Key Points
Question
Does intra-articular administration of sprifermin structurally modify cartilage, as measured by cartilage thickness on quantitative magnetic resonance imaging, in patients with knee osteoarthritis?
Findings
In this dose-finding trial including 549 participants randomized to 30 μg or 100 μg of sprifermin every 6 or 12 months vs placebo, there was a significant increase after 2 years in total femorotibial cartilage thickness for 100 μg of sprifermin every 6 months (0.05 mm) and every 12 months (0.04 mm).
Meaning
Compared with placebo, intra-articular administration of 100 μg of sprifermin every 6 or 12 months resulted in improvement in femorotibial joint cartilage thickness after 2 years that was statistically significant, but of uncertain clinical importance; the durability of response also was uncertain.
Abstract
Importance
Sprifermin is under investigation as a disease-modifying osteoarthritis drug.
Objective
To evaluate the effects of sprifermin on changes in total femorotibial joint cartilage thickness in the more symptomatic knee of patients with osteoarthritis.
Design, Setting, and Participants
FORWARD (FGF-18 Osteoarthritis Randomized Trial with Administration of Repeated Doses) was a 5-year, dose-finding, multicenter randomized clinical trial conducted at 10 sites. Eligible participants were aged 40 to 85 years with symptomatic, radiographic knee osteoarthritis and Kellgren-Lawrence grade 2 or 3. Enrollment began in July 2013 and ended in May 2014; the last participant visit occurred on May 8, 2017. The primary outcome at 2 years and a follow-up analysis at 3 years are reported.
Interventions
Participants were randomized to 1 of 5 groups: intra-articular injections of 100 μg of sprifermin administered every 6 months (n = 110) or every 12 months (n = 110), 30 μg of sprifermin every 6 months (n = 111) or every 12 months (n = 110), or placebo every 6 months (n = 108). Each treatment consisted of weekly injections over 3 weeks.
Main Outcomes and Measures
The primary end point was change in total femorotibial joint cartilage thickness measured by quantitative magnetic resonance imaging at 2 years. The secondary end points (of 15 total) included 2-year change from baseline in total Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores. The minimal clinically important difference (MCID) is unknown for the primary outcome; for total WOMAC score in patients with hip and knee osteoarthritis, the absolute MCID is 7 U (95% CI, 4 to 10 U) and the percentage MCID is 14% (95% CI, 9% to 18%).
Results
Among 549 participants (median age, 65.0 years; 379 female [69.0%]), 474 (86.3%) completed 2-year follow-up. Compared with placebo, the changes from baseline to 2 years in total femorotibial joint cartilage thickness were 0.05 mm (95% CI, 0.03 to 0.07 mm) for 100 μg of sprifermin administered every 6 months; 0.04 mm (95% CI, 0.02 to 0.06 mm) for 100 μg of sprifermin every 12 months; 0.02 mm (95% CI, −0.01 to 0.04 mm) for 30 μg of sprifermin every 6 months; and 0.01 mm (95% CI, −0.01 to 0.03 mm) for 30 μg of sprifermin every 12 months. Compared with placebo, there were no statistically significant differences in mean absolute change from baseline in total WOMAC scores for 100 μg of sprifermin administered every 6 months or every 12 months, or for 30 μg of sprifermin every 6 months or every 12 months. The most frequently reported treatment-emergent adverse event was arthralgia (placebo: n = 46 [43.0%]; 100 μg of sprifermin administered every 6 months: n = 45 [41.3%]; 100 μg of sprifermin every 12 months: n = 50 [45.0%]; 30 μg of sprifermin every 6 months: n = 40 [36.0%]; and 30 μg of sprifermin every 12 months: n = 48 [44.0%]).
Conclusions and Relevance
Among participants with symptomatic radiographic knee osteoarthritis, the intra-articular administration of 100 μg of sprifermin every 6 or 12 months vs placebo resulted in an improvement in total femorotibial joint cartilage thickness after 2 years that was statistically significant, but of uncertain clinical importance; there was no significant difference for 30 μg of sprifermin every 6 or 12 months vs placebo. Durability of response also was uncertain.
Trial Registration
ClinicalTrials.gov Identifier: NCT01919164
This randomized clinical trial compares the effects of 2 doses of sprifermin, a recombinant human fibroblast growth factor 18, vs placebo on changes in total femorotibial joint cartilage thickness in the knees of patients with osteoarthritis.
Introduction
Osteoarthritis most commonly affects the knee joints.1 Symptomatic knee osteoarthritis is associated with physical disability, reduced quality of life, and increased mortality in older adults.1,2
Osteoarthritis is characterized by articular cartilage loss, joint tissue remodeling, and inflammatory changes in the synovial membrane.3 Articular cartilage loss is an accepted measure of structural disease progression, and is assessed directly by magnetic resonance imaging (MRI) or indirectly by radiographs.4,5 An MRI provides a more accurate quantitative assessment of cartilage thickness,4 and can predict the likelihood of future knee replacement with comparable or greater accuracy than radiographs.6
Disease-modifying osteoarthritis drugs should target structural disease progression and symptoms.7 Osteoarthritis therapies primarily target symptoms, and no disease-modifying osteoarthritis drugs have been approved in the United States or Europe. Sprifermin is a recombinant human fibroblast growth factor 18, and has been investigated as a potential anabolic intra-articular disease-modifying osteoarthritis drug. In rat osteoarthritis models, sprifermin induces proliferation of hyaline cartilage–producing articular chondrocytes, stimulates synthesis of hyaline extracellular matrix in vitro and ex vivo,8,9,10 and increases knee joint cartilage thickness.11
In a dose-ascending phase 1 study, sprifermin showed no measurable systemic effects or safety concerns in patients with osteoarthritis scheduled for total knee replacement,12 and there were no safety concerns in a 1-year phase 1b proof-of-concept study. The latter showed statistically significant dose-dependent effects on total and lateral compartment cartilage thickness in patients receiving 100 μg of sprifermin vs placebo, but failed to meet its primary end point of change from baseline in central medial femorotibial compartment cartilage thickness.13
The phase 2 study reported herein examined joint structural changes in cartilage and evaluated the efficacy and adverse events for 2 doses and 2 frequencies of intra-articular injections of sprifermin among participants with symptomatic radiographic knee osteoarthritis.
Methods
The study protocol was approved by independent ethics committees or institutional review boards at all 10 study sites. Written informed consent was obtained from all participants, and the study was performed in accordance with the ethical principles of the Declaration of Helsinki.14
Study Design and Participants
FORWARD (FGF-18 Osteoarthritis Randomized Trial with Administration of Repeated Doses) was a 5-year, dose-finding, double-blind, placebo-controlled, multicenter randomized clinical trial. The study protocol appears in Supplement 1 and the statistical analysis plan appears in Supplement 2. The study design appears in eFigure 1 in Supplement 3.
Eligible participants were aged 40 to 85 years; had symptomatic radiographic primary femorotibial knee osteoarthritis (medial, lateral, or both compartments); had Kellgren-Lawrence grades of 2 or 3 for radiographic severity in the more symptomatic knee; had medial minimum joint space width of 2.5 mm or greater; had knee pain lasting 6 months or longer; had symptoms requiring pain medication for more than 50% of the days during the month prior to screening; and had a pain score of 4 to 9 points in response to question 1 (How much pain have you had [in the more symptomatic knee, over the past 48 hours] when walking on a flat surface?) on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scale (an 11-point pain numerical rating scale with a score range of 0-10; worst score: 10) at screening and baseline after a pain medication washout period of at least 5 half-lives. The exclusion criteria were secondary osteoarthritis; malalignment greater than 5° on radiograph; surgery planned for the more symptomatic or contralateral knee within 2 years; and use of a trial-incompatible concomitant medication.
Randomization and Interventions
Participants were enrolled between July 29, 2013, and May 12, 2014. The last participant visit during the 2-year follow-up analyses occurred on May 5, 2016; and the last visit of the 3-year follow-up analysis occurred on May 8, 2017. Computer-generated randomization was performed in block sizes of 5, stratified by country, and coordinated centrally. An interactive web response system was used to assign a blinded treatment kit number to each participant at each visit for administration of sprifermin. The investigator or a suitably qualified designee administered study treatment to the more symptomatic knee using an ultrasound-guided intra-articular injection.
Participants were randomized 1:1:1:1:1 to 5 treatment groups: 100 μg of sprifermin administered every 6 months or every 12 months; 30 μg of sprifermin administered every 6 months or every 12 months; and placebo administered every 6 months. Sprifermin treatments were administered in cycles either every 6 months (at months 0, 6, 12, and 18) or every 12 months (at months 0 and 12, alternating with placebo [phosphate-buffered saline] at months 6 and 18) (Figure 1). Placebo treatment cycles were administered every 6 months. Each cycle consisted of 3 injections of sprifermin or placebo over 3 weeks (eTable 1 in Supplement 3). The total study duration was 5 years and the primary outcome was evaluated at 2 years after baseline. Outcomes also were evaluated 3 years after baseline and are reported herein. Participants were followed up until 5 years after baseline (eFigure 1 in Supplement 3).
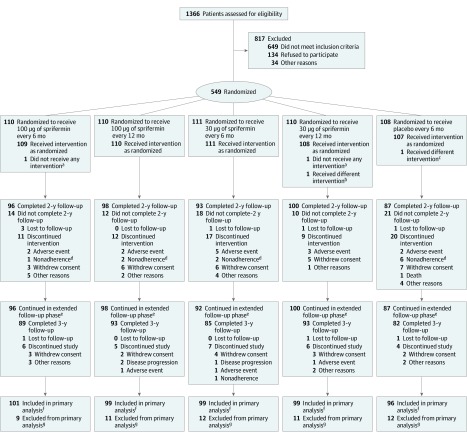
Figure 1. Study Disposition at Year 2.
aParticipant was not included in any adverse event analyses.
bParticipant was randomized to 30 μg of sprifermin every 12 months, but received 100 μg of sprifermin at visit 3. Actual treatment is set as 100 μg of sprifermin every 12 months for the adverse event analyses and as 30 μg of sprifermin every 12 months for the other analyses.
cParticipant was randomized to placebo, but received 30 μg of sprifermin at visit 7. Actual treatment was set as 30 μg of sprifermin every 12 months for the adverse event analyses and as placebo for the other analyses.
dNonadherence refers to protocol nonadherence.
eParticipants may not be included in the primary analysis population (due lack of quantitative magnetic resonance imaging measurement), but may still be included in the year 3 analysis (in the other efficacy and adverse event analyses).
fParticipants may be included in the primary analysis population even if they discontinued treatment.
gThere were no quantitative magnetic resonance imaging measurements up to year 2.
Imaging
Quantitative MRI analyses (blinded to treatment and order of the image acquisition) of the femorotibial cartilage subregions were performed centrally at Chondrometrics GmbH using version 3.0 of Chondrometrics Platform software. Segmentation of medial and lateral femorotibial cartilage plates (tibia and weight-bearing femur) was conducted on coronal fast low-angle shot 3D T1-weighted gradient echo fat saturated sequences, which has been described.13,15
All MRI data were acquired at baseline and before the first injection of each cycle at the 6-, 12-, and 18-month visits and at the 24- and 36-month visits using 1.5- or 3-Tesla clinical MRI scanners. The test-retest reliability analyses for MRI using the coronal fast low-angle shot MRI sequences have been reported,16,17 and have demonstrated MRI reproducibility and sensitivity for detecting changes in cartilage thickness in patients with osteoarthritis.
Fixed flexion radiographs of the knee were obtained using the SynaFlexer Plexiglass positioning frame and were acquired prior to treatment and at 12, 24, and 36 months. Knee minimum joint space width was measured in the medial and lateral femorotibial compartments using the BioClinica knee joint space width tool, which is a component of the StudyDirect reading system.18 Full imaging methods appear in Supplement 3.
Outcomes
The study protocol (Supplement 1) and statistical analysis plan (Supplement 2) are inconsistent regarding the categorization of the sprifermin serum and synovial level end points or the neutralizing antibodies against sprifermin end points. These discrepancies are described in eTable 2 in Supplement 3.
Primary End Point
The primary end point was change in total femorotibial joint cartilage thickness in the more symptomatic knee from baseline to 2 years measured by quantitative MRI. The minimal clinically important difference for the primary end point is unknown.
Secondary End Points
The reported secondary end points include mean 2-year changes from baseline in cartilage thickness in the medial and lateral femorotibial compartments measured by quantitative MRI; cartilage volume in the total, medial, and lateral femorotibial joint measured by quantitative MRI; minimum joint space width in the medial and lateral compartments by radiography; WOMAC total score and pain, function, and stiffness subscale scores (range, 0-100; 0 represents no symptoms; 100 represents extreme symptoms); and sprifermin serum levels (assessed at baseline and at weeks 2, 28, 54, 80, and 104).19 The unreported secondary end points include the 20-m walk test, the patient global assessment, and sprifermin synovial fluid levels.
The minimal clinically important difference is only known for the WOMAC scores. The minimal clinically important difference for the WOMAC total score is 7 U (95% CI, 4-10 U) and the minimal clinically important difference percentage is 14% (95% CI, 9%-18%); pain score, 9 (95% CI, 6-12); function score, 6 (95% CI, 3-9); and stiffness score, 7 (95% CI, 6-9).20 There were discrepancies between the protocol and the statistical analysis plan for the definitions of the secondary outcomes. These discrepancies are summarized in eTable 2 in Supplement 3.
Adverse Events
The end points consisted of adverse events, treatment-emergent adverse events, and serious adverse events; the development of binding and neutralizing antibodies against sprifermin (at baseline and at weeks 3, 26, 29, 52, 55, 78, 81, and 104); and the incidence of acute inflammatory reactions (defined as a pain increase of ≥30 mm on a 100-mm visual analog scale with self-reported joint swelling within 3 days following intra-articular injection21).
Concomitant medications, vital sign measurements, electrocardiogram, laboratory parameters, and physical examinations were recorded throughout the study. An independent data and safety monitoring committee performed periodic reviews of adverse events. Additional adverse events appear in eTable 3 in Supplement 3.
Post Hoc and Exploratory Outcomes
Post hoc quantitative MRI analyses were conducted to determine the mean change in cartilage thickness from baseline to 2 years for the central medial and central lateral femorotibial compartments; and the medial femorotibial compartment in participants with predominantly medial disease.
End points examined during the 3-year analysis were not prespecified and are considered exploratory. Three-year efficacy end points were change from baseline in total, medial, lateral, central medial, and central lateral femorotibial joint cartilage thickness measured by quantitative MRI; medial and lateral minimum joint space width measured by radiography; and WOMAC total score.
Statistical Analysis
To detect an overall treatment effect and a linear dose-response relationship over 2 years for the primary and secondary end points, a sample size of 545 randomized participants (109 per group) was required to provide 90% power. For the primary end point, the minimum treatment effect for a dose-response relationship was 0.03 mm for 100 μg of sprifermin administered every 6 months; 0.01 mm for 100 μg of sprifermin administered every 12 months; 0.01 mm for 30 μg of sprifermin administered every 6 months; −0.01 mm for 30 μg of sprifermin administered every 12 months; and −0.03 mm for placebo administered every 6 months.
The assumptions were based on previous studies using similar analysis techniques, such as mean cartilage loss of 0.02 mm per year observed in patients with knee osteoarthritis in the Osteoarthritis Initiative (relevant only for the placebo effect),17,22 and difference of 0.04 mm in total cartilage thickness between the 100 μg of sprifermin group and the placebo group in the sprifermin phase 1b study.13 The minimal clinically important difference for the primary end point is unknown.
The treatment effect on efficacy end points was analyzed with mixed models and repeated measurement for absolute change from baseline, including baseline value, treatment group, visit, visit × treatment group interaction, and geographical region (as a fixed effect). An additional post hoc analysis (model 2) was performed, which included site within a country as a random effect and the number of days since baseline as a numerical variable. The prespecified significance level was .05 using a 2-sided P value for all tests. Statistical significance was based on 2 P values for overall treatment effect and dose response. The P values from a pairwise comparison were considered supportive.
Dunnett adjustment for multiple comparisons was used within the mixed model for each treatment vs placebo overall (for the whole period) and at each visit. The SAS code for this adjustment is included in the statistical analysis plan (§11 in Supplement 2). Participants were analyzed according to their randomization group for efficacy end points. The intent-to-treat (ITT) population included all randomized participants for nonquantitative MRI end points; and the primary analysis population included all ITT participants with quantitative MRI measurements at baseline and 1 or more posttreatment quantitative MRI measurement up to year 2 for all MRI end points.
Adverse event end points were analyzed according to the actual treatment received (subset of participants in the ITT population who received ≥1 dose of trial treatment). To be conservative, as prespecified in the statistical analysis plan, any participants randomized to placebo who received an injection with active treatment were included in the active group for the adverse event analyses. No imputation of missing data was performed independently of the statistical methods. However, using within-participant correlation in the mixed models, some information was used when the treatment effect was estimated. No interim analyses were performed. Because of the potential for type I error due to multiple comparisons, findings for the analyses of secondary end points should be interpreted as exploratory. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Participants
Among 1366 participants screened, 549 (40.2%) were randomized and included in the ITT analyses (Figure 1). The primary analysis set consisted of 494 participants and the adverse event analysis set consisted of 547 participants. One participant randomized to the placebo group received one 30-μg injection of sprifermin. In the ITT population, 18.4% of participants (81 of 441) receiving sprifermin and 25.9% of participants (28 of 108) receiving placebo discontinued treatment within 2 years, and 12.2% (54 of 441) and 19.4% (21 of 108), respectively, discontinued the study (Figure 1 and eTable 4 in Supplement 3).
Baseline characteristics by treatment group are reported in the Table. The median patient age was 65 years (age range, 40-84 years). Most participants were women (69%), white (80%), and had a Kellgren-Lawrence grade of 2 for radiographic severity in the more symptomatic knee (69%).
Table. Demographic and Baseline Characteristics of the Intent-to-Treat Population.
| 100 μg of Sprifermin | 30 μg of Sprifermin | Placebo Every 6 mo (n = 108) |
|||
|---|---|---|---|---|---|
| Every 6 mo (n = 110) |
Every 12 mo (n = 110) |
Every 6 mo (n = 111) |
Every 12 mo (n = 110) |
||
| Age, median (range), y | 66.0 (44-84) | 65.0 (40-80) | 65.0 (41-80) | 66.5 (41-80) | 64.5 (41-83) |
| Sex, No. (%) | |||||
| Female | 73 (66.4) | 77 (70.0) | 80 (72.1) | 73 (66.4) | 76 (70.4) |
| Male | 37 (33.6) | 33 (30.0) | 31 (27.9) | 37 (33.6) | 32 (29.6) |
| Body mass index, median (Q1-Q3)a | 29.4 (25.46-33.32) | 27.9 (24.84- 32.14) | 28.2 (25.03-31.99) | 28.8 (25.87-32.58) | 29.2 (25.30-33.64) |
| Kellgren-Lawrence grade for radiographic severity, No. (%)b,c | |||||
| 2 | 78 (70.9) | 77 (70.0) | 77 (69.4) | 73 (66.4) | 74 (68.5) |
| 3 | 32 (29.1) | 33 (30.0) | 34 (30.6) | 37 (33.6) | 34 (31.5) |
| Time since diagnosis, median (Q1-Q3), yb | 6.0 (4.0-11.0) | 7.0 (3.0-11.0) | 7.0 (4.0-12.0) | 6.0 (4.0-10.0) | 7.0 (4.0-12.0) |
| Medial minimum joint space width, mean (SD), mmb | 4.2 (1.1) | 4.3 (1.2) | 4.2 (1.3) | 4.1 (1.1) | 4.2 (1.3) |
| Total femorotibial joint cartilage thickness, mean (SD), mmb,d | 1.81 (0.26) | 1.81 (0.27) | 1.84 (0.29) | 1.78 (0.22) | 1.81 (0.27) |
| Response to WOMAC question 1, mean (SD)e | 5.8 (1.4) | 5.5 (1.2) | 5.6 (1.4) | 5.6 (1.4) | 5.6 (1.4) |
| Laterality, No./total No. (%)f | |||||
| Predominately medial | 64/84 (76.2) | 64/88 (72.7) | 61/83 (73.5) | 62/86 (72.1) | 53/78 (67.9) |
| Predominantly lateral | 16/84 (19.0) | 18/88 (20.5) | 19/83 (22.9) | 20/86 (23.3) | 19/78 (24.4) |
| Medial and lateral | 4/84 (4.8) | 6/88 (6.8) | 3/83 (3.6) | 4/86 (4.7) | 6/78 (7.7) |
Abbreviation: WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Calculated as weight in kilograms divided by height in meters squared.
In the more symptomatic knee.
Grading system progresses from grade 0 to 4. Grade 2 indicates the presence of osteophytes and possible joint space narrowing on radiographic analysis; grade 3, multiple osteophytes, definite joint space narrowing, sclerosis, and possible bony deformity.
Calculated as the total volume divided by the total surface area (ie, average cartilage thickness) in the primary analysis population.
The question asked was “How much pain have you had [in the more symptomatic knee, over the past 48 hours] when walking on a flat surface?” The WOMAC is a self-administered questionnaire with questions scored on a scale of 0 to 10 (0, no pain; 10, extreme pain).
Subset of participants with available readings.
Primary End Point
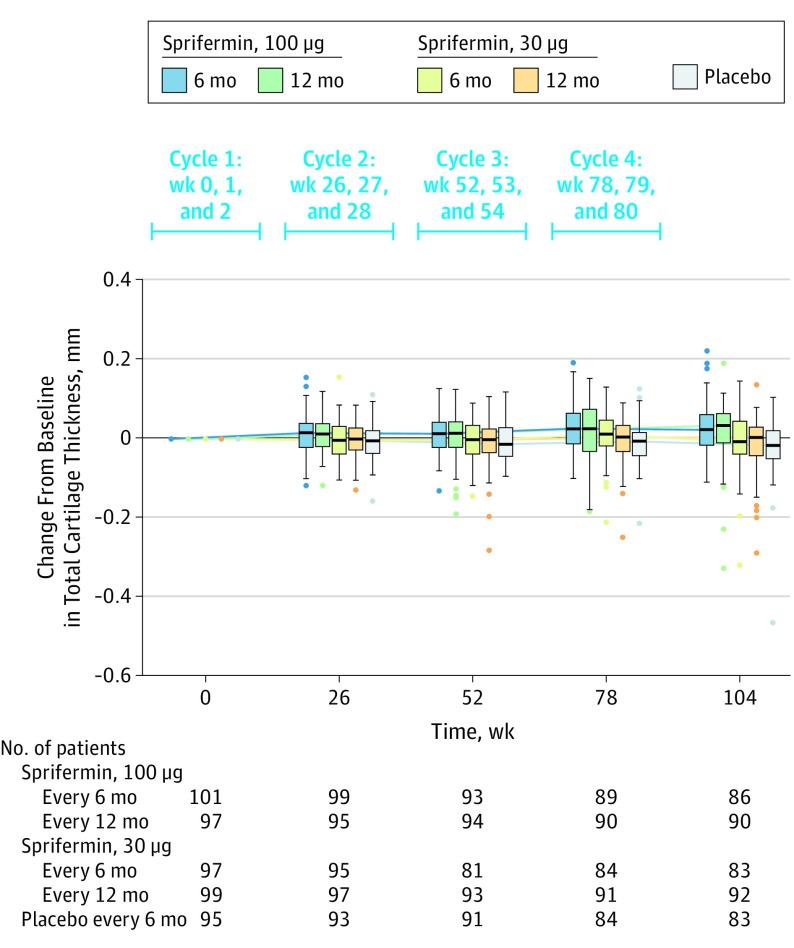
Statistically significant, dose-dependent increases in total femorotibial joint cartilage thickness were observed over 2 years with sprifermin vs placebo (P < .001 for both treatment effect and dose response). There was a mean increase in total femorotibial joint cartilage thickness from baseline to 2 years in the group administered 100 μg of sprifermin every 6 months (0.03 mm; difference vs placebo, 0.05 mm [95% CI, 0.03 to 0.07 mm]) and in the group administered 100 μg of sprifermin every 12 months (0.02 mm; difference vs placebo, 0.04 mm [95% CI, 0.02 to 0.06 mm]) compared with a mean loss in cartilage thickness (−0.02 mm) observed in the placebo group.
Two-year changes in cartilage thickness showed no significant difference vs placebo in the group administered 30 μg of sprifermin every 6 months (0 mm; difference vs placebo, 0.02 [95% CI, −0.01 to 0.04 mm]) or in the group administered 30 μg of sprifermin every 12 months (−0.01 mm; difference vs placebo, 0.01 mm [95% CI, −0.01 to 0.03 mm]) (Figure 2 and eFigure 2 in Supplement 3).
Figure 2. Change From Baseline in Total Femorotibial Joint Cartilage Thickness Over 2 Years in the Primary Analysis Population.
Each cycle consists of 3 once-weekly intra-articular injections of the specified dose over 3 consecutive weeks. Total femorotibial joint cartilage thickness was calculated as the total volume divided by the total surface area (ie, average cartilage thickness). The box plot inner horizontal lines indicate median; boxes, interquartile range (25th and 75th percentiles); vertical whiskers, 1.5 interquartile range beyond the 25th and 75th percentiles; and dots, more extreme values.
Secondary End Points
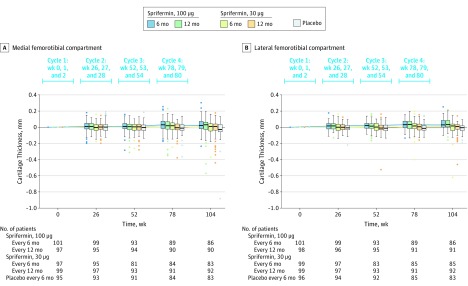
Statistically significant dose-dependent increases in cartilage thickness over 2 years were observed in the medial and lateral femorotibial compartment (Figure 3 and eFigure 3 in Supplement 3). Consistent increases in cartilage volume were observed over 2 years (eFigure 4 in Supplement 3).
Figure 3. Change From Baseline in Medial and Lateral Femorotibial Joint Cartilage Thickness Over 2 Years in the Primary Analysis Population.
Medial femorotibial compartment quantitative MRI cartilage thickness was calculated as the medial cartilage volume divided by medial surface area. Lateral femorotibial compartment quantitative MRI cartilage thickness was calculated as the lateral cartilage volume divided by lateral surface area. The box plot inner horizontal lines indicate median; boxes, interquartile range (25th and 75th percentiles); vertical whiskers, 1.5 interquartile range beyond the 25th and 75th percentiles; and dots, more extreme values.
Statistically significant dose-dependent effects in joint space width were observed in the lateral compartment (P = .004 for treatment effect; P < .001 for dose response), but not for the medial compartment. The 2-year change from baseline in joint space width for the lateral compartment was significantly different among the groups receiving 100 μg of sprifermin administered every 6 months (0.19 mm; difference vs placebo, 0.26 mm [95% CI, 0.12 to 0.41 mm]) and every 12 months (0.19 mm; difference vs placebo, 0.26 mm [95% CI, 0.12 to 0.40 mm]) and the placebo group (−0.07 mm). In contrast, the 2-year change from baseline in joint space width for the lateral compartment was not significantly different among the groups receiving 30 μg of sprifermin administered every 6 months (0.01 mm; difference vs placebo, 0.08 mm [95% CI, −0.08 to 0.25 mm]) and every 12 months (−0.03 mm; difference vs placebo, 0.04 mm [95% CI, −0.13 to 0.20 mm]) and the placebo group (−0.07 mm) (eFigure 5 in Supplement 3).
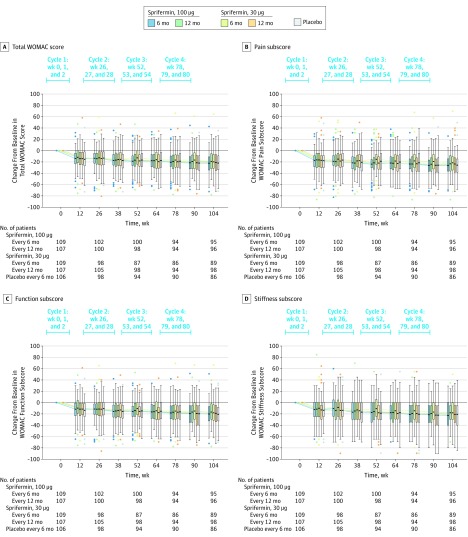
Compared with placebo, there were no statistically significant differences in mean absolute change from baseline for total WOMAC score in the group administered 100 μg of sprifermin every 6 months (−0.06 [95% CI, −5.76 to 5.65]), 100 μg of sprifermin every 12 months (3.65 [95% CI, −1.99 to 9.28]), 30 μg of sprifermin every 6 months (2.58 [95% CI, −3.47 to 8.64]), or 30 μg of sprifermin every 12 months (1.29 [95% CI, −4.53 to 7.10]). Similarly, no significant differences were observed in mean absolute change from baseline for the WOMAC pain, function, or stiffness subscale scores (Figure 4 and eFigure 6 in Supplement 3). Pain medication use was similar in the sprifermin groups compared with the placebo group (eTable 5 in Supplement 3).
Figure 4. Change From Baseline in Total WOMAC Score and in the WOMAC Subscale Scores Over 2 Years in the Intent-to-Treat Population.
The box plot inner horizontal lines indicate median; boxes, interquartile range (25th and 75th percentiles); vertical whiskers, 1.5 interquartile range beyond the 25th and 75th percentiles; and dots, more extreme values. WOMAC indicates Western Ontario and McMaster Universities Osteoarthritis Index.
All sprifermin serum concentrations were below the lower limit (100 pg/mL) of quantification.
Adverse Events
Treatment-emergent adverse events were reported by more than 90% of participants. Treatment-emergent adverse events were reported by 99 participants (90.8%) in the group receiving 100 μg of sprifermin administered every 6 months; by 101 (91.0%) in the group receiving 100 μg of sprifermin every 12 months; by 100 (90.1%) in the group receiving 30 μg of sprifermin every 6 months; by 106 (97.2%) in the group receiving 30 μg of sprifermin every 12 months; and by 99 (92.5%) in the placebo group.
Treatment-emergent adverse events were mostly mild or moderately severe and considered unrelated to the treatment by the site investigators. The most frequent treatment-emergent adverse events were musculoskeletal and connective tissue disorders (arthralgia and back pain), infections and infestations (upper respiratory infection and nasopharyngitis), vascular disorders (hypertension), and nervous system disorders (headache) (eTable 6 in Supplement 3). The most frequently reported treatment-emergent adverse event was arthralgia (placebo: n = 46 [43.0%]; 100 μg of sprifermin every 6 months: n = 45 [41.3%]; 100 μg of sprifermin every 12 months: n = 50 [45.0%]; 30 μg of sprifermin every 6 months: n = 40 [36.0%]; and 30 μg of sprifermin every 12 months: n = 48 [44.0%]).
Serious adverse events were reported by 25 participants (22.9%) in the group receiving 100 μg of sprifermin every 6 months; by 17 (15.3%) in the group receiving 100 μg of sprifermin every 12 months; by 22 (19.8%) in the group receiving 30 μg of sprifermin every 6 months; by 18 (16.5%) in the group receiving 30 μg of sprifermin administered every 12 months; and by 27 (25.2%) in the placebo group. Treatment-emergent serious adverse events were reported by 13 participants (11.9%) in the group receiving 100 μg of sprifermin every 6 months; by 12 (10.8%) in the group receiving 100 μg of sprifermin every 12 months; by 14 (12.6%) in the group receiving 30 μg of sprifermin every 6 months; by 15 (13.8%) in the group receiving sprifermin 30 μg every 12 months; and by 12 (11.2%) in the placebo group.
No serious adverse events were considered related to study treatment by site investigators. One death occurred, which was considered unrelated to study treatment (gastric cancer in the placebo group). Local treatment-emergent adverse events were similar among treatment groups and most frequently consisted of arthralgia, joint swelling, and injection-site pain (eTable 6 in Supplement 3). Less than 1% of participants experienced a local treatment-emergent serious adverse event.
Antisprifermin Antibodies
Of participants who tested positive for binding antibodies, the titer range was from 1.9 U (lowest possible) to 3.11 U; specifically, 6 participants were in the group receiving 100 μg of sprifermin administered every 6 months, 8 in the group receiving 100 μg of sprifermin every 12 months, 6 in the group receiving 30 μg of sprifermin every 6 months, 12 in the group receiving 30 μg of sprifermin every 12 months, and 6 in the placebo group. Other participants tested positive for neutralizing antibodies, with titers ranging from 1.10 U (lowest possible) to 2.30 U; specifically, 1 participant was in the group receiving 100 μg of sprifermin administered every 6 months, 4 in the group receiving 100 μg of sprifermin every 12 months, none in the group receiving 30 μg of sprifermin every 6 months, 5 in the group receiving 30 μg of sprifermin every 12 months, and 2 in the placebo group.
Acute Inflammatory Reactions
Acute inflammatory reactions were reported by 24 participants (22.2%) in the group receiving 100 μg of sprifermin administered every 6 months, 25 (22.9%) in the group receiving 100 μg of sprifermin every 12 months, 18 (16.4%) in the group receiving 30 μg of sprifermin every 6 months, 21 (19.3%) in the group receiving 30 μg of sprifermin every 12 months, and 14 (13.5%) in the placebo group. None led to treatment discontinuation (eTable 7 in Supplement 3). The majority of patients did not have more than 2 acute inflammatory reactions over 4 cycles of treatment; however, 1 patient in the group receiving 100 μg of sprifermin every 12 months had 3 acute inflammatory reactions during cycle 3.
Post Hoc Analyses
Increased Cartilage Thickness in the Central Medial and Lateral Femorotibial Compartments
Compared with placebo, statistically significant dose-dependent increases in cartilage thickness over 2 years were observed in the central medial and central lateral femorotibial compartments (eFigure 7 in Supplement 3).
Medial Femorotibial Cartilage Thickness in Participants With Predominantly Medial Disease
Among 304 participants who had predominantly medial femorotibial compartment disease at baseline, 2-year changes from baseline in the medial femorotibial compartment were consistent with the results of the primary analysis population (eFigure 8 in Supplement 3).
Statistical Analysis Model 2
The primary outcome data were analyzed further using an additional statistical model (model 2) that included site within a country as a random effect and the number of days since baseline as a numerical variable. Analyses of change from baseline in total cartilage thickness over 2 years using model 2 were consistent with the original model and estimated a similar effect size for active treatment groups vs placebo (eTable 8 in Supplement 3).
Exploratory 3-Year Efficacy and Adverse Events
A total of 442 participants (80.5%) completed 3 years of follow-up. An overall decrease in mean total femorotibial joint cartilage thickness was observed in all study groups between years 2 and 3 (eFigure 9 in Supplement 3). Statistically significant differences between sprifermin and placebo in 3-year changes from baseline (0.05 mm [95% CI, 0.03-0.07 mm]) were observed for the group receiving 100 μg of sprifermin every 6 months.
Compared with placebo, no significant differences were observed in mean absolute change from baseline in total WOMAC scores in any sprifermin group (eFigure 10 in Supplement 3). Other 3-year findings appear in Supplement 3 (eTable 9 [patient reason and time to study withdrawal], eTable 10 [adverse events], and eFigure 11 [change from baseline in cartilage thickness and joint space width in other compartments]).
Discussion
This study demonstrated that intra-articular administration of 100 μg of sprifermin every 6 or 12 months, compared with placebo, resulted in statistically significant increases in total femorotibial joint cartilage thickness after 2 years among participants with symptomatic radiographic knee osteoarthritis. No significant difference vs placebo was observed with 30 μg of sprifermin administered every 6 or 12 months.
Osteoarthritis is characterized by cartilage degradation due to imbalance between catabolism (degradation) and anabolism. Progressive cartilage loss can be measured as a decline in cartilage thickness by MRI or a reduction in joint space width by radiography.3 Prevention of cartilage loss, or increasing cartilage thickness, can be considered a disease-modifying quality, and is among the criteria required for an agent to qualify as a disease-modifying osteoarthritis drug, in addition to improved symptoms and clinical outcomes such as knee replacement. Although most available therapies focus on symptom alleviation, treatments that halt or delay disease progression may prevent worsening of symptoms.23
This study investigated whether sprifermin had an effect on cartilage thickness in participants with knee osteoarthritis. Increased cartilage thickness on quantitative MRI was observed after treatment with 100 μg of sprifermin in all compartments investigated.24,25 The effect on cartilage thickness was supported by significant reductions in lateral minimum joint space width narrowing vs placebo. In an analysis of participants from the Osteoarthritis Initiative, the total femorotibial cartilage thickness change over 2 years in those who received knee replacement within 7 years of follow-up was −0.07 mm compared with −0.03 mm in those who did not (difference of 0.04 mm).22 The data reported herein showed that 100 μg of sprifermin administered every 6 months was associated with a 0.05-mm increase in cartilage thickness compared with placebo, the clinical significance of which remains to be determined. As with the prior sprifermin phase 1 studies, the rates of treatment-emergent adverse events in this phase 2 study were similar across treatment groups. There were no treatment-related deaths.
This study was not designed primarily to assess clinical benefit. The clinical outcome that was investigated was the WOMAC score; however, there was no significant effect of sprifermin on change in the WOMAC scores. The WOMAC total score and the pain, function, and stiffness subscale scores improved in all study groups. The association of change in cartilage thickness with change in osteoarthritis symptoms is unknown. Absence of significantly different WOMAC scores also was reported in clinical trials of intra-articular steroid vs saline (placebo) injections.26,27,28 It is possible that using intra-articular saline injections as a control may act as an active placebo, masking symptomatic improvements associated with sprifermin in this study.
Furthermore, approximately 90% of participants in each treatment group took at least 1 pain medication during the 2-year treatment period. Although the overall use of pain medications was similar among treatment groups, it is possible that the use of pain medication may have confounded the evaluation of symptoms and function. Analysis of a more select group of participants, who have baseline characteristics associated with rapid structural and symptomatic progression of knee osteoarthritis, may indicate symptom differences vs placebo and should be investigated in future studies.
Using both quantitative MRI and radiographic analyses, with the former being used to assess the primary end point, was a strength of this study. Even though radiographs are an acceptable method of measuring disease progression in osteoarthritis clinical trials, the imaging data from this study and others29,30,31 suggest that an MRI may be more sensitive for detecting subtle structural modification, including changes in cartilage thickness. The greater sensitivity of MRI may explain the observed difference in the medial femorotibial compartment by quantitative MRI; however, no differences were found in change of minimum joint space width in the medial compartment, a measurement that has been recommended by the US Food and Drug Administration.32
Limitations
This study has several limitations. First, there were multiple secondary end points without adjustment for type I error. Second, due to the 18-month treatment duration, the magnitude of observed structural changes may not represent clinically meaningful benefits to participants during the follow-up periods of this study. It is unknown whether increased articular cartilage thickness with sprifermin will lead to reduced risk of knee replacement, delayed time to knee replacement, or both. However, cartilage loss has been associated with an increased risk of this outcome.22,29,33
Conclusions
Among participants with symptomatic radiographic knee osteoarthritis, the intra-articular administration of 100 μg of sprifermin every 6 or 12 months vs placebo resulted in an improvement in total femorotibial joint cartilage thickness after 2 years that was statistically significant, but of uncertain clinical importance; there was no significant difference for 30 μg of sprifermin every 6 or 12 months vs placebo. Durability of response also was uncertain.
Trial protocol
Statistical analysis plan
eTable 1. Schedule of enrollment, intervention and assessments
eTable 2. Consistencies and discrepancies between endpoints listed in the clinical trial protocol vs the statistical analysis plan
eTable 3. Pre-specified endpoints
eTable 4. Patient reason and time to treatment withdrawal in 2-year period
eTable 5. Patient pain medication use by highest strength analgesic solutions classification (ITT population)
eTable 6. Treatment-emergent adverse events by severity, serious adverse events, and most frequent treatment-emergent adverse events over 2 years (adverse event population)
eTable 7. Incidence of acute inflammatory reactions by cycle (adverse event population)
eTable 8. Treatment effect of sprifermin in the total femorotibial joint vs placebo at 2 years using primary analysis (model 1) and alternative statistical model (model 2)
eTable 9. Patient reason and time to study withdrawal in 3-year period
eTable 10. Adverse events, serious adverse events, and most frequent adverse events over 3 years (adverse event population)
eFigure 1. FORWARD study design
eFigure 2. Mean change from baseline in total femorotibial joint cartilage thickness over 2 years (primary analysis population)
eFigure 3. Mean change from baseline in medial and lateral femorotibial compartment cartilage thickness over 2 years (primary analysis population)
eFigure 4. Mean change from baseline in cartilage volume in the total femorotibial joint, and in the medial femorotibial and lateral femorotibial compartments over 2 years (primary analysis population)
eFigure 5. Mean change from baseline in medial and lateral mJSW over 2 years (ITT population)
eFigure 6. Mean change from baseline in total WOMAC, WOMAC pain, WOMAC function and WOMAC stiffness over 2 years (ITT population)
eFigure 7. Mean change from baseline in central medial and central lateral femorotibial compartment cartilage thickness over 2 years (primary analysis population)
eFigure 8. Mean change from baseline in cartilage thickness in the medial femorotibial compartment in participants with predominant medial disease over 2 years (primary analysis population)
eFigure 9. Mean change from baseline in total femorotibial joint cartilage thickness over 3 years (primary analysis population)
eFigure 10. Mean change from baseline in total WOMAC score over 3 years (ITT population)
eFigure 11. Mean change from baseline in medial femorotibial compartment, lateral femorotibial compartment, central medial femorotibial compartment and central lateral femorotibial compartment cartilage thickness (by quantitative MRI; primary analysis population) and medial and lateral minimum joint space width (by x-ray) over 3 years (ITT population)
Additional information
eReferences
Data sharing statement
References
- 1.Cross M, Smith E, Hoy D, et al. . The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330. doi: 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 2.Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:d1165. doi: 10.1136/bmj.d1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karsdal MA, Michaelis M, Ladel C, et al. . Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage. 2016;24(12):2013-2021. doi: 10.1016/j.joca.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ, Altman RD, Cicuttini F, et al. . OARSI Clinical Trials Recommendations: Knee imaging in clinical trials in osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):698-715. doi: 10.1016/j.joca.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 5.McAlindon TE, Driban JB, Henrotin Y, et al. . OARSI clinical trials recommendations: design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):747-760. doi: 10.1016/j.joca.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 6.Eckstein F, Boudreau R, Wang Z, et al. ; OAI Investigators . Comparison of radiographic joint space width and magnetic resonance imaging for prediction of knee replacement: a longitudinal case-control study from the Osteoarthritis Initiative. Eur Radiol. 2016;26(6):1942-1951. doi: 10.1007/s00330-015-3977-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt KD, Mazzuca SA. Lessons learned from nine clinical trials of disease-modifying osteoarthritis drugs. Arthritis Rheum. 2005;52(11):3349-3359. doi: 10.1002/art.21409 [DOI] [PubMed] [Google Scholar]
- 8.Gigout A, Guehring H, Froemel D, et al. . Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthritis Cartilage. 2017;25(11):1858-1867. doi: 10.1016/j.joca.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 9.Reker D, Kjelgaard-Petersen CF, Siebuhr AS, et al. . Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo. J Transl Med. 2017;15(1):250. doi: 10.1186/s12967-017-1356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sennett ML, Meloni GR, Farran AJE, Guehring H, Mauck RL, Dodge GR. Sprifermin treatment enhances cartilage integration in an in vitro repair model. J Orthop Res. 2018;36(10):2648-2656. doi: 10.1002/jor.24048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore EE, Bendele AM, Thompson DL, et al. . Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005;13(7):623-631. doi: 10.1016/j.joca.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 12.Dahlberg LE, Aydemir A, Muurahainen N, et al. . A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin Exp Rheumatol. 2016;34(3):445-450. [PubMed] [Google Scholar]
- 13.Lohmander LS, Hellot S, Dreher D, et al. . Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014;66(7):1820-1831. doi: 10.1002/art.38614 [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 15.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27(6):737-744. doi: 10.1109/TMI.2007.907323 [DOI] [PubMed] [Google Scholar]
- 16.Eckstein F, Kunz M, Schutzer M, et al. . Two year longitudinal change and test-retest-precision of knee cartilage morphology in a pilot study for the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2007;15(11):1326-1332. doi: 10.1016/j.joca.2007.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckstein F, Mc Culloch CE, Lynch JA, et al. ; OA Initiative Investigators Group . How do short-term rates of femorotibial cartilage change compare to long-term changes? four year follow-up data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20(11):1250-1257. doi: 10.1016/j.joca.2012.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacey T, Haslam J Automated radiographic measurements in the medial tibiofemoral compartment of the knee: intra-operator reproducibility. Paper presented at: Proceedings of the ACR/ARHP Scientific Meeting; November 9, 2007; Boston, MA. [Google Scholar]
- 19.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840. [PubMed] [Google Scholar]
- 20.Bellamy N, Hochberg M, Tubach F, et al. . Development of multinational definitions of minimal clinically important improvement and patient acceptable symptomatic state in osteoarthritis. Arthritis Care Res (Hoboken). 2015;67(7):972-980. doi: 10.1002/acr.22538 [DOI] [PubMed] [Google Scholar]
- 21.Chevalier X, Giraudeau B, Conrozier T, Marliere J, Kiefer P, Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005;32(7):1317-1323. [PubMed] [Google Scholar]
- 22.Kwoh C, Guehring H, Hannon M, Aydemir A. Clinical relevance of structural measures in knee osteoarthritis: baseline values and change from baseline discriminate patients subsequently receiving knee replacement. Arthritis Rheumatol. 2017;69(suppl 10):A1207. [Google Scholar]
- 23.Arthritis Foundation The voice of the patient. https://www.arthritis.org/Documents/Sections/Science/OA-Voice-of-the-Patient-Report.pdf. Accessed June 8, 2018.
- 24.Hayashi D, Felson DT, Niu J, et al. . Pre-radiographic osteoarthritic changes are highly prevalent in the medial patella and medial posterior femur in older persons: Framingham OA study. Osteoarthritis Cartilage. 2014;22(1):76-83. doi: 10.1016/j.joca.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross KD, Niu J, Stefanik JJ, et al. . Breaking the Law of Valgus: the surprising and unexplained prevalence of medial patellofemoral cartilage damage. Ann Rheum Dis. 2012;71(11):1827-1832. doi: 10.1136/annrheumdis-2011-200606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raynauld JP, Buckland-Wright C, Ward R, et al. . Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377. doi: 10.1002/art.10777 [DOI] [PubMed] [Google Scholar]
- 27.McAlindon TE, LaValley MP, Harvey WF, et al. . Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967-1975. doi: 10.1001/jama.2017.5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Moskowitz RW, Nuki G, et al. . OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162. doi: 10.1016/j.joca.2007.12.013 [DOI] [PubMed] [Google Scholar]
- 29.Pelletier JP, Cooper C, Peterfy C, et al. . What is the predictive value of MRI for the occurrence of knee replacement surgery in knee osteoarthritis? Ann Rheum Dis. 2013;72(10):1594-1604. doi: 10.1136/annrheumdis-2013-203631 [DOI] [PubMed] [Google Scholar]
- 30.Raynauld JP, Martel-Pelletier J, Haraoui B, et al. ; Canadian Licofelone Study Group . Risk factors predictive of joint replacement in a 2-year multicentre clinical trial in knee osteoarthritis using MRI: results from over 6 years of observation. Ann Rheum Dis. 2011;70(8):1382-1388. doi: 10.1136/ard.2010.146407 [DOI] [PubMed] [Google Scholar]
- 31.Wildi LM, Raynauld JP, Martel-Pelletier J, et al. . Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann Rheum Dis. 2011;70(6):982-989. doi: 10.1136/ard.2010.140848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conaghan PG, Hunter DJ, Maillefert JF, Reichmann WM, Losina E. Summary and recommendations of the OARSI FDA Osteoarthritis Assessment of Structural Change Working Group. Osteoarthritis Cartilage. 2011;19(5):606-610. doi: 10.1016/j.joca.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirth W, Hunter DJ, Nevitt MC, et al. . Predictive and concurrent validity of cartilage thickness change as a marker of knee osteoarthritis progression: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2017;25(12):2063-2071. doi: 10.1016/j.joca.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eTable 1. Schedule of enrollment, intervention and assessments
eTable 2. Consistencies and discrepancies between endpoints listed in the clinical trial protocol vs the statistical analysis plan
eTable 3. Pre-specified endpoints
eTable 4. Patient reason and time to treatment withdrawal in 2-year period
eTable 5. Patient pain medication use by highest strength analgesic solutions classification (ITT population)
eTable 6. Treatment-emergent adverse events by severity, serious adverse events, and most frequent treatment-emergent adverse events over 2 years (adverse event population)
eTable 7. Incidence of acute inflammatory reactions by cycle (adverse event population)
eTable 8. Treatment effect of sprifermin in the total femorotibial joint vs placebo at 2 years using primary analysis (model 1) and alternative statistical model (model 2)
eTable 9. Patient reason and time to study withdrawal in 3-year period
eTable 10. Adverse events, serious adverse events, and most frequent adverse events over 3 years (adverse event population)
eFigure 1. FORWARD study design
eFigure 2. Mean change from baseline in total femorotibial joint cartilage thickness over 2 years (primary analysis population)
eFigure 3. Mean change from baseline in medial and lateral femorotibial compartment cartilage thickness over 2 years (primary analysis population)
eFigure 4. Mean change from baseline in cartilage volume in the total femorotibial joint, and in the medial femorotibial and lateral femorotibial compartments over 2 years (primary analysis population)
eFigure 5. Mean change from baseline in medial and lateral mJSW over 2 years (ITT population)
eFigure 6. Mean change from baseline in total WOMAC, WOMAC pain, WOMAC function and WOMAC stiffness over 2 years (ITT population)
eFigure 7. Mean change from baseline in central medial and central lateral femorotibial compartment cartilage thickness over 2 years (primary analysis population)
eFigure 8. Mean change from baseline in cartilage thickness in the medial femorotibial compartment in participants with predominant medial disease over 2 years (primary analysis population)
eFigure 9. Mean change from baseline in total femorotibial joint cartilage thickness over 3 years (primary analysis population)
eFigure 10. Mean change from baseline in total WOMAC score over 3 years (ITT population)
eFigure 11. Mean change from baseline in medial femorotibial compartment, lateral femorotibial compartment, central medial femorotibial compartment and central lateral femorotibial compartment cartilage thickness (by quantitative MRI; primary analysis population) and medial and lateral minimum joint space width (by x-ray) over 3 years (ITT population)
Additional information
eReferences
Data sharing statement