Abstract
Interictal and ictal discharges represent two different forms of abnormal brain activity associated with epilepsy. Ictal discharges closely parallel seizure activity, but depending on the form of epilepsy, interictal discharges may or may not be correlated with the frequency, severity, and location of seizures. Recent voltage-imaging studies in slices of piriform cortex indicated that interictal-like discharges are generated in a two-stage process. The first stage consists of a sustained, low-amplitude depolarization (plateau activity) lasting the entire latent period prior to discharge onset. Plateau activity takes place at a site distinct from the site of discharge onset and serves to sustain and amplify activity initiated by an electrical stimulus. In the second stage a rapidly accelerating depolarization begins at the onset site and then spreads over a wide region. Here, we asked whether ictal-like discharges can be generated in a similar two-stage process. As with interictal-like activity, the first sign of an impending ictal-like discharge is a sustained depolarization with a plateau-like time course. The rapidly accelerating depolarization that signals the start of the actual discharge develops later at a separate onset site. As found previously with interictal-like discharges, local application of kynurenic acid to the plateau site blocked ictal-like discharges throughout the entire slice. However, in marked contrast to interictal-like activity, blockade of synaptic transmission at the onset site failed to block the ictal-like discharge. This indicates that interictal- and ictal-like discharges share a common pathway in the earliest stage of their generation and that their mechanisms subsequently diverge.
Keywords: epilepsy, epileptiform activity, ictal activity, voltage imaging, piriform cortex, glutamate receptors
The question of how interictal and ictal activities are related is of fundamental importance to our understanding of the genesis of seizures (Prince and Connors, 1986;Dichter and Ayala, 1987; Fisher, 1989). Interictal activity consists of isolated synchronous electrical discharges, each lasting a few hundred milliseconds. Ictal activity accompanies seizures and consists of prolonged paroxysmal discharges with distinct temporal phases. In some forms of simple focal epilepsy, interictal discharges help indicate the site of seizure origin, and in these cases the onset of seizures may actually be a transition from interictal to ictal activity (Prince et al., 1983; Dichter and Ayala, 1987). However, in some animal models (Sherwin, 1978; Engel and Ackermann, 1980; Gotman, 1984) and human epilepsies (Engel et al., 1981) ictal and interictal discharges can arise from different locations, with little correlation between interictal activity and the frequency and severity of seizures (Dichter and Ayala, 1987; Fisher, 1989). In hippocampal slices interictal-like discharges can be dissociated experimentally from ictal-like discharges. These studies suggested that in contrast to interictal-like activity, some forms of ictal-like activity can occur in the absence of synaptic transmission (Konnerth et al., 1986; Jensen and Yaari, 1988;Schweitzer et al., 1992). This raises important questions about the extent to which parallel versus divergent mechanisms underlie these two forms of paroxysmal activity.
Recent voltage-imaging studies in slices of piriform cortex (PC) revealed that interictal-like discharges can be preceded by a low-amplitude, sustained depolarization (plateau activity) (Demir et al., 1999). Plateau activity was observed during the latent period that precedes discharges evoked by near-threshold electrical stimulation. It begins a few milliseconds after stimulation and is confined to the boundary region between the endopiriform nucleus (En) and layer III of the overlying PC. This site is distinct from the site of discharge onset, situated at a deeper location within the En and layer VI of adjacent neocortex [agranular insula (AI) in anterior slices and anterior perirhinal cortex (PRha) in posterior slices] (Demir et al., 1998). This work suggested that in slices of PC the generation of interictal-like discharges occurs in two stages, with plateau activity representing the first stage and actual onset representing the second stage.
Ictal-like activity can also be elicited in slices of PC (Hoffman and Haberly, 1989). The above questions about the relationship between ictal and interictal activity prompted us to determine whether ictal-like discharges develop in the same two-stage progression as interictal-like discharges. We found that ictal-like discharges were preceded by plateau activity similar to that which precedes the onset of interictal-like discharges. As with interictal-like discharges, local application of kynurenic acid at the site of plateau activity blocked ictal-like discharges. However, in contrast to interictal-like discharges, ictal-like discharges could not be blocked by inhibiting synapses at the site of onset. This suggests that plateau activity is capable of serving as a precursor to both of these types of epileptiform activity. However, by the time of onset, ictal-like discharges diverge from interictal-like discharges in being insensitive to focal blockade of synaptic transmission.
MATERIALS AND METHODS
Piriform cortex slices. Adult male Sprague Dawley rats weighing 175–240 gm were used to prepare PC slices. A vibratome was used to cut slices at a thickness of 350 μm along a near-coronal plane perpendicular to the brain surface (Hoffman and Haberly, 1991,1993; Demir et al., 1998). The physiological saline [artificial CSF (ACSF)] for slicing and recording contained 124 mm NaCl, 5 mm KCl, 26 mm NaHCO3, 1.2 mm KH2PO4, 2.4 mm CaCl2, 1.3 mmMgSO4, and 10 mm glucose bubbled with 95% O2 and 5% CO2(carbogen). Experiments with bath application of CoCl2 were performed with ACSF buffered by HEPES (10 mm) and lacking phosphate, to avoid precipitation (see Fig. 7). Slices were defined as anterior, intermediate, or posterior depending on the stereotaxic levels stated previously (Demir et al., 1998).
Fig. 7.
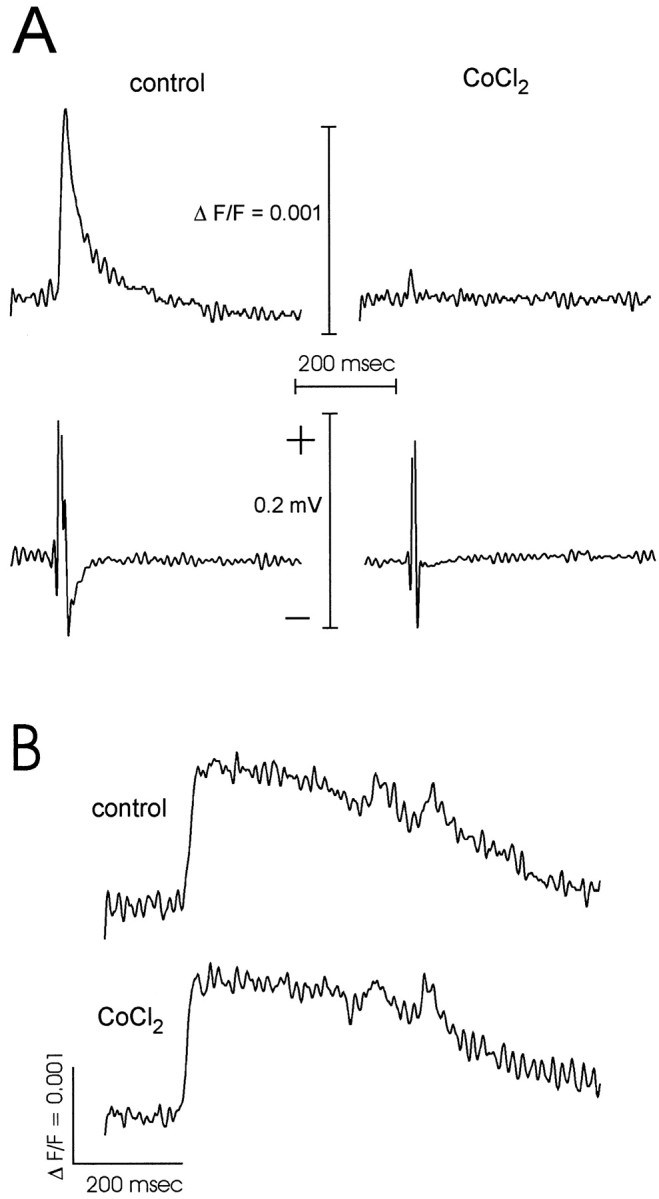
Effects of CoCl2 on synaptic transmission and ictal-like discharges. A, Fluorescence (top) and extracellular field potentials (bottom) were recorded from En in a control slice. Responses evoked by 400 μA applied in deep layer III of the PC were almost completely abolished by bath application of 2 mmCoCl2. (KH2PO4 was omitted from the ACSF for both control and CoCl2). Eachtrace represents an average of signals from six neighboring detectors averaged over 10 trials. B,Fluorescence traces from the En show an ictal-like discharge evoked by a stimulus of 47 μA applied in layer Ib.Traces show discharges before (top) and immediately after (bottom) application of 10 mm CoCl2 and 50 μm bicuculline to the site of discharge onset.
Epileptiform activity. Ictal-like discharges were generated by perfusing slices with a low-Cl− ACSF in which 93% of the Cl− in the ACSF described above was replaced by isethionate. Slices were incubated in this low-Cl− ACSF at 34°C, starting 1.5–2 hr before recording and continuing throughout an experiment. Although previous incubation of slices in low-Cl− ACSF was not necessary to elicit ictal-like activity, the appearance of ictal-like discharges took time, and it was advantageous to begin experiments soon after staining because the voltage-sensitive dye was gradually washed out during hours of perfusion with saline. The low-Cl−method of eliciting ictal-like activity is based on a positive shift in the Nernst potential for Cl− resulting in increased excitability. This increase is thought to result primarily from the reversal of Cl−-dependent GABAA receptor-mediated inhibitory synaptic potentials. However, additional effects of low Cl− may include a decrease in the free cation concentration in the extracellular space (Chamberlin and Dingledine, 1988), with excitatory effects resulting from partial removal of charge screening. Furthermore, low Cl− is thought to have effects on synaptic transmission (Traynelis and Dingledine, 1989), and the reversal of voltage- and Ca2+-dependent Cl− conductances could also contribute to the increase in excitability (Madison et al., 1986; Owen et al., 1986).
Interictal-like activity was generated using the induction and disinhibition protocols described previously (Demir et al., 1998). Disinhibition was achieved by simple addition of 5–10 μmbicuculline during experiments to block GABAAreceptors. Induction used the same low-Cl− ACSF used during recordings of ictal-like activity as described above, but with a major difference that slices were returned to control saline for recordings. Thus, in this model, a period of spontaneous bursting activity in low-Cl− ACSF transformed, or induced, a persistent change in excitability that was manifest after return to control ACSF (Hoffman and Haberly, 1989; Stasheff et al., 1989).
Epileptiform discharges were evoked by electrical stimulation of slices with a saline-filled glass pipette (20–50 μm tip diameter). Current pulses 200 μsec in duration were delivered with a stimulus isolator. Ictal-like discharges are generally defined as lasting >2 sec (Rasmussen et al., 1996; Traub et al., 1996; Rutecki and Yang, 1998). However, we found that the duration varied with stimulus current (see Fig. 2), and with currents near the threshold for discharge generation, events were sometimes as short as 1 sec. Because these discharges were obtained under the same conditions as those lasting much longer and showed clear qualitative differences with interictal-like discharges, we treated them as ictal-like.
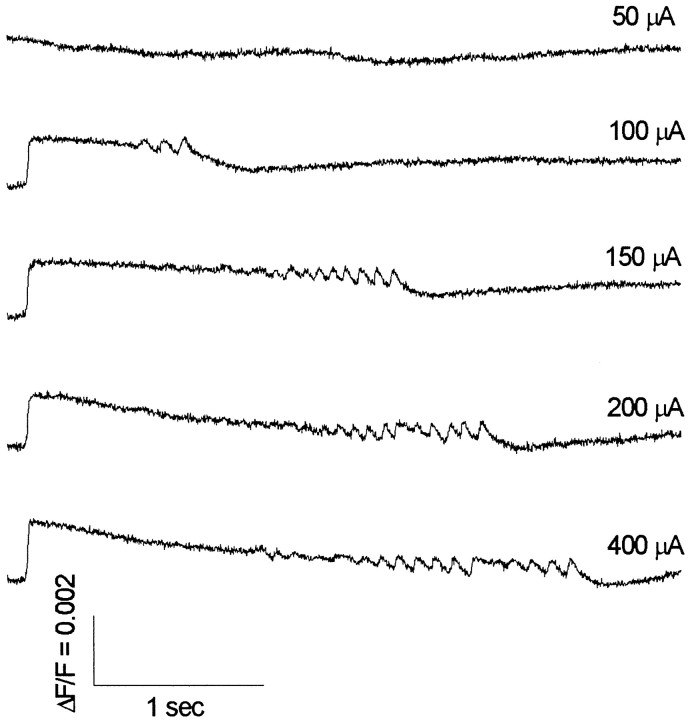
Fig. 2.
Fluorescence traces show responses from En in an intermediate PC slice elicited by increasing stimulus currents (indicated above the traces on the right) applied in layer Ib. The duration of ictal-like discharges increased with increasing stimulus current.Traces represent averages of six neighboring detectors.
The threshold stimulus for discharge generation was determined by careful variation of stimulus intensity. For experiments in which drugs were tested for their ability to block epileptiform discharges, stimulus intensities ∼10% above threshold were used to avoid subthreshold responses. During these experiments, the threshold intensity was regularly determined because it showed a tendency to change over the course of a long experiment (3–6 hr). After evoking an ictal-like discharge, at least 2 min was always allowed for recovery before attempting to evoke another. This interval was necessary because there was a refractory period for ictal-like discharges in PC slices reminiscent of the postictal depression seen during seizures in animals (Ayala et al., 1970).
Voltage imaging. The voltage-sensitive fluorescent dye RH414 (Molecular Probes, Eugene, OR) was used to image voltage. Slices were incubated for 30–45 min in 200 μm RH414 in ACSF bubbled with carbogen. Fluorescence images were recorded with a 464-element, hexagonally arranged, photodiode-fiber optic camera (Chien and Pine, 1991). The tissue was illuminated in an upright epifluorescent microscope equipped with a 100 W tungsten–halogen light source through a Zeiss Fluar 5× objective (numerical aperture, 0.25). This objective produced images in which the distance between neighboring photodetector fields was 144 μm. The optical and electronic instrumentation follows that of Wu and Cohen (1993); a detailed description along with a schematic of the setup can be found in a previous report from this laboratory (Demir et al., 1998). The output of each photodetector was individually amplified to a final level of 0.2 V/pA of photocurrent, digitized, and read into a Pentium computer. Fluorescent signals were high-pass filtered with a 500 msec time constant and low-pass filtered with a corner frequency of 500 Hz. A CCD camera served to take transilluminated video images of slices, which were read into the computer with a frame grabber (Data Translation, Marlboro, MA).
Data acquisition and analysis. Optical signals were acquired and analyzed with the computer program Neuroplex (RedShirt Imaging, Fairfield, CT). Fluorescence traces were overlaid on video images with programs written in IDL (Research Systems, Boulder, CO) (Jackson and Scharfman, 1996; Demir et al., 1998). The contours for the site of discharge onset were prepared from real-time-imaging data by determining the photodetector fields where the fluorescence change had risen to within 50 or 70% of its maximum amplitude. This cutoff excludes regions with plateau activity because with near-threshold stimulus currents, plateau activity has an amplitude ≤25% of the maximum amplitude of interictal-like discharges (Demir et al., 1999), as well as ictal-like discharges (present results). Before the preparation of contours, the fluorescence intensity of each detector was normalized to the intensity range seen by that detector. Another program written in IDL superimposed the contours onto video images. Contours for plateau activity were prepared manually by marking detectors in which a sustained depolarization during the latent period could clearly be seen above the background noise (Demir et al., 1999).
Local drug application. Kynurenic acid (5 and 10 mm), bicuculline methiodide (50 μm), and CoCl2 (10 mm) were dissolved in a 0.9% NaCl solution and topically applied from a micropipette (tip diameter, 3–5 μm) inserted into a slice with a micromanipulator at selected sites. A Picospritzer (General Valve, Fairfield, NJ) connected to the drug-application micropipette provided pressure pulses (0.5 sec; 20–25 psi) for ejection of solution. The effectiveness of this approach in delivering a drug to specific sites was discussed previously (Demir et al., 1999). Localization was tested in every experiment by visualization of fluorescein (0.1 mg/ml) included in the drug solution. Because the optical filter set used for RH414 excluded fluorescein fluorescence, this did not interfere with optical recordings of voltage. As in previous studies from this laboratory (Demir et al., 1999), before drug application epileptiform events were evaluated two or three times for stability of discharge latency. Drug application experiments at each site were repeated at least three times.
Histology. After optical recordings, slices were preserved by fixation in 4% paraformaldehyde, resectioned with a freezing microtome at 60 μm, and counterstained with cresyl violet. Photographs of these Nissl-stained sections helped to identify anatomical structures during analysis of imaging data.
Drugs. Bicuculline methiodide was obtained from Sigma (St. Louis, MO), and kynurenic acid was from Aldrich (Milwaukee, WI).
RESULTS
Ictal-like discharges
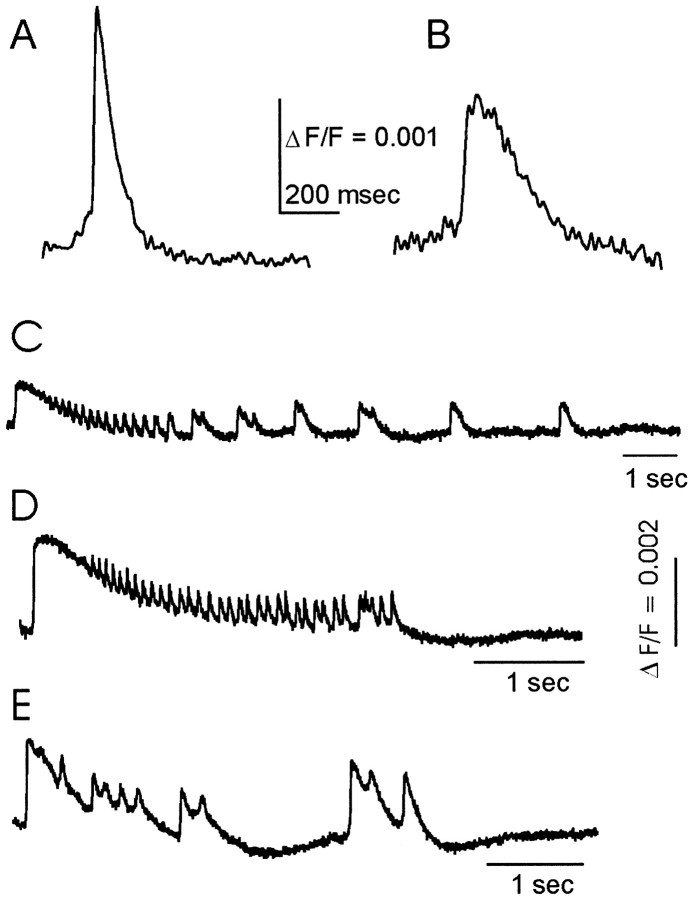
Either interictal- or ictal-like discharges were elicited using the appropriate protocols described in Materials and Methods. Interictal-like discharges in the induced (Fig.1A) and disinhibited (Fig. 1B) models generally lasted 100–300 msec [seeDemir et al. (1998) for comparisons between these two models]. Ictal-like discharges were elicited from slices pretreated and perfused with low-Cl− ACSF (Fig.1C–E). These electrical events were similar in amplitude but considerably longer in duration than were interictal-like discharges. Although the duration of ictal-like discharges was variable (Fig. 1C–E), the initial phase generally consisted of a sustained depolarization. This initial depolarization was sometimes followed by relatively brief voltage oscillations (Fig.1C,E). Ictal-like discharges first appeared at discrete and well defined sites of onset (discussed below) and then spread throughout the entire slice.
Fig. 1.
Optical recordings of interictal- and ictal-like discharges. A, B, Interictal-like discharge in an induced slice (A; previous bursting in low-Cl− ACSF) and a disinhibited slice (B; with 10 μm bicuculline).C–E, Ictal-like discharges in anterior (C), intermediate (D), and posterior (E) PC slices. All fluorescence (F) traces represent averages of six neighboring detectors. Note that the instrumentation uses a high-pass filter with a 500 msec time constant (see Materials and Methods), and this produces some attenuation of the longer events ofC–E. Stimulus was applied to layer Ib of the PC in alltraces; stimulus currents were 175 μA (A), 65 μA (B), 250 μA (C), 275 μA (D), and 60 μA (E).
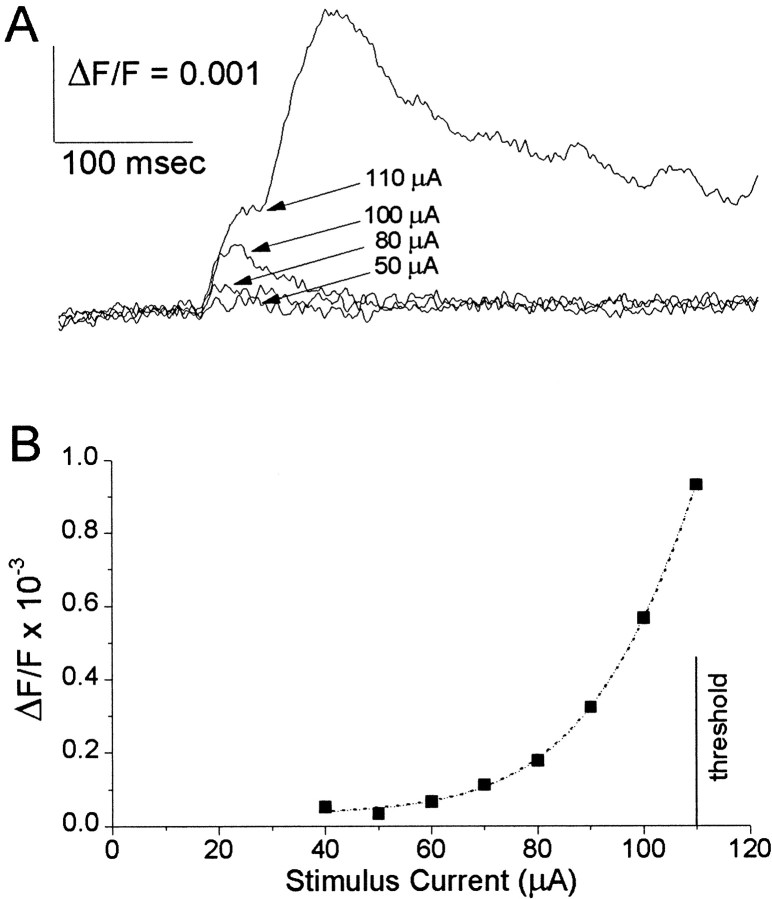
An intriguing feature of evoked ictal-like discharges in PC slices was that their duration increased as the stimulus current was increased above threshold (Fig. 2). Discharges in response to threshold stimuli were generally briefer (Fig.3). Superimposed sub- and suprathreshold responses in Figure 3 show that ictal-like discharges have an all-or-none character and appear with substantial latency after a threshold stimulus. As with interictal-like activity (Hoffman and Haberly, 1989, 1991; Demir et al., 1998), increasing the stimulus current decreased the latency to ictal-like discharge onset (data not shown). To address the question of how the initiation of ictal-like discharges compares with the initiation of interictal-like discharges, we used near-threshold or slightly suprathreshold stimulus currents to produce latent periods of sufficient duration to observe latent period activity. This enabled us to focus on the events leading up to the onset of an ictal-like discharge.
Fig. 3.
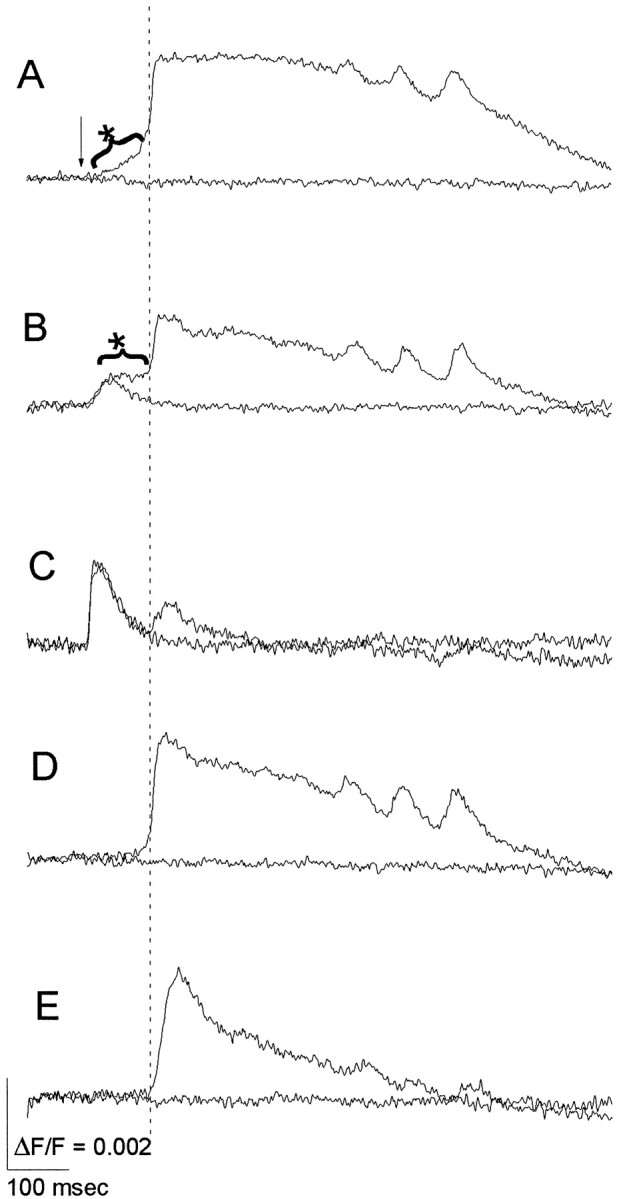
Plateau activity preceding ictal-like discharges. Sub- and suprathreshold responses are superimposed to illustrate the all-or-none character of ictal-like discharges. The time scales are expanded relative to those in Figures 1 and 2 to show the early events before discharge onset. A, A trace from the site of onset shows onset activity (asterisk–curved bracket), characterized by a ramp-like depolarization leading up to a discharge. B, A trace from the site of plateau activity shows plateau activity (asterisk–curved bracket), characterized by a low-amplitude, maintained depolarization. C, Atrace from superficial layer III of the PC is close enough to the site of stimulation to show a rapidly decaying local response. D, E, Responses from deep (D) and superficial (E) layers of the neighboring transitional neocortex between AI and PRha show the ictal-like discharge emerging suddenly from a flat baseline. The discharges in B–E appear after a delay relative to the discharge in the site of onset (A). The verticaldashedline helps to view latency differences. These traces were taken from sites labeledA–E in a subsequent figure (see Fig.5B2). Electrical stimulation (135 μA) was applied in layer Ib at the time indicated by the arrow inA (see Fig. 5B2 for site). Alltraces represent averages of four neighboring detectors.
Plateau activity precedes ictal-like discharges
Electrical activity during the latent period preceding interictal-like discharges has been described previously for the two different experimental models used in Figure 1, A andB (Demir et al., 1999). In both the disinhibition model (bicuculline treatment) and the induction model (previous spontaneous bursting in low-Cl− ACSF), a similar spatiotemporal pattern was seen during the latent period between electrical stimulation and discharge onset. Plateau activity, as mentioned in the introductory remarks, is characterized by an approximately constant, low-amplitude depolarization, beginning a few milliseconds after stimulation and continuing until discharge onset. Onset activity is a rapidly accelerating depolarization that appears at the site of discharge onset (Hoffman and Haberly, 1993), beginning ∼20–50 msec before onset and leading directly to the discharge (Demir et al., 1999). These two forms of latent period activity appeared in sequence at two distinct locations. Plateau activity was generally located at the boundary of En and deep layer III of the PC. Onset activity generally had a deeper location in En and adjoining layer VI of the neocortex. Plateau activity serves to sustain and amplify electrical activity initiated by a stimulus. Onset activity is driven by projections from the site of plateau activity, providing feedback to that site to contribute to the amplification process. Onset activity builds up directly into the epileptiform discharge. Blockade of activity at either site, with either kynurenic acid or CoCl2, blocks the generation of interictal discharges (Demir et al., 1999).
In the present study the electrical activity observed before the onset of an ictal-like discharge was similar to that observed in the two interictal models described above. A ramp-like depolarization resembling the onset activity of interictal-like discharges appeared at the site of onset of ictal-like discharges (Fig. 3A,asterisk–curved bracket). A sustained depolarization resembling the plateau activity of interictal-like discharges was seen at a site different from the site of discharge onset (Fig.3B, asterisk–curved bracket). Thus, onset and plateau activities were each seen at distinct locations that were very similar to the locations where these forms of activity were seen in the interictal models (detailed mapping of locations presented below).
No significant latent-period activity was seen outside of the onset and plateau sites. A rapidly activating and decaying local response could be seen near the stimulus electrode (positioned in superficial layer III of the PC; Fig. 3C), and this was followed by an ictal-like discharge that spread from the site of onset. At other sites far from the site of stimulation, where no local response could be detected, discharges arose abruptly from baseline without any preceding activity (Fig. 3D,E). The onset of these events thus resembled that of the rapid-onset interictal-like events described byHoffman and Haberly (1993). The amplitude of the fluorescence change associated with ictal-like discharges was smaller in amplitude and briefer in duration in superficial layers (Fig. 3C,E) than in deep layers of the PC (Fig. 3A,B) or in neighboring AI or PRha (Fig. 3D). Thus, the spatial patterns for ictal- and interictal-like discharges were similar in showing a decreasing amplitude toward the cortical surface (Demir et al., 1998).
We showed previously that stimulus currents below the threshold for interictal-like discharges evoked depolarizations at the same site where plateau activity was observed. These subthreshold depolarizations were smaller in amplitude than plateau activity. After peaking, subthreshold responses always decayed smoothly to baseline, and never showed a plateau-like time course (Demir et al., 1999). Under ictal-like conditions, subthreshold responses at the site of plateau activity showed a similar low-amplitude, and a similar smooth decay to baseline (Figs. 3B,4A). The fact that these subthreshold responses failed to show the sustained time course of plateau activity indicates that, as was found previously with interictal-like discharges, plateau activity could not be dissociated from the ictal-like discharge.
Fig. 4.
Subthreshold responses at the site of plateau activity. A, Superimposed fluorescencetraces are shown from the site of plateau activity in response to increasing stimulus intensities. Responses were evoked by stimulation in layer Ib with the indicated current.Traces represent averages of seven neighboring detectors. Note that the subthreshold responses were not flat like plateau activity but decayed immediately after peaking. Note further that the amplitude does not increase linearly with increasing stimulus intensities. B, A stimulus–response plot illustrates this nonlinearity more clearly. The peak response amplitudes fromA were plotted versus stimulus current. The nonlinearity is emphasized by a sigmoidal fit.
Under the conditions used to generate ictal-like discharges, the amplitudes of subthreshold responses at the site of plateau activity showed a strongly nonlinear, graded increase with stimulus current (n = 5; Fig. 4). This trend could be seen in superimposed traces from the site of plateau activity (Fig.4A), as well as in the stimulus–response plot in Figure 4B. This sigmoidal behavior was not seen in control slices, in which stimulus–response plots were linear. The stimulus–response behavior under conditions used to generate interictal-like activity depended on the choice of model. Plots were linear in the induction model (Demir et al., 1999) and nonlinear in the disinhibited model (R. Demir et al., unpublished observations).
Location of onset and plateau activity during ictal-like discharges
Sites of latent-period activity were determined by making contours using a 50–70% of maximum cutoff for the onset site and a manual trace-by-trace examination for the plateau site (see Materials and Methods). In anterior slices, the site of onset of ictal-like discharges was confined to the En and to layer VI of adjacent AI (n = 4; Fig.5A2 pink-shaded region). In contrast to interictal-like discharges in anterior slices (induced model), in which the site of onset was confined to the dorsalmost part of En and neighboring layer VI of AI (Demir et al., 1998), the site of onset of ictal-like events occupied a larger portion of the En. The site of plateau activity in anterior slices encompassed part of the En and deep layer III of the PC (n = 4; Fig.5A2, blue-shaded region); a tiny portion of AI layers V and VI also consistently showed plateau activity. This site was similar to the site of plateau activity for interictal-like discharges in the induced model (. However, with ictal-like discharges there was a smaller contribution from the deep layers of neighboring AI and a larger contribution from deep layer III of the PC. Overall, the region showing plateau activity was larger for ictal-like activity than for interictal-like activity. Thus, for both onset activity and plateau activity, larger regions were seen for ictal-like discharges than for interictal-like discharges.
Fig. 5.
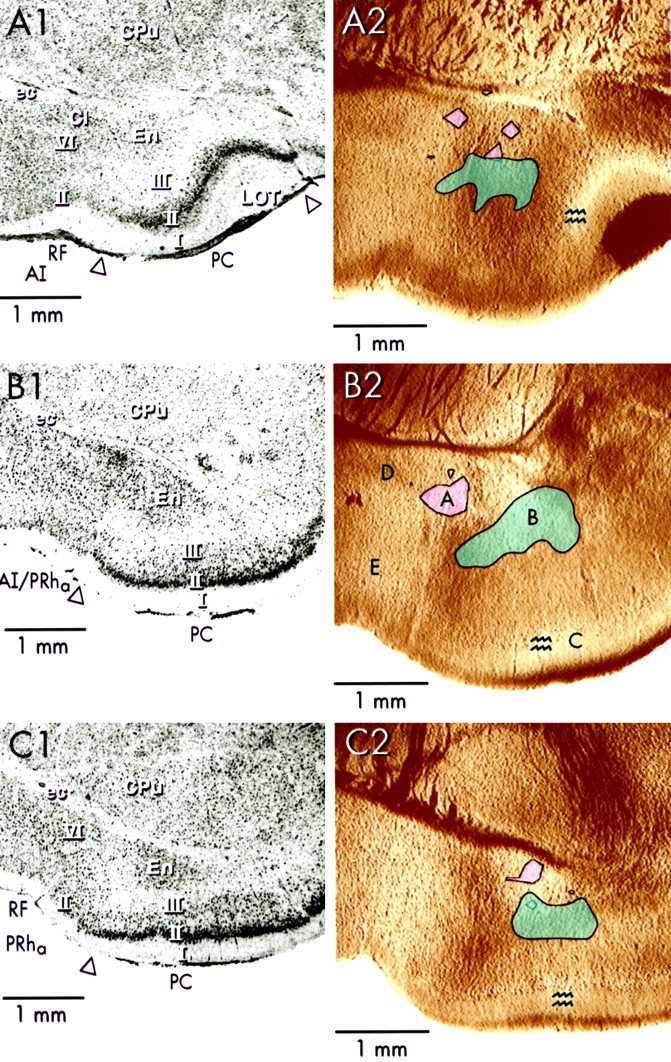
Site of plateau activity associated with ictal-like discharges. Nissl-stained photographs (A1–C1) were taken from the same slices shown inA2–C2. Sites of discharge onset (pinkshading) and plateau activity (blueshading) are indicated on video images from anterior (A1, A2), intermediate (B1, B2), and posterior (C1, C2) PC slices. Stimulus sites are indicated by paralleljaggedlines in A2–C2. Stimulus currents were 100 μA (A1, A2), 135 μA (B1, B2), and 63 μA (C1, C2).AI, Agranular insula; Cl, claustrum;CPu, caudate-putamen; ec, external capsule; En, endopiriform nucleus; LOT,lateral olfactory tract; PC, piriform cortex;PRha, anterior perirhinal cortex;RF, rhinal fissure. Open arrowheads mark the PC/neocortex boundary.
In posterior slices, the site of onset for ictal-like discharges was confined to the En (n = 4; Fig. 5C2,pink-shaded region), and this resembled the site of onset of interictal-like discharges in the disinhibited model in posterior slices. In contrast, the site of onset of interictal-like discharges in the induced model also included layer VI of the neighboring PRha (Demir et al., Demir et al., 1998). Under ictal-like conditions, the site of plateau activity in posterior PC slices consisted of parts of the En and deep layer III of the PC (n = 4; Fig. 5C1,C2, blue-shaded region), which was similar to the site of plateau activity for interictal-like discharges in the disinhibited model (. In the induced model the site of plateau activity did not extend into deep layers of the neighboring PRha.
In intermediate slices, the site of onset was in the dorsal edge of the En, and the site of plateau activity was in the boundary region between En and deep layer III of the PC (Fig. 5B1,B2). Adjoining neocortex was not involved in either form of latent-period activity. Similar sites of onset and plateau activity were observed for both models of interictal-like activity (Demir et al., Demir et al., 1998, 1999). These sites are summarized for ictal-like discharges in Table1 for anterior, intermediate, and posterior slices of the PC.
Table 1.
Plateau and onset sites for ictal-like discharges
| PC level | Onset site | Plateau site |
|---|---|---|
| Anterior (n = 4) | En and small portion of layer VI of AI | En, small portions of layers V and VI of adjoining AI, small portions of layer III of PC |
| Intermediate (n = 4) | Dorsal portion of En | Adjoining parts of En and deep layer III of PC |
| Posterior (n = 4) | En only | En and adjoining portion of deep layer III of PC |
The onset and plateau sites associated with ictal-like activity are summarized (see Fig. 5).
Blockade of plateau activity
Previous work established a requirement for excitatory synaptic transmission at the sites of plateau and onset activity in the genesis of interictal-like discharges (Demir et al., 1999). Because we found the same forms of latent-period activity in the low-Cl− model, we investigated the role of synaptic transmission at these sites in the generation of ictal-like discharges. Kynurenic acid (a broad-spectrum excitatory amino acid receptor antagonist) was applied locally to inhibit synaptic transmission in specific regions. The method of local drug application used in this study was the same as that used previously, in which localization of drug was checked by observing fluorescein. Drug localization was further verified by showing no effect on graded responses at short distances and no blockade of epileptiform activity when the drug application pipette was moved 500 μm from a critical site (Demir et al., 1999).
The sites of discharge onset and plateau activity were first mapped by analysis of imaging data while the experiment was ongoing. Kynurenic acid was then applied to the site of plateau activity, and an electrical stimulus found previously to be suprathreshold for the generation of an ictal-like discharge was reapplied. Blockade was seen in every experiment (n = 8; Fig.6A1b), with the ictal-like response recovering within a few minutes (Fig.6A1c).
Fig. 6.
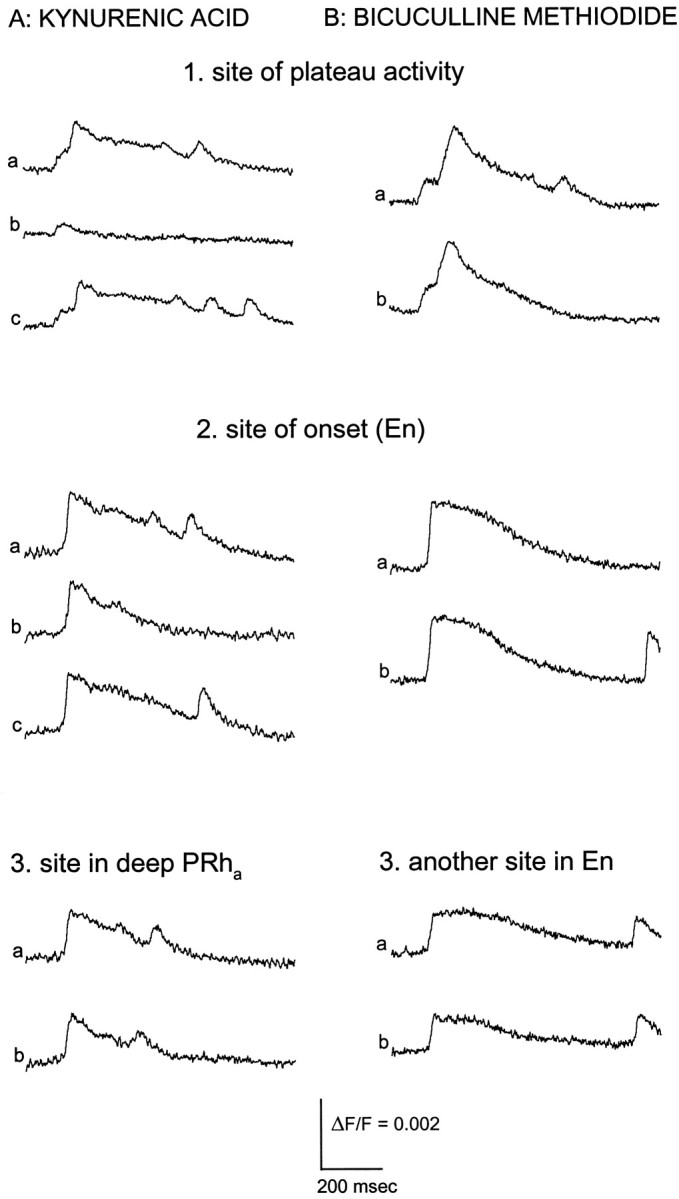
Blockade of ictal-like activity. A, Kynurenic acid (5 mm) was locally applied at several sites to determine the role of excitatory synaptic transmission in the generation of ictal-like discharges. Traces inA1 show responses from the site of plateau activity in an intermediate PC slice before (A1a), immediately after (A1b), and a few minutes after (A1c) kynurenic acid application. The ictal-like discharge was blocked by kynurenic acid application at this site (A1b) and recovered completely after washout (A1c). When kynurenic acid was applied at the site of onset, ictal-like activity (A2a) was not blocked, although the response was usually attenuated locally (A2b) and recovered to full size after a few minutes (A2c). Application of kynurenic acid at a site in deep AI/PRha(A3) did not block the ictal-like discharge.B, Bicuculline methiodide (50 μm) was applied to several sites to test whether reversed inhibitory potentials play a role in the generation of ictal-like discharges. Local application of bicuculline at the site of plateau activity (B1a,b), the site of onset (B2a,b), and another site in En (B3a,b) did not block ictal-like activity. Traces in A andB were taken from two different slices and show responses from the sites of drug application (indicatedabove each set of traces).Traces are averages of four neighboring detectors. Ictal-like discharges were evoked by stimulation in layer Ib at 140 μA (A1), 110 μA (A2), 95 μA (A3), 67 μA (B1), 55 μA (B2), and 52.5 μA (B3).
In contrast to the results seen for application at the plateau site, application of kynurenic acid at the site of onset did not block ictal-like discharges. The signals emanating from this site were attenuated, but the latency-to-discharge onset was unaffected (n = 6; Fig. 6A2b). Applying a higher concentration of kynurenic acid (10 mm) to the onset site also failed to block ictal-like discharges (n = 4). In four of the experiments in which kynurenic acid was applied to the site of onset, the data were examined to see whether the site of onset moved to a new location. In three of these experiments the site of onset was essentially the same, and in the fourth it moved slightly. Ictal-like discharges could still be evoked when kynurenic acid was applied at other locations in En outside the sites of onset and plateau activity (n = 6; data not shown) and at other nearby sites in PRha(n = 7; Fig. 6A3).
The site of plateau activity was the only location in PC slices where the sensitivity of ictal-like discharges to local excitatory amino acid receptor blockade could be demonstrated. Thus, plateau activity appears to be necessary for both ictal- and interictal-like discharges. In contrast, although activity at the onset site was similar in appearance at the start of both ictal- and interictal-like discharges, application of kynurenic acid to this site blocked only interictal-like discharges (Demir et al., 1999).
In the low-Cl− ACSF used here to generate ictal-like discharges, Cl−-mediated inhibitory synaptic potentials are reversed and therefore have an excitatory instead of inhibitory effect on postsynaptic cells. Thus, GABAA receptor activation could contribute to epileptiform activity under these conditions. To test this hypothesis we attempted to block GABAA receptors with the antagonist bicuculline using the same local application method used to block glutamate receptors. Application of bicuculline to the site of plateau activity (n = 4; Fig. 6B1), site of onset (n = 4; Fig. 6B2), elsewhere in the En (n = 4; Fig.6B3), and other nearby sites in neighboring neocortex (n = 4; data not shown) failed to block the ictal-like discharges. To test the possibility that either excitatory amino acid receptors or depolarizing GABAA receptors can initiate an ictal-like discharge, we applied 10 mm kynurenic acid together with 50 μm bicuculline in the same pipette and again saw no blockade when these drugs were injected at the site of onset (n = 2; data not shown).
To test the role of synaptic transmission more generally, we used the Ca2+ channel blocker CoCl2. Like kynurenic acid, CoCl2 blocked interictal-like discharges when applied to the site of onset (Hoffman and Haberly, 1993, 1996; Demir et al., 1999). We specifically tested the action of CoCl2 on evoked responses at the site of onset, to make sure that it was an effective reagent in the blockade of synaptic transmission at that location. Bath application of 2 mm CoCl2 blocked synaptic potentials at the site of onset, recorded either with fluorescence or with an extracellular field electrode (Fig.7A). Ictal-like discharges could still be evoked when 10 mmCoCl2 together with 50 μmbicuculline was applied at the site of onset (n = 3; Fig. 7B). In these experiments the same blocker solution was tested at two to four locations within the onset site, but the discharges were not blocked. These results indicate that local blockade of chemical synapses at the site of onset does not prevent the generation of ictal-like activity.
DISCUSSION
The experiments presented here showed that ictal-like discharges in PC slices are preceded by plateau activity with a time course and location similar to that of plateau activity preceding interictal-like discharges (Demir et al., 1999). Plateau activity in the ictal model showed an approximately constant amplitude throughout the entire latent period, never occurred in isolation from an ictal-like discharge, and was spatially restricted to similar locations in anterior, intermediate, and posterior slices. Furthermore, synaptic blockade at the site of plateau activity blocked ictal-like discharges throughout the slice, as demonstrated previously in interictal models. Thus, plateau activity is required for the generation of both interictal- and ictal-like discharges in PC slices. Because ictal-like activity in this slice preparation resembles electrical activity during seizures in the neocortex (Ayala et al., 1970), the sustained, plateau-like depolarizations described here may contribute to the onset of seizures. The requirement of plateau activity for the genesis of both interictal- and ictal-like events is important, because it implies that both types of epileptiform activity share common mechanisms at an early stage of their genesis. Because plateau activity may serve as a precursor for ictal spikes, pharmacological interventions directed toward curtailing plateau activity could be useful in controlling seizures.
The finding that ictal- and interictal-like activities have a common precursor, namely, plateau activity, provides an interesting perspective on the relationship between interictal spikes and seizures. The onset of seizures is often thought of as a transition from interictal to ictal activity (Dichter and Ayala, 1987). One important issue in this regard is whether interictal spikes can serve as direct progenitors of seizure activity (Prince et al., 1983). However, the relationship between interictal and ictal activity suggested by the present results is one of bifurcation rather than transitional sequence. Factors in effect during plateau activity may determine whether the ensuing discharge will be interictal or ictal in character. By altering the ionic environment, the volume of extracellular space, and the functional states of ion channels and synapses, interictal spikes could modify conditions so that subsequent plateau activity leads to ictal rather than interictal discharges.
Locations of onset and plateau activity
The sites of onset and plateau activity were identified previously in two interictal models, in slices from anterior, intermediate, and posterior PC (Demir et al., 1998, 2000). Here we found that these forms of activity occurred at similar sites in ictal-like discharges (Fig. 5, Table 1), but with some subtle and interesting differences. As with interictal-like activity, the onset site of ictal-like activity included En, and the plateau site included adjoining parts of En and deep layer III of the PC. However, in the induced model, adjoining deep layers of neocortex contributed to both onset and plateau activity, in both anterior and posterior PC slices. By contrast, neocortex did not contribute to these early stages of interictal discharges in the disinhibited model (Demir et al., 1998, 2000). Thus, ictal-like activity in anterior PC slices has a site distribution resembling that of interictal-like activity in the induced model, but in posterior PC slices, ictal-like activity has a site distribution resembling that of interictal-like activity in the disinhibited model. Interestingly, neither onset nor plateau activity was seen in neocortex in intermediate slices, for either ictal- or interictal-like activity. We have suggested previously that the differences between the onset sites in the induced and disinhibited models may be caused by a nonuniform distribution of GABAA receptors and/or synaptic circuitry along the rostrocaudal axis of the En and neighboring neocortex (Demir et al., 1998). Similar regional differences may be relevant to variations in onset and plateau sites associated with ictal-like behavior.
Threshold and subthreshold behavior
Epileptiform discharges are generally all-or-none, and that was the case here for ictal-like discharges. However, subthreshold responses at the site of plateau activity had a nonlinear dependence on stimulus current (Fig. 4). Similar subthreshold nonlinearity was seen in the disinhibited interictal model (. but in control and induced slices, stimulus–response plots were linear (Demir et al., Demir et al., 1999). This suggests the presence of stronger amplification processes in the disinhibited interictal model and the low-Cl− ictal model. Di- and polysynaptic potentials described by Tseng and Haberly (1989) could amplify responses and contribute to this nonlinear behavior.
We were surprised to see that the duration of an ictal-like discharge increased with increasing stimulus current (Fig. 2). This result stands in contrast with interictal-like discharges, which have the same duration regardless of stimulus strength. Increasing the stimulus current also shortens the latency to discharge onset, and this may be relevant to the character of the ensuing discharge. A prolonged latent period, which will be accompanied by prolonged plateau activity, could influence the state of a slice at the time of onset. Modeling studies suggested that both dendritic depolarizations and ectopic axonal spikes can contribute to ictal discharge initiation (Traub et al., 1996). If the relative magnitudes of these two processes change with time, then the duration of the subsequent discharge may depend on which of these two processes predominates at the time of onset. Because of the complex relationship between cellular mechanisms and network phenomenon, computer-modeling studies such as those conducted by Traub and coworkers will probably be necessary to test these hypotheses.
Synaptic blockade and discharge onset
Although the finding that plateau activity is a precursor for both interictal- and ictal-like discharges implies common mechanisms at the earliest stage of genesis, an important difference was seen at the onset. Interictal-like discharges could be abolished by synaptic blockade at the onset site (Demir et al., 1999), but ictal-like discharges could not. Bicuculline methiodide also failed to block ictal-like discharges when applied to the site of onset, suggesting that inverted inhibitory synaptic potentials caused by the reversal of the Cl− gradient do not provide the excitatory drive for discharge initiation. Thus, it is unlikely that inverted inhibitory synaptic potentials simply substitute for excitatory glutamatergic synapses.
One possible explanation for the failure of local synaptic blockade at the onset site to suppress ictal-like discharges is the larger size of the onset site relative to that of interictal-like discharges. This may reflect the involvement of a network of neurons extending over a larger region. This suggests that if we were able to perfuse the entire onset region with drug we might block ictal-like discharges. However, the plateau site was also larger, and local drug application there still blocked discharges. Onset activity was observed in a smaller region than plateau activity for both interictal-like (Demir et al., 1999) and ictal-like (Fig. 5) discharges. Furthermore, kynurenic acid was used at twice the concentration used in all other experiments (10 vs 5 mm), but onset still occurred at essentially the same location.
Another possible explanation for why kynurenic acid, bicuculline, and mixtures of bicuculline with kynurenic acid or CoCl2 failed to block ictal-like discharges when applied to the site of onset is that nonsynaptic mechanisms play a significant role in the initiation process. Kynurenic acid slightly reduced the amplitude of the fluorescence change at the site of onset (Fig. 6A2b), suggesting that excitatory synapses contribute to the observed depolarization, but this depolarization still developed into a spreading epileptiform discharge. Nonsynaptic neuronal interactions play roles in many brain functions, as well as pathological conditions such as epilepsy (Jefferys, 1995). Computer simulations in the hippocampus have suggested that nonsynaptic mechanisms can make significant contributions to ictal activity (Traub et al., 1996). Hippocampal slices can generate synchronous neuronal discharges when synaptic transmission is blocked (Taylor and Dudek, 1982). Furthermore, ictal-like discharges can be generated without functional chemical synapses, but interictal-like activity is eliminated under these conditions (Konnerth et al., 1986; Jensen and Yaari, 1988; Schweitzer et al., 1992). This implies that ictal- and interictal-like paroxysmal activities differ in their dependence on synaptic transmission. The present results are consistent with this general trend. The nonsynaptic mechanisms proposed to play roles in epileptiform activity include electrotonic coupling through gap junctions (Dudek et al., 1986), field effects or ephaptic interactions (Traub et al., 1985; Dudek et al., 1986), voltage-dependent Cl− conductances (Chamberlin and Dingledine, 1988), and elevated [K+]o (Yaari et al., 1986). Further studies using local application methods in PC slices should help evaluate the roles of these diverse processes in the generation of seizure-like activity.
Regardless of the relative contributions of these mechanisms, it is significant that ictal-like discharges diverge from interictal-like discharges at the time of onset. Before onset, both types of epileptiform activity depend on plateau activity, which in turn depends on excitatory amino acid receptor-mediated synaptic transmission. Ictal- and interictal-like activities thus start off on a common pathway, apparently using similar mechanisms. The juncture at which ictal-like activity becomes resistant to synaptic blockade may represent an important point of divergence between ictal- and interictal-like discharges.
Footnotes
Support for this research was provided by National Institutes of Health Grants NS37212 to M.B.J. and NS19865 to L.B.H.
Correspondence should be addressed to Dr. Meyer Jackson, Department of Physiology, SMI 127, University of Wisconsin Medical School, 1300 University Avenue, Madison, WI 53706. E-mail:MJACKSON@PHYSIOLOGY.WISC.EDU.
REFERENCES
- 1.Ayala GF, Matsumoto H, Gumnit RJ. Excitability changes and inhibitory mechanisms in neocortical neurons during seizures. J Neurophysiol. 1970;33:73–85. doi: 10.1152/jn.1970.33.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlin NL, Dingledine R. GABAergic inhibition and the induction of spontaneous epileptiform activity by low chloride and high potassium in the hippocampal slice. Brain Res. 1988;445:12–18. doi: 10.1016/0006-8993(88)91068-2. [DOI] [PubMed] [Google Scholar]
- 3.Chien C-B, Pine J. Voltage-sensitive dye recording of action potentials and synaptic potentials from sympathetic microcultures. Biophys J. 1991;60:697–711. doi: 10.1016/S0006-3495(91)82099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demir R, Haberly LB, Jackson MB. Voltage imaging of epileptiform activity in slices from rat piriform cortex: onset and propagation. J Neurophysiol. 1998;80:2727–2742. doi: 10.1152/jn.1998.80.5.2727. [DOI] [PubMed] [Google Scholar]
- 5.Demir R, Haberly LB, Jackson MB. Sustained and accelerating activity at two discrete sites generate epileptiform discharges in slices of piriform cortex. J Neurosci. 1999;19:1294–1306. doi: 10.1523/JNEUROSCI.19-04-01294.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demir R, Haberly LB, Jackson MB (2000) Characteristics of plateau activity during the latent period prior to epileptiform discharges in slices from rat piriform cortex. J Neurophysiol, in press. [DOI] [PubMed]
- 7.Dichter MA, Ayala GF. Cellular mechanisms of epilepsy: a status report. Science. 1987;237:157–164. doi: 10.1126/science.3037700. [DOI] [PubMed] [Google Scholar]
- 8.Dudek FE, Snow RW, Taylor CP. Role of electrical interactions in synchronization of epileptiform bursts. In: Delgado-Escueta AV, Ward AA Jr, Woodbury DM, Porter RJ, editors. Basic mechanisms of epilepsies. Raven; New York: 1986. pp. 593–617. [PubMed] [Google Scholar]
- 9.Engel J, Jr, Ackermann RF. Interictal EEG spikes correlate with decreased, rather than increased, epileptogenicity in amygdaloid kindled rats. Brain Res. 1980;190:543–548. doi: 10.1016/0006-8993(80)90296-6. [DOI] [PubMed] [Google Scholar]
- 10.Engel J, Jr, Rausch R, Lieb JP, Kuhl DE, Crandall PH. Correlation of criteria used for localizing epileptic foci in patients considered for surgical therapy of epilepsy. Ann Neurol. 1981;9:215–224. doi: 10.1002/ana.410090303. [DOI] [PubMed] [Google Scholar]
- 11.Fisher RS. Animal models of epilepsies. Brain Res Rev. 1989;14:245–278. doi: 10.1016/0165-0173(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 12.Gotman J. Relationships between triggered seizures, spontaneous seizures, and interictal spiking in the kindling model of epilepsy. Exp Neurol. 1984;84:259–273. doi: 10.1016/0014-4886(84)90223-1. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman WH, Haberly LB. Bursting induces persistent all-or-none EPSPs by an NMDA-dependent process in piriform cortex. J Neurosci. 1989;9:206–215. doi: 10.1523/JNEUROSCI.09-01-00206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman WH, Haberly LB. Bursting-induced epileptiform EPSPs in slices of piriform cortex are generated by deep cells. J Neurosci. 1991;11:2021–2031. doi: 10.1523/JNEUROSCI.11-07-02021.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman WH, Haberly LB. Role of synaptic excitation in the generation of bursting-induced epileptiform potentials in the endopiriform nucleus and piriform cortex. J Neurophysiol. 1993;70:2550–2561. doi: 10.1152/jn.1993.70.6.2550. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman WH, Haberly LB. Kindling-induced epileptiform potentials in piriform cortex slices originate in the underlying endopiriform nucleus. J Neurophysiol. 1996;76:1430–1438. doi: 10.1152/jn.1996.76.3.1430. [DOI] [PubMed] [Google Scholar]
- 17.Jackson MB, Scharfman HE. Positive feedback from hilar mossy cells to granule cells in the dentate gyrus revealed by voltage-sensitive dye and microelectrode recording. J Neurophysiol. 1996;76:601–616. doi: 10.1152/jn.1996.76.1.601. [DOI] [PubMed] [Google Scholar]
- 18.Jefferys JGR. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- 19.Jensen MS, Yaari Y. The relationship between interictal and ictal paroxysms in an in vitro model of focal hippocampal epilepsy. Ann Neurol. 1988;24:591–598. doi: 10.1002/ana.410240502. [DOI] [PubMed] [Google Scholar]
- 20.Konnerth A, Heinemann U, Yaari Y. Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro. I. Development of seizure-like activity in low extracellular calcium. J Neurophysiol. 1986;56:409–423. doi: 10.1152/jn.1986.56.2.409. [DOI] [PubMed] [Google Scholar]
- 21.Madison DV, Malenka RC, Nicoll RA. Phorbol esters block a voltage-sensitive chloride current in hippocampal pyramidal cells. Nature. 1986;321:695–697. doi: 10.1038/321695a0. [DOI] [PubMed] [Google Scholar]
- 22.Owen DG, Segal M, Barker JL. Voltage-clamp analysis of a Ca-and voltage-dependent chloride conductance in cultured mouse spinal neurons. J Neurophysiol. 1986;55:1115–1135. doi: 10.1152/jn.1986.55.6.1115. [DOI] [PubMed] [Google Scholar]
- 23.Prince DA, Connors BW. Mechanisms of interictal epileptogenesis. In: Delgado-Escueta AV, Ward AA Jr, Woodbury DM, Porter RJ, editors. Basic mechanisms of the epilepsies. Raven; New York: 1986. pp. 275–299. [PubMed] [Google Scholar]
- 24.Prince DA, Connors BW, Benardo LS. Mechanisms underlying interictal-ictal transitions. In: Delgado-Escueta AV, Wasterlain CG, Treimen DM, Porter RJ, editors. Advances in neurology: status epilepticus. Raven; New York: 1983. pp. 177–187. [PubMed] [Google Scholar]
- 25.Rasmussen PA, Yang Y, Rutecki PA. Propofol inhibits epileptiform activity in rat hippocampal slices. Epilepsy Res. 1996;25:169–175. doi: 10.1016/s0920-1211(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 26.Rutecki PA, Yang Y. Ictal epileptiform activity in the CA3 region of hippocampal slices produced by pilocarpine. J Neurophysiol. 1998;79:3019–3029. doi: 10.1152/jn.1998.79.6.3019. [DOI] [PubMed] [Google Scholar]
- 27.Schweitzer JS, Patrylo PR, Dudek FE. Prolonged field bursts in the dentate gyrus: dependence on low calcium, high potassium, and nonsynaptic mechanisms. J Neurophysiol. 1992;68:2016–2025. doi: 10.1152/jn.1992.68.6.2016. [DOI] [PubMed] [Google Scholar]
- 28.Sherwin I. Interictal-ictal transition in the feline penicillin epileptogenic focus. Electroencephalogr Clin Neurophysiol. 1978;45:525–534. doi: 10.1016/0013-4694(78)90296-1. [DOI] [PubMed] [Google Scholar]
- 29.Stasheff SF, Anderson WW, Clark S, Wilson WA. NMDA antagonists differentiate epileptogenesis from seizure expression in an in vitro model. Science. 1989;245:648–651. doi: 10.1126/science.2569762. [DOI] [PubMed] [Google Scholar]
- 30.Taylor CP, Dudek FE. Synchronous neural afterdischarges in rat hippocampal slices without active chemical synapses. Science. 1982;218:810–812. doi: 10.1126/science.7134978. [DOI] [PubMed] [Google Scholar]
- 31.Traub RD, Dudek FE, Taylor CP, Knowles WD. Simulation of hippocampal afterdischarges synchronized by electrical interactions. Neuroscience. 1985;14:1033–1038. doi: 10.1016/0306-4522(85)90274-x. [DOI] [PubMed] [Google Scholar]
- 32.Traub RD, Borck C, Colling SB, Jefferys GR. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–891. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 33.Traynelis SF, Dingledine R. Modification of potassium-induced interictal bursts and electrographic seizures by divalent cations. Neurosci Lett. 1989;98:194–199. doi: 10.1016/0304-3940(89)90509-0. [DOI] [PubMed] [Google Scholar]
- 34.Tseng GF, Haberly LB. Deep neurons in piriform cortex. I. Morphology and synaptically evoked responses including a unique high-amplitude paired shock facilitation. J Neurophysiol. 1989;62:369–385. doi: 10.1152/jn.1989.62.2.369. [DOI] [PubMed] [Google Scholar]
- 35.Wu JY, Cohen LB. Fast multisite optical measurements of membrane potential. In: Mason WT, editor. Fluorescent and luminescent probes for biological activity. Academic; London: 1993. pp. 389–404. [Google Scholar]
- 36.Yaari Y, Konnerth A, Heinemann U. Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro. II. Role of extracellular potassium. J Neurophysiol. 1986;56:424–438. doi: 10.1152/jn.1986.56.2.424. [DOI] [PubMed] [Google Scholar]