Supplemental Digital Content is available in the text
Keywords: Africa, ageing, blood pressure, Cameroon, epidemiology, hypertension, low-income and middle-income countries, noncommunicable disease, review
Objective:
To estimate national and geography-based variations in blood pressure and burden of hypertension in Cameroon, generally called ‘miniature Africa’.
Methods:
PubMed, Medline, EMBASE, CINHAL, Web of Science, Popline, Scopus and BDSP were searched through November 2018, for hypertension studies among Cameroonians aged at least 18 years. Hypertension was measured as SBP at least 140 mmHg or DBP at least 90 mmHg. Random-effects meta-analysis was used.
Results:
Twenty studies involving 46 491 participants met inclusion criteria. Overall hypertension prevalence was 30.9% [95% confidence interval (CI) 27.0–34.8]: 29.6% (24.1–35.1) and 32.1% (27.2–37.1) in 1994–2010 and 2011–2018, respectively. Of hypertensive participants, only 24.4% (18.9–30.0) – 31.6% (21.0–42.3) and 20.8% (14.0–27.7) in 1994–2010 and 2011–2018, respectively – were aware of their status, 15.1% (10.6–19.6) were taking antihypertensive medications and 8.8% (5.7–11.9) – 10.4% (7.5–13.3) and 8.3% (4.4–12.3) in 1994–2010 and 2011–2018, respectively – were controlled. Hypertension prevalence varied by sex: 34.3% (30.0–38.6) for men and 31.3% (26.5–36.1) for women; ethnicity: from 3.3% (0.4–6.2) among Pygmies to 56.6% (49.4–63.8) among Bamileke; urbanity: 25.4% (17.1–33.7) for rural and 31.4% (27.3–35.5) for urban dwellers; agroecological zone: from 35.1% (28.9–41.3) in Tropical highlands to 28% (20.1–35.9) in Guinea-Savannah; and subnational region: from 36.3% (27.8–44.9) in the West to 17.1% (9.9–44.2) in the South.
Conclusion:
Cameroon's hypertension prevalence is high and increasing whereas awareness, treatment and control are low and declining. Emerging patterns call urgently for effective campaigns to raise hypertension awareness alongside strategies for hypertension prevention and BP control.
INTRODUCTION
Hypertension or high blood pressure remains a leading cause of the global burden of disease (GBD) and death [1–4], and is a major risk factor for cerebrovascular, cardiovascular and renal morbidity and mortality [5–8]. Hypertension is also causally linked or clustered with behavioural factors (harmful use of alcohol, obesity, insufficient physical activity, high salt/sodium intake, tobacco use) and stress, which are high-risk factors for noncommunicable diseases (NCD) [1–3,5–8]. Over 74% of African countries, including Cameroon, have no guidelines for the management of hypertension [9]. How blood pressure (BP) distribution and hypertension prevalence, awareness, treatment and control rates vary over time countrywide and across population subgroups in Cameroon are unknown. National and subnational estimates of BP levels and burden of hypertension from population-based studies and knowledge of their geography-based differences are needed for effective hypertension prevention and BP control [7,8,10,11]. No previous studies have examined the BP levels and the prevalence, awareness, treatment and control of hypertension separately by place of residence and for the 10 subnational regions nested within all five agroecological zones of Cameroon.
Home to around 25 million people [12], Cameroon is part of Central Africa, has a historic divide between its Anglophone and Francophone populations, and is a trade route between the economies of West and Central Africa. It marks the meeting point of different sub-continental regions of Africa. This is evident in the country's diverse geography, languages, cultures, epidemiologic profiles, dietary patterns and ethnic ancestries of the population, making Cameroon a ‘miniature Africa’ [13–18]. Cameroon's 10 regions (Adamawa, Centre, East, Far-North, Littoral, North, North-West, South, South-West and West) are nested within five agroecological zones (sub-humid, humid, tropical highlands, guinea savanna and sudano-sahelian) reproduced in all 44 sub-Saharan African countries [16]. Population-level disparities found in Cameroon may, therefore, be seen on a larger scale in different African settings [9–12,15,19].
In Cameroon, stroke (4.6%) and ischaemic heart disease (3.8%) are top killers among all NCD, and fifth and sixth causes of death, respectively [20]. In such settings, the distribution and drivers of hypertension-related disease and their population impact are expected to be geographically heterogeneous. Geography-based differences contributing to hypertension disparities include differences in environment (physical, social, ethnocultural, economic and religious), neighbourhood characteristics, urbanization rates, lifestyles, food security and consumption patterns, healthcare, mounting socioeconomic inequality, adoption of recommended nonpharmacological or pharmacological hypertension management, population aging, health-promoting resources and soil potassium and sodium concentrations [5–7,21–23].
Few studies have considered such geography-based differences, to help design strategies to reduce or eliminate hypertension disparities at global, national and subnational levels [7,8,11]. Like many countries, Cameroon still lacks reliable estimates for adequate national surveillance. Hypertension-related indicators are repeatedly reported in different ways in studies often with suboptimal sample sizes for subnational analysis, which hampers assessing progress at national and subnational levels. The current investigation aimed to review the national and subnational burden of hypertension in Cameroon and identify knowledge gaps. For informing efforts to prevent and control cerebrovascular and cardiovascular disease in high-burden countries like Cameroon [5,12,17,20,23], this review addressed the question: what is the epidemiological landscape and control of hypertension among community dwellers aged 18 years or older in Cameroon?
METHODS
This review was registered in PROSPERO (registration number CRD42017054950). Details on literature search and selection, data extraction and management, evaluation of studies, statistical analysis and definitions used, were published in the review protocol [24]. The methods are summarized below.
Literature search strategy
This study complied with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) [25] and the checklist of items in the meta-analysis of observational studies in epidemiology [26]. The review included all types of population-based studies published by December 31, 2016 and updated through November 30, 2018, in English or French that examined hypertension in Cameroon. Eight bibliographic databases (PubMed, Medline, EMBASE, CINHAL, Web of Science, Popline, Scopus and ‘Banque de données en santé publique’ [BDSP]) were searched. Grey literature was also identified, and experts consulted, to minimize publication bias across studies.
Study selection
Studies included those on hypertension, population-based, regardless of design, conducted among community dwellers aged 18 years or older, involving at least 10 participants, and containing numerical information on the diagnosis, prevalence, awareness, treatment and/or control of hypertension in Cameroon. Hospital/primary healthcare/clinic-based studies, studies of pregnant women and studies without a description of how patients with hypertension were identified, were excluded. If there was more than one report for the same study, the one with the most detailed information for our research question was included.
Data extraction and risk of bias in individual studies
Two reviewers independently extracted details of included studies into a database, and integrated them into a synthesis table made available to all review team members. Authors’ names, data collection and publication dates, study design, sample size and study population description were extracted. Additionally, all relevant raw data on the presence versus absence of at least one of the outcomes in the total number of participants with/without a given exposure variable (2 × 2 table) were extracted. Disagreements between the two reviewers were resolved either by consensus or arbitration as appropriate with a third review team member. The sample size was categorized into less than 800 versus higher categories, given standard practices in sample size for prevalence studies that assume a conventional multiplier for power of 0.80 with a two-sided significance of 0.05 [27,28]. Two data collection periods (1994–2010 versus 2011–2018) were specified so as to compare the BP and hypertension data since the Cameroonian sectoral health strategy launched in 2011 – combatting NCD including hypertension being a public health priority – with prior data when health strategies focused on communicable diseases [12]. Primary study authors were contacted by E-mail up to three times, to obtain additional information about missing relevant data given the research question. The original pooled data reorganized participants into predefined homogeneous subgroups suitable for meta-analysis [29], by study (sample size, period of data collection and study quality score) and participant (sex, age, ethnic ancestry, area of residence, agroecological zone and subnational region) factors [24]. The Downs and Black checklist, covering four risk-of-bias domains – reporting, external validity, internal validity (bias and confounding) and power – was used to appraise the quality of included studies [30]. Quality score cut-offs used to categorize studies were: excellent (26–28), good (20–25), fair (15–19), or poor (≤14) [30].
Outcome definitions
The World Hypertension League (WHL)'s definitions and recommended analyses [31,32] were adopted. The distributions of SBP and DBP were summarized by the mean SBP and DBP and their associated statistics. Hypertension prevalence was defined as the proportion of participants who had SBP at least 140 mmHg or DBP at least 90 mmHg. Hypertension awareness prevalence was defined as the proportion of participants with hypertension who reported either having been diagnosed with hypertension by a health professional or who reported taking medication for high BP. Hypertension treatment prevalence was defined as the proportion of participants with hypertension who reported taking medication for high BP. The prevalence of controlled hypertension was defined as the proportion of participants with hypertension who both reported taking medication for high BP and had SBP less than 140 mmHg and DBP less than 90 mmHg.
Data synthesis and statistical analysis
The unit of analysis was the individual study (data point). Data were pooled across studies using random-effects meta-analysis [29]. Pooled estimates were reported overall and tracked over time. Subgroup meta-analyses preplanned in the review protocol were performed by population-level (sex, age and ethnic origin) and geography-based (area of residence, agroecological zone and subnational region) factors. Sensitivity analyses pre-planned were performed to explore the impact of excluding or including studies in meta-analysis based on methodological quality and sample size. Age-standardized estimates were calculated using the WHO standard population [33] that has a relatively young distribution similar to that of a typical African country like Cameroon [12] (Supplemental Figure 1). The standard χ2 test (Q test) (significance level of P < 0.1) and I2 statistic (significance level of I2 > 50%) were used to test between-study heterogeneity [29,34]. The presence of publication bias was examined using funnel plots, a useful and popular way of investigating publication bias [35]. To test for the publication bias under alternative assumptions about the association between effect size estimate and measure of study size or precision (i.e. standard error), the two most popular statistical tests were used: the Begg's test (an adjusted rank correlation test) [36] and the Egger's test (uses a linear regression approach) [37]. A generous minimum number of studies for the performance of a funnel plot to be warranted is about 10 [38]. Following the WHL's recommendations [31,32] and standard reporting of mean BP and hypertension prevalence, awareness, treatment and control [39,40], each pooled estimate included its 95% confidence interval (CI). Analyses were performed with STATA 12.1 (Stata Corp, College Station, Texas, USA).
RESULTS
Systematic review
The literature search yielded 1121 publications from Web of Science (292), EMBASE (281), PubMed (164), Scopus (156), Medline (137), CINHAL (16), Popline (7) and BDSP (6). A total of 368 publications remained after removing duplicates. On screening titles for relevance, 169 publications were excluded. We then assessed 199 full texts, and excluded 73 reviews, editorials, case reports, animal/plant studies or publications unrelated to hypertension-related outcomes. An additional 86 hospital/primary healthcare/clinic-based publications were excluded. In total 40 population-based studies were assessed, and after applying the criteria for age eligibility, numerical information and multiple publications of the same study, 20 studies were retained for analysis (Fig. 1).
FIGURE 1.
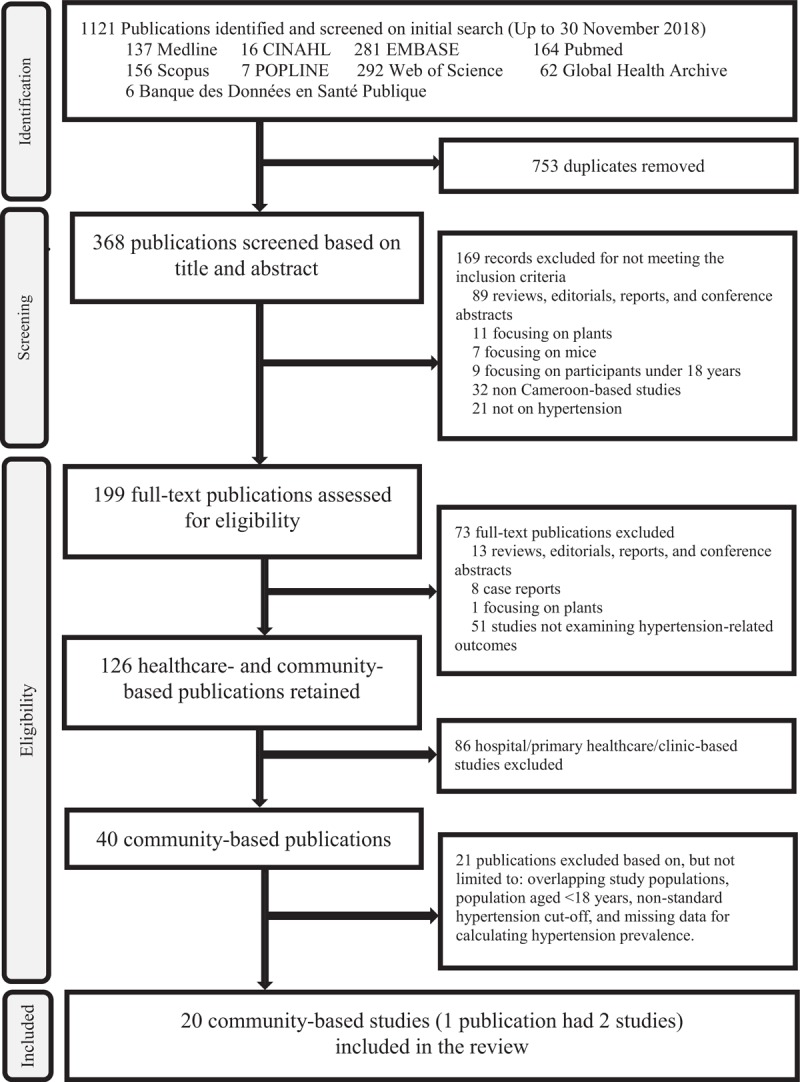
Flow chart of study selection.
Characteristics of the included studies
The 20 studies [41–59] used objective measure of the hypertension (Supplemental Table 1). They were all cross-sectional (two in French), were fielded and published between 1994 and 2018, and involved 46 491 rural and urban community dwellers aged 18 years or older (53.0% women; 21 717 and 24 774 participants in the 1994–2010 and 2011–2018 periods, respectively) residing in all Cameroon's 10 regions and five agroecological zones (Supplemental Table 2 and Supplemental Table 3). The mean time from data collection to study publication was 3.9 (±3.4) years. Sample sizes varied from 181 to 15 470 participants. Most studies involved mixed ethnic origins (17); some focused on specific ethnic ancestries: Bamileke (1), Bantu (1), Fulbe (2), Mbororo (1) and Pygmy (1). The 20 studies were fielded in rural (7; 2 being in rural areas only) and urban (18; 13 being in urban areas only) communities. One publication used two studies [46]. There were no community interventions. Nine and eight of the 20 studies included Yaoundé (capital city) and Douala (main economic city) of Cameroon, respectively. The study quality scores ranged from 8 [45] to 18 [57].
Results of random-effects meta-analyses
For each outcome, the data points and resulting pooled samples generated from raw data in primary studies and utilized in meta-analyses to compute overall pooled estimates and separately pooled estimates for each subgroup are provided in Supplemental Table 3.
Overall pooled estimates and tracked changes between 1994–2010 and 2011–2018
Table 1 shows overall and separately pooled estimates for 1994–2010 and 2011–2018; there were high heterogeneity across studies (Supplemental Figures 1–7). The overall mean SBP and mean DBP were 129.2 (95% CI 125.0–133.3) mmHg (P = 0.000, I2 = 99.5%) and 80.0 (77.9–82.1) mmHg (P = 0.000, I2 = 99.4%), respectively (Supplemental Figure 2). The overall crude hypertension prevalence was 30.9% (27.0–34.8) (P = 0.000, I2 = 98.8%) – increasing from 29.6% (24.1–35.1) in 1994–2010 (P = 0.000, I2 = 98.7%) to 32.1% (27.2–37.1) in 2011–2018 (P = 0.000, I2 = 97.9%) (Supplemental Figure 3). Studies that had meta-analysable and similar age groups data had older people [mean age of 39.9 (±4.7) years] than the general adult Cameroonian population and the WHO standard population; not surprisingly, the overall age-standardized hypertension prevalence was substantially lower than its crude counterpart [23.5% (21.4–25.6) (P = 0.000, I2 = 94.4%) versus 32.4% (27.6–37.3) (P = 0.000, I2 = 98.9%), respectively)] (Supplemental Figure 4). The pooled crude estimates from older age structures in these studies likely overstated the actual hypertension prevalence rates in the general population aged 18 years or older in Cameroon.
Table 1.
Blood pressure and hypertension prevalence, awareness, treatment and control in Cameroon: estimates and changes between 1994–2010 and 2011–2018
| Blood pressure (mmHg) | Hypertension (%) | |||||||
| SBP (95% CI) | DBP (95% CI) | Prevalence (95% CI) | Prevalence in studies with suitable age group data | Awareness (95% CI) | Treatment (95% CI) | Control (95% CI) | ||
| Crude (95% CI) | Age-standardized (95% CI) | |||||||
| Overall | 129.2 (125.0–133.3) | 80.0 (77.9–82.1) | 30.9 (27.0–34.8) | 32.4 (27.6–37.3) | 23.5 (21.4–25.6) | 24.4 (18.9–30.0) | 15.1 (10.6–19.6) | 8.8 (5.7–11.9) |
| Period of data collection | ||||||||
| 1994–2010 | 128.5 (122.5–134.4) | 80.2 (76.8–83.7) | 29.6 (24.1–35.1) | 29.4 (21.3–37.5) | 21.7 (19.4–24.1) | 31.6 (21.0–42.3) | 16.0 (5.5–26.5) | 10.4 (7.5–13.3) |
| 2011–2018 | 129.6 (125.3–133.9) | 79.9 (78.4–81.5) | 32.1 (27.2–37.1) | 35.0 (28.5–41.4) | 25.0 (21.0–28.9) | 20.8 (14.0–27.7) | 14.8 (7.8–21.8) | 8.3 (4.4–12.3) |
CI, confidence interval.
Among people with hypertension, 24.4% (18.9–30.0) (P = 0.000, I2 = 97.6%) were aware of their status – falling from 31.6% (21.0–42.3) in 1994–2010 (P = 0.000, I2 = 96.3%) to 20.8% (14.0–27.7) in 2011–2018 (P = 0.000, I2 = 96.9%) (Supplemental Figure 5); 15.1% (10.6–19.6) (P = 0.000, I2 = 95.1%) were taking prescribed antihypertensive medications – decreasing from 16.0% (5.5–26.5) in 1994–2010 (P = 0.000, I2 = 96.3%) to 14.8% (7.8–21.8) in 2011–2018 (P = 0.000, I2 = 95.8%) (Supplemental Figure 6); and only 8.8% (5.7–11.9) had achieved control (P = 0.000, I2 = 85.2%) – declining from 10.4% (7.7–13.3) in 1994–2010 (1 study) to 8.3% (4.4–12.3) in 2011–2018 (three studies) (Supplemental Figure 7).
Results of subgroup meta-analyses
Results of subgroup analyses by population-level factors (sex, age group and ethnic ancestry) (Table 2) and geography-based characteristics (area of residence, agroecological zone and subnational region) (Table 3) showed noticeable disparities in BP levels and burden of hypertension over time in Cameroon. Compared with women, men had higher mean SBP [131.9 (127.4–136.4) mmHg] and mean DBP [80.4 (79.2–81.7) mmHg], higher crude hypertension prevalence (34.3%; 30.0–38.6) and higher age-standardized hypertension prevalence (23.8%; 19.2–28.4). Compared with women, men had lower prevalence of hypertension awareness (19.5%; 14.8–24.2), treatment (12.8%; 7.3–18.2) and control (7.4%; 3.7–11.2). The hypertension prevalence rose steadily from 24.0% (20.4–27.6) at age less than 35 to 66.4% (46.7–86.2) at age at least 55; likewise, the hypertension awareness prevalence increased from 8.0% (4.6–11.4) at age less than 35 to 37.2% (12.9–61.5) at age at least 55 years. The hypertension treatment prevalence also rose from 3.50% (1.2–5.8) at age less than 35 to 25.2% (14.3–36.1) at age at least 55 years, and the hypertension control prevalence increased from 1.2% (0.1–2.6) at age less than 35 years to 14.4% (8.5–20.2) at age at least 55 years. Mean SBP/DBP were the highest among Bamileke people (135.7/84.6 mmHg) and the lowest among Pygmies (107.0/71.0 mmHg), and community dwellers of Bamileke ancestry also stood out with the highest hypertension prevalence (56.6%) whereas the Pygmies had the lowest prevalence (3.3%).
Table 2.
Blood pressure and hypertension prevalence, awareness, treatment and control by demographic characteristics
| Blood pressure (mmHg) | Hypertension (%) | |||||||
| SBP (95% CI) | DBP (95% CI) | Prevalence (95% CI) | Prevalence in studies with suitable age group data | Awareness (95% CI) | Treatment (95% CI) | Control (95% CI) | ||
| Crude (95% CI) | Age-standardized (95% CI) | |||||||
| Sex | ||||||||
| Male | 131.9 (127.4–136.4) | 80.4 (79.2–81.7) | 34.3 (30.0–38.6) | 38.0 (30.8–45.3) | 23.8 (19.2–28.4) | 19.5 (14.8–24.2) | 12.8 (7.3–18.2) | 7.4 (3.7–11.2) |
| Female | 127.6 (123.2–131.9) | 79.4 (77.6–81.2) | 31.3 (26.5–36.1) | 35.6 (27.2–44.1) | 21.3 (18.1–24.5) | 27.1 (19.8–34.4) | 16.5 (10.3–22.6) | 10.3 (7.0–13.5) |
| Age group (in years) | ||||||||
| < 35 | – | – | 24.0 (20.4–27.6) | – | – | 8.0 (4.6–11.4) | 3.5 (1.2–5.8) | 1.2 (0.1–2.6) |
| 35–44 | – | – | 35.3 (30.6–40.0) | – | – | 16.1 (12.0–20.1) | 9.5 (6.3–12.7) | 4.3 (0.5–8.0) |
| 45–54 | – | – | 54.4 (50.3–58.5) | – | – | 28.5 (22.4–34.6) | 17.9 (14.6–21.2) | 9.7 (0.7–18.6) |
| ≥ 55 | – | – | 66.4 (46.7–86.2) | – | – | 37.2 (12.9–61.5) | 25.2 (14.3–36.1) | 14.4 (8.5–20.2) |
| Ethnic group | ||||||||
| Bamileke | 135.7 (132.3–139.1) | 84.6 (82.0–87.3) | 56.6 (49.4–63.8) | – | – | – | – | – |
| Bantu | 119.0 (116.3–121.7) | 78.0 (75.9–80.1) | 28.0 (20.8–35.2) | – | – | – | – | – |
| Fulbe | 131.1 (125.3–136.9) | 80.2 (79.5–80.9) | 35.6 (29.4–41.7) | 35.6 (29.4–41.7) | 27.5 (23.8–31.1) | 18.6 (14.5–22.7) | 5.4 (3.0–7.8) | – |
| Mbororo | 127.9 (126.4–129.4) | 80.4 (79.5–81.3) | 30.6 (27.6–33.6) | 30.6 (27.6–33.6) | 24.2 (21.4–27.0) | – | – | – |
| Pygmy | 107.0 (105.1–108.9) | 71.0 (69.2–72.8) | 3.3 (0.4–6.2) | – | – | – | – | – |
| Mixed | 129.7 (124.9–132.0) | 80.2 (77.6–82.7) | 29.9 (25.8–34.1) | 31.8 (26.5–37.0) | 22.9 (20.7–25.1) | 25.2 (19.1–31.2) | 17.1 (12.5–21.7) | 8.8 (5.7–11.9) |
CI, confidence interval.
Table 3.
Blood pressure and hypertension prevalence, awareness, treatment and control by geography-based characteristics
| Blood pressure (mmHg) | Hypertension (%) | |||||||
| SBP (95% CI) | DBP (95% CI) | Prevalence (95% CI) | Prevalence in studies with suitable age group data | Awareness (95% CI) | Treatment (95% CI) | Control (95% CI) | ||
| Crude (95% CI) | Age-standardized (95% CI) | |||||||
| Area of residence | ||||||||
| Rural | 123.2 (117.3–129.6) | 76.7 (73.7–79.8) | 25.4 (17.1–33.7) | 31.7 (28.9–34.4) | 24.6 (22.8–26.3) | 22.3 (14.7–29.9) | 11.7 (5.4–28.9) | 6.1 (3.0–9.2) |
| Urban | 129.1 (124.8–133.4) | 80.5 (78.8–82.1) | 31.4 (27.3–35.5) | 33.0 (27.6–38.4) | 23.6 (21.3–25.9) | 25.8 (19.4–32.1) | 14.6 (10.1–19.1) | 9.7 (5.8–13.6) |
| Agroecological zone | ||||||||
| Guinea savannah | 130.2 (127.0–133.5) | 81.3 (79.0–83.7) | 28.0 (20.1–35.9) | 25.9 (15.1–36.6) | 20.7 (12.6–28.7) | 14.1 (9.5–18.7) | – | – |
| Humid | 132.4 (126.8–138.0) | 82.3 (80.8–83.7) | 32.1 (24.3–40.0) | 35.5 (6.8–64.1) | 26.1 (14.8–37.4) | 22.5 (15.8–29.2) | 13.9 (9.6–18.1) | 3.9 (0.1–7.7) |
| Sub-humid | 125.4 (119.9–130.8) | 78.3 (75.0–81.5) | 29.1 (23.5–34.6) | 32.7 (27.5–37.9) | 22.9 (20.3–25.4) | 27.8 (17.9–37.8) | 16.8 (9.4–24.3) | 7.6 (2.1–13.1) |
| Sudano-sahelian | 132.9 (130.9–135.0) | 81.3 (78.8–83.7) | 33.4 (28.7–38.1) | 38.5 (35.3–41.7) | 29.1 (26.1–32.1) | 18.6 (13.1–24.1) | 8.1 (2.8–13.4) | – |
| Tropical highlands | 130.8 (123.2–138.4) | 81.1 (79.0–83.2) | 35.1 (28.9–41.3) | 45.1 (37.1–53.2) | 28.1 (22.5–33.6) | 25.3 (15.6–35.1) | 23.2 (11.6–34.7) | 19.1 (5.3–32.8) |
| Subnational region | ||||||||
| Adamawa | 130.2 (127.0–133.5) | 79.9 (79.1–80.6) | 28.0 (20.1–35.9) | 25.9 (15.1–36.6) | 20.7 (12.6–28.7) | 14.1 (9.5–18.7) | – | – |
| Centre | 127.9 (122.2–133.5) | 78.6 (76.0–81.1) | 31.8 (26.0–37.5) | 33.6 (25.6–41.6) | 22.4 (19.1–25.6) | 31.4 (23.7–39.0) | 16.8 (9.4–24.3) | 7.6 (2.1–13.1) |
| East | 130.2 (126.9–133.6) | 79.7 (79.2–80.1) | 30.8 (29.3–32.2) | 30.5 (27.9–33.1) | 24.3 (21.9–26.7) | – | – | – |
| Far North | 133.0 (131.0–135.1) | 79.8 (79.1–80.5) | 35.5 (29.5–41.5) | 38.5 (35.3–41.7) | 29.1 (26.1–32.0) | 18.6 (14.5–22.7) | 5.4 (3.0–7.8) | – |
| Littoral | 132.4 (126.6–138.2) | 81.6 (79.2–84.0) | 32.3 (23.3–41.2) | 35.5 (6.8–64.1) | 26.1 (14.8–37.4) | 23.9 (21.7–26.2) | 11.5 (9.8–13.1) | 2.2 (1.1–3.4) |
| North | 131.9 (130.1–133.7) | 79.5 (78.4–80.6) | 30.6 (28.1–33.1) | – | – | 23.0 (20.0–26.0) | 10.8 (8.6–13.0) | – |
| North-West | 131.8 (130.4–133.1) | 80.5 (78.1–82.9) | 34.9 (28.6–41.2) | – | – | 23.0 (20.0–26.0) | 10.8 (8.6–13.0) | – |
| South | 119.5 (95.1–143.9) | 75.3 (67.0–83.6) | 17.1 (9.9–44.2) | – | – | – | – | – |
| South-West | 131.9 (130.1–133.7) | 79.5 (78.4–80.6) | 30.9 (28.8–33.0) | – | – | 28.9 (23.0–34.8) | 20.6 (15.4–25.9) | 6.1 (3.0–9.2) |
| West | 130.8 (122.2–139.4) | 80.3 (78.2–82.3) | 36.3 (27.8–44.9) | 41.4 (39.1–43.7) | 25.7 (23.7–27.8) | 16.1 (6.1–26.1) | 14.8 (12.2–17.4) | 12.4 (10.0–14.8) |
CI, confidence interval.
We estimated pooled mean SBP/DBP to be: 123.2/76.7vmmHg for rural inhabitants versus 129.1/80.5 mmHg for their urban counterparts; lowest for the subhumid zone (125.4/78.3 mmHg) and highest for the Sudano-sahelian zone (132.9/81.3 mmHg); and lowest for the South region (119.5/75.3 mmHg) and highest for the Far North region (133.0/79.8 mmHg). The hypertension prevalence was higher in urban (31.4%) than rural (25.4%), highest in the tropical highlands agroecological zone (35.1%) – where the majority of inhabitants are people of Bamileke descent – and lowest in the Guinea-savannah (28.0%), and highest the West region (36.3%) – which is mostly populated by dwellers of Bamileke ancestry – and lowest in the South region (17.1%). The prevalence of undiagnosed, untreated and uncontrolled hypertension was uniformly high in the different demographic and geographic subgroups for which meta-analysable data were available. Specifically, the high burden of undiagnosed hypertension was generalized nationwide, higher among rural (77.7%) than urban (74.2%) dwellers, highest among inhabitants of the Guinea-savannah zone (85.9%) and lowest among those of the sub-humid zone (72.2%).
Sensitivity meta-analyses
We explored sources of heterogeneity with respect to the methodological quality and sample size of the included studies (Table 4). Studies of higher quality score had lower pooled mean SBP/DBP (125.4/79.0 mmHg), lower crude hypertension prevalence (28.7%), higher burden of undiagnosed hypertension (82.2%) and uncontrolled hypertension (91.7%) than studies with lower quality score. However, for the 11 studies with suitable age group data for which meta-analysis was conducted, estimates of crude hypertension prevalence were robustly similar between studies of lower (32.9%) and higher (31.5%) quality score; likewise, the age-standardized hypertension prevalence was 23.6 and 23.3% for studies of higher and lower quality score, respectively. These findings suggested that the age distribution of the participants in included studies partly explained the between-study heterogeneity. Mean BP values and burden of hypertension by sample size depicted no consistent patterns: studies of sample size less than 800 or 800–1999 were not more likely to report more extreme results compared with studies of sample size at least 2000.
Table 4.
Blood pressure and hypertension prevalence, awareness, treatment and control by study quality score and sample size
| Blood pressure (mmHg) | Hypertension (%) | |||||||
| SBP (95% CI) | DBP (95% CI) | Prevalence (95% CI) | Prevalence in studies with suitable age group data | Awareness (95% CI) | Treatment (95% CI) | Control (95% CI) | ||
| Crude (95% CI) | Age-standardized (95% CI) | |||||||
| Quality score | ||||||||
| <15 | 131.6 (124.9–138.2) | 80.7 (77.9–83.5) | 32.3 (26.2–38.4) | 32.9 (23.9–41.9) | 23.3 (20.0–26.6) | 29.4 (23.4–35.3) | 14.8 (9.6–20.0) | 9.4 (3.6–15.2) |
| 15–19 | 125.4 (119.7–131.1) | 79.0 (76.6–81.4) | 28.7 (25.3–32.1) | 31.5 (28.3–34.7) | 23.6 (20.9–26.2) | 17.8 (11.9–23.7) | 15.7 (3.8–27.6) | 8.3 (4.1–12.5) |
| Sample size | ||||||||
| <800 | 127.7 (117.6–137.7) | 80.6 (76.8–84.4) | 31.9 (25.3–38.5) | 32.4 (18.8–46.0) | 19.2 (15.9–22.5) | 19.8 (2.0–37.6) | 20.6 (15.4–25.9) | 6.1 (3.0–9.2) |
| 800–1999 | 130.4 (122.4–138.4) | 79.8 (77.1–82.5) | 32.0 (24.7–39.3) | 34.3 (27.5–41.1) | 24.7 (21.5–28.0) | 27.9 (18.6–37.1) | 13.8 (5.0–22.6) | 11.6 (9.6–13.5) |
| ≥2000 | 129.6 (121.1–138.1) | 79.5 (74.2–84.9) | 27.9 (19.9–36.0) | 30.7 (22.1–39.3) | 24.8 (21.3–28.3) | 22.9 (13.9–31.8) | 14.8 (6.8–22.9) | 6.5 (5.0–8.0) |
CI, confidence interval.
Publication bias
Visual inspection of the funnel plots indicated substantial asymmetry (Supplemental Figures 8–13). For the mean SBP, the Egger's test (P = 0.805) and the Begg's test (P = 0.951) did not indicate evidence of publication bias; similarly, for the mean DBP (Egger, P = 0.151; Begg, P = 0.246). These tests fail to find evidence of publication bias for the prevalence of hypertension for all 20 included studies (Egger, P = 0.539; Begg, P = 0.581) or for the studies, which had suitable age group data for age-standardized hypertension prevalence (Egger, P = 0.627; Begg, P = 0.640); they also did not indicate evidence of publication bias for the prevalence of hypertension awareness (Egger, P = 0.550; Begg, P = 0.755), and for the prevalence of hypertension treatment (Egger, P = 0.231; Begg, P = 0.452). For the prevalence of hypertension control, the Begg's test found no evidence of publication bias (P = 0.806), but the Egger's test did (P = 0.029); however, the small number of studies (<10) used may account for the discrepancy in these test results.
DISCUSSION
Reliable, large-scale, population-based data on hypertension in Africa are scarce. Using nationwide pooled data on 46 491 participants covering all regions and agroecologies of Cameroon, this study provided improved estimates and tracked changes in BP distribution and hypertension prevalence, awareness, treatment and control over time. It used the WHL's definitions and recommended analyses [31,32] to pinpoint agroecologies, regions and population subgroups with heightened BP and at risk for developing cardiovascular disease (CVD).
This review found high mean BP values and prevalence of hypertension contrasting with strikingly low prevalence of hypertension awareness, treatment and control observed in Cameroon. Moreover, the proportion of people aged 18 years or older living with hypertension in Cameroon has increased over time, currently affecting 32% of adults; among these people with hypertension, 76% were unaware of their hypertension status, 85% were not taking antihypertensive medication and 91% had uncontrolled BP. This reflects epidemiological processes unfolding in Africa [60] and its countries of sub-Saharan Africa [10], East Africa [61–66], Central Africa [67–70], North Africa [61,71,72], West Africa [39,61,73–75] and Southern Africa [61,76]. Our estimates are quite close to those reported in sub-Saharan Africa where the hypertension prevalence was 30%; among people with hypertension, 73% were unaware of their status, 82% were not receiving treatment and 93% had uncontrolled BP [10]; but our analysis adds an in-depth picture of the geographic and demographic variability around the population means. These estimates concur with those on hypertension in Africa [10,77,78] and worldwide with about one-third of adults in most communities being hypertensive [6]. The burden of hypertension in Cameroon and in much of Africa, contrasts with the markedly higher prevalence of hypertension awareness, treatment and control reported in high-income countries [4,5,11], and other low-income and middle-income countries [21,79].
We found higher hypertension prevalence for men than women as is generally the case in Africa [77,78], excepting a few countries where the prevalence is higher in women: Algeria (31.6 versus 25.7%), Botswana (37.0 versus 28.8%) and Mali (25.8 versus 16.6%) [60]. A higher prevalence of hypertension was found in urban versus rural areas of Cameroon. This finding is in accordance with those generally reported in high-income, middle-income and low-income countries [21], and in other African countries [39,40,60,61,78]. The structurally higher levels of hypertension in urban than in rural settings has been attributed mainly to contextual and behavioural factors associated with urban environments, including unhealthy dietary patterns and sedentary lifestyle [60]. In contrast, most rural locations generally favor intense physical activities including walking for long periods and rigorous farming coupled with better nutrition, rich in locally produced fruits and vegetables [39,78,80]. There were also remarkable disparities in mean BP values and burden of hypertension across all regions. These regional disparities may reflect differences in urbanization rates, lifestyle factors, food security and consumption patterns, behavioural and stress-related factors, ethnic composition of the population, socioeconomic inequality, population aging, health-promoting resources and soil potassium and sodium concentrations [5,7,8,11,22]. That so many individuals’ hypertension was untreated and uncontrolled may also reflect the high burden of undiagnosed hypertension. The latter is consistent with enduring constraints in access to quality and affordable healthcare, poor public health facilities network coverage, scarcity of health professionals, frequent rupture of stocks of NCD drugs, very limited accessibility to inexpensive and/or essential medicines [81], limited hypertension knowledge among health professionals and patients [6,8,10] and poor adherence to medication among hypertensive patients under treatment.
To our knowledge, this is the first study that attempted to generate nationally and subnationally pooled data, to pinpoint ethnic disparities in hypertension and geography-based variation of BP distribution and hypertension statistics for all five agroecological zones and 10 regions, in both rural and urban areas, in Cameroon. This study revealed that: hypertension prevalence is high and increasing over time in Cameroon; and the burden of undiagnosed, untreated and uncontrolled hypertension in community-dwelling residents is extremely high nationwide and widespread across diverse population subgroups, across all five agroecological zones and 10 subnational regions, and areas of residence. This situation is compounded by the fact that many patients cannot afford the out-of-pocket payment, which impedes their accessibility to healthcare or adherence to treatment in various communities. These new and improved indicators reflect the state of response to the growing burden of hypertension in Cameroon, and most likely of the prevailing situation in much of Africa. Such statistics are crucial for understanding the geography and public health significance of hypertension, and provide data for evaluating the effects of public health policies and programs for population health promotion. Policymakers and public health practitioners may use these geography-based data on BP distribution and hypertension mapping in planning hypertension awareness programs, and to determine the best manner to allocate resources for reducing hypertension risk and hypertension-related diseases and deaths nationwide and across regions. Structural changes are needed to address hypertension in Cameroon where education and screening may not be sufficient without parallel efforts to improve hypertension treatment and control. The most direct implication of these findings is that Cameroon needs a universal, rather than a targeted, approach to hypertension and that the obstacles to control must be determined [12,17,82].
This review also highlighted salient ethnic differences in BP values and the burden of hypertension. Further research is needed to tease out the distinctive features that predispose people of Bamileke descent to higher BP values and burden of hypertension irrespective of geographic location in Cameroon [42,49,51] and probably in other countries in the world. Such research should be designed to assess epidemiological, genetic, socioeconomic, cultural, behavioral, lifestyle and environmental factors affecting BP levels, hypertension awareness and treatment [83], individual responses to pharmacological therapy [84], emotional stress in sub-Saharan Africans [85] and gene–environment interactions [86].
This review had some limitations. First, the evaluation of blood pressure was done on a single occasion, which tends to overestimate the prevalence of hypertension [6,40,53]. Second, the data were unavailable for all outcomes in included studies. This limitation was addressed by showing the number of studies and samples used for each separately pooled estimate. Third, there was considerable between-study heterogeneity, due in part to differences in study methodology and population age distribution. However, the patterns of estimates were generally robust across population-level and geography-based factors. Thus, emerging patterns may represent true disparities across subgroups where substantial disparities in access to healthcare exist. Fourth, all studies were cross-sectional and there was no community-based intervention study to examine the feasibility of reducing or eliminating hypertension disparities. This highlights the need for community-engaged hypertension disparities research using life-course public health approaches [87,88].
In conclusion, this review produced much needed population-level data nationwide and yielded pooled estimates, which tracked changes over time and showed their variation by sex, ethnic ancestry, area of residence, agroecological zone and subnational region. It identified noticeable geography-based disparities in BP values and hypertension in Cameroon, known as miniature Africa. These disparities are embedded in the high burden of undiagnosed, untreated and uncontrolled hypertension simultaneously with the rising burden of hypertension nationwide. These contrasting patterns call for effective campaigns to raise hypertension awareness together with strategies for hypertension prevention and control, and a better policy response.
ACKNOWLEDGEMENTS
Ethical approval was not required for this study. The first and senior author, B.K.D., conceptualized the review, conducted the analyses and interpretation of data, and drafted the manuscript. All authors contributed to the manuscript editing and revision, and approved the final version of the manuscript.
Sources of support: This study was supported by a Project Development Initiative grant from the Université de Montréal. The Université de Montréal had no role in the work.
Conflicts of interest
Members of the review team who were authors of the studies reviewed were allowed to participate in discussions and appraisal of evidence from published work, including their own studies and recused themselves from voting on evidence statements and recommendations related to their studies. J.C.T. reported grants from Amarin, AstraZeneca, Esperion, Ionis, Merck, personal fees from Pfizer and Sanofi, personal fees and minor equity interest from DalCor, grants and personal fees from Servier, as well as a patent Pharmacogenomics-guided CETP inhibition pending, all of which were outside the submitted work. All the other review team members declared no conflicts of interest.
Supplementary Material
Footnotes
Abbreviations: BP, blood pressure; GBD, global burden of disease; HBP, high blood pressure; NCD, noncommunicable disease; WHL, World Hypertension League
REFERENCES
- 1.Lloyd-Sherlock P, Ebrahim S, Grosskurth H. Is hypertension the new HIV epidemic? Int J Epidemiol 2014; 43:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wall HK, Hannan JA, Wright JS. Patients with undiagnosed hypertension: hiding in plain sight. JAMA 2014; 312:1973–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen MH, Spencer S. A global perspective on hypertension: a Lancet Commission. Lancet 2015; 386:637–638. [DOI] [PubMed] [Google Scholar]
- 4.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 2017; 317:165–182. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 2014; 32:3–15. [DOI] [PubMed] [Google Scholar]
- 7.Mensah GA, Cooper RS, Siega-Riz AM, Cooper LA, Smith JD, Brown CH, et al. Reducing cardiovascular disparities through community-engaged implementation research: A National Heart, Lung, and Blood Institute Workshop Report. Circ Res 2018; 122:213–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine. A population-based policy and systems change approach to prevent and control hypertension. Washington DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 9.Dzudie A, Rayner B, Ojji D, Schutte AE, Twagirumukiza M, Damasceno A, et al. PASCAR Task Force on Hypertension. Roadmap to achieve 25% hypertension control in Africa by 2025. Glob Heart 2018; 13:45–59. [DOI] [PubMed] [Google Scholar]
- 10.Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of undiagnosed hypertension in sub-Saharan Africa: a systematic review and meta-analysis. Hypertension 2015; 65:291–298. [DOI] [PubMed] [Google Scholar]
- 11.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016; 134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministry of Public Health. Projections Démographiques et Estimations des Cibles Prioritaires des Différents Programmes et Interventions de Santé. Yaounde: Ministry of Public Health, Republic of Cameroon; 2016. [Google Scholar]
- 13.Louis FJ, Mpoudi-Ngole E, Leloup M, Louis JP. Cameroun, Afrique en miniature: situation sanitaire en 1995. Med Trop 1995; 55:313–322. [PubMed] [Google Scholar]
- 14.Tchawa P. Le Cameroun: une « Afrique en miniature »? Cahiers d’Outre-Mer 2012; 259:319–338. [Google Scholar]
- 15.Amougou JA, Eone N, Bell JM, Abossolo SA, Batha RA, Fondze GB. Characterization of the agro-meteorological systems of family agriculture in Cameroon. World Wide J Multidisciplinary Res Dev 2016; 2:51–56. [Google Scholar]
- 16.Alliance for a Green Revolution in Africa (AGRA). Africa Agriculture Status Report: climate change and smallholder agriculture in Sub-Saharan Africa. Nairobi: AGRA; 2014. [Google Scholar]
- 17.Ministry of Public Health. Health sector strategy 2016–2020. Yaounde: Ministry of Public Health, Republic of Cameroon; 2016. [Google Scholar]
- 18.Caprioli G, Fiorini D, Maggi F, Nicoletti M, Ricciutelli M, Toniolo C, et al. Nutritional composition, bioactive compounds and volatile profile of cocoa beans from different regions of Cameroon. Int J Food Sci Nutr 2016; 67:422–430. [DOI] [PubMed] [Google Scholar]
- 19.Kuate Defo B. Demographic, epidemiological, and health transitions: are they relevant to population health patterns in Africa? Glob Health Action 2014; 7:22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Cameroon: WHO Statistical Profile. Available at: http://www.who.int/gho/countries/cmr.pdf?ua=1 [Accessed 10 August 2017]. [Google Scholar]
- 21.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, et al. PURE (Prospective Urban Rural Epidemiology) Study investigators. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013; 310:959–968. [DOI] [PubMed] [Google Scholar]
- 22.Sun H. Association of soil potassium and sodium concentrations with spatial disparities of prevalence and mortality rates of hypertensive diseases in the USA. Environ Geochem Health 2018; 40:1513–1524. [DOI] [PubMed] [Google Scholar]
- 23.Alwan A, Maclean DR, Riley LM, d’Espaignet ET, Mathers CD, Stevens GA, et al. Monitoring and surveillance of chronic non-communicable diseases: progress and capacity in high-burden countries. Lancet 2010; 376:1861–1868. [DOI] [PubMed] [Google Scholar]
- 24.Kuate Defo B, Mbanya JC, Tardif JC, Ekundayo O, Perreault S, Potvin L, et al. Diagnosis, prevalence, awareness, treatment, prevention, and control of hypertension in Cameroon: protocol for a systematic review and meta-analysis of clinic-based and community-based studies. JMIR Res Protoc 2017; 6:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med 2009; 151:W65–W94. [PMC free article] [PubMed] [Google Scholar]
- 26.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 27.Lwanga SK, Lemeshow S. Sample size determination in health studies: a practical manual. Geneva: World Health Organization; 1991. [Google Scholar]
- 28.Lemeshow S, Levy PS. Sampling of populations: methods and applications. New York: John Wiley & Sons Inc; 1999. [Google Scholar]
- 29.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. New York: Wiley; 2000. [Google Scholar]
- 30.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gee ME, Campbell N, Sarrafzadegan N, Jafar T, Khalsa TK, Mangat B, et al. Standards for the uniform reporting of hypertension in adults using population survey data: recommendations from the World Hypertension League Expert Committee. J Clin Hypertens (Greenwich) 2014; 16:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell N, Ordunez P, Jaffe MG, Orias M, DiPette DJ, Patel P, et al. Implementing standardized performance indicators to improve hypertension control at both the population and healthcare organization levels. J Clin Hypertens (Greenwich) 2017; 19:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad O, Boschi-Pinto C, Lopez A, Murray C, Lozano R, Inoue M. Age standardization of rates: a new WHO standard. GPE Discussion Paper Series-EIP/GPE/EBD No. 31. Geneva: World Health Organization; 2001. [Google Scholar]
- 34.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 35.Deeks JJ, Higgins JPT, Altman DG. Higgins JP, Green S. Chapter 9: analysing data and undertaking meta-analyses. Cochrane handbook for systematic reviews of interventions. Chichester (UK): John Wiley & Sons; 2008. 243–296. [Google Scholar]
- 36.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 37.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006; 333:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adeloye D, Basquill C, Aderemi AV, Thompson JY, Obi FA. An estimate of the prevalence of hypertension in Nigeria: a systematic review and meta-analysis. J Hypertens 2015; 33:230–242. [DOI] [PubMed] [Google Scholar]
- 40.Jessen N, Damasceno A, Silva-Matos C, Tuzine E, Madede T, Mahoque R, et al. Hypertension in Mozambique: trends between 2005 and 2015. J Hypertens 2018; 36:779–784. [DOI] [PubMed] [Google Scholar]
- 41.Arrey WT, Dimala CA, Atashili J, Mbuagbaw J, Monekosso GL. Hypertension, an emerging problem in rural Cameroon: prevalence, risk factors, and control. Int J Hypertens 2016; 2016:5639146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen E, Boetsch G, Palstra FP, Pasquet P. Social valorisation of stoutness as a determinant of obesity in the context of nutritional transition in Cameroon: the Bamileke case. Soc Sci Med 2013; 96:24–32. [DOI] [PubMed] [Google Scholar]
- 43.Cooper R, Rotimi C, Ataman S, McGee D, Osotimehin B, Kadiri S, et al. The prevalence of hypertension in seven populations of West African origin. Am J Public Health 1997; 87:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dzudie A, Kengne A, Muna W, Ba H, Menanga A, Kouam Kouam C, et al. Prevalence, awareness, treatment and control of hypertension in a self-selected sub-Saharan African urban population: a cross-sectional study. BMJ Open 2012; 2:e001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ewane ME, Mandengue SH, Ahmadou G, Moumbe Tamba S, Dzudie A, Luma HN. Dépistage des maladies cardiovasculaires et des facteurs de risque dans une cohorte de 270 Camerounais: effets des activités physiques et sportives. Médecine des Maladies Métaboliques 2011; 5:655–658. [Google Scholar]
- 46.Fezeu L, Kengne AP, Balkau B, Awah PK, Mbanya JC. Ten-year change in blood pressure levels and prevalence of hypertension in urban and rural Cameroon. J Epidemiol Community Health 2010; 64:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fouda AA, Lemogoum D, Manga JO, Dissongo J, Tobbit R, Moyo DF, et al. Epidémiologie de l’obésité en milieu du travail à Douala, Cameroun. Rev Med Brux 2012; 33:131–137. [PubMed] [Google Scholar]
- 48.Kamadjeu RM, Edwards R, Atanga JS, Unwin N, Kiawi EC, Mbanya JC. Prevalence, awareness and management of hypertension in Cameroon: findings of the 2003 Cameroon Burden of Diabetes Baseline Survey. J Hum Hypertens 2006; 20:91–92. [DOI] [PubMed] [Google Scholar]
- 49.Katte JC, Dzudie A, Sobngwi E, Mbong EN, Fetse GT, Kouam CK, et al. Coincidence of diabetes mellitus and hypertension in a semi-urban Cameroonian population: a cross-sectional study. BMC Public Health 2014; 14:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaze FF, Halle MP, Mopa HT, Ashuntantang G, Fouda H, Ngogang J, et al. Prevalence and risk factors of chronic kidney disease in urban adult Cameroonians according to three common estimators of the glomerular filtration rate: a cross-sectional study. BMC Nephrol 2015; 16:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaze FF, Meto DT, Halle MP, Ngogang J, Kengne AP. Prevalence and determinants of chronic kidney disease in rural and urban Cameroonians: a cross-sectional study. BMC Nephrol 2015; 16:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kengne AP, Awah PK, Fezeu L, Mbanya JC. The burden of high blood pressure and related risk factors in urban sub-Saharan Africa: evidences from Douala in Cameroon. Afr Health Sci 2007; 7:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kingue S, Ngoe CN, Menanga AP, Jingi AM, Noubiap JJ, Fesuh B, et al. Prevalence and risk factors of hypertension in urban areas of Cameroon: a Nationwide Population-Based Cross-Sectional Study. J Clin Hypertens (Greenwich) 2015; 17:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kufe CN, Klipstein-Grobusch K, Leopold F, Assah F, Ngufor G, Mbeh G, et al. Risk factors of impaired fasting glucose and type 2 diabetes in Yaounde, Cameroon: a cross sectional study. BMC Public Health 2015; 15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kufe NC, Ngufor G, Mbeh G, Mbanya JC. Distribution and patterning of non-communicable disease risk factors in indigenous Mbororo and non-autochthonous populations in Cameroon: cross sectional study. BMC Public Health 2016; 16:1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lemogoum D, Van de Borne P, Lele C, Damasceno A, Ngatchou W, Amta P, et al. Prevalence, awareness, treatment, and control of hypertension among rural and urban dwellers of the Far North Region of Cameroon. J Hypertens 2018; 36:159–168. [DOI] [PubMed] [Google Scholar]
- 57.Lemogoum D, Ngatchou W, Lele C, Okalla C, Leeman M, Degaute JP, et al. Association of urinary sodium excretion with blood pressure and risk factors associated with hypertension among Cameroonian pygmies and bantus: a cross-sectional study. BMC Cardiovasc Disord 2018; 18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mbouemboue OP, Derew D, Tsougmo JON, Tamanji AT. A Community-based assessment of hypertension and some other cardiovascular disease risk factors in Ngaoundere, Cameroon. Int J Hypertens 2016; 2016:4754636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nkondjock A, Bizome E. Dietary patterns associated with hypertension prevalence in the Cameroon defence forces. Eur J Clin Nutr 2010; 64:1014–1021. [DOI] [PubMed] [Google Scholar]
- 60.van de Vijver S, Akinyi H, Oti S, Olajide A, Agyemang C, Aboderin I, et al. Status report on hypertension in Africa - consultative review for the 6th Session of the African Union Conference of Ministers of Health on NCD's. Pan Afr Med J 2013; 16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hendriks ME, Wit FW, Roos MT, Brewster LM, Akande TM, de Beer IH, et al. Hypertension in Sub-Saharan Africa: cross-sectional surveys in four rural and urban communities. PLoS One 2012; 7:e32638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nahimana M-R, Nyandwi A, Muhimpundu MA, Olu O, Condo JU, Rusanganwa A, et al. A population-based national estimate of the prevalence and risk factors associated with hypertension in Rwanda: implications for prevention and control. BMC Public Health 2018; 18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guwatudde D, Mutungi G, Wesonga R, Kajjura R, Kasule H, Muwonge, et al. The epidemiology of hypertension in Uganda: findings from the National non-communicable diseases risk factor survey. PloS One 2015; 10:e0138991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mufunda J, Mebrahtu G, Usman A, Nyarango P, Kosia A, Ghebrat Y, et al. The prevalence of hypertension and its relationship with obesity: results from a national blood pressure survey in Eritrea. J Hum Hypertens 2006; 20:59–65. [DOI] [PubMed] [Google Scholar]
- 65.Edwards R, Unwin N, Mugusi F, Whiting D, Rashid S, Kissima J, et al. Hypertension prevalence and care in an urban and rural area of Tanzania. J Hypertens 2000; 18:145–152. [DOI] [PubMed] [Google Scholar]
- 66.Musinguzi G, Nuwaha F. Prevalence, awareness and control of hypertension in Uganda. PLoS One 2013; 8:e62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pires JE, Sebastiao YV, Langa AJ, Nery SV. Hypertension in Northern Angola: prevalence, associated factors, awareness, treatment and control. BMC Publ Health 2013; 13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katchunga PB, M’Buyamba-Kayamba JR, Masumbuko BE, Lemogoum D, Kashongwe ZM, Degaute JP, et al. Hypertension in the adult Congolese population of Southern Kivu: results of the vitaraa study. Presse Med 2011; 40:e315–e323. [DOI] [PubMed] [Google Scholar]
- 69.Ngoungou EB, Aboyans V, Kouna P, Makandja R, Ecke Nzengue JE, Allogho CN, et al. Prevalence of cardiovascular disease in Gabon: a population study. Arch Cardiovasc Dis 2012; 105:77–83. [DOI] [PubMed] [Google Scholar]
- 70.Matenga JA, Allain TJ, Wilson AO, Adamchak DJ, Senzanje B, Mushangi E, et al. Hypertension management in Zimbabwe – awareness, treatment and blood pressure control: a community-based study. S Afr Med J 1997; 87:1371–1373. [PubMed] [Google Scholar]
- 71.Nejjari C, Arharbi M, Chentir MT, Boujnah R, Kemmou O, Megdiche H, et al. Epidemiological trial of hypertension in North Africa (ETHNA): an international multicentre study in Algeria, Morocco and Tunisia. J Hypertens 2013; 31:49–62. [DOI] [PubMed] [Google Scholar]
- 72.Ministry of Health and Population, El-Zanaty and Associates, and ICF International. Egypt Health Issues Survey. Cairo: Ministry of Health and Population; 2015. [Google Scholar]
- 73.Soubeiga JK, Millogo T, Bicaba BW, Doulougou B, Kouanda S. Prevalence and factors associated with hypertension in Burkina Faso: a countrywide cross-sectional study. BMC Public Health 2017; 17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Houinato DS, Gbary AR, Houehanou YC, Djrolo F, Amoussou M, Segnon-Agueh J, et al. Prevalence of hypertension and associated risk factors in Benin. Rev Epidémiol Santé Publique 2012; 60:95–102. [DOI] [PubMed] [Google Scholar]
- 75.Agyemang C, Bruijnzeels M, Owusu-Dabo E. Factors associated with hypertension awareness, treatment, and control in Ghana, West Africa. J Hum Hypertens 2006; 20:67–71. [DOI] [PubMed] [Google Scholar]
- 76.Kandala NB, Tigbe W, Manda SO, Stranges S. Geographic variation of hypertension in sub-saharan Africa: a case study of South Africa. Am J Hypertens 2013; 26:382–391. [DOI] [PubMed] [Google Scholar]
- 77.Addo J, Smeeth L, Leon DA. Hypertension in sub-Saharan Africa: a systematic review. Hypertension 2007; 50:1012–1018. [DOI] [PubMed] [Google Scholar]
- 78.Twagirumukiza M, De Bacquer D, Kips J, de Backer G, Stichele RV, Van Bortel L. Current and projected prevalence of arterial hypertension in sub-Saharan Africa by sex, age and habitat: an estimate from population studies. J Hypertens 2011; 29:1243–1252. [DOI] [PubMed] [Google Scholar]
- 79.Pereira M, Lunet N, Azevedo A, Barros H. Differences in prevalence, awareness, treatment and control of hypertension between developing and developed countries. J Hypertens 2009; 27:963–975. [DOI] [PubMed] [Google Scholar]
- 80.Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet 2010; 376:1775–1784. [DOI] [PubMed] [Google Scholar]
- 81.Hogerzeil HV, Liberman J, Wirtz VJ, Kishore SP, Selvaraj S, KiddellMonroe R, et al. Promotion of access to essential medicines for noncommunicable diseases: practical implications of the UN political declaration. Lancet 2013; 381:680–689. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization and the United Nations Children's Fund (UNICEF). A vision for primary health care in the 21st century. Towards universal health coverage and the sustainable development goals. Geneva: World Health Organization; 2018. [Google Scholar]
- 83.Bennett A, Parto P, Krim SR. Hypertension and ethnicity. Curr Opin Cardiol 2016; 31:381–386. [DOI] [PubMed] [Google Scholar]
- 84.Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci 2014; 348:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malan L, Malan NT. Emotional stress as a risk for hypertension in sub-Saharan Africans: are we ignoring the odds? Adv Exp Med Biol 2017; 2:497–510. [DOI] [PubMed] [Google Scholar]
- 86.James GD. Understanding blood pressure variation and variability: biological importance and clinical significance. Adv Exp Med Biol 2017; 2:3–19. [DOI] [PubMed] [Google Scholar]
- 87.Committee on Valuing Community-Based, Non-Clinical Prevention Programs; Board on Population Health and Public Health Practice; Institute of Medicine. An integrated framework for assessing the value of community-based prevention. Washington: National Academies Press; 2012. [PubMed] [Google Scholar]
- 88.Zanchetti A. The approach to hypertension: from childhood to old age. J Hypertens 2016; 34:1885–1886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


