Abstract
We have compared the membrane response of rat primary sensory neurons to capsaicin and noxious heat, using electrophysiological and ion flux measurements. Our aim was to determine whether, as recently proposed, the same molecular entity accounts for excitation by both types of stimulus.
The properties of the ion channels activated by heat and capsaicin show many similarities but also important differences. The calcium permeability of heat-activated channels is lower than that of capsaicin-activated channels. Distinct single channels respond to heat or capsaicin, and only a few show dual sensitivity. At the whole-cell level, individual cells invariably show dual sensitivity, but the amplitudes of the responses show little correlation.
We conclude that distinct molecular entities, which are both likely to be derived from the VR1 gene product, account for the membrane responses to heat and capsaicin.
Keywords: sensory neurons, capsaicin, heat, VR1, whole-cell, single-channel
Primary afferent neurons that respond to noxious stimuli belong mainly to the class of C-polymodal nociceptors. They are sensitive to various types of stimulus, including chemicals such as capsaicin (collectively known as vanilloids), temperatures in the noxious range (>45°C), and mechanical stimuli of moderate to high intensity (Szolcsanyi et al., 1988; Campbell and Meyer, 1996). Studies on the membrane responses of these neurons to vanilloids (Bevan and Szolcsanyi, 1990; Bevan and Docherty, 1993; Oh et al., 1996) have shown that these compounds act by opening a specific class of nonselective cation channels. The discovery of specific binding of a potent vanilloid agonist, resiniferatoxin, to the membranes of dorsal root ganglion neurons (Szallasi and Blumberg, 1990), and of a competitive vanilloid antagonist, capsazepine (Bevan et al., 1992), proved the existence of a specific receptor, which was recently cloned and designated VR1 (Caterina et al., 1997). Membrane responses to noxious heat had previously been recorded from sensory neurons grown in culture (Cesare and McNaughton, 1996; Kirschstein et al., 1997; Reichling and Levine, 1997), and work by Caterina et al. (1997) and Tominaga et al. (1998) showed that when VR1 was expressed in oocytes or in a mammalian cell line, these cells showed sensitivity to noxious heat as well as to vanilloids, leading to the suggestion that the same molecular entity—the VR1 gene product—functions as the “receptor” for both vanilloids and noxious heat. VR1 was shown to function as a membrane ion channel, gated by vanilloid agonists and by noxious heat, and to be expressed mainly in small-diameter sensory neurons (Tominaga et al., 1998; Michael and Priestley, 1999), leading to the suggestion that VR1 functions as a physiological heat transducer in sensory nerve terminals.
Consistent with this hypothesis, we reported recently that all of the small- to medium-sized rat dorsal root ganglion (DRG) neurons that respond to temperatures in the noxious range [average threshold 45.3°C, designated low-threshold (LT) cells] are also capsaicin-sensitive, although there is a second class of heat-sensitive neurons, larger in size, with a threshold of 51°C [high-threshold (HT) cells], which are insensitive to capsaicin, (Nagy and Rang, 1999) and presumably possess a heat-sensitive mechanism distinct from VR1. This could correspond to VRL-1, a VR1 homolog that codes for a high-threshold heat-activated channel that is insensitive to capsaicin (Caterina et al., 1999).
The present study was designed to test the hypothesis that heat and capsaicin activate the same membrane channels in low-threshold cells, by comparing the membrane responses of rat sensory neurons to these stimuli at the whole-cell and single-channel level. We conclude that although there are close similarities, the channels activated by heat and capsaicin are in fact different, possibly representing molecular variants of the VR1 gene product.
Preliminary reports of some of this work have been published previously (Nagy and Rang, 1997, 1998).
MATERIALS AND METHODS
Cell preparation. Cultures of DRG neurons were prepared according to Lindsay (1988). Adult rats (150–200 gm) were killed with CO2, and DRGs from all segments were removed and collected in Ham's Nutrient Mixture F14 (Imperial Laboratories) culture medium containing l-glutamine (2 mm; Life Technologies, Gaithersburg, MD), penicillin/streptomycin (Life Technologies; 50 IU/ml penicillin and 50 μg/ml streptomycin), and 4% Ultroser G (Life Technologies). Ganglia were then incubated in culture medium containing collagenase (Type IV, 0.16–0.32 IU/ml; Sigma, St. Louis, MO) for 3 hr at 37°C in a 3% CO2 incubator. After several washes DRG neurons were dissociated by trituration with a fire-polished Pasteur pipette. Cells were spun through sterile 15% bovine serum albumin (Sigma) and plated on glass coverslips coated with poly-dl-ornithine. DRG neurons were cultured for 1–5 d in F14 medium containing 50 ng/ml nerve growth factor (NGF) (Promega, Madison, WI) at 37°C in the presence of 3% CO2.
Superior cervical ganglion (SCG) neurons were cultured according toLeaney et al. (1997). Sixteen-day-old rats were killed with CO2 and decapitated. The ganglia were dissected, cleaned of connective tissue, and incubated in Leibovitz L-15 culture medium (Sigma) containing collagenase (Sigma; 400 IU/ml) for 15 min, then transferred to medium containing trypsin (Sigma; 1 mg/ml) for 30 min, incubations being carried out at 37°C in 6% CO2 incubator. Ganglia were washed and triturated with a fire-polished Pasteur pipette. Cells were plated on glass coverslips coated with poly-dl-ornithine and laminin, and cultured for 1–5 d in the presence of 6% CO2 at 37°C in L-15 medium containing NaHCO3 (24 mm), fetal calf serum (0.85%, Life Technologies), glucose (38 mm), l-glutamine (2 mm, Life Technologies), penicillin/streptomycin (100 IU penicillin and 100 μg/ml streptomycin, Life Technologies), and NGF (50 ng/ml, Sigma).
Electrophysiology. Coverslips were placed in a recording chamber superfused by 37 ± 0.5°C bath solution (1 ml/min). Recordings of whole-cell currents under voltage clamp were made by standard techniques with an Axopatch 200A amplifier (Axon Instruments) and PCs running the pCLAMP6 and AxoScope software packages (Axon Instruments). For whole-cell recording, the capacitance transient and the series resistance were compensated to >80% before each recording. Single-channel recordings were made in either the cell-attached or inside-out configuration (Hammill et al., 1981). Electrodes of ∼5 MΩ were pulled from borosilicate glass capillaries (Clark Electromedical Instruments) and fire-polished. For single-channel recordings, electrodes were coated with Sylgard (Dow Corning). Recordings were filtered at 2 kHz, digitized at 4 kHz for whole-cell or 16.6 kHz for single-channel recordings with a DigiData 1200 interface, and stored on hard disk.
The standard bath solution contained (in mm): NaCl 130, KCl 10, MgCl2 1.26, CaCl2 1.26, glucose 10, HEPES 10 (pH 7.4, adjusted with NaOH). For whole recordings, the pipette solution contained (in mm): NaCl 10, KCl 130, MgCl2 1.26, EGTA 1, HEPES 10, (pH 7.4, adjusted with KOH). For ionic-selectivity studies, NaCl in the pipette solution and KCl in the bath solution were omitted, and the KCl in the pipette solution was replaced by 130 mm CsCl. In some experiments either Na+ was replaced by equimolar N-methyl-d-glucamine (NMDG) or the CaCl2 concentration was altered in the bath solution. The holding membrane potential was −60 mV. Reversal potentials of whole-cell currents were established by linear voltage ramps (+40 to −100 mV in 0.5 sec) generated by the pClamp6 software. The potential was held at +40 mV for 0.2 sec at the start of the ramp to inactivate voltage-gated inward currents as far as possible. Cells that showed inflections on the control current–voltage (I–V) curve, indicative of such currents, were not used for estimation of reversal potentials. The relative ion permeabilities were calculated by using the Goldman-Hodgkin-Katz equations (Lewis, 1979; Hille, 1992). The experiments were performed on the low-threshold, capsaicin-sensitive group of cells (Nagy and Rang, 1999), defined as those that produced an inward current of >100 pA in response to 2 μm capsaicin. To test the effect of inhibitors (ruthenium red and capsazepine) on the response of the neurons to capsaicin and heat, brief stimuli (2 sec at 49°C, or 1 sec perfusion with 2 μm capsaicin at 37°C) were applied every 2 min. EGTA (5 μm ) was added to the pipette solution to minimize increases in intracellular calcium concentration and thereby reduce desensitization. The inhibitor was added immediately after the first (control) response, and the second (test) response was used to measure the degree of inhibition. With no inhibitor added, the second response to capsaicin averaged 79.5 ± 11.5% (n = 6) of the first, whereas the second response to heat averaged 82.1 ± 4.4% (n = 7) of the first. The effects of inhibitors were corrected for these desensitization factors.
Single-channel recordings were performed in cell-attached patch or inside-out patch configuration established after giga-seal formation (Hammill et al., 1981). To ensure that the cell was fully depolarized, the bath solution contained (in mm): K-aspartate 115, KCl 15, MgCl2 1.26, HEPES 10, glucose 10, EGTA 1 (pH 7.4, adjusted with KOH). The composition of the pipette solution was (in mm): NaCl 130, KCl 10, MgCl2 1.26, CaCl2 1.26 HEPES 10 (pH 7.4, adjusted with NaOH). The junction potential was compensated, and the pipette potential was set to + 60 mV. The voltage dependence of the single-channel currents was established by applying 200 msec voltage steps to vary the pipette potential between + 100 and −40 mV. Analysis of single-channel records was performed by means of SCAN and EKDIST software (Colquhoun and Sigworth, 1995), kindly provided by Prof. D. Colquhoun (Department of Pharmacology, University College London, UK). Because the mean open time of heat-activated channels was brief (see Results), their amplitudes were often reduced by the low-pass filter that was needed to diminish background noise. By correcting for the characteristics of the filter, the SCAN program gives improved estimates of the amplitude and duration of brief openings.
Heat stimuli were applied by perfusing bath solution at ∼0.5 ml/min through a plastic tube positioned <100 μm from the cell. The solution was passed through a silver heat exchanger the temperature of which was regulated by a feedback-controlled Peltier element. Heat stimulation was applied either as a pulse produced by switching between heat exchangers held at 37°C and 52°C, respectively, or by ramping the temperature of the heat exchanger from 37° to 52°C over 10 sec with a temperature controller/power supply (Marlow Instruments). The temperature of the stimulating solution, which was monitored by means of a fine thermocouple at the tip of the perfusion tube, increased from 37° to 52°C. With “pulse” stimulation, the new temperature was reached within 2–3 sec. Drugs were applied to the cells through the same perfusion system at 37°C, and antagonists were applied for 2 min before the test stimulation.
The longest diameter of the neurons in some experiments was measured by an eyepiece graticule.
45Ca uptake experiments. The method described by Wood et al. (1988) was used to measure the increase in 45Ca uptake evoked by capsaicin or heat. In brief, DRG neuron cultures (∼300 cells per sample) on glass coverslips were washed for 10 min at 37°C in physiological buffer containing no added Ca2+. They were then transferred for 10 min to buffer containing45Ca (∼1 μCi/ml) that either contained capsaicin at a given concentration or was maintained at raised temperature (up to 52°C). The coverslips were then washed three times (2 min washes) in buffer containing 2 mmCaCl2 and placed in plastic counting vials to which 5 ml scintillant was added for determination of the45Ca activity by liquid scintillation counting. The radioactivity in each sample was expressed relative to the mean radioactivity of cells that had been exposed to45Ca-containing buffer at 37°C. Most experiments were run with quadruplicate samples.
The composition of the Ca-free physiological buffer was (in mm): NaCl 135, KCl 5, glucose 10, HEPES 10, brought to pH 7.4 with NaOH.
Cell viability determination. To determine whether cells were killed by raised temperatures, combined staining with two fluorescent markers was used (Viability/cytotoxicity kit, Molecular Probes, Eugene, OR). Intact cells trap calcein AM, producing green fluorescence, whereas ethidium bromide enters only damaged cells, producing orange fluorescence. Cell cultures were exposed to normal physiological buffer (as above, plus CaCl2 2 mm, MgCl2 2 mm) at various temperatures for 10 min, returned to room temperature for 30 min, then stained for 45 min with calcein AM (1 μm), which gives green fluorescence in intact cells, and ethidium bromide (2 μm), which gives orange fluorescence in damaged cells. The dyes were made up in physiological buffer. After they were washed in physiological buffer, the cells were photographed with a fluorescence microscope (Nikon).
Statistical analyses were performed by the Clinstat software package. Data are expressed as mean ± SEM.
RESULTS
Whole-cell recordings
Capsaicin application was used to distinguish low-threshold heat-sensitive DRG cells (Nagy and Rang, 1999). Capsaicin (2 μm for 1.5 sec at 37°C) evoked inward current in 109 of 355 neurons selected at random (31%). Other distinct populations were (1) insensitive cells that responded to neither capsaicin nor heat (163 of 355, 46%) and (2) high-threshold heat-sensitive cells that were insensitive to capsaicin (83 of 355, 23%). The results presented here were obtained from cells of the low-threshold type. The average amplitude of the inward current evoked by capsaicin, in cells clamped at −60 mV, was 1.39 ± 0.11 nA (n = 109). The mean threshold of the heat-evoked current, measured from the temperature at which the inward current appeared during a heat ramp, was 45 ± 0.2°C (n = 109) (Fig.1A,B), and the maximum amplitude recorded at the end of the standard heat ramp (52°C) was 2.22 ± 0.14 nA (n = 109). Capsaicin-sensitive cells were among the small- and medium-sized neurons, with an average diameter of 22.8 ± 0.5 μm (n = 109). DRG neurons classed as heat-insensitive usually showed a small, linearly increasing inward current in response to a temperature ramp, averaging 0.1 nA at the end of the ramp (Fig.1A), which was very similar to the response seen in all superior cervical ganglion neurons tested. This was taken to be a nonspecific response to heating and was not analyzed further.
Fig. 1.
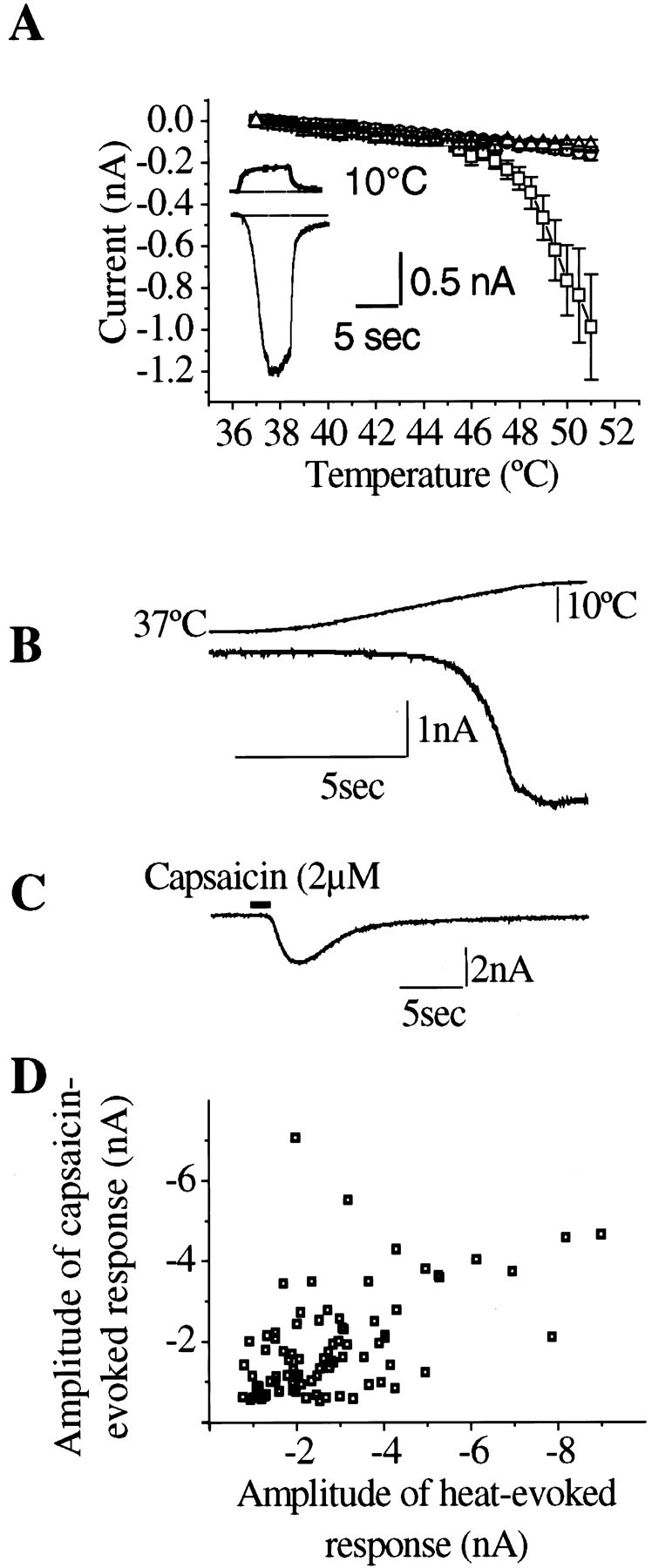
A, Whole-cell voltage-clamp recordings obtained from cultured dorsal root ganglion and superior cervical ganglion neurons reveal a subpopulation of DRG cells that produce inward cationic currents when stimulated by heat in the noxious range. Averaged current temperature plots of noxious heat-sensitive DRG neurons (squares, n = 22), noxious heat-insensitive DRG neurons (triangles,n = 20), and SCG neurons (circles,n = 17). Inset shows recording from a noxious heat-sensitive DRG cell. Top and bottom traces are temperature and current recordings, respectively. Holding potential: −60 mV. B, Heat ramp from 37−52°C (1.75°C/sec) evokes specific heat-activated inward current that increases sharply above 45°C. C, Capsaicin superfusion (2 μm, 2 sec) to the same neuron as B also produces an inward current. D, Relationship between maximum amplitudes of currents evoked by the standard heat ramp stimulation (37−52°C, 1.75°C/sec) and subsequent capsaicin application (2 μm, 2 sec). Correlation coefficient,r2 = 0.53.
Correlation of heat and capsaicin sensitivity
If heat and capsaicin act on the same membrane channels, a close correlation between the amplitudes of the two responses in individual cells would be predicted. Figure 1D shows data for 86 capsaicin-sensitive cells (defined as those giving a response >0.1 nA when tested with 2 μm capsaicin) that were also tested with a standard heat ramp. The correlation between the response amplitudes was weak, although statistically significant (r2 = 0.53; p< 0.001).
Ionic selectivity of channels
For further characterization of the heat- and capsaicin-evoked currents in LT neurons, voltage ramps from +40 to −100 mV were applied before and during the heat- and capsaicin-induced currents. NetI–V curves of the responses were constructed by subtracting the control current from that recorded during the response (Fig. 2A). As reported previously in heat- or capsaicin-sensitive DRG neurons (Cesare and McNaughton, 1996; Oh et al., 1996) and in VR1-transfected human embryonic kidney (HEK) 293 cells (Tominaga et al., 1998), the net currents evoked by capsaicin and by heat show a similar degree of outward rectification, with low slope conductances at potentials negative to −40 mV, and they have similar reversal potentials (Fig.2B,C). TheI–V curves and mean reversal potentials for heat- and capsaicin-activated currents in various ionic solutions are shown in Figure 2 and Table 1. In these experiments, intracellular K+ was replaced by Cs+ to eliminate the large outward K+ current from the controlI–V curve, which makes the determination of the net currents unreliable at positive membrane potentials.
Fig. 2.
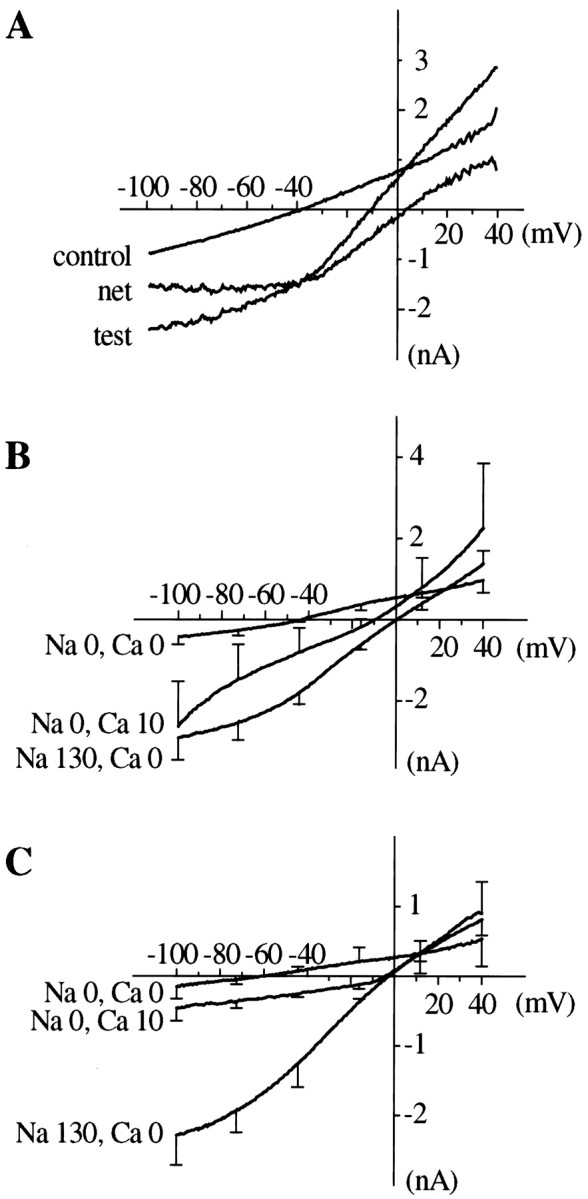
Current–voltage relationship of heat- and capsaicin-evoked currents established by voltage ramps (from +40 to −100 mV, 500 msec). The pipette solution contained Cs+ in place of K+ to eliminate voltage-activated K currents. A, Estimation of the net heat-activated I–V curve by digital subtraction of the control from the test curve. B, Averaged net I–V curves of heat-evoked currents recorded with different ionic composition of the bath solution. C, Averaged netI–V curves of capsaicin-evoked currents recorded with different ionic composition of the bath solution. Error bars show SEM for points at intervals along the curves. Reversal potentials and number of experiments are given in Table 1.
Table 1.
Reversal potentials (mV, mean ± SEM) of heat- and capsaicin-activated currents in various bath solutions
| External cations (mm) | Reversal potential (mV) ± SEM | |||
|---|---|---|---|---|
| Na+ | NMDG+ | Ca2+ | Heat | Capsaicin |
| 130 | 0 | 1.26 | 0.9 ± 1.4 (17) | −0.4 ± 1.1 (8) |
| 130 | 0 | 0 | 2.1 ± 1.4 (13) | −1.7 ± 1.0 (12) |
| 0 | 130 | −54.6 ± 7.8 (3) | −58.4 ± 5.0 (3) | |
| 0 | 130 | 1.26 | −45.2 ± 2.7* (5) | −36.2 ± 2.1* (5) |
| 0 | 130 | 10 | −13 ± 1.4* (4) | −5.0 ± 0.9* (4) |
Internal (pipette) solution contained 130 mmCsCl2. KCl was omitted from external solutions.
*Values for heat and capsaicin differ significantly (p < 0.05). Estimated permeability ratios [based on bi-ionic equations (Hille, 1992, Eq. 1.13 and 13.11)]: for heat: PCs/ PNa/ PNMDG/ PCa= 1.0∶1.08∶0.14∶1.29 (0.95); for capsaicin: PCs/ PNa/ PNMDG/ PCa= 1.0∶0.94∶0.11∶225 (1.94). Estimates of PCa/ PCs shown in brackets were obtained by applying Equation A6 of Lewis (1979), which makes allowance for the estimated permeability of the channels to NMDG.
The reversal potentials of both currents were close to 0 mV when the main extracellular and intracellular cations were Na+ and Cs+, respectively, implying that the permeability of the channel to Na+ and Cs+was similar (Table 1). In the absence of Ca2+, replacement of extracellular Na+ by NMDG reduced the amplitude of the currents, and shifted the reversal potential to approximately −55 mV, with no significant difference between heat- and capsaicin-activated currents, implying that the permeability of the channel to NMDG is similar for both, although much less than the Na+ permeability, as shown by the calculated permeability ratios given in Table 1, which agree well with the values estimated by Cesare and McNaughton (1996). When Na+ was substituted by NMDG, addition of 1.3–10 mm Ca2+ shifted the reversal potential to more positive values, implying a relatively high permeability of the channels to Ca2+. In these solutions, the reversal potential for the capsaicin-activated current was significantly more positive than that of the heat-activated current, showing that the relative Ca2+permeability of the channels is larger in the former case. Exact estimates are difficult, but calculations based on either the simple bi-ionic equation (Hille, 1992, Eq. 1.13 and 13.11), which disregards the contribution of NMDG, or the more complicated Lewis equation (Lewis, 1979, Eq. A6), which takes it into account, show that the Ca2+ permeability associated with capsaicin-activated currents is approximately double that of heat-activated currents, a difference in the same direction as that reported by Tominaga et al. (1998) for responses measured in VR1-transfected HEK293 cells.
Pharmacological properties
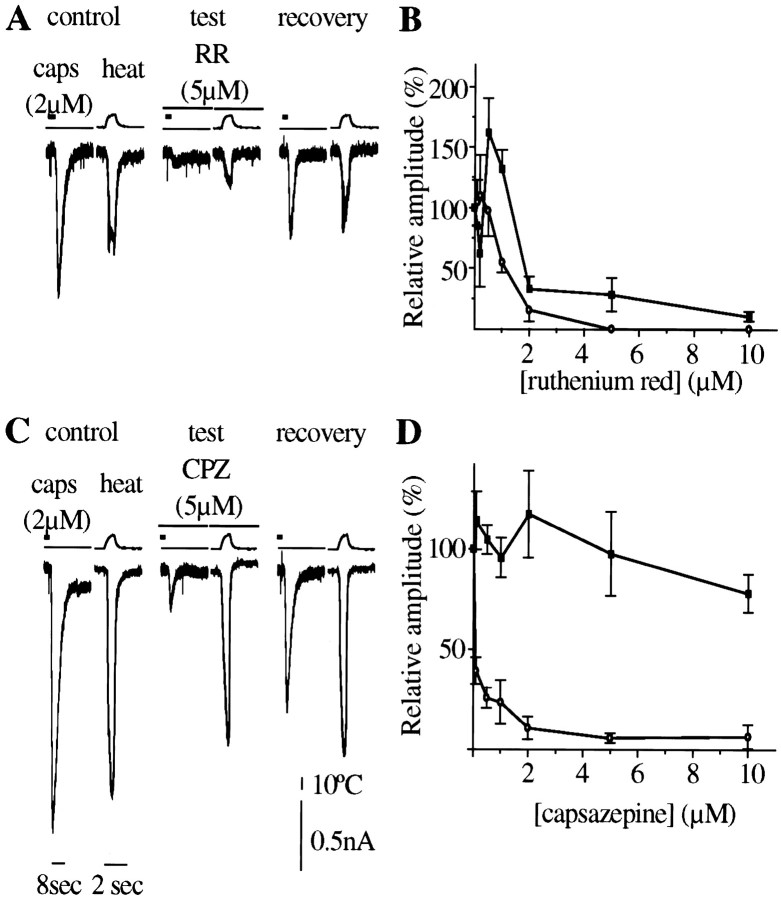
The effect of two known capsaicin antagonists—the competitive antagonist capsazepine (Bevan et al., 1992) and the nonselective channel blocking compound ruthenium red (Dray et al., 1990; Maggi et al., 1993)—on heat-evoked and capsaicin-evoked currents in low-threshold neurons is shown in Figure3.
Fig. 3.
Effect of ruthenium red and capsazepine on capsaicin- and heat-evoked currents. In these experiments both capsaicin-evoked and heat stimulation were brief (2 sec), and 5 mm EGTA was added to the pipette solution to reduce desensitization of subsequent responses. A, Ruthenium red (RR) produces reversible reduction of both capsaicin- and heat-activated currents. B, Dose–response curve of ruthenium red on responses (±SEM) to heat (▪, n = 3–6) and capsaicin (○,n = 3–6) currents. IC50 of RR for both currents is in the same range (1–2 μm), although at low concentrations, RR significantly increased heat-induced current.C, Capsazepine selectively inhibited the capsaicin-evoked currents but had little effect on the heat-induced current. D, Dose–response curve of capsazepine on responses to heat (▪, n = 4–6) and capsaicin (○, n = 4–6).
Ruthenium red inhibited the capsaicin-evoked current with an IC50 close to 1 μm but produced a biphasic effect on the heat-evoked current, increasing it significantly at concentrations below 1 μm and inhibiting it at higher concentrations (Fig. 3C). Capsazepine (Fig.3B,D) strongly inhibited capsaicin-evoked responses (IC50 < 0.5 μm) but was much less active against heat-evoked currents, producing only slight inhibition at 10 μm.
Calcium uptake
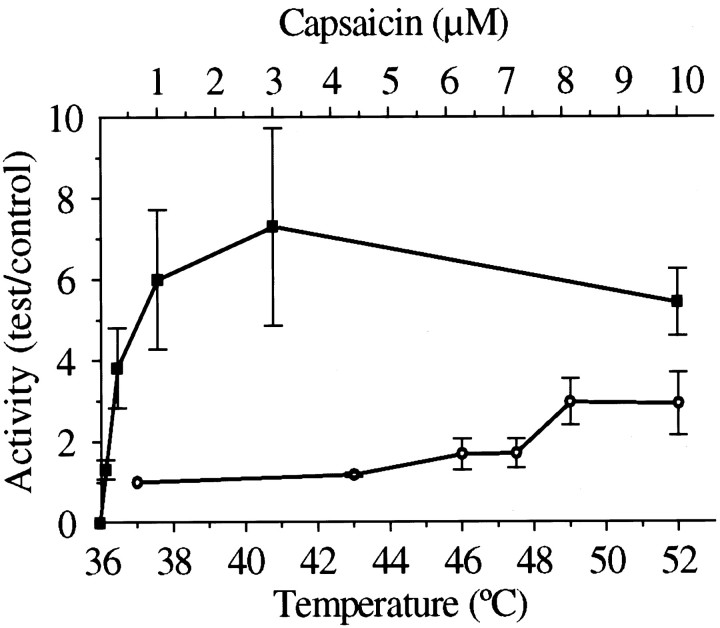
Capsaicin (at 37°C) evoked a graded increase in45Ca uptake, as reported in previous studies (Wood et al., 1988); the maximum effect was an average sevenfold increase over control (Fig. 4). Heating to temperatures above 46°C also produced an increase, maximally threefold at 49°C, a level significantly lower than that achieved by capsaicin.
Fig. 4.
45Ca accumulation, expressed as relative activity (cpmtest/cpmcontrol) ± SEM, by cultured DRG neurons induced by capsaicin (▪, n = 4–6) and heat (○, n = 3–5).
Because of the relatively small response to heat in this assay, the effect of inhibitors was not tested.
Cell viability assay
In electrophysiological studies, brief heating of the cells to 52°C appeared to produce no obvious irreversible effects. We tested for nonspecific cell damage at higher temperatures and longer exposure times, using a fluorescent labeling technique that detects damaged cells by the leakiness of their membranes to hydrophilic dyes (see Materials and Methods). When the cells were exposed to raised temperatures for 10 min in normal physiological buffer and then kept at room temperature for 30 min, all of the neurons appeared viable after a 49°C challenge but were killed at 60°C, graded degrees of damage being evident at intermediate temperatures.
Single-channel recordings
Single-channel activity evoked by heat was observed in 19 of 97 cell-attached patches of DRG cells (Fig.5A,B), and in 25 of 161 excised inside-out patches (Fig.5F). Channel activity was absent at 37°C, and channel events stopped as soon as the temperature was restored to 37°C (Fig. 5A). None of the patches obtained from superior cervical ganglion neurons (n = 37) showed heat-activated channel activity (Fig. 5C). Capsaicin-activated channels were also recorded from 10 of 100 cell-attached patches (capsaicin included in the pipette solution, bath temperature 37°C) and from 18 of 161 inside-out patches. In the majority of the patches (∼70%), both heat and capsaicin induced multiple level of openings at −60 mV membrane potential.
Fig. 5.
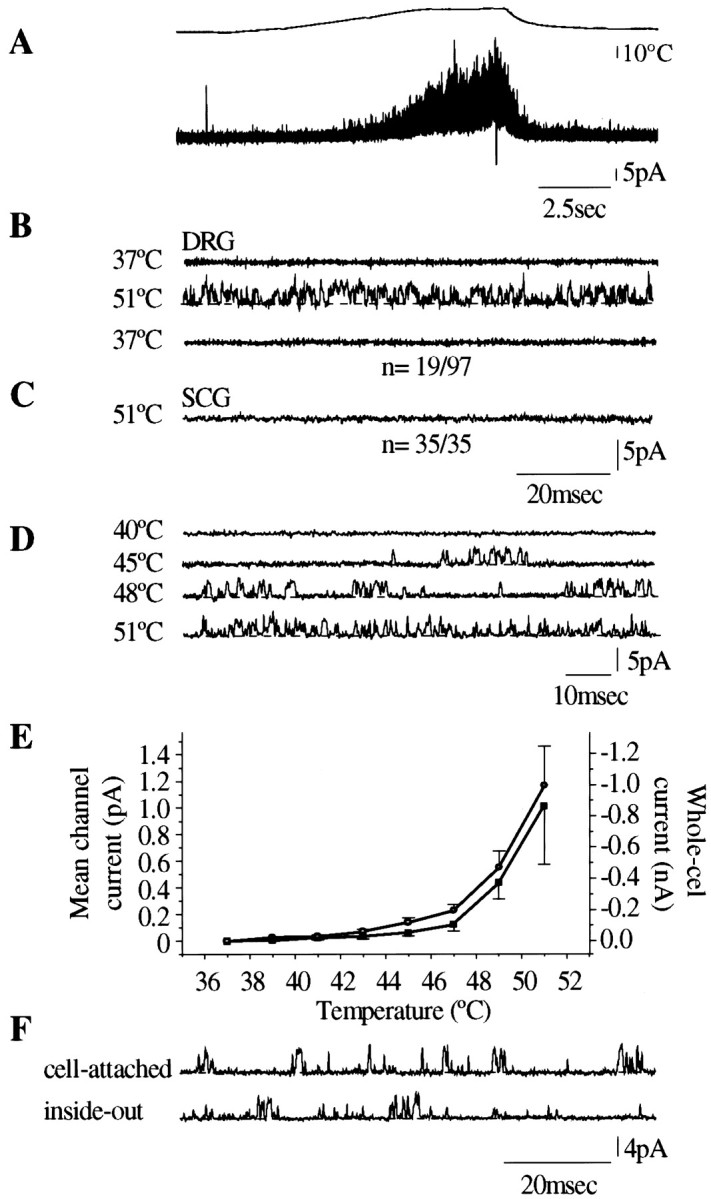
Single-channel recording from membrane patches expressing heat-sensitive ion channels in cell-attached patch and inside-out patch configuration. Bath solution in these experiments contained 130 mm K+, and the pipette solution contained 130 mm Na+. The pipette potential was set to +60 mV. A, Heat ramp from 37°C to 51°C evokes multiple channel activity in cell-attached patch configuration (n = 19 of 97). The activity starts at 44°C and stops when the temperature returns to 37°C.B, Sweeps (100 msec) of a recording from a heat-sensitive membrane patch (cell-attached configuration) showing channel activity (upward deflections) at 51°C. C, SCG neuron membrane patch in cell-attached configuration, showing no heat-activated channel activity. D, Temperature dependence of channel activity in a cell-attached membrane patch stimulated by a slow heat ramp. The dotted line shows shut level. E, Mean single-channel current (▪, mean ± SEM of 8 cell-attached patches) as a function of temperature, compared with the mean whole-cell current (○, mean of 23 cells). F, Heat-induced channel activity at 50°C in the same membrane patch, recorded in cell-attached and inside-out mode.
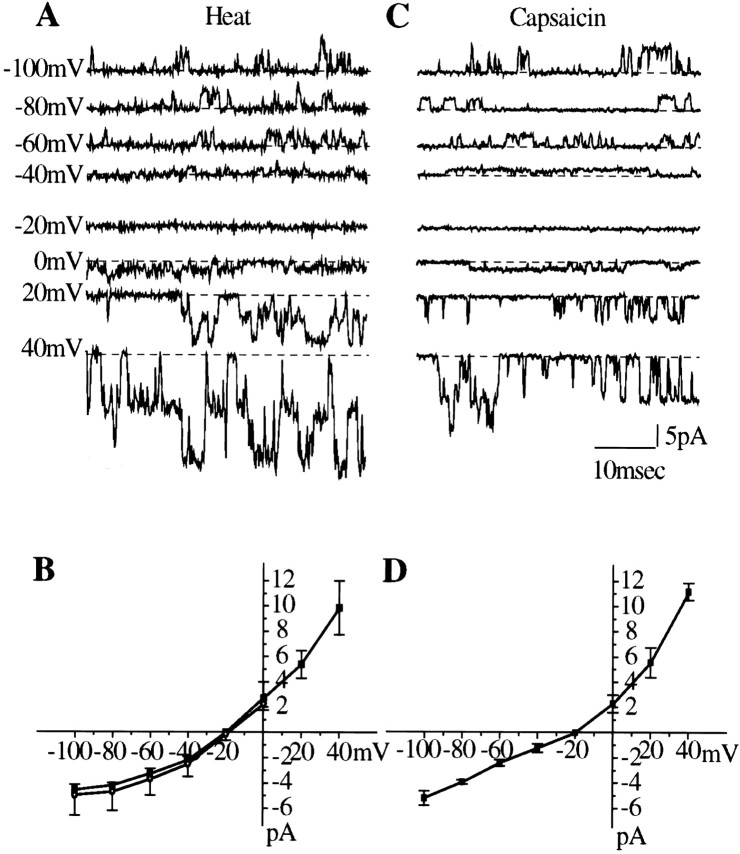
Overall single-channel activity, expressed as the mean channel current, is plotted against temperature in Figure 5E. This curve does not differ significantly from the temperature–current curve obtained in whole-cell recordings. The single-channel current–voltage relationship (Fig.6A,B) was similar to that for whole-cell currents recorded under similar ionic conditions; both curves showed pronounced outward rectification at membrane potentials negative to −40 mV, similar to that of capsaicin-activated channels (Fig. 6C,D). Detailed analysis of single-channel currents by the Scan software package was performed for nine heat-activated (50°C) and 10 capsaicin-activated (1 μm at 37°C) membrane patches in which no multiple openings were observed. Typical amplitude and open and shut time distributions are shown in Figure7. The results are summarized in Table2. This analysis suggested the existence of two distinct amplitude levels for both heat- and capsaicin-activated channels (Fig. 7), the main level being significantly higher for heat (3.4 pA) than for capsaicin (2.8 pA). The low-amplitude channels accounted for an estimated average of 17 and 8% of the openings for heat and capsaicin, respectively, but were not detected in all patches. The open time distributions were well fitted by a single component, the average open time (τopen) of heat-activated channels being significantly shorter (0.41 msec) than that of capsaicin-activated channels (0.69 msec). The shut time distributions, as expected, were more complex and contained more than one component. The distributions were similar for capsaicin and for heat. In both cases, the most numerous events consisted of very brief closures of <1 msec but much longer closures also occurred. Quantitative analysis of the shut time distribution patterns was not performed, because interpretable data would require longer stretches of record and a systematic study with varying temperatures and capsaicin concentrations.
Fig. 6.
Single-channel current–voltage relationship of heat- and capsaicin-gated ion channels studied by applying voltage steps between −100 and +40 mV. Pipette solution contained 130 mm NaCl; bath solution contained 130 mm KCl.A, Recordings of heat-induced single-channel events at different membrane potentials in inside-out patch configuration.B, The single-channel I–Vcurve of heat-activated channels in inside-out patches (▪,n = 3), compared with that for whole-cell currents (open circles, n = 5) recorded with the same internal and external solutions. Data for cell-attached patches (data not shown) were superimposable. The curves show outward rectification and reversal potentials close to −20 mV, different from the values given in Table 1, where Cs+ was the main internal cation. C, Channel activity in an inside-out membrane patch evoked by perfusion of capsaicin (2 μm) to the inside surface at different membrane potentials. D, The I–V curve of capsaicin induced single-channel current (n = 3) showing the same shape and reversal potential as in B. In single-channel recordings, the dotted line indicates shut state.
Fig. 7.
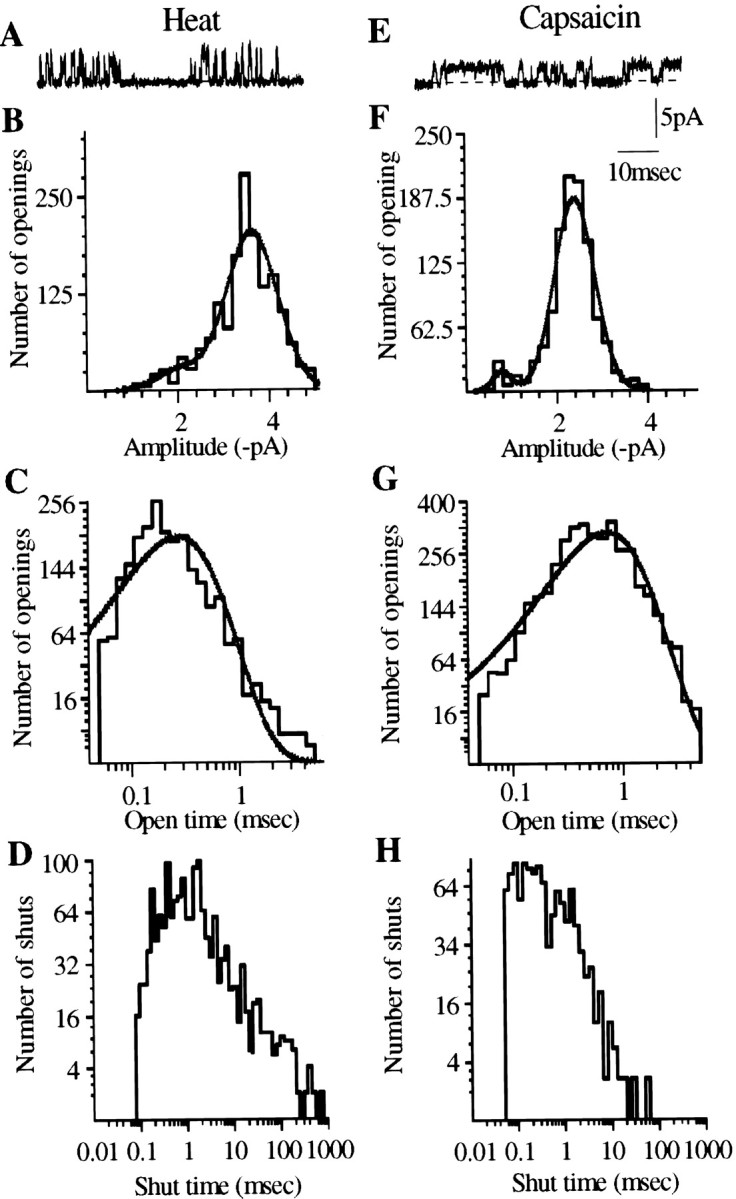
Comparison of the characteristics of single-channel events produced by heat and capsaicin recorded from inside-out patches at −60 mV. Single-channel events were fitted by SCAN and analyzed by EKDIST software. SCAN estimates the amplitudes of single-channel events after correcting for low-pass filtering, and EKDIST plots the frequency distribution of the duration of individual open and closed states. A, E, Single-channel events induced by heat and capsaicin. B,F, Amplitude histogram of channel events, fitted by the maximum likelihood method. Curve fitting reveals a significant number of low-amplitude events, in addition to the main level, for both heat- and capsaicin-evoked activity. Both conductance levels are smaller for capsaicin than for heat (Table 2). C, G, Open time distribution of channel openings induced by heat (C) and capsaicin (G). Both distributions can be fitted by a single component. D,H, Shut time distribution of channel activity produced by heat and capsaicin. In most cases, as here, the fitted distribution indicated the presence of several components, which could not be reliably estimated from the available data. Ordinates of open and shut time distributions are plotted on square root scale, and abscissae are plotted on a logarithmic scale (Colquhoun and Sigworth, 1995).
Table 2.
Characteristics of channels activated by heat (50°C) and capsaicin (1 μm) in inside-out patches
| Stimulus | Amp(1) (pA) | Amp(2) (pA) | % of openings of Amp(1) | % of openings of Amp(2) | Mean open time (msec) |
|---|---|---|---|---|---|
| Heat | 1.8 ± 0.2 | 3.4 ± 0.2 | 16.7 ± 4.5 | 83 ± 4.5 | 0.41 ± 0.04 |
| Capsaicin | 1.2 ± 0.1 | 2.8 ± 0.15 | 8.2 ± 2.1 | 92 ± 2.1 | 0.69 ± 0.06 |
Data show mean ± SEM of records from three patches of each type in which no multiple openings were detected. The pipette potential was +60mV.
Expressing the open-channel characteristics (amplitude and τopen) for heat- and capsaicin-activated channels as ratios gives the following values for inside-out patches: amplitude (heat, 50°C)/amplitude (cap, 37°C) = 1.21; τopen (heat, 50°C)/τopen (cap, 37°C) = 0.59. To test whether these differences in single-channel characteristics could be attributed to the temperature difference rather than to the nature of the activating stimulus, capsaicin-activated channels recorded at 37°C and 50°C were compared in inside-out patches that showed no channel openings in response to heat alone (see below). Two such experiments were analyzed successfully: the ratio amplitude (cap, 50°C)/amplitude (cap,37°C) was 1.57 and 1.39, and the ratio τopen (cap, 50°C)/τopen (cap, 37°C) was 0.81 and 0.53. The difference between capsaicin-activated channels recorded at 37°C and 50°C is similar to the difference between heat- and capsaicin-activated channels in different patches, suggesting that the latter difference could reflect the effect of temperature on the channel properties, without necessarily implying that different channels are activated by the two stimuli.
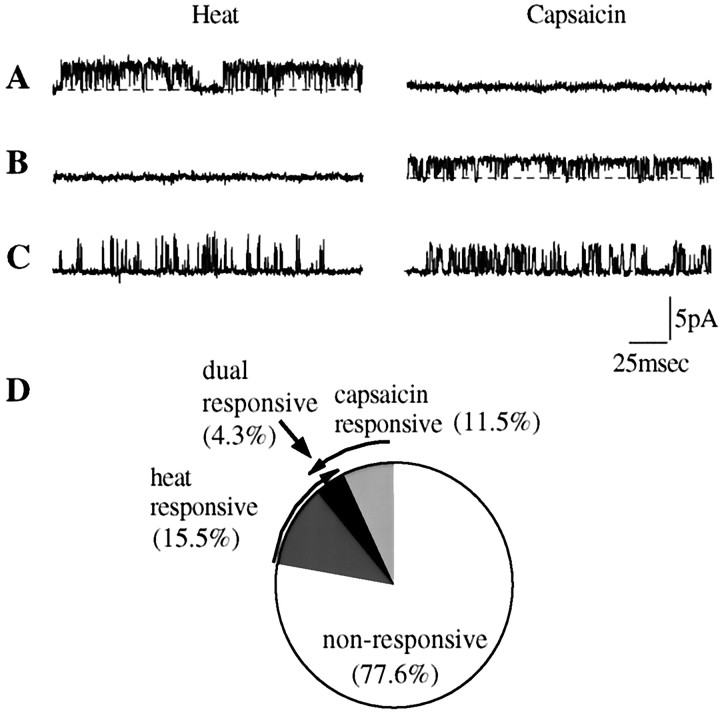
To investigate directly whether heat and capsaicin activate different populations of channels, we performed experiments in which individual inside-out patches were tested successively with both stimuli. These experiments (Fig. 8) showed that of 161 patches, 18 (11%) were sensitive to capsaicin and 25 (15%) were sensitive to heat, but only 7 (4%) responded to both stimuli. Control experiments showed that repeated application of heat or capsaicin in sensitive patches remained effective in opening channels, so the low proportion of dually responding patches could not be explained by desensitization. These data show that distinct channels respond to heat and to capsaicin. The number of dually responding patches (7 of 161) significantly exceeded the number expected by chance occurrence of heat- and capsaicin-sensitive channels in the same patch (χ2 test, 0.01 < p< 0.05), suggesting that some channels exhibit dual sensitivity, but the majority clearly do not.
Fig. 8.
Lack of co-segregation of heat- and capsaicin-sensitivity at the single-channel level. A–C, Records from three inside-out patches that were stimulated consecutively by heat and capsaicin. Patch A responded only to heat, patch B responded only to capsaicin, and patch C responded to both stimuli. Membrane potential was −60 mV. Dotted lines in recordings indicate shut state. D, Pie chart showing percentage of heat- and capsaicin-sensitive membrane patches in a sample of 161 patches.
The characteristics of heat- or capsaicin-evoked channels were similar in patches that responded to only one stimulus and in patches that responded to both (Table 2).
DISCUSSION
The purpose of these experiments was to compare the characteristics of capsaicin- and noxious heat-evoked responses of sensory neurons to throw light on the question of whether both stimuli activate the same ion channels, as suggested recently (Caterina et al., 1997; Tominaga et al., 1998).
Whole-cell electrophysiology
Our results confirm earlier reports (Cesare and McNaughton, 1996;Kirschstein et al., 1997; Reichling and Levine, 1997) that a subset of DRG neurons responds to heat with an increased cation conductance, leading to an inward current at normal resting membrane potential. The characteristics of these currents agree well with those reported byCesare and McNaughton (1996). There are two classes of heat-sensitive DRG neurons: LT cells that also respond to capsaicin and HT cells that are insensitive to capsaicin (Nagy and Rang, 1999). In this study, we have focused on the LT population. The threshold temperature for LT cells was ∼45°C, corresponding to the threshold of cutaneous nociceptors (Campbell and Meyer, 1996). The heat-activated currents in the present study had amplitudes similar to those described byVycklicky et al. (1999) but ∼10 times greater than those reported earlier (Cesare and McNaughton, 1996; Kirschstein et al., 1997;Reichling and Levine, 1997). The reason for this discrepancy is not clear but could be related to differences in experimental technique.Cesare and McNaughton (1996) studied neurons from newborn rats;Kirschstein et al. (1997) used very brief and relatively imprecise heat pulses, whereas Reichling and Levine (1997) recorded responses even at temperatures as low as 30°C, well below the nociceptive threshold.
The reversal potential of the heat-activated current, and its ion selectivity, also agree in general with the results reported by Cesare and McNaughton (1996). Published estimates of the cation permeability of the heat-activated and capsaicin-activated channels of DRG neurons and VR1-transfected HEK cells are summarized in Table3. All give similar values of relative Cs+ and Na+permeability of heat- and capsaicin-activated channels, but the values obtained for Ca2+ permeability vary widely. Our results suggest that PCa/PNa is approximately twofold greater for capsaicin- than for heat-activated channels in DRG neurons, in agreement with results of Caterina et al. (1997) andTominaga et al. (1998) on VR1-transfected HEK cells. Because heat- and capsaicin-evoked responses were necessarily recorded at different temperatures, we cannot exclude the possibility that this could account for the difference in Ca2+ permeability. Alternatively, the nature of the stimulus—chemical or physical—could influence in a subtle way the characteristics of the open channel.
Table 3.
Relative permeabilities (PNa = 1) to Ca2+ and Cs2+ of heat- and capsaicin-activated channels in sensory neurons and in VR1-transfected HEK cells
| Stimulus | Cell | Ion | Reference | |
|---|---|---|---|---|
| Cs2+ | Ca2+ | |||
| Capsaicin | Rat DRG | ∼1 | 0.24 | Oh et al., 1996 |
| Rat DRG | 1.1 | 1.9 | This paper | |
| VR1-transfected HEK cells | 0.85 | 9.6 | Caterina et al., 1997 | |
| Heat | Rat DRG | 1.24 | 1.28 | Cesare and McNaughton, 1996 |
| Rat DRG | 0.9 | 0.9 | This paper | |
| VR1-transfected HEK cells | 3.8 | Tominaga et al., 1998 | ||
We found that capsazepine, a competitive antagonist of capsaicin, strongly antagonized the effect of capsaicin, with an IC50 close to 0.1 μm, but had no effect on heat-evoked currents at concentrations up to 10 μm. This finding agrees with the observation on DRG neurons (Reichling and Levine, 1997), but differs from that of Tominaga et al. (1998) who found that 10 μm capsazepine significantly reduced the heat-induced current in a VR1-expressing cell line. Ruthenium red blocked both responses at similar concentrations, but unexpectedly enhanced the heat response at 0.5 μm, an effect never seen on the capsaicin-induced response. In VR1-expressing non-neuronal cells, ruthenium red reduced both capsaicin- and heat-induced currents (Caterina et al., 1997; Tominaga et al., 1998); however, it did not affect the heat-evoked current in DRG neurons (Reichling and Levine, 1997), but the low-temperature threshold and the Ca2+ dependence of the current studied byReichling and Levine (1997) suggest that those responses are different from those that we have investigated.
Calcium uptake measurements
The temperature-dependent increase in45Ca accumulation by DRG neurons resembles the action of capsaicin and shows a threshold at ∼46°C. The maximal45Ca entry evoked by heat was only approximately one-third of that induced by capsaicin (Fig. 4), and it did not increase with temperature beyond 49°C. The smaller maximal response to heat might have been caused by an adverse effect of high temperature on the ability of intracellular storage mechanisms to sequester calcium. However, the capsaicin-evoked45Ca entry was not affected when the temperature was raised to 49°C. We therefore conclude that the difference in the heat- and capsaicin-evoked maximal45Ca influx could be caused by the relatively lower Ca2+ permeability of the channel during heat activation.
The results of the cell viability tests suggest that DRG neurons retain their integrity well when exposed for 10 min to temperatures up to 52°C, so it is unlikely that nonspecific damage is a major factor in these experiments.
Single-channel recordings
Heat stimulation evoked single-channel activity in one-fifth of the patches taken from DRG cells. The characteristics of this single-channel activity correspond closely with those of the heat-activated currents recorded in the whole-cell configuration. The temperature activation curve is the same shape, with a threshold of 45°C and steep rise at temperatures above this. TheI–V relationship and reversal potential of the single-channel currents also correspond with those of the whole-cell currents, suggesting that the activity of these channels underlies the heat-activated whole-cell current. Channel activity could be recorded from both excised- and cell-attached patches, showing that heat sensitivity is an integral property of the channels and is not secondary to heat-evoked modulation of intracellular signals. This conclusion agrees with the findings of Caterina et al. (1997, 1999) on the heat sensitivity of VR1-transfected cells, but not with the suggestion of Reichling et al. (1997) that heat-activated currents in DRG neurons are secondary to a rise in intracellular calcium concentration.
As with the whole-cell responses, there are strong similarities between the characteristics of heat- and capsaicin-evoked single-channel currents, and our findings on the latter agree well with those of Oh et al. (1996). The I–V curves and reversal potentials for capsaicin and heat agreed closely, although there were significant differences in the mean amplitude and mean open time. Compared with capsaicin-activated channels, heat-activated channels gave currents of larger amplitude and shorter duration. Such differences have not been reported on a VR1-expressing cell line (Tominaga et al., 1998). Studies on the effect of temperature on other types of ion channel [for references, see Chung and Kuyucak (1995)] show that channel conductance often shows Q10values of 1.3–1.5, so the difference in amplitude between capsaicin-activated and heat-activated single-channel events could be explained on this basis. The difference in mean open time could be explained similarly. Where we succeeded in measuring the effect of temperature on the characteristics of capsaicin-activated channels, the results were consistent with this interpretation
The amplitude histograms for both heat- and capsaicin-activated channels show the presence of a small proportion of low-conductance channels, which has not previously been reported. It is difficult to be certain whether these represent distinct channels or a subconductance state of a single type of channel. If there were two distinct types of channel, occasional dual (high plus low) openings would be expected. These were not detected, but typically the total open probability was ∼0.2 (corresponding to ∼0.17 for the large channels and 0.03 for the small channels); the probability of both being open simultaneously would therefore be only ∼0.005, and such events could easily have been missed. Occasional low-high and high-low transitions were detected, as expected if the two levels represent distinct conductance states of the same channels, but a more rigorous analysis would be needed to establish this point. The open-time histograms showed only a single component. Separating the channels into low and high conductance events gave similar mean open times for each, so we were not able to distinguish between them on this basis.
Our most unexpected finding was that most patches responded to either heat or capsaicin, but rarely to both. It was important to be sure that the separation of heat and capsaicin sensitivity was not an artifact attributable to desensitization. Control experiments showed that repetition of either stimulus, although resulting in progressive reduction of the response, never caused complete desensitization. Furthermore, it was frequently found that patches that failed to respond to the first test would respond to the second, after switching from heat to capsaicin, or vice versa. We therefore conclude that distinct channels respond to heat and capsaicin, respectively. The number of dual-sensitive patches somewhat exceeded the number expected by chance. This could reflect the presence of some individual channels that respond to both stimuli or a tendency for channels of both types to aggregate in the same patch of membrane.
The work of Caterina et al. (1997) and Tominaga et al. (1998) shows that transfection of HEK293 cells with the VR1 gene results in the appearance of both heat- and capsaicin-sensitive channels, but it remains to be established whether an individual channel responds to both stimuli. The most economical explanation to reconcile our findings would be that the VR1 gene product may assume different functional states depending on other factors, including splice variants, aggregation with other membrane proteins, the presence of different multimeric species, or the degree of phosphorylation or glycosylation of the channel protein.
The similarities and differences between heat- and capsaicin-evoked responses presented in this paper are summarized in Table4.
Table 4.
Similarities and differences between responses of rat sensory neurons to heat (H) and capsaicin (C)
| Similarities | Differences |
|---|---|
| Co-segregation of C and H responses at whole-cell level | Weak correlation of amplitudes of C and H responses |
| Similar I–V curves (outward rectification) for whole-cell and single-channel currents | |
| Nonselective increase in cation permeability | PCa/PNagreater for C than H responses |
| C and H both elicit Ca uptake | Maximal Ca uptake greater for C than for H |
| C and H both induce single-channel events in excised patches | |
| Similar single-channel characteristics (amplitude, mean open time, substate level) | Segregation of sensitivity to C and H at single-channel level |
Footnotes
Correspondence should be addressed to Istvan Nagy, Department of Anesthetics, Imperial College of Science, Technology and Medicine, School of Medicine, St. Mary's Hospital, London, W2 1NY, UK. E-mail:i.nagy@ic.ac.uk.
REFERENCES
- 1.Bevan SJ, Docherty RJ. Cellular mechanisms of the action of capsaicin. In: Wood JN, editor. Capsaicin in the study of pain. Academic; London: 1993. pp. 27–44. [Google Scholar]
- 2.Bevan SJ, Szolcsanyi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol Sci. 1990;11:330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- 3.Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CSJ, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JN, Meyer RA. Cutaneous nociceptors. In: Belmonte CF, Cervero F, editors. Neurobiology of nociceptors. Oxford UP; Oxford: 1996. pp. 117–145. [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 7.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung S-H, Kuyucak S. Changes in the conductance and kinetics of N-methyl-d-aspartate (NMDA)-receptor activated single channels with temperature. Neurosci Lett. 1995;187:181–184. doi: 10.1016/0304-3940(95)11369-8. [DOI] [PubMed] [Google Scholar]
- 9.Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single channel records. In: Sakmann B, Neher E, editors. Single channel recording. Plenum; New York: 1995. pp. 397–482. [Google Scholar]
- 10.Dray A, Forbes CA, Burgess GM. Ruthenium red blocks the capsaicin-induced increase in intracellular calcium and activation of membrane currents in sensory neurones as well as the activation of peripheral nociceptors in vitro. Neurosci Lett. 1990;110:52–59. doi: 10.1016/0304-3940(90)90786-9. [DOI] [PubMed] [Google Scholar]
- 11.Hammill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 12.Hille B. Ionic channels of excitable membranes. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 13.Kirschstein T, Busselberg D, Treede RD. Coexpression of heat-evoked and capsaicin-evoked inward currents in acutely dissociated rat dorsal root ganglion neurons. Neurosci Lett. 1997;231:33–36. doi: 10.1016/s0304-3940(97)00533-8. [DOI] [PubMed] [Google Scholar]
- 14.Leaney JL, Marsh SJ, Brown DA. A swelling-activated chloride current in rat sympathetic neurones. J Physiol (Lond) 1977;501:555–564. doi: 10.1111/j.1469-7793.1997.555bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis CA. Ion concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol (Lond) 1979;86:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggi CA, Bevan S, Walpole CS, Rang HP, Giuliani S. A comparison of capsazepine and ruthenium red as capsaicin antagonists in the rat isolated urinary bladder and vas deferens. Br J Pharmacol. 1993;108:801–805. doi: 10.1111/j.1476-5381.1993.tb12881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagy I, Rang HP. Noxious heat-activated currents in rat dorsal root ganglion neurons. J Physiol (Lond) 1997;506:153P. [Google Scholar]
- 20.Nagy I, Rang HP. Noxious heat-activated microscopic currents in rat dorsal root ganglion neurones. J Physiol (Lond) 1998;607:29P. [Google Scholar]
- 21.Nagy I, Rang HP. Noxious heat activates all capsaicin-sensitive and also a sub-population of capsaicin-insensitive dorsal root ganglion neurons. Neuroscience. 1999;88:995–997. doi: 10.1016/s0306-4522(98)00535-1. [DOI] [PubMed] [Google Scholar]
- 22.Oh U, Hwang SW, Kim D. Capsaicin activates a non-selective cation channel in cultured rat dorsal root ganglion neurons. J Neurosci. 1996;16:659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichling DB, Levine JD. Heat transduction in rat sensory neurons by calcium-dependent activation of a cation channel. Proc Natl Acad Sci USA. 1997;94:7006–7011. doi: 10.1073/pnas.94.13.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szallasi A, Blumberg PM. Specific binding of resiniferatoxin, an ultrapotent capsaicin analog, by dorsal root ganglion membranes. Brain Res. 1990;524:106–121. doi: 10.1016/0006-8993(90)90498-z. [DOI] [PubMed] [Google Scholar]
- 25.Szolcsanyi J, Anton F, Reeh PW, Handwerker HO. Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Res. 1988;446:262–268. doi: 10.1016/0006-8993(88)90885-2. [DOI] [PubMed] [Google Scholar]
- 26.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 27.Vycklicky L, Vlachova V, Vitaskova Z, Dittert I, Kabat M, Orkand RK. Tempreature coefficient of membrane currents evoked by noxious heat in sensory neurones of the rat. J Physiol (Lond) 1999;517:181–192. doi: 10.1111/j.1469-7793.1999.0181z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood JN, Winter J, James IF, Rang HP, Yeats J, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]