This cohort study compares outcomes and trajectories associated with varying familial risk for autism spectrum disorder across the first 3 years of life in siblings of children with autism spectrum disorder.
Key Points
Question
How does the development of infants with multiplex and single-incidence family risk for autism spectrum disorder (ASD) differ?
Findings
In this longitudinal cohort study of 445 children with multiplex or single-incidence family risk, 68% of children from multiplex families vs 43% of those from single-incidence families had ASD or atypical development at outcome. Children without ASD did not differ in ASD symptoms based on family risk status, but multiplex status was associated with lower cognitive abilities by age 3 years.
Meaning
Infants with a multiplex family history of ASD should be monitored early and often and referred for early intervention services at the first sign of concern.
Abstract
Importance
Autism spectrum disorder (ASD) is a neurodevelopmental disorder associated with different genetic etiologies. Prospective examination of familial-risk infants informs understanding of developmental trajectories preceding ASD diagnosis, potentially improving early detection.
Objective
To compare outcomes and trajectories associated with varying familial risk for ASD across the first 3 years of life.
Design, Setting, and Participants
This longitudinal, prospective cohort study used data from 11 sites in the Baby Siblings Research Consortium database. Data were collected between 2003 and 2015. Infants who were younger siblings of children with ASD were followed up for 3 years. Analyses were conducted in April 2018. Of the initial 1008 infants from the database, 573 were removed owing to missing necessary data, diagnostic discrepancies, or only having 1 older sibling.
Exposures
Number of siblings with ASD.
Main Outcomes and Measures
Outcomes included ASD symptoms, cognitive abilities, and adaptive skills. Diagnosis (ASD or no ASD) was given at 36-month outcome. The no-ASD group was classified as atypical (developmental delays and/or social-communication concerns) or typical for some analyses. Generalized linear mixed models examined developmental trajectories by ASD outcome and familial-risk group.
Results
In the 435 analyzed participants (age range at outcome, 32-43 months; 246 male [57%]), 355 (82%) were from single-incidence families (1 sibling with ASD and ≥1 sibling without ASD) and 80 (18%) were from multiplex families (≥2 siblings with ASD). There were no significant group differences in major demographics. Children from multiplex families were more likely than those from single-incidence families to be classified as having ASD (29 of 80 [36%] vs 57 of 355 [16%]; 95% CI, 9%-31%; P < .001) and less likely as typical (26 of 80 [33%] vs 201 of 355 [57%]; 95% CI, −36% to −13%; P < .001), with similar rates of atypical classifications (25 of 80 [31%] vs 97 of 355 [27%]; 95% CI, −7% to 15%; P = .49). There were no differences in ASD symptoms between multiplex and single-incidence groups after controlling for ASD outcome (95% CI, −0.02 to 0.20; P = .18). During infancy, differences in cognitive and adaptive abilities were observed based on ASD outcome in the single-incidence group only. At 36 months, the multiplex/no-ASD group had lower cognitive abilities than the single-incidence/no-ASD group (95% CI, −11.89 to −2.20; P = .02), and the multiplex group had lower adaptive abilities than individuals in the single-incidence group after controlling for ASD outcome (95% CI, −9.01 to −1.48; P = .02).
Conclusions and Relevance
Infants with a multiplex family history of ASD should be monitored early and often and referred for early intervention at the first sign of concern. Direct examination of genetic contributions to neurodevelopmental phenotypes in infants with familial risk for ASD is needed.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted and repetitive patterns of behavior.1 Recent estimates indicate an ASD prevalence of 1 in 59 children and a typical age at diagnosis of age 4 years.2 Converging evidence suggests that there are multiple genetic pathways to ASD.3,4 One genetic risk group that has been studied widely includes infants with at least 1 older sibling with ASD (familial risk). Prospective studies of these infants have helped to characterize the early emergence of developmental differences associated with later ASD diagnosis, with the identification of these early signs ultimately improving early screening and intervention efforts.5
To overcome the challenge of small sample sizes and to facilitate scientific collaboration in prospective studies of familial-risk infants, the Baby Siblings Research Consortium (BSRC) was formed. Baby Siblings Research Consortium researchers have combined data from common measures across many sites to answer clinically relevant questions about early manifestations of ASD. Baby Siblings Research Consortium research indicates that nearly 20% of infants with familial risk will meet criteria for ASD at age 3 years,6,7 and another approximately 20% will show other developmental atypicalities (eg, developmental delays, subclinical ASD symptoms).8,9 One key question arising from these prospective studies is whether neurodevelopmental outcomes vary based on genetic risk, with variability in risk defined by the number of siblings with ASD. Multiplex ASD (≥2 siblings with ASD) is more commonly associated with the additive risk of common genetic variants and inherited copy number variants,10,11 while single-incidence ASD (1 sibling with ASD) is more often caused by rare de novo copy number variants and mutations.11
Prior BSRC studies have shown that 60% of boys and 30% of girls with multiplex family risk have ASD compared with nearly 30% of boys and 10% of girls with single-incidence family risk.6 Profile analyses of these infants indicated that multiplex status is associated with decreased cognitive scores but no difference in ASD symptoms.6 Family-based studies have demonstrated that individuals without ASD who are siblings of children with ASD from multiplex families have a higher level of subclinical ASD symptoms,12,13 while the degree to which cognitive abilities differ among siblings without ASD based on familial-risk status is less clear.14 No studies have analyzed differences in developmental trajectories in infancy based on multiplex vs single-incidence status, to our knowledge.
Using the BSRC database, we comprehensively examined categorical distinctions and developmental trajectories in social-communication, cognitive, and adaptive skills associated with different levels of familial risk across the first 3 years of life. We sought to answer 3 primary questions: (1) How do rates of typical, atypical (no ASD), and ASD outcomes differ between infants from multiplex and single-incidence families? (2) When and how do developmental trajectories of ASD symptoms, cognitive ability, and adaptive skills across the first 3 years diverge based on familial-risk status and ASD diagnostic outcome? (3) For children without ASD, how do the phenotypic profiles differ at 3-year outcome based on familial-risk status? We expected greater impairment in infants from multiplex families vs single-incidence families, with higher rates of ASD overall, as well as lower developmental and adaptive abilities and higher subclinical ASD symptoms in children without ASD. Results of these analyses can help guide clinicians in earlier and more informed developmental screening and monitoring of infants from multiplex families.
Methods
Participants
Younger siblings of children with ASD were analyzed from an initial sample of 1008 infants from the BSRC database. Participants were removed owing to missing required outcome (n = 110) or older sibling data (n = 8), discrepancies between Autism Diagnostic Observation Schedule (ADOS) score and diagnosis (n = 15), and having only 1 older sibling (n = 404) or multiple siblings from the same family (n = 36). When multiple children from a family participated, only the youngest child was included to maximize information on older siblings. Institutional review board approval and written informed consent for all participants was obtained within each study site.
Children in the multiplex group had 2 or more older siblings with ASD. Unlike previous BSRC studies,6,7 children in the single-incidence group had a single older sibling with ASD and 1 or more older sibling(s) without ASD. Confirmation of older sibling diagnoses and time points varied by study site.
Measures
Autism spectrum disorder symptoms were measured at age 18, 24, and 36 months using the ADOS,15 an observational measure of social-communication and repetitive behaviors. The ADOS yields a calibrated severity score (CSS) ranging from 1 to 10.16,17 The overall CSS was used in longitudinal analyses. The social affect and restricted, repetitive behavior subscale scores were examined in outcome analyses. The Autism Diagnostic Interview–Revised,18 a parent interview, was collected at 36 months in a subset of infants and used as a secondary indicator of ASD symptoms in outcome analyses.
Cognitive abilities were measured at 6, 9, 12, 15, 18, 24, and 36 months, using the Mullen Scales of Early Learning (MSEL).19 The MSEL examines visual reception, fine motor, receptive language, and expressive language, which yield t scores (mean [SD], 50 [10]). An early learning composite (ELC) is also calculated, yielding a standard score (mean [SD], 100 [15]) representing a child’s overall cognitive ability relative to peers. The ELC was used in longitudinal analyses. Subscale scores were analyzed in outcome analyses.
Adaptive skills were assessed at 6, 9, 12, 15, 18, 24, and 36 months in a subset of infants using the Vineland Adaptive Behavior Scales–Second Edition (Vineland-II),20 a parent-report measure. The Vineland-II assesses communication, daily living skills, socialization, and motor skills, which produce standard scores. The adaptive behavior composite score is computed from the first 3 domains, yielding a standard score representing an individual’s overall adaptive ability relative to peers. The adaptive behavior composite score was used in longitudinal analyses. Subscale scores were examined in outcome analyses.
Clinical outcomes were determined following the 36-month assessment. Children were classified as having ASD if they had a clinical best estimate diagnosis of ASD by expert clinicians and an ADOS score at or above the clinical threshold (CSS, ≥4).7 For categorical analyses only, the no-ASD group was split into a typical (MSEL ELC score ≥85 and ADOS CSS <3) and atypical group (MSEL ELC score <85 and/or ADOS CSS ≥3).8,21
Statistical Analyses
Longitudinal trajectories of primary outcome variables (ADOS CSS, MSEL ELC, and Vineland-II adaptive behavior composite) were modeled using generalized linear mixed models (GLMM) with main effects of ASD outcome (ASD vs no ASD), familial-risk status (multiplex vs single incidence), and time, along with their 2-way and 3-way interactions. Participant-specific and site-specific random intercepts were included to model dependency owing to repeated measures within participants and sites. Mullen Scales of Early Learning and Vineland-II scores were modeled using an identity link, while ADOS scores were modeled using a negative binomial GLMM with a log link. Time was modeled as a class variable for ADOS (at 18, 24, and 36 months), with a broken-line model allowing for a slope change at age 18 months for MSEL and linearly for Vineland-II (where a slope change at age 18 months was nonsignificant). Two-way and 3-way interactions between ASD outcome, familial-risk status, and time were found significant in models for Vineland-II and MSEL; however, the final GLMM for ADOS only contained the significant 2-way interaction between ASD outcome and time.
According to the interactions found significant and our hypotheses, we conducted 6 contrasts for MSEL and Vineland-II data at preselected times to evaluate group mean differences between (1) ASD and no-ASD single incidence, (2) ASD and no-ASD multiplex, (3) no-ASD multiplex and single incidence, (4) ASD multiplex and single incidence, (5) ASD multiplex minus no-ASD multiplex and ASD single-incidence minus no-ASD single-incidence, and (6) multiplex and single incidence. Contrasts were conducted at 6, 12, 24, and 36 months for MSEL and 12, 24, and 36 months for Vineland-II (times with most observations). For the final ADOS model, we conducted contrasts between (1) ASD and no-ASD groups at 18, 24, and 36 months and (2) multiplex and single-incidence groups (ages collapsed). We used false discovery rate22 at .05 to adjust for multiple comparisons (46 contrasts). P values were 2-sided, and the significance threshold was less than .05.
Generalized linear mixed models account for correlations between repeated measures within individuals, allowing for fixed and time-varying covariates and automatically handling missing data, thereby producing unbiased estimates as long as observations are missing at random. Accordingly, all available observations from each participant were used in modeling via GLMM.
Results
A total of 435 younger siblings of children with ASD who were enrolled in longitudinal studies across 11 BSRC sites met inclusion criteria (partially overlapping with previous BSRC samples6,7). Multiplex and single-incidence groups were comparable with regard to demographic characteristics (Table 1). The multiplex group had larger families than the single-incidence group. Of the 349 children without ASD, 227 (52.2%) were included in the typical group and 122 (28.0%) were included in the atypical group. Of 435 children, 86 (19.8%) had ASD, and 349 (80.2%) did not have ASD. Within the atypical group, 30 (25.9%) fell into this group owing to lower cognitive scores, 75 (64.7%) owing to elevated ADOS scores, and 11 (9.5%) owing to both factors (eTable in the Supplement).
Table 1. Participant Information by Familial Risk.
| Variable | Mean (SD) | P Value | |
|---|---|---|---|
| Single Incidence (n = 355) | Multiplex (n = 80) | ||
| Male, No. (%) | 200 (56.3) | 46 (57.5) | .85 |
| White, No. (%) | 269 (75.8) | 66 (82.5) | .36 |
| Maternal education of college or higher, No. (%) | 233 (65.7) | 53 (66.7) | .92 |
| Age at birth, y | |||
| Maternal | 34.86 (4.82) | 34.57 (4.87) | .69 |
| Paternal | 37.37 (5.81) | 37.35 (6.26) | .98 |
| No. of children in family | 3.46 (0.79) | 3.83 (1.35) | .02 |
| No. of siblings with autism spectrum disorder | 1.00 (0) | 2.13 (0.44) | <.001 |
| Age first study visit, mo | 6.90 (4.24) | 7.23 (4.14) | .53 |
| Age at diagnostic outcome, mo | 37.28 (1.63) | 37.48 (1.92) | .34 |
36-Month Outcome Classifications Based on Familial-Risk Status
Outcome classifications significantly differed based on familial-risk status (χ2 = 21.10, P < .001). The multiplex group was more likely than the single-incidence group to be classified as having ASD (29 of 80 [36.3%] vs 57 of 355 [16.1%]; 95% CI, 9%-31%; P < .001), less likely to be classified as typical (26 of 80 [32.5%] vs 201 of 355 [56.6%]; 95% CI, −36% to −13%; P < .001), and had similar levels of atypical classifications (25 of 80 [31.3%] vs 97 of 355 [27.3%]; 95% CI, −7% to 15%; P = .49).
Developmental Trajectories Based on Familial-Risk Status and ASD Outcome
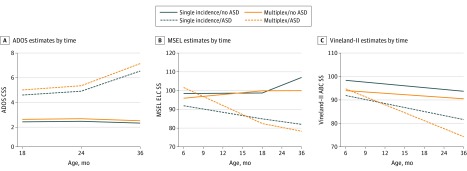
Results from the final GLMMs are summarized below. The Figure depicts modeled developmental trajectories, Table 2 provides detailed sample size information, and Table 3 provides contrast results (eFigure 1 in the Supplement presents raw trajectories).
Figure. Developmental Trajectories of Autism Spectrum Disorder (ASD) Symptoms, Cognitive Abilities, and Adaptive Skills.
Depiction of results from generalized linear mixed models. Autism Diagnostic Observation Schedule (ADOS) measured ASD symptoms (A), Mullen Scales of Early Learning (MSEL) measured cognitive abilities (B), and the Vineland Adaptive Behavior Scales–Second Edition (Vineland-II) measured adaptive skills (C). CSS indicates Calibrated Severity Score; ELC, Early Learning Composite.
Table 2. Participants With Data by Age, Measure, and Group Status.
| Variablea | No. (%) | |||
|---|---|---|---|---|
| Single Incidence | Multiplex | |||
| No ASD | ASD | No ASD | ASD | |
| Total No. of participants | 298 | 57 | 51 | 29 |
| ADOS at age, mo | ||||
| 18 | 214 (71.8) | 39 (68.4) | 36 (70.6) | 23 (79.3) |
| 24 | 260 (87.2) | 54 (94.7) | 44 (86.3) | 27 (93.1) |
| 36 | 298 (100) | 57 (100) | 51 (100) | 29 (100) |
| MSEL at age, mo | ||||
| 6 | 175 (58.7) | 29 (50.9) | 28 (54.9) | 13 (44.8) |
| 9 | 48 (16.1) | 11 (19.3) | 14 (27.4) | 5 (17.2) |
| 12 | 249 (83.6) | 38 (66.7) | 41 (80.4) | 23 (79.3) |
| 15 | 57 (19.1) | 16 (28.1) | 11 (21.5) | 7 (24.1) |
| 18 | 113 (37.9) | 19 (33.3) | 18 (35.3) | 15 (51.7) |
| 24 | 266 (89.3) | 49 (86.0) | 48 (94.1) | 27 (93.1) |
| 36 | 294 (98.7) | 56 (98.2) | 49 (96.1) | 27 (93.1) |
| Vineland-II at age, mo | ||||
| 6 | 38 (12.8) | 9 (12.3) | 5 (9.8) | 2 (6.9) |
| 9 | 38 (12.8) | 9 (12.3) | 4 (7.8) | 3 (10.3) |
| 12 | 148 (49.7) | 26 (45.6) | 21 (41.2) | 14 (48.3) |
| 15 | 42 (14.1) | 13 (22.8) | 7 (13.7) | 3 (10.3) |
| 18 | 177 (59.4) | 28 (49.1) | 29 (56.9) | 17 (58.6) |
| 24 | 187 (62.8) | 37 (64.9) | 28 (54.9) | 18 (62.1) |
| 36 | 210 (70.5) | 39 (68.4) | 30 (58.8) | 17 (58.6) |
| ADI-R at age, mo | ||||
| 36 | 136 (45.6) | 37 (64.9) | 28 (54.9) | 15 (51.7) |
Abbreviations: ADI-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; MSEL, Mullen Scales of Early Learning; Vineland-II, Vineland Adaptive Behavior Scales–Second Edition.
Generalized linear mixed models used all available data to inform estimates.
Table 3. Generalized Linear Mixed Models Contrast Results.
| Group | Contrast | Age, mo | Estimate (SE) | df | t | P Valuea | F |
|---|---|---|---|---|---|---|---|
| Observed ASD Symptoms (ADOS) | |||||||
| NA | ASD vs no ASD | 18 | 0.73 (0.08) | 693 | 8.95 | <.001 | 0.34 |
| 24 | 0.78 (0.07) | 693 | 10.95 | <.001 | 0.42 | ||
| 36 | 1.16 (0.07) | 693 | 17.94 | <.001 | 0.68 | ||
| NA | Multiplex vs single | NA | 0.09 (0.06) | 693 | 1.67 | .18 | 0.06 |
| Cognitive Abilities (MSEL) | |||||||
| Single incidence | ASD vs no ASD | 6 | −6.52 (2.67) | 1304 | −2.45 | .04 | 0.07 |
| 12 | −10.15 (2.02) | 1304 | −5.04 | <.001 | 0.14 | ||
| 24 | −17.53 (2.04) | 1304 | −8.58 | <.001 | 0.24 | ||
| 36 | −25.01 (2.35) | 1304 | −10.65 | <.001 | 0.30 | ||
| Multiplex | ASD vs no ASD |
6 | 5.67 (4.30) | 1304 | 1.32 | .29 | 0.04 |
| 12 | −5.89 (3.17) | 1304 | −1.86 | .13 | 0.05 | ||
| 24 | −18.84 (3.18) | 1304 | −5.92 | <.001 | 0.16 | ||
| 36 | −21.61 (3.81) | 1304 | −5.68 | <.001 | 0.16 | ||
| No ASD | Multiplex vs single |
6 | −2.48 (2.67) | 1304 | −0.93 | .44 | 0.03 |
| 12 | −0.65 (2.05) | 1304 | −0.32 | .79 | 0.01 | ||
| 24 | −1.56 (2.11) | 1304 | −0.74 | .51 | 0.02 | ||
| 36 | −7.05 (2.47) | 1304 | −2.85 | .02 | 0.08 | ||
| ASD | Multiplex vs single |
6 | 9.71 (4.31) | 1304 | 2.26 | .05 | 0.06 |
| 12 | 3.61 (3.16) | 1304 | 1.14 | .36 | 0.03 | ||
| 24 | −2.88 (3.15) | 1304 | −0.91 | .44 | 0.03 | ||
| 36 | −3.65 (3.74) | 1304 | −0.98 | .43 | 0.03 | ||
| ASD – no ASD | Multiplex vs single |
6 | 12.20 (5.06) | 1304 | 2.41 | .04 | 0.07 |
| 12 | 4.26 (3.76) | 1304 | 1.13 | .36 | 0.03 | ||
| 24 | −1.32 (3.79) | 1304 | −0.35 | .78 | 0.01 | ||
| 36 | 3.40 (4.48) | 1304 | 0.76 | .50 | 0.02 | ||
| ASD + no ASD | Multiplex vs single |
6 | 3.61 (2.54) | 1304 | 1.43 | .26 | 0.04 |
| 12 | 1.48 (1.89) | 1304 | 0.79 | .50 | 0.02 | ||
| 24 | −2.22 (1.90) | 1304 | −1.17 | .36 | 0.03 | ||
| 36 | −5.35 (2.25) | 1304 | −2.38 | <.05 | 0.07 | ||
| Adaptive Skills (Vineland-II) | |||||||
| Single incidence | ASD vs no ASD |
12 | −7.59 (1.76) | 843 | −4.32 | <.001 | 0.15 |
| 24 | −9.82 (1.52) | 843 | −6.43 | <.001 | 0.22 | ||
| 36 | −12.05 (1.94) | 843 | −6.21 | <.001 | 0.22 | ||
| Multiplex | ASD vs no ASD |
12 | −2.62 (3.01) | 843 | −0.87 | .45 | 0.03 |
| 24 | −9.38 (2.50) | 843 | −3.75 | .001 | 0.13 | ||
| 36 | −16.14 (3.32) | 843 | −4.87 | <.001 | 0.17 | ||
| No ASD | Multiplex vs single |
12 | −4.14 (1.93) | 843 | −2.14 | .07 | 0.07 |
| 24 | −3.67 (1.61) | 843 | −2.28 | .05 | 0.08 | ||
| 36 | −3.20 (2.12) | 843 | −1.51 | .23 | 0.05 | ||
| ASD | Multiplex vs single |
12 | 0.82 (2.90) | 843 | 0.28 | .79 | 0.01 |
| 24 | −3.23 (2.45) | 843 | −1.32 | .29 | 0.05 | ||
| 36 | −7.29 (3.20) | 843 | −2.28 | .05 | 0.08 | ||
| ASD – no ASD | Multiplex vs single |
12 | 4.96 (3.49) | 843 | 1.42 | .26 | 0.05 |
| 24 | 0.44 (2.93) | 843 | 0.15 | .88 | 0.01 | ||
| 36 | −4.09 (3.84) | 843 | −1.07 | .39 | 0.04 | ||
| ASD + no ASD | Multiplex vs single |
12 | −1.65 (1.74) | 843 | −0.95 | .46 | 0.03 |
| 24 | −2.56 (1.51) | 843 | −1.69 | .18 | 0.06 | ||
| 36 | −5.25 (1.92) | 843 | −2.73 | .02 | 0.08 | ||
Abbreviations: ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; MSEL, Mullen Scales of Early Learning; NA, not applicable; Vineland-II, Vineland Adaptive Behavior Scales–Second Edition.
Reported P values are false discovery rate corrected.
ASD Symptoms
Autism spectrum disorder symptoms differed between the ASD and no-ASD groups, regardless of familial-risk status, at 18, 24, and 36 months. Children with ASD outcomes showed higher levels of ASD symptoms than children without ASD beginning at age 18 months. No differences in ASD symptoms were found between the multiplex and single-incidence groups after controlling for ASD outcome.
Cognitive Abilities
Within the single-incidence group, children with ASD outcomes had lower cognitive abilities than children without ASD at 6, 12, 24, and 36 months. In the multiplex group, the ASD and no-ASD groups did not differ at 6 or 12 months; instead, differences emerged at 24 months, with the ASD group demonstrating lower cognitive abilities than the no-ASD group at 24 and 36 months. Within the no-ASD group, the multiplex group had lower cognitive abilities than the single-incidence group at 36 months; cognitive abilities did not differ based on familial-risk status among children without ASD at earlier ages. In the ASD group, cognitive abilities did not differ between multiplex and single-incidence groups. However, there was an overall difference in cognitive abilities between multiplex and single-incidence groups (ASD + no-ASD contrast) at 36 months. Finally, the difference in cognitive abilities among children with ASD and without ASD differed between the multiplex and single-incidence groups (ASD – no-ASD contrast) at 6 months. Children with ASD outcomes had lower cognitive abilities than those without ASD within the single-incidence group at 6 months, while multiplex children had similar abilities at this age regardless of ASD outcome (Figure, B).
Adaptive Skills
Within the single-incidence group, children with ASD outcomes had lower adaptive abilities than children without ASD at 12, 24, and 36 months. Within the multiplex group, children with and without ASD outcomes showed similar levels of adaptive abilities at 12 months, which then diverged at 24 and 36 months. However, the multiplex and single-incidence groups did not differ significantly within the ASD and no-ASD groups. Likewise, overall familial-risk group differences were mostly nonsignificant. At 36 months, there were overall differences based on familial-risk status; the multiplex group had lower adaptive abilities than the single-incidence group.
36-Month Developmental Profiles Based on Familial-Risk Status in Children Without ASD
Table 4 provides descriptive information and statistical results (eFigure 2 in the Supplement). Results are reported with and without correction for multiple comparisons (13 contrasts). Children without ASD from multiplex and single-incidence groups showed similar levels of social-communication skills and restricted, repetitive behavior scores on the ADOS and Autism Diagnostic Interview–Revised and communication, socialization, daily living, and motor skills on the Vineland-II. However, on the MSEL, the multiplex group had lower visual reception and receptive language scores than the single-incidence group; the difference in receptive language survived false discovery rate correction.
Table 4. Detailed Comparison of 36-Month Outcome Data Across Familial Risk Groups in Children Without ASD.
| Variable | Mean (SD) | P Value | d | ||
|---|---|---|---|---|---|
| Single Incidence | Multiplex | Raw | FDR | ||
| Cognitive abilities (MSEL) | |||||
| Visual reception | 59.14 (12.26) | 54.70 (14.85) | .05 | .22 | 0.33 |
| Fine motor | 50.66 (13.03) | 46.96 (14.32) | .07 | .22 | 0.27 |
| Receptive language | 51.80 (9.91) | 47.61 (8.80) | .003 | .04 | 0.45 |
| Expressive language | 52.28 (9.38) | 50.39 (9.89) | .20 | .29 | 0.20 |
| Observed ASD symptoms (ADOS) | |||||
| Social affect | 2.38 (1.74) | 2.63 (1.68) | .35 | .41 | 0.15 |
| RRB | 3.68 (2.58) | 4.41 (2.48) | .06 | .22 | 0.29 |
| Reported ASD symptoms (ADI-R) | |||||
| Social interaction | 2.66 (2.30) | 3.82 (3.84) | .13 | .29 | 0.37 |
| Communication | 2.39 (2.50) | 3.29 (3.47) | .20 | .29 | 0.30 |
| RRB | 0.73 (1.39) | 1.29 (1.86) | .14 | .29 | 0.34 |
| Adaptive skills (Vineland-II) | |||||
| Communication | 99.57 (13.61) | 96.03 (14.33) | .17 | .29 | 0.25 |
| Daily living skills | 93.47 (13.43) | 90.24 (13.71) | .23 | .30 | 0.24 |
| Socialization | 96.17 (12.03) | 93.85 (17.62) | .46 | .50 | 0.15 |
| Motor skills | 93.90 (11.62) | 93.07 (16.81) | .80 | .80 | 0.06 |
Abbreviations: ADI-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; FDR, false discovery rate; MSEL, Mullen Scales of Early Learning; RRB, restricted, repetitive behavior; Vineland-II, Vineland Adaptive Behavior Scales–Second Edition.
Discussion
Rates of ASD
Children from multiplex families were more than twice as likely to have ASD outcomes as those from single-incidence families. While 57% of the children with only 1 older sibling with ASD were typically developing at age 3 years, only 33% of the children with multiple older siblings with ASD were typically developing at outcome. This finding highlights the first and most important clinical finding of this study: infants with a strong family history of ASD need to be monitored early and often and should be referred for early intervention services at the first sign of concern.
Developmental Trajectories
Longitudinal analyses suggest that group differences over time in ASD symptoms, cognitive abilities, and adaptive skills were mainly attributable to ASD outcome rather than familial-risk status. This was particularly true for ASD symptoms, which differed only based on ASD outcome beginning at age 18 months. Within the single-incidence group, children with ASD outcomes consistently demonstrated lower cognitive abilities than children without ASD beginning at age 6 months and adaptive abilities beginning at age 12 months (earliest ages contrasted). Conversely, multiplex infants showed similar levels of cognitive and adaptive abilities at earlier ages, regardless of ASD outcome, and did not diverge until the second year of life. Multiplex children with ASD outcomes demonstrated a sharp decline in standard scores on measures of early cognitive and adaptive skills in the second and third years of life, reflecting slower growth in these developmental abilities. Neuroimaging studies of familial-risk infants have identified altered trajectories of brain development in the first year, particularly in cortical surface area and neural connectivity.23,24 These studies have not distinguished infants based on multiplex vs single-incidence status, but they support the hypothesis that genetic risk factors lay a foundation for early changes in brain structure and function, which may then cumulatively disturb learning and adaptive behaviors leading to difficulties making expected developmental gains. These neurobiological changes may truly precede behavior; alternatively, our standardized behavioral measures may lack sensitivity to discern subtle changes in development in the first year. Clinically, these results suggest that it may be more challenging to distinguish infants with ASD vs without ASD behaviorally in the context of multiplex status during infancy and early toddlerhood. Further research longitudinally examining biomarkers of risk early in life is needed to determine which infants are most likely to need preemptive intervention in this population.25,26
Profile Analyses
We also detected subtle differences and remarkable similarities between multiplex and single-incidence children without ASD at outcome. Children without ASD did not differ based on familial-risk status in their observed or parent-reported levels of ASD symptoms at age 3 years. This was somewhat surprising given previous research suggesting subclinical ASD symptoms in family members of individuals with ASD (ie, broader autism phenotype), particularly families with multiple affected individuals.13,27 It is possible that our ASD symptom measures, which were designed as clinical diagnostic tools, were not sensitive enough to detect subtle differences in social-communication and repetitive behavior.
However, we did detect differences in cognitive abilities at age 3 years. This finding was primarily explained by differences in receptive language and, to a lesser degree, nonverbal cognitive skills, with no differences in broadly measured expressive language found between the non-ASD multiplex and single-incidence groups. These results are largely consistent with previous research finding deficits in verbal IQ in the unaffected siblings in multiplex but not single-incidence families.14 The likely risk factors for having multiple children with ASD, such as shared genetic variation, vulnerability to genetic mutations, or complex gene-environment interactions (eg, in utero environment), may impact brain development in a more distributed, global way, which then impacts overall development, rather than networks that are more specific to ASD. These findings speak to the need for large, collaborative efforts to examine brain development, genetics, and gene-environment interactions in at-risk infants to understand the neurobiological mechanisms underlying these differences in developmental trajectories and behavior.
Strengths and Limitations
Our study uniquely leveraged a rich data set collected from multiple expert sites to prospectively examine differences associated with multiplex status and diagnostic outcome in a large cohort of infants with elevated familial risk for ASD. Although the sample size was quite large for a study of this kind, the prospective nature led to uneven and occasionally small groups disallowing investigation in some areas of interest (eg, sex) and firm conclusions in others. For instance, the multiplex group was smaller, so comparisons within this group were less powered than those within the single-incidence group. Given the longitudinal, multisite design, there was also some inconsistency among study sites in the ages at which different measures were collected, how older sibling diagnoses were confirmed, and missing data. Statistical models that account for missing data and site differences helped to attenuate possible negative effects. The use of already collected data across multiple sites also required us to choose common broad-based measures that, while highly clinically relevant and well validated, may not have been sensitive enough to detect more subtle differences between children without ASD. Additionally, as is the case across the ASD sibling literature,28 many of the children in the sample had relatively high cognitive scores and came from predominantly white and highly educated families, so these results may not represent the larger population of children with ASD. The most substantial limitation is the lack of genomic data in these infants, which would inform our hypotheses about genetic factors contributing to developmental differences.
Conclusions
Children from multiplex families are more than twice as likely to meet criteria for ASD at age 3 years than children from single-incidence families. Prospectively, single-incidence infants begin to show developmental differences based on later ASD diagnosis by 6 months of age, while multiplex infants with and without ASD outcomes do not differ until the second year of life. Among unaffected children, multiplex risk is associated with lower cognitive abilities but similar levels of ASD symptoms. Results support the need for direct examination of genetic contributions to neurodevelopmental phenotypes in infants with multiplex and single-incidence family risk for ASD. Given their very high rates of ASD and other neurodevelopmental challenges, infants with a strong family history of ASD should be monitored early and often and referred for early intervention at the first sign of developmental concern.
eFigure 1. Depiction of raw data for ASD symptoms, cognitive abilities, and adaptive skills
eFigure 2. 36-month outcome data by familial-risk group in children without ASD
eTable. Participant Information by Outcome Group
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Baio J, Wiggins L, Christensen DL, et al. . Prevalence of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67(6):1-23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramaswami G, Geschwind DH. Genetics of autism spectrum disorder. Handb Clin Neurol. 2018;147:321-329. doi: 10.1016/B978-0-444-63233-3.00021-X [DOI] [PubMed] [Google Scholar]
- 4.Muhle RA, Reed HE, Stratigos KA, Veenstra-VanderWeele J. The emerging clinical neuroscience of autism spectrum disorder: a review. JAMA Psychiatry. 2018;75(5):514-523. doi: 10.1001/jamapsychiatry.2017.4685 [DOI] [PubMed] [Google Scholar]
- 5.Jones EJH, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci Biobehav Rev. 2014;39:1-33. doi: 10.1016/j.neubiorev.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messinger DS, Young GS, Webb SJ, et al. . Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Mol Autism. 2015;6:32. doi: 10.1186/s13229-015-0027-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozonoff S, Young GS, Carter A, et al. . Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488-e495. doi: 10.1542/peds.2010-2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charman T, Young GS, Brian J, et al. . Non-ASD outcomes at 36 months in siblings at familial risk for autism spectrum disorder (ASD): A Baby Siblings Research Consortium (BSRC) study. Autism Res. 2017;10(1):169-178. doi: 10.1002/aur.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messinger D, Young GS, Ozonoff S, et al. . Beyond autism: a baby siblings research consortium study of high-risk children at three years of age. J Am Acad Child Adolesc Psychiatry. 2013;52(3):300-308.e1. doi: 10.1016/j.jaac.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klei L, Sanders SJ, Murtha MT, et al. . Common genetic variants, acting additively, are a major source of risk for autism. Mol Autism. 2012;3(1):9. doi: 10.1186/2040-2392-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leppa VM, Kravitz SN, Martin CL, et al. . Rare inherited and de novo CNVs reveal complex contributions to ASD risk in multiplex families. Am J Hum Genet. 2016;99(3):540-554. doi: 10.1016/j.ajhg.2016.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frazier TW, Youngstrom EA, Hardan AY, Georgiades S, Constantino JN, Eng C. Quantitative autism symptom patterns recapitulate differential mechanisms of genetic transmission in single and multiple incidence families. Mol Autism. 2015;6:58. doi: 10.1186/s13229-015-0050-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdts JA, Bernier R, Dawson G, Estes A. The broader autism phenotype in simplex and multiplex families. J Autism Dev Disord. 2013;43(7):1597-1605. doi: 10.1007/s10803-012-1706-6 [DOI] [PubMed] [Google Scholar]
- 14.Oerlemans AM, Hartman CA, Franke B, Buitelaar JK, Rommelse NN. Does the cognitive architecture of simplex and multiplex ASD families differ? J Autism Dev Disord. 2016;46(2):489-501. doi: 10.1007/s10803-015-2572-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lord C, Risi S, Lambrecht L, et al. . The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205-223. doi: 10.1023/A:1005592401947 [DOI] [PubMed] [Google Scholar]
- 16.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39(5):693-705. doi: 10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: separating severity of social affect and restricted and repetitive behaviors. J Autism Dev Disord. 2014;44(10):2400-2412. doi: 10.1007/s10803-012-1719-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutter M, Le Couteur A, Lord C. ADI-R Autism Diagnostic Interview Revised. Manual. Los Angeles, CA: Western Psychological Services; 2013. [Google Scholar]
- 19.Mullen EM. Mullen Scales Of Early Learning. CirclePines, MN: American Guidance Service; 1995. [Google Scholar]
- 20.Sparrow SS, Cicchetti VD, Balla AD. Vineland Adaptive Behavior Scales. 2nd ed Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- 21.Chawarska K, Shic F, Macari S, et al. . 18-Month predictors of later outcomes in younger siblings of children with autism spectrum disorder: a Baby Siblings Research Consortium study. J Am Acad Child Adolesc Psychiatry. 2014;53(12):1317-1327.e1. doi: 10.1016/j.jaac.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289-300. [Google Scholar]
- 23.Hazlett HC, Gu H, Munsell BC, et al. ; IBIS Network; Clinical Sites; Data Coordinating Center; Image Processing Core; Statistical Analysis . Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348-351. doi: 10.1038/nature21369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis JD, Evans AC, Pruett JR Jr, et al. ; Infant Brain Imaging Study Network . The emergence of network inefficiencies in infants with autism spectrum disorder. Biol Psychiatry. 2017;82(3):176-185. doi: 10.1016/j.biopsych.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green J, Pickles A, Pasco G, et al. ; British Autism Study of Infant Siblings (BASIS) Team . Randomised trial of a parent-mediated intervention for infants at high risk for autism: longitudinal outcomes to age 3 years. J Child Psychol Psychiatry. 2017;58(12):1330-1340. doi: 10.1111/jcpp.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones EJH, Dawson G, Kelly J, Estes A, Webb SJ. Parent-delivered early intervention in infants at risk for ASD: effects on electrophysiological and habituation measures of social attention. Autism Res. 2017;10(5):961-972. doi: 10.1002/aur.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwichtenberg AJ, Young GS, Sigman M, Hutman T, Ozonoff S. Can family affectedness inform infant sibling outcomes of autism spectrum disorders? J Child Psychol Psychiatry. 2010;51(9):1021-1030. doi: 10.1111/j.1469-7610.2010.02267.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacrey LR, Zwaigenbaum L, Szatmari P, et al. . Brief report: characteristics of preschool children with ASD vary by ascertainment. J Autism Dev Disord. 2017;47(5):1542-1550. doi: 10.1007/s10803-017-3062-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Depiction of raw data for ASD symptoms, cognitive abilities, and adaptive skills
eFigure 2. 36-month outcome data by familial-risk group in children without ASD
eTable. Participant Information by Outcome Group