/L1 therapy, treatment options are limited to chemotherapies that have modest activity.7 Thus, there is an urgent need for effective and tolerable therapies in patients with locally advanced and metastatic urothelial carcinoma after treatment with platinum and anti–PD-1/L1 therapies.
Enfortumab vedotin is an investigational antibody–drug conjugate that is comprised of a fully human monoclonal antibody conjugated to the clinically validated microtubule-disrupting agent, monomethyl auristatin E (MMAE), via a protease-cleavable linker.8,9 Enfortumab vedotin targets Nectin-4, a transmembrane protein that belongs to the Nectin family of cell adhesion molecules involved in cellular processes associated with oncogenesis.8,10-12 Nectin-4 is highly expressed in several solid tumors, including urothelial, breast, gastric, and lung carcinomas. Expression is weak to moderate in normal skin.8,13-16 Enfortumab vedotin binds to cells that express Nectin-4 with high affinity, triggering the internalization and release of MMAE in target cells. MMAE disrupts microtubule networks, leading to cell-cycle arrest and apoptotic death of Nectin-4–expressing cells.
The phase I dose escalation and expansion study EV-101 (ClinicalTrials.gov identifier: NCT02091999) demonstrated that enfortumab vedotin, administered on days 1, 8, and 15 of every 28-day cycle, has antitumor activity in previously treated patients with metastatic urothelial carcinoma, including those who received platinum-based chemotherapy and anti–PD-1/L1 therapy.17 Pharmacokinetic data from this study demonstrate a half-life of approximately 2 days, which supports this dosing schedule.18 EV-201, a two-cohort, single-arm, phase II study, was designed to establish the efficacy and safety of enfortumab vedotin in patients with locally advanced or metastatic urothelial carcinoma who were previously treated with anti–PD-1/L1 therapy. Cohort 1 enrolled patients who were previously treated with both platinum chemotherapy and an anti–PD-1/L1 therapy, whereas Cohort 2 continues to enroll patients who were previously treated only with an anti–PD-1/L1 therapy. Here, we report results from EV-201 Cohort 1.
METHODS
Study Participants
Patients with locally advanced or metastatic urothelial carcinoma who were previously treated with anti–PD-1/L1 therapy and age 18 years or older were eligible to enroll if they experienced progression during or after their most recent therapy, had an Eastern Cooperative Oncology Group performance status score of 1 or less, and had adequate baseline organ function. Patients with ongoing sensory or motor neuropathy grade 2 or greater, active CNS metastases, or uncontrolled diabetes were excluded. Uncontrolled diabetes was defined as hemoglobin A1C of 8% or greater or hemoglobin A1C of 7% to less than 8% with associated diabetes symptoms—polyuria or polydipsia—that were not otherwise explained. There were no limits for prior lines of therapy, including taxanes. Full eligibility criteria are available in the protocol (Data Supplement).
Trial Design
EV-201 is a global, single-arm, two-cohort, phase II multicenter study that was designed to assess the efficacy and safety of enfortumab vedotin (Fig 1). Cohort 1 enrolled platinum- and anti–PD-1/L1–treated patients with Eastern Cooperative Oncology Group performance status scores of 1 or less. Platinum treatment was defined as platinum-containing chemotherapy in the neoadjuvant and/or adjuvant setting with recurrent or progressive disease within 12 months of completion, or platinum in the locally advanced or metastatic setting.
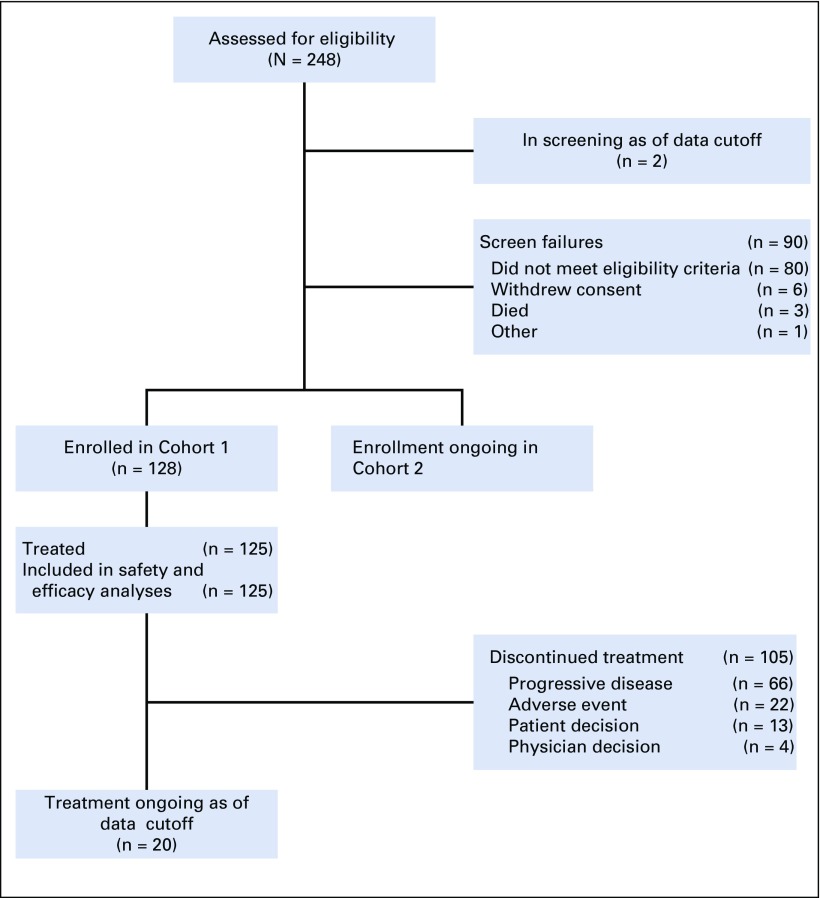
FIG 1.
CONSORT diagram. Three patients were discontinued from the study before receiving study treatment; 1 due to clinical deterioration, 1 per patient decision, and 1 due to low hemoglobin levels after screening and enrollment. This latter patient met all eligibility criteria, including adequate hemoglobin level and was enrolled in the study; however, the patient’s hemoglobin levels were subsequently found to be low and the investigator withdrew the patient from the study as a result.
Treatment
Patients received enfortumab vedotin 1.25 mg/kg intravenously over approximately 30 minutes on days 1, 8, and 15 of each 28-day cycle. Weight-based dosing was calculated using the patient’s actual body weight, with a maximum dose of 125 mg. Dose modifications were permitted to manage treatment-related hematologic and nonhematologic toxicities and are outlined in the protocol (Data Supplement). Treatment continued until disease progression, unacceptable toxicity, consent withdrawal, or investigator decision. Additional details are provided in the protocol.
Assessments
Efficacy of enfortumab vedotin was assessed by appropriate imaging (computed tomography or magnetic resonance imaging) every 8 weeks (± 1 week), then every 12 weeks (± 1 week) after 1 year. Time points for response assessments were calculated from cycle 1, day 1. Complete or partial responses, as defined by RECIST version 1.1,19 were confirmed with repeat scans 4 to 5 weeks after initial response and assessed by blinded independent central review (BICR) and investigator.
Safety assessments included physical and eye examinations, routine chemistry, and hematologic laboratory tests. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Per protocol, certain adverse events observed in the EV-201 study were prespecified for assessment and analysis as composite terms and were observed until resolved, returned to baseline, or became chronic and adequately characterized. These events are summarized here in composite terms of peripheral neuropathy, rash, infusion-related reactions, and hyperglycemia. Expression levels of Nectin-4 and PD-L1 were assessed using validated immunohistochemical assays in archival or fresh tumor samples (Data Supplement).
End Points
The Primary end point was confirmed objective response rate as assessed by BICR. Data cutoff was to be at least 6 months after the last patient in Cohort 1 received his or her first dose. Key secondary end points were duration of response and progression-free survival by BICR and investigator; objective response rate by investigator; and overall survival, safety, and tolerability.
Trial Oversight
The EV-201 trial was designed by the sponsors, with contributions from a steering committee of study investigators. Study protocol and amendments were approved by site independent review boards or ethics committees and conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Committee on Harmonization. Written informed consent was obtained from all patients. Safety was monitored by an independent data-monitoring committee and the sponsor. Data were analyzed by sponsor statisticians and interpreted by authors and the sponsor.
Statistical Analysis
Objective response rate and its two-sided 95% CI were calculated using the Clopper-Pearson method. For time-to-event end points, median survival time was estimated using the Kaplan-Meier method and the associated 95% CI was calculated using the complementary log-log transformation.
With 100 patients in Cohort 1, there is a 98% chance of observing ORR with lower-limit of the exact 95% CI excluding a historical response rate of 10%,20 if the true ORR is 25%. The complete statistical analysis plan is available along with the protocol in the Data Supplement.
RESULTS
Study Participants
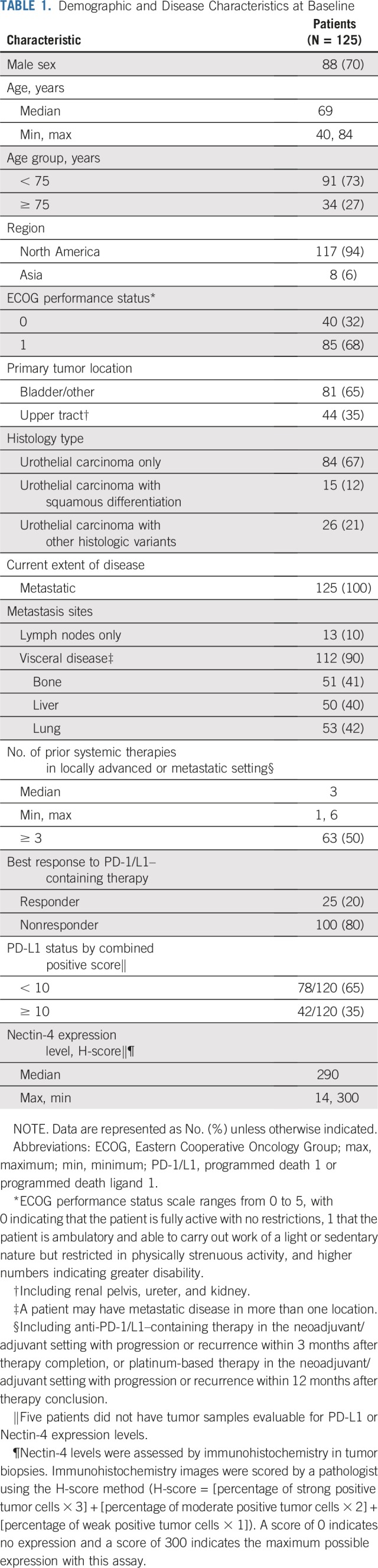
There were 51 sites in the United States and Japan during the enrollment of Cohort 1 (October 8, 2017 to July 2, 2018). A total of 128 patients with metastatic urothelial carcinoma who were previously treated with platinum and anti–PD-1/L1 therapy were enrolled. Three patients withdrew before treatment and 125 were treated with enfortumab vedotin. As of March 1, 2019, median follow-up was 10.2 months (range, 0.5 to 16.5 months). Twenty patients (16%) remain on treatment and 45 patients (36%) are in follow-up for progression or survival. Median duration of treatment was 4.6 months, and maximum duration was 15.6 months and ongoing at data cutoff. All patients who were treated had metastatic disease. Demographic and disease characteristics were representative of patients with metastatic urothelial carcinoma (Table 1 and Appendix Table A1, online only). Median age was 69 years (range, 40 to 84 years), with 27% age 75 years or older. Eighty-one percent of patients had one or more adverse prognostic factor.21 Visceral metastases were present in 90% of patients and 40% had liver metastases. Patients were heavily pretreated, with a median of three systemic therapies (range, one to six therapies) for locally advanced or metastatic disease; 26% received taxanes. Patients with only one previous therapy received platinum and anti–PD-1/L1 therapy in combination. Additional details are available in Appendix Table A1. Most patients (80%) did not respond to prior anti–PD-1/L1 therapy. All tumor biopsy samples from the 120 patients who had adequate tissue for testing had detectable Nectin-4 expression.
TABLE 1.
Demographic and Disease Characteristics at Baseline
Efficacy
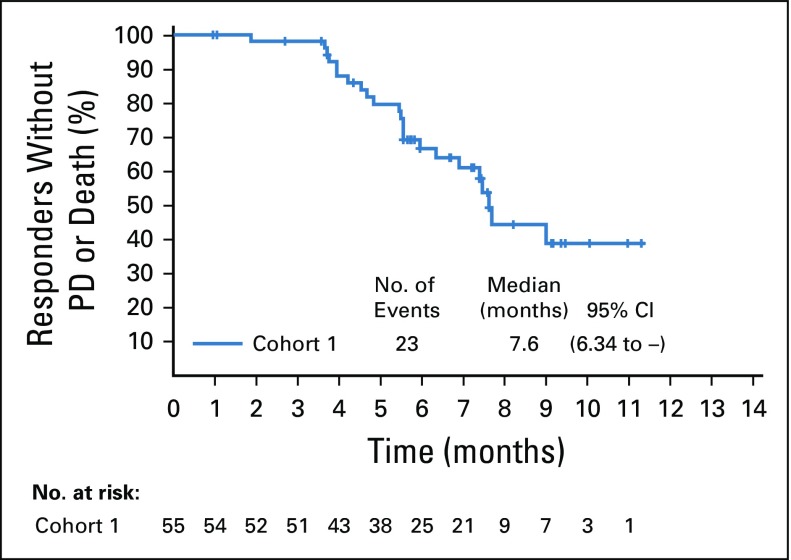
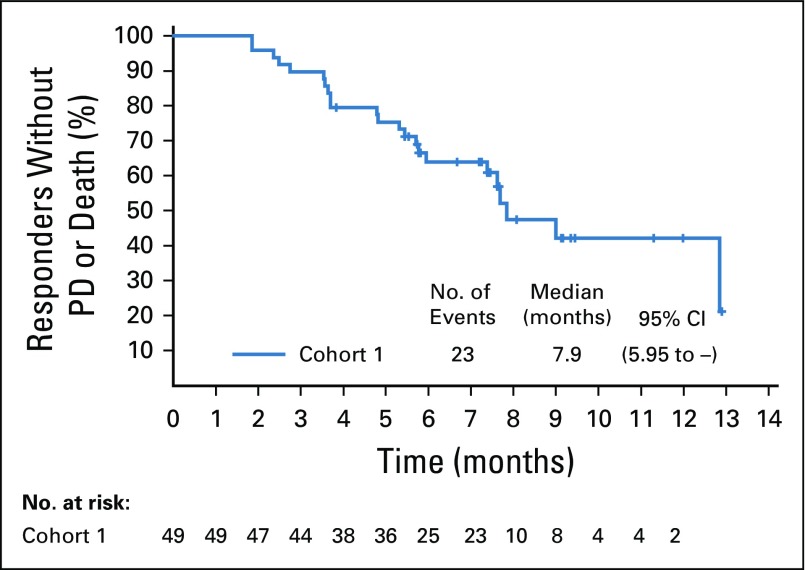
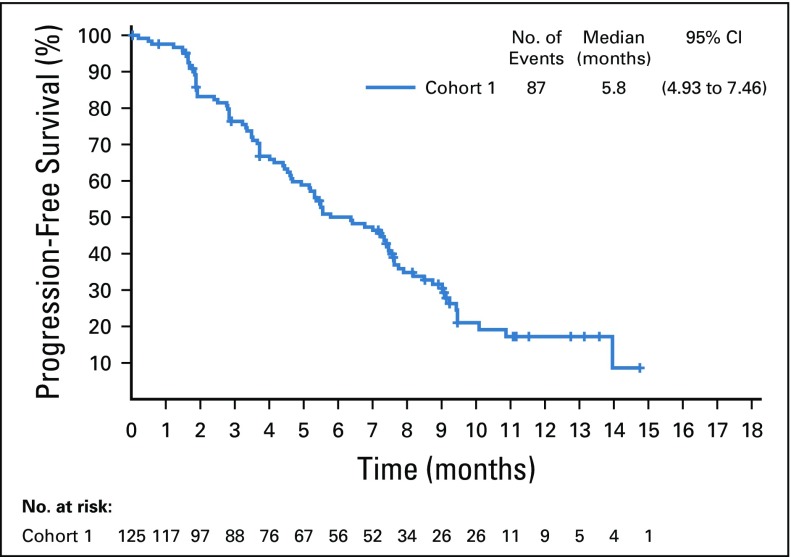
Confirmed objective response rate was 44% (95% CI, 35.1% to 53.2%) as assessed by BICR, including a 12% complete response rate (Table 2). Median time to response was 1.84 months (range, 1.2 to 9.2 months), with most responses identified by the first disease assessment. Median duration of response was 7.6 months (range, 0.95 to 11.30+; 95% CI, 4.93 to 7.46; Appendix Fig A1, online only). At the time of analysis, 44% of all responders had ongoing responses. Duration of response ranged from 3.6+ to 11.3+ months for patients with complete responses (Fig 2A). Investigator-assessed responses, including objective response rate, duration of response, tumor reduction, and progression-free survival, were similar to those assessed by BICR (Data Supplement; Appendix Table A2, online only; and Appendix Figs A2, A3, and A4, online only).
TABLE 2.
Summary of Responses Per Blinded Independent Central Review

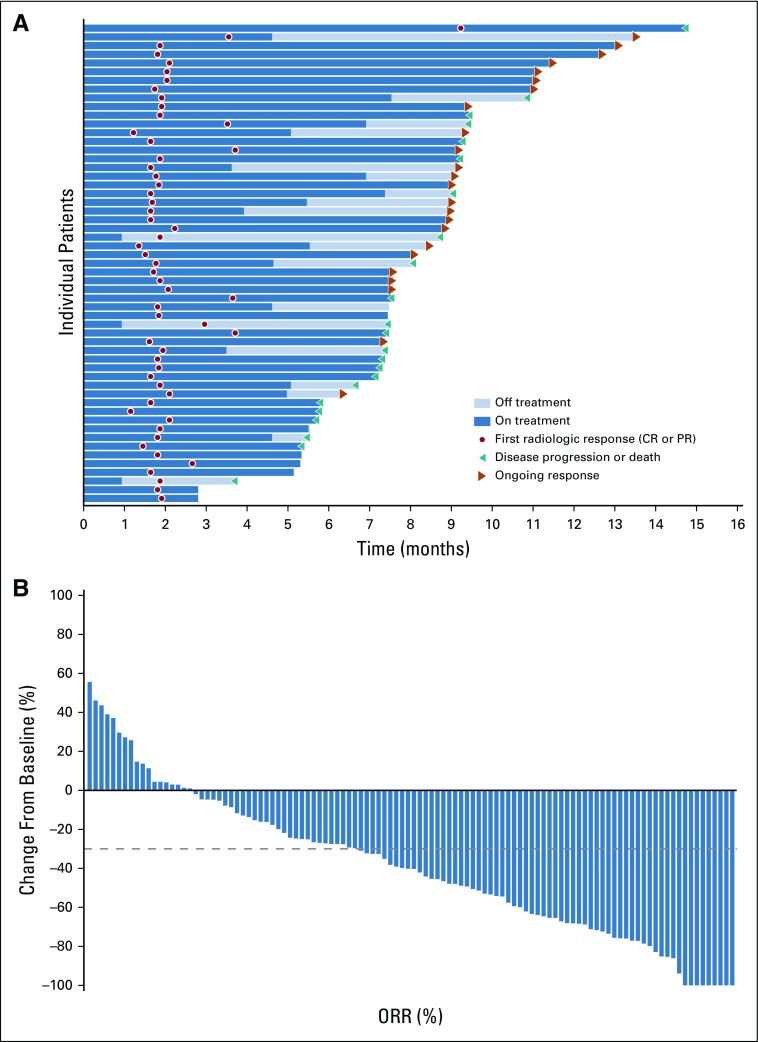
FIG 2.
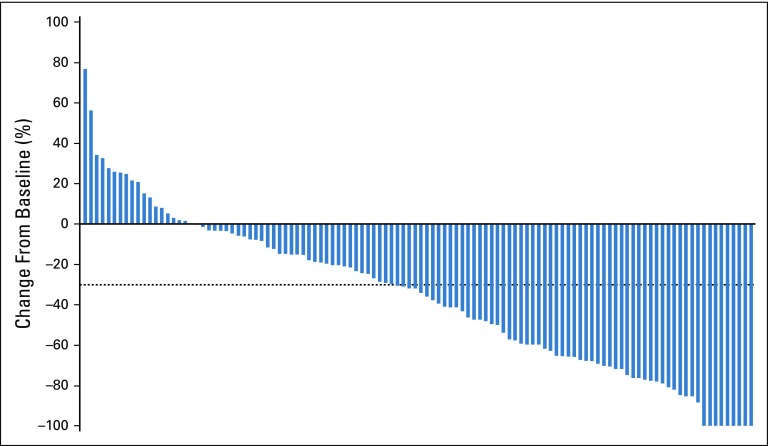
Response among patients with metastatic urothelial carcinoma per blinded independent central review. (A) Swimmer plot of the objective responses (n = 55) (according to RECIST v1.1.) from the start of treatment to disease progression, as determined by blinded independent central review, or death. At the time of analysis, 44% of responders had ongoing responses. (B) Waterfall plot of the best percentage of change from baseline in the sum of the diameters of target lesions as identified per RECIST v1.1. Target lesions were reduced in 84% of patients (92 of 110) who were evaluable—that is, had target lesions and adequate postbaseline assessment). Dashed line indicates threshold for partial response (−30%), but is not necessarily indicative of response. CR, complete response; ORR, overall response rate; PR, partial response.
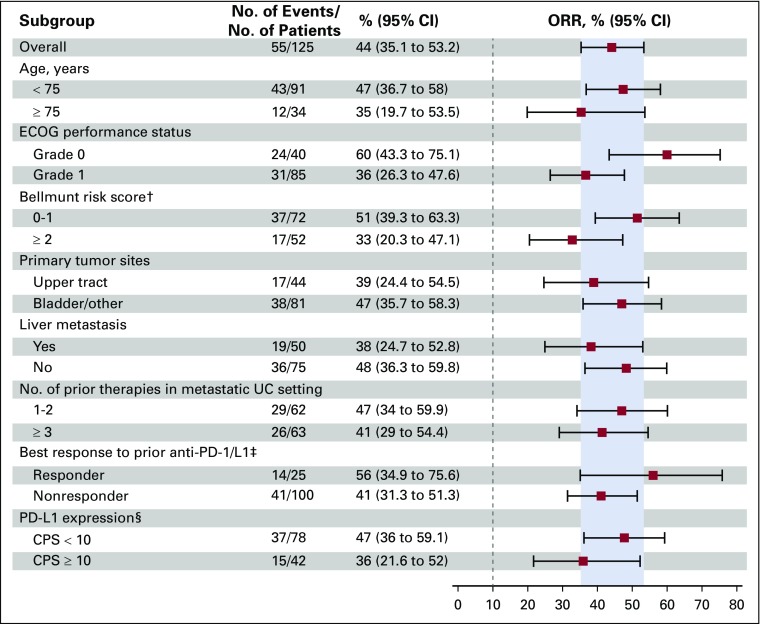
Responses across all subgroups analyzed were consistent with overall study results. Objective responses occurred regardless of patients’ responses to prior anti–PD-1/L1 therapy (56% in responders and 41% in nonresponders). Similar responses were observed in patients with poor prognostic characteristics, including liver metastases (38%), and three or more prior lines of therapy (41%; Fig 3).
FIG 3.
Objective response in key prespecified subgroups per blinded independent central review. This prespecified subgroup analysis was performed on the full analysis set of all patients who received any amount of enfortumab vedotin (N = 125). Historical control response rate is 10%, as indicated by dashed line.20 The programmed death ligand 1 (PD-L1) combined positive score (CPS) was defined as the percentage of tumor and infiltrating immune cells with PD-L1 expression of the total number of tumor cells. The upper tract was defined as the renal pelvis, ureter, and kidney. Data are given as No. (%), unless otherwise noted. (†) Bellmunt risk score was not available for 1 patient. (‡) Anti-PD-1 or anti-PD-L1 therapy. (§) Five patients did not have tumor samples evaluable for PD-L1 expression levels. ECOG, Eastern Cooperative Oncology Group; ORR, objective response rate; UC, urothelial carcinoma.
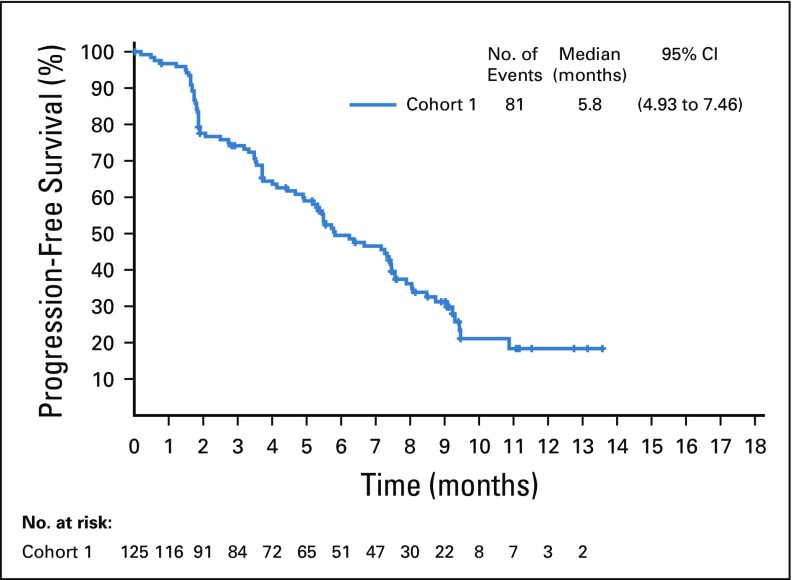
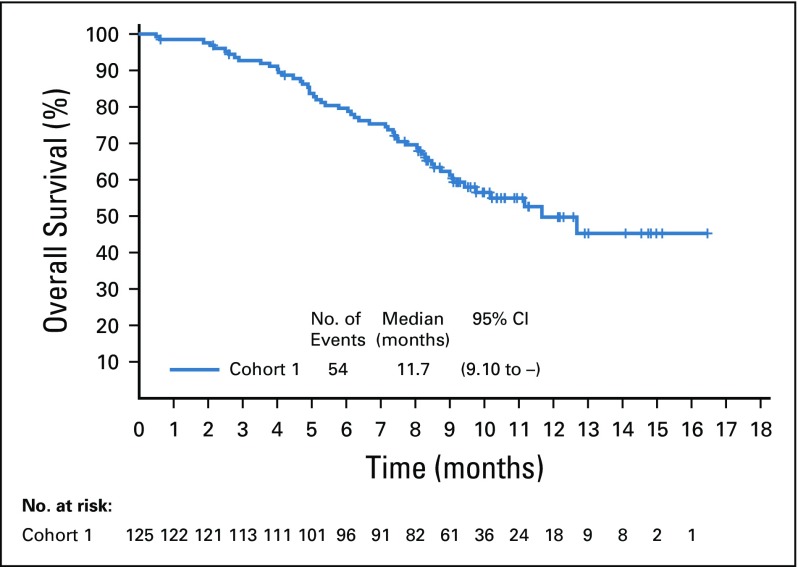
Target lesions were reduced in a majority of evaluable patients (84%; Fig 2B). Estimated median progression-free survival was 5.8 months (95% CI, 4.9 to 7.5 months; Appendix Fig A5, online only), and estimated median overall survival was 11.7 months (95% CI, 9.1 months to not reached; Appendix Fig A6, online only).
Safety
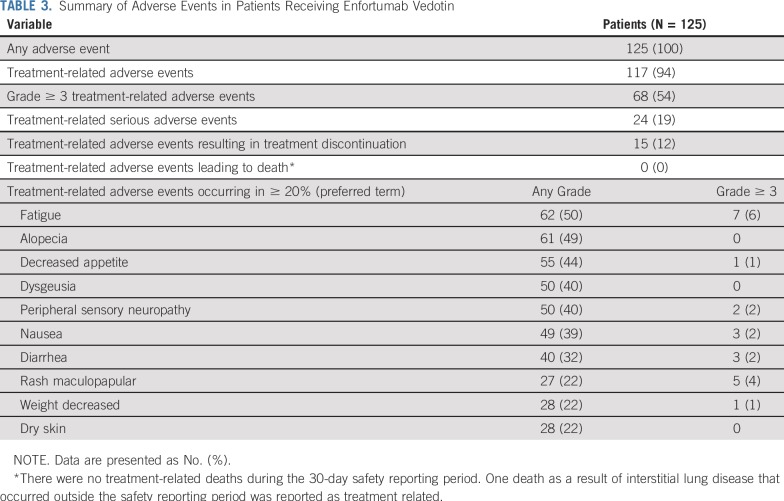
The most common treatment-related adverse events were fatigue (50% all grade and 6% grade ≥ 3), alopecia (49% all grade), decreased appetite (44% all grade and 1% grade ≥ 3), dysgeusia (40% all grade and none grade ≥ 3), and peripheral sensory neuropathy (40% all grade and 2% grade ≥ 3; Table 3). The most common grade 3 or greater treatment-related adverse events were neutropenia (8%), anemia (7%), and fatigue (6%). Febrile neutropenia (4%) was the most common serious treatment-related adverse event; there was no routine growth factor use. A full listing of adverse events is available in Appendix Tables A3 and A4 (online only). Treatment-related adverse events led to dose reductions in 32% of patients and discontinuation in 12% of patients. Peripheral sensory neuropathy was the most common treatment-related adverse event that led to dose reduction (9%) and discontinuation (6%).
TABLE 3.
Summary of Adverse Events in Patients Receiving Enfortumab Vedotin
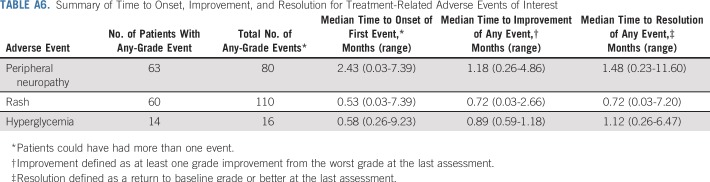
Peripheral neuropathy, rash, hyperglycemia, and infusion-related reactions were prespecified for analysis as composite terms (Appendix Table A5, online only). A summary of time to onset and time to resolution for these events is available in Appendix Table A6 (online only). Treatment-related peripheral neuropathy occurred in 50% of patients, almost all (94%) of which were grade 2 or less. Peripheral sensory neuropathy was more common (44%) than motor neuropathy (14%). Of the 42 patients with peripheral neuropathy at enrollment, 20 (48%) did not experience worsening from baseline. Most patients (76%) with peripheral neuropathy had resolution or ongoing grade 1 peripheral neuropathy at last follow-up.
Treatment-related rash—as a composite term—occurred in 48% of patients, most of which were low grade (75% grade ≤ 2) with onset in the first treatment cycle. Two patients discontinued treatment as a result of rash, one of whom experienced a grade 3 rash reported as Stevens-Johnson syndrome. Onset of symptoms for this event was 4 days after the initial dose and the rash resolved after the discontinuation of enfortumab vedotin and treatment with systemic corticosteroids. Of all patients who experienced rash, 73% experienced complete resolution and 20% had some improvement at last follow-up. Most patients (75%) with ongoing rash had grade 1 at last follow-up. Three patients had infusion site extravasation, of which two cases were considered serious. All patients with extravasation recovered completely and were able to continue treatment.
Treatment-related hyperglycemia occurred in few patients (11%), regardless of known hyperglycemia at baseline. Nineteen patients had hyperglycemia at baseline and, of these, 68% did not develop treatment-related events. Of patients without hyperglycemia at baseline, 8% developed treatment-related hyperglycemia. Hyperglycemia in seven of 14 patients with these events was grade 2 or less. The single patient with grade 4 hyperglycemia did not have known baseline hyperglycemia and, per protocol, treatment was discontinued. The patient later recovered and had no ongoing need for insulin or oral hypoglycemic agents. This was the only discontinuation as a result of hyperglycemia. Among patients who experienced hyperglycemia, 57% achieved complete resolution and 14% experienced some improvement.
There were no treatment-related deaths during the 30-day safety reporting period. One death as a result of interstitial lung disease that occurred outside the safety reporting period was reported as treatment related. This death was confounded by prolonged high-dose corticosteroid use and suspected Pneumocystis jiroveci pneumonia.
DISCUSSION
In patients with metastatic urothelial carcinoma who were previously treated with both platinum chemotherapy and anti–PD-1/L1 therapy, enfortumab vedotin treatment led to a 44% objective response rate, including a 12% complete response rate and a 7.6-month duration of response. Most responses to enfortumab vedotin occurred rapidly. Although this was a single-arm study, which limits interpretation, responses observed here with enfortumab vedotin were remarkably consistent with the prior phase I study EV-101.17
In the control arms of recent randomized phase III trials in the postplatinum setting, objective response rates in patients who were treated with antimicrotubule agents ranged from 11% to 13%, including 3% complete responses.5,6 Unlike these phase III trials, which primarily enrolled patients with only prior platinum therapy, patients who received enfortumab vedotin in this study were more heavily pretreated, with one half of patients receiving three or more lines of therapy, one of which was an anti-PD-1/L1 therapy. In a subset of patients who were previously treated with both platinum and anti–PD-1/L1 therapy from a randomized phase III trial, docetaxel had a 10.5% response rate.23 Although the single-arm nature of EV-201 limits the ability to compare the activity of enfortumab vedotin with standard antimicrotubule chemotherapy, differences in observed response rates (44%) and complete response rates (12%), as well as the consistent results across EV-101 and EV-201, suggest that enfortumab vedotin possesses antitumor effects significantly beyond conventional chemotherapy. In fact, the objective response rate of enfortumab vedotin monotherapy in this study is similar to that of gemcitabine and carboplatin in the first-line setting, which suggests that treatment earlier in the disease course should be explored in clinical trials.3
Enfortumab vedotin also had consistent clinical activity across all subgroups analyzed, including patients with traditionally challenging features, such as liver metastases or other poor prognostic factors. Responses were observed regardless of previous response to anti–PD-1/L1 therapy. These data demonstrate the ability of enfortumab vedotin to elicit responses across a broad range of patients with different disease characteristics.
Enfortumab vedotin was generally well tolerated in this patient population; most treatment-related adverse events were of mild to moderate severity. No single treatment-related adverse event grade 3 or greater occurred in 10% or more of patients, and there were relatively few discontinuations because of a treatment-related adverse event. One treatment-related death occurred outside of the safety reporting period and there were no other treatment-related deaths.
Peripheral neuropathy observed with enfortumab vedotin was generally low grade and manageable. Most patients who developed peripheral neuropathy had either resolution or symptoms ongoing at grade 1 at last follow-up. Peripheral neuropathy is a known toxicity associated with MMAE-containing antibody–drug conjugates, such as brentuximab vedotin24; however, these two MMAE-containing antibody–drug conjugates have distinct targets in different patient populations. Therefore, on-target toxicities are expected to differ.
Because enfortumab vedotin targets Nectin-4, which is expressed in skin,8 rash is an anticipated on-target toxicity. Rashes observed with enfortumab vedotin were generally low grade and manageable, often demonstrating a maculopapular and diffuse appearance. Management included topical corticosteroids, oral antihistamines, and, in some cases, systemic corticosteroids, as well as enfortumab vedotin dose reductions and delays. Nearly all patients with rash had resolution or improvement and most ongoing treatment-related rashes were grade 1 at last follow-up. The one reported case of Stevens-Johnson syndrome may have been confounded by the direct effects of enfortumab vedotin on Nectin-4 in skin. Hyperglycemia was much less common than rash or peripheral neuropathy, and most patients experienced resolution or improvement at last follow-up. Treatment-related hyperglycemia occurred regardless of known hyperglycemia at baseline and the underlying etiology remains unclear but is not likely to be an on-target effect.
An ongoing phase III trial comparing enfortumab vedotin monotherapy with single-agent chemotherapy in patients with prior platinum and anti-PD-1/L1 therapy may establish the survival benefit of enfortumab vedotin in this patient population (EV-301; ClinicalTrials.gov identifier: NCT03474107). The EV-201 study is also actively enrolling a second cohort (Cohort 2) of patients who have received prior anti–PD-1/L1 therapy and are cisplatin ineligible without prior platinum treatment to determine if a similar benefit will be observed. In addition, enfortumab vedotin is being evaluated in a broader population of patients with urothelial carcinoma, including in the first-line setting where it is being studied in combination with anti–PD-1 and/or platinum-based therapies (EV-103; ClinicalTrials.gov identifier: NCT03288545). In this study, enfortumab vedotin is administered on days 1 and 8 of a 21-day cycle to coincide with the administration of the other agents. Nectin-4 is also expressed in other tumor types, and enfortumab vedotin may be explored in other solid tumors.8
In conclusion, enfortumab vedotin is the first antibody–drug conjugate targeting Nectin-4 in clinical development, and the antitumor activity observed in EV-201 validates Nectin-4 as a therapeutic target in urothelial carcinoma. In Cohort 1 patients who previously received platinum and anti–PD-1/L1 therapies, enfortumab vedotin has a 44% objective response rate and a 12% complete response rate. Data reported here demonstrate that enfortumab vedotin has the potential to change the treatment landscape of metastatic urothelial carcinoma.
ACKNOWLEDGMENT
Writing assistance was provided by Heather Brignull, PhD, and Candice L. Willmon, PhD, of Seattle Genetics.
Appendix
This appendix has been provided by the authors to give readers additional information about their work.
EV-201 Investigators
The following investigators (listed by country) participated in the EV-201 study:
France: Yohann Loriot; Germany: Jens Bedke; Italy: Andrea Necchi; Japan: Satoshi Fukasawa, Satoshi Fukasawa, Yasuhiro Hashimoto, Junichi Inokuchi, Hiro-omi Kanayama, Takahiro Kojima, Ryuichi Mizuno, Kazuo Nishimura, Wataru Obara, Yoshihiko Tomita, Yoshiaki Yamamoto, Akira Yokomizo, and Kazuhiro Yoshimura; the Netherlands: Michiel van der Heijden; South Korea: Yu Jung Kim, Hyo Jin Lee, Jae Lyun Lee, Se Hoon Park, and Sang Joon Shin; Spain: Ignacio Duran; United States: Leonard Appleman, Arjun Balar, Britt Bolemon, John Burke, Daniel Chong, Jorge Darcourt, Nancy Davis, Christopher DiSimone, Robert Dreicer, Nicholas Farrell, Mark Fleming, Chunkit Fung, Matthew Galsky, Noah Hahn, Elisabeth Heath, Thomas Hutson, William Kelly, Nataliya Mar, Bradley McGregor, Megan McNamara, Amir Mortazavi, Samuel Myrick, Peter O'Donnell, Moshe Ornstein, Chong-Xian Pan, Daniel Petrylak, Joel Picus, David Quinn, Arash Rezazadeh, Jonathan Rosenberg Ian Schnadig, David Shaffer, Parminder Singh, Mark Stein, Jennifer Suga, Nicholas Vogelzang, Jeffrey Yorio, Evan Yu, and Jingsong Zhang.
Methods
Patient populations for analysis.
The full analysis set (FAS) included all patients who were enrolled in the study who received any amount of enfortumab vedotin. The FAS was used as the primary analysis set for efficacy end points. The safety analysis set included all patients who received any amount of enfortumab vedotin and was therefore used for all safety analyses.
Biomarker assessments.
Samples for exploratory biomarkers were collected at protocol-specified timepoints defined in the schedule of events. Biomarker assessments were not used for patient selection.
Nectin-4 levels were assessed by immunohistochemistry in tumor biopsies. Immunohistochemistry images were scored by a pathologist using the H-score method (H-score = [percentage of strong positive tumor cells × 3] + [percentage of moderate positive tumor cells × 2] + [percentage of weak positive tumor cells × 1]). All evaluable patients (120 of 120) had detectable Nectin-4 on archival or fresh tumor samples by immunohistochemistry as determined by H-score. Nectin-4 expression was high, with a median H-score of 290 (range, 14 to 300).
Programmed death ligand 1 levels were assessed in tumor-infiltrating immune cells using DAKO 22C3 immunohistochemistry to determine PD-L1 combined positive score of less than 10 versus 10 or greater in archival or fresh tumor samples. Overall, 78 (65%) of 120 evaluable patients had a programmed death ligand 1 combined positive score of less than 10.
FIG A1.
Kaplan-Meier estimate of duration of response for responders per blinded independent central review. PD, progressive disease.
FIG A2.
Kaplan-Meier estimate of duration of response for responders per investigator assessment. PD, progressive disease.
FIG A3.
Waterfall plot of the best percentage change from baseline of target lesions per investigator. Waterfall plot of the best percentage of change from baseline in the sum of the diameters of target lesions according to RECIST,19 version 1.1, per investigator. Overall, 114 patients were evaluable for target lesion response, and 11 patients were not evaluable. Dashed line indicates approximate threshold for partial response (−30%), but is not necessarily indicative of response. ORR, overall response rate.
FIG A4.
Kaplan-Meier estimate of progression-free survival per investigator in the full analysis set.
FIG A5.
Kaplan-Meier estimate of progression-free survival per blinded independent central review in the full analysis set.
FIG A6.
Kaplan-Meier estimate of overall survival in the full analysis set.
TABLE A1.
Summary of Demographics and Disease Characteristics at Baseline
TABLE A2.
Summary of Responses Per Investigator in the Full Analysis Set

TABLE A3.
Treatment-Related Adverse Events Occurring in ≥ 10% of Patients
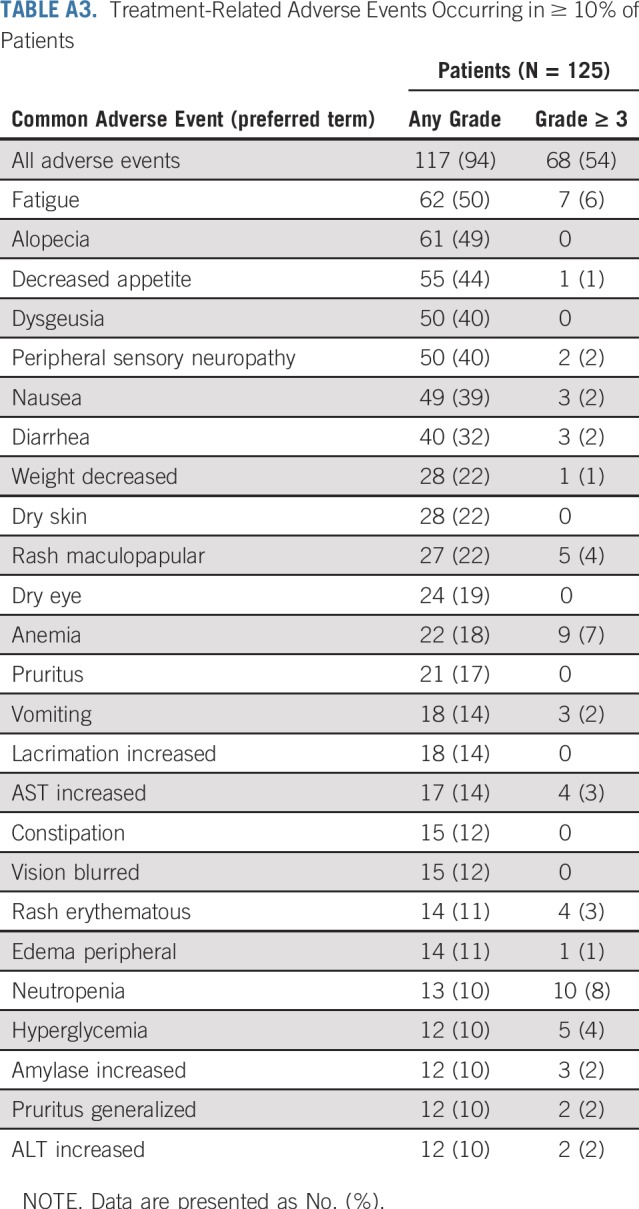
TABLE A4.
All Adverse Events Occurring in ≥ 10% of Patients
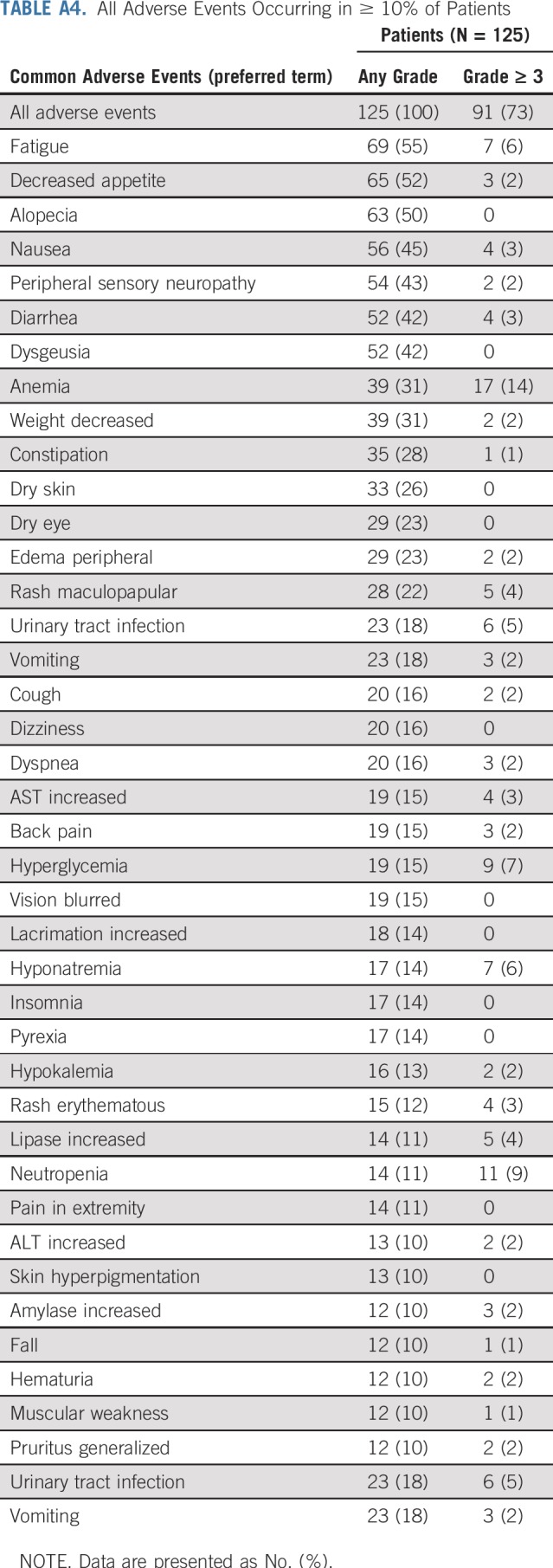
TABLE A5.
Search Terms Used for Composite Adverse Events
TABLE A6.
Summary of Time to Onset, Improvement, and Resolution for Treatment-Related Adverse Events of Interest
Footnotes
Supported by Seattle Genetics and Astellas Pharma.
Clinical trial information: NCT03219333.
Processed as a Rapid Communication manuscript.
See accompanying article on page 2587
AUTHOR CONTRIBUTIONS
Conception and design: Jonathan E. Rosenberg, Elisabeth I. Heath, Elaina M. Gartner, Shang-Ying Liang, Amal Melhem-Bertrandt, Daniel P. Petrylak
Provision of study material or patients: Jonathan E. Rosenberg, Peter H. O'Donnell, Arjun V. Balar, Bradley A. McGregor, Elisabeth I. Heath, Evan Y. Yu, Matthew D. Galsky, Noah M. Hahn, Daniel P. Petrylak
Collection and assembly of data: Jonathan E. Rosenberg, Peter H. O'Donnell, Arjun V. Balar, Bradley A. McGregor, Elisabeth I. Heath, Evan Y. Yu, Matthew D. Galsky, Noah M. Hahn, Elaina M. Gartner, Juan M. Pinelli, Shang-Ying Liang, Amal Melhem-Bertrandt, Daniel P. Petrylak
Data analysis and interpretation: Jonathan E. Rosenberg, Peter H. O’Donnell, Arjun V. Balar, Bradley A. McGregor, Elisabeth I. Heath, Evan Y. Yu, Matthew D. Galsky, Noah M. Hahn, Elaina M. Gartner, Juan M. Pinelli, Shang-Ying Liang, Amal Melhem-Bertrandt, Daniel P. Petrylak
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jonathan E. Rosenberg
Stock and Other Ownership Interests: Merck, Illumina
Honoraria: UpToDate, Bristol-Myers Squibb, AstraZeneca, Medscape, Vindico, Peerview, Chugai Pharma, Gilmore Philips, Research to Practice, Clinical Care Options, Clinical Mind, Intellisphere
Consulting or Advisory Role: Eli Lilly, Merck, Agensys, Genentech, Sanofi, AstraZeneca, MedImmune, Bristol-Myers Squibb, EMD Serono, Seattle Genetics, Bayer, Inovio Pharmaceuticals, BioClin Therapeutics, QED Therapeutics, Adicet Bio, Sensei Biotherapeutics, Fortress Biotech, Pharmacyclics, Western Oncolytics, GlaxoSmithKline, Astellas, Janssen
Research Funding: Genentech (Inst), Oncogenex (Inst), Agensys (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Viralytics (Inst), Genentech (Inst), Incyte (Inst), Seattle Genetics (Inst), Bayer (Inst), AstraZeneca (Inst), QED (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
Travel, Accommodations, Expenses: Genentech, Bristol-Myers Squibb
Peter H. O'Donnell
Stock and Other Ownership Interests: Allergan, PrescriptIQ
Honoraria: Genentech, Merck, AstraZeneca, Astellas Pharma, Seattle Genetics, Inovio Pharmaceuticals, Janssen Biotech, Parexel, Kantar Health, Harrison Consulting Group, Quintiles, OncLive
Consulting or Advisory Role: Merck
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), Genentech (Inst), AstraZeneca (Inst), MedImmune (Inst), Acerta Pharma (Inst), Janssen Pharmaceuticals (Inst), Seattle Genetics (Inst), Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Coinventor on a pending patent for a genomic prescribing system for medication prescribing
Travel, Accommodations, Expenses: Merck, Seattle Genetics, Astellas Pharma
Other Relationship: Advance Medical, Janssen Pharmaceuticals
Arjun V. Balar
Honoraria: Merck, Genentech, AstraZeneca, MedImmune
Consulting or Advisory Role: Genentech, Merck, Cerulean Pharma, AstraZeneca, MedImmune, Pfizer, EMD Serono, Incyte, Seattle Genetics, Astellas Pharma, Nektar
Research Funding: Merck (Inst), Genentech (Inst), AstraZeneca (Inst), MedImmune (Inst), Seattle Genetics
Bradley A. McGregor
Consulting or Advisory Role: Bayer, Seattle Genetics, Astellas Pharma, Exelixis, AstraZeneca, Genentech, Nektar, Janssen Oncology, Pfizer, EMD Serono
Research Funding: Bristol-Myers Squibb (Inst), Exelixis (Inst), Calithera Biosciences (Inst), Seattle Genetics (Inst), Astellas Pharma (Inst)
Elisabeth I. Heath
Honoraria: Bayer, Dendreon, Sanofi, Seattle Genetics, Agensys
Speakers' Bureau: Sanofi
Research Funding: Tokai Pharmaceuticals (Inst), Seattle Genetics (Inst), Agensys (Inst), Dendreon (Inst), Genentech (Inst), Millennium Pharmaceuticals (Inst), Celldex (Inst), Inovio Pharmaceuticals (Inst), Celgene (Inst), Zenith Epigenetics (Inst), Merck (Inst), AstraZeneca (Inst), Esanik (Inst), Oncolys BioPharma (Inst), Curemeta (Inst), Bristol-Myers Squibb (Inst), eFFECTOR Therapeutics (Inst), Fortis (Inst), Astellas Pharma (Inst), Medivation (Inst), Ignyta (Inst), Synta (Inst), Caris Life Sciences (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Merck Sharp & Dohme (Inst), Plexxikon (Inst), Corcept Therapeutics (Inst)
Other Relationship: Caris Centers of Excellence
Evan Y. Yu
Consulting or Advisory Role: Janssen Pharmaceuticals, Bayer, Merck, AstraZeneca, EMD Serono, Incyte, Amgen, Tolmar, QED Therapeutics, Dendreon, Seattle Genetics, Pharmacyclics
Research Funding: Agensys (Inst), Astellas Pharma (Inst), Dendreon (Inst), Genentech (Inst), Bayer (Inst), Merck (Inst), Seattle Genetics (Inst), Daiichi Sankyo
Matthew D. Galsky
Stock and Other Ownership Interests: Rappta Therapeutics
Consulting or Advisory Role: BioMotiv, Janssen Pharmaceuticals, Dendreon, Merck, GlaxoSmithKline, Eli Lilly, Astellas Pharma, Genentech, Bristol-Myers Squibb, Novartis, Pfizer, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Aileron Therapeutics, Dracen, Inovio Pharmaceuticals, NuMab, Dragonfly Therapeutics
Research Funding: Janssen Oncology (Inst), Dendreon (Inst), Novartis (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst), Genentech (Inst)
Patents, Royalties, Other Intellectual Property: Methods and compositions for treating cancer and related methods; application number: 20120322792
Noah M. Hahn
Honoraria: Bladder Cancer Academy, PeerView
Consulting or Advisory Role: Bristol-Myers Squibb, AstraZeneca, MedImmune, Pieris Pharmaceuticals, Inovio Pharmaceuticals, Genentech, Health Advances, Merck, Ferring, Principia Biopharma, Champions Oncology, Taris BioMedical, Seattle Genetics, Astellas Pharma, Incyte, TransMed, Rexahn Pharmaceuticals, CicloMed, Janssen Pharmaceuticals
Research Funding: Genentech (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), MedImmune (Inst), Principia Biopharma (Inst), Acerta Pharma (Inst), Incyte (Inst), Seattle Genetics (Inst), Astellas Pharma (Inst), Astex Pharmaceuticals (Inst), Pieris Pharmaceuticals (Inst)
Elaina M. Gartner
Employment: Seattle Genetics
Stock and Other Ownership Interests: Seattle Genetics
Juan M. Pinelli
Employment: Seattle Genetics, Astellas Pharma
Stock and Other Ownership Interests: Seattle Genetics
Shang-Ying Liang
Employment: Seattle Genetics
Stock and Other Ownership Interests: Seattle Genetics
Amal Melhem-Bertrandt
Employment: Astellas Pharma
Daniel P. Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, TYME
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Eli Lilly, Ada Cap, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis Oncology, Incyte, Janssen Pharmaceuticals, Pharmacyclics, Seattle Genetics, Urogen Pharma
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Eli Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Ada Cap (Inst), Bristol-Myers Squibb (Inst)
Expert Testimony: Celgene, Sanofi
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Cancer Institute . SEER Cancer Stat Facts: Bladder Cancer. Bethesda, MD: National Cancer Institute; 2019. [Google Scholar]
- 2.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 3.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–199. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30:1107–1113. doi: 10.1200/JCO.2011.38.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Bladder cancer (version .2.2019) https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf
- 8.Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016;76:3003–3013. doi: 10.1158/0008-5472.CAN-15-1313. [DOI] [PubMed] [Google Scholar]
- 9.Doronina SO, Toki BE, Torgov MY, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [Erratum: Nat Biotechnol 21:941, 2003] [DOI] [PubMed] [Google Scholar]
- 10.Mandai K, Rikitake Y, Mori M, et al. Nectins and nectin-like molecules in development and disease. Curr Top Dev Biol. 2015;112:197–231. doi: 10.1016/bs.ctdb.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Takai Y, Ikeda W, Ogita H, et al. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- 12.Takai Y, Miyoshi J, Ikeda W, et al. Nectins and nectin-like molecules: Roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhang J, Shen Q, et al. High expression of nectin-4 is associated with unfavorable prognosis in gastric cancer. Oncol Lett. 2018;15:8789–8795. doi: 10.3892/ol.2018.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lattanzio R, Ghasemi R, Brancati F, et al. Membranous nectin-4 expression is a risk factor for distant relapse of T1-T2, N0 luminal-A early breast cancer. Oncogenesis. 2014;3:e118. doi: 10.1038/oncsis.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takano A, Ishikawa N, Nishino R, et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009;69:6694–6703. doi: 10.1158/0008-5472.CAN-09-0016. [DOI] [PubMed] [Google Scholar]
- 16.Pavlova NN, Pallasch C, Elia AE, et al. A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife. 2013;2:e00358. doi: 10.7554/eLife.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg JE, Sridhar SS, Zhang J, et al. Mature results from EV-101: A phase I study of enfortumab vedotin in patients with metastatic urothelial cancer (mUC) J Clin Oncol. 2019;37(abstr 377) [Google Scholar]
- 18.Rosenberg JE, Heath E, Perez R, et al. Interim analysis of a phase I dose escalation trial of ASG-22CE (ASG-22ME; enfortumab vedotin), an antibody drug conjugate (ADC), in patients (Pts) with metastatic urothelial cancer (mUC) Ann Oncol. 2016;27(abstr 788P) [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Vaughn DJ, Broome CM, Hussain M, et al. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol. 2002;20:937–940. doi: 10.1200/JCO.2002.20.4.937. [DOI] [PubMed] [Google Scholar]
- 21.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 22.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 23.Drakaki A, Kirby CJ, Van der Heijden MS, et al. Docetaxel with or without ramucirumab after immune checkpoint inhibition in platinum-refractory metastatic urothelial carcinoma (mUC): Prespecified subgroup analysis from the phase 3 RANGE trial. J Clin Oncol. 2018;36(abstr 434) [Google Scholar]
- 24.Masters JC, Nickens DJ, Xuan D, et al. Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Invest New Drugs. 2018;36:121–135. doi: 10.1007/s10637-017-0520-6. [DOI] [PubMed] [Google Scholar]